Introduction
SARS COV2 infection is likely to cause excessive activation of coagulation, leading to venous and arterial thrombosis. In extreme cases, it can lead to disseminated intravascular coagulation (DIC). This thrombogenic effect can be explained in part by the magnitude of the cytokinic storm as well as by direct viral damage to the endothelium [1]. Several authors have noted the presence of anti-phospholipid antibodies in patients with SARS COV2 infection presenting with atypical arterial or venous thrombosis.
Arterial and venous thrombosis in a same patient is an unusual combination. Mural thrombus of the thoracic aorta is a rare clinical finding in the absence of aneurysm or atherosclerosis. We report the case of a 74-year-old patient who developed thoracic aortic thrombosis in an anatomically healthy artery and concomitant pulmonary embolism with transient elevation of anti-phospholipid antibodies after a SARS COV2 infection [2], [3].
Case report
A 74-year-old patient was hospitalized in our institution for acute dyspnea without chest pain in relation to an exacerbation of chronic obstructive pulmonary disease complicating SARS COV2 pulmonary infection. Symptoms appeared 6 days before entrance. The patient was a former smoker with an estimated 40 pack years smoking. He was not vaccinated against COVID-19 prior to care.
The patient required oxygen therapy, blood pressure was normal and body temperature was 38C°. The initial thoracic computed tomography (CT) scan showed a bi-lobar inferior pulmonary parenchymal injury suggestive of SARS COV2 infection estimated at 25%. There was also a pan-lobular emphysema. Nasopharyngeal Polymerase Chain Reaction test confirmed the presence of a SARS COV2 infection.
A treatment by methylprednisolone 60 mg/24 h associated with terbutaline and ipratropium aerosols was initiated, as well as an antibiotic therapy with Ceftriaxone 1 g/24 h. The patient also received a prophylactic dose of low-molecular-weight heparin (subcutaneous enoxaparin 4000UI/24 h).
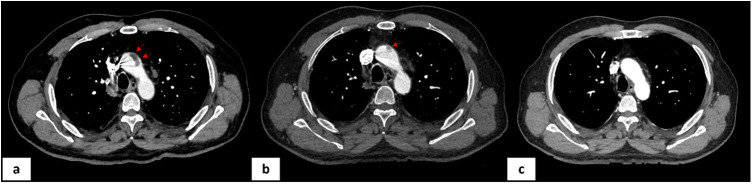
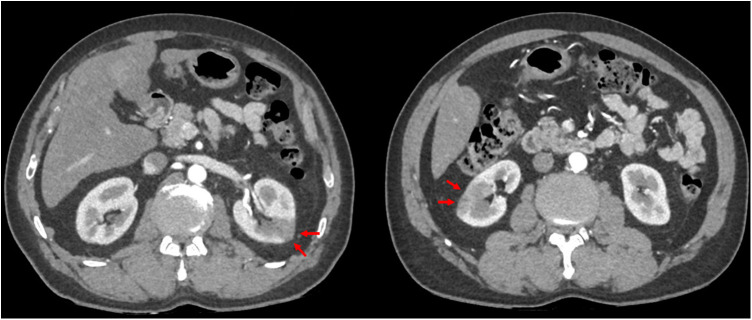
Six days later, the patient reported an oppressive, resting, non-radiating, left latero-thoracic pain. The patient was apyretic with a stable blood pressure and no change in oxygen demand. The ECG was normal. Biological monitoring showed an increase in high sensitive cardiac troponin T to 70 ng/L (N < 14 ng/L), D-dimer to 20 μg/mL, a stable fibrinogen at 5.1 g/L, and platelets at 270 G/L. The echocardiography showed a left ventricular ejection fraction at 55% without wall motion abnormality, without right ventricular dysfunction, pulmonary hypertension or pericardial effusion. A venous Doppler ultrasound performed at the same time did not show any proximal or distal deep vein thrombosis. A CT pulmonary angiogram revealed the presence of a pulmonary embolism in the sub segmental arteries (lingula and inferior left lobe). This imaging also revealed a floating thrombus (11 × 28 mm) in the distal part of the ascending aorta (confirmed by arterial time acquisition) as well as bilateral renal infarct foci of embolic appearance (Figure 1, Figure 2 ).
Figure 1.
Thoracic CT angiography revealing a floating thrombus (11 × 28 mm) in the distal part of the ascending aorta (panel a); partial regression of the thrombus at day four (panel b); disappearance of the thrombus at day ten without underlying obvious aortic disease (panel c).
Figure 2.
Abdominal CT scan with contrast showing the presence of bilateral renal infarcts of embolic origin.
Warfarin was started, overlapped with unfractionated heparin until the INR reached the therapeutic range (between 2 and 3). Aortic CT imaging on the fourth and tenth day showed progressive regression of the thrombus and absence of underlying aortic wall abnormality. The patient was discharged five days later.
A thrombophilia workup showed the presence of circulating lupus-type anticoagulants (LA) and anti-cardiolipin antibodies (IgA type). Anti-beta2GP1 antibodies, anti-nuclear antibodies, protein C and S activity, factor VIII level, and factor II and V mutation tests were normal. The lipid profile showed total cholesterol at 1.97 g/L, LDL cholesterol at 1.36 g/L, and HDL cholesterol at 0.28 g/L. Positive emission tomography (PET) scan did not reveal any aortic wall abnormality.
Normalization of LA and anti-cardiolipin antibodies 12 weeks later invalidated the diagnosis of definite anti-phospholipid syndrome.
These transient thrombotic complications were treated by adjusted dose of warfarin for 6 months, and once the diagnosis of chronic antiphospholipid syndrome was excluded, we introduced a long-term aspirin monotherapy associated with statins. This was done in the hypothesis of an underlying atherosclerotic disease, which could not be visualized by the aortic CT angiography.
Discussion
The concomitant occurrence of venous and arterial thrombosis in a patient is an unusual phenomenon. It may occur in patients with myeloproliferative syndrome (such as essential thrombocythemia), vasculitis and anti-phospholipid syndrome. Several cases have also been reported in patients with cancer [4].
Regarding our patient, cancer or myeloproliferative process was excluded by blood tests and imaging. The PET scan did not reveal any evidence of occult cancer or vasculitis.
Venous and arterial thromboembolic complications have been reported in patients with COVID-19 infection [5]. Nevertheless, the association of these two localizations in the same patient remains a rare event in the absence of DIC.
In a retrospective cohort study of 388 patients hospitalized for symptomatic COVID infection, Lodigiani et al. reported 28 cases of thrombotic events and only one of them with concomitant arterial and venous thrombosis [6].
The location of the arterial thrombosis was also not common. Thoracic aortic thrombosis is a rare condition causing peripheral arterial embolic events. It usually occurs in subjects with atheromatous lesions or vasculitis–related aortic wall damage. Exceptionally, the thrombus may occur in anatomically healthy arteries and is then considered isolated [7].
An extensive meta-analysis conducted in 2013 had gathered 200 cases of aortic mural thrombus in minimally atherosclerotic or normal aorta [8]. The thrombus was located in the ascending aorta or in the aortic arch in half of cases. Most patients (84%) presented with acute lower limb ischemia, 27% presented with visceral ischemia, and 14% with stroke. Regarding our case, we have indeed found peripheral renal emboli. However, in the absence of symptoms, we did not search for subclinical cerebral or lower limb embolism.
Since the start of the pandemic, several cases of aortic thrombosis have been described in patients with SARS-CoV-2 infection, despite prophylactic anticoagulant therapy [9], [10].
Many hypotheses such as endothelial dysfunction, related to direct viral damage to the arterial wall, or secondary to a cytokine storm, have been reported. Endothelial dysfunction is defined by an overexpression of pro-thrombotic factors (tissue factor), vasoactive factors (endothelin-1) and leukocyte adhesion molecules (I-CAM, V-CAM1) on the cell membrane surface [11]. This results in a state of hypercoagulability, which is related to an imbalance between the excessive production of these pro-thrombotic factors and the decrease in fibrinolytic activity.
There is also a parallel between the inflammatory state and an increase in thrombotic risk [12]. This inflammation-related coagulopathy may progress to a DIC, which is characterized by the formation of clots in small and medium-sized vessels associated with hemorrhagic manifestations due to the consumption of platelets and coagulation factors [13].
Circulating lupus-type anticoagulant and anti-cardiolipin antibodies were present at the acute phase of thrombotic events. These autoantibodies are usually associated with an anti-phospholipid syndrome (APS). APS is a multisystem autoimmune disease characterized by recurrent arterial and/or venous thrombosis. Antiphospholipid antibodies interact with platelet membrane phospholipids and endothelial cells, activating coagulation. However, in order to make the diagnosis of APS, the presence of these antibodies must persist twelve weeks after the first test, which was not the case in our patient. Transient elevation of antiphospholipid antibodies or circulating anticoagulants in patients with thrombosis during SARS COV2 infection has been reported previously [2], [3]. The presence of anti-phospholipid antibodies (usually anti-cardiolipin antibodies) during an infectious state may only reflect an intense stimulation of the immune system. Their presence has been demonstrated in various viral infections such as HIV, hepatitis B and C or infections caused by viruses of the Herpesviridae family. Various meta-analyses have attempted to find an association between the presence of these antibodies in viral infections and an increased risk of thrombosis. However, the conclusions of these studies are discordant and there is currently no established causality between the transient presence of these antibodies and an increased thrombotic risk in viral infections [14], [15].
The management of this type of thrombosis remains poorly codified. Initial treatment is based on curative anticoagulation with heparin. Additional surgical management may reduce the risk of local thrombosis recurrence and embolic complications in the context of normal or minimally atherosclerotic aorta [8], [16]. On the other hand, patients with concomitant malignancy, coagulation disorders and sessile thrombi should preferably be treated conservatively as shown in our case report [17].
Long-term treatment must cover both arterial and venous thrombotic disease. Therapeutic anticoagulation for at least 3 months is recommended for all patients with pulmonary embolism. There is no validated long-term antithrombotic therapy in patients following acute aortic syndromes [18]. In case of suspected APS, anti-thrombotic treatment is based on vitamin K antagonists (VKA). Direct oral anticoagulants are not currently indicated in APS [19]. In the presence of an aortic atherosclerotic lesion, a single antiplatelet therapy is recommended with aspirin or clopidogrel [18].
Conclusion
Thrombotic complications of SARS-COV2 infection have been widely described. However, aortic thrombosis and concomitant acute pulmonary embolism is unusual. Transient elevation of anti-phospholipid antibodies may be observed. Management of such cases should include an exhaustive screening of arterial and venous thrombotic risk factors in order to guide treatment options.
Disclosure of interest
The authors declare that they have no competing interest.
Human and animal rights
The authors declare that the work described has not involved experimentation on humans or animals.
Informed consent and patient details
The authors declare that this report does not contain any personal information that could lead to the identification of the patient.
Funding
This work did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
All authors attest that they meet the current International Committee of Medical Journal Editors (ICMJE) criteria for Authorship.
CRediT authorship contribution statement
R. DEMOULIN: Conceptualization, Formal analysis, Investigation, Writing – original draft, Validation.
T. PREVAUTEL: Investigation, Writing – review & editing.
P. SCHMITT: Writing – review & editing, Validation.
N. ROCHE: Writing – review & editing, Validation.
H. GERARD: Writing – review & editing, Validation.
PL. MASSOURE: Conceptualization, Formal analysis, Writing – review, Validation.
References
- 1.Moschonas I.C., Tselepis A.D. SARS-CoV-2 infection and thrombotic complications: a narrative review. J Thromb Thrombolysis. 2021;52:111–123. doi: 10.1007/s11239-020-02374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devreese K.M.J., Linskens E.A., Benoit D., Peperstraete H. Antiphospholipid antibodies in patients with COVID-19: A relevant observation? J Thromb Haemost. 2020;18:2191–2201. doi: 10.1111/jth.14994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe G.D. Common risk factors for both arterial and venous thrombosis. Br J Haematol. 2008;140:488–495. doi: 10.1111/j.1365-2141.2007.06973.x. [DOI] [PubMed] [Google Scholar]
- 5.Priollet P., Yannoutsos A., Mourad J.J. Ten key points that vascular doctors learned very quickly about COVID-19. J Med Vasc. 2020;45:105–106. doi: 10.1016/j.jdmv.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaise S., Appeltants H., Seinturier C., Imbert B., Thony F., Carpentier P.H. Thromboses aortiques «isolées»: analyse rétrospective de 10 observations [“Isolated” thrombi of the aorta: retrospective study of 10 observations] J Mal Vasc. 2005;30:280–290. doi: 10.1016/s0398-0499(05)83844-2. [DOI] [PubMed] [Google Scholar]
- 8.Fayad Z.Y., Semaan E., Fahoum B., Briggs M., Tortolani A., D’Ayala M. Aortic mural thrombus in the normal or minimally atherosclerotic aorta. Ann Vasc Surg. 2013;27:282–290. doi: 10.1016/j.avsg.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Avila J., Long B., Holladay D., Gottlieb M. Thrombotic complications of COVID-19. Am J Emerg Med. 2021;39:213–218. doi: 10.1016/j.ajem.2020.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashi M., Jacquin A., Dakhil B., Zaimi R., Mahé E., Tella E., et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters K., Unger R.E., Brunner J., Kirkpatrick C.J. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res. 2003;60:49–57. doi: 10.1016/s0008-6363(03)00397-3. [DOI] [PubMed] [Google Scholar]
- 12.Levi M., Poll T., van der Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Peters P., Sprynger M., Lancellotti P., Oury C. Coagulopathies, risque thrombotique et anticoagulation dans la COVID-19 [Coagulopathies, thrombotic risk and anticoagulation in COVID-19] Rev Med Liege. 2020;75:86–93. [PubMed] [Google Scholar]
- 14.Sène D., Piette J.-C., Cacoub P. Anticorps antiphospholipide, syndrome des anticorps antiphospholipides et infections virales [Antiphospholipid antibodies, antiphospholipid syndrome and viral infections] Rev Med Interne. 2009;30:135–141. doi: 10.1016/j.revmed.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Wahab N., Talathi S., Lopez-Olivo M.A., Suarez-Almazor M.E. Risk of developing antiphospholipid antibodies following viral infection: a systematic review and meta-analysis. Lupus. 2018;27:572–583. doi: 10.1177/0961203317731532. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y.Y., Yen H.T., Wu C.C., Huang K.R., Sheu J.J., Lee F.Y. Aortic thrombus in a nonaneurysmal ascending aorta. Ann Vasc Surg. 2021;72:617–626. doi: 10.1016/j.avsg.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Tsilimparis N., Hanack U., Pisimisis G., Yousefi S., Wintzer C., Rückert R.I. Thrombus in the non-aneurysmal, non-atherosclerotic descending thoracic aorta-an unusual source of arterial embolism. Eur J Vasc Endovasc Surg. 2011;41:450–457. doi: 10.1016/j.ejvs.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Aboyans V., Bauersachs R., Mazzolai L., Brodmann M., Palomares J.F.R., Debus S., et al. Antithrombotic therapies in aortic and peripheral arterial diseases in 2021: a consensus document from the ESC Working Group on aorta and peripheral vascular diseases, the ESC Working Group on thrombosis, and the ESC Working Group on cardiovascular pharmacotherapy. Eur Heart J. 2021;42:4013–4024. doi: 10.1093/eurheartj/ehab390. [DOI] [PubMed] [Google Scholar]
- 19.Pengo V., Denas G., Zoppellaro G., Jose S.P., Hoxha A., Ruffatti A., et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365–1371. doi: 10.1182/blood-2018-04-848333. [DOI] [PubMed] [Google Scholar]