Abstract
Desmoplastic small round blue cell tumors are a rare and aggressive type of malignant small round blue cell tumor with a limited number of cases published in the literature. They commonly affect males in their second decade of life who usually present with a metastasis picture, resulting in a poor prognosis. We present a case of an intra-abdominal desmoplastic small blue round cell tumor in a 23-year-old male who presented with abdominal pain and bloating. Computed tomography revealed a large intra-abdominal mass which was followed by laparotomy and surgical resection of the abdominal mass with histology confirming the diagnosis. Careful clinical, radiological, and histopathological correlations are crucial to reach an accurate diagnosis which would be followed by surgical cytoreduction with primary, partial or complete tumor removal.
Keywords: Desmoplastic small round blue cell tumors, Small round blue cell, Computed tomography
Introduction
Intra-abdominal desmoplastic small round blue cell tumor desmoplastic small round blue cell tumor (DSRBCT) is a very rare and aggressive tumor. It was first described by Gerald and Rosai in 1989 [1,2] and currently has almost 200 cases reported in the literature. The tumor has a higher incidence in the males with an estimated male to female ratio ranging from 5:1 to 10:1. It is reported that the tumor is more common between the ages of 20 and 30 with a median age of 19 years of age. We describe a case of an intra-abdominal desmoplastic small blue round cell tumor in a young man who presented with a history of abdominal pain and bloating.
Case presentation
A 23-year-old, medically free male, presented to the A/E with a history of abdominal pain, bloating, nausea, and vomiting for the last 3 months, which has in recent days increased in severity. On examination, a central abdominal mass was palpated with mild tenderness felt by the patient. Laboratory tests were unremarkable. A contrast-enhanced computed tomography scan of the abdomen was requested, which revealed a large intra-abdominal heterogeneous, predominantly hypodense mass, centered on the mid/right abdominal mesentery, displaying complex cystic and solid components, showing a large fluid filled center and a solid enhancing wall, measuring about 11.9 × 18 × 16.4 cm (in AP × CC × ML dimensions). The mass is seen extending from the L2 vertebral level to the level of S1 vertebra, displacing the bowel but not causing an obstruction. No intralesional calcifications were seen. The adjacent displaced abdominal vascular structures are patent, with no signs of vascular encasement or invasion. Evidence of minimal omental thickening and fat strandings are noted at the right inferolateral border of the lesion (Fig. 1). The patient underwent an open laparotomy and surgical resection of the abdominal mass, which was sent for histopathology correlation. The slides showed malignant tumor cells with capsules arranged in nests and nodules in a perivascular pattern. The tumor cells were small, round to spindle, exhibiting extensive necrosis and hemorrhage (Fig. 2). Apoptosis and inflammatory cell infiltrates were seen in addition to tumor cells displaying cytoplasmic and dot-like positivity for pan CK,WT-1,Desmin, Cyclin D1 and focal positivity for CD 99 (Figs. 3-Fig. 4, Fig. 5). These findings confirmed the diagnosis: intra-abdominal desmoplastic round blue cell tumor. The patient's case was discussed at the tumor board meeting and the oncology team started his chemo-radiation therapy. Our patient has currently received 5 cycles of chemotherapy, all of which were well-tolerated.
Fig. 1.
Axial and coronal contrast CT abdomen and pelvis showed a large intra-abdominal mass (note the white arrow) located at mid/right side of the abdomen.
Fig. 2.
Photomicrograph (magnification ×40) showed malignant tumor with capsule arranged in nests and nodules separated by fibrous bands.
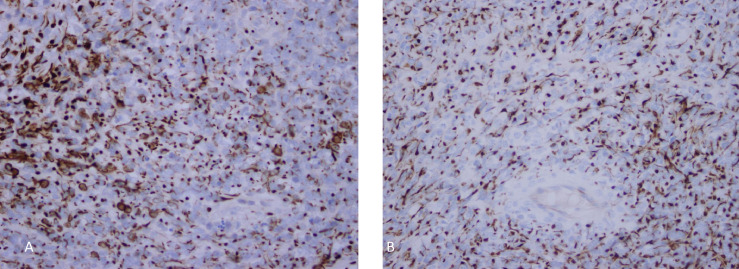
Fig. 3.
Tumor cells showed cytoplasmic and dot-like positivity for pan CK (A) and WT-1 (B) (magnification ×40).
Fig. 4.
Tumor cells showed cytoplasmic and dot-like positivity for desmin (A) and cyclin D1 (B) (magnification ×40).
Fig. 5.
Immunochemistry showed focal positivity for CD 99 (magnification ×40).
Discussion
DSRBCT is a rare and aggressive tumor with an incidence of 0.3-0.7 cases/million worldwide, exhibiting a high mortality rate. Patients usually have an average life expectancy of less than 3 years at diagnosis, and less than 15% of them are given a 5-year life expectancy [3].
Tumors of the small round blue cell family are highly malignant for all age groups and are characterized by their small, round, undifferentiated cells. This malignant family includes, but is not limited to, neuroblastoma, rhabdomyosarcoma, non-Hodgkin's lymphoma, Ewing's sarcoma, peripheral neuro-ectodermal tumor, synovial sarcoma, retinoblastoma, hepatoblastoma and nephroblastoma [4].
DSRBCTs can affect most tissues in a human's body, such as lymph nodes, the peritoneum, diaphragm, spleen, liver, chest wall, skull, spinal cord, large intestine, small intestine, bladder, brain, lungs, testicles, ovaries, and the pelvis. The specific course of the tumor and its histogenesis are unclear. They have been associated with the mesothelium, called mesothelioblastoma, and shown to have a frequent relationship with serous surfaces, immunoreactivity for the WT1 gene, and the tumor's tendency to express epithelial and mesenchymal markers [5,6].
Due to its aggressive nature, more than 40% of patients have distant metastases at the time of diagnosis. These are usually located in the liver, lungs, and lymph nodes with a high frequency of paraneoplastic syndrome affecting the patients [7]. The most common symptoms displayed are the presence of abdominal pain, distension, and a palpable abdominal mass. In around 50% of cases, the clinical symptoms are comparable to processes which cause intestinal pseudo-obstruction, night diaphoresis, weight loss, hematuria, and pleural effusion. The clinical course is aggressive, with multiple local recurrences and distant metastases being common [8,9]. Intestinal obstruction is the most common long-term complication, found in nearly a third of patients [5].
Among the studies conducted regarding the diagnoses of DSRBCT, the abdominopelvic computed tomography scans generally involve one or multiple tumors of well-defined lobulated borders, with hyperdense heterogeneous soft tissue, associated with hypodense areas related to necrosis and hemorrhagic foci, located in the intraperitoneal region, without apparent abdominal organic origin. About 50% may present with adenomegalies and 20% have calcifications. Bellah et al. reported that in 82% of cases, lesions were in the rectum [10,11].
Characteristic findings in MR studies are multiple intraperitoneal neoplasms with intermediate signal in T1* sequences, an increased signal in T2 sequences, and minimal enhancement post contrast introduction. Radiologically, there are no adequate findings specific to confirm the diagnosis, so the clinical/pathological correlation is necessary [5].
Histologically, they are quite variable as well. The tumor may show a pattern of small, round, oval and/or spindled cells, arranged in nests, cords, lines, sheets and trabeculae with hyperchromatic nuclei, a low volume cytoplasm, an increased mitotic rate, and well-defined solid areas, embedded in a desmoplastic stroma. There are less specific morphological patterns, such as papillary, glandular and cribriform patterns with clear, fusiform, pleomorphic, rhabdoid, and basaloid cells forming rosettes or pseudorosettes [7].
Immunophenotypically, it is characterized by a pattern of expression for epithelial, mesenchymal and neuronal markers. This tumor is always positive for desmin, AE1-AE3, epithelial membrane antigen, cytokeratin, and neuron-specific enolase; S100 and CD99 are expressed in certain cases [6,12].
When investigated through radiological imaging, there is a wide range of differential diagnosis which included: mesothelioma, carcinoid tumor, peritoneal carcinomatosis, peritoneal leiomyomatosis, intraperitoneal desmoid tumor, peritoneal lymphomatosis, peritoneal sarcoma and peritoneal tuberculosis [8].
Histopathological differential diagnoses are also broad, and such a multidisciplinary approach to diagnosis must be taken. In pediatric patients, these include: Ewing sarcoma, rhabdomyosarcoma, neuroblastoma, small-cell lymphoma, anaplastic synovial sarcoma and Wilms’ tumor. In adults, the following tumors should be considered: lymphomas, small-cell carcinoma, Merkel-cell carcinoma, neuroendocrine carcinoma, and mesothelioma, among others [7].
Therapeutic options are limited as of the time this paper was written. Surgical cytoreduction with primary, partial or complete tumor removal is preferred and is associated with a better long-term prognosis. However, this is only possible in 60% of cases. Better results are seen in patients when a combined treatment method is used. A treatment plan using exclusively chemotherapy has been associated with a higher rate of toxicity, which worsens patients’ prognosis and life expectancy [5,10].
Conclusion
The intra-abdominal desmoplastic small round cell tumor is a very rare tumor with a complex diagnostic challenge. Clinical, radiological, and histopathological correlations are required to reach a final and accurate diagnosis. Confined knowledge of this tumor pathology and clinical course made it difficult for physicians to agree on the best treatment options for such cases. High clinical suspicion and careful radiological and histopathological correlation is required to reach an accurate diagnosis.
Patient consent
A written informed consent was obtained from the patient. A copy of the informed consent is available and can be provided upon request.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Gerald WL, Rosai J. Case 2. Desmoplastic small cell tumor with divergent differentiation. Pediatr Pathol. 1989;9:177–183. doi: 10.3109/15513818909022347. [DOI] [PubMed] [Google Scholar]
- 2.Gerald WL, Miller HK, Battifora H, Miettinen M, Silva EG, Rosai J. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991;15(6):499–513. [PubMed] [Google Scholar]
- 3.Salako O, Habeebu M, Jimoh MA, Adeniji AA, Adenipekun A, Ajekigbe AT. Desmoplastic small round blue cell tumour of the abdomen—a case report. J West Afr Coll Surg. 2015;5(4):79–89. [PMC free article] [PubMed] [Google Scholar]
- 4.Rajwanshi A, Srinivas R, Upasana G. Malignant small round cell tumors. J Cytol. 2009;26(1):1–10. doi: 10.4103/0970-9371.54861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan I, Shyyan R, Donohue JH, Edmonson JH, Gunderson LL, Moir CR, et al. Intraabdominal desmoplastic small round cell tumors: a diagnostic and therapeutic challenge. Cancer. 2005;104(6):1264–1270. doi: 10.1002/cncr.21282. [DOI] [PubMed] [Google Scholar]
- 6.Benhammane H, Chbani L, Ousadden A, Mouquit O, Tizniti S, Amarti A, et al. Desmoplastic small round cell tumor of the abdomen: a case report and literature review of therapeutic options. Health. 2012;4(4):207–211. [Google Scholar]
- 7.Briseño-Hernández A, Quezada-López D, Corona-Cobián L, Castañeda-Chávez A, Duarte-Ojeda A, Macías-Amezcua M. Cirugía y Cirujanos (English edition), Intra-abdominal desmoplastic small round cell tumour, Elsevier. 2015;83(3):181–270. doi: 10.1016/j.circir.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Martínez S, Silva E. Desmoplastic small round cell tumor of the abdomen: CT findings and radiologic-pathologic correlation in 3 cases. Radiologia. 2009;51:313–317. doi: 10.1016/j.rx.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Koniari K, Mahera H, Nikolaou M, Chatzis O, Glezakou O, Magiasis V, et al. Intraabdominal desmoplastic small round cell tumor: report of a case and literature review. Int J Surg Case Rep. 2011;2(8):293–296. doi: 10.1016/j.ijscr.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas R, Rajeswaran G, Thway K, Benson C, Shahabuddin K, Moskovic E. Desmoplastic small round cell tumour: the radiological, pathological and clinical features. Insights Imaging. 2013;4:111–118. doi: 10.1007/s13244-012-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellah R, Suzuki-Bordalo L, Brecher E, Ginsberg J.P, Maris J, Pawel B.R. Desmoplastic small round cell tumor in the abdomen and pelvis: report of CT findings in 11 affected children and young adults. Am J Roentgenol. 2005;184:1910–1914. doi: 10.2214/ajr.184.6.01841910. [DOI] [PubMed] [Google Scholar]
- 12.Kim H.J, Sohn B.S, Kwon J.E, Kim J.Y, Park K. ThinPrep cytological findings of desmoplastic small round cell tumor with extensive glandular differentiation: a case study. Korean J Pathol. 2013;47:182–187. doi: 10.4132/KoreanJPathol.2013.47.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]