Summary
Background
Effective surveillance strategies are required for patients diagnosed with oesophageal squamous cell carcinoma (OSCC) or adenocarcinoma (OAC) for whom chemoradiotherapy (CRT) is used as a potentially-curative, organ-sparing, alternative to surgery. In this study, we evaluated the safety, acceptability and tolerability of a non-endoscopic immunocytological device (the Cytosponge™) to assess treatment response following CRT.
Methods
This multicentre, single-arm feasibility trial took place in 10 tertiary cancer centres in the UK. Patients aged at least 16 years diagnosed with OSCC or OAC, and who were within 4-16 weeks of completing definitive or neo-adjuvant CRT, were included. Participants were required to have a Mellow-Pinkas dysphagia score of 0-2 and be able to swallow tablets. All patients underwent a single Cytosponge™ assessment in addition to standard of care (which included post-treatment endoscopic evaluation with biopsy for patients undergoing definitive CRT; surgery for those who received neo-adjuvant CRT). The primary outcome was the proportion of consented, evaluable patients who successfully underwent Cytosponge™ assessment. Secondary and tertiary outcomes included safety, study consent rate, acceptance rate, the suitability of obtained samples for biomarker analysis, and the comparative efficacy of Cytosponge™ to standard histology (endoscopy and biopsy or post-resection specimen) in assessing for residual disease. The trial is registered with ClinicalTrials.gov, NCT03529669.
Findings
Between 18th April 2018 and 16th January 2020, 41 (42.7%; 95% confidence interval (CI) 32.7-53.2) of 96 potentially eligible patients consented to participate. Thirty-nine (95.1%, 95% CI 83.5-99.4) successfully carried out the Cytosponge™ procedure. Of these, 37 (95%) would be prepared to repeat the procedure. There were only two grade 1 adverse events attributed to use of the Cytosponge™. Thirty-five (90%) of the completed Cytosponge™ samples were suitable for biomarker analysis; 29 (83%) of these were concordant with endoscopic biopsies, three (9%) had findings suggestive of residual cancer on Cytosponge™ not found on endoscopic biopsies, and three (9%) had residual cancer on endoscopic biopsies not detected by Cytosponge™.
Interpretation
Use of the CytospongeTM is safe, tolerable, and acceptable for the assessment of treatment response following CRT in OAC and OSCC. Further evaluation of Cytosponge™ in this setting is warranted.
Funding
Cancer Research UK, National Institute for Health Research, Medical Research Council.
Keywords: Oesophageal Cancer, Chemoradiation, Radiation, Surveillance, Cytosponge
Research in context.
Evidence before this study
Chemoradiotherapy (CRT) achieves complete pathological response in a significant proportion of patients diagnosed with oesophageal squamous cell carcinoma (OSCC) and oesophageal adenocarcinoma (OAC). These patients may be able to avoid surgery, which is associated with high rates of mortality as well as significant and prolonged morbidity. However locoregional recurrence remains common. Effective post-CRT surveillance strategies are therefore important for detecting patients with early recurrence who may be amenable for salvage resection or other locoregional therapies. However, current endoscopic and cross-sectional imaging approaches are insufficiently sensitive and endoscopy is invasive. The Cytosponge™ is a novel, minimally invasive, non-endoscopic pan-oesophageal immunocytological device that has recently been shown to result in improved detection of Barrett's oesophagus in patients with reflux symptoms. In this setting, it is safe, acceptable and tolerable. There are no prior reports of its use for treatment response assessment following radiotherapy in patients with established malignancy.
Added value of this study
This is the first study to demonstrate that the Cytosponge™ is safe, acceptable to patients and tolerable when used for assessing treatment response following CRT for OSCC and OAC. This work also demonstrates that assessment for residual tumour using the Cytosponge™ appears broadly concordant with assessment of resection or biopsy tissue. For a small number of patients, the Cytosponge™ either identified or missed high-risk features respectively not seen or known to be present on post-treatment biopsy or resection tissue.
Implications of all the available evidence
The Cytosponge™ may provide a novel, safe and acceptable option for response assessment and surveillance in patients with OSCC and OAC who receive CRT. This could be potentially used either as primary tool for oesophageal surveillance (with ‘triggered’ endoscopy for abnormal results) or as an adjunct to endoscopic surveillance. This may facilitate a shift in the standard of care from upfront surgery to the use of organ-sparing CRT, with surgery reserved as a salvage procedure. Phase 2/3 studies are now required to define the role of Cytosponge™ in the non-surgical management of localised oesophageal cancer.
Alt-text: Unlabelled box
Introduction
Oesophageal cancer is a leading cause of cancer-related morbidity and mortality worldwide.1 Two main histopathological subtypes predominate; oesophageal squamous cell carcinoma (OSCC) and oesophageal adenocarcinoma (OAC). Concurrent chemoradiotherapy (CRT) plays a major role in the curative management of both. For patients diagnosed with locoregional OSCC, definitive CRT (dCRT) and neoadjuvant CRT (naCRT) followed by surgical resection are both standards of care and deliver equivalent survival outcomes.2, 3, 4, 5 In the UK, upfront organ-preserving dCRT is used in a majority of patients diagnosed with potentially-curative OSCC.1 Surgery is the standard of care in cases of locoregional OAC; either following naCRT or in combination with peri-operative systemic anti-cancer therapy (SACT).5,6 In contrast, dCRT is presently reserved for patients diagnosed with OAC who are not suitable for surgery due to fitness or as a consequence of the local extent of their disease, or who do not proceed to surgery due to patient or clinician choice.5
Oesophageal resection requires extensive surgery and is associated with substantial post-operative mortality rates of between 1–5%, coupled with significant and lasting morbidity. Short-term post-operative complications are reported in between 30–70% of patients and contribute to prolonged hospitalisation and poorer survival outcomes.7, 8, 9, 10 Health-related quality of life is reduced in the short and long term, and remains impaired for at least ten years following oesophageal cancer surgery.11,12 This is likely to be a consequence of the major, permanent anatomical and physiological changes that follow surgical reconstruction.11
In contrast, CRT offers an organ-sparing treatment approach that achieves complete pathological response in between 25-49% of patients.13 Most patients regain their quality of life shortly after receiving CRT.14 Given this, the use of dCRT followed by active surveillance has been proposed as an alternative to upfront surgery. This may allow a proportion of patients to avoid the high-rates of morbidity and mortality associated with an upfront surgical procedure, with oesophagectomy instead reserved for those with residual disease following CRT. Survival outcomes following the use of this strategy appear promising, though the results of ongoing randomized controlled trials are awaited.15,16 Early use of a brachytherapy boost may also be considered where patients have residual disease but are unfit for salvage resection.17
An effective active surveillance strategy is contingent on the timely and accurate identification of both residual and recurrent disease following CRT. Presently, there is no standard of care for surveillance after CRT in the UK. Options include cross-sectional imaging and invasive endoscopic biopsies and endoscopic ultrasound (EUS). However, all three approaches are resource-intensive and have a poor negative predictive value for identifying persistent or recurrent disease in the early post-treatment period.18, 19, 20 Moreover, frequent endoscopic surveillance may be unacceptable to patients. In view of this, and if an upfront organ preservation strategy is to be pursued for oesophageal cancers, there is a need to develop an accurate, minimally invasive and acceptable surveillance tool for early detection post-CRT recurrence.
One potential novel strategy is frequent oesophageal sampling using the non-endoscopic immunocytological Cytosponge™ device. This is a single-use, non-sterile device comprising of a medical-grade 3cm diameter mesh compressed within a bovine gelatine capsule and attached to a thread, as previously described.21 This may be administered in an out-patient setting, during which the Cytosponge™ is swallowed by a patient and allowed to reach the stomach, where it is left in-situ for five minutes whilst remaining attached to the thread. This time period allows for the gelatine capsule to dissolve within stomach acid, permitting expansion of the Cytosponge™ mesh. This is then drawn back, causing it to collect cells as it moves proximally through the oesophagus towards the mouth.
Being a minimally invasive procedure, Cytosponge™ can be used for primary frequent surveillance of the oesophagus with high risk results then triggering formal evaluation by standard endoscopy. Alternatively, the Cytosponge™, which samples the entire length of oesophageal superficial mucosa, may be a useful adjunct to endoscopic surveillance which is observer-dependent and only samples small segments of the oesophageal mucosa. However, whilst the safety, acceptability and accuracy of the Cytosponge™ is now well established in large cohorts of patients with reflux disease and those diagnosed with Barrett's oesophagus, there are no previous reports of its use in patients who already have a diagnosis of OAC or OSCC and who have received cancer treatment.21, 22, 23
Given this, we conducted a feasibility study to determine the completion rate, safety and acceptability of the use of Cytosponge™ in patients who have recently received CRT.
Methods
Study design and participants
This multicentre single-arm feasibility study took place in ten tertiary UK National Health Service (NHS) cancer centres (Appendix 1, Supp. Table 1). Patients aged 16 years or older with a diagnosis of oesophageal cancer were eligible for inclusion if they were within 4-16 weeks of completion of CRT. As this is a feasibility trial with completion rate, safety and tolerability as primary outcomes, patients who had received either dCRT or naCRT were included in order to facilitate timely recruitment. Participants were required to be physiologically fit for endoscopy/surgery, to have a Mellow-Pinkas dysphagia score of between 0–2 and to be able to swallow tablets. Those known to have oesophageal varices, an oesophageal stent or an oesophageal stricture requiring dilatation were excluded. Patients managed with oral anticoagulants that they were unable to temporarily discontinue and who were not therefore suitable candidates for endoscopic biopsy were also excluded.
The initial study protocol and all subsequent amendments were reviewed and approved by the South Central – Oxford B Health Research Authority Research Ethics Committee (17/SC/0661). Authorisation for the use of the Cytosponge™, which was not CE marked, was provided by the Medicine and Healthcare product Regulatory Agency. The legal manufacturer of the Cytosponge™ Investigational Medical Device (IMD) was Cambridge University Hospitals NHS Foundation Trust, though devices were produced on licence by Europlaz, Essex, UK. All patients provided written informed consent prior to data collection and before undergoing study procedures. An initial target of 50 participants was revised to 40 participants after the study had commenced due to delays in opening participating sites. Data are reported here in compliance with the CONsolidated Standards Of Reporting Trials (CONSORT) Statement.
Procedures
Participants underwent a Cytosponge™ test in an outpatient setting at a single time point 4-16 weeks after receiving their final fraction of radiotherapy. Cytosponge™ test was done in addition to routine care, such that patients who had received dCRT proceeded to their planned post-treatment response assessment endoscopy within the same period, and such that those who received naCRT proceeded to surgery irrespective of the Cytosponge™ test. The Cytosponge™ was not used to dictate clinical management and no longer-term evaluation of patient outcomes was undertaken beyond the assessments outlined here.
Potentially eligible participants were identified from those undergoing or who had recently completed CRT, as well as from relevant multidisciplinary team (MDT) meeting and endoscopy lists. Eligible participants were approached whilst receiving CRT or during their routine post-treatment follow-up. Any reason given by potentially eligible participants for not enrolling in the study was recorded in the screening logs. In the week prior to the Cytosponge™ evaluation, a full medical history and physical examination were documented, as was each patient's dysphagia score as assessed using the Mellow-Pinkas dysphagia scale. Baseline demographics, tumour characteristics and clinical characteristics - including current medications, treatment indication, radiotherapy dose and the use of both induction and concurrent SACT - were collected for each patient.
A repeat assessment of the Mellow-Pinkas dysphagia score was made on the day of Cytosponge™ administration. The Cytosponge™ procedure was carried out by a suitably-trained healthcare professional, who was usually a registered nurse. Wherever possible, this was carried out prior to routine endoscopy or shortly before planned surgical resection. Participants were asked not to eat or drink for four hours prior to attempting to swallow the Cytosponge™, and each was permitted up to three attempts to swallow the capsule. Once the Cytosponge™ had been swallowed, participants were offered an anaesthetic throat spray prior to it being drawn back. Once withdrawn, the Cytosponge™ was placed in SurePath™ preservative fluid (Becton Dickinson, Franklin Lake, NH, USA) and stored at 4°C prior to analysis.
Any immediate complications were recorded post-procedure and all participants who successfully completed the procedure were asked to complete a questionnaire relating to their experience of receiving the Cytosponge™ (Appendix 2). Participants then remained in the study for two further weeks or, for those who had received naCRT, until they underwent surgery if this was prior to the end of the two-week period. Each participant was contacted by telephone at one and two weeks after Cytosponge™ administration. An assessment was made of post-procedure complications, use of concomitant medications and the Mellow-Pinkas dysphagia score. No telephone follow-up appointments occurred following surgery.
Samples collected using the Cytosponge™ were processed to a paraffin embedded cell clot, as previously described.22 Surplus formalin-fixed, paraffin embedded tissue remaining after routine surveillance biopsies undertaken prior to treatment and in the 4-16 week period following dCRT, or from surgical resection in those who received naCRT, was also requested for analysis. All Cytosponge™ specimens and available biopsy and surgical resection specimens were processed in a central laboratory (Addenbrookes Tissue Bank, Cambridge University Hospitals NHS Foundation Trust). Specimens were stained with haematoxylin and eosin, and with the immunohistochemical marker p53, using a Bond RXm Automated Stainer (Leica Biosystems, Newcastle-Upon-Tyne, UK), as has been described previously.23 All biopsy and resection specimens were assessed independently of Cytosponge™ results by one of two experienced Consultant Pathologists with an interest in upper gastrointestinal cancers and extensive experience of Cytosponge (SMa, MO'D). Cytosponge™ samples in which no columnar cells were present were regarded to be low-confidence results and excluded as the device might not have reached the stomach and may not therefore have sampled the full length of the oesophagus.
Details of routine post-CRT response evaluation through endoscopic assessment (patients undergoing dCRT) and surgical resection (patients undergoing naCRT) were recorded for those who specifically consented for this optional component of the study. These may have been performed prior to (in case of endoscopic assessment) or following Cytosponge™ assessment, including beyond the two-week post-procedure monitoring period. Endoscopy attempts were noted regardless of whether they were completed.
Outcomes
The primary outcome was the proportion of patients who had received CRT and who successfully underwent Cytosponge™ assessment after consenting to participate in the study. The secondary outcomes were safety, study consent rate, acceptance rate and the suitability of samples obtained using the Cytosponge™ for biomarker analysis. The planned tertiary objective was the comparative efficacy of Cytosponge™ to standard biopsy and surgical specimen in assessing for residual cancer.
The procedure completion rate was assessed as the proportion of consented, evaluable patients who successfully underwent Cytosponge™ assessment. This was determined for all patients, and separately for those undergoing naCRT and dCRT. A successful procedure was deemed to have occurred where the Cytosponge had been swallowed and subsequently retrieved without the requirement for additional intervention. Evaluable patients were those who had attempted to swallow the Cytosponge™.
Safety outcomes included all adverse events (AEs) and serious adverse events (SAEs) that occurred in the period between Cytosponge™ administration and the two-week follow-up appointment or surgical resection, if this occurred earlier. Both AEs and SAEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. As the Cytosponge™ is an IMD, an additional adverse event categorisation comprising of adverse device effect (ADE), serious adverse device effect (SADE) and unanticipated serious adverse device effect (USADE) was recorded during the same time period. Investigators were also required to report any SADE they were made aware of after each participant had left the study. An ADE as related to the Cytosponge™ was defined as an untoward medical occurrence from insufficiencies or inadequacies in the instructions for use, deployment, implantation, instillation, operation, or any malfunction of the Cytosponge™. ADEs which resulted in any of the characteristics of a SAE were classed as a SADE. Unexpected SADEs were defined as USADEs.
Safety endpoints were assessed by the study Chief Investigator and Trial Management Group. Relatedness of adverse events to use of the Cytosponge™ was assessed by the Principal Investigators (RF, SF, SM, RS, SG, SK, AB, TC, GR, RR). The number and percentage of adverse events is provided, as is their relationship to use of the Cytosponge™.
The study consent rate was defined as the percentage of approached eligible patients who consented to participate in the study. Patients who initially consented to participate but who withdrew this consent prior to the Cytosponge™ procedure were considered to have not consented. The procedure acceptance rate was assessed as the proportion of patients who had undergone the Cytosponge™ procedure who would be prepared to accept the procedure repeatedly if it were to be used for routine post-CRT follow-up. This was determined from a questionnaire (Appendix 2) completed by the patient following use of the Cytosponge™.
The suitability of samples obtained using the Cytosponge™ for biomarker analysis was defined as the percentage of Cytosponge™ samples that exhibited one or both of cytological atypia and p53 abnormality. Atypia was categorised as positive, reactive or negative. Cases of atypia of uncertain significance were regarded to be positive given the relatively greater importance of sensitivity versus specificity in diagnosing disease recurrence. p53 was categorised as aberrant (over- or absent- expression) or wild-type (not aberrant) but it should be noted that the absent pattern is more difficult to establish in Cytosponge samples compared with biopsies/surgical specimens.
To compare Cytosponge™ to standard biopsy/surgical specimen in assessing for residual disease, Cytosponge™ samples were classified as high risk or low risk. Given that p53 is not aberrant in all cases of oesophageal cancer, diagnostic biopsy specimens (where available) were examined in order to evaluate for the baseline tumour p53 status to ascertain whether p53 is informative for that case. ‘High risk’ post-CRT Cytosponge™ samples were defined as those with positive atypia or aberrant p53 expression (where baseline p53 expression was known and abnormal). ‘Low risk’ specimens were defined as those with wild-type p53 expression and that were either negative for atypia, or which demonstrated reactive atypia, which was considered to be secondary to CRT.
Statistical analysis
Statistical analyses were performed by HO'C and CMJ using Stata version 15.0 (StataCorp LLC, College Station, Tx, USA) and GraphPad Prism version 9.4.1 (GraphPad Software, LLC). The statistical analysis protocol is provided online and a summary of the criteria against which each studied outcome was assessed is provided in Supp. Materials. There were no deviations from this a priori plan.
A specific Trial Management Group oversaw the trial. A separate, independent, Radiotherapy and Imaging Trial Oversight Committee provided oversight, monitored for the completeness of data, and evaluated for evidence for treatment harm. This trial was registered with clinicaltrials.gov, reference NCT03529669.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, the writing of the report, or the decision to submit for publication. CMJ, HO'C and SM had full access to the study data. SM had final responsibility for the decision to submit for publication.
Results
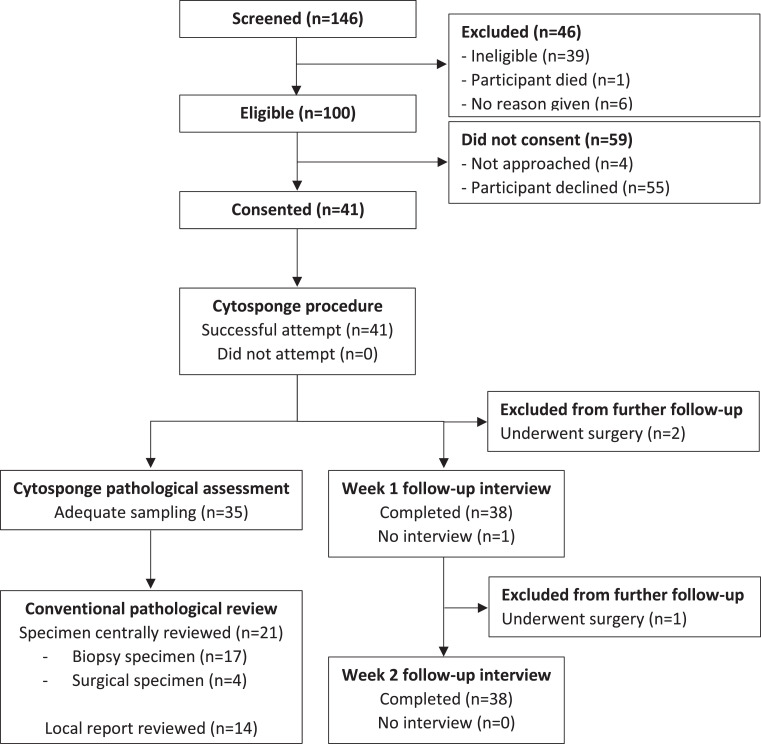
One hundred and forty-six patients were screened between 18th April 2018 and 16th January 2020, with 100 meeting eligibility criteria. Ninety-six eligible patients were approached to enrol in the study. Of these, 41 (42.7%; 95% confidence interval (CI) 32.7-53.2) consented to participate and subsequently attempted the Cytosponge™ procedure and 55 declined (Figure 1). The trial was stopped once the revised target of 40 participants had been reached. Forty three (78%) of the 55 who elected not to participate gave a reason for declining (Supp. Table 2). A majority (n = 26, 47%) of those who choose not to participate reported that they were either too busy to participate in research (n = 12; 22%), that they were not interested in participating in any research (n = 9; 16%) or that they were concerned about travelling to an additional appointment (n = 5; 9%). Only nine (16%) reported a specific concern about the Cytosponge™; one (2%) based on a concern about safety, four (7%) based on pre-existing swallowing difficulty or discomfort, and four (7%) who expressed that they did not like the idea of swallowing the sponge. There were no study withdrawals or serious protocol deviations for those who consented to participate.
Figure 1.
Trial profile. Of 100 who were eligible, 41 (41%) consented to participate and successfully underwent the Cytosponge procedure. Of these, 39 were eligible for week 1 and 38 for week 2 follow-up interviews. Separately, 35 completed Cytosponge samples were available for pathological analysis; the results from which were correlated with 21 centrally reviewed biopsy or surgical specimens, and data drawn from 14 local reports.
A summary of the demographics, baseline tumour characteristics and treatments delivered for study participants is presented in Table 1. A majority (n = 33, 81%) were male, with a median age of 70 (IQR 60-76) years. Most patients (n = 26; 63%) were able to carry out normal activities without restriction (WHO performance status 0) and most (n = 39; 95%) had no dysphagia or were able to swallow at least some solid foods.
Table 1.
Demographics, baseline tumour characteristics and treatments received for study participants. Data are shown as n (%). *All participants were staged as M0. **One participant was staged as Tx. ***One participant was staged as Nx. $Dysphagia score was assessed using the Mellow-Pinkas scale. †Data were not available for six patients, some of whom may not have received induction chemotherapy. Three patients received an alternative regime, as follows: epirubicin/capecitabine/cisplatin (n = 1), 5-FU/leucovorin/oxaliplatin/docetaxel (n = 1), single-agent carboplatin (n = 1). ‡Four patients received definitive single-modality radiotherapy alone and did not therefore receive concurrent chemotherapy. Data for nine patients are missing. Three patients received an alternative regime, as follows: single-agent carboplatin (n = 2), single-agent cisplatin (n = 1).
| Demographic information | Clinical characteristics | ||||||
| Sex | WHO Performance status | ||||||
| Male | 33 (81) | 0 | 26 (63) | ||||
| Female | 8 (20) | 1 | 15 (37) | ||||
| Age distribution, years | Dysphagia score$ | ||||||
| 40–49 | 3 (7) | 0 | 27 (66) | ||||
| 50–59 | 6 (14) | 1 | 12 (29) | ||||
| 60–69 | 12 (29) | 2 | 2 (5) | ||||
| 70–79 | 14 (33) | Treatments received | |||||
| 80–89 | 7 (17) | Induction chemotherapy regime† | |||||
| Tumour characteristics* | Cisplatin/Capecitabine | 22 (24) | |||||
| Histology | Cisplatin/5-fluorouracil | 2 (5) | |||||
| Adenocarcinoma | 16 (39) | Carboplatin/Capecitabine | 1 (2) | ||||
| Squamous cell carcinoma | 25 (61) | Carboplatin/Paclitaxel (weekly) | 4 (10) | ||||
| Tumour site | Carboplatin/Paclitaxel (3-weekly) | 2 (5) | |||||
| Upper thoracic oesophagus | 7 (17) | Radiotherapy dose & fractionation | |||||
| Middle thoracic oesophagus | 8 (19) | 60 Gy in 25 fractions | 6 (14) | ||||
| Lower thoracic oesophagus | 22 (54) | 54 Gy in 27 fractions | 1 (2) | ||||
| Oesophagogastric junction | 4 (10) | 50 Gy in 25 fractions | 27 (64) | ||||
| T-stage** | 45 Gy in 25 fractions | 3 (7) | |||||
| T1 | 2 (5) | 41 Gy in 23 fractions | 4 (10) | ||||
| T2 | 10 (25) | 35 Gy in 25 fractions | 1 (2) | ||||
| T3 | 23 (58) | Concurrent chemotherapy regime‡ | |||||
| T4 | 5 (13) | Cisplatin/Capecitabine | 12 (29) | ||||
| N-stage*** | Carboplatin/Capecitabine | 1 (2) | |||||
| N0 | 14 (35) | Carboplatin/Paclitaxel (weekly) | 10 (24) | ||||
| N1 | 16 (40) | Carboplatin/Paclitaxel (3-weekly) | 2 (5) | ||||
| N2 | 10 (25) | Single-agent carboplatin/cisplatin | 3 (7) | ||||
More patients were diagnosed with OSCC (n = 25, 61%) than OAC, with a majority of tumours (n = 22; 54%) situated in the lower thoracic oesophagus. The median tumour length at diagnosis was 5cm (IQR 2–7 cm). Patients with tumour stages T1-T4 and nodal stages N0-N2 were represented, though few patients had a very early (T1; n = 2, 5%) or very advanced (T4; n = 5, 13%) T-stage. A majority (n = 28; 68%) of patients received dCRT. Reflecting this, 80% of patients received a radiation dose of at least 50Gy. A variety of induction and concurrent systemic anti-cancer therapy regimes were used, whilst four patients received definitive radiotherapy alone.
Thirty-nine patients carried out the Cytosponge™ procedure successfully, with success rates exceeding 90% overall (95.1%, 95%CI 83.5-99.4) and in both the dCRT (26/28; 92.9%, 95%CI 76.5-99.1) and the naCRT (13/13; 100.0%, 95%CI 75-100) groups. A majority (n = 34, 81%) were able to swallow the capsule on their first attempt, whereas five (12%) required two attempts. Only one patient asked for anaesthetic throat spray after swallowing the capsule. No participants bled following the procedure and none required additional intervention to retrieve the Cytosponge™ capsule.
Thirty eight of the 41 patients who attempted the Cytosponge™ procedure completed follow-up at week one and at week two. As summarised in Table 2, most participants reported either no change or an improvement in dysphagia score at week one (n = 37; 97%) and week two (n = 35; 92%). Two grade 1 AEs considered possibly related to the Cytosponge™ procedure were reported during the entire study period; one patient developed a sore throat that started on the day of the procedure and persisted past the study end-date, whilst another developed dyspepsia that resolved within three days of the procedure. One additional reported grade 1 AE and two grade 2 SAEs were considered unlikely to be related to the Cytosponge™ procedure (Supp. Table 3).
Table 2.
Change in dysphagia score at weeks one and two following Cytosponge™ administration. Thirty eight participants completed follow-up at week one and week two, one of whom had attempted the Cytosponge™ procedure but not successfully completed it. Two participants underwent surgery before the week one questionnaire was due. Three participants underwent surgery before the week two questionnaire was due. Data are shown as n (%). *Dysphagia score improved or declined by a single level only in all participants for whom a change was reported.
| Week one (n = 38) | Week two (n = 38) | ||
|---|---|---|---|
| Dysphagia level | |||
| Able to eat normal diet/no dysphagia | 30 (79) | 26 (63) | |
| Able to swallow some solid foods | 6 (16) | 12 (29) | |
| Able to swallow only semi-solid foods | 2 (5) | 0 (0.0) | |
| Change in dysphagia level from pre-procedure baseline | |||
| Improvement* | 6 (16) | 6 (16) | |
| Decline* | 1 (3) | 3 (8) | |
| No change | 31 (82) | 29 (76) | |
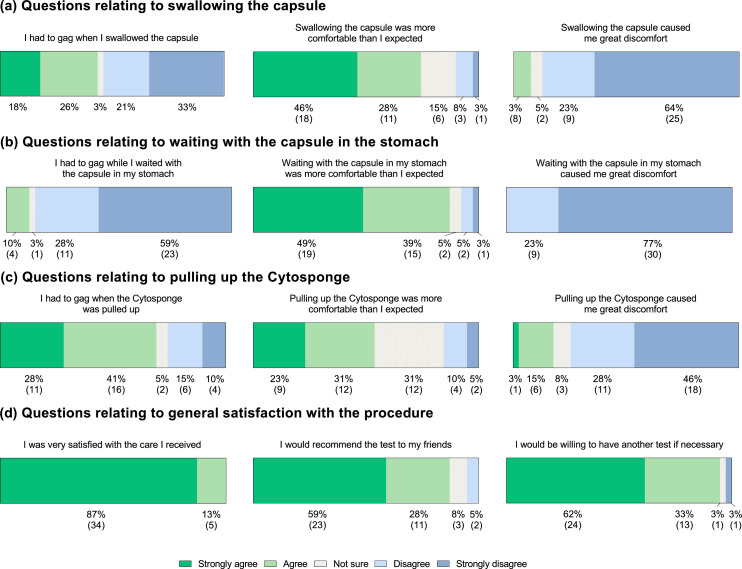
All of the 39 patients who successfully completed the Cytosponge™ procedure completed the study questionnaire (Figure 2, Supp. Fig. 1). Thirty-seven (95%) would be prepared to accept the procedure repeatedly if it were to be used for routine post-CRT follow-up and 34 (87%) would recommend the test to their friends. A majority disagreed that swallowing the capsule (n = 34; 87%), waiting with the capsule in their stomach (n = 39; 100%) and pulling up the Cytosponge™ (n = 29; 74%) caused them great discomfort. However, 27 (69%) had to gag whilst the Cytosponge™ was withdrawn. When asked to grade their test experience from one (worst possible experience) to ten (best possible experience), participants reported a median acceptability score of 7 (interquartile range, IQR, 5-8).
Figure 2.
A summary of post-procedure questionnaire responses. Data are shown as percentage (number). All 39 participants who successfully completed the Cytosponge™ procedure completed the questionnaire; 37 (95%) on the same day as their procedure, one a day later and one a week later. Thirty nine responses were received for each of the questions shown. The proportion of respondents selecting each level of agreement is shown below the relevant section of the bar chart, with the corresponding number of respondents shown in brackets.
Of the 41 patients, 6 (15%) cases were excluded due to Cytosponge™ samples not being available or inadequate sampling, i.e., there were no columnar cells collected suggesting that the stomach and distal oesophagus were not sampled. As a result, 35 Cytosponge™ samples were evaluated for atypia and p53. An endoscopy report was recorded for 26(63%) participants; 21 (81%) of whom had their endoscopy in the 14 days immediately following the Cytosponge™ procedure (overall range -41 to +88 days of Cytosponge™ use). Of the 35 patients for whom Cytosponge™ samples were available, post-treatment (naCRT or dCRT) biopsy (n = 17) or surgical resection (n = 4) samples were available for central histopathological review for 21 patients (Figure 1, Supp. Table 4). In the remaining 14 patients where Cytosponge samples were available, corresponding post-treatment biopsy or surgical resection samples were not available for central histopathological review and locally reported post-treatment histology report was used to determine whether samples demonstrated a residual tumour. Of the evaluable 35 Cytosponge™ samples, 29 (83%) were concordant with biopsy or surgical resection samples (Table 3, Supp. Table 4). This included four patients who were ‘high-risk’ for residual tumour on Cytosponge™ and who also had residual malignant cells on histology from biopsy or surgical resection samples. Six cases were discordant; three cases in which the Cytosponge™ did not sample malignant cells but malignant cells were identified through analyses of surgical resection or biopsy specimens, and three cases in which the Cytosponge™ samples were high risk but surgical resection or biopsy samples did not demonstrate malignancy (Figure 3). All 3 discordant samples which were positive by Cytosponge™ demonstrated atypia, and one of them also demonstrated aberrant p53 overexpression (Supp. Table 4).
Table 3.
Summary table of risk group classification between post-treatment biopsy/resection samples and Cytosponge™.
| Post-treatment biopsy/resection |
||||
|---|---|---|---|---|
| High risk | Low risk | Total | ||
| Cytosponge™ | High risk | 4 | 3 | 7 |
| Low risk | 3 | 25 | 28 | |
| Total | 7 | 28 | 35 | |
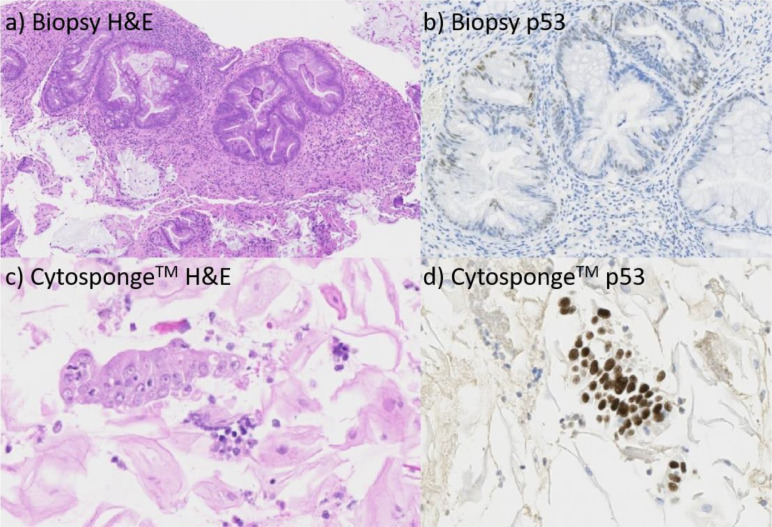
Figure 3.
Haemotoxylin and eosin, and p53, staining of (a,b) post-treatment biopsies and (c,d) Cytosponge™ samples taken from a patient who received chemoradiotherapy for oesophageal adenocarcinoma. The biopsy stains show no atypia and wild-type p53 staining. The Cytosponge™ samples show atypia and p53 overexpression, indicating high risk of residual/recurrent tumour.
Discussion
In this single-arm feasibility study, we have demonstrated the first successful, acceptable and safe application of a non-endoscopic immunocytological device (the Cytosponge™) to assess for residual disease in patients diagnosed with OSCC and OAC who have recently received CRT.
For patients diagnosed with oesophageal cancer, surgical resection is associated with 1-5% mortality as well as significant morbidity, with impairment of health-related quality of life post-oesophagectomy persisting for at least ten years.7, 8, 9, 10, 11, 12 In contrast, most patients regain their quality of life shortly after receiving CRT and complete pathological response is achieved in between 25–49% of patients.13,14 As such, there is growing interest in the use of organ preservation using upfront CRT followed by surveillance and salvage procedures as a means to minimising treatment-related morbidity; with at least three randomised controlled trials planned within this space.15,16 However, the effectiveness of these approaches is contingent on the timely identification of residual and recurrent disease in order to permit salvage resection or reirradiation through a brachytherapy boost or external beam proton therapy.24
At present, identification of residual and recurrent disease following CRT is dependent on endoscopic assessment and cross-sectional imaging. However, both are poor negative predictors of residual disease following CRT. In the preSANO trial, endoscopy and biopsy had a sensitivity of 69% for residual tumour, though this increased to 90% with the use of concomitant bite-on-bite biopsies and fine needle aspiration of suspicious lymph nodes.19 These values exceed that of endoscopic and cross-sectional imaging modalities. In a recent meta-analysis of post-treatment imaging assessment, the sensitivity of endoscopic ultrasound was just 5% (95%CI 1–19%), whilst computed tomography (CT) had a sensitivity of 68% (95%CI 5–99%) and positron emission tomography (PET)-CT a sensitivity of 60% (95%CI 42–76%).20 One key limiting factor is that both endoscopy and cross-sectional imaging rely on the presence of lesions that are macroscopically visible to trigger biopsy and pathological analysis.25 Further, across these current surveillance strategies, the frequency with which repeat assessments for disease recurrence can be made is restricted by radiation exposure, procedural discomfort and the burden placed on healthcare services.
The safety, acceptability and tolerability of the Cytosponge™ has been extensively demonstrated in excess of 3000 patients with gastro-oesophageal reflux, for whom it results in improved detection of Barrett's oesophagus.22 To its advantage, this procedure is less resource-intensive than endoscopic and cross-sectional imaging approaches to surveying the oesophagus, with potential corresponding benefits for patients and health services alike. It is also agnostic to the visibility of cancerous lesions and samples the entire mucosa and therefore does not have the bias that is associated with operator guided endoscopy-directed biopsies. Moreover, it has been shown that of the patients who have persistent disease following CRT, the distribution of residual cancer cells is superficial with 89% having a mucosal/submucosal component which is likely to be picked up with Cytosponge™.26 As such, we propose that use of the Cytosponge™ may provide an opportunity to regularly undertake pan-oesophageal surveillance to assess for disease recurrence following dCRT; either alone or as an adjunct to existing approaches such as through frequent Cytosponge™ sampling and less frequent endoscopic assessment.
In this study, we show that a majority of patients (95.1%; 95%CI 83.5-99.4) in the post-CRT setting are able to successfully swallow the Cytosponge™ device. Given that we were assessing post-CRT acceptability, safety and tolerability, we recruited patients who had received both naCRT and dCRT. Nevertheless, we propose that the Cytosponge™ is used in those who receive dCRT to enable for intensive surveillance as part of an organ-preservation approach, and it is consequently particularly reassuring that the completion rate for this cohort was high at 92.9% (95%CI 76.5-99.1). Despite the presence of more advanced disease, these figures mirror that of the BEST3 trial, in which 95% of 1750 patients with gastro-oesophageal reflux were able to successfully swallow the Cytosponge™ within two attempts.22,27 Notably, however, 90.1% of 2672 patients in a meta-analysis of four prior trials assessing Cytosponge™ use were able to swallow the Cytosponge in one attempt (91.1% in two attempts), which exceeds the 81% able to swallow the device in one attempt here.2 This points to some added difficulty in swallowing the device either due to bulky residual disease or CRT-related inflammation/stricture. It would be of interest to explore whether this is due to a mechanical difficulty with swallowing the sponge or a consequence of discomfort.
We also demonstrate that use of the Cytosponge™ appears broadly acceptable to patients who have recently completed CRT. To this end, firstly, 43% of approached eligible potential participants consented to participate in this study. Whilst this may appear relatively low, only 16% of those who chose not to participate cited a specific concern about the Cytosponge as a reason for doing so, though a further 7% were concerned about using the device in the context of odynophagia or a swallowing difficulty. It is also worth highlighting that participants were aware that they would be assessed by contemporaneous endoscopy or would proceed to a resection regardless of whether they participated or not, such that there is likely to have been no clear perceivable advantage to participating. Secondly, and perhaps more reassuringly, 95% of those who successfully received the Cytosponge™ reported that they would be prepared to repeat the procedure. This is clearly encouraging from the perspective of aiming to use the Cytosponge™ for frequent post-CRT surveillance.
The median overall experience score of 7 (IQR 5-8) described here is lower than in the recently reported BEST3 trial (median score 9, IQR 8-10).3 However, it is broadly in line with an overall median score of 6.0 (IQR 5.0-8.0) reported in a meta-analysis of four prospective trials assessing the safety and acceptability of the Cytosponge™ prior to the BEST3 trial.2,3 It is unclear why the median acceptability score may be lower here than in BEST3. A majority of patients denied peri-procedural discomfort, albeit with 69% finding they had to gag when the device was pulled up through the oesophagus. Nevertheless, 8% and 18% of patients reported some discomfort on respectively swallowing and pulling back the Cytosponge™. This may result from CRT-related inflammation and might at least in part explain the lower median acceptability score, whilst also pointing to a need to ensure that adequate analgesia is available for patients who are assessed with the Cytosponge™ in this context. Notably, only one patient requested anaesthetic throat spray prior to pulling back the Cytosponge™. Further, around 18% of patients reported that they were anxious about having the test and 50% were either unsure or disagreed that the test would benefit their health. Whilst the latter is perhaps not unexpected given that the Cytosponge™ does not yet have a proven benefit in this context, these data do point to a need to ensure that patients receive adequate patient information and counselling prior to assessment using the Cytosponge™. For comparison, It would also have been interesting to ask patients to score overall experience for endoscopy, which is the current gold standard.
Whilst the safety of the Cytosponge™ has been confirmed largely in pre-cancerous (Barrett's) settings,21, 22, 23,27 this is the first formal evaluation of its safety in patients with known oesophageal cancer in the post-radiotherapy setting. Only two minor AEs were reported, both of which occurred in the same patient; one a sore throat and the other dyspepsia. This safety profile is in keeping with that following Cytosponge™ use in patients with gastro-oesophageal reflux,21, 22, 23,27 indicating that there is no added risk from the use of Cytosponge™ shortly after CRT. Adding further reassurance, dysphagia scores deteriorated in just 3% of patients at week one and 8% of patients at week two following use of the Cytosponge™. The deterioration in dysphagia score is unlikely to have been related to the procedure itself.
Beyond demonstrating the safety, acceptability, and tolerability of the Cytosponge™ in the post-CRT setting, its wider use will be contingent on its ability to accurately identify residual and recurrent disease. Here, 15% of completed Cytosponge™ samples were low-confidence, meaning that gastric cells were not sampled, which mirrors the percentage seen in previous analyses of Cytosponge™ use.22 In this context, inadequate columnar sampling may reflect an incomplete swallow through which the Cytosponge™ has been unable to reach the stomach, potentially indicating the presence of a persistent malignant or radiation-related stricture and therefore suggesting the need for endoscopic evaluation.28 Comparison with post-treatment histology was a tertiary outcome and fourteen post-treatment biopsy or resection samples were not available since this was an optional component of the protocol, which limits comparison of p53 and atypia data across samples. Nevertheless, a majority of Cytosponge™ samples (83%) were concordant with biopsy or resection specimens. Of those that were discordant, the Cytosponge™ was low risk when biopsy and surgical specimens were high risk in three cases. It is likely that tumour cells were not sampled in these instances by Cytosponge™. In three further cases, the Cytosponge™ was classed as high-risk but biopsy and surgical specimens were not. Two of these cases exhibited atypia of uncertain significance alongside wild-type p53. Though active malignancy, missed on endoscopy, cannot be excluded, this may also reflect post-CRT inflammation rather than residual cancer. The third high-risk Cytosponge™ case was, however, more convincing, identifying aberrant p53 overexpression and atypia whereas biopsies did not demonstrate residual disease. This is likely to represent missed residual cancer from endoscopic sampling bias.
These exploratory analyses of device efficacy in the post-CRT setting are limited by the absence of long-term follow-up data for any of the study participants. The Cytosponge™ analysis was not done in real time and therefore the procedure was not repeated in the 15% of participants who had low-confidence results. It is possible that these patients are indicative of a subgroup who due to persisting stricture would require endoscopic surveillance, which with dilation would also provide therapeutic benefits for this group. This requires clarification in future work by correlating low-confidence results to the presence or absence of a stricture at a contemporaneous endoscopy.
There are in addition a number of other limitations to this study. Firstly, those who agreed to participate may be a self-selecting group of those with lower levels of odynophagia following CRT, though it is worth noting that only 7% of those who chose not to participate cited swallowing difficulties as their reason. In addition, the eligibility criteria excluded patients with advanced dysphagia grades, and therefore the suitability and utility of Cytosponge™ in that patient cohort remains unknown. It is, however, worth noting that dysphagia scores are known to improve following induction chemotherapy and a clinically significant improvement in eating restriction was seen following CRT in the SCOPE1 trial; indicating that many patients will experience an improvement in dysphagia, which may allow for those who begin with higher dysphagia scores to be sampled post-treatment.4,5 Secondly, these data demonstrate feasibility over a relatively small time period shortly after completion of CRT and does not inform us about patient adherence to a surveillance protocol if multiple visits were mandated. Thirdly, the extent to which the Cytosponge™ could be used alone or as an adjunct to existing and novel surveillance approaches remains unclear, not least given that in previous studies the submucosa has been identified as a not infrequent site of disease recurrence.25 In further work, it would be useful to undertake adequately powered accuracy evaluations for the Cytosponge™ by comparing its risk classification to that obtained through examination of resection specimens, which would form the gold standard against which to assign a kappa statistic. One promising area that requires further exploration is the use of circulating cell free DNA (cfDNA) alongside use of Cytosponge™. Finally, the overall role of the primary non-surgical management of oesophageal cancer also remains uncertain and requires further investigation within the phase 3 trial setting.15 Further work is also required to optimise the use of laboratory biomarkers, clinical factors and the Cytosponge to identify patients who require or who should endoscopic assessment.6
In conclusion, the Cytosponge™ appears safe, tolerable and acceptable for patients diagnosed with OAC and OSCC who have recently received naCRT and dCRT. Further work is required to define the extent to which it may be used as an adjunct or alternative to standard and emerging post-CRT surveillance approaches. If confirmed to be efficacious within the post-CRT setting, the Cytosponge™ may provide a frequent, acceptable and resource-efficient means to improving active surveillance following primary non-surgical treatment, which either alone or in combination with endoscopy or cfDNA and/or cross-sectional imaging, and may in turn allow a significant proportion of patients to avoid the mortality and morbidity associated with surgical resection.
Contributors
R.C.F. and S.M. conceptualised the study, which S.M. sought funding for. Ad.B., An.B., C.M.J., G.R., H.O'C., M.A.H., M.O.D., R.C.F., R.R., R.S., S.F., S.G., S.K., S.L., S.M. and T.D.L.C. contributed to the study design. A.B., G.R., R.C.F., R.R., R.S., S.F., S.G., S.K., S.M. and T.D.L.C. contributed the recruitment and consent of study participants. A.B., C.M.J., D.H., R.C.F., R.H., S.L. and S.M. supported study administration. I.D.B. and B.A. provided training and support to nursing staff who administered the Cytosponge™ device. A.B., D.H., M.O.D. and R.C.F. undertook laboratory analyses. H.O.C. and C.M.J. statistically analysed clinical data. H.O'C., C.M.J. and S.M. have accessed and verified the underlying data. Data were analysed and interpreted by C.M.J., R.S.F. and S.M. C.M.J. prepared data visualisations and the first draft of the manuscript. All authors contributed to subsequent revisions of, and all have approved, the final manuscript. S.M. takes overall responsibility for the conduct of the trial and the reported data.
Data sharing statement
Aggregate data are available on request. The trial protocol, statistical analysis plan and statistical report are available online.
Declaration of interests
The Cytosponge™ technology including the device and TFF3 biomarker has been licensed by the MRC to Covidien (now Medtronic). R.C.F. and M.O.D. are named on patents related to this test. R.C.F. and M.O.D. are shareholders and consultants for Cyted, an early detection company. All other authors declare no competing interests.
Acknowledgements
We are grateful to all participants and the sites in which they were recruited for their participation in this study. The CYTOFLOC trial was funded by the Cancer Research UK (CRUK) Population Research Committee (C28958/A22173). Additional support was provided by the CRUK Oxford Centre and the CRUK/Medical Research Council (MRC) Oxford Institute for Radiation Oncology. Trial management was provided by the Oncology Clinical Trials Office (OCTO) at the University of Oxford (Oxford, UK) as part of the UKCRC Oxford Clinical Trials Research Unit (OCTRU). The Centre for Statistics in Medicine at the University of Oxford provided statistical input. The laboratory of RCF is funded by a Programme Grant from the Medical Research Council (G84369). This research was also supported by the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre (BRC-1215-20014). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. We thank the Human Research Tissue Bank, which is supported by the UK NIHR Cambridge Biomedical Research Centre, from Addenbrooke's Hospital. Cytosponge™ devices were supplied by Cambridge University Hospitals NHS Foundation Trust, which was the legal manufacturer of the devices. Europlaz (Essex, UK) produced the devices under licence. We are also grateful to Joanna Moschandreas for her valuable input in to this work. CMJ is supported by a Clinical Lectureship part-funded by CRUK RadNet Cambridge. SM acknowledges support from the NIHR Oxford Biomedical Research Centre. MAH acknowledges support from the NIHR Biomedical Research Centre at University College London Hospitals NHS Foundation Trust.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101664.
Appendix. Supplementary materials
References
- 1.Kamangar F, Nasrollahzadeh D, Safiri S, et al. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:582–597. doi: 10.1016/S2468-1253(20)30007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J. Clin. Oncol. 2005;23:2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 3.Vellayappan BA, Soon YY, Ku GY, Nang C, Leong CN, Lu JJ, Tey JC. Chemoradiotherapy versus chemoradiotherapy plus surgery for esophageal cancer. Cochrane Database Syst Rev. 2017;8 doi: 10.1002/14651858.CD010511.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J. Clin. Oncol. 2007;25:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 5.Bolger JC, Donohoe CL, Lowery M, Reynolds JV. Advances in the curative management of oesophageal cancer. Br J Cancer. 2022;126(5):706–717. doi: 10.1038/s41416-021-01485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro J, Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 7.Booka E, Takeuchi H, Suda K, et al. Meta-analysis of the impact of postoperative complications on survival after oesophagectomy for cancer. BJS Open. 2018;2:276–284. doi: 10.1002/bjs5.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kataoka K, Takeuchi H, Mizusawa J, et al. Prognostic impact of postoperative morbidity after esophagectomy for esophageal cancer: exploratory analysis of JCOG9907. Ann Surg. 2017;265:1152–1157. doi: 10.1097/SLA.0000000000001828. [DOI] [PubMed] [Google Scholar]
- 9.Baba Y, Yoshida N, Shigaki H, et al. Prognostic impact of postoperative complications in 502 patients with surgically resected esophageal squamous cell carcinoma: a retrospective single-institution study. Ann Surg. 2016;264:305–311. doi: 10.1097/SLA.0000000000001510. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg. 2014;260:259–266. doi: 10.1097/SLA.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 11.Schandl A, Lagergren J, Johar A, Lagergren P. Health-related quality of life 10 years after oesophageal cancer surgery. Eur J Cancer. 2016;69:43–50. doi: 10.1016/j.ejca.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Scarpa M, Valente S, Alfieri R, et al. Systematic review of health-related quality of life after esophagectomy for esophageal cancer. World J Gastroenterol. 2011;17:4660–4674. doi: 10.3748/wjg.v17.i42.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 14.Noordman BJ, Verdam MGE, Onstenk B, et al. Quality of life during and after completion of neoadjuvant chemoradiotherapy for esophageal and junctional cancer. Ann Surg Oncol. 2019;26:4765–4772. doi: 10.1245/s10434-019-07779-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Wilk BJ, Eyck BM, Hofstetter WL, et al. Chemoradiotherapy followed by active surveillance versus standard esophagectomy for esophageal cancer: a systematic review and individual patient data meta-analysis. Ann Surg. 2022;275:467–476. doi: 10.1097/SLA.0000000000004930. [DOI] [PubMed] [Google Scholar]
- 16.Hipp J, Nagavci Schmoor C, Meerpohl J, Hoeppner J, Schmucker C. Post-neoadjuvant surveillance and surgery as needed compared with post-neoadjuvant surgery on principle in multimodal treatment for esophageal cancer: a scoping review. Cancers. 2021;13:429. doi: 10.3390/cancers13030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong Hee Kam S, Rivera S, Hennequin C, et al. Salvage high-dose-rate brachytherapy for esophageal cancer in previously irradiated patients: a retrospective analysis. Brachytherapy. 2015;14:531–536. doi: 10.1016/j.brachy.2015.02.392. [DOI] [PubMed] [Google Scholar]
- 18.Sarkaria IS, Rizk NP, Bains MS, et al. Post-treatment endoscopic biopsy is a poor-predictor of pathologic response in patients undergoing chemoradiation therapy for esophageal cancer. Ann Surg. 2009;249:764–767. doi: 10.1097/SLA.0b013e3181a38e9e. [DOI] [PubMed] [Google Scholar]
- 19.Noordman BJ, Spaander MCW, Valkema R, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol. 2018;19:965–974. doi: 10.1016/S1470-2045(18)30201-8. [DOI] [PubMed] [Google Scholar]
- 20.de Gouw DJJM, Klarenbeek BR, Driessen M, et al. Detecting pathological complete response in esophageal cancer after neoadjuvant therapy based on imaging techniques: a diagnostic systematic review and meta-analysis. J Thorac Oncol. 2019;14:1156–1171. doi: 10.1016/j.jtho.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Ross-Innes CS, Debiram-Beecham I, O'Donovan M, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett's esophagus: a multi-center case-control study. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald RC, di Pietro M, O'Donovan M, et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett's oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet. 2020;396:333–344. doi: 10.1016/S0140-6736(20)31099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadri SR, Lao-Sirieix P, O'Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett's oesophagus in primary care: cohort study. BMJ. 2010;341:c4372. doi: 10.1136/bmj.c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes A, Berman AT, Mick R, et al. A prospective study of proton beam reirradiation for esophageal cancer. Int J Radiat Oncol Biol Phys. 2016;95:483–487. doi: 10.1016/j.ijrobp.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 25.van der Wilk BJ, Eyck BM, Doukas M, et al. Residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer: locations undetected by endoscopic biopsies in the preSANO trial. Br J Surg. 2020;107:1791–1800. doi: 10.1002/bjs.11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shapiro J, ten Kate FJ, van Hagen P, Biermann K, Wijnhoven BP, van Lanschot JJ. Residual esophageal cancer after neoadjuvant chemoradiotherapy frequently involves the mucosa and submucosa. Ann Surg. 2013;258:678–688. doi: 10.1097/SLA.0b013e3182a6191d. discussion 688-689. [DOI] [PubMed] [Google Scholar]
- 27.Januszewicz W, Tan WK, Lehovsky K, et al. Safety and acceptability of esophageal cytosponge cell collection device in a pooled analysis of data from individual patients. Clin Gastroenterol Hepatol. 2019;17:647–656.e1. doi: 10.1016/j.cgh.2018.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.di Pietro M, Modolell I, O'Donovan M, et al. Use of Cytosponge as a triaging tool to upper gastrointestinal endoscopy during the COVID-19 pandemic. Lancet Gastroenterol Hepatol. 2020;5:805–806. doi: 10.1016/S2468-1253(20)30242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.