Abstract
Introduction
Antepartum Tdap remains low despite national recommendations. This prospective observational study aims to identify factors associated with lower antepartum Tdap rates.
Methods
Maternal demographics, personal health beliefs, Tdap vaccination status, and recall of in-office obstetric provider actions were collected from a convenience sample of postpartum women in a New York metropolitan hospital. Bivariate and multivariable logistic regression were used to identify significant factors and adjusted odds ratios (OR) for recorded Tdap; OR > 1 reflects elements with increased odds of not receiving antepartum Tdap, while OR < 1 demonstrates increased odds of receipt.
Results
Surveys were collected (n = 1682) from a study population demographically similar to New York City and more diverse in race/ethnicity than the national population. Demographic analysis showed Hispanic women less likely than white, non-Hispanic women to vaccinate (OR 2.44, CI 1.54–3.88). Health beliefs associated with non-receipt of antepartum Tdap included “It is dangerous for pregnant women to get vaccines” (OR 1.68, CI 1.01–2.77), and “I worry about the safety of the Tdap vaccine” (OR 1.59, CI 1.12–2.24). Obstetric provider actions associated with vaccination included receiving an OB recommendation (OR 0.39, CI 0.23–0.65), getting written information about Tdap (OR 0.44, CI 0.30–0.64), and having Tdap offered in office (OR 0.24, CI 0.15–0.37). Health beliefs associated with antepartum Tdap included “I generally do what my OB/GYN provider recommends” (OR 0.49, CI 0.30–0.80), and “Pregnant women should get the Tdap (pertussis) vaccine” (OR 0.17, CI 0.09–0.33).
Discussion
Maternal race/ethnicity, personal health beliefs, and obstetric provider actions predict antepartum Tdap.
Keywords: Tdap, Vaccine, Pregnancy, Pertussis
Significance
National antepartum Tdap rates remain low. While the public’s attention has shifted to coronavirus disease 2019 (COVID-19), the protective value of antepartum Tdap vaccine should not be overlooked. This prospective, cross-sectional study documents that maternal ethnicity and specific health beliefs around pertussis vaccine safety during pregnancy influence antepartum Tdap acceptance. The Health Belief Model is uniquely applied to antepartum Tdap uptake in a diverse population often underrepresented in the literature. We also reinforce the overall importance of obstetric provider recommendation and offering of Tdap vaccine in the office to help promote a pregnant woman’s decision to accept antepartum Tdap.
Introduction
Infants under one year of age have the highest incidence and experience the greatest risk of complications, hospitalization, and death from pertussis. In 2006, the US Advisory Committee on Immunization Practices (ACIP) recommended that postpartum women and household members receive the Tdap vaccine to create a layer of protection around the infant (Kretsinger et al., 2006). Despite these recommendations, pertussis incidence continued to rise, peaking in 2012 with over 40,000 cases and high mortality in infants under 11 months (Wiley et al., 2013; Bisgard et al., 2004; Wendelboe et al., 2007).
Since 2012, the CDC and American College of Obstetricians and Gynecologists have recommended Tdap vaccination for pregnant women between 27 and 36 weeks gestation for transplacental maternal antibody transfer to the fetus and postpartum protection for the pregnant woman (Havers et al., 2020, Skoff et al., 2017, Perrett et al., 2019, Fernandes et al., 2019). Prior to 2012, 9.7% of women were vaccinated during pregnancy (Ahluwalia et al., 2015). Subsequently, antepartum Tdap increased to 27% in 2014 but remained low at only 54.4% in 2018 (Kahn et al., 2018).
Prior studies have shown that rates of vaccination vary by individuals’ beliefs about vaccines, healthcare, provider actions, and demographic factors, specifically race, socioeconomic status, language, and insurance status (Wong et al., 2015, Housey et al., 2014, Goldfarb et al., 2014, Ahluwalia et al., 2014, Wales et al., 2019, Dorivelu et al., 2019, Zhou et al., 2019, O’Leary et al., 2015, Kriss et al., 2019). Many studies point to provider action as the most important predictor of Tdap vaccination uptake (Kriss et al., 2019; Lindley et al., 2019; Lutz et al., 2018; Psarris et al., 2019; Strassburg et al., 2018).
Our prospective observational study aims to increase the understanding of key factors associated with both Tdap acceptance and hesitancy in a majority non-white population that is often underrepresented in the literature. Our hypothesis was that certain maternal health beliefs about vaccines and particular provider actions in the office would be associated with varied rates of antepartum Tdap vaccine uptake in specific sub-populations with greater vaccine hesitancy.
Materials and Methods
Study Population
A survey was completed by a convenience sample of postpartum women, 18 years and older, in a large New York metropolitan birth hospital on weekdays between March and August 2017. The survey gathered patients’ demographic information, personal health beliefs, and recall of obstetric provider actions in the office. All patients’ Tdap vaccination status was abstracted from the electronic medical record (i.e., obstetric profile), which contained information either directly from the obstetricians’ office records or the patients’ hospital records. These data were categorized as received Tdap during this pregnancy, did not receive Tdap during this pregnancy, and unable to assess. A patient was listed as “unable to assess” if the obstetric profile did not include the vaccination status.
Although all eligible postpartum women were approached, some did not receive the survey due to patient’s lack of availability, provider presence in the patient’s room at the time, or language barriers (surveys were only available in English and Spanish). An information sheet that explained the purpose of the study was given to each participant along with the survey; a verbal explanation of the survey and its voluntary and anonymous nature were provided.
Surveys were color-coded based on the obstetric profile documentation of vaccine status in the electronic medical record, which allowed the research team to maintain participant anonymity while retaining obstetric Tdap vaccination status for analysis. The Feinstein Institute for Medical Research at Northwell Health exempted this study from Institutional Review Board review.
Measures
We adapted published questionnaires, not previously validated, on factors associated with Tdap vaccine uptake in developing our survey (Dempsey et al., 2016; O’Leary et al., 2015). The survey included four questions about participants’ perceptions of vaccine safety in general and about their relationship with their OB/GYN provider using a Likert scale (see Table 4). After a brief description of pertussis and Tdap vaccination, the survey included questions about whether participants were offered written materials, a provider recommendation, or a vaccine by their OB/GYN office (see Table 5). Questions based on the Health Belief Model were used to explore factors that may have influenced the participant’s decision regarding the Tdap vaccine (Becker, 1974). These 15 questions (see Table 4) included 4-point Likert scale statements addressing the 6 domains of the Health Belief Model: perceived benefits of vaccination, perceived severity of pertussis, perceived barriers to Tdap vaccination, perceived susceptibility to pertussis, social norms regarding Tdap vaccination, and self-efficacy (Becker, 1974; Dempsey et al., 2016; O’Leary et al., 2015). Our analyses only included individual questions and responses rather than by domain from the Health Belief Model, as provided in Tables 1, 4 and 5, respectively. Demographic information included history of prenatal care, parity, maternal care provider type, insurance type, age, number of individuals living in household, household income, education, and race/ethnicity. These data were collected to explore them as factors for choosing to be vaccinated with Tdap.
Table 4.
Bivariate analysis of maternal health beliefs influencing her decision to receive antepartum Tdap vaccine
| Characteristic | Vaccinated N = 736 n (%) | Not vaccinated N = 835 n (%) | Unadjusted ORa for not vaccinated (95% CI) | p-value* |
|---|---|---|---|---|
| General questions about participants’ perceptions of vaccine safety | ||||
| I worry about the safety of vaccine IN GENERAL | < 0.0001 | |||
| Disagree | 403 (54.9%) | 331 (45.1%) | ||
| Agree | 318 (39.5%) | 487 (60.5%) | 1.86 (1.52–2.28) | |
| It is dangerous for pregnant women to get vaccines | < 0.0001 | |||
| Disagree | 632 (50.9%) | 609 (49.1%) | – | |
| Agree | 89 (30.1%) | 207 (69.9%) | 2.41 (1.84–3.17) | |
| I feel confident asking my OB/GYN provider about vaccines | 0.0008 | |||
| Disagree | 62 (35.0%) | 115 (65.0%) | – | |
| Agree | 665 (48.5%) | 705 (51.5%) | 0.57 (0.41–0.79) | |
| I generally do what my OB/GYN provider recommends | < 0.0001 | |||
| Disagree | 71 (28.5%) | 178 (71.5%) | – | |
| Agree | 653 (50.4%) | 664 (49.6%) | 0.39 (0.9–0.53) | |
| Questions based on health belief model | ||||
| I worry that I could give pertussis to my baby | < 0.0001 | |||
| Disagree | 212 (39.3%) | 327 (60.7%) | – | |
| Agree | 488 (52.2%) | 446 (47.8%) | 0.59 (0.48–0.74) | |
| Pregnant women should be concerned about the possibility of pertussis in their babies | 0.0002 | |||
| Disagree | 45 (32.6%) | 93 (67.4%) | ||
| Agree | 658 (49.3%) | 676 (50.7%) | 0.50 (0.34–0.72) | |
| The Tdap (pertussis) vaccine is a good way to protect the health of newborn babies | < 0.0001 | |||
| Disagree | 21 (18.4%) | 93 (81.6%) | ||
| Agree | 685 (50.5%) | 671 (49.5%) | 0.22 (0.14–0.36) | |
| My family would probably think getting a Tdap (pertussis) vaccine is a good idea | < 0.0001 | |||
| Disagree | 48 (23.6%) | 155 (76.4%) | – | |
| Agree | 653 (51.3%) | 619 (48.7%) | 0.29 (0.21- 0.41) | |
| I felt that I had enough information about the Tdap (pertussis) vaccine to decide about receiving it | < 0.0001 | |||
| Disagree | 55 (18.6%) | 240 (81.4%) | – | |
| Agree | 649 (54.9%) | 533 (45.1%) | 0.19 (0.14–0.26) | |
| Pregnant women should get the Tdap (pertussis) vaccine | < 0.0001 | |||
| Disagree | 36 (15.2%) | 201 (84.8%) | – | |
| Agree | 664 (54.2%) | 560 (45.8%) | 0.15 (0.10–0.22) | |
| Getting myself vaccinated with the Tdap (pertussis) vaccine will help keep my baby from getting pertussis | < 0.0001 | |||
| Disagree | 27 (15.2%) | 150 (84.8%) | – | |
| Agree | 677 (52.7%) | 607 (47.3%) | 0.16 (0.11–0.25) | |
| I worry about the safety of the Tdap (pertussis) vaccine | < 0.0001 | |||
| Disagree | 424 (47.1%) | 319 (42.9%) | – | |
| Agree | 279 (38.6%) | 444 (61.4%) | 2.12 (1.72–2.61) | |
| I worry that getting the Tdap (pertussis) vaccine will not protect my baby from getting pertussis | < 0.0001 | |||
| Disagree | 528 (53.3%) | 463 (46.7%) | – | |
| Agree | 173 (37.5%) | 288 (62.5%) | 1.90 (1.51–2.38) | |
| It would be really bad if my baby got pertussis | 0.24 | |||
| Disagree | 40 (41.7%) | 56 (58.3%) | – | |
| Agree | 650 (47.8%) | 709 (52.2%) | 0.78 (0.51–1.18) | |
| My friends would probably think getting a Tdap (pertussis) vaccine is a good idea | < 0.0001 | |||
| Disagree | 66 (31.3%) | 145 (68.7%) | - | |
| Agree | 631 (50.6%) | 615 (49.4%) | 0.44 (0.32–0.61) | |
| It would be really bad if I got pertussis DURING my pregnancy | 0.37 | |||
| Disagree | 93 (44.7%) | 115 (55.3%) | – | |
| Agree | 605 (48.1%) | 653 (51.9%) | 0.87 (0.65–1.17) | |
| Getting myself vaccinated with the Tdap (pertussis) vaccine will help keep me from getting pertussis | < 0.0001 | |||
| Disagree | 28 (20.0%) | 112 (80.0%) | – | |
| Agree | 664 (50.5%) | 650 (49.5%) | 0.24 (0.16–0.38) | |
| I worry that someone besides me will give my baby pertussis | 0.92 | |||
| Disagree | 186 (47.6%) | 205 (52.4%) | – | |
| Agree | 512 (47.8%) | 558 (52.2%) | 0.99 (0.78–1.25) | |
| It would be really bad if I got pertussis soon AFTER my pregnancy | 0.09 | |||
| Disagree | 59 (40.7%) | 86 (59.3%) | – | |
| Agree | 637 (48.2%) | 684 (51.8%) | 0.74 (0.52–1.04) | |
Dashed boxes are referent values
*p-value is based on Wald Chi-square test from logistic regression
aUnadjusted odds ratio (OR) > 1 reflects elements associated with increased odds of not receiving antepartum Tdap, while OR < 1 demonstrates decreased odds of non-receipt (i.e., more likely to receive antepartum Tdap vaccine)
Table 5.
Bivariate analysis of maternal recall of obstetric provider actions and receipt of antepartum Tdap vaccine
| Characteristic | Vaccinated N = 736 n (%) | Not vaccinated N = 835 n (%) | Unadjusted OR* for not vaccinated (95% CI) | p-value** |
|---|---|---|---|---|
| Did anyone at your OB/GYN office recommend you receive the Tdap vaccine DURING this most recent pregnancy? | < 0.0001 | |||
| No | 66 (13.6%) | 420 (86.4%) | – | |
| Yes | 662 (62.5%) | 397 (37.5%) | 0.09 (0.07–0.13) | |
| Did your OB/GYN offer to give you the vaccine in their office? | < 0.0001 | |||
| No | 120 (19.0%) | 510 (81.0%) | – | |
| Yes | 610 (67.3%) | 296 (32.7%) | 0.11 (0.09–0.15) | |
| Were you referred to a different location (e.g., Pharmacy, Health Clinic, etc.) to receive the Tdap vaccine? | 0.14 | |||
| No | 626 (47.9%) | 682 (52.1%) | – | |
| Yes | 98 (42.6%) | 132 (57.4%) | 1.24 (0.93–1.64) | |
| Did you receive any written materials in your OB/GYN office describing the importance of the Tdap vaccine during your pregnancy? | < 0.0001 | |||
| No | 183 (25.4%) | 536 (74.6%) | – | |
| Yes | 542 (66.2%) | 77 (33.8%) | 0.17 (0.14–0.22) | |
Dashed boxes are referent values
*Unadjusted odds ratio (OR) > 1 reflects elements associated with increased odds of not receiving antepartum Tdap, while OR < 1 demonstrates decreased odds of non-receipt (i.e., more likely to receive antepartum Tdap vaccine)
**p-value is based on Wald Chi-square test from logistic regression
Table 1.
Demographic characteristics of study participants. N = 1,571
| Characteristic | N (%) |
|---|---|
| Race/Ethnicity | |
| White non-Hispanic | 544 (36.4%) |
| Black non-Hispanic | 274 (18.3%) |
| Asian non-Hispanic | 265 (17.7%) |
| Hispanic | 282 (18.9%) |
| All other non-Hispanic | 131 (8.8%) |
| Age group (years) | |
| 18–24 | 159 (10.7%) |
| 25–34 | 886 (59.8%) |
| ≥ 35 | 437 (29.5%) |
| Received prenatal care during first trimester | |
| Yes | 1430 (93.5%) |
| No | 100 (6.5%) |
| Primiparous | |
| Yes | 622 (40.5%) |
| No | 913 (59.5%) |
| Educational level | |
| High School diploma or equivalent or less | 190 (12.5%) |
| Associate or bachelor degree | 868 (57.2%) |
| More than bachelor degree | 459 (30.3%) |
| Health insurance type | |
| Any private | 1020 (69.1%) |
| Public, no private | 456 (30.9%) |
| Household income | |
| < $25,000 | 122 (10.3%) |
| $25,000-$49,999 | 212 (17.8%) |
| $50,000-$74,999 | 180 (15.2%) |
| $75,000-$149,999 | 401 (33.8%) |
| $150,000 or more | 273 (23.0%) |
| Survey language | |
| English | 1557 (99.1%) |
| Spanish | 14 (0.9%) |
Statistical Analysis
We calculated that 1700 surveys would provide at least 80% power to detect effect sizes (ES) for continuous predictors (differences between vaccinated and unvaccinated participants divided by the standard deviation of this difference) ≥ 0.10 for vaccination rates ≥ 40%. If the proportion of participants who were vaccinated was less (e.g., 30% or 35%), then this sample size would provide at least 80% power for ES ≥ 0.25. For dichotomous outcomes, this sample size provides at least 95% power to detect differences between vaccinated and unvaccinated subjects ≥ 10% (EAST v6, Cytel Inc.).
To assess generalizability, demographics of the study population were compared with the hospital’s overall maternal population during the enrollment period, as well as New York City (NYC) and national maternal populations using descriptive statistics.
Bivariate logistic models were used to explore the relationships between individual survey questions (maternal demographic characteristics, four general questions, 15 questions associated with the Health Belief Model, and four questions on provider actions) and antepartum receipt of the Tdap vaccine according to the obstetric profile. Individual questions and responses are provided in Tables 1, 4 and 5, respectively. A multivariable model was developed, starting with statistically significant (p < 0.10) factors from the bivariate analyses. Factors were eliminated until all were statistically significant at the p < 0.05 level. Race/ethnicity and insurance were included in the final model even if they were not statistically significant because of their theoretical importance in explaining Tdap vaccination receipt. Since our primary objective was to identify factors associated with non-receipt of antepartum Tdap vaccination, adjusted odds ratios (OR) > 1 reflect elements associated with increased odds of not receiving antepartum Tdap, while OR < 1 demonstrate decreased odds of non-receipt (i.e., more likely to receive antepartum Tdap vaccine). Responses to certain demographic characteristic questions were collapsed for analysis to aid in interpretability. Survey language and household size were not included in the analysis due to small numbers and ambiguous survey wording. Respondents with “unable to assess” vaccination status in the Electronic Medical Record (EMR) were excluded in the analysis. Any missing data for certain questions were excluded from analysis. To assess the impact of missing or undocumented Tdap vaccination status, we compared demographics between relevant groups with and without data using two-sample t and Chi-square tests (SAS 9.4, Carey, NC). Preliminary results were presented at the 2018 Pediatric Academic Societies meetings in Toronto. Additional analyses were conducted for drafting the manuscript over the ensuing years with subsequent delay due to the COVID-19 pandemic.
Results
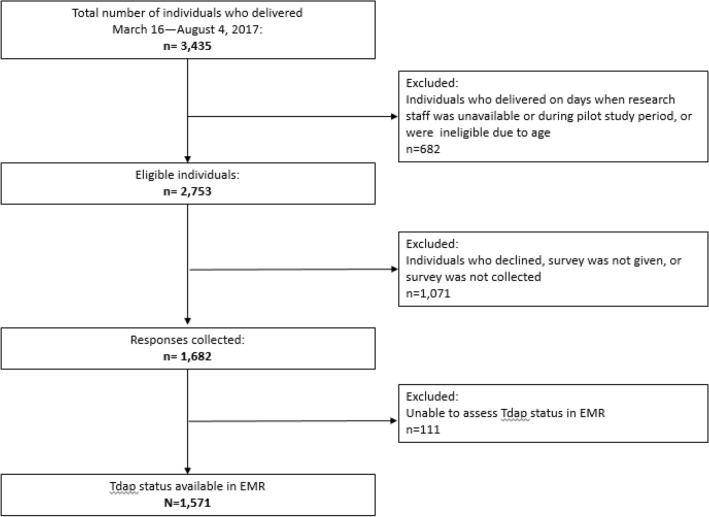
Figure 1 describes the 3435 patients who delivered during the study period (March 16–August 4, 2017); 682 were excluded because they delivered on days research staff were unavailable, they had been part of the pilot study, or they were of a young age. Among the remaining eligible patients (n = 2753; 80.1%), 1071 were not included for the following reasons: they declined study participation, they were not able to be given the survey, or their surveys were not returned. Of the remaining 1682 surveys collected (61.1% overall response rate), Tdap status could not be assessed in the electronic medical record for 111 patients, leaving 1571 for complete analysis.
Fig. 1.
Flow diagram of the study population. Breakdown of study population showing the number of participants, eligible population, and a description of the exclusion criteria. The number of participants is further divided according to the assessment of their EMR obstetric profile
The study group is a diverse population demographically (Table 1), similar to the overall maternity population in the hospital during our data collection period and that of New York City, but more diverse than the national population in regard to race/ethnicity. The percentage of women for whom this was their first pregnancy was also similar to NYC and nationwide statistics. The age and insurance type of the patients in this study population were similar to the hospital population, but differed from the NYC and national populations as a higher percentage had older age, private insurance, higher education, and higher income (data not shown).
The obstetric profile in the electronic medical record revealed 43.8% (n = 736) of respondents received the Tdap vaccination during their current pregnancy, 49.6% (n = 835) did not, and the Tdap vaccination status of 6.6% (n = 111) was unable to be assessed. There were no statistically significant demographic characteristics of the study population compared with the “unable to assess” group which was excluded from the analysis population.
While type of health insurance, self-identified race/ethnicity, total yearly household income, and patient age were significant correlates of vaccination status (p < 0.05) in the bivariate model (Table 2), only self-identified race/ethnicity remained significant in the multivariable model (Fig. 2 and Table 3). Hispanic patients were less likely to receive the vaccine compared with the non-Hispanic groups. When compared with white, non-Hispanic respondents, self-identified Hispanic mothers had more than two times the odds of not being vaccinated.
Table 2.
Bivariate analysis of maternal demographics and receipt of antepartum Tdap vaccine
| Characteristic | Vaccinated N = 736 n (%) | Not vaccinated N = 835 n (%) | Unadjusted ORa for not vaccinated (95% CI) | p-value* |
|---|---|---|---|---|
| Race/Ethnicity | 0.0003 | |||
| White non-Hispanic | 266 (48.9%) | 278 (51.1%) | – | |
| Black non-Hispanic | 118 (43.1%) | 156 (56.9%) | 1.26 (0.94–1.69) | |
| Asian non-Hispanic | 152 (57.4%) | 113 (42.6%) | 0.71 (0.53–0.96) | |
| Hispanic | 112 (39.7%) | 170 (60.3%) | 1.45 (1.08–1.94) | |
| All other non-Hispanic | 55 (42.0%) | 76 (58.0%) | 1.32 (0.90–1.94) | |
| Age group (years) | 0.0077 | |||
| 18–24 | 58 (36.5%) | 101 (63.5%) | – | |
| 25–34 | 441 (49.8%) | 445 (50.2%) | 0.58 (0.41–0.82) | |
| ≥ 35 | 202 (46.2%) | 235 (53.8%) | 0.67 (0.46–0.97) | |
| Received prenatal care during first trimester | 0.14 | |||
| Yes | 681 (47.6%) | 749 (52.4%) | 0.73 (0.48–1.11) | |
| No | 40 (40.0%) | 60 (60.0%) | – | |
| Primiparous | 0.12 | |||
| Yes | 308 (49.5%) | 314 (50.5%) | 0.85 (0.69–1.04) | |
| No | 415 (45.4%) | 498 (54.6%) | – | |
| Educational level | 0.02 | |||
| High School diploma or equivalent or less | 93 (49.0%) | 97 (51.0%) | – | |
| Associate or bachelor degree | 382 (44.0%) | 486 (56.0%) | 1.22 (0.89–1.67) | |
| More than bachelor degree | 239 (52.1%) | 220 (47.9%) | 0.88 (0.63–1.24) | |
| Health insurance type | < 0.0001 | |||
| Any private | 57 (50.7%) | 503 (49.3%) | – | |
| Public, no private | 177 (38.8%) | 279 (61.2%) | 1.62 (1.29–2.03) | |
| Household income | < 0.0001 | |||
| < $25,000 | 42 (34.4%) | 80 (65.6%) | – | |
| $25,000-$49,999 | 83 (39.2%) | 129 (60.8%) | 0.82 (0.51–1.30) | |
| $50,000-$74,999 | 72 (40.0%) | 108 (60.0%) | 0.79 (0.49–1.27) | |
| $75,000-$149,999 | 209 (52.1%) | 192 (47.9%) | 0.48 (0.32–0.74) | |
| $150,000 or more | 151 (55.3%) | 122 (44.7%) | 0.42 (0.27–0.66) | |
| Survey language | 0.41 | |||
| English | 731 (47.0%) | 826 (53.0%) | – | |
| Spanish | 5 (35.7%) | 9 (64.3%) | 1.59 (0.53–4.78) | |
*p-value is based on Wald Chi-square test from logistic regression
aUnadjusted odds ratio (OR) > 1 reflects elements associated with increased odds of not receiving antepartum Tdap, while OR < 1 demonstrates decreased odds of non-receipt (i.e., more likely to receive antepartum Tdap vaccine)
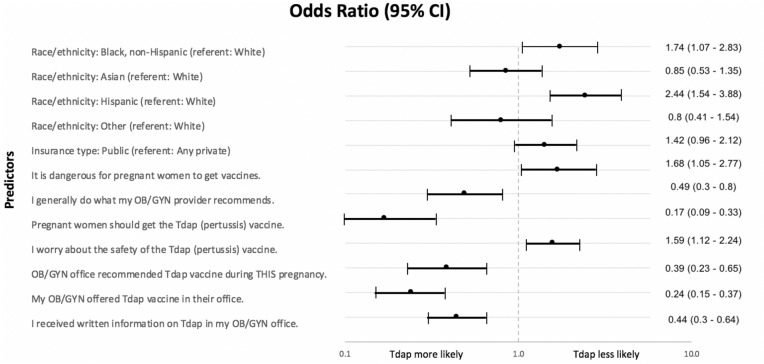
Fig. 2.
Forest plot of multivariable odds ratios for being vaccinated.*Odds ratios of factors significantly associated with receipt/non-receipt of antepartum Tdap vaccination as listed in the multivariable analysis (Table 3)*Adjusted odds ratio (OR) > 1 reflects elements associated with increased odds of not receiving antepartum Tdap, while OR < 1 demonstrates decreased odds of non-receipt (i.e., more likely to receive antepartum Tdap vaccine).
Table 3.
Multivariable analysis of maternal predictors of not receiving antepartum Tdap vaccination
| Characteristic | Adjusted ORa for not being vaccinated (95% CI) | p-value* |
|---|---|---|
| Race/Ethnicity | 0.0001 | |
| White non-Hispanic | – | |
| Black non-Hispanic | 1.74 (1.07–2.83) | |
| Asian non-Hispanic | 0.85 (0.53–1.35) | |
| Hispanic | 2.44 (1.54–3.88) | |
| All other non-Hispanic | 0.80 (0.41–1.54) | |
| Health insurance type | 0.08 | |
| Any private | – | |
| Public, no private | 1.42 (0.96–2.12) | |
| Did anyone at your OB/GYN office recommend you receive the Tdap vaccine DURING this most recent pregnancy? | 0.0003 | |
| No | – | |
| Yes | 0.39 (0.23–0.65) | |
| Did your OB/GYN offer to give you the vaccine in their office? | < 0.0001 | |
| No | – | |
| Yes | 0.24 (0.15–0.37) | |
| Did you receive any written materials in your OB/GYN office describing the importance of the Tdap vaccine during your pregnancy? | < 0.0001 | |
| No | – | |
| Yes | 0.44 (0.30–0.64) | |
| It is dangerous for pregnant women to get vaccines | 0.045 | |
| Disagree | – | |
| Agree | 1.68 (1.01–2.77) | |
| I generally do what my OB/GYN provider recommends | 0.005 | |
| Disagree | – | |
| Agree | 0.49 (0.30–0.80) | |
| Pregnant women should get the Tdap (pertussis) vaccine | < 0.0001 | |
| Disagree | – | |
| Agree | 0.17 (0.09–0.33) | |
| I worry about the safety of the Tdap (pertussis) vaccine | 0.009 | |
| Disagree | – | |
| Agree | 1.59 (1.12–2.24) | |
Dashed boxes are referent values
*p-value is based on Wald Chi-square test from logistic regression
aAdjusted odds ratio (OR) > 1 reflects elements associated with increased odds of not receiving antepartum Tdap, while OR < 1 demonstrates decreased odds of non-receipt (i.e., more likely to receive antepartum Tdap vaccine)
In the bivariate model, many of the health beliefs were significantly associated with whether or not a patient received the Tdap vaccine (Table 4), but only a few remained significant (p < 0.05) in the multivariable model (Fig. 2 and Table 3). While controlling for all covariates in the model, there were four health beliefs that remained significantly associated with vaccination status. If a participant agreed that “it is dangerous for pregnant women to get vaccines” or agreed that they “worry about the safety of the Tdap (pertussis) vaccine,” they had approximately 50 percent higher odds of not being vaccinated. Participants who “generally do what my OB/GYN provider recommends” were twice as likely to be vaccinated, while participants who agreed that “Pregnant women should get the Tdap (pertussis) vaccine” were almost 6 times more likely to be vaccinated compared with those who disagreed with this statement.
In the multivariable model, there were several factors associated with particular office-based obstetric provider actions that remained statistically significant while controlling for the remaining factors. Recall of their provider recommending the Tdap vaccine, receiving written materials on its importance from the OB/GYN office, and offering to give it in the office were significant in both the bivariate (Table 5) and multivariable models (Fig. 2 and Table 3). While controlling for all other covariates, participants whose OB recommended the vaccine had 2.5 times the odds of being vaccinated compared with those who did not receive a recommendation. If the OB offered to give the vaccine in the office, participants had four times the odds of being vaccinated compared with those who were not offered the vaccine in the office. Participants who recalled receiving written Tdap information had twice the odds of being vaccinated compared with those who did not recall receiving written information.
Discussion
This study identified potential barriers to receiving antepartum Tdap vaccine and predictors that increased compliance with Centers for Disease Control and Prevention (CDC) and American College of Obstetricians and Gynecologists (ACOG) guidelines for antepartum Tdap administration (Havers et al., 2020). In the multivariable analysis, the only demographic information that remained statistically significant was race/ethnicity. Since Hispanic participants and non-Hispanic Black participants had significantly lower rates of Tdap vaccination, further research should be conducted to identify any obstacles, perhaps language or cultural, that can be addressed to reach these vulnerable groups.
Several health beliefs were statistically significant in increasing antepartum Tdap receipt, including women doing what their OB/GYN providers recommend and the belief that women should get Tdap vaccine during pregnancy. Participants who worry about the safety of the vaccine and who believe it is dangerous for pregnant women to get vaccines were significantly less likely to accept antepartum Tdap. In addition to addressing the importance of antepartum Tdap vaccination with pregnant women, physicians should also address the safety of all vaccines, especially with women who express reluctance to receive Tdap. This includes pediatricians who can reinforce the importance of antepartum Tdap to the many pregnant women often seen in their offices. Since the information given to patients on maternal vaccination is often affected by personal perceptions, it is essential to educate all healthcare professionals on current guidelines; antenatal care providers must make an additional effort to keep in line with current guidelines (Lumbreras Areta et al., 2022).
Our results emphasize the importance of OB influence on a pregnant woman’s decision to accept antepartum Tdap. Women were more likely to have received the Tdap vaccine antepartum if their provider recommended it, they received written information about it, and the vaccine was available in the office. Prior studies have shown similar results with both influenza and Tdap vaccines during pregnancy (Wong et al., 2015, Housey et al., 2014, Goldfarb et al., 2014, Ahluwalia et al., 2014, Wales et al., 2019, Dorivelu et al., 2019, Zhou et al., 2019, O’Leary et al., 2015, Kriss et al., 2019, Strassburg et al., 2018, Psarris et al., 2019, Lutz et al., 2018, Kriss et al., 2019, Lindley et al., 2019).
There are several strengths to this study, particularly the large sample size and diverse population because of their theoretical importance in health seeking behaviors. In addition, our survey was adapted from the Health Belief Model; while elements of the Health Belief Model had been used to ask about antepartum vaccinations previously (Becker, 1974; Dempsey et al., 2016; O’Leary et al., 2015), the novelty of this study is further enhanced as it is one of the first to apply the Health Belief Model specifically to Tdap. The analysis uses vaccination status from the medical record, which some consider more accurate than patient self-report, although a high level of concordance between the electronic medical record and self-report has recently been shown (Song et al., 2021). Our mixed methods approach of utilizing survey responses to complement vaccination data collected from the EMR offered a unique opportunity to develop a comprehensive picture of the antepartum Tdap landscape.
Our study also has limitations. Although researchers knew a patient’s vaccination status from the obstetric profile in the electronic medical record before administering the survey, an analysis of potential bias done on a portion of the study population (i.e., 40%) shows there was no significant respondent bias in which patients were given the survey. There was a significant difference in vaccination status between the population that took the survey and those who either declined to take or did not complete it. However, respondent bias would theoretically suggest the percentage of patients who received Tdap in this sample is likely higher than that of the general population in the hospital. Thus, patients who received Tdap are likely overrepresented in the sample, which we believe results in an underestimation of effects from the bivariate and multivariable analyses. For example, individuals who view vaccination favorably might have been more likely to be vaccinated and willing to take the survey.
Another potential limitation is that participants who spoke languages other than English and Spanish were excluded. Social desirability bias, which may have had an effect on how patients responded, is an additional limitation. Lastly, the survey relies on the self-report of participant interactions with their OB/GYN, but not from the obstetric providers’ recall or as documented in the medical record. Previous studies have shown that while self-report on vaccine uptake is reliable, it is not perfect, so it is reasonable to conclude that some participants may not fully remember this interaction with their OB/GYN as it occurred (Song et al., 2021; LeardMann et al., 2007).
Given the suboptimal Tdap vaccine uptake and the importance of antepartum Tdap vaccination in protecting women during pregnancy and in the postpartum period, as well as protection for their neonate, it is vital to understand how best to improve antepartum Tdap vaccination rates. While the public’s attention has shifted to COVID-19 (Paguio et al., 2020), the protective value of the Tdap vaccine should not be overlooked. There is a gap in research looking at the impact of COVID-19 on antepartum Tdap rates; a survey conducted by the CDC found that only 55% of the women surveyed had received antepartum Tdap pre-pandemic in 2018 (Lindley et al., 2019). With the notable impact of COVID-19 on childhood vaccinations it is more important than ever to ensure that all pregnant women are receiving the antepartum Tdap vaccine with each pregnancy so that infants are protected from pertussis infection (Bramer et al., 2020; Rowe, et al., 2021; Santoli et al., 2020).
Our study of a diverse population documents that participants’ race/ethnicity, personal health beliefs, and obstetric provider actions predict antepartum Tdap vaccination. Obstetricians should recommend Tdap during each pregnancy, administer it on-site, and provide written information about Tdap vaccine to increase uptake of antepartum Tdap. Pediatricians, other child health professionals, and obstetricians alike should also emphasize Tdap vaccine safety, especially during pregnancy, in encouraging antepartum Tdap vaccination.
Increasing antepartum Tdap vaccination rates can prevent notable morbidity and mortality in pregnant women and young infants and improve compliance with national recommendations. Quality improvement methodology, including implementing standing orders for Tdap vaccination and establishing systems for proper vaccine inventory, can be used to significantly help increase antepartum and postpartum Tdap vaccine administration rates (Bernstein et al., 2017; Jina et al., 2019). Patient counseling by obstetricians during prenatal visits has also been included as a component of successful QI efforts (Jina et al., 2019). Further study of how obstetric providers can target specific patient characteristics and health beliefs in promoting receipt of antepartum Tdap vaccination is necessary, given that racial and ethnic disparities in Tdap access and uptake have the potential to put already vulnerable infants and pregnant women at increased risk of pertussis. Research also is warranted to clarify the understanding of those obstetric providers who might be uncomfortable with offering their patients antepartum Tdap during each pregnancy, and of patients who decline antepartum Tdap.
Authors Contributions
HHB conceptualized, designed, coordinated and supervised data collection, and was responsible for overall direction, supervision and oversight of the study. HHB, STM, and SSC contributed to the conceptualization and study design of the work. HHB and SSC were involved in data acquisition. CS, HHB, and MS were responsible for data analysis and all authors assisted with data interpretation. HHB and STM led the drafting of the initial manuscript with input from all authors, and worked closely with SSC and MS as they led revisions with critical feedback from all authors.
Funding
This work was supported by the Northwell Health Walk/Internal Clinical Care Innovation Grant from the Katz Institute.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code Availability
SAS 9.4 (Carey, NC). Code is available from the biostatistician upon reasonable request.
Declarations
Conflict of interest
Henry H. Bernstein, DO, MHCM, FAAP: Member—Vaccines and Related Biological Products Advisory Committee/FDA; Editor—Office Pediatrics Series, Current Opinion in Pediatrics; Faculty, Masters Program in Healthcare Management/TH Chan Harvard School of Public Health; Data and Safety Monitoring Board member—Takeda; PI—Breastfeeding Promotion grant, New York State Department of Health. No financial disclosures or conflicts of interest were reported by the remaining authors.
Ethical Approval
The Feinstein Institute for Medical Research at Northwell Health exempted our study from Institutional Review Board review.
Consent to Participate
An information sheet that explained the purpose of the study was given to each mother along with the survey; a verbal explanation of the survey and its voluntary and anonymous nature were provided. All patients provided verbal consent before participating in our study.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahluwalia IB, Ding H, D’Angelo D, Shealy K, Singleton J, Liang J, Rosenberg K. Tetanus, diphtheria, pertussis vaccination coverage before, during, and after pregnancy—16 States and New York City, 2011. MMWR. 2015;64(19):522–526. [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia IB, Ding H, Harrison L, D'Angelo D, Singleton JA, Bridges C. Disparities in influenza vaccination coverage among women with live-born infants: PRAMS surveillance during the 2009–2010 influenza season. Public Health Reports. 2014;29:408–416. doi: 10.1177/003335491412900504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M. The health belief model and personal health behavior. Health Education Monographs. 1974;2:324–473. doi: 10.1177/109019817400200407. [DOI] [Google Scholar]
- Bernstein HH, Monty M, Yang P, Cohen A. Increasing Tdap coverage among postpartum women: A quality improvement intervention. Pediatrics. 2017;139(3):e20160607. doi: 10.1542/peds.2016-0607. [DOI] [PubMed] [Google Scholar]
- Bisgard KM, Pascual FB, Ehresmann KR, Miller CA, Cianfrini C, Jennings CE, Rebmann CA, Gabel J, Schauer SL, Lett SM. Infant pertussis: Who was the source? The Pediatric Infectious Disease Journal. 2004;23:985–989. doi: 10.1097/01.inf.0000145263.37198.2b. [DOI] [PubMed] [Google Scholar]
- Bramer CA, Kimmins LM, Swanson R, Kuo J, Vranesich P, Jacques-Carroll LA, Shen AK. Decline in child vaccination coverage during the COVID-19 pandemic—michigan care improvement registry, May 2016–May 2020. MMWR Morbidity and Mortality Weekly Report. 2020;69:630–631. doi: 10.15585/mmwr.mm6920e1. [DOI] [PubMed] [Google Scholar]
- Dempsey AF, Brewer SE, Sevick C, Pyrzanowski J, Mazzoni S, O’Leary ST. Tdap vaccine attitudes and utilization among pregnant women from a high-risk population. Human Vaccines & Immunotherapeutics. 2016;12(4):872–878. doi: 10.1080/21645515.2015.1094594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorivelu K, Boulet SL, Biswas HH, Adams JC, Haddad LB, Jamieson DJ. Predictors of tetanus, diphtheria, acellular pertussis and influenza vaccination during pregnancy among full-term deliveries in a medically underserved population. Vaccine. 2019;37(41):6054–6059. doi: 10.1016/j.vaccine.2019.08.044. [DOI] [PubMed] [Google Scholar]
- Fernandes EG, Sato APS, de Vaz Lima LRA, Rodrigues M, Leite D, de Brito CA, Luna EJ, Carvalhanas TRMP, Ramos MLBN, Sato HR, de Castilho EA. The effectiveness of maternal pertussis vaccination in protecting newborn infants in Brazil: A case-control study. Vaccine. 2019;37(36):5481–5484. doi: 10.1016/j.vaccine.2019.03.049. [DOI] [PubMed] [Google Scholar]
- Goldfarb IT, Little S, Brown J, Riley LE. Use of the combined tetanus-diphtheria and pertussis vaccine during pregnancy. American Journal of Obstetrics and Gynecology. 2014;211:299e1–305. doi: 10.1016/j.ajog.2014.05.029. [DOI] [PubMed] [Google Scholar]
- Havers FP, Moro PL, Hunter P, Hariri S, Bernstein H. Use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines: Updated recommendations of the advisory committee on immunization practices—United States, 2019. MMWR Morbidity and Mortality Weekly Report. 2020;69:77–83. doi: 10.15585/mmwr.mm6903a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housey M, Zhang F, Miller C, Lyon-Callo S, McFadden J, Garcia E, Potter R. Vaccination with tetanus, diphtheria, and acellular pertussis vaccine of pregnant women enrolled in Medicaid—Michigan. MMWR Morbidity and Mortality Weekly Report. 2014;63:839–842. [PMC free article] [PubMed] [Google Scholar]
- Jina A, Wang T, Seyferth E, Cohen A, Bernstein HH. Increasing antepartum Tdap vaccine administration: A quality improvement initiative. Vaccine. 2019;37(28):3654–3659. doi: 10.1016/j.vaccine.2019.05.045. [DOI] [PubMed] [Google Scholar]
- Kahn KE, Black CL, Ding H, Williams WW, Lu P, Fiebelkorn AP, Havers F, D’Angelo DV, Ball S, Fink RV, Delvin R. Influenza and Tdap vaccination coverage among pregnant women—United States, April 2018. MMWR Morbidity and Mortality Weekly Report. 2018;67(38):1055–1059. doi: 10.15585/mmwr.mm6738a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger K, Broder KR, Cortese MM, Joyce MP, Ortega-Sanchez I, Lee GM, Tiwari T, Cohn AC, Slade BA, Iskander JK, Mijalski CM, Brown KH, Murphy TV Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices. Healthcare Infection Control Practices Advisory Committee Preventing tetanus,diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. Morbidity and Mortality Weekly Report. 2006;55(RR-17):1–37. [PubMed] [Google Scholar]
- Kriss JL, Albert AP, Carter VM, Jiles AJ, Liang JL, Mullen J, Rodriguez L, Howards PP, Orenstein WA, Omer SB, Fisher A. Disparities in Tdap vaccination and vaccine information needs among pregnant women in the United States. Maternal and Child Health Journal. 2019;23(2):201–211. doi: 10.1007/s10995-018-2633-8. [DOI] [PubMed] [Google Scholar]
- LeardMann CA, Smith B, Smith TC, Wells TS, Ryan MAK. Smallpox vaccination: Comparison of self-reported and electronic vaccine records in the Millennium Cohort Study. Human Vaccines. 2007;3(6):245–251. doi: 10.4161/hv.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley MC, Kahn KE, Bardenheier BH, D’Angelo DV, Dawood FS, Fink RV, Havers F, Skoff TH. Vital Signs: Burden and prevention of influenza and pertussis among pregnant women and infants—United States. MMWR Morbidity and Mortality Weekly Report. 2019;68(40):885–892. doi: 10.15585/mmwr.mm6840e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbreras Areta M, Valiton A, Diana A, Morales M, Wiederrecht-Gasser JS, Chilin A, Quarta S, Jaksic C, Vallarta-Robledo JR, de Tejada BM. Flu and pertussis vaccination during pregnancy in Geneva during the COVID-19 pandemic: A multicentric, prospective, survey-based study. Vaccine. 2022;40(25):3455–3460. doi: 10.1016/j.vaccine.2022.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz CS, Carr W, Cohn A, Rodriguez L. Understanding barriers and predictors of maternal immunization: Identifying gaps through an exploratory literature review. Vaccine. 2018;36(49):7445–7455. doi: 10.1016/j.vaccine.2018.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary ST, Pyrzanowski J, Brewer SE, Barnard J, Beaty B, Donnelly M, Mazzoni S, Dempsey AF. Influenza and pertussis vaccination among pregnant women and their infants’ close contacts. The Pediatric Infectious Disease Journal. 2015;34:1244–1249. doi: 10.1097/INF.0000000000000873. [DOI] [PubMed] [Google Scholar]
- Paguio JA, Yao JS, Dee EC. Silver lining of COVID-19: Heightened global interest in pneumococcal and influenza vaccines, an infodemiology study. Vaccine. 2020;38(34):5430–5435. doi: 10.1016/j.vaccine.2020.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett KP, Halperin SA, Nolan T, Pancorbo CM, Tapiero B, Martinón-Torres F, Stranak Z, Virta M, Vanderkooi OG, Kosina P, Pardilla MBE, García IC, Zuccotti GV, Kostanyan L, Meyer N, Ceregido MA, Cheuvart B, Kuriyakose SO, Fernández MM, Zambrano MAR, García AM, de la Fuente JEA, Marín MDC, de la Calle F-M, Espinar YR, Marchisio PG, Manzoni P, Mesaros N. Immunogenicity, transplacental transfer of pertussis antibodies and safety following pertussis immunization during pregnancy: Evidence from a randomized, placebo-controlled trial. Vaccine. 2019 doi: 10.1016/j.vaccine.2019.10.105. [DOI] [PubMed] [Google Scholar]
- Psarris A, Sindos M, Theodora M, Antsaklis P, Pergialiotis V, Loutradis D, Daskalakis G. Routine immunizations during pregnancy, doctors’ compliance and patient hesitancy: A two-stage study on vaccination uptake. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2019;243:36–40. doi: 10.1016/j.ejogrb.2019.10.012. [DOI] [PubMed] [Google Scholar]
- Rowe SL, Leder K, Perrett KP, Romero N, Nolan TM, Stephens N, Cowie BC, Cheng AC. Maternal vaccination and infant influenza and pertussis. Pediatrics. 2021;148(3):e2021051076. doi: 10.1542/peds.2021-051076. [DOI] [PubMed] [Google Scholar]
- Santoli JM, Lindley MC, DeSilva MB, Kharbanda EO, Daley MF, Galloway L, Gee J, Glover M, Herring B, Kang Y, Lucas P, Noblit C, Tropper J, Vogt T, Weintraub E. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration—United States, 2020. MMWR Morbidity and Mortality Weekly Report. 2020;69:591–593. doi: 10.15585/mmwr.mm6919e2. [DOI] [PubMed] [Google Scholar]
- Skoff TH, Blain AE, Watt J, Scherzinger K, McMahon M, Zansky SM, Kudish K, Cieslak PR, Lewis M, Shang N, Martin SW. Impact of the US maternal tetanus, diphtheria, and acellular pertussis vaccination program on preventing pertussis in infants <2 months of age: A case-control evaluation. Clinical Infectious Diseases. 2017;65(12):1977–1983. doi: 10.1093/cid/cix724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A, Sherin M, Cleary S, Spino C, Bernstein HH. Maternal self-report of tetanus diphtheria pertussis vaccination during pregnancy correlates with patient-specific electronic medical records. Journal of Pediatrics. 2021;234:220–226. doi: 10.1016/j.jpeds.2021.03.015. [DOI] [PubMed] [Google Scholar]
- Strassburg ER, Power M, Schulkin J, Stark LM, Mackeen AD, Murtough KL, Paglia MJ. Patient attitudes toward influenza and tetanus, diphtheria, and acellular pertussis vaccination in pregnancy. Vaccine. 2018;36:4548–4554. doi: 10.1016/j.vaccine.2018.05.121. [DOI] [PubMed] [Google Scholar]
- Wales DP, Khan S, Suresh D, Ata A, Morris B. Factors associated with Tdap vaccination receipt during pregnancy: A cross-sectional study. Public Health. 2019;179:38–44. doi: 10.1016/j.puhe.2019.10.001. [DOI] [PubMed] [Google Scholar]
- Wendelboe AM, Njamkepo E, Bourillon A, Floret DD, Gaudelus J, Gerber M, Grimprel E, Greenberg D, Halperin S, Liese J, Muños-Rivas F, Teyssou R, Guiso N, Van Rie A. Transmission of Bordetella pertussis to young infants. The Pediatric Infectious Disease Journal. 2007;26:293–299. doi: 10.1097/01.inf.0000258699.64164.6d. [DOI] [PubMed] [Google Scholar]
- Wiley KE, Zuo Y, Macartney KK, McIntyre PB. Sources of pertussis infection in young infants: A review of key evidence informing targeting of the cocoon strategy. Vaccine. 2013;31:618–625. doi: 10.1016/j.vaccine.2012.11.052. [DOI] [PubMed] [Google Scholar]
- Wong CY, Thomas NJ, Clarke M, Boros C, Tuckerman J, Marshall HS. Maternal uptake of pertussis cocooning strategy and other pregnancy related recommended immunizations. Human Vaccines & Immunotherapeutics. 2015;11:1165–1172. doi: 10.1080/21645515.2015.1019188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Xu J, Black CL, Ding H, Cho B, Lu P, Lindley MC. Trends in Tdap vaccination among preivately insured preganant women in the United States, 2009–2016. Vaccine. 2019;37(14):1972–1977. doi: 10.1016/j.vaccine.2019.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
SAS 9.4 (Carey, NC). Code is available from the biostatistician upon reasonable request.