Abstract
This cross-sectional study investigated the association of metabolic syndrome (MetS) with sarcopenia defined by absolute low muscle mass (aLMM) and absolute low muscle strength (aLMS), or sarcopenia defined by relative low muscle mass (rLMM) and relative low muscle strength (rLMS). The cut-off values for men and women were as follows: aLMM, appendicular muscle mass in kg/height2 was <7.0 kg/m2 and <5.7 kg/m2; rLMM, appendicular muscle mass/body weight ×100 was <28.64% and <24.12%; aLMS, handgrip strength was <28 kg and <18 kg; and rLMS, handgrip strength/body weight ×100 was 51.26% and 35.38%. Among 207 men and 164 women, 41.5% men and 57.3% women had MetS, 14.0% men and 6.1% women had sarcopenia as defined by aLMM and aLMS, and 14.0% men and 22.0% women had sarcopenia defined by rLMM and rLMS. Compared with non-sarcopenia, adjusted OR of sarcopenia defined by aLMM and aLMS for the prevalence of MetS was 0.79 (95% CI 0.38–1.67), whereas that of sarcopenia defined by rLMM and rLMS for the prevalence of MetS was 20.6 (95% CI 7.81–54.3). Sarcopenia defined by rLMM and rLMS was associated with the risk of prevalence of MetS, whereas sarcopenia defined by aLMM and aLMS was not.
Keywords: sarcopenia, muscle, obesity, metabolic syndrome, diabetes
Introduction
Metabolic syndrome (MetS), which is one of the global health problems, is reported to be associated with a high risk of cardiovascular disease.(1,2) It has been reported that several factors such as chronic inflammation, oxidative stress, and insulin resistance are associated with the mechanism for MetS.(3,4)
Sarcopenia, which is mainly characterized as absolute muscle mass, power, and function,(5) is now the focus because of higher risk of cardiovascular disease and mortality.(6–9) In patients with diabetes, declining insulin signaling and higher insulin resistance accelerate muscle catabolism(5) and resulting in accelerated reduction of muscle mass and strength.(10) Thus, it is an important target for treatment of diabetes, because the muscle is a major organ of glucose metabolism.(11) In fact, it has been reported that diets, including total energy, proteins, vitamins, and fatty acids, are associated with the sarcopenia in patients with diabetes.(12–14)
On the other hand, relative muscle mass loss, defined as weight-adjusted skeletal muscle mass index (SMI) (SM/Wt), has been described as a risk factor for type 2 diabetes (T2D)(15) and has been associated with insulin resistance.(16) In addition, we and other researchers revealed the relationship between relative muscle mass loss and nonalcoholic fatty liver disease (NAFLD)(17,18) or nonalcoholic steato-hepatitis (NASH)(19,20) which are closely related to MetS.(21) Furthermore, some studies revealed that there was a relationship between relative muscle mass loss and MetS.(22,23)
Previous studies have not revealed whether sarcopenia defined by absolute muscle mass and absolute muscle strength, or sarcopenia defined by the relative muscle mass and relative muscle strength is associated more with the prevalence of MetS. Hence, the objective of this cross-sectional study was to clarify the association of MetS with sarcopenia defined by absolute muscle mass and absolute muscle strength, or sarcopenia defined by relative muscle mass and relative muscle strength in patients with T2D.
Materials and Methods
Study participants
We are conducting an ongoing cohort study KAMOGAWA-DM cohort study.(24) This ongoing study was approved by the research ethics committee of Kyoto Prefectural University of Medicine (No. RBMR-E-466-6) and was carried out in accordance with the Declaration of Helsinki, and informed consent was obtained from all the participants. We performed a post-hoc analysis of the previous cross-sectional study, and details of this study has been described previously.(25) All patients provided details of their demographics, including medication usage and medical history. We excluded patients who did not receive bioelectrical impedance analysis (BIA) and with no data on handgrip strength.
Patients were classified as non-smokers or smokers by a self-administered questionnaire. Patients were classified as non-exerciser or exerciser, performing any kind of physical activity at least once a week, by a self-administered questionnaire. Alcohol consumption was defined as ethanol over 20 g/day.(25)
Biochemical analysis
Serum triglycerides, total cholesterol, high-density lipoprotein (HDL) cholesterol, gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were assessed using standard enzymatic methods. Hemoglobin A1c (HbA1c) was expressed as National Glycohemoglobin Standardization Program (NGSP) unit evaluated by high-performance liquid chromatography.
Definition of metabolic syndrome
Metabolic syndrome was diagnosed when patients fulfilled three or more of the following criteria were present:(26) abdominal obesity, waist circumference ≥90 cm in men and ≥80 cm in women; hypertension, a systolic blood pressure >130 mmHg and/or a diastolic blood pressure >85 mmHg and/or usage antihypertension medication; hypertriglyceridemia, triglycerides >1.7 mM and/or usage antihyperlipidemia medication; reduced HDL-C, HDL-C <1.03 mM in men and <1.29 mM in women; hyperglycemia, fasting plasma glucose >5.6 mM and/or under medical treatment (all patients had T2D in this study). Since waist measurements were not available for the study sample, we defined obesity as a percentage of body fat, i.e., >30% for men and >35% for women.(27) In addition, metabolic syndrome score was defined as the existence of each component of MetS (from 0 to 5).
Definition of sarcopenia
Using the multifrequency impedance body composition analyzer, Inbody 720 (InBody Japan, Tokyo, Japan), data on body weight (BW) (kg), appendicular muscle mass (kg), and body fat mass (kg) were obtained. Body mass index (BMI) was calculated as BW (kg)/height2 (m2). The height-adjusted SMI (SM/Ht2) was calculated as follows: SM/Ht2 = (appendicular muscle mass in kg)/(height in m2).(28) The cut-off values for absolute low muscle mass (aLMM), defined as SM/Ht2, were <7.0 kg/m2 for men and <5.7 kg/m2 for women.(28) The weight adjusted SMI (SM/Wt) was calculated as follows: SM/Wt = [(appendicular muscle mass in kg)/(BW in kg)] × 100.(29) The cut-off of relative low muscle mass (rLMM) was defined by a weight-adjusted SMI <28.64% for men and <24.12% for women.(29) Handgrip strength was measured two times with each hand by a handgrip dynamometer (Smedley, Takei Scientific Instruments Co., Ltd., Niigata, Japan), and the maximum value was used for the analysis. The cutoff points for absolute low muscle strength (aLMS) defined by handgrip strength was <28 kg for men and <18 kg for women.(28) Adjusted grip strength was calculated as follows: adjusted grip strength = [(handgrip strength in kg)/(BW in kg)].(29) The cut-off points for relative low muscle strength (rLMS) defined as adjusted grip strength was 51.26% for men and 35.38% for women.(29) Sarcopenia was defined as the presence of both aLMM and aLMS or both rLMM and rLMS.
Statistical analysis
The statistical analyses were performed using the JMP ver. 13.2. software (SAS Institute Inc., Cary, CA), figures were created by GraphPad Prism ver. 8.4.2 software (GraphPad Software, Inc., La Jolla, CA), and p value <0.05 was considered statistically significant. Continuous variables were presented as mean (SD) and categorized variables were presented as percentage (number). The difference between groups was analyzed by Student’s t test or Chi-square test. The difference of SM/Ht2, SM/Wt, handgrip strength, and adjusted grip strength between the presence of MetS were evaluated by Tukey honestly significant difference test and adjusted for age, sex, smoking, alcohol, and exercise. The difference of SM/Ht2, SM/Wt, handgrip strength, and adjusted grip strength among metabolic syndrome score were evaluated by Kruskal-Wallis test and steel-dwass test. In addition, the participants were divided into four groups according to muscle mass and muscle strength stage: normal, aLMM only, aLMS only, or sarcopenia, defined by aLMM and aLMS; and normal, rLMM only, rLMS only, or sarcopenia, defined by rLMM and rLMS. Multivariate logistic regression analyses were performed to investigate the association of MetS with the presence of sarcopenia, the presence of aLMM, aLMS and sarcopenia, or the presence of rLMM, rLMS, and sarcopenia, adjusting for covariates, sex, age, smoking, alcohol, and exercise.
Results
Initially, 383 patients with type 2 diabetes were included in the study. Among them, 12 patients were excluded based on the exclusion criteria (Fig. 1).
Fig. 1.
Flow chart of inclusion and exclusion criteria. BIA, bioelectrical impedance analysis.
The clinical characteristics of 371 patients (207 men and 164 women) are shown in Table 1. Average age and duration of diabetes were 67.0 (11.0) years and 14.6 (9.6) years in men and 66.0 (10.3) years and 13.0 (10.6) years in women. Among them, 41.5% men and 57.3% women had MetS. In addition, 25.6% men and 25.0% women had aLMM, 17.4% men and 29.9% women had rLMM, 24.0% men and 25.0% women had aLMS, and 51.7% men and 48.8% women had rLMS.
Table 1.
Clinical characteristics of study patients
| Men n = 208 |
Women n = 165 |
p | |
|---|---|---|---|
| Age (years) | 67.0 (11.0) | 66.0 (10.3) | 0.339 |
| Duration of diabetes (years) | 14.6 (9.6) | 13.0 (10.6) | 0.137 |
| Systolic blood pressure (mmHg) | 133.4 (18.1) | 135.2 (19.7) | 0.353 |
| Diastolic blood pressure (mmHg) | 79.2 (10.9) | 78.7 (11.7) | 0.67 |
| Height (cm) | 167.1 (6.2) | 153.3 (5.6) | <0.001 |
| Body weight (kg) | 65.8 (10.8) | 60.3 (13.9) | <0.001 |
| Body mass index (kg/m2) | 23.5 (3.6) | 25.7 (6.1) | <0.001 |
| Appendicular lean mass (kg) | 20.2 (3.5) | 16.1 (3.8) | <0.001 |
| SM/Ht2 (kg/m2) | 7.2 (1.0) | 6.8 (1.6) | 0.009 |
| SM/Wt (%) | 30.9 (4.1) | 26.9 (3.9) | <0.001 |
| Handgrip strength (kg) | 33.6 (7.7) | 21.0 (5.1) | <0.001 |
| Adjusted grip strength (%) | 51.9 (12.5) | 35.8 (9.2) | <0.001 |
| Regular exercises | 50.0% (104) | 44.2% (73) | 0.269 |
| Current smoker | 21.2% (44) | 6.1% (10) | <0.001 |
| Habitual alcohol consumption | 22.1% (46) | 0.6% (1) | <0.001 |
| Aspartate aminotransferase (IU/L) | 24.9 (11.4) | 22.1 (9.2) | 0.014 |
| Alanine aminotransferase (IU/L) | 25.6 (17.7) | 22.1 (15.2) | 0.046 |
| Gamma-glutamyltransferase (IU/L) | 40.2 (38.2) | 28.6 (19.7) | <0.001 |
| Triglycerides (mM) | 1.5 (1.0) | 1.4 (0.8) | 0.281 |
| HDL-C (mM) | 1.5 (0.4) | 1.6 (0.4) | 0.003 |
| HbA1c (%) | 7.4 (1.2) | 7.4 (1.4) | 0.923 |
| HbA1c (mM) | 57.1 (13.3) | 56.9 (15.1) | 0.923 |
| Usage of antihypertensive medications | 55.3% (115) | 52.7% (87) | 0.622 |
| Usage of antidyslipidemia medications | 46.6% (97) | 44.9% (74) | 0.731 |
| Usage of antidiabetic oral medications | 76.4% (159) | 75.8% (125) | 0.878 |
| Usage of GLP-1 analog | 13.9% (29) | 20.0% (33) | 0.119 |
| Usage of insulin | 22.6% (47) | 25.5 (42) | 0.52 |
| Obesity (%) | 31.7% (66) | 40.6% (67) | 0.076 |
| Hypertension (%) | 74.5% (155) | 72.7% (120) | 0.696 |
| Hypertriglycerides (%) | 36.5% (76) | 26.1% (43) | 0.031 |
| Low HDL-C (%) | 7.7% (16) | 21.8% (36) | <0.001 |
| Metabolic syndrome score | 2.5 (0.9) | 2.6 (.1.1) | 0.291 |
| Metabolic syndrome (%) | 53.9% (112) | 47.9% (79) | 0.252 |
| aLMM (%) | 8.7% (18) | 67.3% (111) | <0.001 |
| aLMS (%) | 24.0% (50) | 24.9% (41) | 0.856 |
| Sarcopenia defined by aLMM and aLMS (%) | 3.2% (1) | 18.6% (30) | <0.001 |
| rLMM (%) | 26.1% (43) | 23.0% (37) | 0.518 |
| rLMS (%) | 53.3% (88) | 47.8% (77) | 0.32 |
| Sarcopenia defined by rLMM and rLMS (%) | 17.6% (29) | 15.5% (25) | 0.619 |
Data are expressed as mean (SD) or % (number). The difference between groups was analyzed by Student’s t test or Chi-square test. SM/Ht2, height-adjust skeletal muscle mass index; SM/Wt, weight-adjust skeletal muscle mass index; GLP-1, glucagon-like peptide-1; HDL-C, high-density lipoprotein cholesterol; aLMM, absolute low muscle mass; aLMS, absolute low muscle strength; rLMM, relative low muscle mass; rLMS, relative low muscle strength.
The SM/Ht2 of patients with MetS was higher than that of patients without both in men and women, whereas SM/Wt of patients with MetS was lower than that of patients without both in men and women. Moreover, handgrip strength of patients with MetS tended to be higher than that of patients without both in men and women, whereas adjusted grip strength of patients with MetS was lower than that of patients without both in men and women (Table 2).
Table 2.
Unadjusted and adjusted difference of SM/Ht2, SM/Wt, handgrip strength and adjusted grip strength between the presence or absence of metabolic syndrome
| Men | Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|---|
| MetS (−), n = 121 | MetS (+), n = 86 | p | MetS (−), n = 121 | MetS (+), n = 86 | p | ||
| SM/Ht2 (kg/m2) | 7.3 (0.8) | 7.8 (0.8) | <0.001 | 7.3 (7.1–7.5) | 7.8 (7.6–8.0) | <0.001 | |
| SM/Wt (%) | 32.7 (3.5) | 30.0 (3.1) | <0.001 | 33.1 (32.3–33.8) | 30.3 (29.5–31.1) | <0.001 | |
| Handgrip strength (kg) | 32.5 (7.2) | 35.2 (8.1) | 0.011 | 33.9 (32.4–35.5) | 35.2 (33.6–36.9) | 0.186 | |
| Adjusted grip strength (%) | 52.4 (10.4) | 47.8 (9.5) | 0.001 | 54.4 (52.3–56.6) | 48.8 (46.4–51.2) | <0.001 | |
| Women | Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|---|
| MetS (−), n = 70 | MetS (+), n = 94 | p | MetS (−), n = 70 | MetS (+), n = 94 | p | ||
| SM/Ht2 (kg/m2) | 6.1 (0.7) | 6.6 (0.9) | <0.001 | 6.5 (5.7–7.3) | 6.9 (6.1–7.6) | 0.002 | |
| SM/Wt (%) | 27.5 (2.3) | 24.3 (2.5) | <0.001 | 26.0 (23.3–28.6) | 22.8 (20.3–25.4) | <0.001 | |
| Handgrip strength (kg) | 20.5 (4.8) | 21.3 (5.4) | 0.279 | 23.8 (19.1–28.5) | 23.6 (19.0–28.1) | 0.767 | |
| Adjusted grip strength (%) | 39.5 (8.6) | 33.8 (8.3) | <0.001 | 39.5 (30.5–48.5) | 33.3 (24.6–42.0) | <0.001 | |
SM/Ht2, height-adjusted skeletal muscle mass index; SM/Ht, weight-adjusted skeletal muscle mass index; MetS, metabolic syndrome. Adjusted for age, smoking, exercise, and alcohol intake.
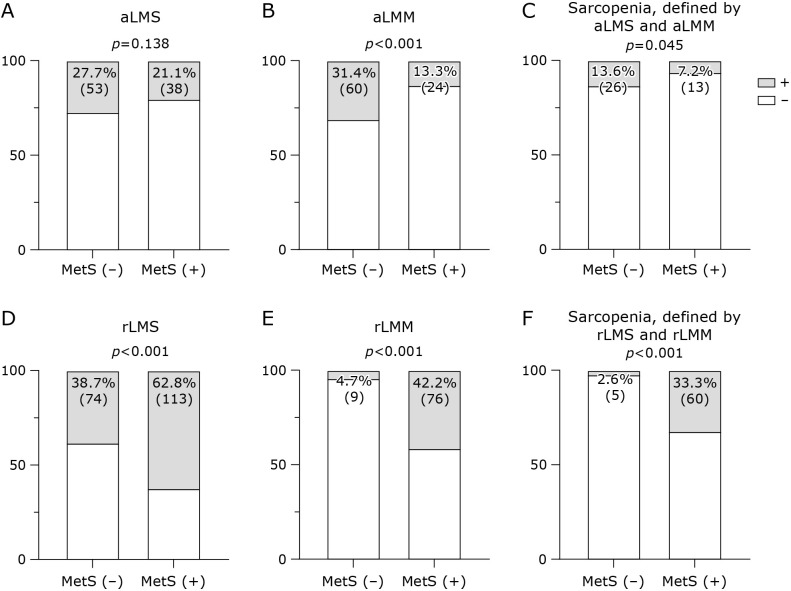
Proportion of aLMM and sarcopenia, defined by aLMM and aLMS in patients with MetS, was lower than those of patients without MetS, and proportion of aLMS in patients with MetS was tended to be lower than that of patients without MetS (Fig. 2). On the other hand, proportions of rLMS, rLMM, and sarcopenia, defined by rLMM and rLMS, in patients with MetS was higher than those of patients without MetS (Fig. 2).
Fig. 2.
Proportion of aLMS, aLMM, sarcopenia, defined by aLMM and aLMS, rLMS, rLMM and sarcopenia, defined by rLMM and rLMS in subjects without and with MetS tended to be lower than that of subjects without MetS. Chi-square tests were performed to evaluate the difference. (A) aLMS, absolute low muscle strength, (B) aLMM, absolute low muscle mass, (C) Sarcopenia, defined by aLMS and aLMM (D) rLMS, relative low muscle strength, (B) rLMM, relative low muscle mass, (C) Sarcopenia, defined by rLMS and rLMM.
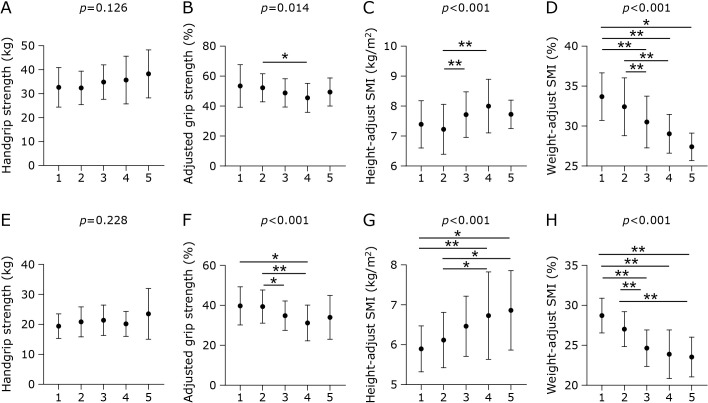
Figure 3 shows the differences in SM/Ht2, SM/Wt, handgrip strength, and adjusted grip strength among metabolic syndrome scores. Interestingly, SM/Ht2 was increased in the order of metabolic syndrome scores, and handgrip strength tended to increase in the order of metabolic syndrome scores in both men and women, whereas SM/Wt and adjusted grip strength decreased in the order of metabolic syndrome scores in both men and women.
Fig. 3.
The difference of height-adjusted skeletal muscle mass index, weight-adjusted skeletal muscle mass index, handgrip strength, and adjusted grip strength among metabolic syndrome scores. The differences were evaluated by Kruskal–Wallis test and steel-dwass test. (A) handgrip strength in men, (B) adjusted grip strength in men, (C) height-adjusted skeletal muscle mass index in men, (D) weight-adjusted skeletal muscle mass index in men, (E) handgrip strength in women, (F) adjusted grip strength in women, (G) height-adjusted skeletal muscle mass index in women, (F) weight-adjusted skeletal muscle mass index in women. *p<0.05 and **p<0.01.
Table 3 shows the ORs of presence of sarcopenia and related factors for the prevalence of MetS. Compared with non-sarcopenia, adjusted OR of sarcopenia defined by aLMM and aLMS for the prevalence of MetS was 0.79 (95% CI 0.38–1.67, p = 0.541) and compared with normal, adjusted ORs of aLMS only, aLMM only, and sarcopenia were 0.83, 0.28, and 0.63, respectively. In contrast, compared with non-sarcopenia, adjusted OR of sarcopenia defined by rLMM and rLMS for the prevalence of MetS was 20.6 (95% CI 7.81–54.3, p<0.001) and compared with normal, adjusted ORs of rLMS only, rLMM only, and sarcopenia were 2.02, 7.02, and 31.2, respectively.
Table 3.
Odds ratios of sarcopenia and related factors for the prevalence of metabolic syndrome
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Normal (n = 235) | Ref | — | Ref | — | |
| aLMS only (n = 52) | 0.84 (0.44–1.59) | 0.589 | 0.83 (0.43–1.58) | 0.569 | |
| aLMM only (n = 45) | 0.28 (0.13–0.60) | <0.001 | 0.28 (0.13–0.60) | <0.001 | |
| Sarcopenia, defined by aLMS and aLMM (n = 39) | 0.62 (0.29–1.33) | 0.22 | 0.63 (0.29–1.37) | 0.244 | |
| Non-sarcopenia (n = 332) | Ref | — | Ref | — | |
| Sarcopenia (n = 39) | 0.78 (0.37–1.63) | 0.504 | 0.79 (0.38–1.67) | 0.541 | |
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Normal (n = 164) | Ref | — | Ref | — | |
| rLMS only (n = 122) | 2.00 (1.20–3.34) | 0.008 | 2.02 (1.20–3.38) | 0.008 | |
| rLMM only (n = 20) | 6.87 (2.11–22.4) | 0.001 | 7.02 (2.14–23.0) | <0.001 | |
| Sarcopenia, defined by rLMS and rLMM (n = 65) | 30.3 (11.1–82.3) | <0.001 | 31.2 (11.4–85.6) | <0.001 | |
| Non-sarcopenia (n = 306) | Ref | — | Ref | — | |
| Sarcopenia (n = 65) | 20.3 (7.72–53.2) | <0.001 | 20.6 (7.81–54.3) | <0.001 | |
aLMM, absolute low muscle mass; aLMS absolute low muscle strength; rLMM, relative low muscle mass; rLMS, relative low muscle strength. Model 1: Adjusted for age and sex. Model 2: Adjusted for age, sex, smoking, exercise, and alcohol intake.
Discussion
This is the first study to investigate the relationship between MetS and sarcopenia in patients with T2D. We observed that handgrip strength and SM/Ht2 of patients with MetS was higher than those without MetS, whereas adjusted grip strength and SM/Wt of patients with MetS was lower than those without MetS. In addition, sarcopenia defined by adjusted grip strength and SM/Wt was associated with the risk of prevalence of MetS, whereas sarcopenia defined by handgrip strength and SM/Ht2 was not associated with the risk of prevalence of MetS.
Previous studies have revealed that rLMM is associated with impaired glucose tolerance,(15,16) NAFLD,(17,18,29) and NASH.(19,20,30)
It is well known that SM/Ht2 and handgrip strength is used as a marker for sarcopenia.(28) However, heavier body weight is associated with more muscle mass, regardless of the fat mass.(17) Thus, there is a possibility that it is not the absolute muscle mass, but the relative muscle mass, which indicated proportion of muscle mass to body weight, is important for metabolic abnormal diseases, including MetS. Decrease in SM/Wt could indicate relative increase of visceral fat and relative decrease of muscle mass. In fact, SM/Wt is reported to be associated with incident diabetes,(15) incident MetS,(22,23) and progression of NAFLD.(31) In addition, previous studies have revealed that SM/Wt, but not SM/Ht2 was associated with insulin resistance.(32–34) In this study, we demonstrated that rLMM, but not aLMM is important for MetS. This result was same as a previous study for NAFLD(35) which is a one of the phenotype of MetS.(21) In addition, we also revealed that low adjusted grip strength, which indicates rLMS but not low handgrip strength, is associated with the prevalence of MetS. Previous studies have revealed that low adjusted grip strength is associated with the prevalence of NAFLD(29) and incident diabetes.(36)
This study also showed that SM/Ht2 increased in the order of metabolic syndrome score, whereas adjusted grip strength and SM/Wt decreased in the order of metabolic syndrome score. These results suggest that accumulation of metabolic abnormalities is associated with a decline in relative lower muscle mass and strength. Taking these findings into account, we should focus on relative low mass and strength in the clinical setting of MetS. Further studies are needed to understand the association between relative lower muscle mass and strength, and absolute lower muscle mass and strength.
The limitations of this study were as follows. First, this is a cross-sectional study; therefore, the causal relationship is unclear. Second, we did not use dual-energy X-ray absorptiometry, although the accuracy of the body composition analyzer has been previously validated.(37) Finally, this study included the Japanese population only; thus, the generalizability of this study to non-Japanese populations, especially non-Asian populations, is unclear.
Conclusion
In conclusion, we discovered that sarcopenia defined by SM/Wt and adjusted grip strength was associated with the risk of prevalence of MetS, whereas sarcopenia defined by SM/Ht2 and handgrip strength was not associated with the risk of prevalence of MetS. Our findings have potential clinical significance because the different definitions for sarcopenia could substantially influence study results, in relation to MetS.
Author Contributions
Conceptualization, YH; data curation, MT, YH, AK, and RS; formal analysis, MT and YH; investigation, YH, MH, AK, RS, TOkamura, NK, TOsaka, HO, NN, SM, TS, EU, MA, MY, and MF; project administration, YH, MH, and EU; supervision, MF; visualization, YH; writing–original draft, MT; and writing–review and editing, YH, MH, AK, RS, TOkamura, NK, TOsaka, HO, NN, SM, TS, EU, MA, MY, and MF.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
YH reports grants from Asahi Kasei Pharma and personal fees from Sanofi K.K., Novo Nordisk Pharma Ltd., Daiichi Sankyo Co. Ltd., and Mitsubishi Tanabe Pharma Corp. outside the submitted work. Dr. Hamaguchi reports grants from Astellas Pharma Inc., Eli Lilly Japan K.K., Mitsubishi Tanabe Pharma Corp., Sanofi K.K., Asahi Kasei Pharma, Novo Nordisk Pharma Ltd., Takeda Pharma Co. Ltd., Kyowa Kirin Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Daiichi Sankyo Co. Ltd., and Sumitomo Dainippon Pharma Co. Ltd. outside the submitted work. MA reports honoraria from AstraZeneca plc, Abbott Japan Co., Ltd., Novo Nordisk Pharma Ltd., Kowa Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., and Sumitomo Dainippon Pharma Co., Ltd. TOsaka reports grants from Combi Corp., and personal fees from Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., MSD K.K., Novo Nordisk Pharma Ltd., Daiichi Sankyo Co. Ltd., Takeda Pharma Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Mitsubishi Tanabe Pharma Corp., Kyowa Kirin Co. Ltd., Kowa Pharma Co. Ltd., Ono Phama Co., Ltd., AstraZeneca plc, Toa Eiyo Corp., outside the submitted work. TS has received personal fees from Eli Lilly Japan K.K., Mitsubishi Tanabe Pharma Co., Kowa Pharma Co., Ltd., Astellas Pharma Inc., Takeda Pharma Co., Ltd., Sanofi K.K., Taisho Toyama Pharma Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Kissei Pharma Co., Ltd., MSD K.K., Novo Nordisk Pharma Ltd., Ono Pharma Co., Ltd. outside the submitted work. EU received grant support from the Astellas Foundation for Research on Metabolic Disorders and the Japanese Study Group for Physiology and Management of Blood Pressure, donated fund Laboratory of Diabetes therapeutics is an endowment department, supported with an unrestricted grant from Ono Pharma. Co., Ltd., and received personal fees from Kyowa Kirin Co. Ltd., AstraZeneca plc, Nippon Boehringer Ingelheim Co. Ltd., Daiichi Sankyo Co. Ltd., Astellas Pharma Inc., Kowa Pharma Co. Ltd., MSD K.K., Novo Nordisk Pharma Ltd., Takeda Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corp., Sumitomo Dainippon Pharma Co. Ltd., and Taisho Toyama Pharma Co., Ltd. outside the submitted work. MY reports personal fees from Daiichi Sankyo Co. Ltd., AstraZeneca plc, MSD K.K., Kowa Pharma Co. Ltd., Ono Phama Co., Ltd., Takeda Pharma Co. Ltd., Kowa Pharma Co. Ltd., Sumitomo Dainippon Pharma Co. Ltd., and Kyowa Kirin Co. Ltd. outside the submitted work. MF received grants from Sanofi K.K., Daiichi Sankyo Co. Ltd., Mitsubishi Tanabe Pharma Corp., Astellas Pharma Inc., Kowa Pharma Co. Ltd., Kissei Phama Co. Ltd., Ono Pharma Co. Ltd., Takeda Pharma Co. Ltd., Eli Lilly Japan K.K., Sanwa Kagagu Kenkyusho Co., Ltd., Teijin Pharma Ltd., Novo Nordisk Pharma Ltd., Kyowa Kirin Co., Ltd., MSD K.K., Sumitomo Dainippon Pharma Co., Ltd., Nippon Chemiphar Co., Ltd., Terumo Corp., Abbott Japan Co., Ltd., Johnson & Johnson K.K. Medical Co., Taisho Pharma Co., Ltd., and Nippon Boehringer Ingelheim Co. Ltd. and received honoraria from Arkray Inc., Sanofi K.K., Daiichi Sankyo Co. Ltd., Takeda Pharma Co. Ltd., Abbott Japan Co., Ltd., Novo Nordisk Pharma Ltd., Teijin Pharma Ltd., Mitsubishi Tanabe Pharma Corp., Nipro Corp., MSD K.K., Medtronic Japan Co. Ltd., Kyowa Kirin Co. Ltd., Mochida Pharma Co. Ltd., Ono Pharma Co. Ltd., Kissei Pharma Co., Ltd., Sumitomo Dainippon Pharma Co. Ltd., Astellas Pharma Inc., Sanwa Kagaku Kenkyusho Co. Ltd., Kowa Pharma Co. Ltd., Taisho Pharma Co., Ltd., Bayer Yakuhin, Ltd., AstraZeneca plc, Eli Lilly Japan K.K., and Nippon Boehringer Ingelheim Co., Ltd. outside the submitted work. The other authors have nothing to disclose.
References
- 1.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002; 288: 2709–2716. [DOI] [PubMed] [Google Scholar]
- 2.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010; 56: 1113–1132. [DOI] [PubMed] [Google Scholar]
- 3.Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, Desouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity (Silver Spring) 2006; 14: 2127–2131. [DOI] [PubMed] [Google Scholar]
- 4.Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem 2014; 395: 203–230. [DOI] [PubMed] [Google Scholar]
- 5.Umegaki H. Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr Gerontol Int 2016; 16: 293–299. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto Y, Kaji A, Sakai R, et al. Sarcopenia is associated with blood pressure variability in older patients with type 2 diabetes: a cross-sectional study of the KAMOGAWA-DM cohort study. Geriatr Gerontol Int 2018; 18: 1345–1349. [DOI] [PubMed] [Google Scholar]
- 7.Landi F, Cruz-Jentoft AJ, Liperoti R, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing 2013; 42: 203–209. [DOI] [PubMed] [Google Scholar]
- 8.Liu P, Hao Q, Hai S, Wang H, Cao L, Dong B. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: a systematic review and meta-analysis. Maturitas 2017; 103: 16–22. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi F, Hashimoto Y, Kaji A, et al. Sarcopenia is associated with a risk of mortality in people with type 2 diabetes mellitus. Front Endocrinol (Lausanne) 2021; 12: 783363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morley JE, Malmstrom TK, Rodriguez-Mañas L, Sinclair AJ. Frailty, sarcopenia and diabetes. J Am Med Dir Assoc 2014; 15: 853–859. [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009; 32 (Suppl 2): S157–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura Y, Omura T, Toyoshima K, Araki A. Nutrition management in older adults with diabetes: a review on the importance of shifting prevention strategies from metabolic syndrome to frailty. Nutrients 2020; 12: 3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamura T, Miki A, Hashimoto Y, et al. Shortage of energy intake rather than protein intake is associated with sarcopenia in elderly patients with type 2 diabetes: a cross-sectional study of the KAMOGAWA-DM cohort. J Diabetes 2019; 11: 477–483. [DOI] [PubMed] [Google Scholar]
- 14.Okamura T, Hashimoto Y, Miki A, et al. Reduced dietary omega-3 fatty acids intake is associated with sarcopenia in elderly patients with type 2 diabetes: a cross-sectional study of KAMOGAWA-DM cohort study. J Clin Biochem Nutr 2020; 66: 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Son JW, Lee SS, Kim SR, et al. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: findings from the KoGES. Diabetologia 2017; 60: 865–872. [DOI] [PubMed] [Google Scholar]
- 16.Takamura T, Kita Y, Nakagen M, et al. Weight-adjusted lean body mass and calf circumference are protective against obesity-associated insulin resistance and metabolic abnormalities. Heliyon 2017; 3: e00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto Y, Osaka T, Fukuda T, Tanaka M, Yamazaki M, Fukui M. The relationship between hepatic steatosis and skeletal muscle mass index in men with type 2 diabetes. Endocr J 2016; 63: 877–884. [DOI] [PubMed] [Google Scholar]
- 18.Hong HC, Hwang SY, Choi HY, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology 2014; 59: 1772–1778. [DOI] [PubMed] [Google Scholar]
- 19.Koo BK, Kim D, Joo SK, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017; 66: 123–131. [DOI] [PubMed] [Google Scholar]
- 20.Petta S, Ciminnisi S, Di Marco V, et al. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2017; 45: 510–518. [DOI] [PubMed] [Google Scholar]
- 21.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005; 143: 722–728. [DOI] [PubMed] [Google Scholar]
- 22.Park SJ, Ryu SY, Park J, Choi SW. Association of sarcopenia with metabolic syndrome in Korean population using 2009–2010 Korea National Health and Nutrition Examination Survey. Metab Syndr Relat Disord 2019; 17: 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park BS, Yoon JS. Relative skeletal muscle mass is associated with development of metabolic syndrome. Diabetes Metab J 2013; 37: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakai R, Hashimoto Y, Ushigome E, et al. Late-night-dinner is associated with poor glycemic control in people with type 2 diabetes: The KAMOGAWA-DM cohort study. Endocr J 2018; 65: 395–402. [DOI] [PubMed] [Google Scholar]
- 25.Kaji A, Hashimoto Y, Sakai R, et al. Frequent usage of convenience stores is associated with low diet quality. Nutrients 2019; 11: 1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimura Y, Wakabayashi H, Nagano F, et al. Sarcopenic obesity is associated with activities of daily living and home discharge in post-acute rehabilitation. J Am Med Dir Assoc 2020; 21: 1475–1480. [DOI] [PubMed] [Google Scholar]
- 28.Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020; 21: 300–307.e2. [DOI] [PubMed] [Google Scholar]
- 29.Gan D, Wang L, Jia M, et al. Low muscle mass and low muscle strength associate with nonalcoholic fatty liver disease. Clin Nutr 2020; 39: 1124–1130. [DOI] [PubMed] [Google Scholar]
- 30.Osaka T, Hashimoto Y, Fukuda T, Tanaka M, Yamazaki M, Fukui M. Relationship between skeletal muscle mass and hepatic fibrosis in patients with type 2 diabetes. Diabetes Metab 2017; 43: 184–186. [DOI] [PubMed] [Google Scholar]
- 31.Osaka T, Hashimoto Y, Okamura T, et al. Reduction of fat to muscle mass ratio is associated with improvement of liver stiffness in diabetic patients with non-alcoholic fatty liver disease. J Clin Med 2019; 8: 2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furushima T, Miyachi M, Iemitsu M, et al. Comparison between clinical significance of height-adjusted and weight-adjusted appendicular skeletal muscle mass. J Physiol Anthropol 2017; 36: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim TN, Park MS, Lee EJ, et al. Comparisons of three different methods for defining sarcopenia: an aspect of cardiometabolic risk. Sci Rep 2017; 7: 6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishida K, Hashimoto Y, Kaji A, et al. Creatinine/(cystatin C × body weight) ratio is associated with skeletal muscle mass index. Endocr J 2020; 67: 733–740. [DOI] [PubMed] [Google Scholar]
- 35.Peng TC, Wu LW, Chen WL, Liaw FY, Chang YW, Kao TW. Nonalcoholic fatty liver disease and sarcopenia in a Western population (NHANES III): the importance of sarcopenia definition. Clin Nutr 2019; 38: 422–428. [DOI] [PubMed] [Google Scholar]
- 36.Brown EC, Buchan DS, Madi SA, Gordon BN, Drignei D. Grip strength cut points for diabetes risk among apparently healthy U.S. adults. Am J Prev Med 2020; 58: 757–765. [DOI] [PubMed] [Google Scholar]
- 37.Kim M, Shinkai S, Murayama H, Mori S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr Gerontol Int 2015; 15: 1013–1022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.