Abstract
While most tissues exhibit their greatest growth during development, adipose tissue is capable of additional massive expansion in adults. Adipose tissue expandability is advantageous when temporarily storing fuel for use during fasting, but becomes pathological upon continuous food intake, leading to obesity and its many comorbidities. The dense vasculature of adipose tissue provides necessary oxygen and nutrients, and supports delivery of fuel to and from adipocytes under fed or fasting conditions. Moreover, the vasculature of adipose tissue comprises a major niche for multipotent progenitor cells, which give rise to new adipocytes and are necessary for tissue repair. Given the multiple, pivotal roles of the adipose tissue vasculature, impairments in angiogenic capacity may underlie obesity-associated diseases such as diabetes and cardiometabolic disease. Exciting new studies on the single-cell and single-nuclei composition of adipose tissues in mouse and humans are providing new insights into mechanisms of adipose tissue angiogenesis. Moreover, new modes of intercellular communication involving micro vesicle and exosome transfer of proteins, nucleic acids and organelles are also being recognized to play key roles. This review focuses on new insights on the cellular and signaling mechanisms underlying adipose tissue angiogenesis, and on their impact on obesity and its pathophysiological consequences.
Keywords: Endothelial, Adipocyte, ADSC, Diabetes, Hypoxia, Collagen, Extracellular matrix
Functions of adipose tissues
The defining feature of adipose tissue is the presence of adipocytes, which have the unique capacity to accumulate large amounts of lipid within specialized droplets, and give adipose depots their characteristic appearance. Despite a similar morphology, adipocytes comprise a heterogenous population of cells, and different types of adipocytes in each depot enable specialized functions [1–3]. For example, thermogenic adipocytes in adipose depots that surround central organs play a critical role in maintaining core body temperature. In another example, subcutaneous adipose tissue (SqAT), the largest depot, is best able to expand in response to increased food intake. Other depots are more restricted in their ability to expand, and have other functions, including immunological surveillance by mesenteric adipose tissue [4], and regulation of vessel tone by perivascular adipose tissue [1]. The roles of other adipose tissue depots, such as those in the bone marrow, are still being defined [5, 6].
In adults, SqAT mass is closely associated with metabolic disease risk. Paradoxically, the ability to expand superficial SqAT, particularly in the lower body, is associated with a lessened risk of metabolic disease. In the early 1980s Smith et al. analyzed 930 men and women and concluded that, for a given body mass index (BMI), visceral adiposity, reflected by waist circumference, was associated with increased insulin resistance and risk of developing type 2 diabetes and cardiovascular disease [7]. However, it is now recognized that visceral adiposity is a surrogate for the many sites within the body that accumulate fat when the SqAT cannot expand. In a recent review, Piche et al. [8] discuss the idea that pathogenic obesity, defined as causing prejudice to health, can no longer be evaluated solely by the body mass index. Even individuals who have normal weight are at high risk of metabolic disease if they accumulate fat in normally lean tissues such as liver, heart, and skeletal muscle. Conversely, individuals who are overweight or obese can be at much lower risk of metabolic disease if they have the ability to expand their SqAT mass, particularly in the gluteal-femoral area, and therefore protect other organs [8, 9].
The prevailing hypothesis explaining the paradoxical association between increased superficial SqAT mass and protection from metabolic disease is that this depot is optimally suited to store lipids. Under chronic caloric excess, energy is converted into fat through de-novo lipogenesis in the liver, and is transported via lipoproteins to adipose tissue for storage. In the absence of sufficient functional SqAT, fat accumulates in other adipose depots and in lean tissues, leading to cellular stress, inflammation and insulin resistance, setting the stage for the development of metabolic disease [10].
The urgency of understanding and mitigating metabolic disease is high; global deaths and disability-adjusted life years doubled from 1990 to 2017 [11], and a recent study found that genetically predicted higher BMI was associated with increased risk of type 2 diabetes mellitus, multiple circulatory disease outcomes including ischemic heart disease, asthma, chronic obstructive pulmonary disease, five digestive system diseases including non-alcoholic liver disease, three musculoskeletal system diseases, and multiple sclerosis as well as cancers of the digestive system (six cancer sites), uterus, kidney, and bladder [12]. Understanding the factors that control the development of SqAT and its capacity to protect lean organs from lipotoxicity is critically important in our efforts to mitigate metabolic disease pathogenesis.
Mechanisms of adipose tissue expansion
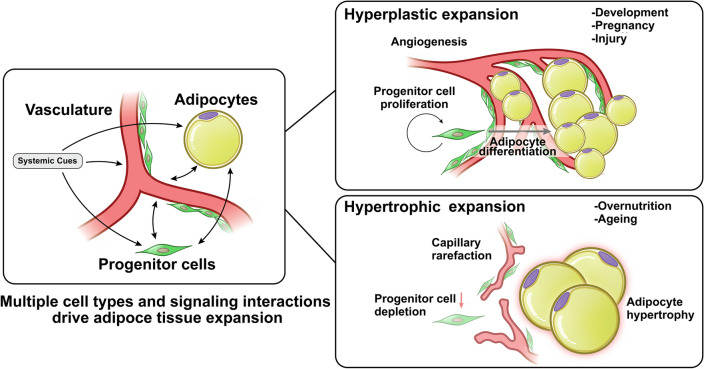
Adipose tissue can expand through two different mechanisms: hyperplasia and hypertrophy. Hyperplasia entails the de-novo differentiation of adipocytes from progenitor cells, resulting in an enlarged adipose tissue mass comprised of numerous, small adipocytes (Fig. 1). A second mechanism of adipose tissue expansion is through hypertrophy, where the size of each existing adipocyte increases to accommodate increasing amounts of fuel, resulting in an enlarged tissue mass composed of fewer, large adipocytes. These two very different expansion modes predominate under different circumstances; hyperplastic adipose tissue expansion occurs mostly during development, and is likely to be defined by genetic mechanisms that influence the number of progenitor cells and their ability to differentiate into appropriate adipocyte subtypes [13]. Hypertrophic expansion occurs mostly post-developmentally, and is dependent on the ability of existing adipocytes to capture and retain circulating lipids [14]. As discussed below, however, both hyperplasia and hypertrophy can contribute to adipose tissue expansion in adults, and the predominance of each mechanism can vary between individuals and between depots. Importantly, the degree to which hypertrophy predominates is associated with higher metabolic disease risk [15].
Fig. 1.
Modes of adipose tissue expansion. Adipose tissue expansion involves numerous cell types, including endothelial cells of the vasculature, adipocytes and multipotent progenitor cells. Under some conditions, angiogenesis, progenitor cell proliferation and adipocyte differentiation lead to hyperplastic expansion, forming tissue containing multiple small adipocytes. Under other conditions, angiogenesis fails, leading to capillary rarefaction, impaired multipotent progenitor cells proliferation and adipocyte hypertrophy. Hypertrophic expansion is strongly associated with metabolic disease risk
Hyperplastic and hypertrophic growth must be accompanied by concomitant increases in supporting structures, including vasculature and innervation, but the mechanisms underlying angiogenic expansion during hyperplastic or hypertrophic growth are fundamentally different. Critically, as detailed below, angiogenesis precedes hyperplastic expansion, ensuring an adequate blood supply to the developing tissue, but follows hypertrophic adipocyte growth and is often insufficient, leading to hypoxia and tissue dysfunction [16, 17]. The mechanisms of angiogenesis operating under either hyperplastic or hypertrophic adipose tissue growth must be considered when evaluating the effects of pro- or anti-angiogenic perturbations on adipose tissue mass and its consequences to systemic metabolism. As discussed further below, suppression of angiogenesis during development, or during hyperplastic or hypertrophic growth in adults, could have very different effects on adipose tissue composition and function. Thus, understanding angiogenic mechanisms accompanying hyperplastic or hypertrophic expansion is critical for conceptualizing therapeutic approaches to metabolic disease centered on adipose tissue angiogenesis.
Angiogenesis during hyperplastic adipose tissue expansion
Adipose tissue development during embryogenesis involves the emergence of lipid-laden adipocytes differentiating from progenitor cells from distinct embryonic layers [18]. In multiple species including pig, rodents and humans, distinct stages of adipose tissue development have been delineated: First proliferation of primitive blood vessels is seen within loose connective tissue, second, mesenchymal cells within the vascular matrix accumulate fine fat vacuoles in their cytoplasm, and finally, clear adipocytes and fat lobules are formed [19–21]. More recent morphological analysis of epicardial adipose tissue during human development [22] reveals similar stages, with the appearance of mesenchyme at 33–35 gestational days, followed by angiogenesis at 42–45 gestational days, followed by appearance of multilocular adipocytes in primitive fat lobules, and finally by appearance of unilocular adipocytes in definitive fat lobules. Thus, in all morphological studies of adipose tissue development, including multiple species and depots, angiogenesis precedes the appearance of lipid-laden adipocytes.
Despite its predominance during development, hyperplastic adipose tissue expansion can also occur in adult animals. In some depots, for example the mouse epididymal fat pad, adipocyte formation continues after birth and is also preceded by rapid expansion of a dense vascular network within the tip of the fat pad [23, 24]. The cellularity of the developing tissue is determined by the rate of proliferation of adipocyte progenitors, which is responsive to dietary lipid composition [13]. In another example, the expansion of the interscapular brown fat depot in rodents in response to cold acclimation involves the generation of new brown adipocytes, and is critically dependent on sympathetic nerve activity and angiogenesis [25, 26]. In a different context, sympathetic innervation has been shown to activate the angiogenic switch that fuels exponential tumor growth [27]. Analogously, the adrenergic response to cold may induce an angiogenic switch that allows proliferation and differentiation of brown adipocyte progenitors, but this has not been confirmed in either mice or humans.
The interdependency between blood vessel expansion and hyperplastic adipose tissue expansion can be explained by lineage tracing studies revealing that adipocyte progenitors are found to be tightly associated with the vasculature [28–33]. Direct observation of embryonic mouse adipose tissue development also revealed proliferating preadipocytes residing as clusters distributed along the growing adipose vasculature [34]. A functional relationship between the microvasculature development and adipocyte progenitor proliferation is supported by the finding that culture conditions that promote angiogenesis also promote the proliferation of mesenchymal progenitors, which are tightly associated with emergent capillary sprouts [35, 36].
In human adults, the capacity for adipocyte hyperplasia varies markedly between depots and between subjects. For example, in response to experimental overfeeding in healthy, normal-weight adults abdominal SqAT adipocyte size increased and correlated with relative upper-body fat gain, but lower-body fat responded by hyperplasia [15]. Because measurements of adipocyte size in human tissue samples can vary dependent on histological technique, hyperplasia is defined as the proportion of adipocytes falling in the lower distribution of adipocyte size, relative to total body adiposity [37–42]. A positive correlation between adipocyte hyperplasia and metabolic health has been observed in many studies [43–45].
The mechanisms underlying individual variation in hyperplastic adipose tissue expansion are not known, but angiogenic factors may play critical roles. Genes involved in angiogenic patterning affect adipose tissue function [46]; for example, forkhead box C2 (FOXC2) in adipose tissue affects angiogenesis and vascular patterning [47], and is associated with adipose tissue function and obesity [48]. The apelin/apelin receptor signaling pathway, discovered as a vasculogenic guiding factor in zebra fish [49], is an important regulator of insulin sensitivity, glucose utilization and diabetes risk [50–52], and indirect evidence suggests apelin regulates adipose tissue vasculature and adipocyte size [53].
In addition to developmental patterning, adipose tissue cellularity is responsive to systemic signals that regulate growth and metabolic needs of the body. Among the central mechanisms that regulate body size and metabolism is the insulin-like growth factor-1 (IGF-1) signaling pathway [54]. IGF-1 is expressed around capillaries, in small fat cells, and in fibroblasts in fetal adipose tissue [55], and strongly stimulates endothelial cell proliferation and angiogenesis [56–61]. In vivo, mice in which insulin and IGF-1 signaling were abrogated in a tissue-selective manner demonstrated a critical need for IGF-1 signaling for adipose tissue development [62]. Insight into the role of IGF-1 in human adipose tissue comes from patients with deficiency of growth hormone (GH), which induces IGF-1 [63]. Adipose tissue of adult individuals with GH deficiency contain extremely large adipocytes, accompanied by decreased tissue concentration of vascular endothelial growth factor-A (VEGFA), stromal cell-derived factor (SDF1), angiopoietin 2 and BDNF, consistent with impaired angiogenesis [40]. Similarly, children with GH deficiency have an increased mean adipocyte volume but a reduced number of fat cells which normalized upon GH treatment [64]. In vitro, inhibition of IGF-1 receptor signaling completely abrogates proliferation of mesenchymal progenitors and endothelial cells from human adipose tissue [56].
Special conditions of hyperplastic adipose tissue growth in adults are pregnancy [65] and injury, for example following myocardial infarction. Adipose tissue hyperplasia in pregnancy is associated with placental mechanisms regulating adipose tissue IGF-1 bioavailability, which are associated with protection from gestational diabetes [56, 66]. Generation of epicardial adipose tissue following injury has been found to be critically dependent on IGF-1 receptor signaling [67]. In aggregate, in vitro and in vivo evidence in both mice and humans points of a critical role of IGF-1 signaling in mediating adipose tissue angiogenesis and hyperplastic adipose tissue expansion.
Angiogenesis and hypertrophic adipose tissue expansion
In the absence of hyperplastic expansion, excess caloric intake leads to adipocyte hypertrophy (Fig. 1). In mice, high calorie, high fat diets or hyperphagia lead to adipocyte hypertrophy in the epidydimal adipose depot [68–70], which is accompanied by disruption in capillary architecture and blood flow, leading to hypoxia [71]. Similar thinning and disruption of blood vessel architecture is seen in response to high calorie, high fat diet in mouse brown fat [72]. Adipocyte hypertrophy in response to overfeeding is also seen in humans, observed preferentially in abdominal adipocytes [37, 73–75]. Most studies find that increased adipocyte size is accompanied by capillary rarefaction in both SqAT and visceral adipose tissue (VAT), and is enhanced in subjects with Type 2 diabetes mellitus (T2DM) [74, 76]. In general, the degree of hypertrophy of abdominal adipocytes correlated inversely with metrics of insulin sensitivity.
The studies above concur in finding a strong correlation between adipocyte hypertrophy and impaired capillary architecture, but the cause-effect associations are not understood. One possibility is that adipocyte hypertrophy causes capillary insufficiency, by for example by failing to elicit adequate angiogenic signaling or by creating a niche that is non-permissive for angiogenesis, resulting in capillary rarefaction relative to adipose mass [77–79]. Alternatively, high caloric intake may directly disrupt angiogenic pathways and capillary function, and these alterations may cause adipocyte hypertrophy, for example by limiting removal of fatty acids from the extracellular space. A third possibility is that overfeeding affects both adipocyte and the capillary functions, leading to the observed phenotype of enlarged adipocytes and disrupted capillary networks. Mechanistically, VEGF-A165b has been reported to act as an anti-angiogenic factor increased in obesity [80], and an anti-angiogenic effect of hypertrophied cells has been reported to operate via TWIST-SLIT2 signaling [81]. Moreover, as further discussed below, hypertrophied adipocytes produce collagen types that can impair angiogenesis [77, 82, 83].
Impaired angiogenesis, originating through effects on the vasculature, on the adipocyte, or on both is likely to be a contributor to metabolic dysfunction associated with adipocyte hypertrophy in obesity [84]. This possibility is supported by findings that experimentally enhancing angiogenesis, for example through enforced overexpression of VEGFA, increases capillary density, reduces adipocyte size, and alleviates the metabolic deficits associated with adipocyte hypertrophy [85–87]. These findings suggest that approaches to improve the angiogenic capacity of hypertrophied adipose tissue in obesity may be of therapeutic value. In support of this view, PPARγ activation has been shown to indirectly elicit angiogenesis [88–90], and nanoparticles that leverage PPARγ activators to elicit angiogenesis in adipose tissue have shown to produce metabolic improvements in mice after experimental overfeeding [91]. Further research to identify pro- and anti-angiogenic mechanisms that accompany adipocyte hypertrophy will help identify additional targets that can elicit similar beneficial effects.
What do the transcriptomes of adipose tissue cells tell us about angiogenesis?
Multiple cell types are likely to regulate angiogenesis in adipose tissue. While adipocytes occupy most of the volume, they represent less than 50% of the total cell composition of adipose tissue, the remainder including progenitor cells, endothelial cells, fibroblasts and immune cells [92]. The large volume and buoyancy of adipocytes makes them difficult to separate from the stromovascular components, complicating their analysis. Nevertheless, exciting recent progress in single-cell and single-nuclei transcriptomics conducted on adipose depots [93–96], provides a new grasp on potential mechanisms of adipose tissue angiogenesis. It is important to note that these insights are bioinformatically-predicted and are not experimentally proven; nevertheless, they provide a valid framework for deriving new hypotheses on the cellular composition of adipose tissues and on the cell–cell interactions important for function. In this section we explore available single-cell and single nuclei studies with a focus on the data we believe to be relevant to our understanding of adipose tissue angiogenesis and the effects of obesity.
In a recent comprehensive study, Emont et al. provided single cell and single nuclei RNA transcriptomic data from subcutaneous and visceral depots of both humans and mice, allowing inter-depot and inter-species comparisons [93]. Its is important to note that single-cell nuclei RNASeq is restricted to transcripts that remain in the nucleus, thus levels of gene expression in these data sets are influenced by post-transcriptional kinetics and may not reflect total transcript expression for any particular gene. Nevertheless, concordance between single-cell and single nuclei RNASeq is sufficient to allow classification of cells and estimation of cell proportions within the tissue. An interesting aspect of the comparison between mouse and human depots data pertains to the proportion of all cells in adipose tissue that correspond to endothelial cells. The proportion of endothelial cells, as percent of all cells sequenced, appears to be significantly higher in human than mouse adipose tissue (~ 0.2% in human, 0.03–0.06% in mouse tissues). This species-dependent difference may be attributable at least in part to a higher overall infiltration of mouse adipose tissue depots by immune cells. This finding of a comparatively low proportion of endothelial cells (~ 0.01%) in mouse fat was also seen by Sarvari et. al. [97].
Differences in the relative proportion of endothelial cells are also seen between depots within the same species. In humans with BMI < 30, the proportion of endothelial cells relative to all tissue cells was ~ 0.2% in SqAT and ~ 0.07% in visceral adipose tissue, attributable in part by the presence of mesothelial cells in visceral adipose tissue. In both depots, the proportion of endothelial cells relative to all cells in the tissue decreased to approximately half in individuals with BMI > 40 (Emont et al., Extended Data Fig. 1). The observed decrease in the relative proportion of endothelial cells in response to obesity may be relevant to tissue homeostasis, as it may translate into a deficit in perfusion relative to tissue requirements. The lower proportion of endothelial cells is also consistent with capillary rarefaction and microvascular damage in response to obesity reported by other methods [78].
In mice, high fat diet feeding for 13 weeks resulted in large infiltration of visceral (epidydimal) adipose depot by immune cells, with consequent proportional decrease in endothelial cells (Extended Data Fig. 2 in Emont, Jacobs et al.) A similar massive immune cell infiltration after prolonged high-fat diet was seen in study by Sarvari et al. [97]. Sarvari et al. further analyzed mesothelial and endothelial cells, finding that they induced a large proportion of the inflammatory genes induced by high-fat diet [97]. These data highlight that overfeeding has a significant effect on the mouse adipose tissue endothelium, with likely consequences for angiogenesis.
Fig. 2.
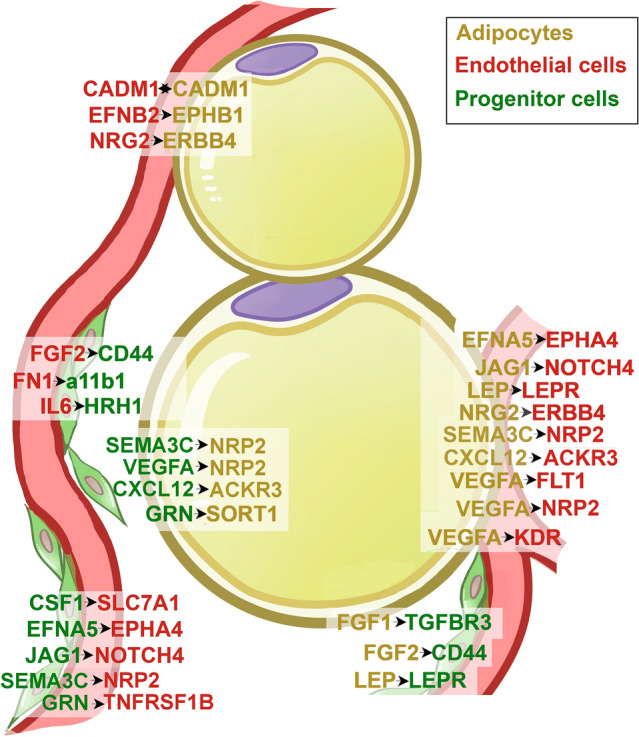
Predicted interactions between adipocytes, endothelial cells and multipotent progenitor cells in human subcutaneous adipose tissue, not including collagens. Cell type interaction analysis was performed using expression data from Emont et al. [93]. The human adipose tissue single-cell/single-nuclei Seurat object (human_all.rds) was retrieved and used to identify the top 5000 most variable features across the dataset using the Seurat FindVariableFeature function. The gene symbol of the top 5000 variable features were translated into Ensembl gene ID using biomaRt (Ensembl Genes 106, hsapiens_gene_ensembl dataset). Normalized count tables for the top features with cells from human SqAT of donors with BMI ≤ 30 or BMI > 40 were exported into tab delimited files for CellPhoneDB analyses. Interaction analysis with CellPhoneDB v3.0.0 was executed in python 3.8.3 environment using the statistical_analysis method and default settings (database release v2.0.0). Gene symbols are depicted in yellow, green or red as a function of their enrichment in adipocytes, multipotent progenitor cells or endothelial cells, respectively. The direction of the interaction (ligand → receptor) is depicted by the arrows
Endothelial cells in human visceral and subcutaneous adipose depots were analyzed in depth by Vijay et al. [96], who analyzed the stromal-vascular fraction of adipose tissue from 12 visceral and 13 abdominal subcutaneous samples derived from 14 individuals with an average BMI of 41 [96]. They identify three types of endothelial cells, one which expressed classical endothelial markers, another expressing genes involved in lipid metabolism, and a third population expressing LYVE1, a marker of lymphatic endothelial cells. Their studies reveal that lymphatic vasculature represents 78% of endothelial cells in visceral adipose tissue from obese subjects. Lymphatic vasculature appears in response to inflammation, and its enhancement by genetic overexpression of VEGD in mice decreased inflammation in response to high fat diet feeding [98]. These results suggest that both angiogenesis and lymph-angiogenesis might be affected by obesity, with distinct physiological consequences such as increased inflammation.
Information on the responses of adipocytes to obesity can also be inferred from these studies. Extracted data from Supplementary Table 5 from Emont et al. [93], which represents the average expression of genes in adipocyte clusters from mouse or human depots, either under different dietary conditions (mouse) or from individuals with different BMIs (human) shows 8 adipocyte genes to be decreased in expression level across both visceral and subcutaneous depots of both human and mouse, while no genes are increased across all depots and species (Table 1). The genes that show decreased expression correspond to ADIPOQ, VEGFA, IGF1, FGFR1, MET, NAMPT, SCTR and PRLR. It is striking that three of these correspond to secreted proteins/growth factors (ADIPOQ, VEGFA, IGF1) which have been directly associated with angiogenesis. Further experiments to confirm that these levels of expression translate into decreased protein secretion from adipocytes are necessary to determine whether these findings might be functionally significant.
Table 1.
Genes affected by obesity across depots and species
| Gene | Mouse | Human | ||||||
|---|---|---|---|---|---|---|---|---|
| Subcutaneous | Visceral | Subcutaneous | Visceral | |||||
| Normal diet | High fat diet | Normal diet | High fat diet | BMI < 30 | BMI > 40 | BMI < 30 | BMI > 40 | |
| ADIPOQ | 4.18 | 1.35 | 4.66 | 1.62 | 9.81 | 4.93 | 13.71 | 5.19 |
| VEGFA | 6.11 | 4.55 | 6.01 | 3.42 | 1.54 | 1.15 | 1.43 | 0.80 |
| IGF1 | 5.67 | 4.13 | 7.44 | 2.63 | 4.56 | 1.21 | 5.02 | 0.86 |
| FGFR1 | 6.60 | 3.63 | 5.25 | 3.23 | 1.58 | 1.00 | 0.99 | 0.76 |
| MET | 1.74 | 1.02 | 1.91 | 1.06 | 0.18 | 0.10 | 0.32 | 0.14 |
| NAMPT | 1.22 | 0.88 | 1.09 | 0.73 | 1.76 | 0.54 | 0.74 | 0.30 |
| SCTR | 2.02 | 0.31 | 1.35 | 0.68 | 0.42 | 0.27 | 0.44 | 0.29 |
| PRLR | 0.35 | 0.15 | 3.68 | 0.93 | 0.02 | 0.01 | 0.04 | 0.01 |
Values were extracted from Supplementary Table 5 from Emont et al., representing average expression of genes in adipocyte clusters from mouse or human depots under different dietary conditions (mouse) or from individuals with different BMIs (human) [93]. Genes shown are all decreased by obesity. No genes were found to be increased by obesity across both depots in both species
Transcriptomic data at single cell resolution can also be used to predict potential interactions between different cell types by examining the expression levels of genes for receptor/ligand pairs. CellPhoneDB is a repository of ligands, receptors and their interactions based on public resources and manual curation of specific families of proteins involved in cell–cell communication, which also considers the subunit architecture for both ligands and receptors to represent heteromeric complexes [99]. Using this resource, Emont et al. [93] report potential interaction networks between cell types in human adipose tissue (Supplementary Table 4 in Emont et al.) including endothelial and progenitor cells. These may be physiologically relevant for angiogenesis given the known perivascular localization of progenitor cells [28–33]. To reveal which predicted molecular interactions might underlie endothelial-progenitor cell networks, we re-analyzed the primary data and find multiple collagen/integrin and growth-factor/receptor pairs classically associated with angiogenesis, but for which very little or no information exists in the context of adipose tissue (Table 2).
Table 2.
Predicted interactions between multipotent progenitor cells and endothelial cells in human subcutaneous and visceral adipose tissues
| Progenitor cells to endothelial cells | Endothelial cells to progenitor cells | ||||||
|---|---|---|---|---|---|---|---|
| SAT | VAT | SAT | VAT | ||||
| COL1A2_a1b1 | 1.079 | COL1A2_a1b1 | 0.568 | COL4A2_a11b1 | 0.434 | COL15A1_a11b1 | 0.181 |
| COL3A1_a1b1 | 0.881 | COL6A3_a1b1 | 0.537 | COL4A1_a11b1 | 0.415 | COL15A1_a1b1 | 0.127 |
| COL6A3_a1b1 | 0.685 | COL5A2_a1b1 | 0.458 | COL15A1_a11b1 | 0.277 | FGF2_CD44 | 0.181 |
| COL1A1_a1b1 | 0.68 | COL4A2_a1b1 | 0.445 | COL8A1_a11b1 | 0.243 | FN1_a11b1 | 0.222 |
| COL6A2_a1b1 | 0.585 | COL6A2_a1b1 | 0.443 | COL21A1_a11b1 | 0.188 | KDR_VEGFC | 0.203 |
| COL5A2_a1b1 | 0.491 | COL3A1_a1b1 | 0.428 | CXCL2_DPP4 | 0.147 | TEK_ANGPT1 | 0.288 |
| COL4A2_a1b1 | 0.414 | COL4A1_a1b1 | 0.396 | FGF2_CD44 | 0.358 | TNFRSF1B_GRN | 0.148 |
| COL4A1_a1b1 | 0.413 | COL12A1_a1b1 | 0.348 | FN1_a11b1 | 0.34 | ||
| COL14A1_a1b1 | 0.347 | COL1A1_a1b1 | 0.32 | IL6_HRH1 | 0.166 | ||
| COL12A1_a1b1 | 0.346 | COL15A1_a1b1 | 0.276 | NRP2_SEMA3C | 0.348 | ||
| COL15A1_a1b1 | 0.29 | COL14A1_a1b1 | 0.217 | TNFRSF1B_GRN | 0.196 | ||
| COL6A6_a1b1 | 0.27 | COL21A1_a1b1 | 0.213 | ||||
| CSF1_SLC7A1 | 0.303 | COL4A5_a1b1 | 0.213 | ||||
| EFNA5_EPHA4 | 0.261 | COL6A6_a1b1 | 0.211 | ||||
| JAG1_NOTCH4 | 0.118 | COL5A3_a1b1 | 0.19 | ||||
| JAG1_NOTCH4 | 0.25 | ||||||
| ACKR3_CXCL12 | 0.132 | ||||||
Cell type interaction analysis was performed using expression data from Emont et al. [93]. The human adipose tissue single-cell/single-nuclei Seurat object (human_all.rds) was retrieved and used to identify the top 5000 most variable features across the dataset using the Seurat FindVariableFeature function. The gene symbol of the top 5000 variable features were translated into Ensembl gene ID using biomaRt (Ensembl Genes 106, hsapiens_gene_ensembl dataset). Normalized count tables for the top features were exported into tab delimited files for CellPhoneDB analyses. Interaction analysis with CellPhoneDB v3.0.0 was executed in python 3.8.3 environment using the statistical_analysis method and default settings (database release v2.0.0). Values refer to the total mean of the individual partner average expression values in the interacting pair of cell types. Significant interactions (P < 0.05) are distinguished on the basis of the value distribution of all predicted ligand-receptor pairs after random permutation of the cluster labels in all cell types analyzed [99]. a1b1 = Integrin α1β1 complex; a11b1 = Integrin α11β1 complex
Collagens play complex and critical roles in adipose tissue, and collagen composition strongly impacts adipose tissue function [82, 83, 100]. In addition to structural roles, collagens interact with receptors either as full-length proteins or via bioactive fragments released by limited proteolysis [101, 102]. The collagen-receptor pairs predicted to occur on the basis of single cell sequencing offer a window into the role of the extracellular matrix in establishing depot- and metabolic state-dependent adipose tissue functions.
In addition to collagen-integrin networks, predicted interactions between adipocytes, progenitor cells and endothelial cells (Fig. 2) that may be relevant for angiogenesis include interactions between VEGFA in adipocytes and its receptors KDR, FLT1 and NRP2 in endothelial cells, interactions between Jagged1 and Notch4 between adipocytes and endothelial cells, and also between progenitor and endothelial cells. These later could potentially modulate the effects of VEGFA to regulate angiogenesis [103–105]. Another potential signaling pathway that to our knowledge has not been previously associated with adipose tissue involves ephrin (EPH) receptor tyrosine kinases and their ligands (EFN), which mediate cell signaling during normal and oncogenic development [106, 107]. Ephrins and Ephrin receptors can interact with numerous other signaling pathways, for example with the FGFR1 receptor and E-cadherin, potentially affecting angiogenesis. Neuropilin-semaphorin interactions predicted between progenitor cells and adipocytes play critical roles in axonal guidance, and have been implicated in cardiovascular development [108, 109].
Interestingly, the number of predicted cell–cell interactions between endothelial cells, lymphatic endothelial cells, progenitor cells (ASPC), smooth muscle cells, pericytes and adipocytes is higher in adipose tissue from individuals with BMI > 40 compared to < 30 (Fig. 4A in Emont, et al. [93]). We re-analyzed the source data to determine predicted interactions between adipocytes and endothelial cells that underlie these differences (Table 3). The results reveal interactions between collagens and integrins enhanced in obesity, consistent with data obtained previously using other approaches; for example, enhanced levels of COL4A1 have been associated with obesity and correlated with metabolic disease severity [110], and COL6 and its cleavage product endotrophin have been found to affect adipocyte differentiation, lipolysis, and inflammation [83, 100, 101, 111]. Predicted interactions between adipocytes and endothelial cells from obese subjects involve COL4A4, COL4A5 and COL4A3, which are major components of basement membranes [112], and COL11A1, a fibrillar collagen necessary for skeletal development and recently implicated in cancer cell invasiveness [113–115]. A large number of predicted interactions by collagens expressed in endothelial cells appear solely in obesity, including COL1A1, COL1A2, COL3A1, COL5A2, COL6A2, COL6A3, COL12A1 and COL15A1. Interestingly, expression of COL1A1 in endothelial cells is associated with a senescent phenotype [116]. Obesity-induced alterations in collagen type expression in adipocytes and endothelial cells, and the interactions resulting from these changes, may underlie the changes in vascularization seen in obesity [82, 83]. Testing these hypotheses and those involving other signaling pathways in the context of specific cell types is an exciting direction for further study.
Table 3.
Predicted interactions between adipocytes and endothelial cells in human subcutaneous adipose tissues
| Adipocytes to endothelial cells | Endothelial cells to adipocytes | ||||
|---|---|---|---|---|---|
| Interacting_pair | BMI < 30 | BMI > 40 | Interacting_pair | BMI < 30 | BMI > 40 |
| COL4A2_a1b1 | 0.852 | 0.888 | COL4A2_a1b1 | 0.499 | 0.66 |
| COL4A1_a1b1 | 0.789 | 0.774 | COL4A1_a1b1 | 0.48 | 0.625 |
| VEGFA_FLT1 | 0.587 | 0.472 | TEK_ANGPT1 | 0.403 | 0.771 |
| EFNA5_EPHA4 | 0.571 | 0.726 | NRG2_ERBB4 | 0.387 | 0.668 |
| COL5A2_a1b1 | 0.45 | 0.671 | COL15A1_a1b1 | 0.342 | 0.63 |
| COL8A1_a1b1 | 0.374 | 0.396 | COL8A1_a1b1 | 0.308 | 0.571 |
| COL12A1_a1b1 | 0.3 | 0.437 | EFNB2_EPHB1 | 0.259 | 0.309 |
| COL15A1_a1b1 | 0.276 | 0.405 | COL21A1_a1b1 | 0.253 | 0.517 |
| JAG1_NOTCH4 | 0.272 | NRP2_SEMA3C | 0.25 | 0.793 | |
| COL5A3_a1b1 | 0.26 | 0.331 | FLT1 | 0.21 | 0.243 |
| COL24A1_a1b1 | 0.256 | 0.35 | NRP2_VEGFA | 0.207 | 0.249 |
| LEP_LEPR | 0.226 | 0.279 | CADM1_CADM1 | 0.121 | 0.197 |
| VEGFA_KDR | 0.21 | 0.243 | COL3A1_a1b1 | 0.847 | |
| CADM1_CADM1 | 0.121 | 0.197 | COL1A2_a1b1 | 0.804 | |
| NRG2_ERBB4 | 0.119 | COL1A1_a1b1 | 0.704 | ||
| FGF1_TGFBR3 | 0.386 | NRG3_ERBB4 | 0.693 | ||
| FGFR2_EPHA4 | 0.332 | COL5A2_a1b1 | 0.565 | ||
| COL11A1_a1b1 | 0.304 | COL6A2_a1b1 | 0.516 | ||
| COL4A4_a1b1 | 0.247 | COL6A3_a1b1 | 0.504 | ||
| COL4A5_a1b1 | 0.22 | COL12A1_a1b1 | 0.473 | ||
| COL4A3_a1b1 | 0.218 | FN1_a11b1 | 0.358 | ||
| VEGFD_KDR | 0.149 | GRN_SORT1 | 0.327 | ||
| THBS1_a3b1 | 0.147 | FLT4_PDGFC | 0.322 | ||
| VEGFD_FLT4 | 0.079 | COL15A1_a11b1 | 0.309 | ||
| EFNB2_EPHA4 | 0.271 | ||||
| COL21A1_a11b1 | 0.196 | ||||
| FGF2_FGFR2 | 0.172 | ||||
| TIMP1_FGFR2 | 0.14 | ||||
In bold are interactions that are predicted to occur only in the obese state
Cell type interaction analysis was performed using data from Emont et al. [93]. The human adipose tissue single-cell/single-nuclei Seurat object (human_all.rds) was retrieved and used to identify the top 5000 most variable features across the dataset using the Seurat FindVariableFeature function. The gene symbol of the top 5000 variable features were translated into Ensembl gene ID using biomaRt (Ensembl Genes 106, hsapiens_gene_ensembl dataset). Normalized count tables for the top features were exported into tab delimited files for CellPhoneDB analyses. Interaction analysis with CellPhoneDB v3.0.0 was executed in python 3.8.3 environment using the statistical_analysis method and default settings (database release v2.0.0). Values refer to the total mean of the individual partner average expression values in the interacting pair of cell types. In bold are interactions that are predicted to occur only in the obese state
Mechanisms of inter-cellular communication
In addition to well understood endocrine mechanisms involving interaction between secreted hormones and their cell surface receptors, extracellular vesicles have emerged as important mediators of cell-to-cell communication. These structures represent a heterogeneous population of membrane bound vesicles generated via diverse mechanisms. Micro vesicles are generated at the plasma membrane through outward budding and fission, while exosomes are produced inside the cell by inward budding of the endosomal membrane. Exosomes are thus very small vesicular structures contained within multivesicular structures within the endosomal system, which are expelled into the pericellular space upon fusion [117, 118]
The relevance of these pathways in adipose tissue was demonstrated in key studies by Thomous et al. [119], in which mice with an adipocyte-specific knockout of the miRNA-processing enzyme Dicer displayed major decreases in circulating exosomal miRNAs, and revealed adipocytes as a major source of these structures. They also demonstrated functional, inter-tissue communication, where exosomes from brown adipose tissue could modulate expression of genes in liver [119]. Pan et al. [120] reported that secretion of exosomal microRNA-34a by adipocytes inhibits M2 macrophage polarization, resulting in measurable differences in adipose tissue inflammation. Additional support for the physiological relevance of adipose tissue-derived exosomal microRNAs was provided by studies in which the exosome composition in obese mice was found to be different from lean mice, and exosomes from obese mice were reported to induce insulin resistance in lean mice [121]. Moreover, the number and composition of exosomes in human blood is also altered in obesity and in response to bariatric surgery and weight loss [122]. These studies imply that impaired adipose tissue vascularization in obesity may have consequences on systemic exosome transport and metabolic regulation at distal organs. Recent exciting advancements are helping to understand how specific microRNA sequences are involved in cell-specific retention or exosome packaging to mediate their systemic functions [123]
In addition to microRNAs, exosomes and other extracellular vesicles carry lipids, proteins, and other nucleic acids including DNA, mRNA, and long non-coding RNAs [117, 118]. In adipose tissue, exosomal transfer of lipid from adipocytes to macrophages [124] accounted for ~ 1% of adipocyte lipid content per day, and was increased in obese animals. Contents of these exosomes were capable of inducing differentiation of hematopoietic progenitors into macrophage-like cells, revealing their potential to modulate macrophage differentiation and function. In a dramatic example of protein transfer via exosomes in adipose tissue, Crewe et al. found high levels of caveolin 1 protein in adipocytes in which the caveolin 1 gene was knocked out. Despite complete absence of mRNA, the levels of caveolin were high and the protein retained functionality. The presence of caveolin in knockout adipocytes was attributable to exosomal [125] transfer of the protein from endothelial cells. In addition to establishing a mechanisms for endothelial-adipocyte communication, the finding of efficient micro vesicle mediated transfer from endothelial cells to adipocytes suggest that this mechanisms may operate to transfer multiple macromolecular components from the circulation into the adipose tissue interstitial space, through mechanisms involving endocytosis, exosome formation and release. These mechanisms can alleviate the constraint imposed by the tight, non-fenestrated features of the adipose tissue endothelium [126].
Exosome and micro vesicle mediated signaling mechanisms have been specifically implicated in the regulation of adipose tissue angiogenesis. Multiple reports have described pro-angiogenic roles of exosomes derived from adipose tissue progenitor cells [127–131], with some studies identifying exosome-associated microRNAs that could mediate the observed effects. In an early example, Liang et al. [132] found that exosomes secreted by human adipose tissue progenitor cells could be taken up by endothelial cells and promote angiogenesis in vitro and in vivo. Mechanistically, they propose that miR-125a, enriched in exosomes, repressed the expression of angiogenic inhibitor delta-like 4 (DLL4), resulting in enhanced angiogenesis. The finding of miR-125a in exosomes produced by adipose tissue progenitor cells, and its pro-angiogenic effect on human umbilical endothelial cells was confirmed by Pi et al., [133] who also find an effect of these exosomes to inhibit PTEN. In other contexts, miR-125 has been shown to have anti-angiogenic effects [134–136], for example, deletion of miR-125a in zebrafish resulted in a hyper-branching phenotype [134]. Many mechanistic questions related to exosome signaling in angiogenesis remain to be answered, including the regulation of exosome production, exosome composition, and recognition and processing by target cells. The potential for providing a complete understanding of mechanisms of adipose tissue angiogenesis, as well as potential therapeutic uses of exosomes, will continue to fuel this field forward.
Angiogenesis targeted therapies for obesity: pro- or anti-?
Substantial evidence reviewed above points to the need for angiogenesis for adequate adipose tissue development, needed to prevent lipotoxicity and metabolic disease. Therefore, limiting adipose tissue angiogenesis during the development of obesity would be expected to occur at the expense of metabolic health. Nevertheless, several reports of anti-angiogenic treatment leading not only to lower adiposity, but to improved metabolic parameters exist [137–142] (Table 4). For example, in an early report, improved obesity and metabolism in leptin-deficient ob/ob mice was seen to occur in response to the angiogenesis inhibitor TNP-450 [137]. The drug was introduced into 4–5-week-old mice that had not developed advanced obesity, and resulted in lower body weight gain during the ensuing 12 weeks of treatment. However, the drug also led to a significant decrease in food intake, confounding the interpretation of the role of the TNP-450 anti-angiogenic effect. In complementary studies, While et al. verified the effect of TNP-470 to decrease food intake, and reported an aggravation of glucose tolerance in mice exposed to the drug [143].
Table 4.
Summary of reports on adipose tissue angiogenesis and metabolic outcomes
| Authors | Approach | Model | Outcome |
|---|---|---|---|
| Brakenhielm et al. [137] | Pharmacological, TNP-450 (MetAP2 inhibitor) | Ob/Ob mouse | Decreased weight gain, decreased food intake |
| White et al. [143] | Pharmacological, TNP-450 (MetAP2 inhibitor) | Ob/Ob mouse | Decreased weight gain, decreased food intake, impaired glucose tolerance |
| Park et al. [140] | Pharmacological, ALS-L1023 | Diet-induced obese mouse | Decreased weight gain |
| Siddik et al. [141] | Pharmacological, BL6 (MetAP2 inhibitor) | Cultured adipocytes | Decreased glucose uptake, decreased lipid uptake |
| Pottorf et al. [145] | Pharmacological, ZGN-1258 (MetAP2 inhibitor) | Bardett–Beidle mouse model | Decreased weight gain, decreased food intake |
| Wang et al. [142] | Pharmacological, AARP (CTT peptide-endostatin mimic-kringle 5) | Diet-induced obese mouse | Decreased weigh gain, increased locomotor activity, increased thermogenesis |
| Robciuc et al. [86] | Genetic, AAV-mediated VEGFB transduction | Diet-induced obese mouse | Improved glucose metabolism, improved adipose tissue vascularization, increased thermogenic adipose tissue |
| Park et al. [85] | Genetic, increased VEGFA expression through Adiponectin-Cre | Doxicicline inducible, diet-induced obese mouse | Decreased weight gain, increased thermogenic adipose tissue |
| Jin et al. [147] | Genetic, repressed VEGF expression through AP2-cre | Doxicicline inducible | Decreased weight gain, improved glucose tolerance, improved insulin sensitivity, increased thermogenic adipose tissue |
| Seki et al. [149] | Genetic, increased VEGFA bioavailabillity in adipose tissue | Anti-VEGFR1 neutralizing antibodies; genetic deletion of VEGFR1 | Decreased weight gain, increased thermogenic adipose tissue |
Studies on a different anti-angiogenic compound, ALS-L1023 revealed lower weight gain in response to the drug, but this effect was abrogated by pair feeding, revealing decreased food intake as a confounding factor [140]. In a more recent study, Siddik et al. [141] showed that BL6, an inhibitor of methionine aminopeptidase 2 (MetAP2) that impairs endothelial cell proliferation, had additional autonomous effects to inhibit lipid accumulation and stimulate glucose uptake by cultured adipocytes. These results raise the possibility that the effect of small molecule inhibitors of angiogenesis to decrease obesity may be attributable to effects other than those on endothelial cells; further advancement of these therapies will require elucidation of their multiple actions to define those specifically resulting in weight loss [144, 145].
Another class of angiogenesis inhibitors that have been tested in the context of obesity include AARP (CTT peptide-endostatin mimic-kringle 5), a multitarget fusion protein designed against tumor angiogenesis [142]. This drug ameliorated weight gain in C57BL6 mice in response to high fat, high calorie diet, and careful assessments demonstrated that food consumption was unaffected. Strikingly, energy expenditure was significantly higher in mice exposed to the drug, manifested as increased locomotor activity as well as increased temperature. The increase in energy expenditure elicited by AARP could be traced to a strong induction of thermogenic adipocytes in subcutaneous and interscapular depots. Adipose tissue browning is well known to contribute to increased energy expenditure and protection from obesity [146]. Thus, it remains unclear whether the protection from weight gain achieved by AARP treatment is due to its potential anti-angiogenic actions, to its stimulation of adipose tissue browning, or to a combination of both effects.
The pleiotropic effects of inhibitors confound our understanding of the specific consequences of angiogenesis inhibition on obesity and associated metabolic dysfunction. Genetic approaches targeting specific angiogenic mechanisms are less ambiguous, but have also revealed underlying complexity. VEGFA is a potent stimulator of endothelial cell proliferation and angiogenesis, and is expressed by adipocytes and progenitor cells in adipose tissue. Numerous groups have reported that increased expression [85, 86, 147, 148] or enhanced bioavailability [149] of VEGFA in adipose tissue mitigates obesity and its metabolic consequences. In elegant, temporally controlled experiments, Park et al. [85] used a doxycycline-inducible, adipocyte-specific, VEGFA-overexpressing mouse to find that VEGFA overexpression triggered angiogenesis early as 2 days postinduction. However, in all studies angiogenic induction by VEGFA also resulted in formation of thermogenic beige adipocytes, which as mentioned above have cell-autonomous, systemic metabolic effects [36, 85].
Conversely, deficiency in adipose tissue VEGFA signaling results in aggravation of metabolic dysfunction [87, 148]. A role for VEGFB in adipose tissue has also been reported [147]; deletion of adipocyte VEGFB driven by CRISPR-Cas9 resulted in adipocyte hypertrophy, expanded adipose tissue mass, and exacerbation of metabolic dysfunction in response to high fat diet [150]. Conversely, overexpression of VEGFB resulted in amelioration of metabolic effects, and thermogenic induction in adipose tissue, similar to that seen in response to VEGFA [86].
Paradoxically, the use of pharmacological, systemic inhibitors of VEGFA do not recapitulate the metabolic dysfunction elicited by adipose tissue-specific loss of VEGFA; treatment of mice with a neutralizing antibody to VEGFA did not exacerbate the metabolic dysfunction in hyperphagic ob/ob mice, which on the contrary trended to an improvement in glucose levels [151]. Similarly, Wu et al. found that administration of the VEGFA-neutralizing monoclonal antibody B.20-4.1, led to amelioration of high-fat diet-induced insulin resistance, principally due to improved insulin sensitivity in the liver and associated with decreased inflammatory markers [152] These results indicate that systemic inhibition of VEGFA affects multiple organs and tissues leading to improved glucose homeostasis, dominating over the local effect on adipose tissue, which would be expected to impair systemic metabolism.
Genetic models targeting angiogenic factors other than VEGFA and VEGFB support a beneficial effect of adipose tissue angiogenesis independently of browning (Table 4). An et al. [153] found that overexpression of Angiopoietin-2 resulted in enhanced adipose tissue vascularization, decreased fibrosis and inflammation, decreased adipocyte size and improved metabolic parameters in response to high-fat diet, with no detectable induction of thermogenic markers. Conversely, Ang-2 neutralization led to larger adipocyte size and exacerbation of high-fat diet-induced metabolic changes. A similar result was seen in another model, where enhancing endothelial cell proliferation by increasing PI-3 kinase signaling led to enhanced vascularization of adipose tissue and improvements in metabolic parameters [154]. In aggregate, these results suggest that approaches leveraging tissue-specific targeting to elicit angiogenesis in adipose tissue [91] constitute an exciting therapeutic strategy to ameliorate obesity and metabolic disease.
Conclusions
The increased prevalence of metabolic diseases and their deleterious effects on human health and wellbeing emphasizes the importance of understanding cellular and molecular mechanisms underlying adipose tissue development and its relationship to systemic metabolism. Adipose tissue angiogenesis is at the heart of these mechanisms, as it controls adipocyte metabolism, establishes communication between adipose tissue and the rest of the body, and is critically required for progenitor cell proliferation and tissue remodeling. The application of single-cell and single-nuclei sequencing has opened our horizons on the role of specific signaling pathways operating between endothelial cells, adipocytes and progenitor cells, and their derangements in obesity. Findings revealing new signaling mechanisms consisting of macrovesicles and their contents are also opening up new modes for potential intervention, and results of multiple studies pointing to beneficial effects of enhancing adipose tissue angiogenesis are providing direction for further efforts.
Author contributions
SC and ZYR prepared the results in Tables 1, 2, 3, 4 and Fig. 2; JSR prepared Fig. 1; all authors wrote and reviewed the manuscript.
Funding
This work was supported by United States National Institutes of Health Grants DK089101 and DK123028 to SC and GM135751 to JSR.
Data availability
The datasets analyzed for this study were generated by Emont et al. [92], and are publicly available. Methods used to analyze the datasets are described fully within this article.
Declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stieber C, et al. Human perivascular adipose tissue as a regulator of the vascular microenvironment and diseases of the coronary artery and aorta. J Cardiol Cardiovasc Sci. 2019;3(4):10–15. doi: 10.29245/2578-3025/2019/4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford ST, et al. Methylome and transcriptome maps of human visceral and subcutaneous adipocytes reveal key epigenetic differences at developmental genes. Sci Rep. 2019;9(1):9511. doi: 10.1038/s41598-019-45777-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zwick RK, et al. Anatomical, physiological, and functional diversity of adipose tissue. Cell Metab. 2018;27(1):68–83. doi: 10.1016/j.cmet.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ha CWY, et al. Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell. 2020;183(3):666–683 e17. doi: 10.1016/j.cell.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Meng Y, Yu X. The unique metabolic characteristics of bone marrow adipose tissue. Front Endocrinol (Lausanne) 2019;10:69. doi: 10.3389/fendo.2019.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tencerova M, Ferencakova M, Kassem M. Bone marrow adipose tissue: role in bone remodeling and energy metabolism. Best Pract Res Clin Endocrinol Metab. 2021;35(4):101545. doi: 10.1016/j.beem.2021.101545. [DOI] [PubMed] [Google Scholar]
- 7.Krotkiewski M, et al. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Investig. 1983;72(3):1150–62. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piche ME, Tchernof A, Despres JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126(11):1477–1500. doi: 10.1161/CIRCRESAHA.120.316101. [DOI] [PubMed] [Google Scholar]
- 9.Smith U. Abdominal obesity: a marker of ectopic fat accumulation. J Clin Investig. 2015;125(5):1790–1792. doi: 10.1172/JCI81507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray SL, Vidal-Puig AJ. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr Rev. 2007;65(6 Pt 2):S7–12. doi: 10.1301/nr.2007.jun.S7-S12. [DOI] [PubMed] [Google Scholar]
- 11.Dai H, et al. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the global burden of disease study. PLoS Med. 2020;17(7):e1003198. doi: 10.1371/journal.pmed.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson SC, Burgess S. Causal role of high body mass index in multiple chronic diseases: a systematic review and meta-analysis of Mendelian randomization studies. BMC Med. 2021;19(1):320. doi: 10.1186/s12916-021-02188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meln I, et al. Dietary calories and lipids synergistically shape adipose tissue cellularity during postnatal growth. Mol Metab. 2019;24:139–148. doi: 10.1016/j.molmet.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serra MC, et al. High adipose LPL activity and adipocyte hypertrophy reduce visceral fat and metabolic risk in obese, older women. Obesity (Silver Spring) 2015;23(3):602–607. doi: 10.1002/oby.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinonen S, et al. Adipocyte morphology and implications for metabolic derangements in acquired obesity. Int J Obes (Lond) 2014;38(11):1423–1431. doi: 10.1038/ijo.2014.31. [DOI] [PubMed] [Google Scholar]
- 16.Cifarelli V, et al. Decreased adipose tissue oxygenation associates with insulin resistance in individuals with obesity. J Clin Investig. 2020;130(12):6688–6699. doi: 10.1172/JCI141828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lempesis IG, et al. Oxygenation of adipose tissue: a human perspective. Acta Physiol (Oxf) 2020;228(1):e13298. doi: 10.1111/apha.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebo ZL, Rodeheffer MS. Assembling the adipose organ: adipocyte lineage segregation and adipogenesis in vivo. Development. 2019 doi: 10.1242/dev.172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997;4(2):211–232. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- 20.Poissonnet CM, Burdi AR, Bookstein FL. Growth and development of human adipose tissue during early gestation. Early Hum Dev. 1983;8(1):1–11. doi: 10.1016/0378-3782(83)90028-2. [DOI] [PubMed] [Google Scholar]
- 21.Poissonnet CM, Burdi AR, Garn SM. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum Dev. 1984;10(1–2):1–11. doi: 10.1016/0378-3782(84)90106-3. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Miguelsanz J, et al. Early appearance of epicardial adipose tissue through human development. Nutrients. 2021;13(9):2906. doi: 10.3390/nu13092906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho SW, et al. Engineered adipose tissue formation enhanced by basic fibroblast growth factor and a mechanically stable environment. Cell Transplant. 2007;16(4):421–434. doi: 10.3727/000000007783464795. [DOI] [PubMed] [Google Scholar]
- 24.Han J, et al. The spatiotemporal development of adipose tissue. Development. 2011;138(22):5027–5037. doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]
- 25.Xue Y, et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 2009;9(1):99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Lee YH, et al. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J. 2015;29(1):286–299. doi: 10.1096/fj.14-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zahalka AH, et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 2017;358(6361):321–326. doi: 10.1126/science.aah5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang W, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vishvanath L, et al. Pdgfrbeta+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 2016;23(2):350–359. doi: 10.1016/j.cmet.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry DC, Jiang Y, Graff JM. Emerging roles of adipose progenitor cells in tissue development, homeostasis, expansion and thermogenesis. Trends Endocrinol Metab. 2016;27(8):574–585. doi: 10.1016/j.tem.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Y, et al. A PPARgamma transcriptional cascade directs adipose progenitor cell-niche interaction and niche expansion. Nat Commun. 2017;8:15926. doi: 10.1038/ncomms15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long JZ, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19(5):810–820. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran KV, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15(2):222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong KY, et al. Perilipin+ embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development. 2015;142(15):2623–2632. doi: 10.1242/dev.125336. [DOI] [PubMed] [Google Scholar]
- 35.Gealekman O, et al. Control of adipose tissue expandability in response to high fat diet by the insulin-like growth factor-binding protein-4. J Biol Chem. 2014;289(26):18327–18338. doi: 10.1074/jbc.M113.545798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Min SY, et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med. 2016;22(3):312–318. doi: 10.1038/nm.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tchoukalova YD, et al. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci USA. 2010;107(42):18226–18231. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye RZ, et al. Fat cell size: measurement methods, pathophysiological origins, and relationships with metabolic dysregulations. Endocr Rev. 2022;43(1):35–60. doi: 10.1210/endrev/bnab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, et al. Fat cell size and adipokine expression in relation to gender, depot, and metabolic risk factors in morbidly obese adolescents. Obesity (Silver Spring) 2014;22(3):691–697. doi: 10.1002/oby.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ukropec J, et al. Adipokine protein expression pattern in growth hormone deficiency predisposes to the increased fat cell size and the whole body metabolic derangements. J Clin Endocrinol Metab. 2008;93(6):2255–2262. doi: 10.1210/jc.2007-2188. [DOI] [PubMed] [Google Scholar]
- 41.Maffeis C, et al. Fat cell size, insulin sensitivity, and inflammation in obese children. J Pediatr. 2007;151(6):647–652. doi: 10.1016/j.jpeds.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 42.Andersson DP, et al. Abdominal subcutaneous adipose tissue cellularity in men and women. Int J Obes (Lond) 2017;41(10):1564–1569. doi: 10.1038/ijo.2017.148. [DOI] [PubMed] [Google Scholar]
- 43.Fried SK, Lee MJ, Karastergiou K. Shaping fat distribution: new insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity (Silver Spring) 2015;23(7):1345–1352. doi: 10.1002/oby.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eriksson-Hogling D, et al. Adipose tissue morphology predicts improved insulin sensitivity following moderate or pronounced weight loss. Int J Obes (Lond) 2015;39(6):893–898. doi: 10.1038/ijo.2015.18. [DOI] [PubMed] [Google Scholar]
- 45.Michaud A, et al. Abdominal subcutaneous and omental adipocyte morphology and its relation to gene expression, lipolysis and adipocytokine levels in women. Metabolism. 2014;63(3):372–381. doi: 10.1016/j.metabol.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Gesta S, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA. 2006;103(17):6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue Y, et al. FOXC2 controls Ang-2 expression and modulates angiogenesis, vascular patterning, remodeling, and functions in adipose tissue. Proc Natl Acad Sci USA. 2008;105(29):10167–10172. doi: 10.1073/pnas.0802486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cederberg A, et al. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106(5):563–573. doi: 10.1016/S0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 49.Helker CS, et al. The hormonal peptide Elabela guides angioblasts to the midline during vasculogenesis. eLife. 2015 doi: 10.7554/eLife.06726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaves-Almagro C, et al. Apelin receptors: from signaling to antidiabetic strategy. Eur J Pharmacol. 2015;763(Pt B):149–159. doi: 10.1016/j.ejphar.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 51.Hu H, et al. Apelin/APJ system as a therapeutic target in diabetes and its complications. Mol Genet Metab. 2016;119(1–2):20–27. doi: 10.1016/j.ymgme.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 52.Li C, et al. The role of apelin-APJ system in diabetes and obesity. Front Endocrinol (Lausanne) 2022;13:820002. doi: 10.3389/fendo.2022.820002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawane M, et al. Apelin inhibits diet-induced obesity by enhancing lymphatic and blood vessel integrity. Diabetes. 2013;62(6):1970–1980. doi: 10.2337/db12-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Samerria S, Radovick S. The role of insulin-like growth factor-1 (IGF-1) in the control of neuroendocrine regulation of growth. Cells. 2021;10(10):2664. doi: 10.3390/cells10102664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim HS, Richardson RL, Hausman GJ. The expression of insulin-like growth factor-1 during adipogenesis in vivo: effect of thyroxine. Gen Comp Endocrinol. 1998;112(1):38–45. doi: 10.1006/gcen.1998.7126. [DOI] [PubMed] [Google Scholar]
- 56.Rojas-Rodriguez R, et al. PAPPA-mediated adipose tissue remodeling mitigates insulin resistance and protects against gestational diabetes in mice and humans. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.aay4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bid HK, et al. Potent inhibition of angiogenesis by the IGF-1 receptor-targeting antibody SCH717454 is reversed by IGF-2. Mol Cancer Ther. 2012;11(3):649–659. doi: 10.1158/1535-7163.MCT-11-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin S, et al. IGF-1 promotes angiogenesis in endothelial cells/adipose-derived stem cells co-culture system with activation of PI3K/Akt signal pathway. Cell Prolif. 2017;50(6):e12390. doi: 10.1111/cpr.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shigematsu S, et al. IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocr J. 1999;46(Suppl):S59–62. doi: 10.1507/endocrj.46.Suppl_S59. [DOI] [PubMed] [Google Scholar]
- 60.Xu S, et al. Dexamethasone interferes with osteoblasts formation during osteogenesis through altering IGF-1-mediated angiogenesis. J Cell Physiol. 2019 doi: 10.1002/jcp.28157. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Q, et al. lncRNA NR2F1-AS1 promotes breast cancer angiogenesis through activating IGF-1/IGF-1R/ERK pathway. J Cell Mol Med. 2020;24(14):8236–8247. doi: 10.1111/jcmm.15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boucher J, et al. Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nat Commun. 2012;3:902. doi: 10.1038/ncomms1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garten A, Schuster S, Kiess W. The insulin-like growth factors in adipogenesis and obesity. Endocrinol Metab Clin North Am. 2012;41(2):283–95, v–vi. doi: 10.1016/j.ecl.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Wabitsch M, et al. Mitogenic and antiadipogenic properties of human growth hormone in differentiating human adipocyte precursor cells in primary culture. Pediatr Res. 1996;40(3):450–456. doi: 10.1203/00006450-199609000-00014. [DOI] [PubMed] [Google Scholar]
- 65.Moore BJ, et al. Maternal brown fat metabolism returns to control level by four weeks postweaning in rats. J Nutr. 1989;119(12):1992–1998. doi: 10.1093/jn/119.12.1992. [DOI] [PubMed] [Google Scholar]
- 66.Rojas-Rodriguez R, et al. Human adipose tissue expansion in pregnancy is impaired in gestational diabetes mellitus. Diabetologia. 2015;58(9):2106–2114. doi: 10.1007/s00125-015-3662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zangi L, et al. Insulin-like growth factor 1 receptor-dependent pathway drives epicardial adipose tissue formation after myocardial injury. Circulation. 2017;135(1):59–72. doi: 10.1161/CIRCULATIONAHA.116.022064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jo J, et al. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol. 2009;5(3):e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mandenoff A, Lenoir T, Apfelbaum M. Tardy occurrence of adipocyte hyperplasia in cafeteria-fed rat. Am J Physiol. 1982;242(3):R349–R351. doi: 10.1152/ajpregu.1982.242.3.R349. [DOI] [PubMed] [Google Scholar]
- 70.Wang QA, et al. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19(10):1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nishimura S, et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Investig. 2008;118(2):710–721. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimizu I, et al. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Investig. 2014;124(5):2099–2112. doi: 10.1172/JCI71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salans LB, Horton ES, Sims EA. Experimental obesity in man: cellular character of the adipose tissue. J Clin Investig. 1971;50(5):1005–1011. doi: 10.1172/JCI106570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alligier M, et al. Visceral fat accumulation during lipid overfeeding is related to subcutaneous adipose tissue characteristics in healthy men. J Clin Endocrinol Metab. 2013;98(2):802–810. doi: 10.1210/jc.2012-3289. [DOI] [PubMed] [Google Scholar]
- 75.McLaughlin T, et al. Adipose cell size and regional fat deposition as predictors of metabolic response to overfeeding in insulin-resistant and insulin-sensitive humans. Diabetes. 2016;65(5):1245–1254. doi: 10.2337/db15-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belligoli A, et al. Characterization of subcutaneous and omental adipose tissue in patients with obesity and with different degrees of glucose impairment. Sci Rep. 2019;9(1):11333. doi: 10.1038/s41598-019-47719-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spencer M, et al. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab. 2011;96(12):E1990–E1998. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paavonsalo S, et al. Capillary rarefaction in obesity and metabolic diseases-organ-specificity and possible mechanisms. Cells. 2020;9(12):2683. doi: 10.3390/cells9122683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pasarica M, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58(3):718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karki S, et al. WNT5A regulates adipose tissue angiogenesis via antiangiogenic VEGF-A165b in obese humans. Am J Physiol Heart Circ Physiol. 2017;313(1):H200–H206. doi: 10.1152/ajpheart.00776.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hunyenyiwa T, et al. Obesity inhibits angiogenesis through TWIST1-SLIT2 signaling. Front Cell Dev Biol. 2021;9:693410. doi: 10.3389/fcell.2021.693410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khan T, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29(6):1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pasarica M, et al. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94(12):5155–5162. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Investig. 2017;127(1):74–82. doi: 10.1172/JCI88883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park J, et al. VEGF-A-expressing adipose tissue shows rapid beiging and enhanced survival after transplantation and confers IL-4-independent metabolic improvements. Diabetes. 2017;66(6):1479–1490. doi: 10.2337/db16-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robciuc MR, et al. VEGFB/VEGFR1-induced expansion of adipose vasculature counteracts obesity and related metabolic complications. Cell Metab. 2016;23(4):712–724. doi: 10.1016/j.cmet.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sung HK, et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013;17(1):61–72. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 88.Ashoff A, et al. Pioglitazone prevents capillary rarefaction in streptozotocin-diabetic rats independently of glucose control and vascular endothelial growth factor expression. J Vasc Res. 2012;49(3):260–266. doi: 10.1159/000335214. [DOI] [PubMed] [Google Scholar]
- 89.Gealekman O, et al. Effect of rosiglitazone on capillary density and angiogenesis in adipose tissue of normoglycaemic humans in a randomised controlled trial. Diabetologia. 2012;55(10):2794–2799. doi: 10.1007/s00125-012-2658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li M, et al. Decreased ANGPTL4 impairs endometrial angiogenesis during peri-implantation period in patients with recurrent implantation failure. J Cell Mol Med. 2020;24(18):10730–10743. doi: 10.1111/jcmm.15696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xue Y, et al. Preventing diet-induced obesity in mice by adipose tissue transformation and angiogenesis using targeted nanoparticles. Proc Natl Acad Sci USA. 2016;113(20):5552–5557. doi: 10.1073/pnas.1603840113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Corvera S. Cellular heterogeneity in adipose tissues. Annu Rev Physiol. 2021;83:257–278. doi: 10.1146/annurev-physiol-031620-095446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Emont MP, et al. A single-cell atlas of human and mouse white adipose tissue. Nature. 2022;603(7903):926–933. doi: 10.1038/s41586-022-04518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Merrick D, et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science. 2019 doi: 10.1126/science.aav2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schwalie PC, et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature. 2018;559(7712):103–108. doi: 10.1038/s41586-018-0226-8. [DOI] [PubMed] [Google Scholar]
- 96.Vijay J, et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab. 2020;2(1):97–109. doi: 10.1038/s42255-019-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sarvari AK, et al. Plasticity of epididymal adipose tissue in response to diet-induced obesity at single-nucleus resolution. Cell Metab. 2021;33(2):437–453 e5. doi: 10.1016/j.cmet.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Chakraborty A, et al. Vascular endothelial growth factor-D (VEGF-D) overexpression and lymphatic expansion in murine adipose tissue improves metabolism in obesity. Am J Pathol. 2019;189(4):924–939. doi: 10.1016/j.ajpath.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Efremova M, et al. Cell PhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat Protoc. 2020;15(4):1484–1506. doi: 10.1038/s41596-020-0292-x. [DOI] [PubMed] [Google Scholar]
- 100.Tam CS, et al. Adipose tissue remodeling in children: the link between collagen deposition and age-related adipocyte growth. J Clin Endocrinol Metab. 2012;97(4):1320–1327. doi: 10.1210/jc.2011-2806. [DOI] [PubMed] [Google Scholar]
- 101.Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Investig. 2012;122(11):4243–4256. doi: 10.1172/JCI63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karamanos NK, et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021;288(24):6850–6912. doi: 10.1111/febs.15776. [DOI] [PubMed] [Google Scholar]
- 103.Boareto M, et al. Jagged mediates differences in normal and tumor angiogenesis by affecting tip-stalk fate decision. Proc Natl Acad Sci USA. 2015;112(29):E3836–E3844. doi: 10.1073/pnas.1511814112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tan E, Asada HH, Ge R. Extracellular vesicle-carried Jagged-1 inhibits HUVEC sprouting in a 3D microenvironment. Angiogenesis. 2018;21(3):571–580. doi: 10.1007/s10456-018-9609-6. [DOI] [PubMed] [Google Scholar]
- 105.Wen Z, et al. The microvascular niche instructs T cells in large vessel vasculitis via the VEGF-Jagged1-Notch pathway. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aal3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cecchini A, Cornelison DDW. Eph/Ephrin-based protein complexes: the importance of cis interactions in guiding cellular processes. Front Mol Biosci. 2021;8:809364. doi: 10.3389/fmolb.2021.809364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kania A, Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol. 2016;17(4):240–256. doi: 10.1038/nrm.2015.16. [DOI] [PubMed] [Google Scholar]
- 108.Valdembri D, et al. Class 3 semaphorins in cardiovascular development. Cell Adh Migr. 2016;10(6):641–651. doi: 10.1080/19336918.2016.1212805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang WJ, et al. Semaphorin-3C signals through neuropilin-1 and PlexinD1 receptors to inhibit pathological angiogenesis. EMBO Mol Med. 2015;7(10):1267–1284. doi: 10.15252/emmm.201404922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reggio S, et al. Increased basement membrane components in adipose tissue during obesity: links with TGFbeta and metabolic phenotypes. J Clin Endocrinol Metab. 2016;101(6):2578–2587. doi: 10.1210/jc.2015-4304. [DOI] [PubMed] [Google Scholar]
- 111.Oh J, et al. Type VI collagen and its cleavage product, endotrophin, cooperatively regulate the adipogenic and lipolytic capacity of adipocytes. Metabolism. 2021;114:154430. doi: 10.1016/j.metabol.2020.154430. [DOI] [PubMed] [Google Scholar]
- 112.Cosgrove D, Liu S. Collagen IV diseases: a focus on the glomerular basement membrane in Alport syndrome. Matrix Biol. 2017;57–58:45–54. doi: 10.1016/j.matbio.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Y, et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell. 1995;80(3):423–430. doi: 10.1016/0092-8674(95)90492-1. [DOI] [PubMed] [Google Scholar]
- 114.Styrkarsdottir U, et al. Meta-analysis of Icelandic and UK data sets identifies missense variants in SMO, IL11, COL11A1 and 13 more new loci associated with osteoarthritis. Nat Genet. 2018;50(12):1681–1687. doi: 10.1038/s41588-018-0247-0. [DOI] [PubMed] [Google Scholar]
- 115.Wu YH, et al. COL11A1 activates cancer-associated fibroblasts by modulating TGF-beta3 through the NF-kappaB/IGFBP2 axis in ovarian cancer cells. Oncogene. 2021;40(26):4503–4519. doi: 10.1038/s41388-021-01865-8. [DOI] [PubMed] [Google Scholar]
- 116.Crespo-Garcia S, et al. Pathological angiogenesis in retinopathy engages cellular senescence and is amenable to therapeutic elimination via BCL-xL inhibition. Cell Metab. 2021;33(4):818–832 e7. doi: 10.1016/j.cmet.2021.01.011. [DOI] [PubMed] [Google Scholar]
- 117.van Niel G, et al. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022 doi: 10.1038/s41580-022-00460-3. [DOI] [PubMed] [Google Scholar]
- 118.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 119.Thomou T, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pan Y, et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Investig. 2019;129(2):834–849. doi: 10.1172/JCI123069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Castano C, et al. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci USA. 2018;115(48):12158–12163. doi: 10.1073/pnas.1808855115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hubal MJ, et al. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring) 2017;25(1):102–110. doi: 10.1002/oby.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garcia-Martin R, et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601(7893):446–451. doi: 10.1038/s41586-021-04234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Flaherty SE, 3rd, et al. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science. 2019;363(6430):989–993. doi: 10.1126/science.aaw2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Crewe C, et al. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell. 2018;175(3):695–708 e13. doi: 10.1016/j.cell.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ioannidou A, Fisher RM, Hagberg CE. The multifaceted roles of the adipose tissue vasculature. Obes Rev. 2022;23(4):e13403. doi: 10.1111/obr.13403. [DOI] [PubMed] [Google Scholar]
- 127.An Y, et al. Exosomes from adipose-derived stem cells (ADSCs) overexpressing miR-21 promote vascularization of endothelial cells. Sci Rep. 2019;9(1):12861. doi: 10.1038/s41598-019-49339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xue C, et al. Exosomes derived from hypoxia-treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. Stem Cells Dev. 2018;27(7):456–465. doi: 10.1089/scd.2017.0296. [DOI] [PubMed] [Google Scholar]
- 129.Li X, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):1–14. doi: 10.1038/s12276-018-0058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Han YD, et al. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun. 2018;497(1):305–312. doi: 10.1016/j.bbrc.2018.02.076. [DOI] [PubMed] [Google Scholar]
- 131.Lopatina T, et al. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014;12:26. doi: 10.1186/1478-811X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liang X, et al. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129(11):2182–2189. doi: 10.1242/jcs.170373. [DOI] [PubMed] [Google Scholar]
- 133.Pi L, et al. Exosomal microRNA-125a-3p from human adipose-derived mesenchymal stem cells promotes angiogenesis of wound healing through inhibiting PTEN. Mol Cell Biochem. 2022;477(1):115–127. doi: 10.1007/s11010-021-04251-w. [DOI] [PubMed] [Google Scholar]
- 134.Wade SM, et al. Dysregulated miR-125a promotes angiogenesis through enhanced glycolysis. EBioMedicine. 2019;47:402–413. doi: 10.1016/j.ebiom.2019.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hou P, et al. PinX1 represses renal cancer angiogenesis via the mir-125a-3p/VEGF signaling pathway. Angiogenesis. 2019;22(4):507–519. doi: 10.1007/s10456-019-09675-z. [DOI] [PubMed] [Google Scholar]
- 136.Che P, et al. miR-125a-5p impairs endothelial cell angiogenesis in aging mice via RTEF-1 downregulation. Aging Cell. 2014;13(5):926–934. doi: 10.1111/acel.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brakenhielm E, et al. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94(12):1579–1588. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 138.Kim J, et al. The angiogenesis inhibitor ALS-L1023 from lemon-balm leaves attenuates high-fat diet-induced nonalcoholic fatty liver disease through regulating the visceral adipose-tissue function. Int J Mol Sci. 2017;18(4):846. doi: 10.3390/ijms18040846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ngo DT, et al. Antiangiogenic actions of vascular endothelial growth factor-A165b, an inhibitory isoform of vascular endothelial growth factor-A, in human obesity. Circulation. 2014;130(13):1072–1080. doi: 10.1161/CIRCULATIONAHA.113.008171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Park BY, et al. Reduction of adipose tissue mass by the angiogenesis inhibitor ALS-L1023 from Melissa officinalis. PLoS ONE. 2015;10(11):e0141612. doi: 10.1371/journal.pone.0141612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Siddik MAB, et al. A MetAP2 inhibitor blocks adipogenesis, yet improves glucose uptake in cells. Adipocyte. 2019;8(1):240–253. doi: 10.1080/21623945.2019.1636627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang H, Shi Y, Gu J. A multitarget angiogenesis inhibitor, CTT peptide-endostatin mimic-kringle 5, prevents diet-induced obesity. J Mol Med (Berl) 2020;98(12):1753–1765. doi: 10.1007/s00109-020-01993-w. [DOI] [PubMed] [Google Scholar]
- 143.White HM, Acton AJ, Considine RV. The angiogenic inhibitor TNP-470 decreases caloric intake and weight gain in high-fat fed mice. Obesity (Silver Spring) 2012;20(10):2003–2009. doi: 10.1038/oby.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goya Grocin A, Kallemeijn WW, Tate EW. Targeting methionine aminopeptidase 2 in cancer, obesity, and autoimmunity. Trends Pharmacol Sci. 2021;42(10):870–882. doi: 10.1016/j.tips.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 145.Pottorf TS, et al. MetAP2 inhibition reduces food intake and body weight in a ciliopathy mouse model of obesity. JCI Insight. 2020 doi: 10.1172/jci.insight.134278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Czech MP. Mechanisms of insulin resistance related to white, beige, and brown adipocytes. Mol Metab. 2020;34:27–42. doi: 10.1016/j.molmet.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jin H, et al. VEGF and VEGFB play balancing roles in adipose differentiation, gene expression, and function. Endocrinology. 2018;159(5):2036–2049. doi: 10.1210/en.2017-03246. [DOI] [PubMed] [Google Scholar]
- 148.Elias I, Franckhauser S, Bosch F. New insights into adipose tissue VEGF-A actions in the control of obesity and insulin resistance. Adipocyte. 2013;2(2):109–112. doi: 10.4161/adip.22880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Seki T, et al. Ablation of endothelial VEGFR1 improves metabolic dysfunction by inducing adipose tissue browning. J Exp Med. 2018;215(2):611–626. doi: 10.1084/jem.20171012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen Y, et al. Adipose vascular endothelial growth factor B is a major regulator of energy metabolism. J Endocrinol. 2020;244(3):511–521. doi: 10.1530/JOE-19-0341. [DOI] [PubMed] [Google Scholar]
- 151.Sun K, et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA. 2012;109(15):5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu LE, et al. Systemic VEGF-A neutralization ameliorates diet-induced metabolic dysfunction. Diabetes. 2014;63(8):2656–2667. doi: 10.2337/db13-1665. [DOI] [PubMed] [Google Scholar]
- 153.An YA, et al. Angiopoietin-2 in white adipose tissue improves metabolic homeostasis through enhanced angiogenesis. eLife. 2017 doi: 10.7554/eLife.24071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Monelli E, et al. Angiocrine polyamine production regulates adiposity. Nat Metab. 2022;4(3):327–343. doi: 10.1038/s42255-022-00544-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed for this study were generated by Emont et al. [92], and are publicly available. Methods used to analyze the datasets are described fully within this article.