Abstract
Background
Vitamin D deficiency is a common side effect of imatinib mesylate (IM) therapy. Transporter polypeptides involved in the disposition of IM may be required for maintenance of adequate vitamin D concentrations.
Objective
The aim of the present work is to study the association between the plasma concentrations of IM and plasma 25-hydroxyvitamin (25[OH]) D3 with transporter genotypes in patients with chronic myelogenous leukemia.
Methods
A total of 77 adult patients with chronic myelogenous leukemia treated with IM participated in this study. Peak and trough plasma IM and 25(OH) vitamin D3 concentrations were measured and compared to the results of single nucleotide polymorphisms of the efflux transporting gene ABCB1-1236 C>T and the uptake transporting gene OATP1B3-334 T>G. Multiple linear regressions were used to examine the associations between 25(OH) vitamin D3 concentrations and a number of patient characteristics, including responses to therapy. Binary logistic regression analysis was used to predict odd ratios for the clinical response to IM.
Results
Plasma 25(OH) vitamin D3 concentration quartile values were: 25%, 8.2 ng/mL; 50%, 9.8 ng/mL; and 75%, 12 ng/mL. High IM peak concentration, being OATP1B3-334 T>G (TT), and/ or ABCB1-1236 C>T (CT) are associated with lower concentrations of 25(OH) vitamin D3. Moreover, IM peak concentration, IM trough concentration, and plasma concentration of 25(OH) vitamin D3 were associated with the clinical response to IM.
Conclusions
vitamin D, IM concentration, as well as the genotype of OATP1B3-334 T>G, had an influence on the response of patients with chronic myelogenous leukemia.
Keywords: 25(OH) vitamin D3, CML, Imatinib, TKI, Uptake-efflux genes
Graphical abstract
Introduction
Imatinib mesylate was the first selective tyrosine kinase inhibitor (TKI) used for the successful treatment of chronic myelogenous leukaemia (CML).1 However, long-term imatinib therapy has been associated with several adverse effects, including growth limitation,2,3 changes in bone mineral metabolism,4 changes in growth hormone, and deficiency of vitamin D.5 Vitamin D deficiency in patients treated with imatinib may also be caused by a lack of vitamin D consumption, limited sunshine exposure, and medication interactions. However, there is currently limited published research on the role of transporter polypeptides in imatinib-associated vitamin D deficiency5 or on the effects of this deficiency on the clinical response to TKIs in patients with CML.
Cytochrome P450 enzymes are responsible for the primary metabolism of TKIs but the TKIs are carried into and out of cells by different members of the solute carrier and adenosine triphosphate-binding cassette (ABC) families.6,7 Also, these P450 enzymes have a role in both the effectiveness of imatinib therapy and in the synthesis of vitamin D because imatinib decreases P450-associated production of calcidiol and calcitriol.8 It has also been observed that imatinib interferes with CYP27B1 in the kidney, inhibits vitamin D metabolism, and results in vitamin D (calcitriol) insufficiency in patients treated with imatinib.9
It has been suggested that organic cation transporters (OCTs) such as OCT1 and OCTN2, as well as the organic anion transporting polypeptides (OATPs) such as OATP1B3 and OATP1A2 may be involved in the absorption of imatinib by cells that overexpress these transporters.10,11 Vitamin D3 and the vitamin D receptor (VDR) have been shown to regulate the expression of a variety of intestinal membrane transporter genes. Multidrug resistance protein 1 (ABCB1), ABCC2, and ABCC4 mRNA and protein levels were increased by vitamin D3 therapy.12
For example, colorectal and bladder cancers have been linked to low circulating levels of plasma 25-hydroxyvitamin (25[OH]) vitamin D3.13 Even in the context of hematological malignancies, such as leukemia and lymphoma, low 25(OH) vitamin D3 concentrations were associated with a worse outcome.14, 15, 16 Higher levels of 25(OH) vitamin D3 in the blood have been shown to protect against the development of chronic lymphocytic leukemia.17 VDR overexpression has also been found in malignant hematopoietic cells. These findings suggest that vitamin D may have important antitumor effects.15
There are many reasons, including therapy-associated mucositis, that some patients with cancer may not absorb sufficient vitamins from their diet properly. Chemotherapy-induced activation of CYP3A4 or other metabolising enzymes may result in the conversion of 25(OH) vitamin D3 into inert molecules such as 24,25(OH) vitamin D3.18 Published reports suggest that vitamin D deficiency may have clinically important implications. In Philadelphia chromosome-positive leukemia, adequate 25(OH) vitamin D3 concentrations were positively correlated with molecular response.19 A significant decrease in circulating 25(OH) vitamin D3 concentrations in patients with acute leukemia experiencing relapse compared with those in complete remission has also been observed.19 In the present study, we aimed to investigate the relationship between the plasma concentrations of imatinib and 25(OH) vitamin D3 in patients with CML and to evaluate their association with the clinical response to imatinib therapy. In addition, the effect of transporting gene polymorphisms on 25(OH) vitamin D3 concentrations was investigated.
Patients and Methods
Design and sampling
This study was designed as an observational study. A total of 77 patients from the Haematological Outpatient Clinic of the National Cancer Institute, Cairo University, were recruited into this study. The eligible patients were diagnosed with CML. Their age was 18 years or older and they had been treated with imatinib for at least 30 days (to reach a steady state) before study. Peak and trough plasma imatinib concentrations were determined by HPLC-MS/MS as previously described.20 Plasma concentrations of 25(OH) vitamin D3 were determined using an ELISA kit. Single nucleotide polymorphisms of genes involved in imatinib and 25(OH) vitamin D3 uptake and efflux transporters were assessed using restriction fragment length polymorphisms as previously described.20 All other laboratory tests were performed in the clinical laboratory of the National Cancer Institute in Cairo, Egypt, using routine validated methods. These laboratory tests included haemoglobin, white blood cells (WBCs), platelets, aspartate transaminase (AST), alanine transaminase (ALT), creatinine, and other routine biochemical parameters. The study protocol was approved by the institutional review board of the National Cancer Institute of Cairo University, Egypt, and all patients gave written informed consent in compliance with the Declaration of Helsinki (revised 2013). The institutional review board acceptance number for this study is IRB00004025.
Response assessment
All the clinical response data were extracted from the patient files using routine validated methods.21 Over the course of therapy, optimum and poor responses to treatment are monitored using cytogenetic (karyotype and/or Fish) and molecular (BCR-ABL1 mRNA metaphases) indicators, which revealed optimal and unsatisfactory responses, along with treatment failure.
Measurement of 25(OH) vitamin D3 concentrations
Plasma 25(OH) vitamin D3 concentrations were determined using an ELISA kit (Sunlong Biotech, China) and a Tecan plate reader infinite F 50 (Tecan Group Ltd, Mannedorf, Switzerland). The protocol followed the manufacturer's instructions.
Measurement of imatinib mesylate trough and peak concentrations
All patients had been treated with imatinib for at least 30 days (to reach a steady state). Whole blood samples were collected into EDTA-containing tubes just before subsequent drug administration (trough sample) and 2 hours after imatinib administration (peak sample). The plasma was separated by centrifugation at 2500 × g for 10 minutes. The LC-MS/MS system used for plasma separation consisted of an ABSCIEX Q TRAP 3200 mass spectrometer (ABSCIEX, Berlin, Germany) equipped with an ESI interface linked to an Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, California). The method is described in detail in a previously published study.20 Multiple reaction monitoring with ion transitions: m/z 494:394 for imatinib was used for quantification.
Pharmacogenetic analysis
The polymerase chain reaction-restriction fragment length polymorphism method was used for the genotyping. Genotyping data for the efflux transporting gene ABCB1-1236 C>T and the uptake transporting gene OATP1B3-334 T>G was extracted as previously described.20
Statistical analysis
Demographic and clinical data were subjected to descriptive analysis. The 25(OH) vitamin D3 plasma concentration quartiles were determined. The χ2 test, ANOVA test (for normally distributed variables) with Bonferoni adjustment when appropriate, and the Kruskal-Wallis H test (for nonparametric analysis) were used to examine the associations between 25(OH) vitamin D3 quartiles and other patient demographic, genetic, and laboratory data. Pearson correlation coefficients between plasma 25(OH) vitamin D3 and all patient characteristics were calculated.
Multiple linear regression analysis was used to determine the role of various genetic polymorphisms and other factors on the 25(OH) vitamin D3 plasma concentrations measured. The binary logistic regression method was used to predict the dependent variable (response to imatinib). If it was found that the regression coefficients were statistically significant, the 95% CIs were established for the likelihood ratio. Observed versus predicted frequency differences were assessed using the Hosmer-Lemeshow goodness-of-fit test. The statistical program IBM SPSS Statistics (IBM-SPSS Inc, Armonk, New York) was used to perform all statistical tests with a significance threshold of P ˂ 0.05.
Results
Baseline Characteristics
Seventy-seven patients with CML (41 women and 36 men) were treated orally with 400 mg/d imatinib. Patients enrolled in this study had a mean (SD) age of 39 (9) years (range = 20–60 years). The mean (SD) patient weight was 84 (19) kg (range = 44–135 kg). The median levels of hemoglobin, WBCs, platelets, AST, ALT, and creatinine are all shown in Table 1. Mean (SD) imatinib peak and trough concentrations were 2968 (1072) ng/mL and 1500 (690) ng/mL, respectively. Table 1 depicts the frequency distribution of the efflux transporter ABCB1-1236 C>T and the uptake transporting polypeptides OATP1B3-334 T>G, as well as their relationship with plasma 25(OH) vitamin D3 quartiles. The frequency distribution of ABCB1-1236 C>T among our patients was 15.5% (CC), 37.7% (CT), and 46.8% (TT). The frequency distribution of the gene variant OATP1B3-334 T>G among our patients was: 7.8% had the homozygous wild type of TT allele, 29.8% had the heterozygous TG allele, and 62.4% had the variant type GG.
Table 1.
Baseline characteristics of patients by quartile of plasma 25-hydroxyvitamin D3.
| Characteristic | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P value | |
|---|---|---|---|---|---|---|
| ABCB1-1236 C>T† | CC | 1 (8.3) | 2 (16.7) | 5 (41.7) | 4 (33.3) | 0.026* |
| TT | 8 (27.6) | 5 (17.2) | 8 (27.6) | 8 (27.6) | ||
| CT | 10 (27.8) | 10 (27.8) | 9 (25.0) | 7 (19.4) | ||
| OATP1B3-334 T>G† | TT | 6 (100) | 0 (0) | 0 (0) | 0 (0) | 0.03* |
| GG | 7 (14.6) | 14 (29.2) | 17 (35.4) | 10 (20.8) | ||
| TG | 6 (26.1) | 3 (13) | 5 (21.7) | 9 (39.1) | ||
| Response† | Good response | 13 (52.0) | 3 (12.0) | 7 (28.0) | 2 (8.0) | 0.142 |
| Bad response | 6 (11.5) | 14 (26.9) | 15 (28.8) | 17 (32.7) | ||
| Gender† | Female | 10 (24.4) | 11 (26.8) | 9 (22) | 11 (26.8) | 0.81 |
| Male | 9 (25.0) | 6 (16.7) | 13 (36.1) | 8 (22.2) | ||
| Age‡, y | Total: 39 (9.8) | 37 (7) | 41 (8) | 41 (11) | 38 (11) | 0.445 |
| Weight‡, kg | Total: 84 (19) | 88 (16) | 82± 15 | 94 (21) | 72 (16) | 0.008* |
| Hg†, g/dL | Total: 11.7 (1.8) | 11.3 (2.5) | 11.5 (1.5) | 12 (1.6) | 11.7 (1.9) | 0.75 |
| WBCs‡, /mm3 | Total: 8 (21) | 6.6 (5) | 5.4 (1.5) | 14 (39) | 6.8 (5.8) | 0.52 |
| Platelets‡, /mm3 | Total: 206 (115) | 175 (66) | 241 (189) | 228 (101) | 179 (65) | 0.198 |
| AST‡, IU | Total: 25 (13) | 24 (6) | 24 (7) | 23 (7) | 29 (25) | 0.618 |
| ALT‡, IU | Total: 21 (11) | 24 (14) | 21 (11) | 20 (10) | 18 (8) | 0.521 |
| Creatinine‡, mg/dL | Total: 0.88 (0.2) | 0.9 (0.2) | 0.86 (0.2) | 0.85 (0.2) | 0.9 (0.2) | 0.286 |
| Imatinib peak level‡, ng/mL | Total: 2968 (1072) | 3490 (1219) | 3095 (1059) | 2843 (953) | 2476 (856) | 0.051 |
| Imatinib trough level‡, ng/mL | Total: 1501 (690) | 1694 (674) | 1718 (789) | 1327 (705) | 1307 (517) | 0.11 |
| Duration of imatinib therapy‡ months | Total: 27.5 (22) | 24 (18) | 32 (25) | 27 (22) | 25 (21) | 0.97 |
ALT = alanine aminotransferase; AST = alanine aminotransferase; Hg = hemoglobin; WBCs = white blood cells.
Statistically significant p-value less than 0.05.
Values are presented as n (%).
Values are presented as mean (SD).
Distribution of 25(OH) vitamin D3 concentrations
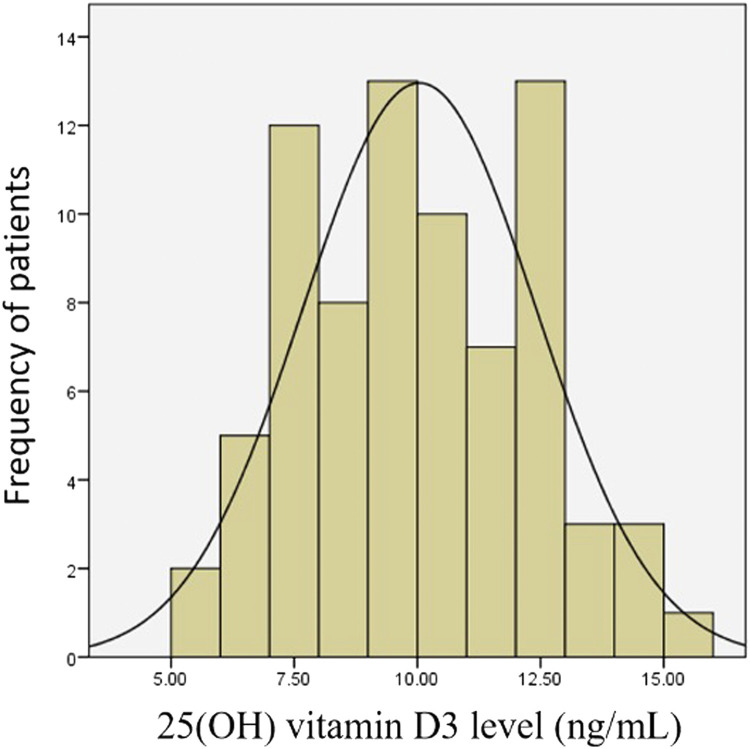
Plasma 25(OH) vitamin D3 concentrations measured in these patients were normally distributed as shown in (Figure). All of the patients’ concentrations were below the laboratory's lower limit of normal (<20 ng/mL) of 25(OH) vitamin D3. The 25(OH) vitamin D3 concentration quartiles were: 25%, 8.2 ng/mL; 50%, 9.8 ng/mL; and 75%, 12 ng/mL.
Figure.
Distribution of plasma 25-hydroxyvitamin (25[OH]) vitamin D3 concentrations in the study cohort (N = 77).
Patients' characteristics versus quartiles of 25(OH) vitamin D3 concentrations are shown in Table 1. The relationships between 25(OH) vitamin D3 concentration and each of the demographic and clinical characteristics of our patients showed no significant difference for all parameter, including gender, duration of therapy, hemoglobin level, WBCs, platelets, AST, ALT, creatinine concentration, imatinib peak concentration, and imatinib trough concentration (P > 0.05). However, there was a significant relationship between a patient's weight and the concentration of 25(OH) vitamin D3. There were also significant relationships between 25(OH) vitamin D3 and the heterozygote-type CT of ABCB1-1236 C>T, and the wild-type GG of OATP1B3-334 T>G (P ˂ 0.05) (Table 1). Vitamin D concentrations showed nonsignificant correlation with all of the patients’ laboratory and demographic data except for patient's weight and imatinib peak concentration. There were also significant negative correlations between both 25(OH) vitamin D3 concentration and weight as well as imatinib peak concentration (r = –0.261 and –0.304, respectively; P < 0.05).
Variables associated with vitamin D concentration
For studying variables associated with vitamin D concentration, multiple linear regression analyses were performed with 25(OH) vitamin D3 as the dependent variable. In Table 2, the results of the multiple linear regression analyses are presented; imatinib peak, ABCB1-1236 C>T and OATP1B3-334 T>G were the independent factors tested for prediction of 25(OH) vitamin D3. Both OATP1B3-334T>G (TT) and ABCB1- 1236 C>T (CT) had negative associations with 25(OH) vitamin D3 concentration. Moreover, high imatinib peak concentration also had a negative association with 25(OH) vitamin D3 concentrations. The plasma concentration of 25(OH) vitamin D3 decreased by 0.001 ng/mL for every 1 ng/mL increase in imatinib peak concentration (95% CI, –0.001 to 0.0). For patients having OATP1B3-334 T>G (TT), the plasma concentration of 25(OH) vitamin D3 was decreased by 4.46 ng/mL compared with patients having OATP1B3-334 T>G (GG) (95% CI, –2.575 to –0.824). For patients having ABCB1-1236 C>T (CT), the plasma concentration of 25(OH) vitamin D3 decreased by ∼1 ng/mL compared with patients having ABCB1-1236 C>T (TT) (95% CI, –0.97 to –1.031) (Table 2).
Table 2.
Relationship of plasma 25-hydroxyvitamin Dvitamin D3 levels to selected patient characteristics.
| Model | Unstandardized coefficient | Significance | 95% CI |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Imatinib peak level, ng/mL | –0.001 | 0.018 | –0.001 | 0.000 |
| OATP1B3-334 T>G (TT) | –4.459 | 0.000 | –6.343 | –2.575 |
| ABCB1-1236 C>T (CT) | –0.989 | 0.039 | –1.928 | –0.049 |
Statistically significant p-value less than 0.05.
Variables associated with response to imatinib
Good clinical responses were achieved in 52 (67.5%) patients. These good responses included patients who achieved complete molecular response (CMR), major molecular response (MMR), or complete cytogenetic response. A binary logistic regression model was used to predict the response to imatinib based on independent factors (predictors).
Table 3 shows the parameters used in the regression models, their SE, and the odds ratios with 95% CIs. The results show that imatinib peak, imatinib trough, as well as the second, third, and fourth quartiles of 25(OH) vitamin D3 compared with the first quartile, were independent factors that had a substantial influence on imatinib response.
Table 3.
Odds ratio for response to imatinib by quartile of plasma 25-hydroxyvitamin D (25[OH]) vitamin D3 levels and plasma imatinib levels.
| B Unstandardized coefficient | Significance | Odds ratio | 95% CI |
||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Imatinib peak, ng/mL | –0.001 | 0.015 | 0.999 | 0.998 | 1.000 |
| Imatinib trough, ng/mL | 0.003 | 0.001 | 1.003 | 1.001 | 1.005 |
| 25(OH) vitamin D3 | |||||
| Quartile 2 | 2.748 | 0.005 | 15.615 | 2.325 | 104.866 |
| Quartile 3 | 2.717 | 0.003 | 15.141 | 2.457 | 93.318 |
| Quartile 4 | 3.637 | 0.001 | 37.968 | 4.152 | 347.166 |
Statistically significant p-value less than 0.05.
The response to imatinib decreased by ∼1 time for every 0.001 ng/mL increase in the imatinib peak concentration (95% CI, 0.998–1.00). Also, the response to imatinib increased by ∼1 time for every 0.003 ng/mL increase in the imatinib trough concentration (95% CI, 1.001–1.005) (Table 3).
It was found that the response to imatinib was increased by 15.6, 15.1, and 38 times in patients in the second, third, and fourth vitamin D quartiles in comparison to the 25(OH) vitamin D3 first quartile (95% CI, 2.325–104.866, 2.457–93.318, and 4.152–347.166), respectively.
Discussion
In this study, the influence of imatinib concentrations on plasma 25(OH) vitamin D3 concentrations was examined across multiple strata of the characteristics of 77 patients with CML. The Endocrine Society has defined 25(OH) vitamin D3 deficiency as concentrations below 20 ng/mL, whereas insufficiency was defined as 25(OH) vitamin D3 concentrations between 21 and 29 ng/mL.22 All of our patients were deficient because all had 25(OH) vitamin D3 concentrations <20 ng/mL. Patients with CML, as well as many healthy individuals, are at risk for vitamin D insufficiency.23 Insufficient 25(OH) vitamin D3 levels are associated with decreased chances of recurrence-free survival in patients with cancer.24 Patients with acute leukemia who relapsed were found to have lower blood 25(OH) vitamin D3 concentrations than those who were completely free of disease.19 The prevalence of 25(OH) vitamin D3 insufficiency in hematological malignancies was high and there was a strong correlation between the lowest blood concentrations and the most aggressive phases of the disease, as well as with a poor response to treatment.25 Furthermore, the present study showed that high 25(OH) vitamin D3 concentrations were associated with low imatinib peak concentrations. An independent study also found low vitamin D3 concentrations in children with CML treated with imatinib.4 Lack of sun exposure or poor diet are 2 possible alternative or contributing causes, whereas other possibilities include medication interactions or a combination of these causes.26 However, an inhibitory effect of imatinib on the synthesis of calcidiol and calcitriol in human keratinocytes has been reported.8
The development of some diseases, including cancer, can be influenced significantly by variations in an individual patient's DNA. Vitamin D3 concentrations and the VDR have been shown to regulate the expression of a variety of intestinal membrane transporter genes. Because vitamin D3 therapy has been shown to enhance mRNA and protein levels of ABCB1, ABCC2, and ABCC4,12 the associations were examined in this study between different genes and 25(OH) vitamin D3 concentrations. ABCB1-1236 C>T, OATP1B3-334 T>G, age, and weight all showed significant associations with 25(OH) vitamin D3 concentrations.
When multiple linear regression analysis was performed with 25(OH) vitamin D3 as the dependent variable, it was found that OATP1B3-334 T>G (TT) had a positive association with 25(OH) vitamin D3 concentration. 25(OH) vitamin D3 is a known substrate for OATP2B1 and OATP1B3, providing a pathway for its excretion from the body through the liver.27 This suggests that the intestinal absorption and excretion of 25(OH) vitamin D3 from the body is affected by OATP1B1/OATP1B3-mediated hepatic uptake.27 ABCB1-1236 C>T (CT) was also found to have a negative association with 25(OH) vitamin D3 concentration. Moreover, high imatinib peak concentration had a negative association with 25(OH) vitamin D3 concentration. Hypophosphatemia is common in patients with CML receiving imatinib, and it is linked to a decrease in 25(OH) vitamin D3 (calcidiol) and 1, 25-dihydroxy vitamin D3 (calcitriol).4
The 25(OH) vitamin D3 concentration quartiles were found by binary logistic regression to correlate with the response to imatinib that was independent of the effect of imatinib trough and peak concentrations. In a non–small-cell lung cancer model, vitamin D analogues were found to enhance the anticancer effect of imatinib.28 In comparison to individuals with a CMR, those with only an MMR had lower vitamin D concentrations. Patients using dasatinib and vitamin D supplementation showed a shift from MMR to CMR in patients who were treated with a TKI for a period of at least 4 years.29
Observational studies have also shown that low concentrations of 25(OH) vitamin D3 are associated with an increased risk of multiple types of cancer.30 A 25(OH) vitamin D3 concentration >20 ng/mL at the time of diagnosis and throughout cancer therapy might improve the prognosis in many diseases.31,32 For example, high concentrations of 25(OH) vitamin D3 were associated with reduced development of leukemia.33 Also, It has been found that 25(OH) vitamin D3 concentrations below 20 ng/mL are linked to an increased risk of solid tumors and a higher death rate from these malignancies30,34,35 as well as to increased cancer incidence and death.36,37 It has been reported that 1,25(OH)2 vitamin D stimulates differentiation of mouse myeloid leukemia cells38 and enhances survival in mice injected with murine myeloid leukemia cells.39
Our patients had very low concentrations of 25(OH) vitamin D3. On the other hand, studies in both experimental and clinical settings have shown a synergistic effect between vitamin D supplementation and cancer therapy.40,41 Also, clinical remission of chronic lymphocytic leukemia has been linked to sufficient vitamin D consumption.42 25(OH) vitamin D3 concentrations are affected by the disease, either directly or indirectly; especially if patients with advanced disease are less able to participate in outdoor activities or have suboptimal eating habits.19
Study limitations
There are some limitations in our study, including the fact that we only examined 1 sample of 25(OH) vitamin D3 from each patient without collecting baseline samples. The change in 25(OH) vitamin D3 concentrations over time may be more informative. We also did not collect information on dietary intake, nonprescribed supplements, sun exposure, and so on. Additional therapeutic trials may be too costly to justify unless the possibility of therapeutic benefit is judged to outweigh any costs. However, the fact that increasing quartiles of 25(OH) vitamin D3 concentrations, as well as both peak and trough imatinib concentration were positively correlated with the patients’ response support the need to study or consider vitamin D supplementation in patients with CML treated with imatinib.
Conclusions
The present study found that OATP1B3-334 T>G (TT), ABCB1- 1236 C>T (CT), and high peak imatinib concentration all had a negative correlation with 25(OH) vitamin D3 concentration. These data support the hypothesis that genetic differences in transporter polypeptides play a role in imatinib effects on both vitamin D concentrations and clinical effects.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
The authors thank the National Cancer Institute, Cairo University, for providing facilities used for this work.
M. Hamza, M. Omran, and S. Shouman contributed to the study design, data collection, analysis, interpretation of results and manuscript drafting as well as revision. R. Abdelfattah and H. Moussa contributed to data collection and analysis. All authors approved the final version of the manuscript.
References
- 1.Andolina J.R., Neudorf S.M., Corey S.J. How I treat childhood CML. Blood. 2012;119(8):1821–1830. doi: 10.1182/blood-2011-10-380774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narayanan K.R., et al. Growth failure in children with chronic myeloid leukemia receiving imatinib is due to disruption of GH/IGF-1 axis. Pediatr Blood Cancer. 2013;60(7):1148–1153. doi: 10.1002/pbc.24397. [DOI] [PubMed] [Google Scholar]
- 3.Shima H., et al. Distinct impact of imatinib on growth at prepubertal and pubertal ages of children with chronic myeloid leukemia. J Pediatr. 2011;159(4):676–681. doi: 10.1016/j.jpeds.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Jaeger B.A., et al. Changes in bone metabolic parameters in children with chronic myeloid leukemia on imatinib treatment. Med Sci Monit. 2012;18(12):Cr721–Cr728. doi: 10.12659/MSM.883599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choeyprasert W., et al. Adverse effects of imatinib in children with chronic myelogenous leukemia. Pediatr Int. 2017;59(3):286–292. doi: 10.1111/ped.13136. [DOI] [PubMed] [Google Scholar]
- 6.Neul C., et al. Impact of Membrane Drug Transporters on Resistance to Small-Molecule Tyrosine Kinase Inhibitors. Trends Pharmacol Sci. 2016;37(11):904–932. doi: 10.1016/j.tips.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Gong L., et al. PharmGKB summary: sorafenib pathways. Pharmacogenet Genomics. 2017;27(6):240–246. doi: 10.1097/FPC.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehlig L.M., et al. Inhibitory effects of imatinib on vitamin D₃ synthesis in human keratinocytes. Mol Med Rep. 2015;11(4):3143–3147. doi: 10.3892/mmr.2014.3074. [DOI] [PubMed] [Google Scholar]
- 9.Bikle D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas J., et al. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. 2004;104(12):3739–3745. doi: 10.1182/blood-2003-12-4276. [DOI] [PubMed] [Google Scholar]
- 11.Hu S., et al. Interaction of imatinib with human organic ion carriers. Clin Cancer Res. 2008;14(10):3141–3148. doi: 10.1158/1078-0432.CCR-07-4913. [DOI] [PubMed] [Google Scholar]
- 12.Fan J., et al. Up-regulation of transporters and enzymes by the vitamin D receptor ligands, 1alpha,25-dihydroxyvitamin D3 and vitamin D analogs, in the Caco-2 cell monolayer. J Pharmacol Exp Ther. 2009;330(2):389–402. doi: 10.1124/jpet.108.149815. [DOI] [PubMed] [Google Scholar]
- 13.Mondul A.M., et al. Vitamin D and Cancer Risk and Mortality: State of the Science, Gaps, and Challenges. Epidemiol Rev. 2017;39(1):28–48. doi: 10.1093/epirev/mxx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W., et al. Serum 25-Hydroxyvitamin D Levels and Prognosis in Hematological Malignancies: A Systematic Review and Meta-Analysis. Cellular Physiology and Biochemistry. 2015;35(5):1999–2005. doi: 10.1159/000374007. [DOI] [PubMed] [Google Scholar]
- 15.Kulling P.M., et al. Vitamin D in hematological disorders and malignancies. European Journal of Haematology. 2017;98(3):187–197. doi: 10.1111/ejh.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radujkovic A., et al. Low serum vitamin D levels are associated with shorter survival after first-line azacitidine treatment in patients with myelodysplastic syndrome and secondary oligoblastic acute myeloid leukemia. Clinical Nutrition. 2017;36(2):542–551. doi: 10.1016/j.clnu.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Łuczyńska A., et al. Plasma 25-hydroxyvitamin D concentration and lymphoma risk: results of the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2013;98(3):827–838. doi: 10.3945/ajcn.112.054676. [DOI] [PubMed] [Google Scholar]
- 18.Fakih M.G., et al. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. International journal of colorectal disease. 2009;24(2):219–224. doi: 10.1007/s00384-008-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas X., et al. Serum 25-hydroxyvitamin D levels are associated with prognosis in hematological malignancies. Hematology. 2011;16(5):278–283. doi: 10.1179/102453311X13085644679908. [DOI] [PubMed] [Google Scholar]
- 20.Omran M.M., et al. Association of the Trough, Peak/Trough Ratio of Imatinib, Pyridine–N-Oxide Imatinib and ABCG2 SNPs 34 G>A and SLCO1B3 334 T>G With Imatinib Response in Egyptian Chronic Myeloid Leukemia Patients. Frontiers in Oncology. 2020:10. doi: 10.3389/fonc.2020.01348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baccarani M., et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holick M.F., et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 23.Elkerdany T., Eissa D., Moussa M. Serum 25-hydroxyvitamin D levels in relation to disease status and prognosis in acute myeloid leukemia. The Egyptian Journal of Haematology. 2014;39(2):47–51. [Google Scholar]
- 24.Lee H.J., et al. Low 25(OH) vitamin D3 levels are associated with adverse outcome in newly diagnosed, intensively treated adult acute myeloid leukemia. Cancer. 2014;120(4):521–529. doi: 10.1002/cncr.28368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W., et al. Serum 25-hydroxyvitamin D levels and prognosis in hematological malignancies: a systematic review and meta-analysis. Cell Physiol Biochem. 2015;35(5):1999–2005. doi: 10.1159/000374007. [DOI] [PubMed] [Google Scholar]
- 26.Genc D.B., Ozkan M.A., Buyukgebiz A. Vitamin D in childhood cancer: a promising anticancer agent? Pediatr Endocrinol Rev. 2013;10(4):485–493. [PubMed] [Google Scholar]
- 27.Gao C., et al. Hepatic Transport of 25-Hydroxyvitamin D(3) Conjugates: A Mechanism of 25-Hydroxyvitamin D(3) Delivery to the Intestinal Tract. Drug Metab Dispos. 2018;46(5):581–591. doi: 10.1124/dmd.117.078881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maj E., et al. Vitamin D Analogs Potentiate the Antitumor Effect of Imatinib Mesylate in a Human A549 Lung Tumor Model. International journal of molecular sciences. 2015;16(11):27191–27207. doi: 10.3390/ijms161126016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campiotti L., et al. Vitamin D and tyrosine kinase inhibitors in chronic myeloid leukemia. Internal and Emergency Medicine. 2018;13(8):1337–1339. doi: 10.1007/s11739-018-1957-0. [DOI] [PubMed] [Google Scholar]
- 30.Garland C.F., et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96(2):252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robsahm T.E., et al. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway) Cancer Causes Control. 2004;15(2):149–158. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- 32.Zhou W., et al. Circulating 25-hydroxyvitamin D levels predict survival in early-stage non-small-cell lung cancer patients. J Clin Oncol. 2007;25(5):479–485. doi: 10.1200/JCO.2006.07.5358. [DOI] [PubMed] [Google Scholar]
- 33.Giovannucci E., et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 34.Gorham E.D., et al. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol. 2005;97(1-2):179–194. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Ahonen M.H., et al. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland) Cancer Causes Control. 2000;11(9):847–852. doi: 10.1023/a:1008923802001. [DOI] [PubMed] [Google Scholar]
- 36.Freedman D., Freedman DM, Looker AC, Chang S-C, Graubard BI, et al. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99:1594–1602. doi: 10.1093/jnci/djm204. Journal of the National Cancer Institute99: p. 1594-602. [DOI] [PubMed] [Google Scholar]
- 37.Tangpricha V., et al. Prevalence of vitamin D deficiency in patients attending an outpatient cancer care clinic in Boston. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2004;10(3):292–293. doi: 10.4158/EP.10.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abe E., et al. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A, 1981;78(8):4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honma Y., et al. 1 alpha,25-Dihydroxyvitamin D3 and 1 alpha-hydroxyvitamin D3 prolong survival time of mice inoculated with myeloid leukemia cells. Proc Natl Acad Sci U S A, 1983;80(1):201–204. doi: 10.1073/pnas.80.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deeb K.K., Trump D.L., Johnson C.S. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 41.Dunlap N., et al. 1alpha,25-dihydroxyvitamin D(3) (calcitriol) and its analogue, 19-nor-1alpha,25(OH)(2)D(2), potentiate the effects of ionising radiation on human prostate cancer cells. British journal of cancer. 2003;89(4):746–753. doi: 10.1038/sj.bjc.6601161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Politzer W.M. Long-term clinical remission of chronic lymphocytic leukaemia by dietary means. S Afr Med J. 2005;95(5):321–322. [PubMed] [Google Scholar]