Abstract
Since the discovery of silicate bioactive glass (BG) by Larry Hench in 1969, different classes of BGs have been researched over decades mainly for bone regeneration. More recently, validating the beneficial influence of BGs with tailored compositions on angiogenesis, immunogenicity and bacterial infection, the applicability of BGs has been extended to soft tissue repair and wound healing. Particularly, fibrous wound dressings comprising BG particle reinforced polymer nanofibers and cotton-candy-like BG fibers have been proven to be successful for wound healing applications. Such fibrous dressing materials imitate the physical structure of skin’s extracellular matrix and release biologically active ions e.g. regenerative, pro-angiogenic and antibacterial ions, e.g. borate, copper, zinc, etc., that can provoke cellular activities to regenerate the lost skin tissue and to induce new vessels formation, while keeping an anti-infection environment. In the current review, we discuss different BG fibrous materials meant for wound healing applications and cover the relevant literature in the past decade. The production methods for BG-containing fibers are explained and as fibrous wound dressing materials, their wound healing and bactericidal mechanisms, depending on the ions they release, are discussed. The present gaps in this research area are highlighted and new strategies to address them are suggested.
Keywords: Scaffolds, Fibers, Angiogenesis, Wound healing, Bioactive glass
Highlights.
Wound healing bioactive glass (BG) nano/microfibers are comprehensively reviewed.
BG fibrous wound dressings are made from BG particle reinforced polymer fibers and cotton-candy-like fibers.
Fiber drawing, electrospinning and laser spinning are typical fabrication techniques for BG fibers.
BG fibrous dressings release biologically active ions for angiogenesis and wound healing.
The positive impact of BG fibers on wound healing can be related to their immunogenic impact.
Background
Across the world, skin disruptions and wounds in chronic and even acute form endanger patients’ welfare and indirectly challenge the healthcare systems. For instance, according to the statistics published in 2015 [1], management of chronic wounds costs the National Health Service (NHS) of the UK between £4.5 and £5.1 billion per year. In the UK, 200,000 patients suffer from a chronic wound [2], caused by increasing the incidence of diabetes and obesity and by an aging population. Approximately 1–2% of the general population will experience a chronic wound and up to 25% of diabetic patients will develop an ulcer [3]. To address this crisis, the global advanced wound care market including wound dressings is expected to reach £18.6 billion by 2024 from £14.8 billion in 2019 [4]. Such a market has been growing not only in terms of customer numbers but also technology-wise. To address the dynamic nature of wound healing and its multidimensional objectives, the wound care market is transitioning from classic protective barriers into advanced, bioactive wound dressings, interacting with the wound by stimulating and managing cell migration and the sequence of healing events including inflammation, proliferation and remodeling [5].
The inflammatory phase starts upon fulfillment of hemostasis and whereby pathogens are eliminated from the wound bed. To accomplish this objective, vascular permeability is enhanced via vasodilation, allowing for accumulation of monocytes and neutrophils inside the wound milieu [5]. The proliferation phase follows inflammation after nearly 3 days and proceeds with the formation of collagen and ground substance, driven via the activity of fibroblasts. The fibroblasts existing in the wound bed and those originating from blood, proliferate and migrate, whereby forming wound granulation tissue alongside a new extracellular matrix (ECM). Moreover, some fibroblasts are differentiated into myofibroblasts to engender wound closure [6]. Over the course of the proliferation step, endothelial cells promptly grow and trigger vascularization within the granulation tissue. Eventually, the remodeling (maturation) of the wound tissue results in formation of normal tissue after 2–3 weeks [7].
Compared to the classic dressings made as foams, films, hydrogels and sponges, fibrous wound dressings are an emerging class with distinct advantages. Fibrous dressings provide notable structural resemblance with the ECM in terms of porosity, morphology and mechanical properties [8–10]. Apart from structural similarity, fibrous systems can be made of inorganic and organic materials, providing the necessary biochemical cues for the cells involved in the wound healing process. Among the proposed fibrous materials for wound healing applications, bioactive glass (BG), either as a filler or as the main fiber material, has proven to be a promising candidate with distinct advantages for wound healing, angiogenesis and antibacterial activity through the release of supportive biologically active ions.
BGs are highly bioactive inorganic materials with different compositions, which can be shaped into different physical forms (particulate and fibrous), allowing their implementation as a novel class of wound dressing materials [10–12]. BGs are primarily renowned for their well-investigated potential for bone repair [13,14]. Additionally, in recent years, they have been considered for soft tissue repair [11]. In this regard, the original silicate BG developed by Hench, i.e. 45S5 bioactive glass [15], has been extensively investigated and proposed for various clinical applications. For instance, a fibrous structure made of 45S5 BG has been employed for the treatment of soft tissue ulceration and skin repair [10]. This BG can offer a controlled ion release and ion exchange process and induces formation of a hydroxyapatite (HA) layer upon immersion in the body fluid [10]. As a result, better healing conditions are realized for a soft tissue such as skin, via activation and upregulation of healing factors, including antigen hematopoietic form precursor (CD44), fibroblast growth factor receptor precursor (N-sam), vascular cell adhesion protein precursor, vascular endothelial growth factor (VEGF) precursor, and fibronectin receptor beta subunit [16]. The main cells involved in wound healing are thus provoked to further proliferate and grow when subjected to such factors and accumulate in areas adjacent to the BG surface, thereby forming new skin tissue [10].
In the current review, we aim to highlight the emerging role and significance of BG fibrous materials for wound healing. The involved healing mechanisms are discussed and different types of BGs in terms of composition and form will be introduced, while mentioning the pros and cons of each type. We intend to unravel the available gaps in this research area and propose new solutions to the currently available shortcomings. This might prompt researchers to try new perspectives and approaches. We also discuss commercial BG fibrous dressings and highlight their healing features. It is worthy to note that this topic has been insufficiently studied as reflected in the number of publications coming up in ‘Web of Science’, totaling 26 (bioactive glass fiber) and 18 (bioactive glass + electrospinning + wound healing). This review is thus of relevance to those interested in exploring applications of fibrous BGs in wound healing.
Review
Different types of BGs
BG is a bioactive material that upon in vivo implantation develops a HA surface layer, thereby creating a robust interface with hard tissues (e.g. bone and tooth). Such a HA layer can be also of relevance to enable bonding with soft tissues (e.g. skin) [17]. The chemical composition of BGs can be tailored by inclusion of biologically active ions that provoke particular cellular activities [18,19]. In general, depending on the glass network former, BGs can be classified as silicate BGs, borate BGs and phosphate BGs [19].
Silicate BGs
Silicate BGs were for the first time developed around 50 years ago in the seminal work of Hench and co-workers [20]. Thereafter, the 45S5 BG composition (45 wt.% SiO2, 24.5 wt.% CaO, 24.5 wt.% Na2O, and 6.0 wt.% P2O5) has been extensively investigated for various biomedical applications [21]. From a structural standpoint, this silicate BG comprises a 3D glass-forming SiO2 network [11]. The main compositional characteristics that synergistically bring about bioactivity of 45S5 BG include: (1) inferior SiO2 amount as compared to chemically resistant silicate glasses (e.g. soda-lime glass), (2) relatively high content of CaO and Na2O (glass network modifiers), and (3) high CaO/P2O5 ratio [11]. An alternative silicate BG is the composition 13–93 BG (53% SiO2, 20% CaO, 6% Na2O, 4% P2O5, 12% K2O, 5% MgO in wt.%) [22], which has been developed to exhibit less bioactivity than 45S5 BG and to facilitate fiber fabrication from molten glass [23–25].
Borate BGs
Particular glass-forming systems like borate glasses of certain compositions have also been shown to be bioactive [10]. Borate BG, particularly 13-93B3 glass (54.6% B2O3, 6% Na2O, 22.1% CaO, 7.9% K2O, 1.7% P2O5, 7.7% MgO in mol.%) [26], is more biodegradable and bioactive compared to silicate BGs, thus offering distinct potential for bone and soft tissue repair [27–29]. Borate BGs, thanks to a lower chemical resistance than silicate 45S5 and 1393 BGs, degrade in an even shorter time than silicate BGs and transform majorly to an HA-based material [11,27–29]. For instance, compared to 45S5 BG, 13-93B3 borate glasses have been reported to react with simulated body fluid (SBF) in a five times shorter time [27]. Borate BGs transform to HA in a similar manner to 45S5 silicate BG, yet without formation of the SiO2-rich surface layer [27]. Borate BGs offer promising biological properties, thereby enhancing cell proliferation and cell differentiation in vitro [30,31], and promoting tissue ingrowth in vivo [32]. Their further application as a drug delivery substrate to address the bone infection problem has also been validated [33–35]. Moreover, borate BGs were rapidly considered for wound healing applications [10]. In this regard, cotton-candy-like fibers composed of 13-93B3 glasses have been proven to be effective in healing diabetic ulcers, most likely due to the release of B and Ca ions that can drive the migration process of epidermal cells and govern the wound healing cascade [36,37].
Despite all the therapeutic pros mentioned earlier, borate BGs might induce adverse biological responses, due to the potential toxicity of the released borate ions, (BO3)3−, at relatively high concentrations [11]. As reported by Brown et al. [38], some borate BGs trigger cellular toxicity when tested under ‘static’ in vitro conditions, while remain non-harmful to cells under ‘dynamic’ testing conditions. Substituting silica in 13-93 BG with B2O3 has led to a very popular borate glass, designated as 13-93B3. This glass has been shown to be toxic in vitro against murine MLO-A5 osteogenic cells [32]. In contrast, such a composition has not shown any toxic effects in vivo; on the contrary, the glass encouraged tissue regeneration and ingrowth in rat models [39].
One important characteristic of borate BGs is the tailorability of their degradation rate through partial or total replacement of SiO2 with B2O3 in silicate 45S5 or 13-93 BGs, for instance, to achieve a borosilicate or borate BG [27,28,40]. Moreover, the addition of biologically active ions to a basic borate glass composition provides a useful approach to enhance the biological activity of the BG. A typical example is the addition of Cu ions to 13-93B3 to induce an angiogenic effect [41].
Phosphate BGs
Other than silicate- and borate-based BGs, phosphate BGs, comprising a P2O5 glass-forming network alongside Na2O and CaO modifiers, have also been synthesized for biomedical applications [42,43]. Similar to borate BGs, the degradation rate of phosphate glasses (thus their interaction with cells) can be modulated by tailoring the glass’s chemistry (composition). Such a characteristic further expands the clinical potential of phosphate BGs [11]. The cellular response to phosphate BGs has been tuned via stabilization of the glass network and by controlling their degradation rate, which can be achieved by inclusion of various oxides such as B2O3, TiO2, MgO, ZnO and CuO [44–48]. Phosphate BGs can be formed as microfibers, which enables their applicability in wound healing [49]. For instance, recently, gallium and cerium ion-doped phosphate BG fibers (18MgO–10CaO–24Na2O–45P2O5–3Ga2O3/CeO2 mol.%) have been investigated for wound dressing application [49].
BG-induced wound healing mechanisms
The volume of research on osteogenesis induced by BGs notably prevails over the number of studies dealing with the potentials of BGs for soft tissue regeneration and wound healing. However, the encouraging effect of BGs on angiogenesis has been already validated [50,51]. Given the fact that over the course of the granulation phase, angiogenic rooting of new vessels is of high importance for delivering nutrients and oxygen to the cells present in the wound bed to induce wound healing [52], BG-based therapies that promote angiogenesis can be particularly effective [53]. There are diverse strategies that potentially support angiogenesis, for instance, by biohybrid constructs that contain pro-angiogenic factors such as platelet-derived growth factor (PDGF), VEGF, and basic fibroblast growth factor (bFGF) [54–56]. Yet, this concept is typically costly, might impose undesired biological consequences particularly when it involves supra-physiological doses [57,58], and declines bioactivity [59,60]. In contrast, inorganic pro-angiogenic factors (ions) appear to offer advantages such as optimum stability, low cost and higher clinical safety as compared to the mentioned growth factors [61,62]. For instance, Cu ions have been shown to play a crucial role in the angiogenic response [63] through control of the expression of hypoxia-inducible factor (HIF-1α), thereby simulating hypoxia, that notably contributes to formation of blood vessels [62]. Cu2+ ions provoke the proliferation of endothelial cells and thus angiogenesis through mediation of the release of cytokines and VEGF [64–66]. Additionally, the release of such ions can upregulate growth factor-β (TGF-β), as a pro-angiogenic factor, in diabetic wounds [67,68], which reduces the risk of ischemia in skin flaps [69]. The presence of borate ions in human keratinocyte cultures, even in millimolar concentration, can upregulate matrix metalloproteinases MMP-2 and MMP-9, thereby driving the migration of these cells and promoting remodeling of granulation tissue [70]. As a result, development of BG systems releasing such pro-angiogenic ions can be regarded as a promising, simple strategy for wound healing. In this regard, Jung synthesized BG fibrous scaffolds made of 45S5 and 13-93B3 (with and without CuO), that could release pro-angiogenic ions and encourage soft tissue ingrowth in vivo [71]. Based on a histological analysis, it was shown that the soft tissue growing into the Cu-doped 13-93B3 fibrous scaffold contained a larger microvascular density compared to that found in the 45S5 fibrous scaffold [71].
As reported by Wray in 2011 [72], two kinds of borate BG microfiber dressings composed of 13-93B3 and 13-93B3 with 0.4 wt.% CuO were applied for the purpose of healing cutaneous wounds clinically. According to this study, both BG microfiber dressings were able to optimally heal the wounds in 66% of the patients who took part in the study [72]. Interestingly, 13-93B3 dressing promoted re-epithelialization, while the Cu-doped 13-93B3 BG dressing provoked the formation of granulation tissue. As a result, collectively BG microfibers caused tissue regeneration with proper vascularization [72].
Lin et al. [36] quantitatively characterized the angiogenic response of a soft tissue wound to borate BG microfibers (Figures 1b, c, representing the neat and Cu-doped 13-93B3 microfibers, respectively) in comparison to 45S5 silicate BG microfibers (Figure 1a) and sham implant controls. Figures 1d, e show a high magnification image and a camera image of the borate BG microfibers implanted in an animal model, respectively. As shown in Figures 1f–i, 13-93B3 BG microfibers doped with copper (0.4 wt.%) raised angiogenesis more notably than 45S5 BG microfibers and sham controls did. The superior angiogenic behavior of such BG microfibers stems from beneficial biological effects of the released copper and borate ions on endothelial cell proliferation and vessel formation, as mentioned earlier. In general, the ions released from BG microfibers including silica and copper ions might also induce collagen synthesis, thereby forming a fibrous tissue in addition to that resulting from the inflammatory response against the implanted microfibers [64]. Additionally, possible cytotoxicity of the released borate ions over a long time (4 weeks) was investigated via a histological analysis on a kidney tissue obtained from rats after subcutaneous implantation of the borate BG microfibers. This study did not show any sign of long-term histopathological consequences in kidney [36].
Figure 1.
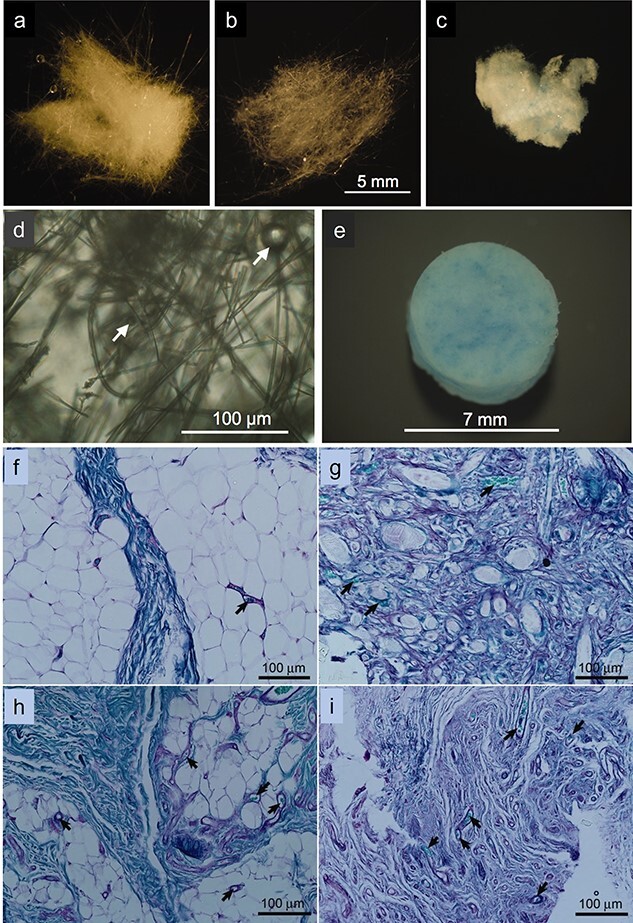
Cotton-like microfibers composed of: (a) 45S5, (b) 13-93B3, and (c) Cu-doped 13-93B3 BGs. (d) Image of 13-93B3 BG microfibers at a high magnification (the arrows mark glass beads). (e) Camera image of the Cu-doped 13-93B3 BG microfiber implant. The periodic acid Schiff (PAS)-stained sections of soft tissue exposed (for 4 weeks) to implanted BG microfibers composed of: (f) none (sham control); (g) 45S5, (h) 13-93B3, and (i) Cu-doped 13-93B3 (the arrows mark the microvessels found in the tissues). Reproduced with permission from [36]. Copyright 2014, John Wiley and Sons
The positive impact of BGs on wound healing can be also related to their promising immunogenic impact. BGs can alter the local microenvironment, thereby modulating the activities of macrophages via physicochemical and biological cues [73,74]. Dong et al. [74] have indicated that the ions released from 45S5 BG particles can not only polarize macrophages as M2 phenotype but also drive them to largely produce anti-inflammatory growth factors. These chemokines and cytokines, such as VEGF, bFGF and TGF-β, are known to play several important roles in the tissue regeneration cycle [75,76]. Among the mentioned growth factors, TGF-β and VEGF, have been shown to employ and attract repairing cells such as fibroblasts and endothelial cells [77], thereby promoting collagen deposition, re-epithelization and vascularization. TGF-β is a cytokine that is involved in all steps of the wound healing process [78]. It provokes the proliferation of fibroblasts and thus the synthesis of ECM. As a result, provisional granulation tissue forms within the wound milieu [79]. Conversely, VEGF and bFGF can support angiogenesis and vascularization during the wound healing process [80]. VEGF as a pro-angiogenic growth factor provokes the migration of endothelial cells and helps them assemble as capillaries [81]. Moreover, bFGF increases the population of new capillaries in the wound bed [82].
Production methods of BG-based fibers
The production method of BG fibers totally depends on the glass processability and melting behavior. For instance, given the narrow sintering window of 45S5 BG, fiber production through typical melt-spinning methods is quite challenging and results in glass crystallization [83]. In contrast, the composition of 13-93BG enables fiber drawing from its melt, though in micron size [84]. As mentioned earlier, nanofibers are more in demand, considering their biomimicry effect and ability to simulate the collagenous ECM in terms of morphology and topography [85]. Therefore, various BG nanofiber production techniques have been developed that suit rheological properties and crystallinity of the BG composition. In this regard, laser spinning and electrospinning are well-known strategies that have advanced the development of BG nanofibers of high quality (desired composition) and quantity.
Laser spinning
Laser spinning allows for production of BG micro/nanofibers in a large quantity [86]. The technique is applicable for any specific, predefined chemical composition of BGs with no need for inclusion of chemical additives or a subsequent thermal treatment [87]. For instance, with respect to 45S5 BG, the only possible nanofiber fabrication technique that can produce amorphous 45S5 BG nanofibers is laser spinning [83], as shown in Figure 2a. In this method, nanofibers are made via laser irradiation on a 45S5 BG monolith, resulting in formation of a small bath of molten glass, that is later spun (stretched and cooled) by a gas jet emanating from a supersonic nozzle [88]. As a result of fast cooling that hinders crystallization, an amorphous BG non-woven nanofiber is created that comprises nanofibers as small as 200 nm to 300 nm in diameter. The laser spinning process is highly time-efficient and BG nanofibers can be produced in a few microseconds. In addition to 45S5 BG, Quintero et al. [88] succeeded in producing 52S4.6 silicate BG nanofibers (52.27 SiO2, 0.45 Al2O3, 24.71 CaO, 2.30 P2O5, 20 Na2O, 0.21 K2O, 0.01 Fe2O3, 0.03 TiO2 mol.%), as well. Interestingly, the nanofibers could be promptly transformed to hydroxycarbonate apatite (HCA) tubes (Figure 2b) upon immersion in SBF, thanks to their nanoscale diameter (size) and composition [88].
Figure 2.
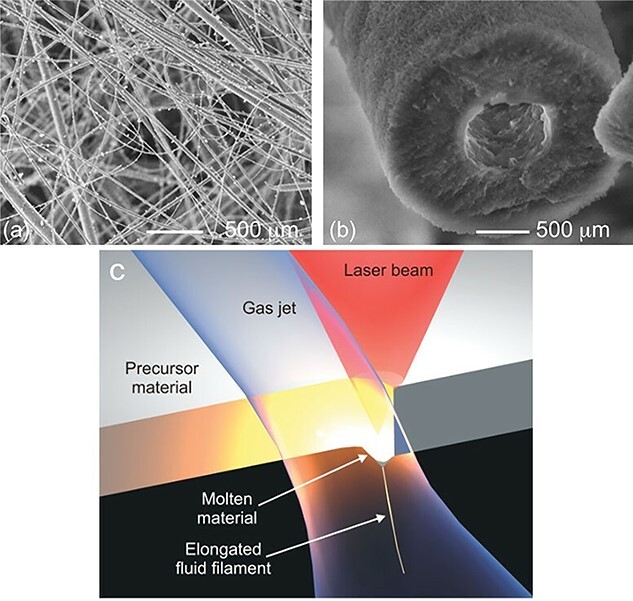
Scanning electron microscopy images showing: (a) 45S5 BG nanofibers produced through the laser spinning process, (b) conversion of a 45S5 BG nanofiber to a hydroxycarbonate apatite tube after immersion in simulated body fluid for 48 h. (c) The laser spinning method that employs a powerful laser to get a small fraction of a precursor material melted. Meanwhile, a high-velocity gas jet draws and cools the molten material, forming a nanofiber. Reproduced with permission [88]. Copyright 2009, John Wiley and Sons
Figure 2c schematically illustrates the laser spinning process, in which a pendant drop of the molten glass is exposed to a high-velocity gas jet that forcefully stretches and cools the melt [88]. The cooling speed is extremely high and as a result the spun nanofibers are amorphous. The superfast nature of the process involving high elongation forces enables production of nanofibers with extraordinary length/diameter ratios of, e.g. 1,000,000 : 1 in less than one second [88]. The relative movement of the laser beam against the plate of the precursor material, i.e. 45S5 and 52S4.6 BG plates, creates a cut that persistently supplies the melt and thus allows for production of dense BG nanofiber mats in minutes [88].
Electrospinning
Electrospinning enables production of polymeric submicron fiber mats featuring an extraordinary surface area that can be engineered in terms of chemistry and topography, adjustable porosity, and conformability over an extensive range of objects with different shapes and sizes [8,9,89–92]. The technique can be upgraded to produce BG-polymer composite fibers that are characterized with the presence of BG surface nanodomains along the polymer fiber matrix. In this simple approach, as shown in Figures 3a–c, a polymer solution containing BG particles is constantly (with a fixed feed rate) infused through a nozzle electrified by a high DC voltage supplier and is converted to a polymer jet flying toward a grounded collector. The structural properties and morphology of electrospun nanofibers including diameter, surface porosity (roughness), and alignment are predetermined by the device operating parameters, polymer solution properties, and nearby environment conditions [93]. Moreover, multiphasic nanofibers can be produced by electrospinning set-ups containing multichannel and coaxial nozzles (Figures 3b, c, respectively) [94].
Figure 3.
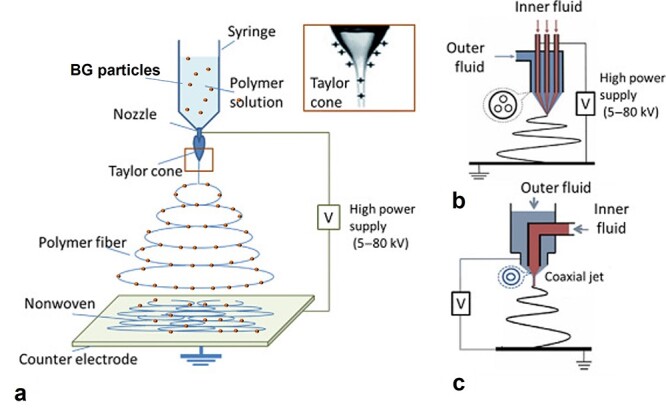
Schematic illustration of electrospinning of a BG particle/polymer suspension in various set-ups with different nozzle configurations: (a) single nozzle, (b) multichannel nozzle, and (c) coaxial nozzle. Reproduced and re-drawn (section a) with permission [94]. Copyright 2019, Elsevier
Electrospinning allows for simple production of nanofibers made of natural and synthetic polymers individually or blended with other polymers. Additionally, depending on the target application and desired structural and biological properties, inorganic–organic composite nanofibers can be developed. In this regard, BG-(bio)polymer suspensions have been electrospun to provide composite nanofiber systems benefitting from a brittle yet bioactive inorganic BG phase alongside the bioinert yet flexible organic (bio)polymer phase [95]. For instance, BG–PCL (polycaprolactone) composite nanofibers have been shown to offer proper bioactivity and to stimulate the secretion of alkaline phosphatase by MC3T3 pre-osteoblast cells adjacent to the nanofibers [96,97]. Considering the different chemical nature of the inorganic filler (BG) and the organic (polymer) matrix, interfacial bond strength can be insignificant, leading to a poor distribution of the BG phase and low mechanical properties. One promising solution in this respect is the use of a coupling agent. For instance, 3-glycidoxypropyltrimethoxysilane has been employed to induce formation of a covalent bond between BG particles and gelatine, thereby creating flexible, yet mechanically robust BG-gelatine composite fibers [98]. A large variety of BG-containing biopolymer electrospun fibers has been developed for wound healing application [99–103].
BG nano/microfibers can also be synthesized through a combination of sol–gel process and electrospinning [104]. Such nanofibers are electrospun in a similar manner as polymer nanofibers, though a polymer is added to the inorganic sol. The first electrospun silicate BG nanofibers (as small as 84 nm in diameter) were made of 70 mol.% SiO2, 25 mol.% CaO, and 5 mol.% P2O5 through a combination process comprising sol–gel and electrospinning [83,105]. To enable electrospinning, viscosity of the sol was tailored by addition of polyvinyl butyral/ethanol solution in a small amount. The 70S30C BG nanofibers were also electrospun with inclusion of polyvinyl alcohol in the sol [105]. More sophisticated versions of BG nanofibers such as those made as hollow mesoporous fibers (∼600 nm in diameter) have also been synthesized using high molecular weight poly(ethylene oxide) (PEO) as the phase separation (and then sacrificial) agent [106]. Recently, cotton-like Cu ion-doped BG nanofibers have been developed by a similar sol electrospinning technique [107].
BG-based fibers for wound healing
BG nanoparticle reinforced polymeric nanofibers
Despite all the merits that BGs show for bone regeneration and wound healing such as formation of a calcium phosphate layer in exposure to physiological liquids, enhancing osteointegration, and release of therapeutic ions stimulating different cellular pathways, their application is restricted by their insufficient mechanical properties. As a solution for this shortcoming, BGs have been combined with biodegradable synthetic or natural polymers to create malleable yet bioactive composite nanofibers that can potentially be applied as a wound dressing material.
BG/natural (and blend) polymer nanofibers
The production of natural polymer nanofibers from biological wastes or bioresources for biomedicine has always been appealing, due not only to their biomimicry but also to their suitable biocompatibility and biodegradability. For instance, fish collagen has been proven to be a biocompatible biomolecule that poorly induces antigenic response and offers promising wound healing effects [108]. However, it is highly costly and thus rarely applicable in biomedicine, unless it is synthesized from economical resources. In this regard, fish collagen can be potentially extracted from biowastes largely produced in fish processing units [109]. The as-prepared collagen per se cannot be used as a wound healing material, due to its insufficient thermomechanical properties, leading to fast degradation at the physiological temperature of the human body. One optimum solution for such a bottleneck can be the combination of fish collagen and BG (nano)particles to create a composite system with improved structural and therapeutic properties. In this regard, Zhou et al. [101] developed composite nanofibers composed of BG and Tilapia fish collagen. The as-developed nanofibers were shown to possess improved tensile strength and the ability to inactivate Staphylococcus aureus bacteria. Moreover, such nanofibers could raise skin regeneration in the wound bed, indicating their wound healing potential. Very recently, Jana et al. [100] synthesized a microfibrous wound dressing made of fish collagen, derived from Rohu (Labeo rohita) skin, coupled with a novel formulation of BG doped with Cu and Co. As shown in Figure 4, the structure and composition of the microfibrous dressing were encouraging for human dermal fibroblasts (HDFs) to adhere, spread and proliferate on such a cytocompatible and nontoxic platform. In vivo testing with animal models (rabbits) also confirmed an improved wound healing behavior in the presence of the doped BG reinforced fish collagen microfibers. Particularly, enhanced wound closure, homogenously formed epidermis, larger wound maturity, and proper deposition of ECM components including mature elastin and collagen were observed. Additionally, neovascularization was obvious in the wounds treated with the doped BG/fish collagen microfibers, most likely due to the bioactivity of BG and the supportive role of Cu and Co ions along with fish collagen [100]. Eggshell membrane (ESM) is a connective tissue that comprises a thin (60–80 μm) layer of collagen fibers [110]. ESM is in fact a porous biopolymeric fibrous network consisting of protein fibers (80–85%), thereof ∼10% are made of collagen (types I, V and X) [111]. In addition to its fascinating porous structure, ESM provides a proper antibacterial activity that is necessary for wound healing [112]. Employing the interesting characteristics of ESM, Li et al. [113] devised a Cu-doped BG coated ESM for wound healing application. The 5 mol.% Cu-BG/ESM material could provoke angiogenesis by upregulation of VEGF and HIF-1α, in human umbilical vein endothelial cells (HUVECs). Moreover, thanks to the sustained release of Cu2+ ions, the system was successful in inactivation of Escherichia coli bacteria.
Figure 4.
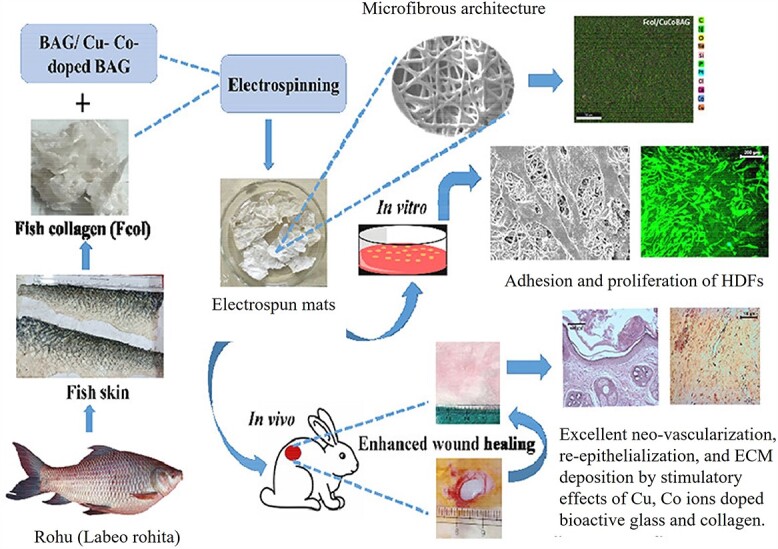
Schematic illustration of the preparation procedure of bioactive glass (BG) (here shown as BAG; Cu- and Co-doped) reinforced fish collagen electrospun fiber mats with improved human dermal fibroblast (HDF) cell response and enhanced in vivo wound healing reflected in neovascularization, re-epithelialization, and extracellular matrix (ECM) deposition. Reproduced with permission from [100]. Copyright 2022, ACS
Plant-derived natural polymers, e.g. cellulose, have also been researched to be applied as a carrier for BG and cooperatively for production of composite nanofiber wound dressings. Cellulose is the most abundant natural polysaccharide that can be derived from green resources such as plants, wood, fungi, seaweed and bacteria [114–116]. Thanks to negligible toxicity and carcinogenicity, biocompatibility and biodegradability, cellulose is regarded as a high-potential wound dressing material. Additionally, it can maintain moisture, adequately absorb exudates, expedite granulation and encourage wound healing via fibrogenesis [115,117]. As a derivative of cellulose, methylcellulose (MC) has been electrospun blended with PCL, which assures desirable electrospinning of MC. The MC/PCL blend nanofibers were incorporated with borate BG and Manuka honey to develop a wound dressing material with antibacterial properties [99]. In vitro tests based on human keratinocytes (HaCaT cells) and HDFs confirmed that the nanofibrous system can be potentially applied as a wound dressing material [99]. BG nanoparticles have also been employed to reinforce cellulose acetate nanofibers (100–200 nm in diameter) to develop a broad spectrum antibacterial wound dressing material that accelerates wound healing [118]. Another polysaccharide that has been widely studied for biomedical applications is chitosan. Chitosan is also biodegradable, nontoxic, low cost and abundant. Additionally, it accelerates tissue regeneration and induces hemostasis [119]. Moreover, chitosan shows antibacterial activity that is attributed to its capability in binding with sialic acid in phospholipids, thereby challenging the transport of microbiological substances [120]. Such important biological characteristics render chitosan an ideal candidate for fabrication of biomedical systems for wound healing, tissue engineering, drug delivery, among other applications [121]. In a recent study by Sergi et al. [122], chitosan/PEO blend fibers, cross-linked with genipin, were incorporated with several types of BG including 45S5 BG, Sr- and Mg-doped BG and Zn-doped BG. The release of therapeutic ions such as Sr, Mg and Zn ions from chitosan/PEO fibers was reported to potentially raise tissue regeneration. Sr ions can provoke cell proliferation and angiogenesis [50,123], whereas Mg ions can improve migration and proliferation of microvascular cells [124]. Furthermore, Zn ions cause better wound healing and angiogenesis conditions in the wound bed [125,126]. Cerium (Ce)-doped BG particles have also been embedded into chitosan/PEO nanofibers to create antibacterial nanofiber wound dressings with improved mechanical properties, matching those of skin [98]. Ce-doped BGs have been shown to effectively inactivate gram-negative bacteria such as Escherichia coli, particularly at Ce concentrations exceeding 5 mol.% [127]. Silk fibers, as the building blocks of the commercial suture Mersilk®, are comprised of a fibroin core encased by sericin, which is an antigenic gum-like protein [128]. Moreover, to confer polymer sutures with bioactivity and antibacterial activity, they have been coated with Ag-doped BG (60% SiO2, 2% Ag2O, 34% CaO, 4% P2O5 in mol.%) via a conventional slurry-dipping approach. The as-coated suture could show limited bacterial adhesion, thus a promising antibacterial effect against Staphylococcus epidermidis [128].
BG/synthetic polymer nanofibers
Synthetic polymers have also been proposed for construction of BG incorporated nano/microfibrous wound dressings. Such a type of polymers is outstanding due to their largely known processing techniques (e.g. electrospinning), desirable physicochemical characteristics and scalability [9]. Such advantages can be crucial for production of wound dressing materials at large scale and in a cost-effective manner. Despite such merits, synthetic polymers are typically bioinert and thus can challenge the removal of wound dressings made thereof after the wound is healed [89]. This shortcoming can be addressed by blending with natural, biodegradable polymers and by incorporation of bioactive (inorganic) materials such as BG. For instance, insufficient number of cell recognition sites on PCL nanofibers and their poor bioactivity are crucial bottlenecks that can be addressed by addition of BG particles. In this regard, silicate (13-93) and borosilicate (13-93BS) BG nanoparticles have been incorporated into poly(glycerol-sabacate)(PGS)/PCL blend nanofibers to create a wound healing material [129]. Thanks to the pro-angiogenetic activity of the ions released from the BG particles, the BG-reinforced PGS/PCL nanofibers can be employed as a wound dressing material. PCL fibers containing pro-angiogenic Co-containing BG particles were also developed to stimulate wound healing without formation of a HCA layer [130]. Co ions were released steadily with a release rate governed by Mg concentration of the BG. As a result of the dissolution of BG, in an in vitro study with primary human fibroblasts, HIF-1α was stabilized and VEGF was notably upregulated. Therefore, the composite fibers can potentially activate the HIF pathway, thereby stimulating angiogenesis [130]. In general, as the current literature review indicates, natural polymers seem to be more appealing for the development of BG-incorporated polymer fibers meant for wound healing. Among the few synthetic polymers proposed for such a research objective, undoubtedly, PCL is the most studied polymer. Table 1 tabulates the studies that deal with BG-incorporated PCL nano/microfibers developed for wound healing that have been carried out in the past five years [131–137]. In addition to PCL, bioresorbable poly(glycolide-l-lactide) (PGLA) fibers, constituting Vicryl® (polyglactin 910) surgical sutures, have been coated with Ag-doped BG (60% SiO2, 2% Ag2O, 34% CaO, 4% P2O5, in mol.%) to achieve bioactivity and antimicrobial and antibacterial properties [138]. In a recent study [139], such PGLA fibers were also coated with composite coatings of Zn-doped BG and Ag-doped mesoporous BG-incorporated PCL or chitosan. The inclusion of ordered mesoporous BG particles can potentially allow for further loading of the system with drugs (e.g. anti-inflammatories or antibiotics) or growth factors, exploiting such mesoporous BG particles as drug carriers. Moreover, by implementation of the composite coating, the BG particles are stabilized on the fiber surface and the release of antibacterial ions can be properly tuned.
Table 1.
BG reinforced PCL fibers for wound healing (studies reported after 2017)
| BG type | Polymer carrier | Improved biological properties | Reference |
|---|---|---|---|
| 45S5, Sr- and Mg-substituted BG (BGMS10), and Zn-substituted BG (BGMS-2Zn) | PCL | Improved cell adhesion and proliferation, higher wound healing rate | [131] |
| 13-93B3 | PCL | Improved cell (human adipose-derived mesenchymal stem cells) proliferation | [132] |
| B and Co co-doped bioactive glass nanoparticles | PCL | Upregulated VEGF and enhanced angiogenesis | [133] |
| 77S | PCL | Improved cell (human skin fibroblast) adhesion and proliferation | [134] |
| Ag2O- and CoO-doped BG nanoparticles | PCL | Enhanced angiogenesis and antibacterial activity | [135] |
| 45S5+ Cu nanoparticles | PCL | Improved cytocompatibility | [136] |
| 58S | collagen/chitosan-coated PCL | Improved cell (human dermal fibroblast) proliferation and antibacterial activity | [137] |
BG bioactive glass, PCL polycaprolactone, VEGF vascular endothelial growth factor
BG nano/microfibers
Cotton-wool-like BG fiber mats are an interesting type of inorganic fibers made of various BG compositions. They are typically made through a sol–gel process and by hydrolysis of alkoxide precursors, allowing bottom-up formation of a silicate glass network (gel) under ambient temperature [140]. After drying and calcination, nanoporous glasses in the form of a cotton-wool mat remain. Recently, such BG fiber scaffolds have been developed via electrospinning of a BG sol [141]. The BG composition could be formulated as (100-x)SiO2 – xCaO (x = 0, 10, 20, 30, and 40 mol.%). It was shown that the sol’s Ca content and relative humidity in the electrospinning chamber determine the morphology (and quality) of the cotton-wool-like fibers [141]. Taking 80S20C and 70SiO2-30CaO (70S30C) as two main BG compositions, the BG fibers co-cultured with HDF induced a similar cellular metabolic activity to that of the control sample (i.e. tissue culture polystyrene). Conversely, in the presence of such BG fibers, HDFs secreted a higher level of VEGF compared to the control [141].
In a very recent study, Ju et al. [142] synthesized antibacterial Ag-doped 70S30C BG fibers with a 3D cotton-wool-like structure through a combination of a sol–gel process and electrospinning. The as-prepared BG fibers can simulate the fibrous architecture of the skin’s ECM, and also enable moisture control and platelet aggregation in the wound bed [72,142]. Moreover, they release Ag and Ca/Si ions for antibacterial and wound healing purposes, respectively. Particularly, Ca ions upregulate fibrin and thrombin in the wound bed during the early stages of clot formation [143] and control the expression of various genes involved in epithelial migration [72]. Conversely, Si ions provoke proliferation of endothelial cells and upregulate the expression of VEGF and bFGF by fibroblasts, thereby enhancing angiogenesis [124,144]. In the field of silicate cotton-like fibers, recently, Cu-doped nanofibers were developed [96,107]. Other than silicate BGs, borate BGs, e.g. 13–93B3, have also been processed as cotton-candy-like fibers (however, not by electrospinning but via a glass melting process). Yang et al. [145] compared the in vitro behavior of 45S5 silicate BG fibers with that of 13-93B3 and 1605 borate BG fibers (the latter contains ZnO and CuO as dopants). According to this study, borate BG fibers were shown to release ions faster. Moreover, glass conversion and formation of HA in such fibers take place more promptly compared to silicate BG fibers. In general, borate BG fibers were proven to be more effective in terms of wound healing than silicate BG fibers. Such BG fibers have been shown to encourage the healing of full-thickness skin defects, thanks to the release of B and Ca ions that stimulate epidermal cell migration and angiogenesis, and govern the wound healing cascade [10]. B ions, in particular, drive the translation of encoding mRNA growth factors that trigger angiogenesis and wound healing including TGF-β and VEGF [146]. The BG nanofibers have been shown to offer an antibacterial activity, due to the increase of pH from 7 up to 9 and promotion of osmotic pressure of the tissue liquids by ionic dissolution [102]. Zhao et al. [41] developed Cu-doped borate BG microfibers (with the composition of 6Na2O, 8K2O, 8MgO, 22CaO, 54B2O3, 2P2O5; mol.%) that could release Cu, B and Ca ions into physiological medium, whereby enhancing the migration of HUVECs, tubule formation and secretion of VEGF. Moreover, these dissolution products could upregulate the expression of angiogenic genes in fibroblasts. Interestingly, it was shown that full thickness skin defects treated with such Cu-doped BG fibers achieved superior healing conditions reflected in larger collagen deposition, maturity and orientation. In addition to doping of the BG fibers (or particles) with pro-angiogenic elements, incorporation of angiogenic growth factors including VEGF, bFGF and PDGF into the tissue regenerating materials can be a second strategy to raise angiogenicity [54–56]. However, the implementation of growth factors imposes high costs and undesired biological consequences, e.g. in supra-physiological doses [57,58,147] and the incorporated factors might lose their bioactivity [59,60]. Therefore, with respect to BG-based fibers, doping of the BG particles with copper, for instance, can be regarded a superior strategy compared to incorporation of growth factors. Similarly, Ag-doped borate BG fibers (with the composition of 1–2 B2O3, 68–69 SiO2, ~0.001 Ag2O, and 29–30 CaO; mol.%) have been shown to offer an antibacterial activity and support the wound healing process [148].
Despite promising therapeutic effects of BG fibers, their mechanical mismatch with underlying skin tissue might cause an adverse effect on cellular behavior. As a proven fact, mechanical forces can control cell and tissue phenotype [149]. Cells employ an active contact sensing mechanism, whereby they respond to the stiffness of the underlying surface [150,151]. Specifically, dermal fibroblast cells respond to the substrate mechanics by altering their gene expression level, leading to differential ECM synthesis or their phenotypic transformation into myofibroblasts [152]. Moreover, migration of human bone marrow-derived mesenchymal stem cells, which accumulate and improve cutaneous wound healing [153], is governed by the mechanical properties of the ECM and depending on the matrix stiffness different differentiated phenotypes are generated [154]. Therefore, proper modulation of mechanical properties of a skin substitute or a dressing material can be vital in provision of an encouraging microenvironment for wound healing. One strategy to adjust the stiffness of the BG fiber dressings to match the underlying skin’s elastic properties could be hybridization of BG fibers with softer polymeric materials. This approach is not only efficient in terms of mechanical modulation, but also prevents uncontrolled release of ions from the BG fibers exposed to biological fluids. It has been shown that the Cu-doped borate BG fibers can release boron and Cu2+ ions in a non-tailored manner [155] and even trigger an initial burst release [156], thereby causing prompt degradation of the fibers and inducing transient biotoxicity [157]. Therefore, incorporation of BG fibers into a polymer matrix could be a more advantageous alternative for a BG fiber mat. In this regard, Hu et al. [158] developed vitamin E loaded Cu-doped borate BG microfibers incorporated poly(lactic-co-glycolic acid) wound dressings. According to in vitro tests, Cu2+ ions and vitamin were released in a sustained manner, thereby stimulating the secretion of VEGF in HUVECs and angiogenesis-linked genes in fibroblasts thus inducing better tubule formation. Moreover, the composite wound dressing was encouraging toward epithelialization and wound closure, collagen remodeling, and vessel sprouting in vivo.
State-of-the-art and current challenges
BG (nano)fibers were originally investigated for the repair and restoration of hard tissues, mainly bone, thanks to their remarkable potential in formation of a surface HA layer. In recent years, BG fibers have also been proposed for development of soft tissue engineering scaffolds as well as wound dressings. It has been proven that the BG phase (depending on the composition) can release biologically active ions, thereby improving angiogenesis, which is an important prerequisite for wound healing. In this regard, bioresorbable borate-based BG fibers, commercially known as Mirragen®, have been successfully tested for chronic wound healing with commercial success. Mirragen® Advanced Wound Matrix (BBGFM) fabricated by ETS Wound Care (Rolla, Missouri) based on fibers (with composition: 53B2O3–6Na2O–12K2O–5MgO–20CaO–4P2O5 in wt.%) has been so designed to be degraded within a wound bed in days or weeks, depending on the wound exudate and healing rate [159].
Despite their superior bioactivity, BGs are predominantly fragile and suffer from low fracture toughness, particularly when formed as fibrous meshes. A promising solution can be the combination of BG particles with a supporting flexible polymeric phase. Synthetic and natural biodegradable polymers have been shown to perform properly as a carrier for BG (nano)particles and maintain ECM biomimicry. Moreover, hybridization of BG particles with a variety of polymers enables development of wound dressing materials with mechanical compatibility with natural skin tissue. Such dressing materials would be elastic, pliable and robust, thus protecting the wounded skin against mechanical damage. The mechanical properties of BG/polymer nanofibrous structures impact cellular activities thus tissue regeneration, because the cell–material interactions are largely dependent on the applied shear stresses and the mechanical signaling channels that govern the cells’ migration, proliferation and differentiation [85]. Therefore, mechanical properties of a wound dressing made from BG-based fibers must match the mechanical properties of the skin tissue to distribute comparable biomechanical signals [160]. As derived from the literature [161], human skin is as stiff as 0.1 to 10 MPa when exposed to tensile forces. Inorganic BG fibers are trivially much stiffer than skin but the BG-incorporated polymer (e.g. BG/PCL [137]) fibers can be so developed to properly match the skin’s elastic modulus. These properties can be adjusted in BG/polymer nanofibers to match that of the skin tissue through proper selection of polymer matrix, the quantity of the BG filler, and surface chemistry of the filler (in the presence/absence of coupling agents), which affects the physicochemical interaction (bonding) between the BG particles and the polymer. The fiber diameter could be also regarded as an influential factor, as it determines the arrangement of polymer chains in a confined area with a low free volume, thereby intensifying the entanglement of the polymer chains and their interaction with BG particles that would act as a physical barrier against mobility of the polymer chains.
Currently, doped (e.g. with Cu) and non-doped borate BG nanofibers are employed for wound healing applications. Depending on the specific healing objective, ranging from inflammation and proliferation to angiogenesis, there is a need for new customized formulations that can cover the entire wound healing process with a smart function triggered by exclusive environmental factors. For instance, pH and temperature can act as stimuli to induce the healing ion release, thereby governing the healing process in a controlled manner. In this regard, various ions can play a boosting role in each wound healing step including inflammation, proliferation and remodeling. Additionally, antibacterial ions can provide an extra functionality to BG-containing wound dressings as proposed also for sutures.
Regarding the metal ions doped into BG materials, it should be borne in mind that different metal ions perform only in a well-defined concentration range and if exceeded, the metal ion would engender undesired side effects. For instance, exceeding the optimum concentration range, Cu ions might generate free radicals, which are toxic to nerve cells, and raise the chance of neurodegenerative diseases [162]. Therefore, it is vital to notice the biosafety of metal ion-doped BG materials in the future, particularly given the complex nature of the human body’s internal physiological environment. In this regard, the design of BG-based fibers needs to be properly conducted to allow the release of ions in a controlled manner and within the safety and therapeutic relevant range.
Laser spinning and electrospinning are two main techniques for production of high-quality BG nanofibers. Electrospinning also enables production of hollow BG nanotubes and BG/polymer hybrid nanofibers. However, scalable production of BG nanofibers is still a challenge that potentially restricts their wide, commercial applications.
With respect to in vivo testing of BG nanofibers for wound healing, there is still a need of reliable animal models and novel skin on chip models. The currently used animal models such as mice, rats and rabbits do not realistically indicate the biological performance of the studied material in the human skin. Particularly, wound healing in mice is governed by myofibroblast-mediated contraction through an extensive subcutaneous striated muscle layer called the panniculus carnosus that is absent in humans [163]. Therefore, the biological results using this animal model cannot be extended directly to humans.
Conclusions
Despite the well-known application potential of BG-based materials in hard tissue engineering, they are relatively new in the field of soft tissue repair and, in particular, as wound healing materials. In this context, BG fibers offer promising properties such as angiogenicity, immunogenicity and antibacterial activity, thus notably encouraging the skin repair cascade. This field is still in its infancy and further research in the future, particularly in relation to cotton-candy-like BG fibers, which are less studied compared to BG/polymer fibers, could pave the way toward the development of high-potential products for the dynamic wound dressing market. The successful studies reviewed in this paper indicate that bioactive glasses in the form of flexible (nano)fibers have a crucial role to play in the further progress of the field of antibacterial wound-healing biomaterials.
Abbreviations
bFGF: Basic fibroblast growth factor; BG: Bioactive glass; ECM: Extracellular matrix; ESM: Eggshell membrane; HA: Hydroxyapatite; HCA: Hydroxycarbonate apatite; HDF: Human dermal fibroblasts; HIF: Hypoxia-inducible factor; HUVEC: Human umbilical vein endothelial cells; MC: Methylcellulose; PCL: Polycaprolactone; PDGF: Platelet-derived growth factor; PEO: Poly(ethylene oxide); PGLA: Poly(glycolide-l-lactide); PGS: Poly(glycerol sebacate); SBF: Simulated body fluid; VEGF: Vascular endothelial growth factor
Acknowledgements
Meng Li gratefully acknowledges the China Scholarship Council (CSC) (No. 202006290028) for financial support.
Contributor Information
Shahin Homaeigohar, School of Science and Engineering, University of Dundee, Dundee DD1 4HN, United Kingdom.
Meng Li, Institute of Biomaterials, Department of Materials Science and Engineering, University of Erlangen-Nuremberg, 91058 Erlangen, Germany.
Aldo R Boccaccini, Institute of Biomaterials, Department of Materials Science and Engineering, University of Erlangen-Nuremberg, 91058 Erlangen, Germany.
Funding
Not applicable.
Authors’ contributions
Conception and design: SH, ARB; collection and assembly of data: SH, ML; manuscript writing: SH, ML, ARB; manuscript revision: SH, ML, ARB; final approval of manuscript: SH, ML, ARB.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare no conflict of interest.
Data availability
Not applicable.
References
- 1. Guest JF, Ayoub N, Mcilwraith T, Uchegbu I, Gerrish A, Weidlich D, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;512:e009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tronci G. The application of collagen in advanced wound dressings. In: Rajendran S (eds). Advanced Textiles for Wound Care. Duxford, United Kingdom: Woodhead Publishing-Elsevier, 2019, 363–89. [Google Scholar]
- 3. Naves CCLM. The diabetic foot: a historical overview and gaps in current treatment. Adv Wound Care. 2016;55:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. http://www.marketsandmarkets.com/Market-Reports/wound-care-market-371.html.
- 5. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;343:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Brit J Dermatol. 2015;1732:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haukipuro K, Melkko J, Risteli L, Kairaluoma MI, Risteli J. Synthesis of type-I collagen in healing wounds in humans. Ann Surg. 1991;2131:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Homaeigohar S, Monavari M, Koenen B, Boccaccini AR. Biomimetic biohybrid nanofibers containing bovine serum albumin as a bioactive moiety for wound dressing. Mater Sci Eng C-Mater. 2021;123:111965. [DOI] [PubMed] [Google Scholar]
- 9. Homaeigohar S, Tsai TY, Zarie ES, Elbahri M, Young TH, Boccaccini AR. Bovine serum albumin (BSA)/polyacrylonitrile (PAN) biohybrid nanofibers coated with a biomineralized calcium deficient hydroxyapatite (HA) shell for wound dressing. Mater Sci Eng C-Mater. 2020;116:111248. [DOI] [PubMed] [Google Scholar]
- 10. Zhou J, Wang H, Zhao SC, Zhou N, Li L, Huang WH, et al. In vivo and in vitro studies of borate based glass micro-fibers for dermal repairing. Mater Sci Eng C-Mater. 2016;60:437–45. [DOI] [PubMed] [Google Scholar]
- 11. Rahaman MN, Day DE, Bal BS, Fu Q, Jung SB, Bonewald LF, et al. Bioactive glass in tissue engineering. Acta Biomater. 2011;76:2355–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gatti AM, Valdre G, Andersson OH. Analysis of the in vivo reactions of a bioactive glass in soft and hard tissue. Biomaterials. 1994;153:208–12. [DOI] [PubMed] [Google Scholar]
- 13. Gerhardt LC, Boccaccini AR. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials. 2010;37:3867–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El-Rashidy AA, Roether JA, Harhaus L, Kneser U, Boccaccini AR. Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater. 2017;62:1–28. [DOI] [PubMed] [Google Scholar]
- 15. Hench LL. The story of Bioglass. J Mater Sci Mater Med. 2006;1711:967–78. [DOI] [PubMed] [Google Scholar]
- 16. Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J Biomed Mater Res. 2001;552:151–7. [DOI] [PubMed] [Google Scholar]
- 17. Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;2715:2907–15. [DOI] [PubMed] [Google Scholar]
- 18. Hoppe A, Guldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;3211:2757–74. [DOI] [PubMed] [Google Scholar]
- 19. Mehrabi T, Mesgar AS, Mohammadi Z. Bioactive glasses: a promising therapeutic on release strategy for enhancing wound healing. ACS Biomater Sci Eng. 2020;610:5399–430. [DOI] [PubMed] [Google Scholar]
- 20. Hench LL, Splinter RJ, Allen W, Greenlee T. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res. 1971;56:117–41. [Google Scholar]
- 21. Hench LL. Bioceramics -- from concept to clinic. J Am Ceram Soc. 1991;747:1487–510. [Google Scholar]
- 22. Qazi TH, Hafeez S, Schmidt J, Duda GN, Boccaccini AR, Lippens E. Comparison of the effects of 45S5 and 1393 bioactive glass microparticles on hMSC behavior. J Biomed Mater Res A. 2017;10510:2772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fu Q, Rahaman MN, Bal BS, Brown RF, Day DE. Mechanical and in vitro performance of 13-93 bioactive glass scaffolds prepared by a polymer foam replication technique. Acta Biomater. 2008;46:1854–64. [DOI] [PubMed] [Google Scholar]
- 24. Fu Q, Rahaman MN, Bal BS, Huang W, Day DE. Preparation and bioactive characteristics of a porous 13-93 glass, and fabrication into the articulating surface of a proximal tibia. J Biomed Mater Res A. 2007;82a1:222–9. [DOI] [PubMed] [Google Scholar]
- 25. Deliormanli AM. Preparation, in vitro mineralization and osteoblast cell response of electrospun 13-93 bioactive glass nanofibers. Mater Sci Eng C. 2015;53:262–71. [DOI] [PubMed] [Google Scholar]
- 26. Boccaccini AR, Brauer DS, Hupa L. Aldo R Boccaccini, Delia S Brauer, Leena Hupa (eds). Bioactive Glasses: Fundamentals, Technology and Applications. Cambridge, United Kingdom: Royal Society of Chemistry, 2016. [Google Scholar]
- 27. Huang WH, Day DE, Kittiratanapiboon K, Rahaman MN. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J Mater Sci Mater Med. 2006;177:583–96. [DOI] [PubMed] [Google Scholar]
- 28. Yao AH, Wang DP, Huang WH, Fu Q, Rahaman MN, Day DE. In vitro bioactive characteristics of borate-based glasses with controllable degradation behavior. J Am Ceram Soc. 2007;901:303–6. [Google Scholar]
- 29. Fu QA, Rahaman MN, Fu HL, Liu X. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation. J Biomed Mater Res A. 2010;95a1:164–71. [DOI] [PubMed] [Google Scholar]
- 30. Marion CM, Liang W, Liang W, Reilly GC, Day DE, Rahaman M, Mao JJ. Borate glass supports the In vitro osteogenic differentiation of human mesenchymal stem cells. Mech Adv Mater Struct 2005;12:239–46. [Google Scholar]
- 31. Fu HL, Fu Q, Zhou N, Huang WH, Rahaman MN, Wang DP, et al. In vitro evaluation of borate-based bioactive glass scaffolds prepared by a polymer foam replication method. Mater Sci Eng C-Mater. 2009;297:2275–81. [Google Scholar]
- 32. Fu QA, Rahaman MN, Bal BS, Bonewald LF, Kuroki K, Brown RF. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. II. In vitro and in vivo biological evaluation. J Biomed Mater Res A. 2010;95a1:172–9. [DOI] [PubMed] [Google Scholar]
- 33. Liu X, Xie ZP, Zhang CQ, Pan HB, Rahaman MN, Zhang X, et al. Bioactive borate glass scaffolds: in vitro and in vivo evaluation for use as a drug delivery system in the treatment of bone infection. J Mater Sci Mater Med. 2010;212:575–82. [DOI] [PubMed] [Google Scholar]
- 34. Jia WT, Zhang X, Luo SH, Liu X, Huang WH, Rahaman MN, et al. Novel borate glass/chitosan composite as a delivery vehicle for teicoplanin in the treatment of chronic osteomyelitis. Acta Biomater. 2010;63:812–9. [DOI] [PubMed] [Google Scholar]
- 35. Zhang X, Jia WT, Gua YF, Wei XA, Liu X, Wang DP, et al. Teicoplanin-loaded borate bioactive glass implants for treating chronic bone infection in a rabbit tibia osteomyelitis model. Biomaterials. 2010;3122:5865–74. [DOI] [PubMed] [Google Scholar]
- 36. Lin YN, Brown RF, Jung SB, Day DE. Angiogenic effects of borate glass microfibers in a rodent model. J Biomed Mater Res A. 2014;10212:4491–9. [DOI] [PubMed] [Google Scholar]
- 37. Dalisson B, Barralet J. Bioinorganics and wound healing. Adv Healthc Mater. 2019;818ARTN:1900764. [DOI] [PubMed] [Google Scholar]
- 38. Brown RF, Rahaman MN, Dwilewicz AB, Huang W, Day DE, Li Y, et al. Effect of borate glass composition on its conversion to hydroxyapatite and on the proliferation of MC3T3-E1 cells. J Biomed Mater Res A. 2009;88a2:392–400. [DOI] [PubMed] [Google Scholar]
- 39. Jung S, Day DE, Brown RF, Bonewald L. Potential toxicity of bioactive borate glasses in-vitro and in-vivo. Ceram Eng Sci Proc. 2013;33:65–74. [Google Scholar]
- 40. Huang WH, Rahaman MN, Day DE, Li YD. Mechanisms for converting bioactive silicate, borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solution. Phys Chem Glasses-B. 2006;476:647–58. [Google Scholar]
- 41. Zhao SC, Li L, Wang H, Zhang YD, Cheng XG, Zhou N, et al. Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials. 2015;53:379–91. [DOI] [PubMed] [Google Scholar]
- 42. Ahmed I, Lewis M, Olsen I, Knowles JC. Phosphate glasses for tissue engineering: Part 1. Processing and characterisation of a ternary-based P(2)O(5)-CaO-Na(2)O glass system. Biomaterials. 2004;253:491–9. [DOI] [PubMed] [Google Scholar]
- 43. Ahmed I, Lewis M, Olsen I, Knowles JC. Phosphate glasses for tissue engineering: Part 2. Processing and characterisation of a ternary-based P(2)O(5)-CaO-Na(2)O glass fibre system. Biomaterials. 2004;253:501–7. [DOI] [PubMed] [Google Scholar]
- 44. Navarro M, Ginebra MP, Planell JA. Cellular response to calcium phosphate glasses with controlled solubility. J Biomed Mater Res A. 2003;67a3:1009–15. [DOI] [PubMed] [Google Scholar]
- 45. Saranti A, Koutselas I, Karakassides MA. Bioactive glasses in the system CaO-B2O3-P2O5: preparation, structural study and in vitro evaluation. J Non-Cryst Solids. 2006;3525:390–8. [Google Scholar]
- 46. Ahmed I, Collins CA, Lewis MP, Olsen I, Knowles JC. Processing, characterisation and biocompatibility of iron-phosphate glass fibres for tissue engineering. Biomaterials. 2004;2516:3223–32. [DOI] [PubMed] [Google Scholar]
- 47. Abou Neel EA, Ahmed I, Pratten J, Nazhat SN, Knowles JC. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials. 2005;2615:2247–54. [DOI] [PubMed] [Google Scholar]
- 48. Shu C, Wenjuan Z, Xu GH, Wei Z, Wei J, Dongmei W. Dissolution behavior and bioactivity study of glass ceramic scaffolds in the system of CaO-P2O5-Na2O-ZnO prepared by sol-gel technique. Mater Sci Eng C-Mater. 2010;301:105–11. [Google Scholar]
- 49. Lapa A, Cresswell M, Campbell I, Jackson P, Goldmann WH, Detsch R, et al. Ga and Ce ion-doped phosphate glass fibres with antibacterial properties and their composite for wound healing applications. J Mater Chem B. 2019;745:7246–6. [DOI] [PubMed] [Google Scholar]
- 50. Gorustovich AA, Roether JA, Boccaccini AR. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences. Tissue Eng Part B-Rev. 2010;162:199–207. [DOI] [PubMed] [Google Scholar]
- 51. Durand LAH, Vargas GE, Vera-Mesones R, Baldi A, Zago MP, Fanovich MA, et al. In vitro human umbilical vein endothelial cells response to ionic dissolution products from lithium-containing 45S5 bioactive glass. Materials. 2017;10:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mohammad G, Pandey H, Tripathib K. Diabetic wound healing and its angiogenesis with special reference to nanoparticles. Dig J Nanomater Bios. 2008;3:203–8. [Google Scholar]
- 53. Edwards JV, Howley PS. Human neutrophil elastase and collagenase sequestration with phosphorylated cotton wound dressings. J Biomed Mater Res A. 2007;83a2:446–54. [DOI] [PubMed] [Google Scholar]
- 54. Nomi M, Atala A, De Coppi P, Soker S. Principals of neovascularization for tissue engineering. Mol Asp Med. 2002;236:463–83. [DOI] [PubMed] [Google Scholar]
- 55. Madeddu P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp Physiol. 2005;903:315–26. [DOI] [PubMed] [Google Scholar]
- 56. Tan Q, Chen B, Yan X, Lin Y, Xiao ZF, Hou XL, et al. Promotion of diabetic wound healing by collagen scaffold with collagen-binding vascular endothelial growth factor in a diabetic rat model. J Tissue Eng Regen Med. 2014;83:195–201. [DOI] [PubMed] [Google Scholar]
- 57. Thawani JP, Wang AC, Than KD, Lin CY, La Marca F, Park P. Bone morphogenetic proteins and cancer: review of the literature. Neurosurgery. 2010;662:233–46. [DOI] [PubMed] [Google Scholar]
- 58. Laurencin CT, Ashe KM, Henry N, Kan HM, Lo KWH. Delivery of small molecules for bone regenerative engineering: preclinical studies and potential clinical applications. Drug Discov Today. 2014;196:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hanft J, Pollak R, Barbul A, Cv G, Kwon P, Gray S, et al. Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. J Wound Care. 2008;171:30–7. [DOI] [PubMed] [Google Scholar]
- 60. Uchi H, Igarashi A, Urabe K, Koga T, Nakayama J, Kawamori R, et al. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur J Dermatol. 2009;195:461–8. [DOI] [PubMed] [Google Scholar]
- 61. Barralet J, Gbureck U, Habibovic P, Vorndran E, Gerard C, Doillon CJ. Angiogenesis in calcium phosphate scaffolds by inorganic copper ion release. Tissue Eng Part A. 2009;157:1601–9. [DOI] [PubMed] [Google Scholar]
- 62. Gerard C, Bordeleau LJ, Barralet J, Doillon CJ. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials. 2010;315:824–31. [DOI] [PubMed] [Google Scholar]
- 63. Barbucci R, Lamponi S, Magnani A, Piras FM, Rossi A, Weber E. Role of the hyal-Cu (II) complex on bovine aortic and lymphatic endothelial cells behavior on microstructured surfaces. Biomacromolecules. 2005;61:212–9. [DOI] [PubMed] [Google Scholar]
- 64. Hu GF. Copper stimulates proliferation of human endothelial cells under culture. J Cell Biochem. 1998;693:326–35. [DOI] [PubMed] [Google Scholar]
- 65. Harris ED. A requirement for copper in angiogenesis. Nutr Rev. 2004;622:60–4. [DOI] [PubMed] [Google Scholar]
- 66. Feng WK, Ye F, Xue WL, Zhou ZX, Kang YJ. Copper regulation of hypoxia-inducible factor-1 activity. Mol Pharmacol. 2009;751:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Borkow G, Gabbay J, Lyakhovitsky A, Huszar M. Improvement of facial skin characteristics using copper oxide containing pillowcases: a double-blind, placebo-controlled, parallel, randomized study. Int J Cosmetic Sci. 2009;316:437–43. [DOI] [PubMed] [Google Scholar]
- 68. Gorter RW, Butorac M, Cobian EP. Examination of the cutaneous absorption of copper after the use of copper-containing ointments. Am J Ther. 2004;116:453–8. [DOI] [PubMed] [Google Scholar]
- 69. Giavaresi G, Torricelli P, Fornasari PM, Giardino R, Barbucci R, Leone G. Blood vessel formation after soft-tissue implantation of hyaluronan-based hydrogel supplemented with copper ions. Biomaterials. 2005;2616:3001–8. [DOI] [PubMed] [Google Scholar]
- 70. Chebassier N, El Houssein O, Viegas I, Dreno B. In vitro induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 expression in keratinocytes by boron and manganese. Exp Dermatol. 2004;138:484–90. [DOI] [PubMed] [Google Scholar]
- 71. Jung SB. Borate based bioactive glass scaffolds for hard and soft tissue engineering. Ph.D. thesis, Missouri University of Science and Technology, Missouri, United States, 2010. Available online at: https://scholarsmine.mst.edu/doctoral_dissertations/2075.
- 72. Wray P. 'Cotton candy' that heals? Borate glass nanofibers look promising. Am Ceram Soc Bull. 2011;904:25–9. [Google Scholar]
- 73. Zhu YL, Ma ZJ, Kong LZ, He YH, Chan HF, Li HY. Modulation of macrophages by bioactive glass/sodium alginate hydrogel is crucial in skin regeneration enhancement. Biomaterials. 2020;256:120216. [DOI] [PubMed] [Google Scholar]
- 74. Dong X, Chang J, Li HY. Bioglass promotes wound healing through modulating the paracrine effects between macrophages and repairing cells. J Mater Chem B. 2017;526:5240–50. [DOI] [PubMed] [Google Scholar]
- 75. Sakurai E, Anand A, Ambati BK, Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophth Vis Sci. 2003;448:3578–85. [DOI] [PubMed] [Google Scholar]
- 76. Bussolino F, Wang JM, Defilippi P, Turrini F, Sanavio F, Edgell CJ, et al. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature. 1989;3376206:471–3. [DOI] [PubMed] [Google Scholar]
- 77. Tsukamoto Y, Helsel WE, Wahl SM. Macrophage production of fibronectin, a chemoattractant for fibroblasts. J Immunol. 1981;1272:673–8. [PubMed] [Google Scholar]
- 78. Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care (New Rochelle). 2013;25:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;3035659:848–51. [DOI] [PubMed] [Google Scholar]
- 80. Pieper JS, Hafmans T, Wachem PB, Luyn MJA, Brouwer LA, Veerkamp JH, et al. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J Biomed Mater Res. 2002;622:185–94. [DOI] [PubMed] [Google Scholar]
- 81. Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;395:733–42. [DOI] [PubMed] [Google Scholar]
- 82. Tsuboi R, Rifkin DB. Recombinant basic fibroblast growth-factor stimulates wound-healing in healing-impaired Db/Db mice. J Exp Med. 1990;1721:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jones JR. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013;91:4457–86. [DOI] [PubMed] [Google Scholar]
- 84. Pirhonen E, Niiranen H, Niemela T, Brink M, Tormala P. Manufacturing, mechanical characterization, and in vitro performance of bioactive glass 13-93 fibers. J Biomed Mater Res B. 2006;77b2:227–33. [DOI] [PubMed] [Google Scholar]
- 85. Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;3105751:1135–8. [DOI] [PubMed] [Google Scholar]
- 86. Quintero F, Mann AB, Pou J, Lusquinos F, Riveiro A. Rapid production of ultralong amorphous ceramic nanofibers by laser spinning. Appl Phys Lett. 2007;9015:153109. [Google Scholar]
- 87. Taygun ME, Boccaccini A. Nanoscaled bioactive glass particles and nanofibers. In: Heimo Ylänen (ed). Bioactive Glasses. Duxford, United Kingdom: Woodhead Publishing-Elsevier, 2018, 235–83. [Google Scholar]
- 88. Quintero F, Pou J, Comesana R, Lusquinos F, Riveiro A, Mann AB, et al. Laser spinning of bioactive glass nanofibers. Adv Funct Mater. 2009;1919:3084–90. [Google Scholar]
- 89. Homaeigohar S, Boccaccini AR. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020;107:25–49. [DOI] [PubMed] [Google Scholar]
- 90. Homaeigohar S, Strunskus T, Strobel J, Kienle L, Elbahri M. A flexible oxygenated carbographite nanofilamentous buckypaper as an amphiphilic membrane. Adv Mater Interfaces. 2018;58:1800001. [Google Scholar]
- 91. Abrigo M, McArthur SL, Kingshott P. Electrospun nanofibers as dressings for chronic wound care: advances, challenges, and future prospects. Macromol Biosci. 2014;146:772–92. [DOI] [PubMed] [Google Scholar]
- 92. Rahmati M, Mills DK, Urbanska AM, Saeb MR, Venugopal JR, Ramakrishna S, et al. Electrospinning for tissue engineering applications. Prog Mater Sci. 2021;117:100721. [Google Scholar]
- 93. Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;283:325–47. [DOI] [PubMed] [Google Scholar]
- 94. Zheng Y. 3-Fabrication on bioinspired surfaces. In: Bioinspired Design of Materials Surfaces. Elsevier, 2019, 99–146. [Google Scholar]
- 95. Felgueiras HP, Amorim MTP. Alina-Maria Holban, Alexandru Mihai Grumezescu (eds). Production of polymer–bioactive glass nanocomposites for bone repair and substitution. In: Materials for Biomedical Engineering. Amsterdam, Netherlands: Elsevier, 2019, 373–96. [Google Scholar]
- 96. Kouhi M, Morshed M, Varshosaz J, Fathi MH. Poly (epsilon-caprolactone) incorporated bioactive glass nanoparticles and simvastatin nanocomposite nanofibers: preparation, characterization and in vitro drug release for bone regeneration applications. Chem Eng J. 2013;228:1057–65. [Google Scholar]
- 97. Jo JH, Lee EJ, Shin DS, Kim HE, Kim HW, Koh YH, et al. In vitro/in vivo biocompatibility and mechanical properties of bioactive glass nanofiber and poly(epsilon-caprolactone) composite materials. J Biomed Mater Res B. 2009;91b1:213–20. [DOI] [PubMed] [Google Scholar]
- 98. Gao CX, Gao Q, Li YD, Rahaman MN, Teramoto A, Abe K. In vitro evaluation of electrospun gelatin-bioactive glass hybrid scaffolds for bone regeneration. J Appl Polym Sci. 2013;1274:2588–99. [Google Scholar]
- 99. Saatchi A, Arani AR, Moghanian A, Mozafari M. Cerium-doped bioactive glass-loaded chitosan/polyethylene oxide nanofiber with elevated antibacterial properties as a potential wound dressing. Ceram Int. 2021;477:9447–61. [Google Scholar]
- 100. Schuhladen K, Raghu SN, Liverani L, Neščáková Z, Boccaccini AR. Production of a novel poly (ɛ-caprolactone)-methylcellulose electrospun wound dressing by incorporating bioactive glass and Manuka honey. J Biomed Mater Res B Appl Biomater. 2021;1092:180–92. [DOI] [PubMed] [Google Scholar]
- 101. Jana S, Datta P, Das H, Ghosh PR, Kundu B, Nandi SK. Engineering vascularizing electrospun dermal grafts by integrating fish collagen and ion-doped bioactive glass. ACS Biomater Sci Eng. 2022;82:734–52. [DOI] [PubMed] [Google Scholar]
- 102. Zhou T, Sui BY, Mo XM, Sun J. Multifunctional and biomimetic fish collagen/bioactive glass nanofibers: fabrication, antibacterial activity and inducing skin regeneration in vitro and in vivo. Int J Nanomedicine. 2017;12:3495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ma WB, Yang XY, Ma L, Wang XG, Zhang L, Yang GJ, et al. Fabrication of bioactive glass-introduced nanofibrous membranes with multifunctions for potential wound dressing. RSC Adv. 2014;4104:60114–22. [Google Scholar]
- 104. Nagrath M, Alhalawani A, Yazdi AR, Towler MR. Bioactive glass fiber fabrication via a combination of sol-gel process with electro-spinning technique. Mater Sci Eng C Mater. 2019;101:521–38. [DOI] [PubMed] [Google Scholar]
- 105. Lu H, Zhang T, Wang XP, Fang QF. Electrospun submicron bioactive glass fibers for bone tissue scaffold. J Mater Sci Mater Med. 2009;203:793–8. [DOI] [PubMed] [Google Scholar]
- 106. Hong YL, Chen XS, Jing XB, Fan HS, Gu ZW, Zhang XD. Fabrication and drug delivery of ultrathin mesoporous bioactive glass hollow fibers. Adv Funct Mater. 2010;209:1503–10. [Google Scholar]
- 107. Liverani L, Reiter T, Zheng K, Neščáková Z, Boccaccini AR. Copper-doped cotton-like malleable electrospun bioactive glass fibers for wound healing applications. Mater Lett: X. 2022;14:100133. [Google Scholar]
- 108. Jafari H, Lista A, Siekapen MM, Ghaffari-Bohlouli P, Nie L, Alimoradi H, et al. Fish collagen: extraction, characterization, and applications for biomaterials engineering. Polymers. 2020;1210:2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Savedboworn W, Kittiphattanabawon P, Benjakul S, Sinthusamran S, Kishimura H. Characteristics of collagen from Rohu (Labeo rohita) skin. J Aquat Food Prod Technol. 2017;263:248–57. [Google Scholar]
- 110. Devi PS, Banerjee S, Chowdhury SR, Kumar GS. Eggshell membrane: a natural biotemplate to synthesize fluorescent gold nanoparticles. RSC Adv. 2012;230:11578–85. [Google Scholar]
- 111. Balaz M. Eggshell membrane biomaterial as a platform for applications in materials science. Acta Biomater. 2014;109:3827–43. [DOI] [PubMed] [Google Scholar]
- 112. Ahlborn G, Sheldon BW. Identifying the components in eggshell membrane responsible for reducing the heat resistance of bacterial pathogens. J Food Protect. 2006;694:729–38. [DOI] [PubMed] [Google Scholar]
- 113. Li JY, Zhai D, Lv F, Yu QQ, Ma HS, Yin JB, et al. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016;36:254–66. [DOI] [PubMed] [Google Scholar]
- 114. Frenot A, Henriksson MW, Walkenstrom P. Electrospinning of cellulose-based nanofibers. J Appl Polym Sci. 2007;1033:1473–82. [Google Scholar]
- 115. Portela R, Leal CR, Almeida PL, Sobral RG. Bacterial cellulose: a versatile biopolymer for wound dressing applications. Microb Biotechnol. 2019;124:586–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Nasatto PL, Pignon F, Silveira JLM, Duarte MER, Noseda MD, Rinaudo M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers. 2015;75:777–803. [Google Scholar]
- 117. Vijayakumar V, Samal SK, Mohanty S, Nayak SK. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int J Biol Macromol. 2019;122:137–48. [DOI] [PubMed] [Google Scholar]
- 118. Sharaf SS, El-Shafei AM, Refaie R, Gibriel AA, Abdel-Sattar R. Antibacterial and wound healing properties of cellulose acetate electrospun nanofibers loaded with bioactive glass nanoparticles; in-vivo study. Cellulose. 2022;298:4565–77. [Google Scholar]
- 119. Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;317:603–32. [Google Scholar]
- 120. Liu HQ, Zhao YC, Cheng S, Huang N, Leng YX. Syntheses of novel chitosan derivative with excellent solubility, anticoagulation, and antibacterial property by chemical modification. J Appl Polym Sci. 2012;1244:2641–8. [Google Scholar]
- 121. Jin J, Song M, Hourston DJ. Novel chitosan-based films cross-linked by genipin with improved physical properties. Biomacromolecules. 2004;51:162–8. [DOI] [PubMed] [Google Scholar]
- 122. Sergi R, Cannillo V, Boccaccini AR, Liverani L. A new generation of electrospun fibers containing bioactive glass particles for wound healing. Materials. 2020;1324:5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Baino F, Novajra G, Miguez-Pacheco V, Boccaccini AR, Vitale-Brovarone C. Bioactive glasses: special applications outside the skeletal system. J Non-Cryst Solids. 2016;432:15–30. [Google Scholar]
- 124. Kargozar S, Baino F, Hamzehlou S, Hill RG, Mozafari M. Bioactive glasses: sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018;364:430–44. [DOI] [PubMed] [Google Scholar]
- 125. Brauer DS. Bioactive glasses-structure and properties. Angew Chem Int Ed. 2015;5414:4160–81. [DOI] [PubMed] [Google Scholar]
- 126. Naseri S, Lepry WC, Nazhat SN. Bioactive glasses in wound healing: hope or hype? J Mater Chem B. 2017;531:6167–74. [DOI] [PubMed] [Google Scholar]
- 127. Goh YF, Alshemary AZ, Akram M, Kadir MRA, Hussain R. In-vitro characterization of antibacterial bioactive glass containing ceria. Ceram Int. 2014;401:729–37. [Google Scholar]
- 128. Pratten J, Nazhat SN, Blaker JJ, Boccaccini AR. In vitro attachment of Staphylococcus epidermidis to surgical sutures with and without Ag-containing bioactive glass coating. J Biomater Appl. 2004;191:47–57. [DOI] [PubMed] [Google Scholar]
- 129. Luginina M, Schuhladen K, Orru R, Cao G, Boccaccini AR, Liverani L. Electrospun PCL/PGS composite fibers incorporating bioactive glass particles for soft tissue engineering applications. Nanomaterials. 2020;105:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Solanki AK, Lali FV, Autefage H, Agarwal S, Nommeots-Nomm A, Metcalfe AD, et al. Bioactive glasses and electrospun composites that release cobalt to stimulate the HIF pathway for wound healing applications. Biomater Res. 2021;251:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sergi R, Cannillo V, Boccaccini AR, Liverani L. Incorporation of bioactive glasses containing Mg, Sr, and Zn in electrospun PCL fibers by using benign solvents. Appl Sci-Basel. 2020;1016:5530. [Google Scholar]
- 132. Kolan KCR, Li J, Roberts S, Semon JA, Park J, Day DE, et al. Near-field electrospinning of a polymer/bioactive glass composite to fabricate 3D biomimetic structures. Int J Bioprinting. 2019;51:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chen S, Galuskova D, Kankova H, Zheng K, Michalek M, Liverani L, et al. Electrospun PCL fiber mats incorporating multi-targeted B and Co co-doped bioactive glass nanoparticles for angiogenesis. Materials. 2020;1318:4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lin ZF, Gao WD, Ma LM, Xia H, Xie WH, Zhang Y, et al. Preparation and properties of poly(epsilon-caprolactone)/bioactive glass nanofibre membranes for skin tissue engineering. J Bioact Compat Pol. 2018;332:195–209. [Google Scholar]
- 135. Moura D, Souza MT, Liverani L, Rella G, Luz GM, Mano JF, et al. Development of a bioactive glass-polymer composite for wound healing applications. Mater Sci Eng C. 2017;76:224–32. [DOI] [PubMed] [Google Scholar]
- 136. Akturk A, Erol-Taygun M, Goller G, Kucubayrak S. Optimization and characterization of poly(epsilon-caprolactone) nanofiber mats doped with bioactive glass and copper metal nanoparticles. Chem Pap. 2021;7511:5929–43. [Google Scholar]
- 137. Molavi AM, Sadeghi-Avalshahr A, Nokhasteh S, Naderi-Meshkin H. Enhanced biological properties of collagen/chitosan-coated poly(epsilon-caprolactone) scaffold by surface modification with GHK-Cu peptide and 58S bioglass. Prog Biomater. 2020;91-2:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Blaker JJ, Nazhat SN, Boccaccini AR. Development and characterisation of silver-doped bioactive glass-coated sutures for tissue engineering and wound healing applications. Biomaterials. 2004;257-8:1319–29. [DOI] [PubMed] [Google Scholar]
- 139. Ciraldo FE, Schnepf K, Goldmann WH, Boccaccini AR. Development and characterization of bioactive glass containing composite coatings with ion releasing function for antibiotic-free antibacterial surgical sutures. Materials. 2019;123:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Li R, Clark AE, Hench LL. An investigation of bioactive glass powders by sol-gel processing. J Appl Biomater. 1991;24:231–9. [DOI] [PubMed] [Google Scholar]
- 141. Norris E, Ramos-Rivera C, Poologasundarampillai G, Clark JP, Ju Q, Obata A, et al. Electrospinning 3D bioactive glasses for wound healing. Biomed Mater. 2020;151:015014. [DOI] [PubMed] [Google Scholar]
- 142. Ju Q, Zenji T, Macon ALB, Norris E, Poologasundarampillai G, Obata A, et al. Silver-doped calcium silicate sol-gel glasses with a cotton-wool-like structure for wound healing. Biomater Adv. 2022;134:112561. [DOI] [PubMed] [Google Scholar]
- 143. Lansdown ABG. Calcium: a potential central regulator in wound healing in the skin. Wound Repair Regen. 2002;105:271–85. [DOI] [PubMed] [Google Scholar]
- 144. Zhai WY, Lu HX, Chen L, Lin XT, Huang Y, Dai KR, et al. Silicate bioceramics induce angiogenesis during bone regeneration. Acta Biomater. 2012;81:341–9. [DOI] [PubMed] [Google Scholar]
- 145. Yang QB, Chen SS, Shi HL, Xiao H, Ma YF. In vitro study of improved wound-healing effect of bioactive borate-based glass nano-/micro-fibers. Mater Sci Eng C Mater. 2015;55:105–17. [DOI] [PubMed] [Google Scholar]
- 146. Dzondo-Gadet M, Mayap-Nzietchueng R, Hess K, Nabet P, Belleville F, Dousset B. Action of boron at the molecular level -- effects on transcription and translation in an acellular system. Biol Trace Elem Res. 2002;851:23–33. [DOI] [PubMed] [Google Scholar]
- 147. Patra CR, Kim JH, Pramanik K, d'Uscio LV, Patra S, Pal K, et al. Reactive oxygen species driven angiogenesis by inorganic nanorods. Nano Lett. 2011;1111:4932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Saha S, Bhattacharjee A, Rahaman SH, Ray S, Marei MK, Jain H, et al. Prospects of antibacterial bioactive glass nanofibers for wound healing: an in vitro study. Int J Appl Glas Sci. 2020;112:320–8. [Google Scholar]
- 149. Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;15:E131–8. [DOI] [PubMed] [Google Scholar]
- 150. Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;791:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Clark RAF, Ghosh K, Tonnesen MG. Tissue engineering for cutaneous wounds. J Invest Dermatol. 2007;1275:1018–29. [DOI] [PubMed] [Google Scholar]
- 152. Chiquet M, Reneda AS, Huber F, Fluck M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;221:73–80. [DOI] [PubMed] [Google Scholar]
- 153. Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;1962:245–50. [DOI] [PubMed] [Google Scholar]
- 154. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;1264:677–89. [DOI] [PubMed] [Google Scholar]
- 155. Aboud H, Wagiran H, Hussin R, Ali H, Alajerami Y, Saeed MA. Thermoluminescence properties of the Cu-doped lithium potassium borate glass. Appl Radiat Isotopes. 2014;90:35–9. [DOI] [PubMed] [Google Scholar]
- 156. Lawrence BJ, Maase EL, Lin HK, Madihally SV. Multilayer composite scaffolds with mechanical properties similar to small intestinal submucosa. J Biomed Mater Res A. 2009;88a3:634–43. [DOI] [PubMed] [Google Scholar]
- 157. Shailajha S, Geetha K, Vasantharani P, Kadhar SPSA. Effects of copper on the preparation and characterization of Na-Ca-P borate glasses. Spectrochim Acta A. 2015;138:846–56. [DOI] [PubMed] [Google Scholar]
- 158. Hu HR, Tang Y, Pang LB, Lin CL, Huang WH, Wang DP, et al. Angiogenesis and full-thickness wound healing efficiency of a copper-doped borate bioactive glass/poly(lactic-co-glycolic acid) dressing loaded with vitamin E in vivo and in vitro. ACS Appl Mater Inter. 2018;1027:22939–50. [DOI] [PubMed] [Google Scholar]
- 159. Armstrong DG, Orgill DP, Galiano RD, Glat PM, DiDomenico LA, Carter MJ, et al. A multi-centre, single-blinded randomised controlled clinical trial evaluating the effect of resorbable glass fibre matrix in the treatment of diabetic foot ulcers. Int Wound J. 2022;194:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Chouhan D, Chakraborty B, Nandi SK, Mandal BB. Role of non-mulberry silk fibroin in deposition and regulation of extracellular matrix towards accelerated wound healing. Acta Biomater. 2017;48:157–74. [DOI] [PubMed] [Google Scholar]
- 161. Sun FZ, Nordli HR, Pukstad B, Gamstedt EK, Chinga-Carrasco G. Mechanical characteristics of nanocellulose-PEG bionanocomposite wound dressings in wet conditions. J Mech Behav Biomed. 2017;69:377–84. [DOI] [PubMed] [Google Scholar]
- 162. Brewer GJ. Avoiding Alzheimer's disease: the important causative role of divalent copper ingestion. Exp Biol Med. 2019;2442:114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Grada A, Mervis J, Falanga V. Research techniques made simple: animal models of wound healing. J Invest Dermatol. 2018;13810:2095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


