Abstract
Background
Malignant pleural mesothelioma (MPM) is an aggressive neoplasm and often acquires chemoresistance by increasing stemness in tumour tissue, thereby generating cancer stem cells (CSCs). CSCs escape treatment by deploying metabolic pathways to trigger dormancy or proliferation, also gaining the ability to exit and re-enter the cell cycle to hide their cellular identity.
Methods
We employed various cellular and biochemical assays to identify the role of the glycolytic enzyme PFKFB3, by knocking it down and pharmacologically inhibiting it with PFK158, to determine its anticancer effects in vitro and in vivo by targeting the CSC population in MPM.
Results
Here, we have identified PFKFB3 as a strategic player to target the CSC population in MPM and demonstrated that both pharmacologic (PFK158) and genetic inhibition of PFKFB3 destroy the FAK-Stat3-SOX2 nexus resulting in a decline in conspicuous stem cell markers viz. ALDH, CD133, CD44, SOX2. Inhibition of PFKFB3 accumulates p21 and p27 in the nucleus by decreasing SKP2. Lastly, PFK158 diminishes tumour-initiating cells (TICs) mediated MPM xenograft in vivo.
Conclusions
This study confers a comprehensive and mechanistic function of PFKFB3 in CSC maintenance that may foster exceptional opportunities for targeted small molecule blockade of the TICs in MPM.
Subject terms: Mesothelioma, Cancer stem cells
Background
The metabolic activity is frequently enhanced and constitutively activated by an increase in the utilisation of glucose and glutamine as a resultant phenotype of both genetic deregulations and plasticity maintenance [1, 2]. Increased glycolysis is recognised as a prerequisite for malignancy [3] and 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) is a crucial rate-limiting [4] enzyme that is often positively associated with neoplastic progression [5–10]. PFKFB3 acts as a double-edged sword. The nuclear PFKFB3 is associated with an increase in cell proliferation via cyclin-dependent kinases (CDKs) [11] as well as it is critical for the recruitment of homologous recombination (HR) proteins to enhance HR activity and cell survival upon radiation [12]. On other hand, PFKFB3 acetylation by cisplatin impairs the activity of the nuclear localisation signal (NLS) and resulting in cytoplasmic accumulation of PFKFB3 and triggers glycolysis [13]. However, the function of PFKFB3 in cancer stem cell (CSC) maintenance is yet to be established.
To reorganise cellular anabolic requirements, pluripotent stem cells rely on high glycolytic flux with preferential production of lactate to gain stemness traits [14] instead of oxidative phosphorylation (OXPHOS). However, cells continuously modify their metabolic dependency in response to extracellular cues and nutrient availability [15]. CSCs can shift between OXPHOS and glycolysis depending upon the availability of nutrients and oxygen to maintain homoeostasis and, thereby, facilitate tumour growth and maintain the pool of minimal residual disease (MRD) [16].
Mesothelioma is rare cancer that begins in the mesothelial lining of different internal organs of the body. Malignant pleural mesothelioma (MPM) that originates in the pleural lining surrounding the lungs represents ~75 to 80% of mesotheliomas. Due to its late diagnosis, the overall 5-year survival rate of MPM is only 10% [17] and is often associated with resistance to chemotherapy [18]. The presence of MRD following chemotherapy and suboptimal debulking are enriched in the CSC population in tumours is associated with tumour resistance and recurrence. The well-established CSC markers CD133, CD44 and SOX2 have been shown to be involved in the expansion of chemoresistant cells [19]. In parallel, the CD133+ cells show the glycolytic phenotype in other cancers [20].
In our earlier study, we showed that PFKFB3 is highly expressed in MPM and by therapeutically targeting it with the glycolysis inhibitor PFK158 resulted in an upsurge of macropinocytosis and ER stress that leads to MPM cell death [10]. Here, the main objective of this study is to determine if targeting PFKFB3 by PFK158 results in the altered expression of the small CSC population in MPM. The experimental results demonstrate that PFKFB3 inhibition decreases CSC markers, reduces CSC population, altered FAK-Stat3- SOX2 signalling and restores p27 in the nucleus. Cyclin-dependent kinase inhibitor 1B (p27Kip1) is often downregulated in most human epithelial cancers with poor prognosis. Restoration of p27 levels and/or its nuclear localisation is often associated with a good prognosis [21] and response to molecular therapies [22]. However, ectopic expression of nuclear PFKFB3 is often inversely related to p27 phosphorylation leading to its ubiquitination and subsequent degradation [11]. In addition, p27 can suppress SOX2 directly in iPSCs [23]. As we previously reported knockdown or pharmacological inhibition of PFKFB3 activity sensitises cells to platinum-based cell death. Using a minimal number of MPM tumour-initiating cells (TIC) and PFK158 treatment resulted in a significant reduction of TIC-induced CSC markers in vivo. Taken together this is the first report in MPM targeting the CSC population by inhibiting PFKFB3.
Methods
Reagents
We obtained PFK158 from Gossamer Bio (San Diego, CA) [10] and was dissolved in PBS containing 40% capitasol as recommended. Reagents and antibodies used in this study are listed in Supplementary Information S1.
Cell culture
Human sarcomatoid MPM cell lines, NCI-H28, NCI-H2052 and epithelioid cell lines NCI-H226, NCI-H2452 were purchased from ATCC. All cell lines were authenticated by STR profiling and validated with the ATCC data bank. Another epithelioid cell line EMMeso was gifted by Dr. Tobias Peikert, Mayo Clinic. The cells were maintained in what media as adherent culture as described before [10].
To enrich the CSC population the cells were cultured for 2 weeks in suspension in serum-free RPMI-1640 supplemented with EGF (20 ng/ml), bFGF (20 ng/ml) and 1× nitrogen supplements (Gibco, Thermo Fisher Scientific) as described [24].
Generation of PFKFB3 downregulated stable MPM clones
To generate the PFKFB3-knockdown clones, we followed the protocol stated elsewhere [10]. We used 3’UTR-targeting shRNA (sh55) and ORF-targeting shRNA (sh59) to stably knock down PFKFB3. The shRNA oligo sequences are:
HBE55-NM_004566.1-3406s1c1 CGGGTGCATGATTGTGCTTAA 3’UTR
HBE59- NM_004566.2-706s1c1 CCACCAATACTACTAGAGAGA.
Quantitative real-time PCR
cDNA synthesis was done via Quantitect (Qiagen) and quantitative real-time PCR (qRT-PCR) was performed using SYBR-Green PCR Master Mix (Bio-Rad) following the manufacturer’s protocol, with specific primers for human SOX2, CD133 and CD44 and β-actin (Supplementary Information S2). Normalisation was done using housekeeping β-actin primers [24].
Detection of cell surface protein expression
The MPM cells untreated or were treated with variable doses of PFK158 for 24 h (unless mentioned otherwise), washed once with ice-cold sterile PBS and were co-labelled with PE-CD133 (Miltenyi Biotec), FITC-CD44 and APC-CD90 (BD Biosciences) for 30 min at 4 °C in the dark. Cells were washed twice with 1% FBS containing PBS and finally resuspended in the same for analysis on the BD FACSAria II Flow Cytometer.
Aldefluor assay
To identify the cell population with ALDH1 enzymatic activity, we used the Aldefluor kit (Stem Cell Technologies) [25] following the manufacturer’s instructions. In brief, 1 × 106 cells were resuspended in 1 ml aldefluor assay buffer and labelled as ‘test’ (control). In parallel, the ‘DEAB-control’, contained 5 µl diethylaminobenzaldehyde (DEAB) inhibitor. Following the addition of 5 µl of the activated aldefluor reagent to the “test” sample, 500 µl of the cell suspension was taken out and put in the DEAB-control tube containing the inhibitor. Cells were incubated for 30 min at 37 °C, washed and resuspended in 500 µl aldefluor assay buffer and were analysed on the BD FACSAria II Flow Cytometer.
Subcellular fractionation study
The cells (H28 and EMMeso) were treated with either vehicle or PFK158 (5 μM) for 24 h and fractionated into the cytosol and nuclear portions using a NE-PER Kit (Thermo Scientific, USA) according to the manufacturer’s protocol. Briefly, the cells were washed after treatment, incubated in cytosol extraction reagent and subsequently centrifuged. The supernatant served as the cytosolic fraction. The pellet was solubilized in nuclear extraction buffer, centrifuged and the supernatant contained the nuclear fraction. Western blot analyses were performed with these subcellular fractions with a proper subcellular loading control.
Immunoblot and immunoprecipitation assay
Immunoblot analysis was carried out as previously described [26]. Briefly, parental, PFKFB3 knockdown or CSC-enriched MPM cells (1 × 106) were either untreated or treated (with PFK158, dose- and time-dependent manner) and 40 μg of proteins were separated in SDS–PAGE (4–12.5% gradient gel) followed by electrotransfer onto nitrocellulose membrane, blocked with 2–5% TBS–BSA, probed overnight with primary antibodies (Supplementary information) at 4 °C and washed with 0.1% Tween-20-containing TBS. Immunocomplexes were identified with fluorophore-conjugated secondary antibodies (LI-COR). The membrane was washed and target proteins were identified by the LI-COR OdysseyFc Imaging System (Nebraska, USA).
For detection of the protein complex, the cell lysates containing around 350 μg of protein were incubated with the anti-Stat3 antibody (1:100) overnight at 4 °C on a nutator. The next day, 15 μl of 50% protein-A-agarose beads were added and thoroughly mixed at 4 °C for 4 h on a nutator. The immunoprecipitates were washed at least thrice with ice-cold PBS-T (0.1% tween 20%), collected and precipitated beads were loaded into the sample buffer, subjected to electrophoresis on 4–12.5% SDS–PAGE and blotted using an anti-Stat3 or anti-SOX2 antibody.
Confocal and phase-contrast microscopy
Confocal imaging was performed as previously described [27]. Briefly, MPM cells were grown in four-well chambered slide for the preferred time with the presence and absence of PFK158 and fixed with 4% paraformaldehyde at 4 °C for 10 min. Cells were then washed with 0.5% PBS–BSA and followed by blocking with 3% PBS–BSA at 37 °C for 1 h. Subsequently, cells were probed with the primary antibody in 3% PBS–BSA (1:200 dilution) at 4 °C overnight. Later incubated with fluorophore-labelled secondary antibody in 3% PBS–BSA (1:200 dilution) at 37 °C for 1 h. Subsequently, cells were mounted in a mounting medium (1.5 μg/ml) (Vectashield, USA) and visualised by using Zeiss-LSM 710 confocal microscope. Quantification of the fluorescence was done using Image-J software.
For the immunofluorescence imaging of CSC-enriched spheroid MPM, cells were plated on poly-l lysine-coated four-well chambered slide to adhere before processing for the general confocal imaging as stated above.
Cell cycle analysis via flow cytometry
Cell cycle analysis by flow cytometry was done as mentioned [28]. PFKFB3 knockdown and non-targeted control MPM cells (1 × 106 cells/well) were processed according to the manufacturer’s protocol (BD Biosciences, San Jose, CA). The cells were analysed using a BD FACSAria II Flow Cytometer. A minimum of 2 × 104 cells per sample was evaluated, and the cell distribution percentage analysed for each phase of the cell cycle and the percentage of population calculated with CellQuestPro software (BD Biosciences).
Lactate-dehydrogenase assay
Spheroid culture-induced H28CSC and EMMesoCSC cells were propagated to check the release of LDH. The LDH released into the medium was transferred to a new 96-well plate and mixed with a reaction mixture according to the manufacturer’s protocol (Pierce, USA). After a 30-min room temperature incubation, reactions were stopped by adding the stop solution. Absorbance at 490 and 680 nm was measured using a plate-reading spectrophotometer to determine LDH activity.
Determination of tumour-initiating stem-like cells for in vivo MPM xenograft study
Animal experiments complied with the guidelines of the Institutional Animal Care and Use Committee (IACUC) at the Mayo Foundation, following approved protocols. Eighteen female athymic homozygous nude mice (nu/nu, 4–6 weeks old) were obtained from Jackson Laboratories, USA. After 7 days of acclimatisation, the mice were randomly assigned to three groups (n = 6). The parental cells were grown on adherent 2D plates. In parallel, EMMeso cells were grown in suspension to enrich for stem cell-like population (for 2 weeks) as described in 'Methods', cell culture. Subsequently, 5000 cells henceforth known as tumour-initiating cells (TICs) were injected into the right flank of the fore-limb of mice and treated as follows [Group A—adherent parental cells, Group B—TICs treated with captisol only and Group C—TICs treated with 30 mg/kg PFK158, twice a week, 2 weeks). Mice were kept under constant observation for the formation of palpable tumours. On day 35 after postinoculation, palpable tumours were seen in Group B and C mice and treatment started for those groups as mentioned. On day 49 of postinoculation, all mice were sacrificed and subsequently tumour weight and tumour volume were determined. Tumours were preserved either in formalin or −80 °C or Trump’s fixative.
Immunohistochemistry (IHC) study
For the IHC study, freshly incised tumours were fixed and cryo-preserved, embedded in paraffin and microtome-sectioned. Tissue sections were stained with anti-PFKFB3 and anti-CD133 antibodies. The IHC score was done by using an Image-J plugin “IHC profiler” [https://sourceforge.net/projects/ihcprofiler/].
Statistical analyses
All results were shown as mean ± standard deviation (SD). Data were obtained from at least three individual sets of experiments. All statistical analyses were performed using the GraphPad Prism 9 software (San Diego, CA). Data were analysed using either non-linear regression or t test or one-way ANOVA as appropriate, where ***P < 0.0001, **P < 0.001, *P < 0.01 considered as statistically significant unless otherwise mentioned. P > 0.05 considered as non-significant (ns).
Results
PFKFB3 inhibition downregulates stem cell markers in MPM
We have previously shown that PFK158 inhibits MPM cells driving them into nutrient stress-induced methuosis [10]. As most of the chemotherapeutics have a tendency of enrichment of CSCs and putative CSCs in MPM demonstrate resistance to cisplatin and pemetrexed [19, 29, 30], we furthermore intended to appraise whether PFKFB3 inhibition could target the CSC population in MPM, we treated MPM cell lines with 2.5 µM PFK158 for 24 h. The q-PCR data showed that primarily pharmacological inhibition of PFKFB3 decreased SOX2, CD133 and CD44 transcript levels in H28 (Fig. 1a), SOX2 and CD133 in H2052 (Supplementary Fig. S-1). However, the genetic knockdown of PFKFB3 could not accomplish the same fate for CD44 at the transcription level. Furthermore, flow cytometric analysis also revealed that CD133 decreased drastically in H28 and H2052 cells even in a lower concentration of PFK158 (1.25 μM); however, the decrease was only significant 2.5 and 5 μM in H2452 and H226, respectively. CD44 also shows a significant decrease at 5 μM dose of PFK158 in all the cell lines, except H2452 (Fig. 1b). However, a significant decrease in CD133 marker was observed in almost all of the tested cell lines compared to CD44. Western blots and their densitometric analysis also corroborated with the flow cytometry data which further confirmed PFK158 mediated dose-dependent significant decrease in CD133 and CD44 (Fig. 1c).
Fig. 1. PFKFB3 inhibition downregulates stem cell markers in MPM.
a Pharmacological and genetic inhibition of PFKFB3 by PFK158 and shRNA respectively downregulate the expression of stem cell-associated marker genes, assessed by real-time quantitative PCR. Data present mean ± SD, n = 3 independent experiments, one-way ANOVA; **P < 0.001, *P < 0.01. b Flow cytometry analysis for CD133 and CD44 stem-cell surface markers in H28, H226, H2052 and H2452 after dose-dependent PFK158 treatment for 24 h. Values are normalised to the untreated sample for each cell line and shown as percentage expression of the gated cell population using flow cytometry with at least 50,000 events counted. All experiments were done in technical replicates and at least three independent experiments. One-way ANOVA; **P < 0.001, *P < 0.01. c Left: Western blot analysis of CD133 and CD44 in a dose-dependent manner in H28 and H2052. GAPDH served as a control. Right, densitometric quantification of the western blot. The figure is a representative image of at least three independent experiments.
PFKFB3 inhibition diminishes ALDH + CD133 + cell population
ALDH has emerged as a stem cell marker in tumours and as a potential therapeutic target [31, 32]. Accumulating evidence shows an increase in ALDH expression in response to chemotherapy and promotes chemoresistance in CSCs. A high level of ALDH is often associated with an increase in glucose uptake resulting in a higher rate of glycolysis [33]. Conversely, CD133 is known as one of the important biomarkers of stem cells [34]. Our flow cytometric analysis showed significant downregulation of ALDH+CD133-, ALDH-CD133+ or both ALDH+CD133+ population (Fig. 2). Genetic knockdown of PFKFB3 by shRNA in H28 cells showed ~5% ALDH + population decrease with sh55 and almost 35% with sh59 cells compared to NTC cells (Fig. 2a, b). Similarly, pharmacological inhibition of PFKFB3 with PFK158 showed a significant dose-dependent decrease in ALDH+CD133+ population in the tested cell lines (Fig. 2c).
Fig. 2. PFKFB3 inhibition diminishes the ALDH+ CD133+ cell population.
a FACS analysis was performed after preparing the samples according to the protocol described in 'Methods' on H28NTC and two targeted shRNA clones of the same cells. Cells are first gated with Aldefluor positivity and subsequently monitored for the CD133 expression singly or ALDH-positive population. Bargraphs of FACS analysis of three independent experiments in H28NTC and PFKFB3-knockdown clones sh55 and sh59 (b) and PFK158-treated H28, H226 and H2052 in a concentration-dependent manner (c). Data are shown as percentage expressions of the gated cell population with at least 50,000 events and are presented as mean ± SD. One-way ANOVA; **P < 0.001, *P < 0.01.
PFK158 modulates FAK-Stat3-SOX2 axis against stemness
To further understand the molecular players involved in PFK158 mediated stem cell population depletion, we primarily concentrated on FAK which has been reported both in modulating intrinsic glucose levels [35] and also associated with mesothelioma CSCs [36]. Our western blot analysis showed that PFK158 inhibits the expression of phospho-FAKY397 in both dose (Fig. 3a) and time-dependent (Fig. 3b) manner. Our real-time PCR data showed SOX2, one of the major transcription factors involved in stem cell maintenance, decreased after PFKFB3 inhibition (Fig. 1b). Since Stat3 might be a crucial nexus in between FAK and SOX2, we assessed the levels of these two proteins in their active form. Active Stat3 (Y705) was also similarly downregulated in PFK158-treated cells. Likewise, the protein level of SOX2 was also reduced (Fig. 3a, b). The densitometric bar diagram of western blots of SOX2, phospho and total FAK, phospho and total Stat3 showed a significant decrease in both dose and time-dependent manner (Supplementary Fig. S-4A, S-4B). In addition, PFKFB3-knockdown H28 cells also demonstrated comparable molecular occurrence in Fig. 3c and Supplementary Fig. S-4C.
Fig. 3. PFK158 works on the FAK-Stat3-SOX2 axis.
Western blot analysis of molecular players contributing to PFK158 mediated stem cell population reduction in H28 and H2052 in dose-dependent (a), in a time-dependent manner in H28 (b) and PFKFB3-knockdown H28 clones with non-targeted control (c). Left: A decrease in Stat3-SOX2 interaction after PFK158 treatment as evident by immunoprecipitation with anti-Stat3 and subsequent immunoblotting with anti-Stat3 and anti-SOX2 in H226. Right: A bar graph of quantification of SOX2-Stat3 interaction normalised to the input (d) and (e). Left: H28 PFKFB3-knockdown clones sh55 and sh59 with its non-targeted control (NTC). Right: A bar graph of quantification of SOX2-Stat3 interaction normalised to the NTC2. Confocal microscopic images also revealed decreased interaction of Stat3 and SOX2 (f) after PFK158 treatment in an increasing manner for 24 h and (g) H28 knockdown clones. The lower panels of (f, g) show corrected total cell fluorescence (CTCF) of at least five different fields as mean ± SD. Images were taken in ×20 magnification.
PFKFB3 inhibition disrupts the SOX2-Stat3 interaction
As Stat3 and SOX2 both are both localised in the nucleus, to decipher their function, we evaluated if they interacted with each other. Immunoprecipitation assay with anti-Stat3 and anti SOX2 followed by immunoblotting with Stat3 and Sox 2 correspondingly confirmed their weakened interaction upon both pharmacological (Fig. 3d) and genetic (Fig. 3e and Supplementary Fig. S-4D) PFKFB3 inhibition. Furthermore, the confocal microscopic image analysis both in PFK158-treated (Fig. 3f) and PFKFB3-knockdown H28 cells (Fig. 3g) showed impaired colocalization of these two proteins compared to untreated and NTC cells respectively. The lower panel of 3f and 3g correspondingly demonstrate their significant decrease in fluorescence intensity.
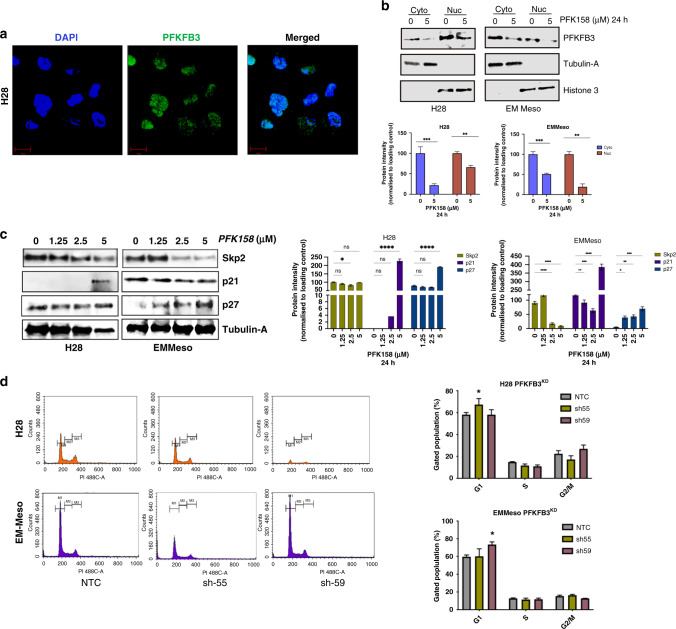
MPM cells show nuclear localisation of PFKFB3
Numerous pieces of evidence showed the nuclear localisation of PFKFB3 and its restricted function in tumour proliferation via cyclin-dependent kinases [11]. Our data also showed a significant amount of PFKFB3 in the H28 cell nucleus (Fig. 4a). The subcellular fractionation study, demonstrated in Fig. 4b (upper and lower panel), showed that PFK158 treatment can reduce both the nuclear and cytosolic pool of PFKFB3. Hence, it not only inhibits glycolysis but probably also restricts cell proliferation impeding DNA damage repair and cell cycle arrest. We have previously reported that PFK158 treatment arrests MPM cells in the G1/S phase [10]. As p21 and p27 are potent suppressors of the G1/S transition and activator of apoptosis, correspondingly we checked the level of these two modulators. Data showed that PFK158 treatment increased p21 significantly in H28 but not in EMMeso. Nevertheless, the p27 level, in comparison to p21, was significantly increased in EMMeso (Fig. 4c). Furthermore, cell cycle analysis in PFKFB3-knockdown clones also showed almost G1/S arrest (8–12%) sh55 clone of H28 and sh59 clone of EMMeso (Fig. 4d). These results confirm that the role of PFKFB3 is not only important for the regulation of glycolysis in the cytoplasm but it also acts as a regulator of the cell cycle in the nucleus and hence confers an anti-apoptotic effect in MPM. Here worth mentioning that in the generation of stably PFKFB3-knockdown cell lines with respective shRNA, the plasmid (containing the sequence for shRNA) could be inserted randomly anywhere in the genome. This can alter the expression of other genes and eventually can modify the proteome of the cells as well. This could be a reason why we found diverse outcomes with different shRNA in two different MPM cell lines. Moreover, H28 and EMMeso lines are not from the same lineage and have different proteome pools. Taken altogether, these can result in a G1/S block with two distinct shRNAs targeting the same protein but into two different cell lines.
Fig. 4. MPM cells show the nuclear localisation and function of PFKFB3.
a A representative fluorescent microscopic image of nuclear localisation of PFKFB3 in H28 cells. b Upper: Subcellular fractionation followed by immunoblotting of PFKFB3 with proper cytosolic (Tubulin-A) and nuclear (Histone3) control in H28 and EMMeso cells. Lower: Bar diagram of PFKFB3 amount normalised to the respective subcellular control. c Left: Western blot analysis of the alteration of some nuclear proteins involved in cell cycle regulation in H28 and EMMeso cells after PFK158 treatment for 18 h. Right: Graphical representation of quantification of proteins normalised to control. c Cell cycle analysis through PI staining followed by flow cytometry in H28 and EMMeso NTC, sh55 and sh59 clones. Representative data of three individual sets of experiments. d The quantitative measurement of cell cycle phases in both the cell lines. Data showed the percentage of the gated population of each phase. **P < 0.001, *P < 0.01.
Platin drug-induced CSC enrichment is a recognised fact in various cancers [37]. We have previously reported that PFK158 alone and in combination with carboplatin is enough to reduce the MPM tumour growth in vivo and to sensitise these tumour cells to carboplatin in combination [10]. Our additional study now shows PFKFB3-knockdown H28 clones are also highly sensitive to platin drugs (Supplementary Fig. S-2). This may open a notion that PFKFB3 downregulation can lead to a reduction in platin induced CSC population.
Enrichment of CSC population characterised by stem cell surface CD markers and ALDHhiCD133+ in MPM
Aiming next to whether PFK158 can decrease the stem cell population not only in heterogeneous adherent MPM cell culture but also in vitro enriched stem cells/ tumour-initiating cells (TICs) grown as spheroid cultures. Our pilot study of enrichment of TICs showed growth factors and nitrogen supplemented (see 'Methods') special culture media can promote spheroid formation in 7 days for H28, however, it took almost 15 days to form spheroids in EMMeso cells (Fig. 5a). The enrichment of TICs in terms of CD44, CD133 and CD90 increased by nearly 32%, 18% and 20% in H28, respectively (Fig. 5b). However, the upsurge of these CD markers was almost 40%, 30% and 25% correspondingly in EMMeso (Fig. 5c). Furthermore, ALDH+ only as well as ALDHhiCD133+ populations also were markedly upregulated (Fig. 5d). Enriched TICs also showed a higher glycolytic rate. These cell populations demonstrated significantly higher intracellular LDH activity in both H28 and EMMeso (2–2.5-fold increase) as measured spectrophotometrically (Fig. 5e).
Fig. 5. Enrichment and characterisation of MPM-CSC population.
a The phase-contrast image of H28 (upper) and EMMeso (lower) spheroid culture grown in specific growth factor and nitrogen supplemented media (see 'Methods'). Quantitative analysis of flow cytometric evaluation of enrichment of CD markers in epithelial adherent culture and cancer cancer cancer stem cell-like population in the spheroid culture both in H28 (b) and EMMeso (c). d Bar diagram of ALDH+ population and CD133+ population in ALDHhi cells showing augmentation of cancer stem cell-like population in the spheroid culture of EMMeso. e Quantitative measurement of lactate-dehydrogenase activity in adherent and the spheroid culture both in H28 and EMMeso. Bar diagram showing a decrease in CD44, CD133 and CD90 in spheroid culture enriched stem cell-like H28 (f) and EMMeso (g) cells after PFK158 treatment. h, i A representative FACS data along with the bar diagram of ALDH+ population and CD133+ population in ALDHhi cells showing augmentation of cancer stem cell-like population in the spheroid culture of EMMeso. **P < 0.001, *P < 0.01.
However, these enriched cell populations are more resistant in comparison to the heterogeneous cultures. PFK158 could target these TICs. Instead of a lower concentration in heterogeneous adherent culture (1.25 µM), a comparatively higher dose of PFK158 (5 µM) could reduce CD44, CD133 and CD90 in a substantial manner (Fig. 5f, g). A similar phenomenon was also observed in the reduction of ALDH+ only as well as in the ALDHhiCD133+ population (Fig. 5h, i). Lowering the FBS in the culture media also enriched CSC-like populations in both sarcomatoid and epithelioid MPM cells (Supplementary Fig. S-3), though expedited enrichment was only observed in controlled growth factor and nitrogen supplemented as described.
Repression of p21/p27 and overexpression of PFKFB3 in spheroid MPM cells regulate the stemness
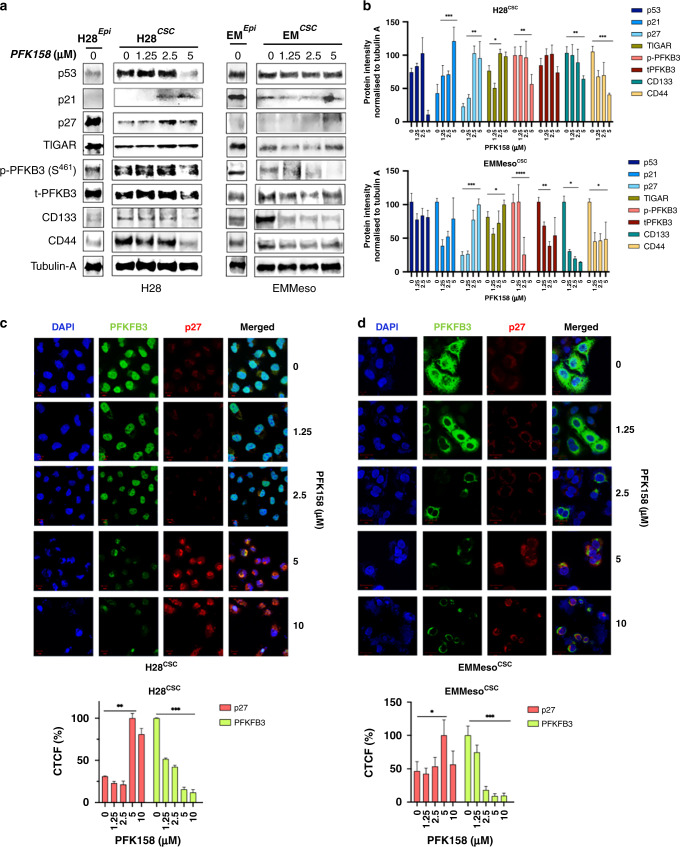
We have already established that the inhibition of PFKFB3 inactivates FAK, Stat3 (Fig. 3a–c) and thereafter decrease in S-phase kinase-associated protein-2 (Skp2) and subsequent upregulation of p21 and p27 (Fig. 4b) in adherent MPM cells. However, how this signalling cascade is modulated in spheroid-enriched CSC-like MPM cells is not well-established. Firstly, we found loss or downregulation of Cip/Kip family cell cycle regulators p21 and p27 in CSC-enriched cells (Supplementary Fig. S-4). Enrichment of phospho- and total-PFKFB3 was also observed in enriched H28 and EMMeso cells, respectively, which further went down after PFK158 treatment (Fig. 6a). Interestingly, TP53-induced glycolysis regulatory phosphatase (TIGAR), a phosphatase regulator of PFKFB3’s kinase activity, is also found to be downregulated in the enriched population with no further change in PFK158 treatment. Collectively, these data show CSC-defined surface CD makers and their subsequent downregulation after PFK158 treatment also warrants its ability to target the enriched stem-like cells. The densitometric quantification of the western blots confirms significant changes in the expression of these markers (Fig. 6b).
Fig. 6. Repression of p21/p27 and overexpression of PFKFB3 in spheroid MPM cells regulate the stemness.
a Western blot analysis of enrichment of cell cycle- and stem cell-related proteins in a spheroid culture of H28 and EMMeso cells and their subsequent modulation after PFK158 treatment in a dose-dependent manner. b Evaluation of protein intensity (normalised to control, Tubulin-A). c, d Confocal microscopic images of PFKFB3 and p27 and their co-localisation in a spheroid culture enriched cancer stem cell-like population in H28 and EMMeso.
While cell cycle regulator p21/p27 is known to be degraded by CDK-mediated phosphorylation-dependent ubiquitination by SKP2. However, any direct interaction between PFKFB3, the target glycolytic enzyme of this study, and p21/p27 are not known. Therefore, in search of any interaction, we performed confocal microscopy imaging. The data revealed inverse spatial expression of PFKFB3 and p27 in CSC- like enriched MPM cell population (Fig. 6c, d). Strikingly, PFKFB3 pool was observed more in the nucleus in H28 and cytoplasm in EMMeso. Thus the major role of PFKFB3 in these two cell types still needs additional study. The lower panel of (Fig. 6c, d) demonstrates the graphical representation of the corrected total cell fluorescence in corresponding confocal images.
Tumour-Initiating stem-like Cells (TIC) in human pleural mesothelioma exhibit upregulation of PFKFB3
We next aimed to investigate the tumour-initiating ability of enriched CSC-like populations in vivo and the ensuing efficacy of PFK158 on that. Initially, we wanted to establish the minimum TIC cell number needed to develop a palpable subQ tumour as described in the method section and outlined in Fig. 7a. Remarkably, there was no tumour formation in the parental control group inoculated with 5000 adherent EMMeso even after the end of the in vivo study on day 49. However, the same number of spheroid-enriched EMMeso cells developed palpable tumour within 35 days in groups B and C which further suggests their minimum number to develop a tumour in immunocompromised mice Fig. 7b. Upon 2-week of PFK158 treatment, an evident reduction in tumour burden (Fig. 7b, right panel), tumour weight (Fig. 7c) and tumour volume (Fig. 7d) were observed. Two substantial marker proteins of the whole study, CD133 and PFKFB3 were further validated by IHC staining in untreated and treated xenograft tumour sections demonstrating a significant decrease in tissue-specific expression respectively (Fig. 7e, f). Furthermore, expression of key modulatory stem cell marker proteins along with total and phospho-PFKFB3 from four randomised xenograft tumour lysates were significantly decreased in the PFK158-treated group compared to the untreated one and consistent with the in vitro data (Fig. 7g, h). Altogether, the in vivo data established a minimum TICs in MPM and revealed the efficacy of PFK158 to inhibit CSC-like cell-mediated tumorigenesis through decreasing stem cell markers and finally reduce the tumour burden.
Fig. 7. Tumour-initiating stem-like cells (TIC) in human pleural mesothelioma exhibit upregulation of PFKFB3.
a Schematic representation of the study design with the establishment of the tumorigenicity of 5000 TIC (epithelial and spheroid-enriched EMMeso). b The reduction in tumour size as reflected in the image of six representative MPM-TIC xenograft tumours, after 2 weeks of the vehicle (40% captisol) and PFK158 treatment (30 mg/kg). The decrease in MPM incised tumour weight (c) and tumour volume (d) after aforesaid treatments. e Representative images of immunohistochemical staining of PFKFB3 and CD133 performed in MPM-TIC xenograft untreated and treated tumour tissue. f The IHC score was done by using an Image-J plugin “IHC profiler” [https://sourceforge.net/projects/ihcprofiler/]. g Immunoblot analysis against CD133, CD44, SOX2, total- and phospho-PFKFB3 (S461) in MPM xenograft tumours. PCNA served as a control. h Densitometric quantification of protein intensity curated in a bar graph where n = 4. Data shown as mean ± SD where ***P < 0.0001, **P < 0.001, *P < 0.01.
Discussion
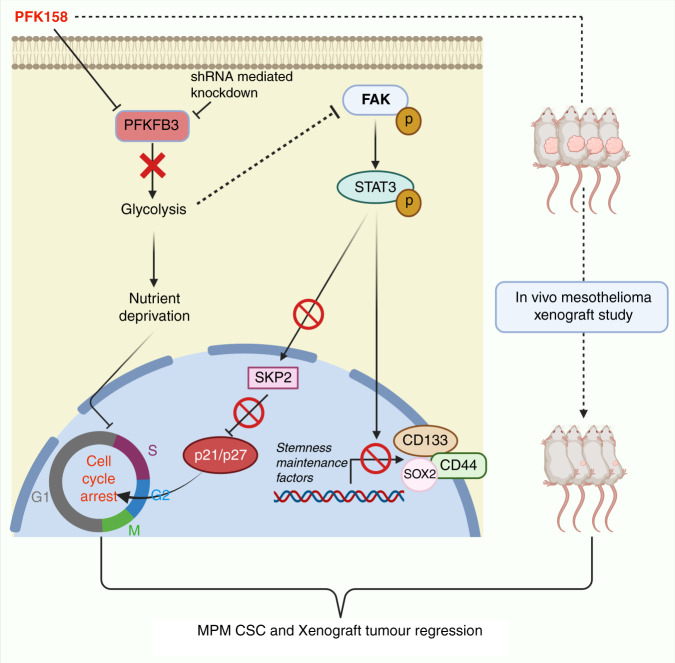
Exploring novel strategies to target cancer metabolic pathways is an emerging field of research, though consideration is being paid to the cellular heterogeneity and metabolism is almost negligible. Diversified cell populations, including CSC, in malignant mass, are often manifested by differential metabolic behaviour which has recently been associated with CSC function [38]. To accord and adapt to transient bioenergetics crises triggered by nutrient deficiency or hypoxia, a tiny CSC population in tumours alters their metabolic priority. Glycolysis has been recognised as the preferred metabolic pathway of CSCs in numerous cancers [39–43]. The key rationale for exploring the role of glycolytic metabolism in CSCs is to determine whether it renders similar self-renewal characteristics shown in normal stem cells. In this study, for the first time, we demonstrated the significant role of a critical glycolytic regulator PFKFB3 in the maintenance of CSC markers in MPM and targeting it can regress the CSCs in MPM (Fig. 8).
Fig. 8. A summarised illustration (BioRender, https://biorender.com) showing the contribution of PFKFB3 in the enrichment of MPM stemness and cell cycle regulation.
PFKFB3 inhibition reduces the CSC population by inactivating FAK-Stat3-SOX2 signalling and restores p27 in the nucleus.
In our previous study, we already showed that an elevated level of PFKFB3 results in a higher glycolytic phenotype in MPM cells [10]. However, its role in the maintenance of stem cell markers in MPM was unknown. A recent study showed a three-fold increase in glycolysis with a simultaneous decrease in oxygen consumption rate (OCR) in CD133+ hepatocellular CSCs compared to CD133− CSCs [20]. Likewise, we also found a reduction in CSC markers in PFK158-treated or PFKFB3-knockdown cells. CD133+ CSCs are known to have self-renewal potential. In parallel, investigations revealed that escalation of glycolysis by upregulating glycolytic enzymes (GLUT1, HK-1 and PDK-1) is essential for cell immortalisation and self-renewal [44]. Though how CD133 expression triggers enhanced glycolysis or vice versa is yet to be established. In parallel, targeting PFKFB3 does not lead to the downregulation of CD44 in MPM cells conspicuously although a phenotypic decrease in glycolysis was observed. Nevertheless, several studies prove that CD44 triggers glycolysis by either upregulating LDHA [45] or by inhibiting the activity of PKM2 via direct interaction [46].
FAK has been shown to play an important role in the self‐renewal and tumour‐initiating ability of CSCs [47]. The FAK inhibitors BI-853520 and VS-6063 (defactinib) inhibit MPM and breast cancer by targeting CSCs [48–50]. However, the latter had been prematurely stopped even after entering into a Phase 2 clinical trial with MPM due to its futility (NCT01870609). Henceforth, FAK is a strategic target, selectively aiming for CSCs in the tumour. FAK is perpetually associated with enhanced glycolysis [51]. Taken together, our data also revealed that inhibiting glycolysis by PFK158 efficiently inactivates FAK in MPM cells. This observation is further corroborated by FAK downregulation in gastric cancer by another PFKFB3 inhibitor, PFK15 [52]. Moreover, our earlier study shows that PFK158 triggers ER stress [10], strikingly this fits with our current observation of PFK158 mediated FAK inhibition as ER stress can decrease FAK activity in endothelial cells [53]. FAK is frequently reported to crosstalk with Stat3 [54–56] which is activated in CD133+ CSC populations in many cancers [57–59]. Substantially, we also observed the downregulation of activated Stat3 in MPM. This represents a new axis of PFKFB3 mediated FAK-Stat3 signalling to target stemness factors.
Moreover, we have also shown that PFKFB3 inhibition arrests MPM cells predominantly in the G1/S phase by decreasing Skp2 and the concomitant increase of p21 and p27 that further supported by another observation where PFKFB3 triggers CDK1 mediated phosphorylation of p27 and its subsequent degradation [11]. In addition, our data also show that nuclear PFKFB3 inversely expresses itself with p27. Collectively, our observation suggests comparable crosstalk of Stat3 depletion with Skp2 downregulation and henceforth increase in p27 and p21 [60].
It is concluded that targeting PFKFB3 elicits various intracellular crosstalks apart from inhibiting glycolysis. Two back to back articles reveal the function of PFKFB3 depending on its spatial expression. In one observation, a unique mechanism of PFKFB3 regulation has been unfolded by platin induced acetylation-mediated cytoplasmic retention of PFKFB3 to amplify glycolysis and finally inhibit apoptosis [13]. Another uncovers a novel function of nuclear PFKFB3 in the radiation-induced HR repair pathway in cancer cells [12]. Conjointly, these reports unveil the variant tasks of PFKFB3 according to its intracellular localisation. We observed sarcomatoid H28 displaying higher nuclear PFKFB3 in comparison to epitheloid EMMeso where it is higher in the cytoplasm. However, PFK158 can target both despite their localisation and initiate a signal cascade to eradicate the CSC population in MPM. Considering the complexity of a tumour niche bearing diverse cell populations with differential metabolic needs, it is challenging to eradicate malignant cells entirely. However, targeting the CSCs population with a metabolic inhibitor might act as a double-edged sword that unlocks a promising strategy to eliminate both CSCs and non-CSCs. Thus, exploiting the metabolic dependencies of constituents of the tumour microenvironment holds substantial clinical potential and can be explored for better therapeutic intervention along with conventional chemotherapy reservoir.
Supplementary information
Acknowledgements
We sincerely acknowledge Dr. Tobias Peikert, Mayo Clinic, Rochester, MN for providing the EMMeso cell line. Our acknowledgement towards the personnel of Microscopy and Cell Analysis Core and Pathology Research Cores Mayo Clinic, Rochester, MN.
Author contributions
Conceptualisation: SSB and VS; formal analysis: SSB; funding acquisition: VS and JRM; investigation: SSB and VS; methodology: SSB and PT; project administration: VS; supervision: JRM and VS; writing—original draft: SSB; writing—review and editing: SSB, PT, JS and VS.
Funding
This work is supported (in part) by the Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (JM and VS) and a generous gift from Samuel and Ilda Conde to JM—Mayo Clinic, Rochester.
Data availability
The data that support the findings of this study are available from the corresponding author on a reasonable request.
Ethics approval and consent to participate
This work was approved by the Mayo Clinic Institutional Review Board (IRB).
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Viji Shridhar, Email: Shridhar.Vijayalakshmi@mayo.edu.
Julian R. Molina, Email: Molina.Julian@mayo.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-01867-7.
References
- 1.De Francesco EM, Sotgia F, Lisanti MP. Cancer stem cells (CSCs): metabolic strategies for their identification and eradication. Biochem J. 2018;475:1611–34. doi: 10.1042/BCJ20170164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo SL, Palacios-Callender M, Frakich N, Carcamo S, Kovacs I, Tudzarova S, et al. Molecular basis for the differential use of glucose and glutamine in cell proliferation as revealed by synchronized HeLa cells. Proc Natl Acad Sci USA. 2011;108:21069–74. doi: 10.1073/pnas.1117500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clem BF, O’Neal J, Tapolsky G, Clem AL, Imbert-Fernandez Y, Kerr DA, 2nd, et al. Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther. 2013;12:1461–70. doi: 10.1158/1535-7163.MCT-13-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi L, Pan H, Liu Z, Xie J, Han W. Roles of PFKFB3 in cancer. Signal Transduct Target Ther. 2017;2:17044. doi: 10.1038/sigtrans.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler R, Bleichert F, Warnke J-P, Eschrich K. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB3) is up-regulated in high-grade astrocytomas. J Neuro-Oncol. 2008;86:257–64. doi: 10.1007/s11060-007-9471-7. [DOI] [PubMed] [Google Scholar]
- 6.Li H-M, Yang J-G, Liu Z-J, Wang W-M, Yu Z-L, Ren J-G, et al. Blockage of glycolysis by targeting PFKFB3 suppresses tumor growth and metastasis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36:7. doi: 10.1186/s13046-016-0481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobarykina AY, Minchenko DO, Opentanova IL, Moenner M, Caro J, Esumi H, et al. Hypoxic regulation of PFKFB-3 and PFKFB-4 gene expression in gastric and pancreatic cancer cell lines and expression of PFKFB genes in gastric cancers. Acta Biochim Pol. 2006;53:789–99. doi: 10.18388/abp.2006_3308. [DOI] [PubMed] [Google Scholar]
- 8.Atsumi T, Chesney J, Metz C, Leng L, Donnelly S, Makita Z, et al. High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res. 2002;62:5881–7. [PubMed] [Google Scholar]
- 9.Mondal S, Roy D, Sarkar Bhattacharya S, Jin L, Jung D, Zhang S, et al. Therapeutic targeting of PFKFB3 with a novel glycolytic inhibitor PFK158 promotes lipophagy and chemosensitivity in gynecologic cancers. Int J Cancer. 2019;144:178–89. doi: 10.1002/ijc.31868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar Bhattacharya S, Thirusangu P, Jin L, Roy D, Jung D, Xiao Y, et al. PFKFB3 inhibition reprograms malignant pleural mesothelioma to nutrient stress-induced macropinocytosis and ER stress as independent binary adaptive responses. Cell Death Dis. 2019;10:725. doi: 10.1038/s41419-019-1916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yalcin A, Clem BF, Simmons A, Lane A, Nelson K, Clem AL, et al. Nuclear targeting of 6-phosphofructo-2-kinase (PFKFB3) increases proliferation via cyclin-dependent kinases. J Biol Chem. 2009;284:24223–32. doi: 10.1074/jbc.M109.016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gustafsson NMS, Färnegårdh K, Bonagas N, Ninou AH, Groth P, Wiita E, et al. Targeting PFKFB3 radiosensitizes cancer cells and suppresses homologous recombination. Nat Commun. 2018;9:3872. doi: 10.1038/s41467-018-06287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F-L, Liu J-P, Bao R-X, Yan G, Feng X, Xu Y-P, et al. Acetylation accumulates PFKFB3 in cytoplasm to promote glycolysis and protects cells from cisplatin-induced apoptosis. Nat Commun. 2018;9:508. doi: 10.1038/s41467-018-02950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folmes CD, Dzeja PP, Nelson TJ, Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 2012;11:596–606. doi: 10.1016/j.stem.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang H, Yang J, Lee E, Cheong JH. Metabolism in embryonic and cancer stemness. Arch Pharm Res. 2015;38:381–8. doi: 10.1007/s12272-015-0558-y. [DOI] [PubMed] [Google Scholar]
- 16.Snyder V, Reed-Newman TC, Arnold L, Thomas SM, Anant S. Cancer stem cell metabolism and potential therapeutic targets. Front Oncol. 2018;8:203. doi: 10.3389/fonc.2018.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer.Net. Mesothelioma: Statistics, 2019; https://www.cancer.net/cancer-types/mesothelioma/statistics.
- 18.Villanova F, Procopio A, Rippo MR. Malignant mesothelioma resistance to apoptosis: recent discoveries and their implication for effective therapeutic strategies. Curr Med Chem. 2008;15:631–41. doi: 10.2174/092986708783885273. [DOI] [PubMed] [Google Scholar]
- 19.Cortes-Dericks L, Carboni GL, Schmid RA, Karoubi G. Putative cancer stem cells in malignant pleural mesothelioma show resistance to cisplatin and pemetrexed. Int J Oncol. 2010;37:437–44. doi: 10.3892/ijo_00000692. [DOI] [PubMed] [Google Scholar]
- 20.Song K, Kwon H, Han C, Zhang J, Dash S, Lim K, et al. Active glycolytic metabolism in CD133(+) hepatocellular cancer stem cells: regulation by MIR-122. Oncotarget. 2015;6:40822–35. doi: 10.18632/oncotarget.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamoto H, Fujishima F, Nakamura Y, Zuguchi M, Ozawa Y, Takahashi Y, et al. Significance of CD133 expression in esophageal squamous cell carcinoma. World J Surg Oncol. 2013;11:51. doi: 10.1186/1477-7819-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–67. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Collado M, Villasante A, Matheu A, Lynch CJ, Cañamero M, et al. p27Kip1 directly represses SOX2 during embryonic stem cell differentiation. Cell Stem Cell. 2012;11:845–52. doi: 10.1016/j.stem.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung D, Khurana A, Roy D, Kalogera E, Bakkum-Gamez J, Chien J, et al. Quinacrine upregulates p21/p27 independent of p53 through autophagy-mediated downregulation of p62-Skp2 axis in ovarian cancer. Sci Rep. 2018;8:2487. doi: 10.1038/s41598-018-20531-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkar S, Dutta D, Samanta SK, Bhattacharya K, Pal BC, Li J, et al. Oxidative inhibition of Hsp90 disrupts the super‐chaperone complex and attenuates pancreatic adenocarcinoma in vitro and in vivo. Int J cancer. 2013;132:695–706. doi: 10.1002/ijc.27687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandal C, Sarkar S, Chatterjee U, Schwartz-Albiez R, Mandal C. Disialoganglioside GD3-synthase over expression inhibits survival and angiogenesis of pancreatic cancer cells through cell cycle arrest at S-phase and disruption of integrin-beta1-mediated anchorage. Int J Biochem Cell Biol. 2014;53:162–73. doi: 10.1016/j.biocel.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar S, Mandal C, Sangwan R, Mandal C. Coupling G2/M arrest to the Wnt/β-catenin pathway restrains pancreatic adenocarcinoma. Endocr Relat Cancer. 2014;21:113–25. doi: 10.1530/ERC-13-0315. [DOI] [PubMed] [Google Scholar]
- 29.Thakur B, Ray P. Cisplatin triggers cancer stem cell enrichment in platinum-resistant cells through NF-kappaB-TNFalpha-PIK3CA loop. J Exp Clin Cancer Res. 2017;36:164. doi: 10.1186/s13046-017-0636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phi LTH, Sari IN, Yang YG, Lee SH, Jun N, Kim KS, et al. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018;2018:5416923. doi: 10.1155/2018/5416923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toledo-Guzman ME, Hernandez MI, Gomez-Gallegos AA, Ortiz-Sanchez E. ALDH as a stem cell marker in solid tumors. Curr Stem Cell Res Ther. 2019;14:375–88. doi: 10.2174/1574888X13666180810120012. [DOI] [PubMed] [Google Scholar]
- 32.Clark DW, Palle K. Aldehyde dehydrogenases in cancer stem cells: potential as therapeutic targets. Ann Transl Med. 2016;4:518. doi: 10.21037/atm.2016.11.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori Y, Yamawaki K, Ishiguro T, Yoshihara K, Ueda H, Sato A, et al. ALDH-dependent glycolytic activation mediates stemness and paclitaxel resistance in patient-derived spheroid models of uterine endometrial cancer. Stem Cell Rep. 2019;13:730–46. doi: 10.1016/j.stemcr.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z. CD133: a stem cell biomarker and beyond. Exp Hematol Oncol. 2013;2:17. doi: 10.1186/2162-3619-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Gao Q, Zhou Y, Dier U, Hempel N, Hochwald SN. Focal adhesion kinase-promoted tumor glucose metabolism is associated with a shift of mitochondrial respiration to glycolysis. Oncogene. 2016;35:1926–42. doi: 10.1038/onc.2015.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.News in brief. FAK inhibitor kills mesothelioma cells. Cancer Discov. 2014;4:OF3. 10.1158/2159-8290.CD-NB2014-093. [DOI] [PubMed]
- 37.Wang L, Liu X, Ren Y, Zhang J, Chen J, Zhou W, et al. Cisplatin-enriching cancer stem cells confer multidrug resistance in non-small cell lung cancer via enhancing TRIB1/HDAC activity. Cell Death Dis. 2017;8:e2746–e2746. doi: 10.1038/cddis.2016.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–28. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Ciavardelli D, Rossi C, Barcaroli D, Volpe S, Consalvo A, Zucchelli M, et al. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis. 2014;5:e1336–e1336. doi: 10.1038/cddis.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE. 2014;9:e84941. doi: 10.1371/journal.pone.0084941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y, Zhou Y, Shingu T, Feng L, Chen Z, Ogasawara M, et al. Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. J Biol Chem. 2011;286:32843–53. doi: 10.1074/jbc.M111.260935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen Y-A, Wang C-Y, Hsieh Y-T, Chen Y-J, Wei Y-H. Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle. 2015;14:86–98. doi: 10.4161/15384101.2014.974419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen CL, Uthaya Kumar DB, Punj V, Xu J, Sher L, Tahara SM, et al. NANOG metabolically reprograms tumor-initiating stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab. 2016;23:206–19. doi: 10.1016/j.cmet.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, Peters G, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65:177–85. doi: 10.1158/0008-5472.177.65.1. [DOI] [PubMed] [Google Scholar]
- 45.Nam K, Oh S, Shin I. Ablation of CD44 induces glycolysis-to-oxidative phosphorylation transition via modulation of the c-Src-Akt-LKB1-AMPKalpha pathway. Biochem J. 2016;473:3013–30. doi: 10.1042/BCJ20160613. [DOI] [PubMed] [Google Scholar]
- 46.Tamada M, Nagano O, Tateyama S, Ohmura M, Yae T, Ishimoto T, et al. Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res. 2012;72:1438–48. doi: 10.1158/0008-5472.CAN-11-3024. [DOI] [PubMed] [Google Scholar]
- 47.Schober M, Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-beta and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad Sci USA. 2011;108:10544–9. doi: 10.1073/pnas.1107807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laszlo V, Valko Z, Ozsvar J, Kovacs I, Garay T, Hoda MA, et al. The FAK inhibitor BI 853520 inhibits spheroid formation and orthotopic tumor growth in malignant pleural mesothelioma. J Mol Med. 2019;97:231–42. doi: 10.1007/s00109-018-1725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pachter JA, Kolev VN, Schunselaar L, Shapiro IM, Bueno R, Baas P, et al. Abstract 4236: FAK inhibitor VS-6063 (defactinib) targets mesothelioma cancer stem cells, which are enriched by standard of care chemotherapy. Cancer Res. 2015;75:4236–4236. doi: 10.1158/1538-7445.AM2015-4236. [DOI] [Google Scholar]
- 50.Kolev VN, Tam WF, Wright QG, McDermott SP, Vidal CM, Shapiro IM, et al. Inhibition of FAK kinase activity preferentially targets cancer stem cells. Oncotarget. 2017;8:51733–47. doi: 10.18632/oncotarget.18517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Gao Q, Zhou Y, Dier U, Hempel N, Hochwald SN. Focal adhesion kinase-promoted tumor glucose metabolism is associated with a shift of mitochondrial respiration to glycolysis. Oncogene. 2016;35:1926–42. doi: 10.1038/onc.2015.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu W, Ye L, Zhang J, Yu P, Wang H, Ye Z, et al. PFK15, a small molecule inhibitor of PFKFB3, induces cell cycle arrest, apoptosis and inhibits invasion in gastric cancer. PLoS ONE. 2016;11:e0163768. doi: 10.1371/journal.pone.0163768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banerjee K, Keasey MP, Razskazovskiy V, Visavadiya NP, Jia C, Hagg T. Reduced FAK-Stat3 signaling contributes to ER stress-induced mitochondrial dysfunction and death in endothelial cells. Cell Signal. 2017;36:154–62. doi: 10.1016/j.cellsig.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Visavadiya NP, Keasey MP, Razskazovskiy V, Banerjee K, Jia C, Lovins C, et al. Integrin-FAK signaling rapidly and potently promotes mitochondrial function through Stat3. Cell Commun Signal. 2016;14:32. doi: 10.1186/s12964-016-0157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao F, Connolly DC. Abstract 2095: FAK mediates Stat3 activation, migration and invasion in ovarian carcinoma cells. Cancer Res. 2014;74:2095–2095. doi: 10.1158/1538-7445.AM2014-2095. [DOI] [Google Scholar]
- 56.Thakur R, Trivedi R, Rastogi N, Singh M, Mishra DP. Inhibition of Stat3, FAK and Src mediated signaling reduces cancer stem cell load, tumorigenic potential and metastasis in breast cancer. Sci Rep. 2015;5:10194. doi: 10.1038/srep10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin L, Fuchs J, Li C, Olson V, Bekaii-Saab T, Lin J. Stat3 signaling pathway is necessary for cell survival and tumorsphere forming capacity in ALDH+/CD133+ stem cell-like human colon cancer cells. Biochem Biophys Res Commun. 2011;416:246–51. doi: 10.1016/j.bbrc.2011.10.112. [DOI] [PubMed] [Google Scholar]
- 58.Won C, Kim BH, Yi EH, Choi KJ, Kim EK, Jeong JM, et al. Signal transducer and activator of transcription 3-mediated CD133 up-regulation contributes to promotion of hepatocellular carcinoma. Hepatology. 2015;62:1160–73. doi: 10.1002/hep.27968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garg N, Bakhshinyan D, Venugopal C, Mahendram S, Rosa DA, Vijayakumar T, et al. CD133+ brain tumor-initiating cells are dependent on Stat3 signaling to drive medulloblastoma recurrence. Oncogene. 2017;36:606–17. doi: 10.1038/onc.2016.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei Z, Jiang X, Qiao H, Zhai B, Zhang L, Zhang Q, et al. Stat3 interacts with Skp2/p27/p21 pathway to regulate the motility and invasion of gastric cancer cells. Cell Signal. 2013;25:931–8. doi: 10.1016/j.cellsig.2013.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on a reasonable request.