Abstract
Background
The systemic immune-inflammation index (SII) is an inflammatory parameter calculated as platelet count × neutrophil count/lymphocyte count in the peripheral blood. In recent years, the prognostic role of the SII in patients with biliary tract cancer (BTC) has been gradually investigated. However, the results were controversial. This meta-analysis aimed to illustrate the prognostic value of the SII in BTC.
Methods
The electronic databases of PubMed, the Web of Science, Embase, and the Cochrane Library were thoroughly retrieved up to April 15, 2022. Pooled hazard ratios (HRs) with 95% confidence intervals (CIs) were used to evaluate the prognostic value of the SII for clinical outcomes. The association between the SII and overall survival (OS) and recurrence-free survival (RFS)/progression-free survival (PFS) was evaluated.
Results
Thirteen studies involving 3515 patients were included in this meta-analysis. The pooled results indicated that an elevated SII was significantly associated with poor OS (HR, 1.77; 95% CI, 1.47–2.14; p<0.001) and RFS/PFS (HR, 1.66; 95% CI, 1.38–1.99; p<0.001) in patients with BTC. Subgroup analysis stratified by country, sample size, and cutoff value showed similar results. The sensitivity analysis and publication bias test confirmed the reliability of our results.
Conclusions
An elevated pretreatment SII was significantly associated with worse OS and RFS/PFS in patients with BTC. Our results suggest that the SII is a valuable and cost-effective prognostic parameter for the treatment of patients with BTC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-022-02783-z.
Keywords: Systemic immune-inflammation index, Biliary tract cancers, Meta-analysis, Prognosis, Risk factors
Background
Biliary tract cancers (BTCs) comprise a heterogeneous group of aggressive malignancies involving the bile ducts and gallbladder [1]. BTC accounts for approximately 3% of all gastrointestinal malignancies and is the second most common primary hepatic malignancy after hepatocellular carcinoma [2]. BTC comprises intrahepatic cholangiocarcinoma (ICC), extrahepatic cholangiocarcinoma (ECC), and gallbladder cancer (GBC) [3]. The histology of BTC is mainly adenocarcinoma. The incidence of cholangiocarcinoma has been increasing worldwide, whereas that of GBC has been decreasing in recent years [4]. Surgical resection is the only method for the long-term survival of patients with resectable BTC [2]. However, approximately 80% of BTC cases are unresectable with clear margins or metastatic when diagnosed [5]. Immunotherapy for BTC has shown promising results. Durvalumab is an anti-PD-L1 inhibitor, and several clinical trials are ongoing to evaluate its efficacy in BTC [6]. The FGFR inhibitor pemigatinib was the first US Food and Drug Administration-approved molecularly targeted therapy for the treatment of cholangiocarcinoma [7]. A recent single study in Italy revealed that the timing of the first radiofrequency ablation significantly affected survival outcomes in ICC in multivariate analysis [8]. The prognosis for unresectable BTC is poor, with a 5-year survival rate of 2% [9]. Prognostic biomarkers are important for the selection of patient management strategies and prediction of clinical outcome prediction [10]. The lack of novel prognostic markers is partially responsible for the poor prognosis of patients with BTC. Therefore, identifying a cost-effective and reliable prognostic marker before treatment is important for BTC treatment.
Prolonged inflammation is a hallmark of cancer [11], and systemic immune responses participate in tumor growth and development [12]. In recent years, many inflammation-related markers have been reported as the prognostic indexes in patients with BTC, such as the neutrophil-to-lymphocyte ratio [13], platelet-to-lymphocyte ratio [14], systemic inflammation response index [15], and systemic immune-inflammation index (SII) [16–18]. The SII was first proposed in 2014 by Hu et al. to predict the prognosis of patients with hepatocellular carcinoma receiving surgical resection [19]. The SII is calculated as neutrophil × platelet/lymphocyte count. Previous studies have shown that an elevated SII is associated with poor prognosis in non-small cell lung cancer (SCLC) [20], colorectal cancer [21], breast cancer [22], and renal cell carcinoma [23]. Many studies have also investigated the prognostic significance of the SII in BTC; however, the results were inconsistent [16–18, 24–33]. Some studies identified the SII as a significant prognostic factor for BTC [27–29], whereas others reported that this association was nonsignificant [16, 33]. For example, Tsilimigras et al. reported that an elevated SII was an independent prognostic marker for overall survival (OS) in patients with ICC (hazards ratio [HR], 1.70; 95% confidence interval [CI], 1.23–2.34; p=0.001) [26]. Moreover, Li et al. also demonstrated that an SII of >510 was an independent predictor of OS (HR, 1.90; 95% CI, 1.42–2.54; p<0.001) in a multicenter study including 1072 patients with GBC [30]. However, some other studies reported that there was no significant difference between the SII and survival of patients with BTC. For example, in a recent study, Ha et al. showed that the SII was not an independent prognostic factor for OS in patients with advanced BTC in multivariate analysis (HR, 0.928; 95% CI, 0.59–1.45; p=0.745) [16]. Therefore, to comprehensively identify the prognostic role of the SII in patients with SII, we performed this meta-analysis.
Methods
Search strategy
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [34] (Supplementary file 1). The protocol for this meta-analysis was registered in INPLASY (registration number, INPLASY202280082) and is available at https://inplasy.com/inplasy-2022-8-0082/. The electronic databases of PubMed, the Web of Science, Embase, and the Cochrane Library were thoroughly retrieved up to April 15, 2022. The following search strategies were applied: (“systemic immune-inflammation index” OR “SII” OR “systemic immune-inflammatory index”) AND (“biliary tract cancer” OR “bile duct cancer” OR “bile duct neoplasms” OR “cholangiocarcinoma” OR “gallbladder cancer” OR “gallbladder carcinoma”). All searches were performed using a combination of MeSH terms and free-test words. Only studies published in English were considered. The references of the retrieved studies were manually examined to identify other potential inclusions.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (i) studies reported the relationship between the SII and survival outcomes of patients with BTC, including OS, progression-free survival (PFS), recurrence-free survival (RFS), and disease-free survival (DFS); (ii) the diagnosis of BTC was pathologically confirmed; (iii) a definite cutoff value of the SII was provided; (iv) the HRs with 95% CIs of prognostic factors could be extracted, or sufficient data were provided to calculate them; and (v) studies were published in English. The exclusion criteria were as follows: (i) nonhuman studies; (ii) reviews, letters, comments, case reports, and meeting abstracts; (iii) studies with overlapping patients; and (iv) studies without sufficient data.
Data extraction and quality assessment
Two independent investigators (BZ and WY) extracted the necessary data from the eligible studies, and all disagreements were resolved through discussion to reach a consensus. The following data were extracted: name of the first author, year of publication, country, study design, sample size, histological type, tumor stage, treatment, follow-up, cutoff value of the SII, survival endpoint, survival analysis type, and HR and 95% CI. The quality of the included studies was evaluated using the Newcastle–Ottawa Scale (NOS) [35]. The NOS scores ranged from 0 to 9, and studies with NOS scores of ≥6 were regarded as high-quality studies.
Statistical analysis
Pooled HRs and 95% CIs were used to evaluate the prognostic value of the SII for clinical outcomes. Heterogeneity across studies was evaluated using the chi-square Q test and I2 index. If low heterogeneity between studies (Ph>0.10, I2 < 50%) was observed, a fixed-effects model was applied for analysis. Otherwise, a random-effects model was used. Subgroup analysis stratified by various clinicopathological factors was performed to identify the source of heterogeneity. Sensitivity analysis was performed by sequentially omitting each study to observe the impact of individual studies on the overall results. Funnel plots and Begg’s and Egger’s tests were used to examine potential publication bias. Stata software (version 12.0; Stata Corporation, College Station, TX, USA) was used for all statistical analyses. Statistical significance was set at p<0.05.
Ethics statement
Ethical approval was not required for this study because the data from this meta-analysis were based on previous studies and no individual patient information was used.
Results
Literature selection
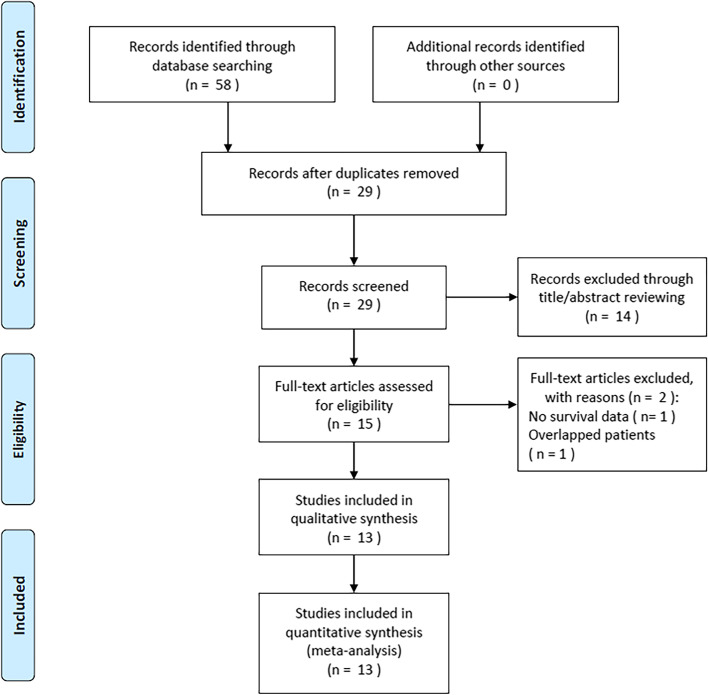
An initial literature search identified 58 records (Fig. 1). After removing duplicate studies, 29 studies remained. Fourteen studies were excluded after reviewing the titles and abstracts, and 15 studies were further evaluated by full-text examination. Subsequently, two studies were eliminated because one study did not provide survival data and the other included overlapping patients. Finally, 13 studies with 3515 patients [16–18, 24–33] were included in this meta-analysis (Fig. 1).
Fig. 1.
Flow diagram of the literature search and selection
Characteristics of included studies
The basic characteristics of the 13 included studies [16–18, 24–33] are shown in Table 1. They were published between 2016 and 2022 and were retrospective studies. Eight studies were performed in China [17, 24, 25, 27–31], two in the USA [18, 26], and one each in Korea [16], Japan [32], and Italy [33]. The total sample size was 3515, ranging from 28 to 1072. The median sample size was 140 patients. Five studies included patients with ICC [18, 24, 26, 27, 31], three studies enrolled patients with ECC [17, 32, 33], three studies recruited patients with GBC [25, 28, 30], and two studies included patients with BTC [16, 29]. All included studies reported the prognostic value of the SII for OS in BTC, five studies reported the association between the SII and RFS [24, 26, 27, 31, 33], and one study showed a correlation between the SII and PFS [29]. The cutoff values of the SII ranged from 447.48 to 1450, and the median value was 600. Nine studies reported the HRs and 95% CIs from a multivariate analysis [16, 24–28, 30–32], and four studies presented the HRs and 95% CIs from univariate analysis [17, 18, 29, 33]. The NOS scores of the included studies ranged from 6 to 8, with a median value of 7, indicating that all included studies were of high quality (Table 1).
Table 1.
Baseline characteristics of included studies in this meta-analysis
| Study | Year | Country | Study period | Study design | Sample size | Age (years) Median (range) |
Sex (M/F) | Histology | TNM stage | Treatment | Follow-up (months) Median (range) |
Cut-off value of SII | Survival endpoints | Survival analysis | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ha, H. | 2016 | Korea | 2004–2009 | Retrospective | 158 | 59.6 (31.3–76.2) | 103/55 | BTC | IV | Chemotherapy | 95.3 | 572.38 | OS | Multivariate | 7 |
| Hu, X. | 2019 | China | 2012–2017 | Retrospective | 113 | 70 | 75/38 | ECC | I–IV | PTBS + 125I | To Sep 2017 | 456 | OS | Univariate | 7 |
| Sellers, C. M. | 2019 | USA | 2005–2016 | Retrospective | 131 | 65 (57–71) | 68/63 | ICC | I–IV | Surgery | 12 | 867 | OS | Univariate | 8 |
| Li, H. | 2020 | China | 2009–2017 | Retrospective | 530 | 57.2 | 256/274 | ICC | I–III | Surgery | 18.0 (1.0-115.4) | 450 | OS, RFS | Multivariate | 7 |
| Sun, L. | 2020 | China | 2003–2017 | Retrospective | 142 | 63 | 60/82 | GBC | I–IV | Surgery | 12 | 600 | OS | Multivariate | 8 |
| Tsilimigras, D. I. | 2020 | USA | 2000–2017 | Retrospective | 688 | 57 (49–65) | 416/272 | ICC | I–III | Surgery | 22.3 | 1,150 | OS, RFS | Multivariate | 8 |
| Zhang, Z. | 2020 | China | 2013–2017 | Retrospective | 128 | 56.19 | 70/58 | ICC | I–III | Surgery | 25.2 | 1,027 | OS, RFS | Multivariate | 7 |
| Chen, H. | 2021 | China | 2012–2020 | Retrospective | 93 | 62 (32–90) | 62/31 | GBC | I–III | Surgery | 14 (2-60) | 824 | OS | Multivariate | 8 |
| Du, F. | 2021 | China | 2016–2019 | Retrospective | 60 | 61 (28–83) | 41/19 | BTC | IV | Immunotherapy | To Apr 2020 | 710 | OS, PFS | Univariate | 6 |
| Li, L. | 2021 | China | 2002–2019 | Retrospective | 1072 | 62 | 414/658 | GBC | I–III | Surgery | 53.8 | 510 | OS | Multivariate | 7 |
| Ren, A. | 2021 | China | 2013–2018 | Retrospective | 28 | 51.5 (46.8–60) | 25/3 | ICC | IV | Liver transplantation | 33.5 | 447.48 | OS, RFS | Multivariate | 8 |
| Terasaki, F. | 2021 | Japan | 2002–2015 | Retrospective | 140 | 71 (39–85) | 109/31 | ECC | I–III | Surgery | 48.2 | 1,450 | OS | Multivariate | 8 |
| Di Martino, M. | 2022 | Italy | 2010–2019 | Retrospective | 232 | 70 | 146/86 | ECC | I–IV | Surgery | 35.8 | 592 | OS, RFS | Univariate | 7 |
M Male, F Female, BTC Biliary tract cancer, ECC Extrahepatic cholangiocarcinoma, ICC Intrahepatic cholangiocarcinoma, GBC Gallbladder cancer, PTBS Percutaneous transhepatic biliary stenting, OS Overall survival, PFS Progression-free survival, RFS Recurrence-free survival, NOS Newcastle-Ottawa Scale
SII and OS in BTC
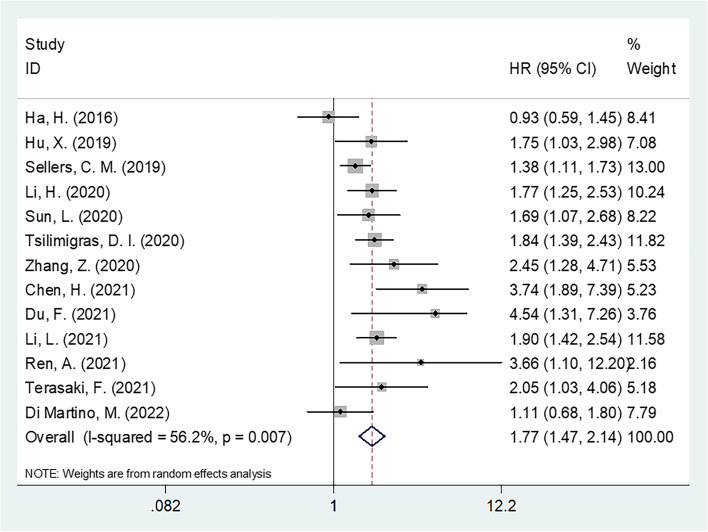
All 13 studies with 3515 patients [16–18, 24–33] showed a connection between the SII and OS in patients with BTC. Because of the significant heterogeneity (I2=56.2% and p for heterogeneity=0.007), a random-effects model was applied. As shown in Table 2 and Fig. 2, the combined results were as follows: HR, 1.77, and 95% CI, 1.47–2.14 (p<0.001), indicating that a high SII was significantly associated with poor OS in BTC. Subgroup analysis demonstrated that the prognostic value of the SII for OS was still significant irrespective of country, sample size, cutoff value, or survival analysis type (Table 2).
Table 2.
Subgroup analysis of the prognostic effect of SII for OS in patients with BTC
| Subgroups | No. of studies | No. of patients | HR (95%CI) | p | Effects model | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2(%) | Ph | ||||||
| Total | 13 | 3515 | 1.77 (1.47–2.14) | <0.001 | Random | 56.2 | 0.007 |
| Countries | |||||||
| Asian | 10 | 2,464 | 1.97 (1.54–2.54) | <0.001 | Random | 54.3 | 0.020 |
| Non-Asian | 3 | 1,051 | 1.47 (1.14–1.90) | 0.003 | Random | 50.9 | 0.130 |
| Sample size | |||||||
| ≤140 | 7 | 693 | 2.31 (1.59–3.36) | <0.001 | Random | 63.7 | 0.011 |
| >140 | 6 | 2,822 | 1.55 (1.25–1.93) | <0.001 | Random | 52.4 | 0.062 |
| Histology | |||||||
| BTC | 2 | 218 | 1.97 (0.42–9.30) | 0.394 | Random | 90.3 | 0.001 |
| ECC | 3 | 485 | 1.49 (1.08–2.05) | 0.014 | Fixed | 23.9 | 0.269 |
| ICC | 5 | 1,505 | 1.65 (1.42–1.92) | <0.001 | Fixed | 36.5 | 0.178 |
| GBC | 3 | 1,307 | 2.00 (1.58–2.51) | <0.001 | Fixed | 48.2 | 0.145 |
| TNM stage | |||||||
| I–III | 6 | 2,651 | 1.96 (1.67–2.30) | <0.001 | Fixed | 0 | 0.483 |
| I–IV | 4 | 618 | 1.42 (1.19–1.69) | <0.001 | Fixed | 0 | 0.524 |
| IV | 3 | 246 | 2.33 (0.72–7.57) | 0.160 | Random | 84.5 | 0.002 |
| Treatment | |||||||
| Surgery | 10 | 3,184 | 1.70 (1.51–1.92) | <0.001 | Fixed | 41.8 | 0.079 |
| Non-surgery | 3 | 331 | 1.81 (0.82–4.04) | 0.144 | Random | 82.0 | 0.004 |
| Cutoff value | |||||||
| ≤600 | 7 | 2,275 | 1.59 (1.35–1.87) | <0.001 | Fixed | 47.8 | 0.074 |
| >600 | 6 | 1,240 | 2.17 (1.55–3.03) | <0.001 | Random | 67.1 | 0.009 |
| Survival analysis | |||||||
| Multivariate | 9 | 2,979 | 1.81 (1.57–2.09) | <0.001 | Fixed | 47.8 | 0.053 |
| Univariate | 4 | 536 | 1.64 (1.10–2.45) | 0.016 | Random | 65.5 | 0.034 |
BTC Biliary tract cancer, ECC Extrahepatic cholangiocarcinoma, ICC Intrahepatic cholangiocarcinoma, GBC Gallbladder cancer, OS Overall survival
Fig. 2.
Forest plot for the relationship SII and OS in BTC patients
SII and RFS/PFS in BTC
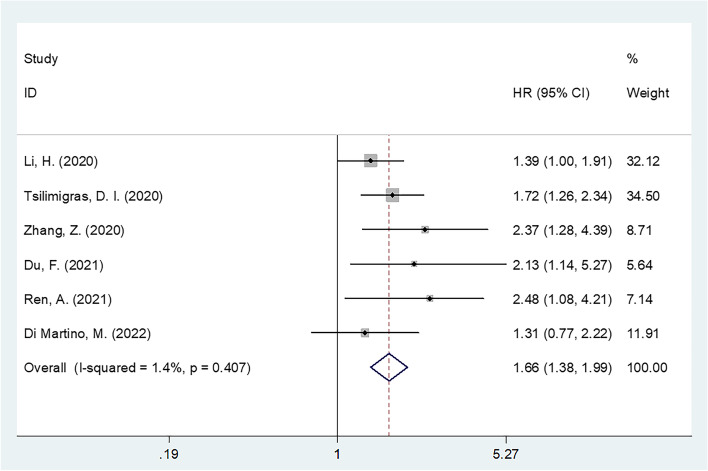
Six studies with 1666 patients [24, 26, 27, 29, 31, 33] showed an association between the SII and RFS/PFS in BTC. Heterogeneity was not significant (I2=1.4% and p for heterogeneity=0.407), and a fixed-effects model was applied. The combined results were as follows: HR, 1.66, and 95% CI, 1.38–1.99 (p<0.001) (Table 3, Fig. 3), which suggested that an elevated SII was associated with poor RFS/PFS in BTC. Subgroup analysis demonstrated that the prognostic role of the SII for RFS/PFS was not affected by country, sample size, or cutoff value (Table 3).
Table 3.
Subgroup analysis of the prognostic effect of SII for RFS/PFS in patients with BTC
| Subgroups | No. of studies | No. of patients | HR (95%CI) | p | Effects model | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2(%) | Ph | ||||||
| Total | 6 | 1666 | 1.66 (1.38–1.99) | <0.001 | Fixed | 1.4 | 0.407 |
| Countries | |||||||
| Asian | 4 | 746 | 1.71 (1.33–2.19) | <0.001 | Fixed | 28.6 | 0.241 |
| Non-Asian | 2 | 920 | 1.60 (1.23–2.09) | 0.001 | Fixed | 0 | 0.384 |
| Sample size | |||||||
| ≤140 | 3 | 216 | 2.34 (1.58–3.46) | <0.001 | Fixed | 0 | 0.955 |
| >140 | 3 | 1450 | 1.51 (1.23–1.85) | <0.001 | Fixed | 0 | 0.539 |
| Histology | |||||||
| BTC | 1 | 60 | 2.13 (0.99–4.57) | 0.054 | - | - | - |
| ECC | 1 | 232 | 1.31 (0.77–2.22) | 0.314 | - | - | - |
| ICC | 4 | 1374 | 1.69 (1.38–2.06) | <0.001 | Fixed | 22.6 | 0.275 |
| TNM stage | |||||||
| I–III | 3 | 1346 | 1.63 (1.32–2.01) | <0.001 | Fixed | 20.5 | 0.284 |
| I–IV | 1 | 232 | 1.31 (0.77–2.22) | 0.314 | Fixed | - | - |
| IV | 2 | 88 | 2.32 (1.39–3.86) | 0.001 | Fixed | 0 | 0.765 |
| Treatment | |||||||
| Surgery | 5 | 1606 | 1.64 (1.36–1.97) | <0.001 | Fixed | 13.9 | 0.325 |
| Non-surgery | 1 | 60 | 2.13 (0.99–4.57) | 0.054 | - | - | - |
| Cut-off value | |||||||
| ≤600 | 3 | 790 | 1.48 (1.15–1.91) | 0.002 | Fixed | 22.9 | 0.273 |
| >600 | 3 | 876 | 1.87 (1.44–2.42) | <0.001 | Fixed | 0 | 0.622 |
| Survival analysis | |||||||
| Multivariate | 4 | 1374 | 1.69 (1.38–2.06) | <0.001 | Fixed | 22.6 | 0.275 |
| Univariate | 2 | 292 | 1.53 (0.99–2.36) | 0.054 | Fixed | 3.6 | 0.308 |
BTC Biliary tract cancer, ECC Extrahepatic cholangiocarcinoma, ICC Intrahepatic cholangiocarcinoma, PFS Progression-free survival, RFS Recurrence-free survival
Fig. 3.
Forest plot for the relationship SII and RFS/PFS in BTC patients
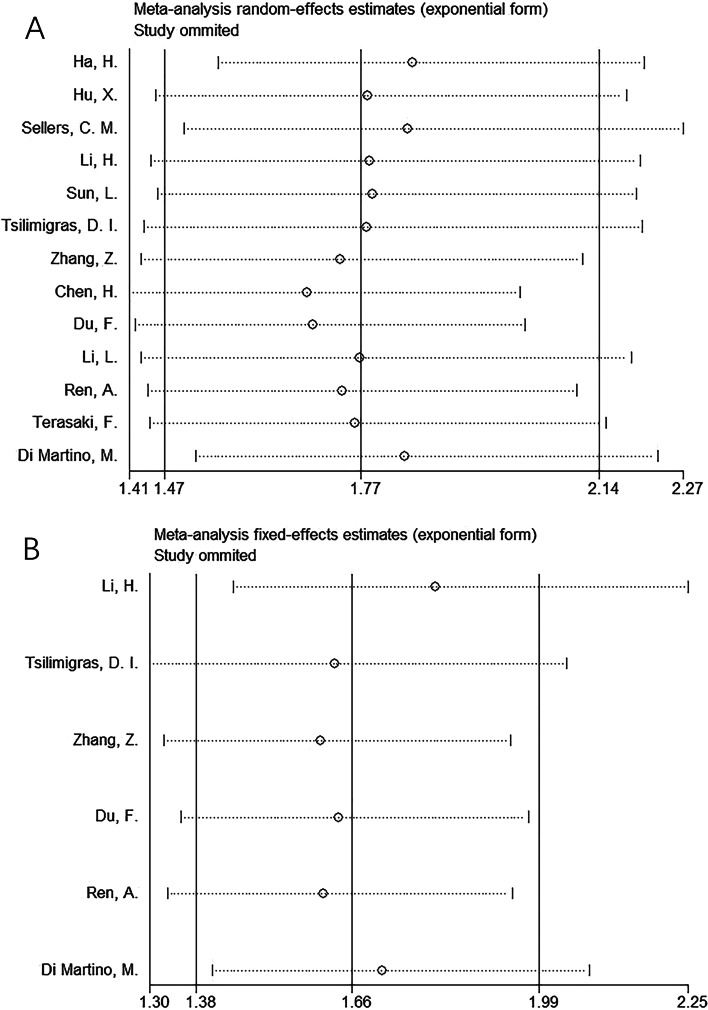
Sensitivity analysis
Sensitivity analysis was performed to explore potential sources of heterogeneity for OS and RFS/PFS. As shown in Fig. 4, the pooled HRs and corresponding 95% CIs were stable in our meta-analysis.
Fig. 4.
Result of sensitivity analyses by omitting one study in each turn for (A) OS and (B) RFS/PFS
Publication bias
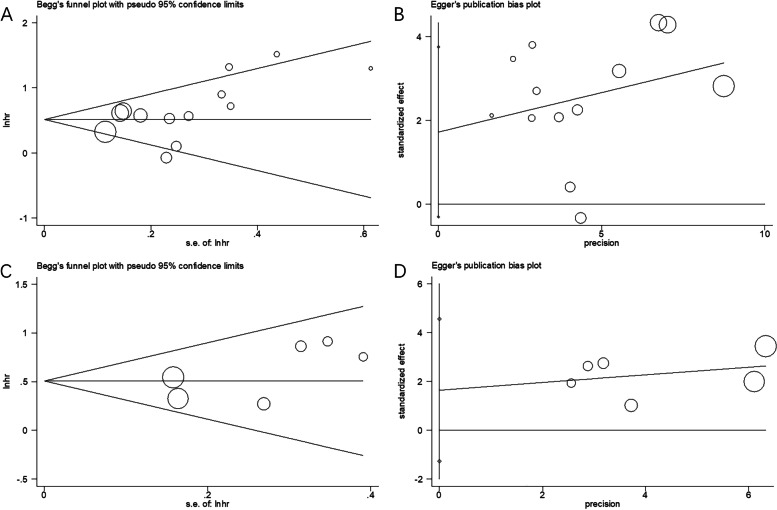
Begg’s and Egger’s tests were conducted to examine the potential publication bias in this meta-analysis. As shown in Fig. 5, the results of Begg’s and Egger’s tests showed that there was no significant publication bias for OS (Begg’s test, p=0.079; Egger’s test, p=0.088) or RFS/PFS (Begg’s test, p=0.260; Egger’s test, p=0.193).
Fig. 5.
Publication bias test by Begg’s funnel plot and Egger’s test. A Begg’ test for OS, p=0.079; B Egger’s test for OS, p= 0.088; C Begg’ test for RFS/PFS, p=0.260; D Egger’s test for RFS/PFS, p= 0.193
Discussion
To our knowledge, this is the first meta-analysis to assess the prognostic value of the SII in stratifying the prognosis of patients with BTC. Many studies have explored the prognostic significance of the SII in patients with BTC [16–18, 24–33]; however, the results have been inconsistent. In the current meta-analysis, we included 13 studies with 3515 patients to shed light on this issue. The results suggested that an elevated SII was significantly associated with worse OS and RFS/PFS in patients with BTC. Moreover, the prognostic value of the SII was not influenced by country, sample size, cutoff value, or survival analysis type in the subgroup analysis. The sensitivity analysis and publication bias test showed that our results were reliable. Taken together, this meta-analysis proposes that the SII could be a promising prognostic biomarker for survival prediction in patients with BTC.
Systemic inflammatory responses can promote tumor invasion and progression by reducing apoptosis and promoting metastasis [36]. The SII is a parameter reflecting inflammatory status and is composed of three inflammatory immune cell counts: neutrophil, lymphocyte, and platelet. The SII was defined as platelet count × neutrophil count/lymphocyte count [37]; therefore, an increase in platelet and neutrophil counts and/or a decrease in lymphocyte counts can lead to a high SII. Neutrophils can promote proliferation and metastasis of BTC through multiple mechanisms [38]. Neutrophils can inhibit the host immune response to cancer cells by suppressing cytotoxic immune cells via the secretion of various cytokines and chemokines [39]. Elevated platelet levels have been shown to accelerate tumor angiogenesis and prevent cytolysis [40]. Platelets can also inhibit tumor cell extravasation by potentiating tumor cell-induced endothelial cell retraction and, therefore, contribute to the promotion of tumor cell proliferation and metastasis [41]. In contrast, lymphocytes play an important role in T cell-mediated antitumor responses. Lymphocytes can change the tumor microenvironment and prevent tumorigenesis and tumor relapse by migrating and infiltrating the tumor microenvironment [42]. Therefore, the SII combines the significance of neutrophil, platelet, and lymphocyte counts and is a promising prognostic biomarker.
In addition to cancer, the SII has also been reported as a significant prognostic marker in other diseases. For example, a recent retrospective cohort study showed that the SII was associated in a J-shaped pattern with all-cause mortality among critically ill patients with acute kidney injury [43]. Another study indicated that patients with heart failure with higher SII values had a shorter survival time [44]. Xia et al. demonstrated that the SII is a potential new diagnostic biomarker in patients with severe COVID-19 in a study including 125 patients diagnosed with COVID-19 [45].
Previous meta-analyses have also investigated the prognostic impact of the SII in a variety of cancer types [46–48]. Li et al. showed that an elevated preoperative SII was significantly associated with worse survival outcomes and adverse pathological features in patients with bladder cancer based on a meta-analysis of 7087 patients [49]. Fu et al. reported that a higher SII value was significantly associated with worse OS and DFS in gastric cancer in a meta-analysis of 11 studies [50]. Another meta-analysis of 3180 patients revealed that a high SII was independently associated with poor survival outcomes in patients with renal cell carcinoma [51]. A recent meta-analysis indicated that a high SII was significantly associated with OS in patients with SCLC [52]. Moreover, a high SII was correlated with extensive-stage SCLC [52]. The results of our meta-analysis are in line with those of the prognostic role of the SII in other solid tumors [49, 51, 52].
This meta-analysis has several limitations. First, all included studies had a retrospective design. Therefore, there may have been a potential selection bias. Second, the population included in this meta-analysis was mainly from Asian countries but is not a good representation of the worldwide population. Third, the cutoff values of the SII varied across the included studies, which may have introduced heterogeneity in the meta-analysis. Therefore, large-scale prospective trails using uniform SII cutoff value are needed to consolidate our findings.
Notably, inherent heterogeneity may have existed in our meta-analysis, and we performed several analyses to reveal the impact of heterogeneity on our results. First, in the data analysis (Tables 2 and 3), we selected a fixed-effects or random-effects model according to the level of heterogeneity. Second, the sensitivity analysis (Fig. 4) showed that the overall results were not influenced by a single study. Third, the publication bias test demonstrated that there was no significant publication bias in our meta-analysis (Fig. 5). Therefore, the aforementioned analysis suggests that there was inherent heterogeneity in our meta-analysis; however, our results were reliable and were not affected by this heterogeneity.
Conclusions
In summary, an elevated pretreatment SII was significantly associated with worse OS and RFS/PFS in patients with BTC. Our results suggest that the SII is a valuable and cost-effective prognostic parameter for the treatment of patients with BTC.
Supplementary Information
Additional file 1. The PRISMA checklist.
Acknowledgements
None.
Abbreviations
- SII
Systemic immune-inflammation index
- BTC
Biliary tract cancers
- HR
Hazards ratio
- CI
Confidence interval
- OS
Overall survival
- PFS
Progression-free survival
- RFS
Recurrence-free survival
- DFS
Disease-free survival
- NOS
Newcastle-Ottawa Scale
Authors’ contributions
Conceptualization: BZ and WY. Methodology: BZ and WY. Software: BZ. Formal analysis: BZ and WY. Writing—original draft preparation: BZ. Writing—review and editing: WY. The authors contributed to the article and approved the submitted final version.
Funding
None.
Availability of data and materials
The information used and analyzed during this study is available from the original literature listed in the reference. The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397(10272):428–444. doi: 10.1016/S0140-6736(21)00153-7. [DOI] [PubMed] [Google Scholar]
- 2.Kone LB, Bystrom PV, Maker AV. Robotic surgery for biliary tract cancer. Cancers (Basel). 2022;14(4):1046. [DOI] [PMC free article] [PubMed]
- 3.Pezzicoli G, Triggiano G, Sergi MC, Mannavola F, Porta C, Tucci M. Biliary tract cancers: moving from the present standards of care towards the use of immune checkpoint inhibitors. Am J Transl Res. 2021;13(8):8598–8610. [PMC free article] [PubMed] [Google Scholar]
- 4.Sasaki T, Takeda T, Okamoto T, Ozaka M, Sasahira N. Chemotherapy for biliary tract cancer in 2021. J Clin Med. 2021;10(14):3108. [DOI] [PMC free article] [PubMed]
- 5.Mukkamalla SKR, Naseri HM, Kim BM, Katz SC, Armenio VA. Trends in incidence and factors affecting survival of patients with cholangiocarcinoma in the United States. J Natl Compr Cancer Netw. 2018;16(4):370–376. doi: 10.6004/jnccn.2017.7056. [DOI] [PubMed] [Google Scholar]
- 6.Rizzo A, Ricci AD, Brandi G. Durvalumab: an investigational anti-PD-L1 antibody for the treatment of biliary tract cancer. Expert Opin Investig Drugs. 2021;30(4):343–350. doi: 10.1080/13543784.2021.1897102. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo A, Ricci AD, Brandi G. Pemigatinib: hot topics behind the first approval of a targeted therapy in cholangiocarcinoma. Cancer Treat Res Commun. 2021;27:100337. doi: 10.1016/j.ctarc.2021.100337. [DOI] [PubMed] [Google Scholar]
- 8.Brandi G, Rizzo A, Dall'Olio FG, Felicani C, Ercolani G, Cescon M, Frega G, Tavolari S, Palloni A, De Lorenzo S, et al. Percutaneous radiofrequency ablation in intrahepatic cholangiocarcinoma: a retrospective single-center experience. Int J Hyperthermia. 2020;37(1):479–485. doi: 10.1080/02656736.2020.1763484. [DOI] [PubMed] [Google Scholar]
- 9.Cowzer D, Harding JJ. Advanced bile duct cancers: a focused review on current and emerging systemic treatments. Cancers (Basel). 2022;14(7):1800. [DOI] [PMC free article] [PubMed]
- 10.Sinniah RS, Shapses MS, Ahmed MU, Babiker H, Chandana SR. Novel biomarkers for cholangiocarcinoma: how can it enhance diagnosis, prognostication, and investigational drugs? Part-1. Expert Opin Investig Drugs. 2021;30(10):1047–1056. doi: 10.1080/13543784.2021.1985461. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21(6):345–359. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamara MG, Templeton AJ, Maganti M, Walter T, Horgan AM, McKeever L, Min T, Amir E, Knox JJ. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur J Cancer (Oxford, England 1990) 2014;50(9):1581–1589. doi: 10.1016/j.ejca.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Cho KM, Park H, Oh DY, Kim TY, Lee KH, Han SW, Im SA, Kim TY, Bang YJ. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and their dynamic changes during chemotherapy is useful to predict a more accurate prognosis of advanced biliary tract cancer. Oncotarget. 2017;8(2):2329–2341. doi: 10.18632/oncotarget.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin B, Hu W, Su S, Xu H, Lu X, Sang X, Yang H, Mao Y, Du S. The prognostic value of systemic inflammation response index in cholangiocarcinoma patients. Cancer Manag Res. 2021;13:6263–6277. doi: 10.2147/CMAR.S317954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha H, Nam AR, Bang JH, Park JE, Kim TY, Lee KH, Han SW, Im SA, Kim TY, Bang YJ, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget. 2016;7(47):76604–76612. doi: 10.18632/oncotarget.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X, Pang Q, Liu H, Qian Z, Jin H, Zhou L, Wang Y, Man Z, Li Z, Yang S. Inflammation-based prognostic scores in patients with extrahepatic bile duct lesions treated by percutaneous transhepatic biliary stenting combined with (125) I seeds intracavitary irradiation. Clin Transl Oncol. 2019;21(5):665–673. doi: 10.1007/s12094-018-1969-2. [DOI] [PubMed] [Google Scholar]
- 18.Sellers CM, Uhlig J, Ludwig JM, Stein SM, Kim HS. Inflammatory markers in intrahepatic cholangiocarcinoma: effects of advanced liver disease. Cancer Med. 2019;8(13):5916–5929. doi: 10.1002/cam4.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 20.Tong YS, Tan J, Zhou XL, Song YQ, Song YJ. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med. 2017;15(1):221. doi: 10.1186/s12967-017-1326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Xu H, Guo X, Zhang J, Ye X, Yang Y, Ma X. Pretreatment inflammatory indexes as prognostic predictors for survival in colorectal cancer patients receiving neoadjuvant chemoradiotherapy. Sci Rep. 2018;8(1):3044. doi: 10.1038/s41598-018-21093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Shi Z, Bai Y, Liu L, Cheng K. Prognostic significance of systemic immune-inflammation index in triple-negative breast cancer. Cancer Manag Res. 2019;11:4471–4480. doi: 10.2147/CMAR.S197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozbek E, Besiroglu H, Ozer K, Horsanali MO, Gorgel SN. Systemic immune inflammation index is a promising non-invasive marker for the prognosis of the patients with localized renal cell carcinoma. Int Urol Nephrol. 2020;52(8):1455–1463. doi: 10.1007/s11255-020-02440-y. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Wang JJ, Zhang M, Ren B, Li JX, Xu L, Wu H. Prognostic significance of systemic immune-inflammation index in patients with intrahepatic cholangiocarcinoma undergoing hepatic resection. World J Gastrointest Oncol. 2020;12(4):467–482. doi: 10.4251/wjgo.v12.i4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L, Jin Y, Hu W, Zhang M, Jin B, Xu H, Du S, Xu Y, Zhao H, Lu X, et al. The impacts of systemic immune-inflammation index on clinical outcomes in gallbladder carcinoma. Front Oncol. 2020;10:554521. doi: 10.3389/fonc.2020.554521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsilimigras DI, Moris D, Mehta R, Paredes AZ, Sahara K, Guglielmi A, Aldrighetti L, Weiss M, Bauer TW, Alexandrescu S, et al. The systemic immune-inflammation index predicts prognosis in intrahepatic cholangiocarcinoma: an international multi-institutional analysis. HPB. 2020;22(12):1667–1674. doi: 10.1016/j.hpb.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Zhou Y, Hu K, Huang Y. Investigating effects of preoperative inflammatory biomarkers on predicting survival outcomes of intrahepatic cholangiocarcinoma after curative resection. World J Surg Oncol. 2020;18(1):272. doi: 10.1186/s12957-020-02053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, Huang Z, Sun B, Wang A, Wang Y, Shi H, Zheng T, Li T, Huang M, Fu W. The predictive value of systemic immune inflammation index for postoperative survival of gallbladder carcinoma patients. J Surg Oncol. 2021;124(1):59–66. doi: 10.1002/jso.26470. [DOI] [PubMed] [Google Scholar]
- 29.Du F, Qiu Z, Ai W, Huang C, Ji J, Xiao X, Zhou J, Fang M, Jiang X, Gao C. Blood tests predict the therapeutic prognosis of anti-PD-1 in advanced biliary tract cancer. J Leukoc Biol. 2021;110(2):327–334. doi: 10.1002/JLB.5MA1220-631R. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Ren T, Liu K, Li ML, Geng YJ, Yang Y, Li HF, Li XC, Bao RF, Shu YJ, et al. Development and validation of a prognostic nomogram based on the systemic immune-inflammation index for resectable gallbladder cancer to predict survival and chemotherapy benefit. Front Oncol. 2021;11:692647. doi: 10.3389/fonc.2021.692647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren A, Li Z, Cheng P, Zhang X, Deng R, Ma Y. Systemic immune-inflammation index is a prognostic predictor in patients with intrahepatic cholangiocarcinoma undergoing liver transplantation. Mediators Inflamm. 2021;2021:6656996. doi: 10.1155/2021/6656996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terasaki F, Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, Ohgi K, Uesaka K. Systemic immune-inflammation index as a prognostic marker for distal cholangiocarcinoma. Surg Today. 2021;51(10):1602–1609. doi: 10.1007/s00595-021-02312-7. [DOI] [PubMed] [Google Scholar]
- 33.Di Martino M, Koh YX, Syn N, Min Chin K, Fernando B, Sánchez Velázquez P, et al. It is the lymph node ratio that determines survival and recurrence patterns in resected distal cholangiocarcinoma. A multicenter international study. Eur J Surg Oncol. 2022;48(7):1576–1584. [DOI] [PubMed]
- 34.Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–W264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 35.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 36.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 37.Kubota K, Ito R, Narita N, Tanaka Y, Furudate K, Akiyama N, Chih CH, Komatsu S, Kobayashi W. Utility of prognostic nutritional index and systemic immune-inflammation index in oral cancer treatment. BMC Cancer. 2022;22(1):368. doi: 10.1186/s12885-022-09439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe A, Harimoto N, Araki K, Kubo N, Igarashi T, Tsukagoshi M, Ishii N, Yamanaka T, Yoshizumi T, Shirabe K. Absolute neutrophil count predicts postoperative prognosis in mass-forming intrahepatic cholangiocarcinoma. Anticancer Res. 2019;39(2):941–947. doi: 10.21873/anticanres.13197. [DOI] [PubMed] [Google Scholar]
- 39.Miyahara Y, Takashi S, Shimizu Y, Ohtsuka M. The prognostic impact of neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) in patients with distal bile duct cancer. World J Surg Oncol. 2020;18(1):78. [DOI] [PMC free article] [PubMed]
- 40.Wojtukiewicz MZ, Sierko E, Hempel D, Tucker SC, Honn KV. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. 2017;36(2):249–262. doi: 10.1007/s10555-017-9673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huong PT, Nguyen LT, Nguyen XB, Lee SK, Bach DH. The role of platelets in the tumor-microenvironment and the drug resistance of cancer cells. Cancers (Basel). 2019;11(2):240. [DOI] [PMC free article] [PubMed]
- 42.Unitt E, Marshall A, Gelson W, Rushbrook SM, Davies S, Vowler SL, Morris LS, Coleman N, Alexander GJ. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006;45(2):246–253. doi: 10.1016/j.jhep.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 43.Jia L, Li C, Bi X, Wei F, Meng J, Sun G, et al. Prognostic value of systemic immune-inflammation index among critically ill patients with acute kidney injury: a retrospective cohort study. J Clin Med. 2022;11(14):3978. [DOI] [PMC free article] [PubMed]
- 44.Yuan M, Ren F, Gao D. The value of SII in predicting the mortality of patients with heart failure. Dis Markers. 2022;2022:3455372. doi: 10.1155/2022/3455372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia W, Tan Y, Hu S, Li C, Jiang T. Predictive value of systemic immune-inflammation index and neutrophil-to-lymphocyte ratio in patients with severe COVID-19. Clin Appl Thromb Hemost. 2022;28:10760296221111391. doi: 10.1177/10760296221111391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y, Gao Y, Wu Y, Lin H. Prognostic value of systemic immune-inflammation index in patients with urologic cancers: a meta-analysis. Cancer Cell Int. 2020;20:499. doi: 10.1186/s12935-020-01590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9(18):3295–3302. doi: 10.7150/jca.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8(43):75381–75388. doi: 10.18632/oncotarget.18856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Cao D, Huang Y, Xiong Q, Tan D, Liu L, Lin T, Wei Q. The prognostic and clinicopathological significance of systemic immune-inflammation index in bladder cancer. Front Immunol. 2022;13:865643. doi: 10.3389/fimmu.2022.865643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu S, Yan J, Tan Y, Liu D. Prognostic value of systemic immune-inflammatory index in survival outcome in gastric cancer: a meta-analysis. J Gastrointest Oncol. 2021;12(2):344–354. doi: 10.21037/jgo-20-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin M, Yuan S, Yuan Y, Yi L. Prognostic and clinicopathological significance of the systemic immune-inflammation index in patients with renal cell carcinoma: a meta-analysis. Front Oncol. 2021;11:735803. doi: 10.3389/fonc.2021.735803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y, Dai M, Zhang Z. Prognostic significance of the systemic immune-inflammation index (SII) in patients with small cell lung cancer: a meta-analysis. Front Oncol. 2022;12:814727. doi: 10.3389/fonc.2022.814727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The PRISMA checklist.
Data Availability Statement
The information used and analyzed during this study is available from the original literature listed in the reference. The datasets analyzed during the current study are available from the corresponding author on reasonable request.