Abstract
Background:
Anti CD19 Chimeric Antigen Receptor (CAR) T-cell therapy has transformed the care of relapsed & refractory aggressive B cell Lymphoma. However, financial toxicity and manufacturing time represent barriers to its widespread implementation.
Objective:
Study applicability, toxicity, and efficacy of a locally produced autologous CD19-directed CAR-T cell product.
Methods:
We performed a phase 1b/2 clinical trial with a point-of-care (POC) CAR T-cell product that contains a CD28 costimulatory domain. Adult patients with aggressive B cell lymphoma or transformed low-grade lymphoma who received at least two prior regimens were eligible.
Results:
A total of 73 patients, with a median age of 49 years, met inclusion criteria. CAR-T production time from apheresis was 10 days (IQR 10-11), negating the need for bridging chemotherapy. Overall and complete response rates were 62.5% and 37.5%. Median progression-free and overall survival were 3.7 and 12.1 months, respectively. Overall and progression-free survival at 12 months were 52.1% (CI: 40.8%-66.5%) and 40% (CI: 30%-53.7%), respectively. Patients who achieved response had longer progression-free and overall survival. Grade 3-4 CRS was observed in 9.5% of the patients, and ICANS grade 3-4 in 21.9%. No deaths occurred due to CAR T-cell toxicity. Fifteen patients (20%) underwent allogeneic stem cell transplantation at a median time of 60 days post CAR T-cell therapy; 8 were alive at last follow-up. Of the six patients that underwent the transplant in complete response 2 deceased due to toxicity.
Conclusions:
POC CAR-T cells are a feasible therapeutic option in aggressive B-cell lymphoma. They provide good efficacy while minimizing production time and the need for bridging therapy.
Keywords: Aggressive B Cell Lymphoma, CAR T-cell, Allogeneic Stem Cell Transplantation, Point of Care
Background
Relapse of aggressive B cell lymphoma (ABCL) poses a significant clinical challenge. Current standard second-line therapy in intent to cure is based on platinum-containing regimens and autologous stem cell transplantation (ASCT) 1. This will achieve roughly 20-30% long-term survival 2. Many of the patients are elderly and therefore are not ASCT candidates. As a result, until recently, these patients were considered incurable.
Transduced T cells expressing chimeric antigen receptor targeting CD19 (CD19 CAR T-cells) became the standard of care for multiply relapsed ABCL. They constitute a form of adoptive immunotherapy which was found to be effective in B acute lymphoblastic leukemia (B-ALL), B cell Non Hodgkin Lymphoma (NHL), and to a somewhat lesser degree in chronic lymphocytic leukemia (CLL) 3. The long-term survival post CAR T-cell therapy in ABCL is reported to be 40%, 4,5 Lympho-depletion, mostly with cyclophosphamide and fludarabine prior to CAR T-cell infusion, is necessary to enhance cellular efficacy prior to CAR T-cell infusion 6. CAR T-cell therapy most common side effects are cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and hematological toxicity. However, additional long-term side effects have been described 7.
Real-world experience with CAR T-cell therapy for ABCL with the FDA-approved products demonstrates similar efficacy and toxicity profile as reported in the clinical trials 8,9.
The COVID-19 pandemic brought significant difficulties with CAR T-cell therapy, from patient selection to timing, manufacturing, and delivery issues10,11. Point-of-care, academic, CAR T-cell products have been shown before to be efficacious 12. The possibility to provide local CAR T-cell product became an advantage during the pandemic.
Since November 2017, we started treating adult patients with relapsed or refractory ABCL with our locally produced anti CD19 CAR T-cell product that contains CD28 costimulatory domain (NCT02772198). Peripheral blood mononuclear cells (PBMC) are isolated, activated and transduced with a gammaretrovirus encoding for a CD19 CAR 13-15. The median turnover time is 10 days (IQR 10-11).
The question of allogeneic stem cell transplantation (allo-SCT) as consolidation for CAR T –cell therapy remains unanswered 16. It is clear though, that most patients will relapse after CAR T-cell therapy and there is an unmet need for this patient population. When we first started our CAR T-cell trial we consolidated responding patients with allo-SCT. Later on we switched to transplanting only patients in partial response or relapse post CAR T-cell therapy. Here we describe the results of our experience with our in house CAR T-cell product in 73 ABCL patients. We also describe the outcomes of the patient population that underwent allo-SCT after CAR T-cell therapy.
Methods
Study design
This is a phase 1b/2 trial (NCT02772198), which was approved by the Sheba Medical Center institutional review board, and the Israeli Ministry of Health. All authors participated in the data analysis and have full access to the clinical data. Individual participant data will not be shared. Inclusion criteria were age above 18 years, failure of at least two prior therapeutic protocols, a CD3 count greater than 250/μL, no immunosuppressive treatment as well as preserved heart, lung kidney and liver function. Patients with uncontrolled rapidly progressing disease or active CNS involvement were excluded, as well as patients with active hepatitis B or C, HIV, and pregnant women13,14. Until the end of 2018, we limited our study to patients aged 55 or lower, since then, advanced age was no longer an exclusion criterion. Bridging chemotherapy was allowed.
CAR T - cell production and Administration
The retroviral supernatant was generated from the CD19 CAR producer line PG13-CD19-CAR-H3, which was provided by the NCI. A plasmid encoding the CD19 CAR containing of the mouse stem-cell virus gamma-retroviral backbone engineered to a scFv derived from the mouse anti-CD19 hybridoma, FMC63, fused to intracellular domains from human CD28 and CD3-ζ, was used for viral vector production. Fresh leukapheresis product was used for CD19 CAR-T cell production. Peripheral blood monuclear cells (PBMC) were isolated from the apheresis product by density gradient with Ficoll-Hypaque. 400×10e6 PBMC were re-suspended at the concentration of 1×10e6 cells per ml. After 2 days, 60×10e6 cells were transduced with the CD19 CAR retroviral vector and the rest were discarded. The CD19 CAR T-cells quality that included cell identity, transduction efficacy, cell count, viability, potency, impurity and replication competent retrovirus (RCR) PCR, was controlled throughout the manufacturing process. Sterility was tested on day 8 (+1). Quality control passed, if no growth was seen following membrane filtration. The test was validated for anaerobic, aerobic and fungal growth. A preliminary result was available on the day of infusion. Mycoplasma test was validated by nested PCR on day 9 (+1) and the result was available before infusion. On the day of infusion, cells were washed, counted and 1×10e6 CD19 CAR expressing cells/kg were re-suspended in 100 mL 0.9% sodium chloride (Baxter,) containing 2.5% human albumin and 300 IU/mL IL-2. The fresh cell product was delivered to the patient for immediate infusion 14. Lymphodepletion included fludarabine 25 mg/m2 × 3 days (days −4 to −2) and cyclophosphamide 900 mg/m2 × 1 day (day −2), followed by infusion (day 0) of 1 × 106 CAR+ transduced cells per kilogram recipient 13,14. This study was initiated at 1x10^6 CAR+ cell/kg based on initial results from previous studies with FMC63-28-zeta CARs (NCI: NCT00924326 and NCT03827343). No dose escalation was planned. Data on CAR T-cell persistence was not prospectively collected.
Response assessment and Definitions
The study's primary endpoints were response on day 28, best response, and safety. Response assessment was done with a PET-CT scan and interpreted according to the Lugano criteria 17. Overall response rate (ORR) was defined as the proportion of subjects with either a CR or PR. Day 28 response was defined as response assessment in the first 28 days (+/− 7 days; whichever is closest to day 28) after CAR T-cell infusion. Best response was defined as best achieved response post-CAR-T infusion. Response was calculated relative to the most recent disease assessment before infusion of CAR T-cells. Time to best response was defined as the time from the date of CAR-T infusion to the date documented the best response. CRS and ICANS were graded as per American Society for Transplantation and Cellular Therapy (ASTCT) guidelines18. Secondary endpoints were overall survival (OS), progression-free survival (PFS), and production feasibility. OS was defined as the time from CART-cell infusion to death of any cause. PFS was defined as the time CAR T -cell infusion to the date of either first documented relapse, progression, or death due to any cause. Patients were censored if they were event-free or received anti-cancer treatment post CAR T.
Statistical analysis
Continuous variables were summarized by number, mean, standard deviation, minimum, median, maximum and sum. Categorical variables were summarized by frequencies, percentages, and two-sided 95% CIs. For time-to-event variables, the Kaplan–Meier method was used for descriptive summaries and Log-Rank test for comparison of survivals. Cox regression was used for multivariate survival analysis. Correlations between categorical and continuous variables and outcomes were done using logistic and linear regression, respectively. The data were analyzed using the R version 3.5.0. Day 0 was defined as the day of CAR T-cell administration.
Results
Patients
From November 2017 until December 2020, we enrolled 73 adult ABCL patients who received CAR T-cells. Patients’ characteristics are depicted in Table 1. Median age was 49 (range: 20-73), 25 of the patients (34%) were older than 55 and 45 (61.6%) were male. The Karnofsky performance status (KPS) was 90-100% in 74%, 70%-80% in 15.1%, and lower in the others. Most patients (72.6%) had stage III/IV disease at apheresis. Twenty-five (34.2%) patients had previous ASCT and 4 (5.5%) had alloSCT. Forty-six patients (63%) had three or more prior lines of therapy. Twelve (16.4%) had bulky disease at aphaeresis, and 6 (8.2%) had a history of CNS involvement. Sixty-three (86.3%) had stable or progressive disease at screening, and 37 (51.4%) were primary refractory.
Table 1.
Patients’ and Disease characteristics
| Patients | |
|---|---|
| Total | 73(%) |
| Age | Median 49 (20-73) |
| Gender: | |
| Male | 45 (61.6) |
| Female | 28 (38.4) |
| KPS: | |
| 90-100% | 54 (74) |
| 70-80% | 11 (15.1) |
| 50-60% | 4 (5.5) |
| 30-40% | 3 (4.1) |
| missing | 1 (1.4) |
| Histological subtype: | |
| DLBCL NOS, de novo | 28(38) |
| Transformed DLBCL (including Richter transformation) | 22(30) |
| PMBCL | 12(16) |
| High grade B cell lymphoma (DHL/NOS) | 7 5/2(9) |
| MCL | 4(5) |
| History of CNS involvement: | |
| Yes | 6(8.2) |
| No | 67 (91.8) |
| Previous ASCT: | |
| Yes | 25 (34.2) |
| No | 48 (65.8) |
| Previous allo-SCT: | |
| Yes | 4 (5.5) |
| No | 69(94.5) |
| Bulky disease at time of apheresis: | |
| Yes | 12 (16.4) |
| No | 61 (83.6%) |
| Number of previous treatment lines: | |
| 2 | 27 (37%) |
| 3 or more | 46 (63%) |
| Stage at apheresis: | |
| I/II | 19 (26%) |
| III/IV | 53 (72.6%) |
| No evidence of disease | 1(1.4%) |
| Disease status at apheresis: | |
| CR | 1 (1.4%) |
| PR | 9 (12%) |
| SD/PD | 63 (86%) |
| Primary refractory: | |
| Yes | 37(51.4%) |
| No | 35(48.6%) |
Abbreviations:
KPS – Karnofsky Performance Status
DLBCL – Diffuse large B Cell Lymphomas
PMBCL – Primary Mediastinal B Cell Lymphoma
MCL- Mantle Cell Lymphoma
DHL – Double Hit Lymphoma
CNS – Central Nervous System
ASCT – Autologous Stem Cell Transplantation
alloSCT – Allogeneic Stem Cell Transplantation
Disease
The cohort included 28 (38%) patients with diffuse large B cell lymphoma (DLBCL), 22 (30%) with transformed low-grade lymphoma, of them 8 patients with richter's transformation (RT), 12 (16%) with primary mediastinal B cell lymphoma (PMBCL), 7 (9%) with high-grade B cell lymphoma (five of them with double-hit lymphoma) and 4 patients (5%) with mantle cell lymphoma (MCL) (Table 1). Cell of origin, which was determined by the Hans criteria, was germinal center in 25 (34.2%) and non-germinal center in 24 (32.9%); 19 status was unknown in 24 cases.
CAR T-cells
CAR T-cell production was done as described 13,14. Patients received the target CAR T-cell dose of 1 x 106/kg except for one patient who received 0.6 X 106/kg CAR T-cells due to insufficient production. Sixty-six (92%) patients did not receive any bridging therapy while 6 (8%) received. The protocols used were: gemcitabine, dexamthadose cisplatinum (GDP) (N = 1), bendamustine and polatuzumab (N = 2), reduced dose Cytoxan, adriamycin, oncovine prednisone (miniCHOP) (N = 1), etoposide, solumedrol, high dose ara-c, and cisplatiunum ESHAP (N =1), mesna, ifosphamide, mitoxantrone, and etoposide (MINE) (N =1). Median time from apheresis to infusion was 10 days (IQR: 10-11).
Toxicity
Toxicity profile and treatment are presented in Table 2. CRS was observed in 62 patients (85%). Forty-seven (64.4%) had grade I CRS, 8 (11%) had grade II, 5(6.8%) had grade III and 2 (2.7%) had grade IV. Median time from CAR T-cell infusion to CRS was 4 days (IQR: 2-5 days) and median duration of CRS was 5 days (IQR: 4-9 days). Sixty-four (87.7%) patients did not require any treatment for the CRS, 5 (6.8%) received tocilizumab and 4 (5.5%) were treated with tocilizumab and corticosteroids. ICANS was observed in 29 patients (39.7%) after a median of 7 days from CAR T-cell infusion (IQR: 6-9 days). ICANS grade was 1 in 9 (12.3%), 2 in 4 (5.5%), 3 in 12 (16.4%) and 4 in 4 (5.5%). Median duration of ICANS was 4 days (IQR: 2-7 days) and 16 (21.9%) patients required treatment with steroids.
Table 2:
Toxicity & Treatment
| Toxicity | N=73 (%) |
|---|---|
| CRS | 62 (85) |
| *Maximal grade | |
| 1 | 47 (64) |
| 2 | 8 (11) |
| 3 | 5 (7) |
| 4 | 2 (3) |
| Time from infusion (days), median (range) | 4 (2-5) |
| Duration (days), median (range) | 5 (4-9) |
| Treatment | 12 (13) |
| Tocilizumab | 5 (7) |
| Tocilizumab + steroids | 4 (6) |
| ICANS | 29 (40) |
| Maximal grade | |
| 1 | 9 (12) |
| 2 | 4 (6) |
| 3 | 12 (16) |
| 4 | 4 (6) |
| Time from infusion (days), median (range) | 7 (6-9) |
| Duration (days), median (range) | 4 (2-7) |
| Treatment | 29 (40) |
| Steroids | 6 (8) |
| Anti-epileptics | 7 (10) |
| Steroids + anti-epileptics | 16 (22) |
Abbreviations:
CRS – Cytokine Release Syndrome
ICANS - Immune Effector Cell-associated Neurotoxicity Syndrome
Severe neutropenia (<0.5k/microliter) was observed in 62 patients (85%). First wave was captured in 53 (73%) patients at a median of 7 days post-CART-cell infusion (range: −3-196). The median duration of the first wave was 9 days (IQR 6-14, range: 1-39). Second wave of neutropenia was observed in 9 (12%) patients, with a median time of 38 days, (IQR 31-76, range: 20-116). Its median duration was 5 days (IQR 3-8, range: 1-34).
Notable adverse events that were captured during the first 28 days after infusion were as follows: one spleen rupture porbably due to disease progression in a patient with PMBCL, one perforation of duodenal ulcer and three cases of death due to disease progression. There were no cases of death that were attributed to CAR T-cell treatment.
Outcome
Response
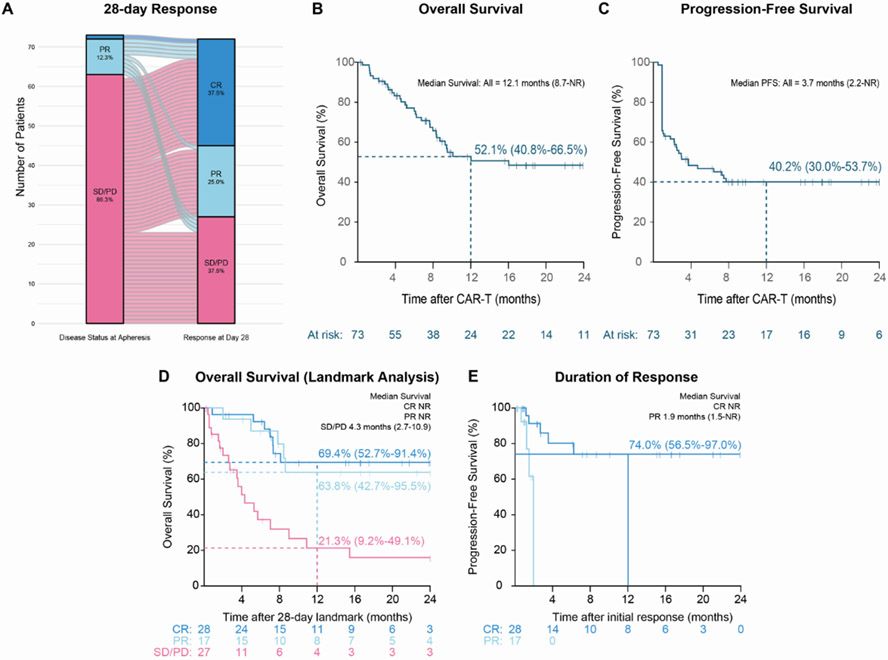
Response data were evaluable for 72 patients, one patient deceased prior to his first response assessment. Overall response at day 28 was observed in 45 patients (62.5%). Complete response (CR) was 37.5% (n=27) and partial response (PR) was 25% (n=18). Stable or progressive disease (SD/PD) was reported in 27 (37.5%) patients (Table 3). The patient who received CAR T-cells in a dose lower than the target of 1 X 106/kg achieved CR 28 days post-infusion without evidence of PD at data cut-off. Best response was CR in 28 patients (38.9%), PR in 17 (23.6%) and SD/PD in 27 (37.5%). Figure 1A presents the disease status pre-CAR T-cell therapy and at day 28. In univariate and multivariate regression analysis, we did not find any correlation between age, performance status, stage, bulky disease, LDH, number of previous treatment lines, refractoriness, and disease status in apheresis to outcome or risk of toxicity. The response at day 28, as well as the best response , in patients with RT was CR in 4/8 and PD in 4/8. Among the four patients in CR, 2/4 were consolidated with alloSCT and the other two did not receive further therapy. Neither of these 4 patients progressed following CAR T-cell therapy.
Table 3:
Response
| *Overall response 28d post CAR T-cell infusion |
N = 72 (%) |
|---|---|
| ORR (CR + PR) | 45 (62.5) |
| CR | 27 (37.5) |
| PR | 18 (25) |
| SD/PD | 27 (37.5) |
Abbreviations:
ORR – Overall Response Rate
CR- Complete Response
PR – Partial Response
SD – Stable Disease
PD – Progressive Disease
Figure 1: Response and Survival outcomes of the study population.
A. Alluvial plot describing disease status at apheresis and at day 28. Most of the patients achieved PR or CR.
B. Overall survival of the study population - one-year OS of 52.1%.
C. Progression-free survival of the study population - one year PFS of 40%.
D. Landmark analysis of the patients that reached their first imaging evaluation pot CAR T-cell infusion. OS is significantly better in the patients that achieved CR or PR vs. SD/PD.
E. Duration of response significantly better in the patients that achieved CR vs. PR.
Survival
Median follow-up is 18 months (11.7-23). Median PFS and OS was 3.7 (2.2-NA) and 12.1 (8.7-NA) months, respectively (figure 1B, C). Land mark analysis included patients that achieved their first response assessment demonstrated median OS of 15.5 months with superior OS to the patients that achieved CR/PR compared to those with SD/PD (1-year OS 67.2% vs. 21.3%, figure 1D). Twenty-eight patients (32%) died due to disease progression and 6 (8%) due to stem cell transplantation complications. In uni and multivariate analysis the factors that were found to predict OS were response (CR/PR HR=0.2 (0.096-0.448), p=6.40E-05), and bulky disease (>10cm) (HR=2.9 (1.301-6.584), p=0.009). Duration of response (DOR) was 65.1% at one year, and was significantly better for patients that achieved CR (figure 1E). Five of the the 8 patients with RT were alive at last follow-up. Among these patients three of them were in CR at day 28. One of the four patients who achieved CR in day 28 deceased later due to alloSCT complications.
Consolidation with alloSCT post CAR T-cell treatment
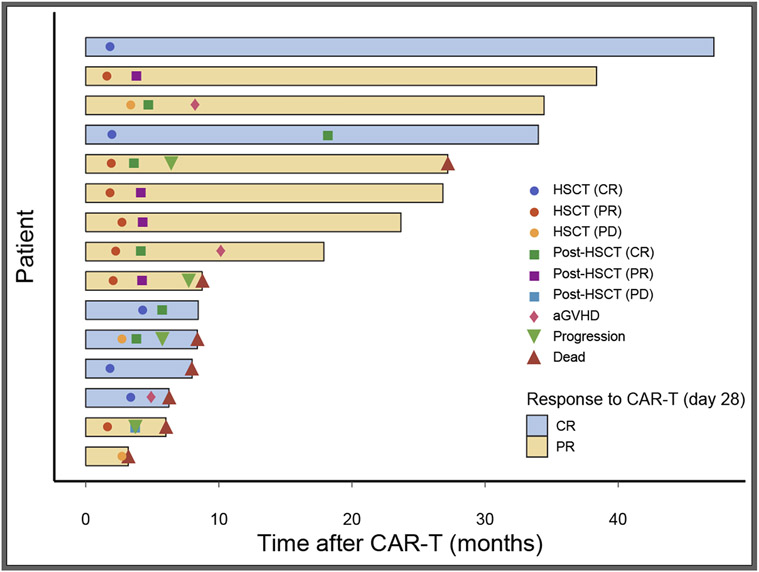
AlloSCT as consolidation post CAR T-cell therapy was performed in 15/73 (20%) of the patients. In the initial study period (first 1.5 years) alloSCT was done per protocol for 12 patients that achieved response to CAR T-cell therapy and later on to relapsed or non-responding patients. Transplant characteristics and complications are presented in table 4. Six (40%) were in CR and 9 (60%) in PR after the CART-cell therapy. The median time to alloSCT was 60 days (range: 48-130) after cell infusion. PD was identified by repeated PET/CT done prior to alloSCT in three (20%) of the patients who responded initially to CAR T-cell therapy. Six patients (40%) had matched sibling donors, 7 (47%) had matched unrelated donors and 2 (13%) had haplo-identical donors. Five patients (53%) had acute graft versus host disease (GVHD) and one chronic GVHD. As evident in the swimmer plot (Figure 2), a total of 8 patients are still alive. Among the 6 patients with complete response following CAR T-cell therapy, 3 deceased, one from PD and 2 due to transplant-related toxicity. Of the 9 patients with PR post CAR T-cell therapy, 4 converted to CR following transplant and 4 died, all from PD, including 2 patients who converted to CR following transplant. The sequence of events for the patients that underwent alloSCT in presented in figure 2.
Table 4:
allo-SCT characteristics
| All | N = 15 (%) |
|---|---|
| Disease status post CAR T-Cell | |
| CR | 6 (40) |
| PR | 9 (60) |
| Disease status at transplantation | |
| CR | 5 (33) |
| PR | 7 (47) |
| PD | 3 (20) |
| Donor type | |
| Matched sibling | 6 (40) |
| Matched unrelated | 7 (47) |
| Haplo | 2 (13) |
| Disease | |
| DLBCL | 10 (67) |
| PMBCL | 2 (13) |
| Richter | 2 (13) |
| DHL | 1 (7) |
| GVHD | |
| acute | 5 (33) |
| chronic | 1 (6) |
| Response | |
| CR | 9 (60) |
| PR | 4 (27) |
| PD | 1 (6) |
| Not evaluable | 1 (6) |
| Death | |
| Disease related | 4 |
| alloSCT related | 3 |
Abbreviations:
CR- Complete Response
PR – Partial Response
PD – Progressive Disease
DLBCL – Diffuse large B Cell Lymphomas
PMBCL – Primary Mediastinal B Cell Lymphoma
DHL – Double Hit Lymphoma
GVHD – Graft Versus Host Disease
HSCT
alloSCT – Allogeneic Stem Cell Transplantation
Figure 2: Swimmer plot of the 15 patients that underwent allo-SCT post CAR-T cell therapy.
Six patients achieved CR and 9 PR post CAR T-cell. Seven patients died post-transplant, of them 3 patients that achieved CR post CAR-T.
Discussion
CAR T-cell therapy revolutionized the treatment of relapsed/refractory (R/R) ABCL. In the pre CAR T-cell era, patients with R/R ABCL, who were unable to undergo an ASCT or whose disease progressed after ASCT, were mostly treated with palliative care and had dismal overall survival 20. The long-term survival for patients treated with commercial CAR T-cells after at least two lines was consistently reported as 35-40% 4,5,21,22.
Our study describes a cohort of 73 patients treated with an academic local CAR T-cell product that includes a CD28 costimulatory domain 13,14. The production efficiency was 98.6 % and all the screened patients were eventually treated with CAR T -cells. The local production and the short vein-to-vein turnover time (10 days) enabled us to avoid bridging therapy in most patients and to include patients that had rapidly progressing disease. Compared to the main three pharma-sponsored trials (ZUMA-1, Juliet, and TRANSCEND NHL 001), we had a younger patient population (median age was 49 years) but more patients with primary refractory disease (50.7%) 4,5,22. ORR and median PFS in our study is slightly lower, but long term PFS and OS are very similar to those reported in the literature. The relative lower ORR and median PFS can be explained by the study population which included patients with rapidly progressing disease and patients with expected poor prognosis such as RT and high grade B cell lymphoma .
As in our analysis, the real-world experience with Axi-cel demonstrated a slightly lower response rate than ZUMA-1 23,24. The real-world experience with Tisa-cel demonstrated similar response rates, albeit in a significantly smaller cohort of patients 23,25.
When the first CAR T-cell products were approved for clinical use many health professionals were discouraged due to the financial toxicity of this procedure 26-28. The cost effectiveness is still not clear. The fact that CAR T-cell therapy may cure only 35-40% of patients with R/R DLBCL means that 60% of them could have received other, significantly cheaper, salvage therapies that would also prolong their lives but not cure them 29. The Point-of-care production is not only faster but also potentially cheaper as it reduces the costs of shipment and bridging chemotherapy. Since currently we are lacking robust tools to predict response to CAR T-cell therapy, lowering the costs of the procedure is extremely important.
We and others demonstrated that achievement of CR predicts better OS 4,5,22. While others found LDH and tumor mass to predict response rate 4,5,22, we could not find any significant clinical predictors of response to CAR T-cell treatment 23,30. Higher grades of CRS or ICANS were also not predictive. The small sample size can explain the difference.
AlloSCT post CAR T-cell therapy for responding patients is debatable 16. During the first 1.5 years of the study period, we recommended alloSCT to all patients who responded to CAR T-cell therapy, and after this period alloSCT was suggested according to physician discretion. In this cohort, a total of 15 responding patients underwent alloSCT as consolidation. Seven patients died after alloSCT, two patients in CR due to transplant complications, and five due to progressive disease. Considering the relatively small numbers of patients treated with consolidative alloSCT after CAR T-cell therapy, based on the current analysis, it appears that this approach did not benefit, and in the meantime, it is generally discouraged.
To summarize, we present the outcome of 73 patients with ABCL that were treated with locally produced CAR T-cell therapy. The point-of-care production made the turnover time much faster and saved shipment and bridging therapy costs. Taking into consideration the amendment made in the age inclusion criteria and the change of alloSCT referral policy in the course of the trial, the long-term outcomes are comparable to those reported with the commercial products. Consolidative alloSCT post CAR T-cell therapy, which was done initially per protocol for 15 responsive patients, did not appear to be beneficial.
Acknowledgments
RS is supported by the Memorial Sloan Kettering Cancer Center Core grant (P30 CA008748) from the National Cancer Institute/National Institutes of Health.
Footnotes
Conflict of Interest
None
Disclosures
RS serves as a consultant for Medexus and MyBiotics.
References
- 1.Hagberg H, Gisselbrecht C, CORAL study group. Randomised phase III study of R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL) followed by high-dose therapy and a second randomisation to maintenance treatment with rituximab or not: an update of the CORAL study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol 2006;17 Suppl 4(suppl 4):iv31–2. [DOI] [PubMed] [Google Scholar]
- 2.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J. Clin. Oncol 2010;28(27):4184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.June CH, Sadelain M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med 2018;379(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 6.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest 2016;126(6):2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019;54(10):1643–1650. [DOI] [PubMed] [Google Scholar]
- 8.Sermer D, Batlevi C, Lia Palomba M, et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020;4(19):4669–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalbert JJ, Arnason JE, Ge W, et al. Real-world treatment patterns among patients with diffuse large B-cell lymphoma (DLBCL) treated with CD19-directed chimeric antigen receptor T-cell therapy (CAR T). J. Clin. Oncol 2020;38(15_suppl):e19351–e19351. [Google Scholar]
- 10.Hu Y, Tan Su Yin E, Yang Y, et al. CAR T-cell treatment during the COVID-19 pandemic: Management strategies and challenges. Curr. Res. Transl. Med 2020;68(3):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz de Landazuri I, Egri N, Muñoz-Sánchez G, et al. Manufacturing and Management of CAR T-Cell Therapy in “COVID-19’s Time”: Central Versus Point of Care Proposals. Front. Immunol 2020;11:573179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghorashian S, Kramer AM, Onuoha S, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat. Med 2019;25(9):1408–1414. [DOI] [PubMed] [Google Scholar]
- 13.Jacoby E, Bielorai B, Avigdor A, et al. Locally produced CD19 CAR T cells leading to clinical remissions in medullary and extramedullary relapsed acute lymphoblastic leukemia. Am. J. Hematol 2018;93(12):1485–1492. [DOI] [PubMed] [Google Scholar]
- 14.Itzhaki O, Jacoby E, Nissani A, et al. Head-to-head comparison of in-house produced CD19 CAR-T cell in ALL and NHL patients. J. Immunother. cancer 2020;8(1):. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avigdor A, Shouval R, Jacoby E, et al. CAR T cells induce a complete response in refractory Burkitt Lymphoma. Bone Marrow Transplant. 2018;53(12):1583–1585. [DOI] [PubMed] [Google Scholar]
- 16.Bouziana S, Bouzianas D. Exploring the Dilemma of Allogeneic Hematopoietic Cell Transplantation after Chimeric Antigen Receptor T Cell Therapy: To Transplant or Not? Biol. Blood Marrow Transplant 2020;26(8):e183–e191. [DOI] [PubMed] [Google Scholar]
- 17.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J. Clin. Oncol 2014;32(27):3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant 2019;25(4):625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. [DOI] [PubMed] [Google Scholar]
- 20.Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematol. Am. Soc. Hematol. Educ. Progr 2011;2011(1):498–505. [DOI] [PubMed] [Google Scholar]
- 21.Yassine F, Iqbal M, Murthy H, Kharfan-Dabaja MA, Chavez JC. Real world experience of approved chimeric antigen receptor T-cell therapies outside of clinical trials. Curr. Res. Transl. Med 2020;68(4):159–170. [DOI] [PubMed] [Google Scholar]
- 22.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. [DOI] [PubMed] [Google Scholar]
- 23.Vitale C, Strati P. CAR T-Cell Therapy for B-Cell non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia: Clinical Trials and Real-World Experiences. Front. Oncol 2020;10:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nastoupil LJ, Jain MD, Spiegel JY, et al. Axicabtagene Ciloleucel (Axi-cel) CD19 Chimeric Antigen Receptor (CAR) T-Cell Therapy for Relapsed/Refractory Large B-Cell Lymphoma: Real World Experience. Blood. 2018;132(Supplement 1):91–91. [Google Scholar]
- 25.Jaglowski S, Hu Z-H, Zhang Y, et al. Tisagenlecleucel Chimeric Antigen Receptor (CAR) T-Cell Therapy for Adults with Diffuse Large B-Cell Lymphoma (DLBCL): Real World Experience from the Center for International Blood & Marrow Transplant Research (CIBMTR) Cellular Therapy (CT) Registry. Blood. 2019;134(Supplement_1):766–766. [Google Scholar]
- 26.Baumgardner JR, Brauer MS, Zhang J, et al. CAR-T therapy and historical trends in effectiveness and cost-effectiveness of oncology treatments. J. Comp. Eff. Res 2020;9(5):327–340. [DOI] [PubMed] [Google Scholar]
- 27.Chicaybam L, Bonamino MH, Invitti AL, et al. Overhauling car t cells to improve efficacy, safety and cost. Cancers (Basel). 2020;12(9):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin JK, Muffly LS, Spinner MA, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult large B-cell lymphoma. J. Clin. Oncol 2019;37(24):2105–2119. [DOI] [PubMed] [Google Scholar]
- 29.Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J. Clin. Oncol 2020;38(2):155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vercellino L, Di Blasi R, Kanoun S, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(22):5607–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]