Abstract
Medicinal plants are being used as an alternative source of health management to cure various human ailments. The healing role is attributed to the hidden dynamic groups of various phytoconstituents, most of which have been recorded from plants and their derivatives. Nowadays, medicinal plants have gained more attention due to their pharmacological and industrial potential. Aromatic compounds are one of the dynamic groups of secondary metabolites (SM) naturally present in plants; and anthraquinones of this group are found to be attractive due to their high bioactivity and low toxicity. They have been reported to exhibit anticancer, antimicrobial, immune-suppressive, antioxidant, antipyretic, diuretic and anti-inflammatory activities. Anthraquinones have been also shown to exhibit potent antiviral effects against different species of viruses. Though, it has been reported that a medicinal plant with antiviral activity against one viral infection may be used to combat other types of viral infections. Therefore, in this review, we explored and highlighted the antiviral properties of anthraquinones of Polygonaceae, Rubiaceae and Asphodelaceae families. Anthraquinones from these plant families have been reported for their effects on human respiratory syncytial virus and influenza virus. They are hence presumed to have antiviral potential against SARS-CoV as well. Thus, anthraquinones are potential candidates that need to be screened thoroughly and developed as drugs to combat COVID-19. The information documented in this review could therefore serve as a starting point in developing novel drugs that may help to curb the SARS-COVID-19 pandemic.
Keywords: Anthraquinones, Polygonaceae, Asphodelaceae, Rubiaceae, Antiviral potential, SARS-COVID-19
1. Introduction
A plethora of diseases and health conditions are being managed using plant-derived medicines as a promising alternative. Throughout human history, plants and their derivatives have been used to treat different human ailments (Kiani et al., 2016). Medicinal plants have a long historical background of usage in the healthcare systems, probably some 4000 years back (Rai et al., 2014). They have been used in traditional medicine for primary healthcare in many developing and developed countries, including Ethiopia (90%), Benin (80%), India (70%), Tanzania (60%), and China (40%) (WHO, 2002; Kassa et al., 2020). In Africa, about 80% of the population uses medicinal plants to manage their diseases (Kassa et al., 2020). Nowadays, approximately 40% of drugs used in the modern systems of medicine and more than 49% of the new medicines recorded by the United States Food and Drug Administration (USFDA) are known to owe their origin to natural resources, including plants (Patil et al., 2016). Interest in plant-derived drugs has increased due to their negligible harmful and deleterious effects. Herbal medicines have been reported as a promising treatment due to their rich secondary metabolite profiles (Kiani et al., 2016).
Aromatic compounds are one of the major groups of secondary metabolites naturally present in plants. They include anthraquinones which contain in their basic structure three benzene rings (9,10-anthracenedione) belonging to the quinone family of naturally occurring secondary metabolites (Duval et al., 2016). Anthraquinones are the largest group of this family and have been found to be interesting due to their high bioactivity and low toxicity (Chien et al., 2015; Malik and Müller, 2016). They are produced by different species, including plants, lichen and fungi. Approximately 200 anthraquinones have been reported from flowering plants (Diaz-Munoz et al., 2018). They are ubiquitous in plants and can be found in different amounts in various plant tissues, including flowers, roots, stems, rhizomes, bark, fruits and leaves. They are stored in their glycosylated form in plant tissues like rhizomes and can be converted into aglycone anthraquinone by oxidation or β-glycosidase (Pandith et al., 2014; Duval et al., 2016). About 700 anthraquinones have been described as dyes, making them the most important group of naturally occurring pigments (Duval et al., 2016). They have attracted more attention these days due to their pharmacological and industrial potential. Apart from their use as dye pigments in cosmetics, pharmaceutical and food industries (Dufossé, 2014; Duval et al., 2016), anthraquinones have been used as pulping catalysts due to their ability to increase the delignification rate in pulping processes (Diaz-Munoz et al., 2018). Due to their cathartic and laxative properties, anthraquinones of plant origin have been found to be used since ancient times (Thomson, 1986). In addition, their pharmacological potential includes anticancer, antimicrobial, immunosuppressive, antioxidant, antipyretic, diuretic, anti-inflammatory and antiviral effects (Bisset, 1994; Chien et al., 2015; Zhao et al., 2015, Singh et al., 2021).
Antiviral agents are any substance that can produce a therapeutic or protective effect in a virus-infected host. The term excludes substances like a virus-containing vaccine or specific antibodies against the virus (Swallow, 1977). High morbidity and mortality of human beings worldwide are attributed to viral infections (Al-Ali and El-Badry, 2010). Currently, the whole world is facing one of the devastating viral outbreaks, COVID-19, a disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) (Tillu et al., 2020). In 2020, the pandemic affected more than 45,667,780 individuals causing over 1,189,499 deaths globally (ECDPC, 2020). In early 2021, approximately 100 million subjects in the world were reported with confirmed SARS-CoV-2 infection and more than 2 million deaths have been attributed to COVID-19 (Wang et al., 2021). To date, an estimated 4.7 million individuals worldwide have died after being exposed to the disease (WHO, 2021). With the declaration of the pandemic, significant efforts have been made in designing, manufacturing and testing a variety of vaccines against COVID-19 worldwide (Spencer et al., 2021). Statistical data have been released to display the impact of immunization on treatment and records of the death toll of the disease (Hall et al., 2021; Vasileiou et al., 2021). Although different mutations have occurred within the viral genome, leading to more virulent variants like delta and omicron (Moona et al., 2021, Torjesen, 2021). To date, no other approved antiviral drug is available to treat novel coronavirus infection except remdesivir (Henss et al., 2021). Thus, a critical necessity has emerged to search for antiviral agents that could help to fight this uncontrollable virus.
Viruses have shown more resistance to prophylaxis or therapy as compared to other pathogens and only a few antiviral drugs are available today (Abd-Alla et al., 2012). To fight against severe viral infections in living organisms, efforts have been devoted to exploring new antiviral agents from natural resources (Mbanga et al., 2010). However, the occurrence of antiviral agents in plants makes them potential candidates for the treatment of viral diseases (Abd-Alla et al., 2012). The search for antiviral agents of plant origin has intensified since 2020 due to the COVID-19 outbreak. Plants and their derivatives have been known to show promising antiviral effects and different phytochemicals have been screened against various pathogenic viruses. Among them, anthraquinones have attracted more attention and have been reported from different plant species including Rubia, Cassia, Galium, Morinda, Rheum and Caprosma (Duval et al., 2016). The majority of anthraquinones with antiviral effects have been reported from plants of Asphodelaceae and Polygonaceae families. It is known that the antiviral effects of a medicinal plant against one viral infection may be demonstrated in other types of viral diseases (Tegen et al., 2021). Hence, in this review, we have gathered vital information on the antiviral potential of anthraquinones isolated from the plants of Asphodelaceae, Rubiaceae and Polygonaceae families. Our goal is to draw the attention of researchers toward the major resources of these naturally occurring anthraquinones which could serve as the reservoirs for the isolation and development of antiviral drugs used in the treatment of various viral infections, including COVID-19.
2. Extraction of anthraquinones from Polygonaceae, Asphodelaceae and Rubiaceae
Different methods such as maceration, soxhlet extraction, microwave assisted extraction, ultrasound-assisted extraction, pressurized liquid extraction and super/subcritical fluid extraction are commonly used for the extraction of naturally occurring anthraquinones (Duval et al., 2016). In this review, we have focused on the methods used for the extraction of anthraquinones from different plant parts of the Polygonaceae, Rubiaceae and Asphodelaceae families.
Flower-peduncles and flowers of Aloe hijazensis (Asphodelaceae) have been used in the preparation of plant extracts. The dried sample of each part has been powdered and extracted by macerating in 80% aqueous methanol. The extract obtained after evaporation of the solvent has been further defatted, resuspended in water and fractionated using solvents of increasing polarity viz. ether, ethyl acetate and n-butanol. The fractions obtained have been used for the isolation of phytochemicals of the plant (Abd-Alla et al., 2012). A similar protocol has been used by Kim et al. (2017) to extract 5 kg of the powder of Aloe vera (Asphodelaceae). The extract has been partitioned successively thrice with n-hexane, chloroform ethyl acetate and butanol. Chloroform and ethyl acetate fractions have been further used for the isolation of the plant secondary metabolites. Successive partition in ethyl acetate and n-butanol has been also used to fractionate aqueous suspension of 80% aqueous ethanol extract prepared by macerating 8.5 kg of the bark powder of Morinda citrifolia L. (Rubiaceae) (Wang et al., 2016). The root powder of Rheum tanguticum (Polygonaceae) has been extracted under reflux in hydro-alcoholic solution (80% ethanol). The extract has been further dissolved in water, mixed with 80% ethanol:acetone, ultrasonicated and used to isolate anthraquinones (Xiong et al., 2011). Hydro-alcoholic extract (95% ethanol) has been prepared by macerating 20 kg of pulverized dried roots of Knoxia valerianoides (Rubiaceae) in 10 L of solvent. The extract has been dissolved in water and partitioned with ethyl acetate (Zhao et al., 2015). The authors have further used the ethyl acetate fraction to isolate the plant secondary metabolites. Ethanol extract of Dianella longifolia (Asphodelaceae) has been prepared by macerating the powdered roots in the solvent at room temperature (Semple et al., 2001). The powder of the tubers of Rumex dentatus and Rheum palmatum (Polygonaceae) has been macerated separately in water and anthraquinones have been quantified from the aqueous extract using HPLC analysis (Liu et al., 1997).
3. Antiviral anthraquinones of Polygonaceae, Rubiaceae and Asphodelaceae
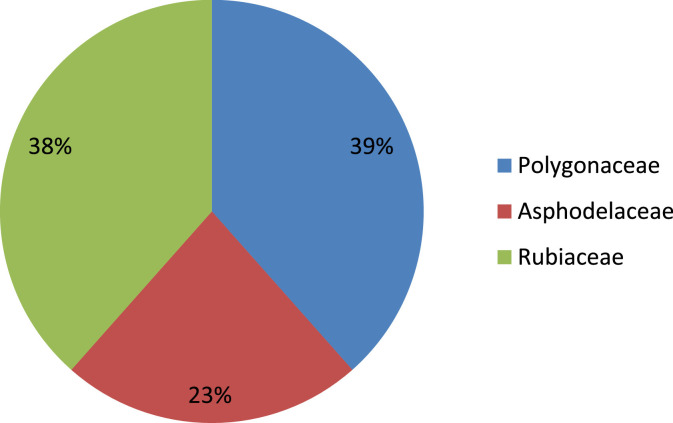
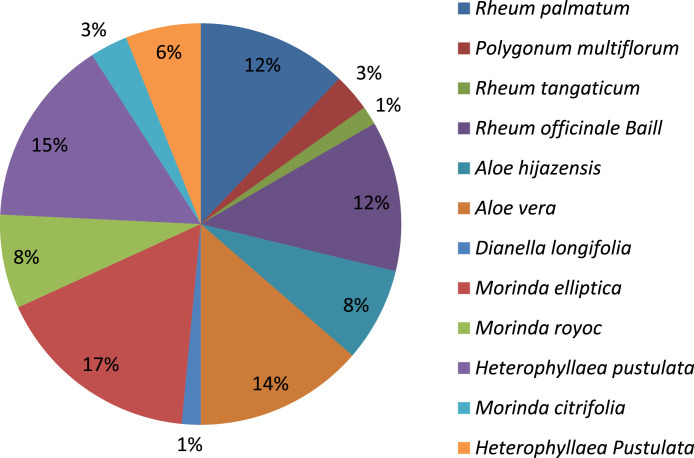
Different plant species of the Asphodelaceae, Polygonaceae and Rubiaceae family have been collected to isolate anthraquinones and screened for antiviral activities. Plants of the Polygonaceae family have been shown with high frequency in the isolation of anthraquinones (Fig. 1 ). Rubiaceae was found as a potent source of anthraquinones with most of the compounds isolated from Morinda elliptica followed by Heterophylleae pustulata (Fig. 2 ).
Fig. 1.
Frequency of anthraquinone-producing species in each family.
Fig. 2.
Occurring frequency of anthraquinones in different species.
3.1. Anthraquinones of Polygonaceae
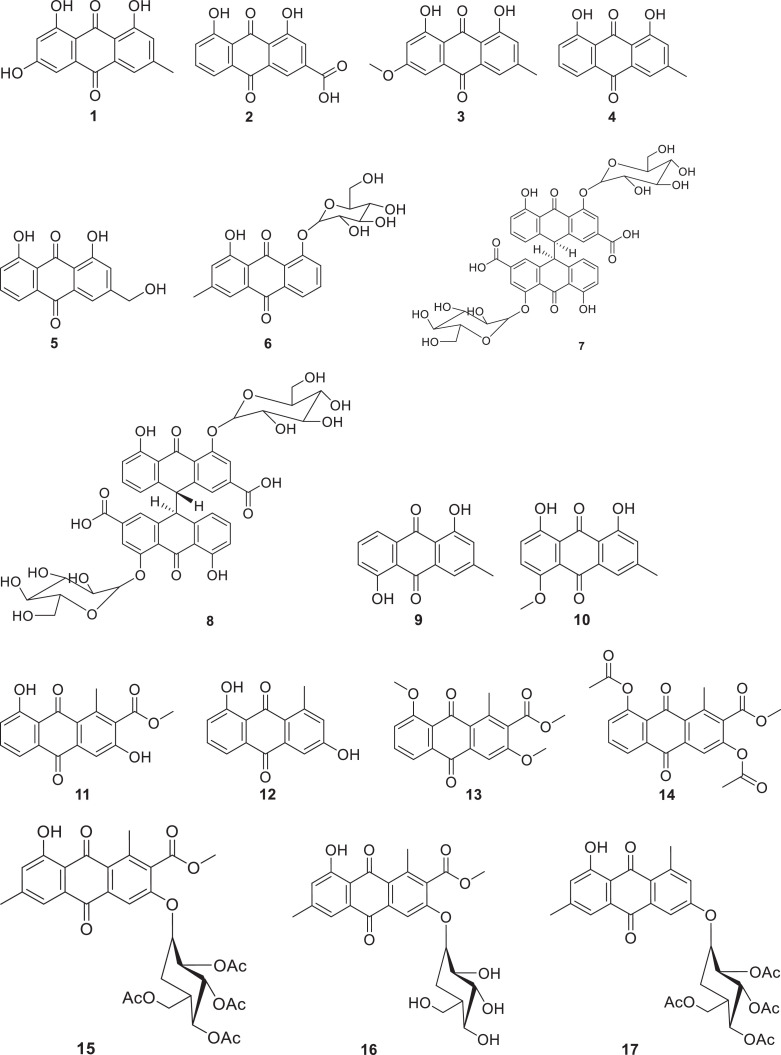
Polygonaceae, commonly known as knotwood or smartweed is a family of flowering plants which contains about 1200 species distributed among 50 different genera (Ammar et al., 2020, Mishra et al., 2018). Anthraquinones have been reported from different genera of the Polygonaceae family including Rheum, Polygonum and Rumex. Among these genera, plants of Rheum and Polygonum species have demonstrated their ability to produce anthraquinones with vital antiviral effects. Emodin (1), a potential antiviral anthraquinone, has been isolated from Rheum palmatum, Rheum tanguticum, Rheum officinale Baill., Polygonum cuspidatum and Polygonum multiflorum (Li et al., 2005; Ho et al., 2007; Xiong et al., 2011, Liu et al.; 2013). In addition, other anthraquinones such as rhein (2), physcion (3), chrysophanol (4), aloe-emodin (5), chrysophanol 8-O-β- D-glucoside (6) have also been reported from Rheum palmatum and Rheum officinale Baill (Li et al., 2007, Esposito et al., 2016). Sennoside A (7) and Sennoside B (8) have been reported in Rheum palmatum and Rheum officinale Baill. (Esposito et al., 2016). Rhein has been also reported as one of the major phytochemicals from Polygonum multiflorum (Ho et al., 2007).
3.2. Antraquinones of Asphodelaceae
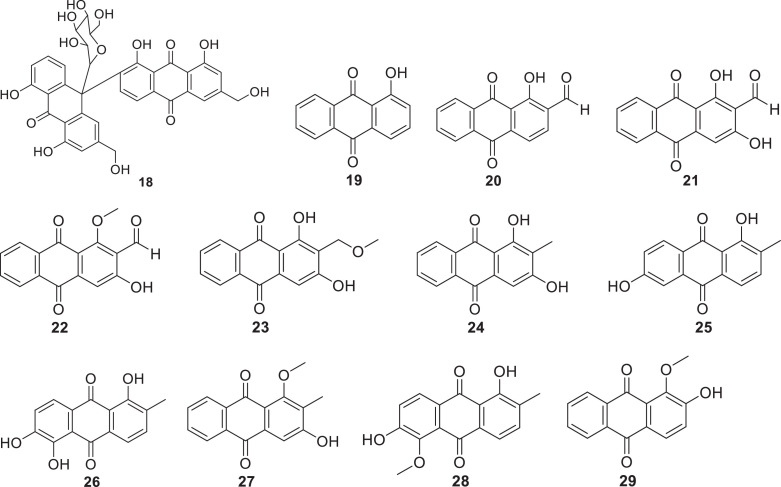
Plants of Asphodelaceae family are flowering plants classified in the order Asparagales (APG, 2016). The family contains approximately 40 genera and 900 species (Christenhusz and Byng, 2016), which are known to produce majorly anthraquinones. This class of secondary metabolites has been isolated from Aloe hijazensis, Aloe vera and Dianella longifolia (Semple et al., 2001; Abd-Alla et al., 2012; Kim et al., 2017; Borges-Argáez et al., 2019). Anthraquinones named chrysophanol, aloe-emodin, ziganein (9), ziganein-5-methyl ether (10) and aloesaponarin I (11) have been isolated along with other compounds such as p-coumaric acid, aloenin, feralolide, alkaloids and barbaloin from the flowers and flower-peduncles of Aloe hijazensis, (Abd-Alla et al., 2012). Aloe vera has demonstrated its capability to biosynthesize anthraquinones, including aloesaponarin I and aloesaponarin II (12), along with their derivatives such as 3,8-dimethoxy-aloesaponarin I (13), 3,8-diacetoxy-aloesaponarin I (14), 3-(2´,3´,4´,6´-Tetra-O-acetyl-β-D-glucopyranosyl-aloesaponarin I (15), 3-glucosyl aloesaponarin I (16) and 3-(2´,3´,4´,6´-Tetra-O-acetyl-β-D-glucopyranosyl-aloesaponarin II (17) (Borges-Argáez et al., 2019). In addition, the plant has been reported to produce aloe-emodin and elgonica dimer A (18) (Kim et al., 2017; Mpiana et al., 2020). Dianella longifolia has been reported to produce chrysophanol (chrysophanic acid) (Semple et al., 2001).
3.3. Anthraquinone of Rubiaceae
Rubiaceae family contains majorly terrestrial plants comprising more than 13000 species distributed in at least 600 genera (Simpson, 2019). Plants of this family are mostly distributed in temperate, tropical and subtropical regions. Some species are of important medicinal value and are widely used to treat diarrhoea, headache, cholera, fever, wounds, eye problem, cancer, typhoid and enlarged spleen (Ali et al., 2000; Jeruto et al., 2011).
Only a few species of the Rubiaceae family have been reported to produce anthraquinones with antiviral activity. Morinda elliptica, Heterophyllaea pustulata and Morinda royoc have been shown to produce a variety of anthraquinones, including 1-Hydroxy-2-methylanthraquinone (19), 2-formyl-1-hydroxyanthraquinone (20), nordamnacanthal (21), damnacanthal (22), lucidin-ω-methyl ether (23), rubiadin (24), soranjidiol (25), morindone (26), rubiadin-1-methyl ether (27), morindone-5-methyl ether (28) and alizarin-1-methyl ether (29) (Ali et al., 2000; Borroto et al., 2008, Konigheim et al., 2012).
4. Antiviral activity of anthraquinones of Polygonaceae, Rubiaceae and Asphodelaceae
To fight against infectious viruses in humans and other living organisms, significant efforts have been made for the discovery of new natural products with antiviral potential. A variety of medicinal plants have been demonstrated for their potential in the treatment of various viral infections and many of them have shown a broad-spectrum antiviral activity (Dao et al., 2011; Perera and Efferth, 2012). Anthraquinones and anthraquinone-like compounds have been reported earlier to represent a novel class of potential antiviral secondary metabolites and hypericin has proven its effectiveness against infectious bronchitis virus (Tang et al., 1990; Anderson et al., 1991; Hudson et al., 1991; Chen et al., 2019). Anthraquinones from different species of Polygonaceae, Asphodelaceae and Rubiaceae have demonstrated their antiviral activity against a variety of viruses including SARS-CoV-2, human immunodeficiency virus (HIV), poliovirus, herpes simplex virus, hepatitis virus, respiratory syncytial virus (RSV) and coxsackievirus (Ho et al., 2007, Li et al., 2007; Xiong et al., 2011; Liu et al., 2015; Esposito et al., 2016; Parvez et al., 2019). Among them, chrysophanol has been reported to exhibit potential antiviral effects against poliovirus type-2 and type-3, and SARS-coronavirus has been shown to be inhibited by aloe-emodin and emodin (Table 1 ).
Table 1.
Antiviral potential of naturally occurring anthraquinones.
| Compound | Plant species | Model | Pharmacological parameters | Refs. |
|---|---|---|---|---|
| Chrysophanol |
Rheum palmatum, Rheum officinale, Aloe vera, Dianella longifolia |
HIV-1 RT-associated RNase H HIV-1 RDDP HBV HBV virion DNA HBsAg HBeAg Poliovirus type-2 Poliovirus type-3 HBV polymerase |
IC50 = 25.0 µM IC50 = 26.5 µM I = 65.5% IC50 = 213.2 µg/mL IC50 = 286 µg/mL IC50 = 322 µg/mL EC50 = 0.21 µg/mL EC50 = 0.02 µg/mL ∆G = – 7.6 kcal/mol |
Li et al. (2007), Semple et al. (2001), Esposito et al. (2016), Parvez et al. (2019) |
| Sennoside A |
Rheum palmatum, Rheum officinale |
HIV-1 RT-associated RNase H HIV-1 RDDP |
IC50 = 1.9 µM IC50 = 5.3 µM |
Esposito et al. (2016) |
| Sennoside B |
Rheum palmatum, Rheum officinale |
HIV-1 RT-associated RNase H HIV-1 RDDP |
IC50 = 2.1 µM IC50 = 2.3 µM |
Esposito et al. (2016) |
| Rhein |
Rheum palmatum, Rheum officinale |
HIV-1 RT-associated RNase H HIV-1 RDDP HBV virion DNA HBsAg HBeAg Human coronaviruses |
IC50 = 60 µM IC50 > 100 µM IC50 = 30.97µg/mL IC50 = 57.10 µg/mL IC50 = 59.91 µg/mL Dose-dependently |
Li et al. (2007), Esposito et al. (2016), Zannella et al. (2021) |
| Aloe-emodin |
Rheum palmatum, Rheum officinale, Aloe vera. Rheum emodi |
HIV-1 RT-associated RNase H HIV-1 RDDP HBV HBV virion DNA HBsAg HBeAg HBV polymerase SARS coronavirus 3CL pro COVID-19 |
IC50 = 23.0 µM IC50 = 21.0 µM I = 81.7% IC50 = 4,626.2 µg/mL IC50 = 715.31 µg/mL IC50 = 5,863.36 µg/mL ∆G = – 8.2 kcal/mol IC50 = 366 µM ∆G = – 36.92 kcal/mol |
Lin et al. (2005), Li et al. (2007), Esposito et al. (2016), Parvez et al. (2019), Rolta et al. (2021) |
| Alizarine | Rheum emodi | COVID-19 | ∆G = – 33.59 kcal/mol |
Rolta et al. (2021) |
| Physcion |
Rheum palmatum, Rheum officinale |
HIV-1 RT-associated RNase H HIV-1 RDDP HBV virion DNA HBsAg HBeAg |
IC50 > 100 µM IC50 > 100 µM IC50 = 159.3 µg/mL IC50 = 253 µg/mL IC50 = 194 µg/mL |
Li et al. (2007), Esposito et al. (2016) |
| Emodin |
Rheum palmatum, Rheum officinale, Rheum tanguticum, Polygonum multiflorum, Rheum emodi Polygonum cuspidatum |
HIV-1 RT-associated RNase H HIV-1 RDDP Herpes simplex virus HBV virion DNA HBsAg HBeAg SARS-CoV-2 COVID-19 CVB4 CVB5 |
IC50 > 100 µM IC50 > 100 µM Dose-dependent manner IC50 = 27.32 µg/mL IC50 = 46.17 µg/mL IC50 = 35.24 µg/mL IC50 = 200 µM ∆G = – 40.60 kcal/mol EC50 = 12.06 µM EC50 = 12.11 µMol/L |
Ho et al. (2007), Li et al. (2007), Xiong et al. (2011), Liu et al. (2013), Esposito et al. (2016), Rolta et al. (2021) |
| Aloin B | Aloe vera | HBV HBVpolymerase |
I = 62% ∆G = – 7.4 kcal/mol |
Parvez et al. (2019) |
| Dantron | Rheum emodi | COVID-19 | ∆G = – 45.48 kcal/mol | Rolta et al. (2021) |
| Chrysophanol 8-O-β-D-glucoside | Rheum palmatum | HBV virion DNA HBsAg HBeAg |
IC50 = 36.98 µg/mL IC50 = 237.4 µg/mL IC50 = 183.41 µg/mL |
Li et al. (2007) |
| Anthrarufin | Rheum emodi | COVID-19 | ∆G = – 34.95 kcal/mol | Rolta et al. (2021) |
| 1,3-dihydroxy-5-methoxy-6-methoxymethyl-2- methyl-9,10-anthraquinone. | Morinda citrifolia | H1N1 | IC50 = 66.1 µM | Wang et al. (2016) |
| 1,3-dihydroxy-5-methoxy-2,6-bismethoxy- methyl-9,10-anthraquinone. | Morinda citrifolia | H1N1 | IC50 = 10.5 µM | Wang et al. (2016) |
HBV: Hepatitis B virus, HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B envelop antigen; I: percentage of inhibition, IC50 or EC50: Concentration of the compound producing 50% inhibition; ∆G: binding energy in docking study; 3CL pro: 3C-like protease
4.1. Herpes simplex virus type-1 (HSV-1) and type-2 (HSV-2)
HSV-1 and HSV-2 are enveloped viruses belonging to the herpes virus family consisting of more than 100 double-stranded DNA viruses (Shukla and Spear, 2001). They are divided into α, β and γ subgroups, and HSV-1 and HSV-2 are of α subfamily with high prevalent infections among humans (Whitley and Roizman, 2001). It is estimated that about 60 to 95% of human adults are infected by type-1 or type-2 herpes virus worldwide. HSV-2 is responsible for genital herpes in humans, in addition to other complications like cold sore, meningitis, eye infections and encephalitis (Zandi et al., 2007; Burcea et al., 2015; Sauerbrei, 2016). The virus can also cause life-threatening malady in immune-compromised persons such as newborns, HIV patients, or victims undergoing immunosuppressive remedies (Shukla and Spear, 2001; Whitley and Roizman, 2001; Gottlieb et al., 2019; Obisesan et al., 2021).
Extract of Aloe vera has been reported for its inhibitory effects on pre- and post-attachment of HSV-2 to the cell, with IC50 values of 428 and 536 µg/mL (Zandi et al., 2007). It has been reported as a potential candidate for the isolation of natural antiviral products that can be used in the development of drugs against HSV infection. 0.5% of the extract and gel of the plant in hydrophilic cream have been demonstrated for their efficacy in the management of genital herpes in males (Syed et al., 1996). Aloe-emodin, an anthraquinone from the plant has been shown to exhibit inhibitory effects on HSV-1 and HSV-2 by blocking nucleic acid biosynthesis leading to immature termination of the viral proteosynthesis (Mpiana et al., 2020). At the concentration of 50 µg/mL, emodin isolated from Rheum tanguticum has been reported to inhibit the replication of both viruses (HSV-1 and type-2 HSV-2) in infected cells with an antiviral index of 2.07 and 3.53, respectively. The compound has been found to increase the survival rate of HSV-infected mice and to prolong significantly the efficacy of HSV elimination from the organs such as the liver, brain, heart and ganglion (Xiong et al., 2011). Nanoparticles containing emodin, aloe-emodin, chrysophanol, rhein and physcion from the plant have shown their ability to inhibit the whole phase of HSV-1 replication. The compounds have been reported to exhibit the inhibition of mRNA expression and proteosynthesis of the viral proteins ICP4 and ICP8. In addition, the nanoparticles have been demonstrated for their ability to decrease the viral load and alleviate pathological changes in the brain tissues of HSV-1-induced mice (Shen et al., 2019).
4.2. Human immunodeficiency virus (HIV)
HIV is a retrovirus that can induce Acquired Immuno-Deficiency Syndrome (AIDS) in humans. Individuals affected by the syndrome are characterized by immune deterioration resulting finally in the failure of the immune system. The virus is the leading cause of morbidity and mortality, mostly in African countries, including those in Sub-Saharan Africa (GDB, 2017). The disease has been reported to be the cause of death of more than 25 million people since its first recognition in 1981. The prevalence of HIV was estimated to 0.6% of the global population in 2006 (Naithani et al., 2008). In 2017, the virus was reported to cause 75% of deaths and 65% of new cases have been further reported, and 71% of people have been declared living with HIV in Sub-Saharan Africa (GDB, 2017, Dwyer-Lindgren et al., 2019).
The focus on the discovery and usage of phytoconstituents as antivirals against HIV has been extensively increased. Extracts of Rheum palmatum L. and Rheum officinale Baill. have shown their ability to inhibit the activity of HIV-1 reverse transcriptase (RT)-associated RNase H with IC50 values of 0.9 and 0.25 µg/mL, respectively (Esposito et al., 2016). Sennosides A and B identified as novel dual functions RT inhibitors have been reported to display potent inhibitory effects on both (RT)-associated DNA Polymerase (RDDP) and RNase H RT-associated functions with IC50 values ranging from 1.9 to 5.3 µM. Chrysophanol and aloe-emodin isolated from the plants have shown moderate inhibitory effects on both enzymes (RT and RDDP) with IC50 values of 21–26 μM (Esposito et al., 2016).
4.3. Hepatitis B virus
Hepatitis B virus (HBV) is an enveloped DNA virus responsible for a major public health problem. The virus has been reported to infect about two billion individuals and 350 million people have been estimated to be chronic carriers of the pathogen worldwide (Ayoola et al., 1988; WHO, 1998). Its infection leads to hepatocellular carcinoma, acute and chronic liver ailments in humans such as liver cirrhosis and hepatitis, leading to the death of more than one million people annually (Ayoola et al., 1988; WHO, 1998; Ott et al., 2012).
Extract of Aloe vera has shown its inhibitory effects against HBV by down-regulating the synthesis of viral antigens by 38.1% at the concentration of 50 µg/mL. The virus has been also found to be inhibited by the ethanolic extract of Rheum palmatum which encumbered the viral DNA production and antigen (HBsAg) expression, with an IC50 value of 212.36 µg/mL (Li et al., 2007; Parvez et al., 2019). The inhibitory effects have been observed with aloe‐emodin, chrysophanol and aloin B isolated from the A. vera at the concentration of 10 µg/mL, displaying the inhibition percentage of 81.7%, 65.5% and 62%, respectively (Parvez et al., 2019). Chrysophanol 8-O-β-D-glucoside from R. palmatum has been reported to significantly inhibit antigen expression and DNA replication in HBV with an IC50 value of 36.98 µg/mL, and has been reported as a promising candidate in the development of antiviral drugs against HBV infections (Li et al., 2007). In a docking study, aloe‐emodin, chrysophanol and aloin B have been shown to bind to the active site of HBV polymerase with high binding energy of –8.2 kcal/mol, –7.6 kcal/mol and –7.4 kcal/mol, respectively, thus forming a stable complex with the enzyme suggesting its possible inhibition by the compounds (Parvez et al., 2019).
4.4. Poliovirus
Poliovirus is an enterovirus and a causative agent of poliomyelitis (or polio) in humans. The virus contains a single-stranded RNA in a non-enveloped capsid. It is of viral serotypes responsible for the damage to the nervous system and induces paralytic disease in patients (Racaniello, 2006). The disease is more prevalent in children of developing countries, mainly in Asia and Africa, where polio immunization is less accessible (Naithani et al., 2008).
In vitro study has displayed the inhibitory effects of chrysophanol (chrysophanic acid) isolated from Dianella longifolia against the replication of poliovirus types-2 and type-3 with an EC50 value of 0.21 and 0.02 µg/mL, respectively (Semple et al., 2001). In vitro antiviral effects have been also investigated and the compound has shown the ability to inhibit the cytopathogenic effect of poliovirus types-2 and type-3 in buffalo and green monkey kidneys with EC50 equal to 210 and 20 µg/mL, respectively. The compound has been also found to attenuate the replication of the virus at its early stage (Yusuf et al., 2019)
4.5. Human respiratory syncytial virus
Human respiratory syncytial virus (RSV) is a negative-sense single-stranded RNA virus (Lin et al., 2021) which is the most frequent cause of infection of the lower part of the respiratory tract. The infection is most common in children under the age group of 5 years, causing mainly bronchiolitis and pneumonia (Shi et al., 2017; Aranda and Polack, 2019). The virus is responsible for infection in about 33 million cases annually worldwide and infants younger than one year are more exposed to the disease (Shi et al., 2017). The virus is also reported to infect the older age group and patients with chronic cardiopulmonary disease and immunocompromised individuals, causing severe illness (Varga and Braciale, 2013).
Emodin, an active ingredient isolated from hydroalcoholic extract of Rheum palmatum has demonstrated its inhibitory effects against RSV. The compound has been shown to inhibit the virus significantly with an effective concentration (EC50) of 13.06 µmol/L. In addition, emodin has been demonstrated to decrease mRNA expression of cytokine IFN-α (Lin et al., 2021), suggesting that the compound could be developed as an antiviral candidate against human respiratory viral infection. Rhein isolated from the same plant has shown its capability to alleviate the infection and injury in lungs caused by the virus in RSV-induced mice. The compound has been reported to inhibit the immune inflammatory response of the infected animals. Hence, rhein has been suggested to be a promising treatment for RSV infection and to further prevent lung tissue damage (Shen et al., 2020).
4.6. Coxsackievirus B4 and B5
Coxsackievirus is a non-enveloped, single-stranded RNA virus belonging to the family of Picornaviridae (Muckelbauer et al., 1995, Liu et al., 2013). Coxsackievirus B4 (CVB4) is an Enterovirus responsible for a wide range of diseases, including pancreatitis, myocarditis, aseptic, hepatitis, meningitis, pneumonia and necrotizing enterocolitis, and is fatal to newborns (Crowell and Landau, 1997). Coxsackievirus B5 (CVB5) is highly prevalent in some countries and is commonly associated with diseases such as myocarditis and encephalitis in immunocompromised children. It has been also reported to induce central nervous system illness in elderly people (Zhong et al., 2009; Trallero et al., 2010).
Emodin, an anthraquinone isolated from Polygonum cuspidatum has been shown to block the penetration and replication of CVB4 in Hep-2 cells in a dose and time-dependent manner with an EC50 value of 12.06 μM. In addition, the compound has been also shown to increase the survival rate and prolong the mean time of death in CVB4-induced mice (Liu et al., 2013). Emodin isolated from Rheum palmatum has shown inhibitory activity on CVB5 in Hep-2 cells with an EC50 value of 12.11 µmol/L (Liu et al., 2015).
4.7. Influenza virus
Influenza is an enveloped negative-strand RNA virus (Palese and Shah, 2007) responsible for the most common respiratory infections in humans with high morbidity and mortality (Wright et al., 2007). The virus was recorded as the worst pandemic in 1918 and has led to the death of about 50 million people worldwide (Taubenberger and Morens, 2008, Hutchinson, 2018). Influenza A virus (IAV) and influenza B virus (IBV) are typically virulent, causing the death of about 290 000 – 650 000 individuals a year globally (Hutchinson, 2018).
Rhein has been reported to inhibit the entry and replication of IAV in cells, in addition to its ability to decrease IAV-induced oxidative stress and to activate NF-κB, Akt, TLR4, p38 and JNK MAPK pathways (Wang et al., 2018). Rheum officinale Baillon, Aloe vera and Polygonum cuspidatum have been demonstrated to display high inhibitory effects against IAV and their activity has been assigned to their phytoconstituents, including anthraquinones. Naturally occurring anthraquinones like emodin, aloe-emodin-8-O-β-D-glucopyranoside, chrysophanol-8-O-glucoside and emodin-1-O-β-D-glucopyranoside have been shown to significantly suppress IAV replication even at the lowest concentration of 3.125 μg/mL (Bei et al., 2021). Aloe vera extract and its constituents, including aloe-emodin have been also reported to inhibit IAV. The compound has shown a high affinity with the IAV M2 proton channel protein in the docking study, with a binding energy of 5.47 kcal/mol (Choi et al., 2019).
4.8. Japanese encephalitis virus
Japanese encephalitis virus (JEV) is a positive single-strand RNA virus belonging to the Flaviviridae family. The virus is more prevalent in many countries of the western Pacific and Asian continents. About 67,900 people are infected by the virus annually and approximately 75% of them are children age group 0–14 (Zheng et al., 2012). The virus is of high morbidity and mortality, causing severe central nervous system ailments like encephalitis and aseptic meningitis (Unni et al. 2011).
Extracts of R. palmatum and its anthraquinones viz. aloe-emodin and chrysophanol have been demonstrated with a potent inhibitory effect on the virus with IC50 values ranging from 0.46 µg/mL to 51.41 µg/mL. Aloe-emodin has been reported as the most potent virucidal compound with an IC50 value of 0.46 µg/mL (Chang et al.,2014). The compound and methanol extract of the plant have shown a high therapeutic index and thus could be developed as potential antiviral candidates against JEV.
4.9. SARS coronavirus
Severe acute respiratory syndrome coronavirus (SARS-CoV) is an enveloped single-stranded positive-sense RNA virus that has been reported to be responsible for progressive respiratory failure and death in up to 10% of infected patients in 2003 (Ksiazek et al., 2003; Peiris et al., 2003). Currently, the world is facing the COVID-19 pandemic, a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Tillu et al., 2020). In early 2021, more than 100 million subjects globally have been reported with confirmed SARS-CoV-2 infection and more than 2 million deaths have been attributed to COVID-19 (Wang et al., 2021). To date, no antiviral drug is available to treat novel coronavirus infection except remdesivir.
Many reviews have already mentioned the potent inhibitory effects of emodin on the interaction of S protein with angiotensin-converting enzyme-2 (ACE-2) reported by Ho and colleagues (Nazir et al., 2013; Zhang and Liu, 2020). In addition, the authors showed that rhein from Polygonaceae exhibited slight inhibition of the enzyme interaction with S protein (Ho et al., 2007). In a study by Zannella and colleagues (2021), rhein has shown its capability to inhibit the infection and replication of different human coronaviruses (HCoV-229E, HCoV-OC43 and SARS-CoV-2). The compound has exhibited higher inhibitory effects against HCoV-OC43 and SARS-CoV-2 by blocking the viral life cycles completely at a higher concentration of 200 µg/mL. At the same concentration (200 µg/mL), 90% of inhibition has been observed with the compound against HCoV-229E. Aloe-emodin has been demonstrated with its anti-SARS coronavirus effects by inhibiting the cleavage activity of 3C-like protease dose-dependently, with an IC50 value of 366 µM (Lin et al., 2005). Docking study showed the ability of a variety of anthraquinones and their derivatives, including chrysophanol 8-O-β-D-glucoside and emodin 8-glucoside to bind to SARS-CoV-2 proteins such as 3C-like protease, spike protein and papain-like protease, with torososide B and 1,3,6-trihydroxy-2-methyl-9,10-anthraquinone-3-O-(6′-O-acetyl)-β-d-xylopyranosyl-(1→2)-β-D-glucopyranoside, showing the highest binding affinity with papain-like protease and 3C-like protease, respectively (Khanal et al., 2020). In silico study has been reported showing a high-affinity binding of anthraquinones of R. emodi including aloe-emodin, anthrarufin, alizarine, dantron and emodin to SARS-CoV-2 at the active sites of RNA binding domain of nucleocapsid phosphoprotein. The compounds have been found to bind to all three active sites with the binding energy varying from –25.45 Kcal/mol to – 45.48 Kcal/mol (Rolta et al., 2021), and hence could be screened thoroughly and released as potential drug candidates to treat COVID-19.
5. Conclusion
This paper is an investigation of plants of three different families known to produce natural anthraquinones which are well known for their antiviral potential. The review revealed that plants of Polygonaceae, Asphodaceae and Rubiaceae are a potential source of anthraquinones which have demonstrated their antiviral potential against a variety of infectious viruses. Still, only a few numbers of this group of secondary metabolites have been screened for their effects against coronavirus. Many anthraquinones from the plants of these families have shown their effects against viruses that infect the respiratory tract, including human respiratory syncytial virus and influenza virus which infect the respiratory tract like coronavirus and can be potential candidates in the screening and development of drugs against COVID-19. Further, many anthraquinones have demonstrated their capacity to inhibit coronavirus in vitro as well as in silico and constitute potently targeted naturally occurring phytochemicals that could be developed as anti-COVID-19 drugs. Hence, this review is a repertoire of natural antiviral anthraquinones reported from Polygonaceae, Asphodaceae and Rubiaceae that could serve as potential leads for antiviral drugs discovery, especially in combating coronavirus and in the development of other new antiviral agents.
Funding
No specific grant was provided for this investigation
Ethics approval
Not applicable.
Consent to participate (include appropriate statements)
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
CRediT authorship contribution statement
Augustin Ntemafack: Conceptualization, Writing – original draft. Rahul Vikram Singh: Data curation, Writing – original draft. Sabeena Ali: Data curation, Writing – review & editing. Jules-Roger Kuiate: Investigation, Writing – original draft. Qazi Parvaiz Hassan: Investigation, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no conflict of interests.
Edited by: K. Dolezal
References
- Abd-Alla H.I., Abu-Gabal N.S., Hassan A.Z., El-Safty M.M., Shalaby N.M. Antiviral activity of Aloe hijazensis against some haemagglutinating viruses infection and its phytoconstituents. Arch. Pharm. Res. 2012;35(8):1347–1354. doi: 10.1007/s12272-012-0804-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ali K.H., El-Badry A.A. Anti-viral activity of two Labiatae plants [Naana (Hassoi, Habak) and basil (Rahan)] of Al-Madiah Al-Munawarah. J. Med. Bio-med. Sci. 2010;2:67–73. [Google Scholar]
- Ali A.M., Ismail N.H., Mackeen M.M., Yazan L.S., Mohamed S.M., Ho A.S., Lajis N.H. Antiviral, cytotoxic and antimicrobial activities of anthraquinones isolated from the roots of Morinda elliptica. Pharm. Biol. 2000;38(4):298–301. doi: 10.1076/1388-0209(200009)3841-AFT298. [DOI] [PubMed] [Google Scholar]
- Ammar S., Abidi J., Vlad Luca S., Boumendjel M, Skalicka‐Woźniak K., Bouaziz M. Untargeted metabolite profiling and phytochemical analysis based on RP‐HPLC‐DAD‐QTOF‐MS and MS/MS for discovering new bioactive compounds in Rumex algeriensis flowers and stems. Phytochem. Anal. 2020;3:616–635. doi: 10.1002/pca.2928. [DOI] [PubMed] [Google Scholar]
- Anderson D.O., Weber N.D., Wood S.G., Hughes B.G., Murray B.K., North J.A. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antivir. Res. 1991;16:185–196. doi: 10.1016/0166-3542(91)90024-l. [DOI] [PubMed] [Google Scholar]
- APG: Angiosperm Phylogeny Group An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016;181(1):1–20. [Google Scholar]
- Aranda S.S., Polack F.P. Prevention of pediatric respiratory syncytial virus lower respiratory tract illness: perspectives for the next decade. Front. Immunol. 2019:1006. doi: 10.3389/fimmu.2019.01006. 7,10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoola E.A., Balayan M.S., Deinhardt F., Gust I., Kure Maynaed A.W., Nayak N.C., Brodley D.W., Ferguson M., Melnic Purcell J., Zuckerman A.J. Progress in the control of viral memorandum from a WHO meeting. Bull. W. H. O. 1988;66:443–455. [PMC free article] [PubMed] [Google Scholar]
- Bei Y., Tia B, Li Y., Guo Y., Deng S., Huang R., Zeng H., Li R., Wang G.F., Dai J. Anti-influenza A virus effects and mechanisms of emodin and its analogs via regulating PPARα/γ-AMPK-SIRT1 pathway and fatty acid metabolism. BioMed. Res. Int. 2021 doi: 10.1155/2021/9066938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset N.G. CRC Press; Boca Ratόn: 1994. Herbal Drugs and Phytopharmaceutical. [Google Scholar]
- Borges-Argáez R., Chan-Balan R., Cetina-Montejo L., Ayora-Talavera G., Sansores-Peraza P., Gómez-Carballo J., Cáceres-Farfán M. In vitro evaluation of anthraquinones from Aloe vera (Aloe barbadensis Miller) roots and several derivatives against strains of influenza virus. Ind. Crops. Prod. 2019;132:468–475. doi: 10.1016/j.indcrop.2019.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto J., Coll J., Rivas M., Blanco M., Concepción O., Tandrón Y.A., Hernández M., Trujillo R. Anthraquinones from in vitro root culture of Morinda royoc L. Plant. Cell. Tissue. Organ. Cult. 2008;94(2):181–187. [Google Scholar]
- Burcea M., Gheorghe A., Pop M. Incidence of herpes simplex virus keratitis in HIV/AIDS patients compared with the general population. J. Med. Life. 2015;8(1):62–63. [PMC free article] [PubMed] [Google Scholar]
- Chang S.J., Huang S.H., Lin Y.J., Tsou Y.Y., Lin C.W. Antiviral activity of Rheum palmatum methanol extract and chrysophanol against Japanese encephalitis virus. Arch. Pharm. Res. 2014;37(9):1117–1123. doi: 10.1007/s12272-013-0325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Feng R., Muhammad I., Abbas G., Zhang Y., Ren Y., Huang X., Zhang R., Diao L., Wang X., Li G. Protective effects of hypericin against infectious bronchitis virus induced apoptosis and reactive oxygen species in chicken embryo kidney cells. Poult. Sci. 2019;98(12):6367–6377. doi: 10.3382/ps/pez465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S.C., Wu Y.C., Chen Z.W., Yang W.C. Naturally occurring anthraquinones: chemistry and therapeutic potential in autoimmune diabetes. Evid-Based Complement. Altern. Med. 2015 doi: 10.1155/2015/357357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.G., Lee H., Kim Y.S., Hwang Y.H., Oh Y.C., Lee B., Moon K.M., Cho W.K., Ma J.Y. Aloe vera and its components inhibit influenza A virus-induced autophagy and replication. Am. J. Chin. Med. 2019;47(06):1307–1324. doi: 10.1142/S0192415X19500678. [DOI] [PubMed] [Google Scholar]
- Christenhusz M.J.M., Byng J.W. The number of known plant species in the world and its annual increase. Phytotaxa. 2016;261(3):201–217. doi: 10.11646/phytotaxa.261.3.1. [DOI] [Google Scholar]
- Crowell R.L., Landau B.J. A short history and introductory background on the coxsackieviruses of group B. Curr. Top. Microbiol. Immunol. 1997;233:1–11. doi: 10.1007/978-3-642-60687-8_1. [DOI] [PubMed] [Google Scholar]
- Dao T.T., Nguyen P.H., Lee H.S., Kim E., Park J., Lim S.I., Oh W.K. Chalcones as novel influenza A (H1N1) neuraminidase inhibitors from Glycyrrhiza inflata. Bioorg. Med. Chem. Lett. 2011;21:294–298. doi: 10.1016/j.bmcl.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Diaz-Munoz G., Miranda I.L., Sartori S.K., de Rezende D.C., Diaz M.A. Anthraquinones: an overview. Stud. Nat. Prod. Chem. 2018;58:313–338. [Google Scholar]
- Dufossé L. Anthraquinones, the Dr Jekyll and Mr Hyde of the food pigment family. Food Res. Int. 2014;65:132–136. doi: 10.1016/j.foodres.2014.09.012. [DOI] [Google Scholar]
- Duval J., Pecher V, Poujol M, Lesellier E. Research advances for the extraction, analysis and uses of anthraquinones: a review. Ind. Crops. Prod. 2016;94:812–833. [Google Scholar]
- Dwyer-Lindgren L., Cork M.A., Sligar A., Steuben K.M., Wilson K.F., Provost N.R., Mayala B.K., VanderHeide J.D., Collison M.L., Hall J.B., Biehl M.H. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature. 2019;570(7760):189–193. doi: 10.1038/s41586-019-1200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDPC: European Centre for Disease Prevention and Control . European Centre for Disease Prevention and Control; Solna, Sweden: 2020. COVID-19 Situation Update Worldwide.https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases [Google Scholar]
- Esposito F., Carli I., Del Vecchio C., Xu L., Corona A., Grandi N., Piano D., Maccioni E., Distinto S., Parolin C., Tramontano E. Sennoside A, derived from the traditional Chinese medicine plant Rheum L., is a new dual HIV-1 inhibitor effective on HIV-1 replication. Phytomedicine. 2016;23(12):1383–1391. doi: 10.1016/j.phymed.2016.08.001. [DOI] [PubMed] [Google Scholar]
- GBD Causes of death collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2017;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S.L., Giersing B., Boily M.C., Chesson H., Looker K.J., Schiffer J., Spicknall I., Hutubessy R., Broutet N. WHO HSV vaccine impact modelling meeting working group. Modelling efforts needed to advance herpes simplex virus (HSV) vaccine development: key findings from the World Health Organization consultation on HSV vaccine impact modelling. Vaccine. 2019;37(50):7336–7345. doi: 10.1016/j.vaccine.2017.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall V.J., Foulkes S., Saei A., Andrews N., Oguti B., Charlett A., Wellington E., Stowe J., Gillson N., Atti A., Islam J. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henss L., Auste A., Schürmann C., Schmidt C., von Rhein C., Mühlebach M.D., Schnierle B.S. The green tea catechin epigallocatechin gallate inhibits SARS-CoV-2 infection. J. Gen. Virol. 2021;102(4) doi: 10.1099/jgv.0.001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74(2):92–101. doi: 10.1002/cbdv.201000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J.B., Lopez-Bazzocchi I., Towers G.H. Antiviral activities of hypericin. Antiviral Res. 1991;15:101–112. doi: 10.1016/0166-3542(91)90028-p. [DOI] [PubMed] [Google Scholar]
- Hutchinson E.C. Influenza virus. Trends Microbiol. 2018;26(9):809–810. doi: 10.1016/j.tim.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Kassa Z., Asfaw Z., Demissew S. An ethnobotanical study of medicinal plants in Sheka Zone of Southern nations nationalities and peoples regional state, Ethiopia. J. Ethnobiol. Ethnomed. 2020;16(1):7. doi: 10.1186/s13002-020-0358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeruto P., Mutai C., Catherine L., Ouma G. Phytochemical constituents of some medicinal plants used by the Nandis of South Nandi district, Kenya. J. Anim. Plant. Sci. 2011;9(3):1201–1210. [Google Scholar]
- Khanal P., Patil B.M., Chand J., Naaz Y. Anthraquinone derivatives as an immune booster and their therapeutic option against COVID-19. Nat. Prod. Bioprospect. 2020;10(5):325–335. doi: 10.1007/s13659-020-00260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani S., Minaei S., Ghasemi-Varnamkhasti M. Application of electronic nose systems for assessing quality of medicinal and aromatic plant products: a review. J. Appl. Res. Med. Arom. Plants. 2016;3(1):1–9. [Google Scholar]
- Kim J.H., Yoon J.Y., Yang S.Y., Choi S.K., Kwon S.J., Cho I.S., Jeong M.H., Ho Kim Y., Choi G.S. Tyrosinase inhibitory components from Aloe vera and their antiviral activity. J. Enzyme Inhib. Med. Chem. 2017;32(1):78–83. doi: 10.1080/14756366.2016.1235568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigheim B.S., Beranek M., Comini L.R., Aguilar J.J., Marioni J., Cabrera J.L., Contigiani M.S., Montoya S.C. In vitro antiviral activity of Heterophyllaea pustulata extracts. Nat. Prod. Commun. 2012;7(8):1025–1028. [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Li H.L., Chen H.L, LI H., Zhang K.L., Chen X.Y., Wang X.W., Kong Q.Y., Liu J. Regulatory effects of emodin on NF-κB activation and inflammatory cytokine expression in RAW 264.7 macrophages. Int. J. Mol. Med. 2005;16(1):41–47. [PubMed] [Google Scholar]
- Li Z., Li L.J., Sun Y., Li J. Identification of natural compounds with anti-hepatitis B virus activity from Rheum palmatum L. ethanol extract. Chemotherapy. 2007;53(5):320–326. doi: 10.1159/000107690. [DOI] [PubMed] [Google Scholar]
- Lin G.L., Drysdale S.B., Snape M.D., O'Connor D., Brown A., MacIntyre-Cockett G., Mellado-Gomez E., De Cesare M., Bonsall D., Ansari M.A., Öner D. Distinct patterns of within-host virus populations between two subgroups of human respiratory syncytial virus. Nat. Commun. 2021;12(1):1–11. doi: 10.1038/s41467-021-25265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.W., Tsai F.J., Tsai C.H., Lai C.C., Wan L., Ho T.Y., Hsieh C.C., Chao P.D. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant derived phenolic compounds. Antiviral. Res. 2005;68:36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.Y., Sporer F., Wink M., Jourdane J., Henning R., Li Y.L., Ruppel A. Anthraquinones in Rheum palmatum and Rumex dentatus (Polygonaceae), and phorbol esters in Jatropha curcas (Euphorbiaceae) with molluscicidal activity against the schistosome vector snails Oncomelania, Biomphalaria, and Bulinus. Trop. Med. Int. Health. 1997;2(2):179–188. doi: 10.1046/j.1365-3156.1997.d01-242.x. [DOI] [PubMed] [Google Scholar]
- Liu Z., Ma N., Zhong Y., Yang Z.Q. Antiviral effect of emodin from Rheum palmatum against coxsackievirus B5 and human respiratory syncytial virus in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015;35(6):916–922. doi: 10.1007/s11596-015-1528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wei F., Chen L.J., Xiong H.R., Liu Y.Y., Luo F., Hou W., Xiao H., Yang Z.Q. In vitro and in vivo studies of the inhibitory effects of emodin isolated from Polygonum cuspidatum on Coxsackievirus B4. Molecules. 2013;18(10):11842–11858. doi: 10.3390/molecules181011842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik E.M., Müller C.E. Anthraquinones as pharmacological tools and drugs. Med. Res. Rev. 2016;36(4):705–748. doi: 10.1002/med.21391. [DOI] [PubMed] [Google Scholar]
- Mbanga J., Mangoma N., Saidi B. An evaluation of the antimicrobial activities of Aloe barbadensis, A. chabaudii and A. arborescens leaf extracts used in folklore veterinary medicine in Zimbabwe. J. Anim. Vet. Adv. 2010;9:2918–2923. [Google Scholar]
- Mishra A.P., Sharifi-Rad M., Shariati M.A., Mabkhot Y.N, Al-Showiman S.S., Rauf A., Salehi B., Župunski M., Sharifi-Rad M., Gusain P. Bioactive compounds and health benefits of edible Rumex species-A review. Cell. Mol. Biol. 2018;64(8):27–34. [PubMed] [Google Scholar]
- Moona A.A., Daria S., Asaduzzaman M., Islam M.R. Bangladesh reported delta variant of coronavirus among its citizen: actionable items to tackle the potential massive third wave. Infect. Prevent. Pract. 2021;3(3) doi: 10.1016/j.infpip.2021.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpiana P.T., Tshibangu D.S., Kilembe J.T., Gbolo B.Z., Mwanangombo D.T., Inkoto C.L., Lengbiye E.M., Mbadiko C.M., Matondo A., Bongo G.N., Tshilanda D.D. Aloe vera (L.) Burm. F. as a potential anti-COVID-19 plant: a mini-review of its antiviral activity. Eur. J. Med. Plants. 2020:86–93. [Google Scholar]
- Muckelbauer J.K., Kremer M., Minor I., Diana G., Dutko F.J., Groarke J., Pevear D.C., Rossmann M.G. The structure of coxsackievirus B3 at 3.5 Å resolution. Structure. 1995;3(7):653–667. doi: 10.1016/s0969-2126(01)00201-5. [DOI] [PubMed] [Google Scholar]
- Naithani R., Huma L.C., Holland L.E., Shukla D., McCormick D.L., Mehta R.G., Moriarty R.M. Antiviral activity of phytochemicals: a comprehensive review. Mini Rev. Med. Chem. 2008;8(11):1106–1133. doi: 10.2174/138955708785909943. [DOI] [PubMed] [Google Scholar]
- Nazir S., Sharma M., Saxena M., Abrar M., Ajaz M. Rheum emodi: phytochemistry, bioactive compounds and their biological activity. Int. J. Phytopharmacol. 2013;4(4):272–276. [Google Scholar]
- Obisesan O., Katata-Seru L., Mufamadi S., Mufhandu H. Applications of nanoparticles for herpes simplex virus (HSV) and human immunodeficiency virus (HIV) treatment. J. Biomed. Nanotechnol. 2021;17(5):793–808. doi: 10.1166/jbn.2021.3074. [DOI] [PubMed] [Google Scholar]
- Ott J.J., Stevens G.A., Groeger J., Wiersma S.T. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- Palese P., Shah M.L. Fields Virology. 5th ed. Lippincott, Williams &Wilkins; Philadelphia: 2007. Orthomyxoviridae: the viruses and their replication; pp. 1647–1690. In. ed. DM Knipe, PM Howley. [Google Scholar]
- Pandith S.A., Hussain A., Bhat W.W., Dhar N., Qazi A.K., Rana S., Razdan S., Wani T.A., Shah M.A., Bedi Y.S, Hamid A. Evaluation of anthraquinones from Himalayan rhubarb (Rheum emodi Wall. ex Meissn.) as antiproliferative agents. S. Afr. J. Bot. 2014;95:1–8. [Google Scholar]
- Parvez M.K., Al-Dosari M.S., Alam P., Rehman M., Alajmi M.F., Alqahtani A.S. The anti-hepatitis B virus therapeutic potential of anthraquinones derived from Aloe vera. Phytother. Res. 2019;33(11):2960–2970. doi: 10.1002/ptr.6471. [DOI] [PubMed] [Google Scholar]
- Patil R.H., Patil M.P., Maheshwari V.L. Bioactive secondary metabolites from endophytic fungi: a review of biotechnological production and their potential applications. Stud. Nat. Prod. Chem. 2016;1(49):189–205. [Google Scholar]
- Peiris J.S.M., Lai S.T., Poon L.L.M., Guan Y., Yam L.Y.C., Lim W., Nicholls J., Yee W.K.S., Yan W.W., Cheung M.T., Cheng V.C.C. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera C., Efferth T. Antiviral medicinal herbs and phytochemicals. J. Pharmacogn. 2012;3:45–48. [Google Scholar]
- Racaniello V.R. One hundred years of poliovirus pathogenesis. Virology. 2006;344(1):9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Rai M., Rathod D., Agarkar G., Dar M., Brestic M., Pastore G.M., Junior M.R.M. Fungal growth promotor endophytes: a pragmatic approach towards sustainable food and agriculture. Symbiosis. 2014;62(2):63–79. [Google Scholar]
- Rolta R., Yadav R., Salaria D., Trivedi S., Imran M., Sourirajan A., Baumler D.J., Dev K. In silico screening of hundred phytocompounds of ten medicinal plants as potential inhibitors of nucleocapsid phosphoprotein of COVID-19: an approach to prevent virus assembly. J. Biomol. Struct. Dyn. 2021;39(18):7017–7034. doi: 10.1080/07391102.2020.1804457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbrei A. Optimal management of genital herpes: current perspectives. Infect. Drug Resist. 2016;9:129–141. doi: 10.2147/IDR.S96164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple S.J., Pyke S.M., Reynolds G.D., Flower R.L. In vitro antiviral activity of the anthraquinone chrysophanic acid against poliovirus. Antiviral. Res. 2001;49(3):169–178. doi: 10.1016/s0166-3542(01)00125-5. [DOI] [PubMed] [Google Scholar]
- Shen C., Zhang Z., Xie T., Ji J., Xu J., Lin L., Yan J., Kang A., Dai Q., Dong Y., Shan J. Rhein suppresses lung inflammatory injury induced by human respiratory syncytial virus through inhibiting NLRP3 inflammasome activation via NF-κB pathway in mice. Front. Pharmacol. 2020;10:1600. doi: 10.3389/fphar.2019.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M.X., Ma N., Li M.K., Liu Y.Y., Chen T., Wei F., Liu D.Y., Hou W., Xiong H.R., Yang Z.Q. Antiviral properties of R. tanguticum nanoparticles on herpes simplex virus type I in vitro and in vivo. Front. Pharmacol. 2019;10:959. doi: 10.3389/fphar.2019.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T., McAllister D.A., O'Brien K.L., Simoes E.A., Madhi S.A., Gessner B.D., Polack F.P., Balsells E., Acacio S., Aguayo C., Alassani I. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla D., Spear P.G. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Invest. 2001;108:503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Hussain Y., Luqman S., Meena A. Purpurin: a natural anthraquinone with multifaceted pharmacological activities. Phytother. Res. 2021;35(5):2418–2428. doi: 10.1002/ptr.6965. [DOI] [PubMed] [Google Scholar]
- Spencer, A.J., Morris, S., Ulaszewska, M., Powers, C., Kaliath, R., Bissett, C.D., Truby, A., Thakur, N., Newman, J., Allen, E.R., Lui, C., 2021. The ChAdOx1 vectored vaccine, AZD2816, induces strong immunogenicity against SARS-CoV-2 B. 1.351 and other variants of concern in preclinical studies. 77, 103902. BioRxiv. 10.1101/2021.06.08.447308. [DOI] [PMC free article] [PubMed]
- Simpson M.G. Diversity and classification of flowering plants: eudicots. J. Plant Syst. 2019:285–466. [Google Scholar]
- Swallow D.L. Butterworth Group; London: 1977. Progress in Medicinal Chemistry; p. 120. In: G. P. Ellis and G. B. West (eds) [Google Scholar]
- Syed T.A., Cheema K.M., Ahmad S.A., Unit A.H., Jr. Aloe vera extract 0.5% in hydrophilic cream versus Aloe vera gel for the management of genital herpes in males. A placebo-controlled, double-blind, comparative study. J. Eur. Acad. Dermatol. Venereol. 1996;7:294–295. [Google Scholar]
- Tang J., Colacino J.M., Larsen S.H., Spitzer W. Virucidal activity of hypericin against enveloped and non-enveloped DNA and RNA viruses. Antiviral. Res. 1990;13:313–326. doi: 10.1016/0166-3542(90)90015-y. [DOI] [PubMed] [Google Scholar]
- Taubenberger J.K., Morens D.M. The pathology of influenza virus infections. Ann. Rev. Pathol. Mech. Dis. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegen D., Dessie K., Damtie D. Candidate anti-COVID-19 medicinal plants from Ethiopia: a review of plants traditionally used to treat viral diseases. Evid.-Based Complement. Altern. Med. 2021 doi: 10.1155/2021/6622410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson R.H. Chapman and Hall; London: 1986. Naturally occurring quinones III. Recent advances. [Google Scholar]
- Tillu G., Chaturvedi S., Chopra A., Patwardhan B. Public health approach of ayurveda and yoga for COVID-19 prophylaxis. J. Altern. Complement. Med. 2020;26(5):360–364. doi: 10.1089/acm.2020.0129. [DOI] [PubMed] [Google Scholar]
- Torjesen I. Covid-19: omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ. 2021;375:n2943. doi: 10.1136/bmj.n2943. [DOI] [PubMed] [Google Scholar]
- Trallero G., Avellon A., Otero A., De Miguel T., Pérez C., Rabella N., Rubio G., Echevarria J.E., Cabrerizo M. Enteroviruses in Spain over the decade 1998–2007: virological and epidemiological studies. J. Clin. Virol. 2010;47(2):170–176. doi: 10.1016/j.jcv.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Unni S.K., Růžek D., Chhatbar C., Mishra R., Johri M.K., Singh S.K. Japanese encephalitis virus: from genome to infectome. Microbes. Infect. 2011;13(4):312–321. doi: 10.1016/j.micinf.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Varga S.M., Braciale T.J. The adaptive immune response to respiratory syncytial virus. Curr. Top. Microbiol. Immunol. 2013;372:155–171. doi: 10.1007/978-3-642-38919-1_8. [DOI] [PubMed] [Google Scholar]
- Vasileiou E., Simpson C.R., Shi T., Kerr S., Agrawal U., Akbari A., Bedston S., Beggs J., Bradley D., Chuter A., De Lusignan S. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Wang Z., Wang G., Lau J.Y.N., Zhang K., Li W. COVID-19 in early 2021: current status and looking forward. Signal. Transduct. Target. Ther. 2021;6(1):1–14. doi: 10.1038/s41392-021-00527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Qin X., Chen Z., Ju Z., He W., Tan Y., Zhou X., Tu Z., Lu F., Liu Y. Two new anthraquinones with antiviral activities from the barks of Morinda citrifolia (Noni) Phytochem. Lett. 2016;15:13–15. [Google Scholar]
- Wang Q.W., Su Y., Sheng J.T., Gu L.M., Zhao Y., Chen X.X., Chen C., Li W.Z., Li K.S., Dai J.P. Anti-influenza A virus activity of rhein through regulating oxidative stress, TLR4, Akt, MAPK, and NF-κB signal pathways. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley R.J., Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- WHO, 1998. Hepatitis B. Fact Sheet WHO/204.
- WHO . World Health Organization; Roma, Italy: 2021. World Health Organization Coronavirus (COVID-19) Dashboard. [Google Scholar]
- WHO . WHO; Geneva, Switzerland: 2002. WHO Monographs on Selected Medicinal Plants, Volume 2. [Google Scholar]
- Wright P.F., Neumann G., Kawaoka Y. Fields Virology. 5th ed. Lippincott Williams & Wilkins; Phladelphia: 2007. Orthomyxoviruses; pp. 1691–1740. ed. DM Knipe, PM Howley. [Google Scholar]
- Xiong H.R., Luo J., Hou W., Xiao H., Yang Z.Q. The effect of emodin, an anthraquinone derivative extracted from the roots of Rheum tanguticum, against herpes simplex virus in vitro and in vivo. J. Ethnopharmacol. 2011;133(2):718–723. doi: 10.1016/j.jep.2010.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf M.A., Singh B.N., Sudheer S., Kharwar R.N., Siddiqui S., Abdel-Azeem A.M., Fernandes Fraceto L., Dashora K., Gupta V.K. Chrysophanol: a natural anthraquinone with multifaceted biotherapeutic potential. Biomolecules. 2019;9(2):68. doi: 10.3390/biom9020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi K., Zadeh M.A., Sartavi K., Rastian Z. Antiviral activity of Aloe vera against herpes simplex virus type 2: an in vitro study. Afr. J. Biotechnol. 2007;6(15):1770–1773. [Google Scholar]
- Zannella C., Rinaldi L., Boccia G., Chianese A., Sasso F.C., De Caro F., Franci G., Galdiero M. Regulation of m6A methylation as a new therapeutic option against COVID-19. Pharmaceuticals. 2021;14(11):1135. doi: 10.3390/ph14111135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Zhao S., Han J.T., Wang Y.F., Wang Y.N., Wang C.H. Antiviral anthraquinones from the roots of Knoxia valerianoides. Phytochem. Lett. 2015;11:57–60. [Google Scholar]
- Zheng Y., Li M., Wang H., Liang G. Japanese encephalitis and Japanese encephalitis virus in mainland China. Rev. Med. Virol. 2012;22(5):301–322. doi: 10.1002/rmv.1710. [DOI] [PubMed] [Google Scholar]
- Zhong Q., Yang Z., Liu Y., Deng H., Xiao H., Shi L., He J. Antiviral activity of arbidol against coxsackie virus B5 in vitro and in vivo. Arch. Virol. 2009;154(4):601–607. doi: 10.1007/s00705-009-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.