Abstract
Aryl hydrocarbon receptor-interacting protein (AIP) is a co-chaperone to heat shock proteins and nuclear receptors. Loss-of-function heterozygote germline mutations lead to predisposition to growth hormone- or prolactin-secreting pituitary typically presenting in childhood. Based on these data AIP behaves as a tumour suppressor. However, previously in diffuse large B cell lymphoma and now in this new manuscript in the British Journal of Cancer on colorectal cancer, it seems that high expression of AIP is associated with tumour development and more aggressive disease. AIP, therefore, joins a distinguished group of proteins that can behave both as a tumour suppressor and as an oncogene.
Subject terms: Cancer genetics, Endocrine cancer
Aryl hydrocarbon receptor-interacting protein (AIP) is a relatively little-known co-chaperone of HSP90 and HSC70 that, to date, is best known as a tumour suppressor within the pituitary. Heterozygous loss-of-function mutations in AIP can lead to the development childhood-onset pituitary adenomas (pituitary neuroendocrine tumours), usually secreting excessive amounts of growth hormone and resulting in gigantism [1]. AIP is evolutionarily highly conserved [2] and biallelic loss in animal models leads to embryonic lethality. These data suggest that it has a key role in normal cell function, but currently little is known what the potentially numerous functions of AIP are.
Recently, Solís-Fernández et al. have reported that increased expression of AIP is associated with promoting colorectal cancer and liver metastasis [3]. Examining The Cancer Genome Atlas (TCGA) data, they found that high AIP expression was linked to decreased survival and increased risk of relapse. To explore this observation in more detail, the authors conducted in vitro experiments over-expressing AIP in colon epithelial cells, and found that increased AIP expression resulted in increased migration and was associated with an epithelial-to-mesenchymal transition, a characteristic of metastatic cancer cells. The authors also used an in vivo model of metastatic colorectal cancer by injecting tumour cells over-expressing AIP into the spleen of recipient mice, and found that cells over-expressing AIP had metastasised to the liver and this was associated with decreased survival, thereby recapitulating the findings in humans that increased AIP expression leads to an increased risk of colorectal cancer metastasis and decreased survival.
Although AIP behaves as a tumour suppressor in the pituitary, the publication by Solís-Fernández et al. is not the first time that AIP has been found to be a tumour promoter. Increased expression of AIP was found to be highly expressed in diffuse large B cell lymphoma (DLBCL), and was associated with increased survival of DLBCL cells and decreased survival of patients with this aggressive, difficult-to-treat lymphoma [4]. More recently, high AIP expression was found to be a prognostic marker for cholangiocarcinoma [5].
The tumour suppressor role of AIP in the pituitary is supported by the loss-of-function germline mutations and loss-of-heterozygosity in the tumour tissue. Several tumorigenic mechanisms have been suggested, including stimulation of the tumour suppressor ZAC [6] or the inhibitory G protein Gαi2 [7]. More recently, an exciting mechanism has been put forward which explains not just the tumorigenic effect of AIP deficiency but also the tissue-specific nature of the tumorigenesis [8]: AIP supports the dependence receptor function of RET. AIP is part of a complex with monomeric-intracellular-RET, caspase-3 and PKCδ resulting in a balanced PIT1/CDKN2A-ARF/p53-pathway-induced apoptosis. The specificity is provided by the co-expression of RET and PIT1 in the same cell, only present in somatotroph and some lactotroph cells.
For the oncogenic effects, protein over-expression rather than activating mutations were found to be present, and this is also seen in the TCGA data. However, the precise molecular mechanisms by which excess AIP promotes tumour survival remain unclear. In DLBLC, AIP protected BCL6, a key molecule in B cell survival, from ubiquitin-mediated proteasomal degradation by the E3-ubiquitin ligase FBXO11 by binding to the deubiquitinase UCHL1; this helps to maintain the expression of BCL6 by supporting deubiquitination of BCL6. In colorectal cancer, they found upregulation of cadherin 17 and stimulation of the AKT, SRC and JNK kinase pathways, but it remains unclear if the increased expression of these molecules is via transcriptional or post-transcriptional mechanisms.
AIP has joined a distinguished group of proteins that can behave both as a tumour suppressor and as an oncogene [9, 10] (Fig. 1). Numerous transcription factors, kinases and other proteins have been found to have a similar dual role. These include TP53, PTEN, RUNX1, DNMT1, FOXO1, GLI1, HDAC1, NOTCH1, PAX5, TCF3, MAP3K8, RHOA, PTPN11, and many others [9, 10]. Understanding the context, location and circumstances as to whether a gene is oncogenic or tumour suppressive is important not only for diagnosis and for the potential therapeutic targeting of the gene in question, but also for understanding the molecular pathobiology of the tumour.
Fig. 1. AIP as a double agent.
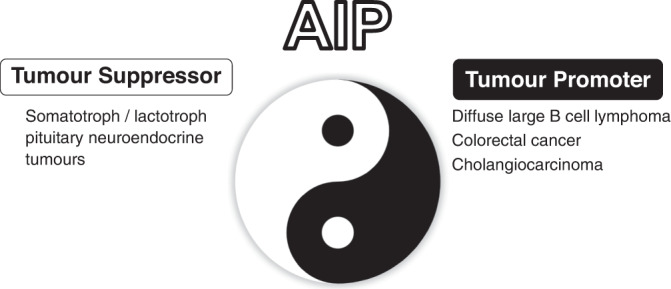
AIP can behave both as a tumour suppressor, as in somatotroph and lactotroph pituitary neuroendocrine tumours, or as an oncogene as in diffuse large B cell lymphomas or colorectal tumours.
As a co-chaperone to heat shock proteins, and as partner of various nuclear receptors and diverse group of other proteins [11, 12], it is now becoming clear that AIP can impact various molecular pathways [11]. Little is known on AIP regulation [13, 14], if it is part of any feedback loops or if any counter-regulation would bring beneficial effects. Further studies are clearly needed to identify the molecules and signalling pathways supported by AIP, and to find out if manipulating AIP can unleash novel therapeutic pathways.
Author contributions
The two authors have written the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Editorial on Solís-Fernández et al. “Aryl hydrocarbon receptor-interacting protein regulates 2 tumorigenic and metastatic properties of colorectal cancer cells driving liver metastasis”.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312:1228–30. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]
- 2.Aflorei ED, Klapholz B, Chen C, Radian S, Dragu AN, Moderau N, et al. In vivo bioassay to test the pathogenicity of missense human AIP variants. J Med Genet. 2018;55:522–9. doi: 10.1136/jmedgenet-2017-105191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solis-Fernandez G, Montero-Calle A, Sanchez-Martinez M, Pelaez-Garcia A, Fernandez-Acenero MJ, Pallares P, et al. Aryl-hydrocarbon receptor-interacting protein regulates tumorigenic and metastatic properties of colorectal cancer cells driving liver metastasis. Br J Cancer. 2022;126:1604–15. [DOI] [PMC free article] [PubMed]
- 4.Sun D, Stopka-Farooqui U, Barry S, Aksoy E, Parsonage G, Vossenkamper A, et al. Aryl hydrocarbon receptor interacting protein maintains germinal center B cells through suppression of BCL6 degradation. Cell Rep. 2019;27:1461–71 e1464. doi: 10.1016/j.celrep.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu H, Zhao H, Wang J, Zhao S, Ma C, Wang D, et al. Potential prognosis index for m(6)A-related mRNA in cholangiocarcinoma. BMC Cancer. 2022;22:620. doi: 10.1186/s12885-022-09665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chahal HS, Trivellin G, Leontiou CA, Alband N, Fowkes RC, Tahir A, et al. Somatostatin analogs modulate AIP in somatotroph adenomas: the role of the ZAC1 pathway. J Clin Endocrinol Metab. 2012;97:E1411–1420. doi: 10.1210/jc.2012-1111. [DOI] [PubMed] [Google Scholar]
- 7.Tuominen I, Heliovaara E, Raitila A, Rautiainen MR, Mehine M, Katainen R, et al. AIP inactivation leads to pituitary tumorigenesis through defective Galphai-cAMP signaling. Oncogene. 2015;34:1174–84. doi: 10.1038/onc.2014.50. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Rendueles AR, Chenlo M, Oroz-Gonjar F, Solomou A, Mistry A, Barry S, et al. RET signalling provides tumorigenic mechanism and tissue specificity for AIP-related somatotrophinomas. Oncogene. 2021;40:6354–68. doi: 10.1038/s41388-021-02009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen L, Shi Q, Wang W. Double agents: genes with both oncogenic and tumor-suppressor functions. Oncogenesis. 2018;7:25. doi: 10.1038/s41389-018-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datta, N, Chakraborty, S, Basu, M & Ghosh, MK Tumor Suppressors Having Oncogenic Functions: The Double Agents. Cells 2020;10:46. [DOI] [PMC free article] [PubMed]
- 11.Trivellin G, Korbonits M. AIP and its interacting partners. J Endocrinol. 2011;210:137–55. doi: 10.1530/JOE-11-0054. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Ramirez LC, Morgan RML, Barry S, D’Acquisto F, Prodromou C, Korbonits M. Multi-chaperone function modulation and association with cytoskeletal proteins are key features of the function of AIP in the pituitary gland. Oncotarget. 2018;9:9177–98. doi: 10.18632/oncotarget.24183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dénes J, Kasuki L, Trivellin G, Colli LM, Takiya CM, Stiles CE, et al. Regulation of aryl hydrocarbon receptor-interacting protein (AIP) protein expression by miR-34a in sporadic somatotropinomas. PLoS One. 2015;10:e0117107. doi: 10.1371/journal.pone.0117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai, F, Chen, S, Yu, X, Zhang, J, Liang, W, Zhang, Y, et al. Transcription factor GTF2B regulates AIP protein expression in growth hormone-secreting pituitary adenomas and influences tumor phenotypes. Neuro Oncol. 10.1093/neuonc/noab291 (2021). [DOI] [PMC free article] [PubMed]


