Abstract
Aims
A nationwide diabetic retinopathy (DR) screening program has been established in Denmark since 2013. We aimed to perform an evaluation of adherence to DR screenings and to examine whether non-adherence was correlated to DR progression.
Methods
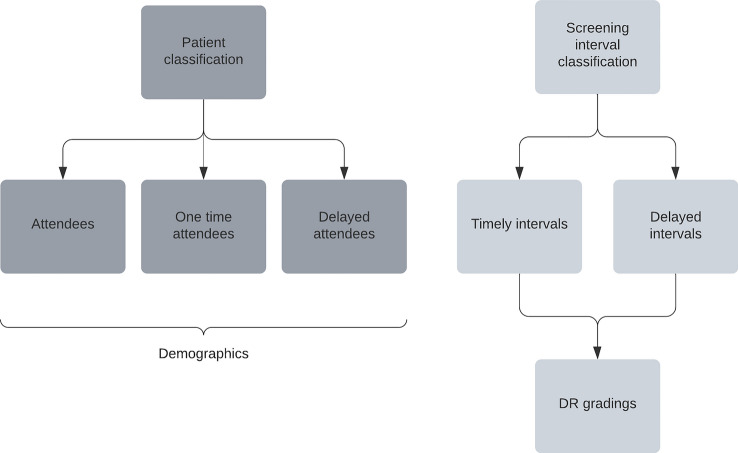
The population consisted of a register-based cohort, who participated in the screening program from 2013 to 2018. We analyzed age, gender, marital status, DR level (International Clinical DR severity scale, none, mild-, moderate-, severe non-proliferative DR (NPDR) and proliferative DR (PDR)), comorbidities and socioeconomic factors. The attendance pattern of patients was grouped as either timely (no delays > 33%), delayed (delays > 33%) or one-time attendance (unexplained).
Results
We included 205,970 patients with 591,136 screenings. Rates of timely, delayed and one-time attendance were 53.0%, 35.5% and 11.5%, respectively. DR level at baseline was associated with delays (mild-, moderate-, severe NPDR and PDR) and one-time attendance (moderate-, severe NPDR and PDR) with relative risk ratios (RRR) of 1.68, 2.27, 3.14, 2.44 and 1.18, 2.07, 1.26, respectively (P < 0.05). Delays at previous screenings were associated with progression to severe NPDR or PDR (hazard ratio (HR) 2.27, 6.25 and 12.84 for 1, 2 and 3+ delays, respectively). Any given delay doubled the risk of progression (HR 2.28).
Conclusions
In a national cohort of 205,970 patients, almost half of the patients attended DR screening later than scheduled or dropped out after first screening episode. This was, in particular, true for patients with any levels of DR at baseline. DR progression in patients with delayed attendance, increased with the number of missed appointments.
Keywords: Complications, Diabetes, Diabetic retinopathy, Attendance, Progression, Screening
Introduction
Diabetic retinopathy (DR) is a frequent complication of diabetes, and sight-threatening DR is among the leading causes of preventable blindness in the working-age population [1]. According to the International Diabetes Federation, the global prevalence of diabetes is 10.5% equivalent to 536.6 million people [2] and amongst patients with diabetes the prevalence of DR is approximately 30% [1]. DR, especially at more severe levels, can have vast physical and emotional consequences for the affected patients, and management of the disease requires many resources from healthcare systems [3]. Diabetic eye screening is a crucial part of disease management for all patients with diabetes. In Denmark, screening is recommended immediately after diagnosis of type 2 diabetes and within five years of diagnosis of type 1 diabetes (at age 12 at the earliest) and lifelong screening is recommended [4]. Non-attendance or delay of scheduled screenings might result in new and potential sight-threatening DR changes, that are not discovered timely, hence delaying proper treatment [5]. Incidence of DR can rise significantly in association with delay of screenings [6]. Still the cause for non- and delayed attendance seems to be multifaceted and optimal attendance might be dependent on both incentives and obstacles being prioritized [7, 8]. No studies have, to our knowledge, examined attendance patterns and the potential health consequences in a population-based cohort.
Denmark has a national tax-funded screening program for DR. It is recommended that patients attend screening at either a practicing ophthalmologist or a hospital-based screening facility. Financial reimbursement is provided regardless of screening site and patients with proliferative DR (PDR) or diabetic macular edema are referred for treatment at the public hospital departments of ophthalmology. Denmark is divided geographically into five regions; the Capital Region of Denmark, Central Denmark Region, North Denmark Region, Region Zealand and Region of Southern Denmark [9]. The regions are responsible for the Danish hospitals and the health services provided by practicing physicians, including practicing ophthalmologists. The capital of Denmark, Copenhagen, is located in the Capital Region of Denmark. Screening is done by either retinal fundus photographs alone or by a combination of photographs and clinical examination. Individualized intervals are planned according to national guidelines [4] and defined by the level of DR as well as glycemic control.
In this study, we aimed to utilize the Danish registers to examine attendance patterns in the Danish nationwide DR screening program, to characterize timely, delayed and one-time attending patients, as well as explore the effects of delayed attendance on DR progression.
Methods and materials
Participants
In this retrospective nationwide cohort study, our population was defined by the data in The Danish Registry of Diabetic Retinopathy (DiaBase), which contains data of all patients who had attended DR screening at least once, from January 2, 2013, to December 30, 2018 [10]. We included data from all 591,136 screening visits by 205,970 patients (Table 1), above 18 years of age.
Table 1.
Characteristics of patients at baseline, according to attendance group
| All, n = 205,970 | Timely attendance, n = 109,135 | Delayed attendance, n = 73,242 | One-time attendance, n = 23,593 | P value | |
|---|---|---|---|---|---|
| Sex, % Male | 116,534 (56.6) | 62,567 (57.3) | 40,610 (55.4) | 13,357 (56.6) | < 0.001 |
| Age, Years (IQR) | 66 (55;73) | 66 (56;73) | 65 (54;73) | 66 (54;74) | < 0.001 |
| Marital status | < 0.001 | ||||
| Never married | 30,904 (15.0) | 16,035 (14.7) | 11,050 (15.1) | 3819 (16.2) | |
| Married | 118,764 (57.7) | 63,833 (58.5) | 42,718 (58.3) | 12,213 (51.8) | |
| Widowed or divorced | 56,302 (27.3) | 29,267 (26.8) | 19,474 (26.6) | 7561 (32.0) | |
| Diabetes type, N (%) | < 0.001 | ||||
| Type 1 diabetes | 16,999 (8.3) | 7492 (6.9) | 8375 (11.4) | 1132 (4.8) | |
| Type 2 diabetes | 153,238 (74.4) | 85,786 (78.6) | 48,791 (66.6) | 18,661 (79.1) | |
| Unknown | 35,733 (17.3) | 15,857 (14.5) | 16,076 (21.9) | 3800 (16.1) | |
| DR level (ICDR), N (%)A | < 0.001 | ||||
| No DR | 171,633 (83.3) | 95,507 (87.5) | 55,464 (75.7) | 20,662 (87.6) | |
| Mild NPDR | 20,964 (10.2) | 9009 (8.3) | 10,157 (13.9) | 1798 (7.6) | |
| Moderate NPDR | 6551 (3.2) | 2405 (2.2) | 3583 (4.9) | 563 (2.4) | |
| Severe NPDR | 1153 (0.6) | 327 (0.3) | 687 (0.9) | 139 (0.6) | |
| PDR | 5165 (2.5) | 1727 (1.6) | 3007 (4.1) | 431 (1.8) | |
| Charlson comorbidity index score, N (%)B | < 0.001 | ||||
| Low | 148,615 (72.2) | 79,792 (73.1) | 51,920 (70.9) | 16,903 (71.6) | |
| Moderate low | 27,728 (13.5) | 12,984 (11.9) | 11,798 (16.1) | 2946 (12.5) | |
| Moderate high | 18,721 (9.1) | 10,252 (9.4) | 6137 (8.4) | 2332 (9.9) | |
| High | 10,906 (5.3) | 6107 (5.6) | 3387 (4.6) | 1412 (6.0) | |
| Screening facility, N (%) | < 0.001 | ||||
| Private practice | 161,418 (78.4) | 89,210 (81.7) | 53,241 (72.7) | 18,967 (80.4) | |
| Hospital | 44,552 (21.6) | 19,925 (18.3) | 20,001 (27.3) | 4626 (19.6) | |
| Region of screening, N (%) | < 0.001 | ||||
| Capital region of Denmark | 53,303 (25.9) | 24,363 (22.3) | 20,908 (28.5) | 8032 (34.0) | |
| Region Zealand | 33,299 (16.2) | 17,531 (16.1) | 11,332 (15.5) | 4436 (18.8) | |
| Central Denmark region | 41,499 (20.1) | 24,581 (22.5) | 12,733 (17.4) | 4185 (17.7) | |
| North Denmark Region | 22,248 (10.8) | 9945 (9.1) | 9761 (13.3) | 2542 (10.8) | |
| Region of Southern Denmark | 55,575 (27.0) | 32,690 (30.0) | 18,488 (25.2) | 4397 (18.6) | |
| Socioeconomic status | |||||
| Income (household net worth), N (%) | < 0.001 | ||||
| Low | 50,484 (24.5) | 23,942 (21.9) | 19,704 (26.9) | 6838 (29.0) | |
| Moderate low | 50,310 (24.4) | 26,383 (24.2) | 18,140 (24.8) | 5787 (24.5) | |
| Moderate high | 50,953 (24.7) | 27,857 (25.5) | 17,640 (24.1) | 5456 (23.1) | |
| High | 52,660 (25.6) | 29,491 (27.0) | 17,711 (24.2) | 5458 (23.1) | |
| Education, N (%) | < 0.001 | ||||
| Lower secondary | 77,796 (37.8) | 40,620 (37.2) | 27,676 (37.8) | 9500 (40.3) | |
| Upper secondary | 85,012 (41.3) | 45,902 (42.1) | 29,880 (40.8) | 9230 (39.1) | |
| Post-secondary | 36,122 (17.5) | 19,103 (17.5) | 13,232 (18.1) | 3787 (16.1) | |
| Occupation, N (%) | < 0.001 | ||||
| Employed or employer | 58,533 (28.4) | 31,016 (28.4) | 21,332 (29.1) | 6185 (26.2) | |
| Student or other | 5179 (2.5) | 2571 (2.4) | 1953 (2.7) | 655 (2.8) | |
| Early retirement | 28,404 (13.8) | 14,608 (13.4) | 10,526 (14.4) | 3270 (13.9) | |
| Retirement | 101,135 (49.1) | 54,663 (50.1) | 34,882 (47.6) | 11,590 (49.1) | |
| Unemployed | 12,715 (6.2) | 6274 (5.7) | 4549 (6.2) | 1892 (8.0) | |
| Ethnic background, N (%) | < 0.001 | ||||
| Danish heritage | 183,476 (89.1) | 98,237 (90.0) | 65,072 (88.8) | 20,167 (85.5) | |
| Other heritage | 22,457 (10.9) | 10,882 (10.0) | 8160 (11.1) | 3415 (14.5) |
Results given as number (%) or median (IQR). AClassification of DR given by the International Clinical Diabetic Retinopathy Severity Scale [30], BExcluding diabetes
Data Sources
We utilized the Danish national registers where all data can be linked on an individualized level. This includes entire medical records, socioeconomic data and prescription medication usage.
Diabase, which defined our population, contains data reported by the screening ophthalmologist, and the database has approximately 100,000 additions annually [11]. From DiaBase, we extracted reported and planned screening dates, DR level according to the International Clinical DR severity scale (ICDR scale, no DR = 0, mild non-proliferative DR (NPDR) = 1, moderate NPDR = 2, severe NPDR = 3 or PDR = 4), screening facility (hospital or practicing ophthalmologists) and geographical region of screening (Capital Region of Denmark, Central Denmark Region, North Denmark Region, Region Zealand and Region of Southern Denmark).
In addition to DiaBase, we utilized the following registers:
The Danish Civil Registry (1968) was used to link data across registries using an individual identification number (CPR number) given to all citizens in Denmark [12]. We extracted date of birth, sex (female or male), status (alive, institutionalized, living in Greenland, living abroad, missing or dead) and marital status (never married, married or divorced/widowed). The Danish National Patient Register (1976) contains information on all patients treated at Danish hospitals. This includes the specific department, diagnoses according to the International Classification of Disease (ICD) version ten codes, surgical procedures, treatments and other procedures [13]. The Danish National Prescription Registry (1994) is a unique pharmacological register and one of the largest of its kind worldwide [14]. The registry contains information on all collected prescriptions of medicine nationwide, connected to CPR number. This includes information on the Anatomical Therapeutic Chemical (ATC) classification of the medication as well as detailed information on all prescriptions. The Danish National Patient Register and The Danish National Prescription Registry were utilized for categorizing diabetes type (type 1, type 2 or unknown) [15] as well as to categorize patients' comorbidities according to the Charlson Comorbidity Index score (CCI, 1 = low, 2 = moderate low, 3 = moderate high or 4 = high) [16]. Furthermore, socioeconomic data were acquired from Statistics Denmark [17]; we extracted information on equivalent household income (low, moderate low, moderate high and high), highest achieved level of education (lower secondary, upper secondary and post-secondary) in accordance with the International Standard Classification of Education (ISCED) [18], affiliation to the labor market (employed, student, unemployed, early retirement or retirement) [19] and ethnicity (Danish or other).
Quantitative variables
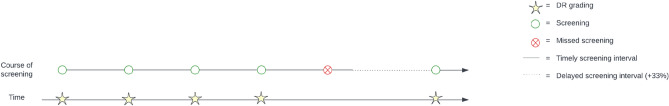
The index date was defined as the first screening date, and delay was calculated according to the next recommended screening interval, as given by the screening physician. Patients were classified as having timely attendance if they were never delayed > 33% and did not miss any screenings during follow-up. Patients were classified as delayed if the actual date of the next screening was registered beyond 33% of the intended interval, e.g., a patient with a recommended interval of 90 days, would therefore be classified as delayed if the next screening date was more than 30 days after the planned screening date (Fig. 1).
Fig. 1.
Timeline illustration of intervals and screening visits with DR gradings, indicating risk time as delayed screening interval
One-time attendance was defined as a patient only participating in screening once, with no follow-up appointments, without apparent reason. Patients with a scheduled next screening date beyond the observation period or who were referred for treatment (for DR or other eye-related illness) and therefore exited the screening program, as well as patients, who disappeared or died before their next screening, were censored at exit date and, thus, only included in the analyses in the periods where they could be clearly classified. DR progression was defined as a worsening in DR to either severe NPDR or PDR in either eye.
Statistical methods
Descriptive data on the population were reported in numerical format with percentages for all variables except age, which was reported in median and interquartile range. Statistical significance was calculated using the Chi2 test. Using a multinomial logistic regression model with relative risk ratio (RRR) calculations, we compared the characteristics of patients with delayed and one-time attendance to patients with timely attendance depending on various exposure variables. The model included a crude, semi-adjusted (age and gender) and fully adjusted multivariable analysis, adjusted for all statistically significant exposure variables from Table 1 (age, gender, marital status, diabetes type, DR level, modified CCI score (excluding diabetes), screening facility, geographical region of screening, income, education length, occupation and ethnic descent). A multivariable Cox regression model with hazard ratios (HRs) was performed to examine a potential risk of progression in DR level that could be associated with delayed screening intervals. Time-varying analyses were utilized to examine each individual screening period. A period was defined as the time from one screening to next screening and could be timely or delayed. Risk time only included delayed periods, and time splitting at missed screening visits was utilized to define delayed periods from timely periods. A patient stopped contributing with risk time, when they attending a screening again, but could contribute again later on, if another > 33% delay occurred (Fig. 1). All analyses were done in Stata 17 (StataCorp, College Station, Texas, USA), and P-values < 0.05 and confidence intervals (CIs) not including 1.0 were considered statistically significant.
Results
Descriptive data
The population (n = 205,970) consisted of 56.6% males, had a median age of 65.7 years (55;73), and 89.1% were of Danish lineage (Table 1, Fig. 2). Baseline prevalence of DR was 16.5% (10.2%, 3.2%, 0.6% and 2.5% for levels 1–4, respectively). Rates of timely attendance, delayed attendance and one-time attendance in the population was 53.0%, 35.5% and 11.5%, respectively. Compared to patients with timely attendance, delayed attendance and one-time attendance were more often observed in females (42.7% vs. 44.6% and 43.4%), non-married patients (14.7% vs. 15.1% and 16.2%) and patients of other ethnic descent than Danish (10.0% vs. 11.1% and 14.5%). Furthermore, compared to patients with timely attendance, patients with delayed attendance had a higher prevalence of DR (12.5% vs. 24.3%), more often type 1 diabetes (6.9% vs. 11.4%) and were screened more frequently at hospitals (18.3% vs. 27.3%). Patients with one-time attendance were more comparable to patients with timely attendance in all three parameters (12.4%, 4.8% and 19.6%). Patients from all five Danish regions were represented, but with varying degrees of adherence. The Central Denmark Region had the highest percentage of attendance, and the North Denmark Region had the lowest (59.2% vs. 44.7%). The North Denmark Region had the highest number of patients with delayed attendance, while the Central Denmark Region had the lowest, within their screened populations (43.9% vs. 30.7%). The highest number of patients with one-time attendance was found in the Capital Region of Denmark and the lowest in the Region of Southern Denmark (15.1% vs. 7.9%). Compared to patients with timely attendance, delayed and one-time attendance were more often observed in patients with lower income (26.9% and 29.0% vs. 21.9%), lower educational level (37.8% and 40.3% vs. 37.2%) and a higher rate of unemployment (6.2% and 8.0% vs. 5.7%).
Fig. 2.
Flowchart with key elements of study design
Main results
Delayed attendance
The multivariable multinomial logistic regression (Table 2) showed that patients with delayed attendance were less likely to be of male gender (RRR 0.94 (95% CI 0.92–0.96)), be older in age (40–59 years (RRR 0.79 (95% CI 0.75;0.85)), 60–79 years (0.76 (95% CI 0.72;0.81)), 80+ years (0.78 (95% CI 0.73;0.84)) and have type 2 diabetes (0.67 (95% CI 0.64;0.70)) compared to patients attending screening at recommended intervals. Having delayed attendance was associated with being either divorced/widowed or married (RRR 1.14 (95% CI 1.10;1.18) and 1.19 (95% CI 1.15;1.23)), having DR level 1–4 (RRR 1.68 (95% CI 1.63;1.74), 2.27 (95% CI 2.14;2.40), 3.14 (95% CI 2.72;3.62), 2.44 (95% CI 2.29;2.61)), a CCI score of 1 (RRR 1.08 (95% CI 1.04;1.11)) and being screened at a hospital based facility (RRR 1.07 (95% CI 1.04;1.10)) in either the Capital Region of Denmark of Denmark (RRR 1.31 (95% CI 1.28;1.35)) or the North Denmark Region (RRR 1.52 (95% CI 1.46;1.58)). Socioeconomically, delayed attendance was mainly associated with having a low income (RRR 1.19 (95% CI 1.16;1.23)), but also being employed (RRR 1.22 (95% CI 1.14;1.31)), in retirement (RRR 1.33 (95% CI 1.23;1.44)) or in early retirement (RRR 1.23 (95% CI 1.14;1.33)).
Table 2.
Multinomial regression showing the risk of delayed and one-time attendance according to exposure variables, reported in relative risk ratios (RRR) with 95% confidence intervals (CI)
| Delayed attendees | One-time attendees | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | RRR crude (95%CI) | RRR semi adjusted (95%CI) | RRR fully adjusted (95%CI) | P-value | n (%) | RRR crude (95%CI) | RRR semi adjusted (95%CI) | RRR fully adjusted (95%CI) | P-value | |
| Sex | ||||||||||
| Male | 40,610 (55.4) | 0.93 (0.91;0.94) | 0.92 (0.90;0.94) | 0.94 (0.92;0.96) | 0.0000 | 13,357 (56.6) | 0.97 (0.94;1.00) | 0.97 (0.94;1.00) | 1.04 (1.01;1.08) | 0.0083 |
| Female | 32,632 (44.6) | Ref | Ref | Ref | 10,236 (43.4) | Ref | Ref | Ref | ||
| Age, Years | ||||||||||
| 18–39 | 5823 (8.0) | Ref | Ref | Ref | 1538 (6.5) | Ref | Ref | Ref | ||
| 40–59 | 21,027 (28.7) | 0.69 (0.66;0.72) | 0.69 (0.66;0.72) | 0.79 (0.75;0.83) | 0.0000 | 7145 (30.3) | 0.89 (0.83;0.94) | 0.89 (0.83;0.94) | 0.70 (0.65;0.75) | < 0.001 |
| 60–79 | 40,223 (54.9) | 0.63 (0.61;0.66) | 0.64 (0.61;0.66) | 0.76 (0.72;0.81) | 0.0000 | 11,865 (50.3) | 0.71 (0.67;0.75) | 0.71 (0.67;0.75) | 0.53 (0.49;0.58) | < 0.001 |
| 80+ | 6169 (8.4) | 0.62 (0.59;0.65) | 0.62 (0.59;0.65) | 0.78 (0.73;0.84) | 0.0000 | 3045 (12.9) | 1.16 (1.08;1.24) | 1.16 (1.08;1.24) | 0.83 (0.75;0.92) | < 0.001 |
| Marital Status | ||||||||||
| Never married | 11,050 (15.1) | Ref | Ref | Ref | 3819 (16.2) | Ref | Ref | Ref | ||
| Married | 42,718 (58.3) | 0.97 (0.95;1.00) | 1.08 (1.05;1.11) | 1.19 (1.15;1.23) | 0.0000 | 12,213 (51.8) | 0.80 (0.77;0.84) | 0.82 (0.78;0.85) | 0.85 (0.81;0.89) | < 0.001 |
| Widowed or divorced | 19,474 (26.6) | 0.97 (0.94;1.00) | 1.11 (1.07;1.15) | 1.14 (1.10;1.18) | 0.0000 | 7561 (32.0) | 1.08 (1.04;1.13) | 1.12 (1.06;1.17) | 1.09 (1.03;1.15) | 0.0014 |
| Diabetes type, N (%) | ||||||||||
| Type 1 diabetes | 8375 (11.4) | Ref | Ref | Ref | 1132 (4.8) | Ref | Ref | Ref | ||
| Type 2 diabetes | 48,791 (66.6) | 0.51 (0.49;0.53) | 0.53 (0.51;0.55) | 0.67 (0.64;0.70) | 0.0000 | 18,661 (79.1) | 1.44 (1.35;1.54) | 1.55 (1.44;1.66) | 1.47 (1.36;1.59) | < 0.001 |
| Unknown | 16,076 (21.9) | 0.91 (0.87;0.94) | 0.94 (0.91;0.98) | 0.99 (0.95;1.04) | 0.7542 | 3800 (16.1) | 1.59 (1.48;1.70) | 1.69 (1.57;1.82) | 1.53 (1.41;1.66) | < 0.001 |
| DR level (ICDR), N (%)A | ||||||||||
| No DR | 55,464 (75.7) | Ref | Ref | Ref | 20,662 (87.6) | Ref | Ref | Ref | ||
| Mild NPDR | 10,157 (13.9) | 1.94 (1.88;2.00) | 1.92 (1.86;1.98) | 1.68 (1.63;1.74) | 0.0000 | 1798 (7.6) | 0.92 (0.88;0.97) | 0.92 (0.87;0.97) | 0.95 (0.90;1.01) | 0.0959 |
| Moderate NPDR | 3583 (4.9) | 2.57 (2.43;2.70) | 2.54 (2.41;2.68) | 2.27 (2.14;2.40) | 0.0000 | 563 (2.4) | 1.08 (0.99;1.19) | 1.08 (0.99;1.19) | 1.18 (1.06;1.30) | 0.0017 |
| Severe NPDR | 687 (0.9) | 3.62 (3.17;4.13) | 3.51 (3.07;4.00) | 3.14 (2.72;3.62) | 0.0000 | 139 (0.6) | 1.96 (1.61;2.40) | 1.96 (1.61;2.39) | 2.07 (1.67;2.57) | < 0.001 |
| PDR | 3007 (4.1) | 3.00 (2.82;3.18) | 2.96 (2.79;3.14) | 2.44 (2.29;2.61) | 0.0000 | 431 (1.8) | 1.15 (1.04;1.28) | 1.15 (1.04;1.28) | 1.26 (1.13;1.42) | < 0.001 |
| Charlson comorbidity index score, N (%)B | ||||||||||
| Low | 51,920 (70.9) | Ref | Ref | Ref | 16,903 (71.6) | Ref | Ref | Ref | ||
| Moderate low | 11,798 (16.1) | 1.40 (1.36;1.44) | 1.41 (1.37;1.45) | 1.08 (1.04;1.11) | 0.0000 | 2946 (12.5) | 1.07 (1.03;1.12) | 1.07 (1.03;1.12) | 1.04 (0.99;1.09) | 0.1280 |
| Moderate high | 6137 (8.4) | 0.92 (0.89;0.95) | 0.96 (0.93;1.00) | 0.87 (0.84;0.90) | 0.0000 | 2332 (9.9) | 1.07 (1.02;1.13) | 1.08 (1.03;1.14) | 1.07 (1.02;1.13) | 0.0068 |
| High | 3387 (4.6) | 0.85 (0.82;0.89) | 0.91 (0.87;0.95) | 0.74 (0.71;0.78) | 0.0000 | 1412 (6.0) | 1.09 (1.03;1.16) | 1.10 (1.04;1.17) | 1.09 (1.02;1.16) | 0.0090 |
| Screening facility, N (%) | ||||||||||
| Practicing Ophthalmologist | 53,241 (72.7) | Ref | Ref | Ref | 18,967 (80.4) | Ref | Ref | Ref | ||
| Hospital | 20,001 (27.3) | 1.68 (1.64;1.72) | 1.65 (1.61;1.69) | 1.07 (1.04;1.10) | 0.0000 | 4626 (19.6) | 1.09 (1.05;1.13) | 1.09 (1.05;1.14) | 0.92 (0.88;0.96) | < 0.001 |
| Region of screening, N (%) | ||||||||||
| Capital region of Denmark | 20,908 (28.6) | 1.33 (1.29;1.37) | 1.31 (1.28;1.35) | 1.30 (1.26;1.34) | 0.0000 | 8032 (34.0) | 1.30 (1.25;1.36) | 1.30 (1.25;1.36) | 1.31 (1.26;1.37) | < 0.001 |
| Region Zealand | 11,332 (15.5) | Ref | Ref | Ref | 4436 (18.8) | Ref | Ref | Ref | ||
| Central Denmark region | 12,733 (17.4) | 0.80 (0.78;0.83) | 0.79 (0.77;0.82) | 0.77 (0.74;0.79) | 0.0000 | 4185 (17.7) | 0.67 (0.64;0.70) | 0.67 (0.64;0.70) | 0.68 (0.65;0.72) | < 0.001 |
| North Denmark region | 9761 (13.3) | 1.52 (1.46;1.57) | 1.52 (1.46;1.57) | 1.52 (1.46;1.58) | 0.0000 | 2542 (10.8) | 1.01 (0.96;1.07) | 1.01 (0.96;1.07) | 1.01 (0.96;1.08) | 0.6338 |
| Region of Southern Denmark | 18,488 (25.2) | 0.87 (0.85;0.90) | 0.87 (0.85;0.90) | 0.84 (0.81;0.87) | 0.0000 | 4397 (18.6) | 0.53 (0.51;0.56) | 0.53 (0.51;0.56) | 0.51 (0.49;0.54) | < 0.001 |
| socioeconomic status | ||||||||||
| income (Household net worth), N (%) | ||||||||||
| Low | 19,704 (26.9) | 1.20 (1.17;1.23) | 1.19 (1.16;1.22) | 1.19 (1.16;1.23) | 0.0000 | 6838 (29.0) | 1.30 (1.25;1.35) | 1.30 (1.25;1.35) | 1.18 (1.13;1.24) | < 0.001 |
| Moderate low | 18,140 (24.8) | Ref | Ref | Ref | 5787 (24.6) | Ref | Ref | Ref | ||
| Moderate high | 17,640 (24.1) | 0.92 (0.90;0.95) | 0.93 (0.90;0.95) | 0.91 (0.89;0.94) | 0.0000 | 5456 (23.2) | 0.89 (0.86;0.93) | 0.89 (0.86;0.93) | 0.92 (0.88;0.96) | < 0.001 |
| High | 17,711 (24.2) | 0.87 (0.85;0.90) | 0.88 (0.86;0.90) | 0.84 (0.82;0.87) | 0.0000 | 5458 (23.2) | 0.84 (0.81;0.88) | 0.84 (0.81;0.88) | 0.85 (0.81;0.89) | < 0.001 |
| Education, N (%) | ||||||||||
| Lower secondary | 27,676 (39.1) | 0.98 (0.96;1.01) | 1.01 (0.98;1.04) | 0.97 (0.94;1.00) | 0.0254 | 9500 (42.2) | 1.18 (1.13;1.23) | 1.19 (1.14;1.24) | 1.11 (1.06;1.16) | < 0.001 |
| Upper secondary | 29,880 (42.2) | 0.94 (0.92;0.97) | 0.95 (0.93;0.98) | 0.94 (0.91;0.96) | 0.0000 | 9230 (41.0) | 1.01 (0.97;1.06) | 1.02 (0.97;1.06) | 0.97 (0.93;1.02) | 0.2127 |
| Post-secondary | 13,232 (18.7) | Ref | Ref | Ref | 3787 (16.8) | Ref | Ref | Ref | ||
| OCCUPATION, N (%) | ||||||||||
| Employed or employer | 21,332 (29.1) | 0.91 (0.85;0.96) | 1.10 (1.03;1.17) | 1.22 (1.14;1.31) | 0.0000 | 6185 (26.2) | 0.78 (0.72;0.86) | 0.76 (0.69;0.83) | 0.93 (0.84;1.03) | 0.1658 |
| Student or other | 1953 (2.7) | Ref | Ref | Ref | 655 (2.8) | Ref | Ref | Ref | ||
| Early retirement | 10,526 (14.4) | 0.95 (0.89;1.02) | 1.10 (1.03;1.19) | 1.23 (1.14;1.33) | 0.0000 | 3270 (13.9) | 1.18 (1.07;1.31) | 1.16 (1.04;1.28) | 0.85 (0.76;0.95) | 0.0043 |
| Retirement | 34,882 (47.6) | 0.95 (0.89;1.01) | 1.20 (1.12;1.28) | 1.33 (1.23;1.44) | 0.0000 | 11,590 (49.1) | 0.88 (0.80;0.97) | 0.84 (0.76;0.93) | 0.87 (0.78;0.98) | 0.0209 |
| Unemployed | 4549 (6.2) | 0.84 (0.79;0.89) | 1.30 (1.21;1.40) | 1.08 (0.99;1.16) | 0.0680 | 1892 (8.0) | 0.83 (0.76;0.91) | 0.77 (0.69;0.85) | 1.03 (0.92;1.16) | 0.5775 |
| Ethnic background, N (%) | ||||||||||
| Danish heritage | 65,072 (88.9) | Ref | Ref | Ref | 20,167 (85.5) | Ref | Ref | Ref | ||
| Other heritage | 8160 (11.1) | 1.13 (1.10;1.17) | 1.08 (1.05;1.12) | 0.98 (0.94;1.01) | 0.2181 | 3415 (14.5) | 1.53 (1.47;1.59) | 1.53 (1.47;1.60) | 1.20 (1.14;1.26) | < 0.001 |
Data are given as numbers (n, (%)) and relative risk ratios (95% CI). Reference group is patients with timely attendance. AClassification of DR given by the International Clinical Diabetic Retinopathy Severity Scale [30], BExcluding diabetes. Semi adjusted model adjusted for sex and age. Fully adjusted model adjusted for all statistically significant variables in Table 1
One-time attendance
One-time attendance was associated with being male (RRR 1.04 (95% CI 1.01;1.08)), divorced or widowed (RRR 1.09 (95% CI 1.03;1.15)), having type 2 diabetes (RRR 1.47 (95% CI 1.36;1.59)), DR level 2–4 ((RRR 1.18 (95% CI 1.06;1.30), 2.07 (95% CI 1.67;2.57), 1.26 (95% CI 1.13;1.42)) or CCI scores of 2 or 3 ((RRR 1.07 (95% CI 1.02;1.13), 1.09 (95% CI 1.02;1.16)) compared to patients attending screening at recommended intervals (Table 2). One-time attending patients were more likely to be screened in the Capital Region of Denmark of Denmark (RRR 1.31 (95% CI 1.26;1.37)). One-time attendance was inversely associated with age (40–59 (RRR 0.70 (95% CI 0.65;0.75)), 60–79 (0.53 (95% CI 0.49;0.58)) and 80+ (0.83 (95% CI 0.75;0.92)). Socioeconomically one-time attendance was associated with a low income (RRR 1.18 (95% CI 1.13;1.24)), lower educational length (RRR 1.11 (95% CI 1.06;1.16)) and other ethnic heritage than Danish (RRR 1.20 (95% CI 1.14;1.26)).
Progression
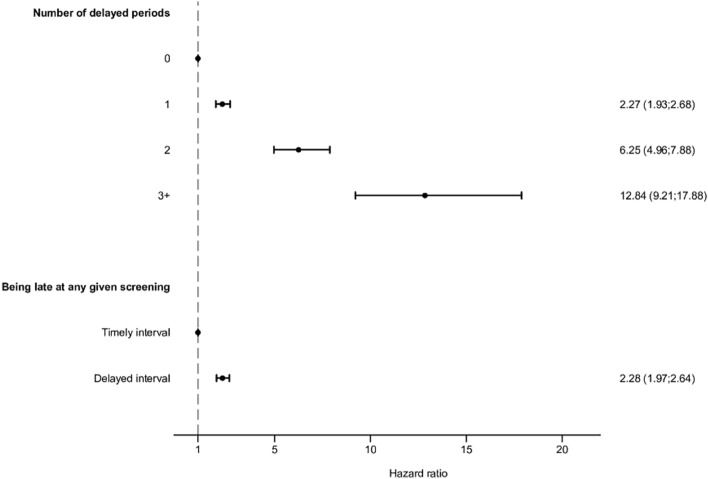
Cox regression analysis (Table 3, Fig. 3) showed that any delay in screening resulted in double the risk of progression to severe NPDR or PDR (2.28 HR (95% CI 1.97;2.64). Patients with past delayed intervals were more likely to experience disease progression to severe NPDR or PDR during follow-up; the risk increased by the number of missed appointments so that patients with delays in 1, 2 or 3+ appointments had increased risks of HR 2.27 (95% CI 1.93;2.68), HR 6.25 (95% CI 4.96;7.88) and HR 12.84 (95% CI 9.21;17.88) for progression, compared to patients who attended screenings timely.
Table 3.
Risk of progression to severe non-proliferative diabetic retinopathy (DR) or proliferative DR according to number of delays, given in hazard ratios (HR) and 95% confidence intervals (CI)
| Events | Risk time | Crude HR (CI 95%) | Semi adjusted HR (CI 95%) | Fully adjusted HR (CI 95%) | P-value | |
|---|---|---|---|---|---|---|
| Number of delayed periods | ||||||
| 0 | 1015 | 324,108.39 | Ref | Ref | Ref | |
| 1 | 670 | 123,844.96 | 2.34 (1.97;2.79) | 2.26 (1.90;2.68) | 2.27 (1.93;2.68) | < 0.001 |
| 2 | 229 | 12,869.24 | 8.80 (6.89;11.24) | 7.40 (5.82;9.41) | 6.25 (4.96;7.88) | < 0.001 |
| 3+ | 75 | 1812.18 | 21.15 (15.13;29.57) | 17.18 (12.29;24.03) | 12.84 (9.21;17.88) | < 0.001 |
| Any given delay | ||||||
| Timely interval | 1321 | 379,180.86 | Ref | Ref | Ref | |
| Delayed interval | 574 | 80,626.27 | 2.09 (1.81;2.42) | 2.07 (1.79;2.39) | 2.28 (1.97;2.64) | < 0.001 |
Data are given as numbers and hazard ratios (confidence interval). Semi adjusted model adjusted for sex and age. Fully adjusted model adjusted for all statistically significant variables in Table 1. AProgressions. BRisk time given in person-days per 1000
Fig. 3.
Forest plot illustrating the findings of Table 3; the risk of progression to severe non-proliferative diabetic retinopathy (DR) or proliferative DR (PDR) according to number of delayed periods and at any given screening Data are given in hazard ratios (HRs) with confidence intervals (CI)
Discussion
This study is, to our knowledge, the most extensive study in the field of attendance to DR screening, utilizing 591,136 screening episodes by 205,970 patients with diabetes in a nationwide cohort. Our research showed that delayed attendance and one-time attendance of DR screenings were associated with younger age, divorce, lower income, screening in the Capital Region of Denmark, as well as higher levels of DR and competing illnesses. Progression to more advanced DR (severe NPDR and PDR) was seen more often in patients with delayed attendance, and the number of delays was correlated to a significantly increased risk of progression. This is in accordance with a study from England [6], in which the number of missed screenings were examined in a retrospective observational study of 62,067 patients in the North East London Diabetes Eye Screening Programme. A 20% increase in the incidence of referable DR was demonstrated in patients that missed ten or more consecutive appointments. We found that patients age 40 years and above were less likely to be delayed or have one-time attendance, compared to the 60–79 age group. Which is in agreement with previous studies from England [20–23], Ireland [24] and Scotland [25] thus confirming a trend across DR screening programs internationally. Delayed attendance was observed to be vastly increasing according to more severe DR levels at baseline compared to patients with no DR. Paradoxically, the patients who needed the timely screenings the most, were the ones who utilized it the least. This, in turn, could also be part of the explanation as to why their DR was in fact of a more severe level. It should be noted that the groups of patients diagnosed with severe NPDR made up a small percentage of the cohort as a whole, and therefore, there might be a larger statistical uncertainty in the results for these patients. Several studies examining the incentives and barriers of patients to DR screening found that a great facilitator to attendance was the knowledge of the potential consequences of non-attendance on vision and DR progression [24, 26–28]. This could be a point of focus to ensure proper communication and dissemination of DR awareness from healthcare professionals to patients with diabetes—in Denmark, as well as internationally. This could also help combat the anxiety that might counterintuitively keep some patients from attending a screening, because the fear of a severe examination result or the possible societal stigma is too overwhelming. Patients who attended screenings at practicing ophthalmologists were more adherent to their given intervals than patients at hospitals. Because of the centralization of larger hospitals in Denmark, access to practicing ophthalmologists might be logistically easier and more accessible to patients, especially in rural areas. Distance to the screening facility has previously been shown as a barrier to screening [8]. To increase the convenience for patients, DR screenings can often be timed with other diabetes-related screenings including podiatry, cardiology and endocrinology appointments at most Danish hospitals. Attendance in the different geographical regions of Denmark varied; although we observed a greater non-adherence in the North Denmark Region, we also observed this in the Capital Region of Denmark of Denmark, where patients were more likely to have both delayed and one-time attendance. This could be due to the more diverse population composition in metropolitan areas, including younger people, with lower incomes. Technical issues, partly due to the implementation of a new electronic medical record system, might also have affected the data received in DiaBase from hospitals in Region Zealand and the Capital Region of Denmark, introducing a potential bias. Patients with type 2 diabetes were more likely to only attend screening once compared to patients with type 1 diabetes. This could be due to the fact that type 2 diabetes often is discovered later in life, and perhaps in relation to other lifestyle-related illnesses; patients might, therefore, not be accustomed to the sudden burden of appointments this entails. One-time attendance could partly be explained by patients with pre-diabetes or who are undergoing a medical investigation to determine a potential diabetes diagnosis, that have been recommended a screening by their general practicing physician. We found a correlation between both delayed and one-time attendance and general comorbidity in regards to higher CCI scores across the regression analysis, indicating that patients who are suffering from competing illnesses might not have the surplus to also keep up screening at timely intervals, or at all. Socioeconomic deprivation in terms of low income and unemployment was seen as risk factor for delayed and one-time attendance. The risk of non-adherence was lower in patients with higher incomes, showcasing a potential distortion and inequality in health care access according to income. Several studies credit socioeconomic deprivation as the leading cause of non-attendance [25, 29], and even though an association in a Danish setting is apparent in regard to low income and non-Danish descent, it might not be as stark due to the generally flatter societal structure as well as the completely tax-funded healthcare system, where no out of pocket expenses are needed. Length of education did not significantly change the odds of delayed or one-time attendance, as seen in previous studies [7].
The inclusion of a large nationwide cohort with a considerable amount of screenings, and detailed, validated register information on an individualized level, is a clear strength of this study. The addition of socioeconomic data ensured the completeness of the characterization of the study population.
As our study focused on adherence to the screening program, and patients attending DR screening at least once, we did not address the issue of patients never attending screening, which might add another dimension. Due to the register-based nature of the study, the subjective reasons for non-adherence were not addressed. This would, however, be important for future reference, as an involvement of patients and a prioritization of their prerogative will be crucial in order to improve attendance.
In conclusion, our study of non-adherence successfully added information on a population basis using a national cohort of patients in the Danish screening program of DR in Denmark. We highlighted younger age, divorce, presence of DR, competing illnesses and low income as the characteristics of patients with delayed and one-time attendance in the Danish screening program and showed twice the risk of progression to severe NPDR and PDR in patients with delayed attendance.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analyses were performed by AST, LMS, LR and SM. The first draft of the manuscript was written by AST, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Funding for this study was obtained by the VELUX Foundation (Grant Number (00028744)), MLJ & GH Foundation and Steno Diabetes Center Odense. The study funders were not involved in the design of the study; the collection, analysis, and interpretation of data; writing the manuscript; and did not impose any restrictions regarding the publication.
Data availability
The datasets created and analyzed during the current study are available from the Danish Health Data Authority and Statistics Denmark, but restrictions apply to the availability of these data, which were used under license from OPEN and Danish Health Data Authority and are not publicly available.
Declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in accordance with the principles of the Declaration of Helsinki. No ethical approvals or informed consent agreements were required, as this is an observational register-based study.
Footnotes
This article belongs to the topical collection Eye Complications of Diabetes, managed by Giuseppe Querques.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wong TY, et al. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. doi: 10.1038/nrdp.2016.12. [DOI] [PubMed] [Google Scholar]
- 2.Sun H, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenwick EK, et al. The impact of diabetic retinopathy: understanding the patients’ perspective. Br J Ophthalmol. 2011;95(6):774–782. doi: 10.1136/bjo.2010.191312. [DOI] [PubMed] [Google Scholar]
- 4.Grauslund J, et al. Evidence-based Danish guidelines for screening of diabetic retinopathy. Acta Ophthalmol. 2018;96(8):763–769. doi: 10.1111/aos.13936. [DOI] [PubMed] [Google Scholar]
- 5.Scanlon PH, Aldington SJ, Stratton IM. Delay in diabetic retinopathy screening increases the rate of detection of referable diabetic retinopathy. Diabet Med. 2014;31(4):439–442. doi: 10.1111/dme.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virk R, et al. How is the risk of being diagnosed with referable diabetic retinopathy affected by failure to attend diabetes eye screening appointments? Eye (Lond) 2021;35(2):477–483. doi: 10.1038/s41433-020-0877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashim RM, Newton P, Ojo O. Diabetic retinopathy screening: a systematic review on patients' non-attendance. Int J Environ Res Public Health. 2018;15(1):157. doi: 10.3390/ijerph15010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham-Rowe E, et al. Barriers to and enablers of diabetic retinopathy screening attendance: a systematic review of published and grey literature. Diabet Med. 2018;35(10):1308–1319. doi: 10.1111/dme.13686. [DOI] [PubMed] [Google Scholar]
- 9.Regional Denmark. Danske Regioner. https://www.regioner.dk/services/in-english/regional-denmark
- 10.Andersen N, et al. The Danish registry of diabetic retinopathy. Clin Epidemiol. 2016;8:613–619. doi: 10.2147/CLEP.S99507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DDD, National yearly report Danish Diabetes Database (DDD, The Danish Adult Diabetes Database, Danish Registry of Childhood and Adolescent Diabetes and The Danish Registry of Diabetic Retinopathy). 2020/2021
- 12.Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt M, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7 Suppl):38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 15.Larsen MEC, et al. Diabetic retinopathy as a potential marker of Parkinson's disease: a register-based cohort study. Brain Commun. 2021;3(4):fcab262. doi: 10.1093/braincomms/fcab262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan H, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 17.Møller B et al (2001) Official statistics in Denmark: Socio economic microdata for research
- 18.UNESCO International Standard Classification of Education, ISCED 2011. UNESCO Institute for Statistics
- 19.Quitzau J, Pedersen U Documentations of statistics, labour and income. Statistics Denmark
- 20.Lawrenson JG, et al. Trends in diabetic retinopathy screening attendance and associations with vision impairment attributable to diabetes in a large nationwide cohort. Diabet Med. 2021;38(4):e14425. doi: 10.1111/dme.14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachdeva A, et al. Diabetic retinopathy screening: study to determine risk factors for non-attendance. Diabetes Prim Care. 2012;14(5):308. [Google Scholar]
- 22.Gulliford MC, et al. Socio-economic and ethnic inequalities in diabetes retinal screening. Diabet Med. 2010;27(3):282–288. doi: 10.1111/j.1464-5491.2010.02946.x. [DOI] [PubMed] [Google Scholar]
- 23.Millett C, Dodhia H. Diabetes retinopathy screening: audit of equity in participation and selected outcomes in South East London. J Med Screen. 2006;13:152–155. doi: 10.1258/096914106778440608. [DOI] [PubMed] [Google Scholar]
- 24.Dervan E, et al. Factors that influence the patient uptake of diabetic retinopathy screening. Ir J Med Sci. 2008;177(4):303–308. doi: 10.1007/s11845-008-0192-5. [DOI] [PubMed] [Google Scholar]
- 25.Leese GP, et al. Screening uptake in a well-established diabetic retinopathy screening program: the role of geographical access and deprivation. Diabetes Care. 2008;31(11):2131–2135. doi: 10.2337/dc08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lake AJ, et al. What factors influence uptake of retinal screening among young adults with type 2 diabetes? A qualitative study informed by the theoretical domains framework. J Diabetes Complicat. 2017;31(6):997–1006. doi: 10.1016/j.jdiacomp.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Hipwell AE, et al. Attitudes, access and anguish: a qualitative interview study of staff and patients' experiences of diabetic retinopathy screening. BMJ Open. 2014;4(12):e005498. doi: 10.1136/bmjopen-2014-005498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Eijk KND et al Diabetic retinopathy screening in patients with diabetes mellitus in primary care: incentives and barriers to screening attendance. Diabetes Res Clin Pract [DOI] [PubMed]
- 29.Waqar S, et al. Cost implications, deprivation and geodemographic segmentation analysis of non-attenders (DNA) in an established diabetic retinopathy screening programme. Diabetes Metab Syndr. 2012;6(4):199–202. doi: 10.1016/j.dsx.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson CP, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets created and analyzed during the current study are available from the Danish Health Data Authority and Statistics Denmark, but restrictions apply to the availability of these data, which were used under license from OPEN and Danish Health Data Authority and are not publicly available.