Abstract
This work is part of a larger study whose main objective was to find a series of promising molecules to be used as glass-ionomer-type materials. The project was divided into 3 successive stages; the results of the first stage have been previously published and were used to continue the study. The molecules evaluated in the second stage were constructed by adding a glycidyl methacrylate molecule to the carboxylic groups of the polyacids selected in the previous stage. The modeling was done using the density functional theory for M06-2X/6-311G(d,p). The results indicate that the addition over the carboxylic groups of the fraction of the molecule, corresponding to itaconic acid, is thermodynamically favored. The final stage was modeled with the M06 functional and consisted of obtaining basic structures of glass-ionomer-type materials, by acid–base reaction between the molecules resulting from the second stage with individual ions of Ca (2 +), Zn (2 +), or Al (+ 3). It was concluded that aluminum atoms generate more compact structures that would correlate with more resistant materials.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00894-022-05211-x.
Keywords: Dental cement, Glycidyl methacrylate, Glass-ionomer, M06, Polyacids
Introduction
The invention of dental cements emerged as an aesthetic alternative to the use of materials such as amalgams [1]. However, there is still a need for improved materials that solve problems such as color stability, thermal expansion, low adhesion to the tooth, lack of mechanical resistance, bacterial penetration, material stability, toxicity, setting, and operation times, among others.
After many studies and changes in research, the approach to solve these problems has been focused on materials referred to as glass-ionomers [2–4]. This is a solid matrix obtained from acid–base reaction between polyacid molecule and a metal. In conventional glass ionomer cements (GIC), the metal comes from the degradation of a glass, the polyacid is a polyelectrolyte that includes mono, di, and tricarboxylic acids and water acts as the reaction medium.
Experimentally, polyacids are obtained by free radical polymerization reaction of the monomers of interest. Reaction is carried out in aqueous solution and in the presence of an initiator. Structurally, polyacids are represented by linear chains containing an excess acid groups [5, 6].
It has been suggested to use polymers that are obtained from the combination of different unsaturated carboxylic acids, for example, acrylic acid with itaconic acid and/or maleic acid, with the purpose of increasing the degree of cross-linking within the molecule and therefore culminating in obtaining of materials with greater hardness [7, 8].
During setting, an acid–base reaction occurs between the polyacid and the ionic leachable glass [9, 10]. Polyacids can be modified with compounds that contain the methacrylate group, so that in addition to the acid–base reaction, they undergo a free radical polymerization reaction [5, 11–13]. The setting process occurs in three successive steps [9]. Step 1 is the decomposition of glass powder. Carboxylic acid ionizes and generates H + protons that can subsequently react with the glass surface, releasing Al3+, Ca2+, Na+, F−, etc. ions. It is emphasized that not all carboxylic groups are ionized. Then, these ions are precipitated as insoluble polyacrylates. Al3+, Ca2+, and Zn2+ form metal salt bridges with free groups (–COO–) which allows cross-linking of the polycarboxylate chains and also the hardening.
Step 2 describes the precipitation-gelation of cations and anions, and the final step is referred to as the maturation phase [9, 10].
This work presents the continuation of the study published by Gaviria [14] (named as stage 1), where the most stable molecules of some basic structures that make up polyacids were chosen to be evaluated as reagents in obtaining glass-ionomer materials.
The starting polyacid monomers were the following: AACA-1, AADH-1, AADOH-3, ABA-1, AG-1, AGA-2, AH-2, EU-2, MBA-1, MG-1, MGA-1, MH-1, NMP-1, NVC-1, and NVP-1, where AACA is N-acryloyl-6-aminocaproic acid, AADH is molecule derived from phenylalanine, AADOH is molecule derived from tyrosine, ABA is acryloyl β-alanine, AG is acryloyl glycine, AGA is acryloyl glutamic acid, AH is acryloyl histidine, EU is eugenol, MBA is methacryloyl β-alanine, MG is methacryloyl glycine, MGA is methacryloyl glutamic acid, MH is methacryloyl histidine, NMP is N-methacryloyl proline, NVC is N-vinylcaprolactam, and NVP is N-vinylpyrrolidone.
AACA-1 means that the AACA is in the first place of the structure made of AACA, acrylic acid (AA), and itaconic acid (IA). On the other hand, EU-2 means that the Eugenol is found in position 2 of the molecular structure that represents the union between an acrylic acid molecule, a eugenol molecule, and an itaconic acid.
Stages 2 and 3 of the project, reported in this work, consist of the study of the reaction of polyacid monomers with a molecule of glycidyl methacrylate (GM) in some of the carboxyl groups of the substrate, according to the nature of the amino acids.
Finally, the most stable molecules were selected and the way their molecular structure is modified by their union with Ca, Zn, and Al ions, to yield the products of the setting reaction, was analyzed.
Methods
In this work, the nomenclature, proposed in the article by Gaviria [15], has been followed to identify the different positions for the addition of the GM group to the COO− functional groups. The products of that reaction were modeled at positions 1, 2, 3, and 4.
Figure 1 lists the COOH groups of the monomer. A base molecule of one of the polyacids is taken as an example. Position 1 corresponds to the COOH group of acrylic acid. Itaconic acid has position 2 that corresponds to the COOH group that is attached to carbon C, and position 3 represents COOH attached to carbon CH2. Position 4 refers to a COOH group of the amino acid derivative.
Fig. 1.
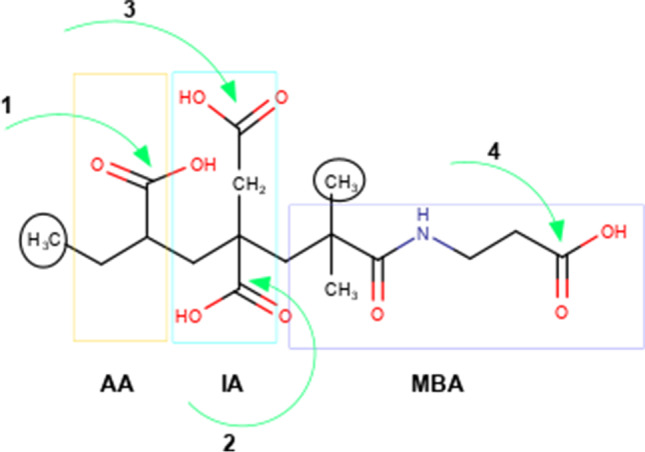
Nomenclature of the acid groups that could react with the GM; the structure of the AA-IA-MBA is shown as an example
All molecules were optimized in the Gaussian 09 computational package [16], using the density functional theory (DFT). The addition stage of the glycidyl methacrylate to the polyacid (stage 2) was investigated using the global-hybrid meta-GGA M06-2X functional [17] with the 6-311G(d,p) basis set [18].
In the optimization of the glass ionomer molecules (stage 3), the M06 functional [17] and the 6-311G(d,p) basis set were used. That functional has been parametrized including both transition metals and nonmetals.
To build the molecular structures of conventional glass ionomers (GICs), the local indices of the condensed Fukui functions () [19–21] were obtained using the finite difference method aiming to find the COOH groups with the highest electrophilic character that preferentially interact with the Zn, Ca, or Al cations.
The Hirshfeld charges [19] for the atoms in the most stable molecules obtained from the reaction of the polyacid with the GM group were calculated using the Multiwfn program [22]. Hirshfeld charges are recommended for the calculation of condensed Fukui functions [22, 23].
The condensed Fukui function for an electrophilic attack on atom A () can be defined as follows:
| 1 |
where ρA is the electronic population of atom A. Taking into account that the atomic charge of A is defined as follows:
| 2 |
(Z is the charge of the atomic nucleus), Eq. 1 can be rewritten as follows:
| 3 |
Geometry optimizations were followed by analytical calculations of frequencies to determine the nature of the stationary point and obtain the thermodynamic parameters of the molecules. To calculate enthalpy an entropy at a temperature T, the difference between the values at that temperature and 0 K has been evaluated according to standard thermodynamics equations, they assume non-interacting particles. A harmonic oscillator model has also been used [24].
Thermal corrections to enthalpy and entropy values have been evaluated at the experimental temperatures (313.15 K and 298 K) to stage 2 and stage 3, respectively, and 1 atm pressure.
Enthalpy, entropy, and free energy are calculated from the partition function with contributions from translation, electronic, rotational, and vibrational motion.
Vibrational frequency calculations were performed at M06/6-311G(d,p) for modified polyacids with glycidyl methacrylate (stage 2). Optimizations of Ca, Zn, and Al atoms were performed under the same conditions.
The effect of the aqueous solution on all the molecules studied was carried out using the polarizable continuous model, specifically the integral equation formalism (IEFPCM) [25].
Results
Addition of glycidyl methacrylate to the polyacid (stage 2)
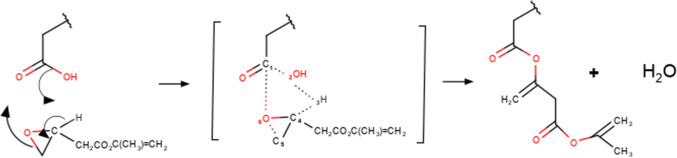
The GM addition reaction to the substrate is thought to occur according to the process depicted in Fig. 2 [15]. The mechanism involves a transition state of six members: The hydroxyl group of the carboxylic acid (OH) binds H-3 to form a water molecule.
Fig. 2.
Reaction mechanism between the polyacid and GM
The results obtained from the calculations in stage 2 are reported in Table 1. Table S1 in the electronic supplementary material shows the energy data of the studied molecules and the optimized molecular structures can be seen in Figure S1.
Table 1.
Relative energy (ΔG) of the glycidyl methacrylate addition reaction products
| Molecule | ΔG (kJ mol−1) | Molecule | ΔG (kJ mol−1) | Molecule | ΔG (kJ mol−1) | Molecule | ΔG (kJ mol−1) |
|---|---|---|---|---|---|---|---|
| AACA-1-GM1 | 92.8 | ABA-1-GM4 | 47.5 | EU-2-GM3 | 0.0 | MH-1-GM2 | 11.4 |
| AACA-1-GM2 | 20.2 | AG-1-GM1 | 0.0 | EU-2-GM4** | – | MH-1-GM3 | 0.0 |
| AACA-1-GM3 | 0.0 | AG-1-GM2 | 1.6 | MBA-1-GM1 | 85.1 | MH-1-GM4 | 88.6 |
| AACA-1-GM4 | 89.1 | AG-1-GM3 | 5.8 | MBA-1-GM2 | 0.0 | NMP-1-GM1 | 56.1 |
| AADH-1-GM1 | 37.6 | AG-1-GM4 | 21.7 | MBA-1-GM3 | 22.1 | NMP-1-GM2 | 0.0 |
| AADH-1-GM2 | 81.3 | AGA-2-GM1 | 60.2 | MBA-1-GM4 | 62.01 | NMP-1-GM3 | 12.5 |
| AADH-1-GM3 | 0.0 | AGA-2-GM2 | 0.0 | MG-1-GM1 | 0.0 | NMP-1-GM4 | 82.5 |
| AADH-1-GM4 | 13.3 | AGA-2-GM3 | 6.4 | MG-1-GM2 | 28.0 | NVC-1-GM1 | 107.44 |
| AADOH-3-GM1 | 59.7 | AGA-2-GM4* | 75.9 (63.5) | MG-1-GM3 | 56.2 | NVC-1-GM2 | 0.0 |
| AADOH-3-GM2 | 7.3 | AH-21-GM1 | 4.7 | MG-1-GM4 | 57.9 | NVC-1-GM3 | 75.6 |
| AADOH-3-GM3 | 0.0 | AH-2-GM2 | 0.0 | MGA-1-GM1 | 16.6 | NVC-1-GM4** | – |
| AADOH-3-GM4 | 150.4 | AH-2-GM3 | 5.5 | MGA-1-GM2 | 0.0 | NVP-1-GM1 | 6.2 |
| ABA-1-GM1 | 39.2 | AH-2-GM4 | 142.8 | MGA-1-GM3 | 29.8 | NVP-1-GM2 | 0.0 |
| ABA-1-GM2 | 0.0 | EU-2-GM1 | 25.8 | MGA-1-GM4* | 145.2 (31.9) | NVP-1-GM3 | 12.9 |
| ABA-1-GM3 | 8.4 | EU-2-GM2 | 28.1 | MH-1-GM1 | 19.7 | NVP-1-GM4** | – |
*Two COOH groups in the molecule of the amino acid derivative that forms the polyacid.
**There are no COOH groups in the structure of the amino acid derivative.
For example, AACA-1-GM2 refers to the polyacid AACA-AA-IA for which the glycidyl methacrylate group has been attached to the carbon at position 2 and AACA-1-GM1 when the addition of that group occurs on the carbon in position 1 (see Fig. 1).
The relative free energy values (ΔG) indicate that the most stable molecules in the GM group addition reaction are as follows: AACA-1-GM3, AADH-1-GM3, AADOH-3-GM3, ABA-1-GM2, AG-1-GM1, AGA-2-GM2, AH-2-GM2, EU-2-GM3, MBA-1-GM2, MG-1-GM1, MGA-1-GM2, MH-1-GM3, NMP -1-GM2, NVC-1-GM2, and NVP-1-GM2.
The results express that approximately 53% of the molecules studied are more stable when the addition of the GM group occurs on the carboxyl group attached to carbon “C” of itaconic acid (position 2), and ≈ 33% of the molecules are more stable when the addition of GM occurs on the carboxyl bonded to the “CH2” carbon also of itaconic acid (position 3). The remaining percentage (≈13%) of the molecules studied have less Gibbs energy, if the addition occurs in the carboxyl group of acrylic acid (position 1).
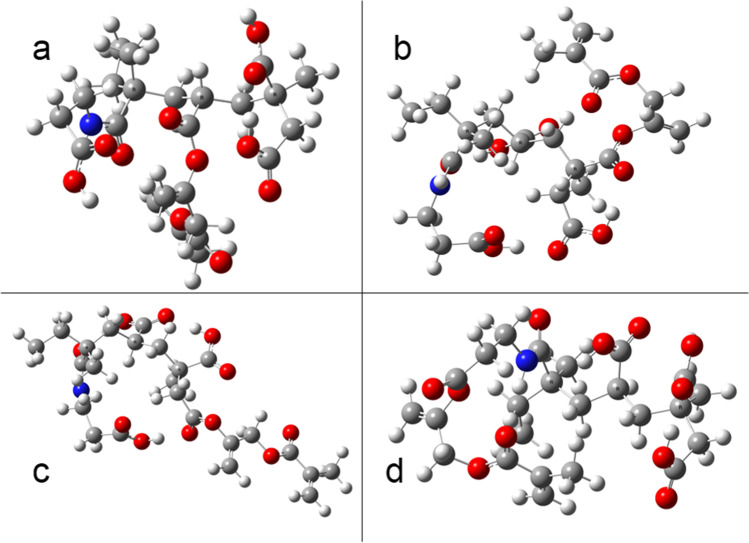
For example, the AADOH-3-GM4 and AH-2-GM4 molecules present higher energy values than AADOH-3-GM3 and AH-2-GM2, up to 150 and 143 kJ mol−1, respectively. Figure 3 shows the optimized structures of the molecules resulting from the addition of GM to the polyacid MBA-1.
Fig. 3.
Optimized molecular structure of the reaction product of MBA-1 with GM in the different carboxyl groups. a Position 1, b position 2, c position 3, d position 4
Thermodynamically, the addition of the GM group has a preference over the carboxyl groups of itaconic acid.
The data show that most of the molecules present the lowest values of electronic energy (the data are collected in the electronic supplementary material) when the GM group is added in positions 2 or 3; 60% of the molecules analyzed have lower free energy because they also have lower enthalpy. It is concluded that positions 2 and 3 allow favorable atomic interactions and reduce unfavorable interactions.
When the addition of GM occurs in position 1, a greater number of molecules have higher entropy values, meaning that the entropic component is favored but at the cost of losing favorable atomic interactions.
Setting reaction for the formation of the glass-ionomer (stage 3)
Based on the results obtained from stage 2, we proceeded to model the structures resulting from the reaction of the selected molecules with the cations of Zn, Ca and Al (glass components), by an acid–base reaction, which is represented in Fig. 4.
Fig. 4.
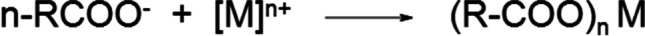
General representation of the acid–base reaction for the formation of the basic structure of the glass ionomer; M: Ca, Zn, Al; n = 2 or 3
Hirshfeld atomic charges and Fukui indices calculated for each atom are given in Table S2 in the electronic supplementary material.
Table 2 shows the average bond distances of the metal with the atoms to which it is attached and the entropies of each optimized molecule.
Table 2.
Bond distances around the metal (Å) and entropy values for the molecule (cal.mol-1K-1) obtained from the molecular modeling of M06/6-311G(d,p)
| Molecule | Average distance (Å)/entropy (cal.mol-1K-1) | Molecule | Average distance (Å)/entropy (cal.mol-1K-1) | Molecule | Average distance (Å)/entropy (cal.mol-1K-1) | |||
|---|---|---|---|---|---|---|---|---|
| AACA-1-GM3 | Ca | 2.46/240 | AGA-2-GM2 | Ca | 2.43/235 | MGA-1-GM2 | Ca | 2.26/244 |
| Zn | 1.92/233 | Zn | 2.02/233 | Zn | 1.89/238 | |||
| Al | 1.82/220 | Al | 1.82/230 | Al | 1.77/230 | |||
| AADH-1-GM3 | Ca | 2.32/256 | AH-2-GM2 | Ca | 2.41/247 | MH-1-GM3 | Ca | 2.48/247 |
| Zn | 1.91/255 | Zn | 1.97/237 | Zn | 1.95/241 | |||
| Al | 1.84/247 | Al | 2.06/229 | Al | 1.76/240 | |||
| AADOH-3-GM3 | Ca | 2.38/256 | EU-2-GM3 | Ca | 2.33/229 | NMP-1-GM2 | Ca | 2.35/222 |
| Zn | 2.07/254 | Zn | 1.83/231 | Zn | 1.90/230 | |||
| Al | 1.73/256 | Al | 1.74/230 | Al | 1.86/243 | |||
| ABA-1-GM2 | Ca | 2.39/220 | MBA-1-GM2 | Ca | 2.44/233 | NVC-1-GM2 | Ca | 2.16/229 |
| Zn | 1.96/224 | Zn | 1.92/226 | Zn | 1.86/222 | |||
| Al | 1.80/217 | Al | 1.80/221 | Al | 1.69/217 | |||
| AG-1-GM1 | Ca | 2.22/224 | MG-1-GM1 | Ca | 2.29/224 | NVP-1-GM2 | Ca | 2.39/203 |
| Zn | 1.89/221 | Zn | 1.89/224 | Zn | 1.93/202 | |||
| Al | 1.76/207 | Al | 1.72/208 | Al | 1.76/198 | |||
From the average bond distances, it can be seen that when the atom involved is calcium, the values obtained are between 2.19 and 2.56 Å; when the metal is replaced by Zn or Al, the average values are in the ranges of 1.83–2.07 and 1.69–2.06 Å, respectively. In addition, molecular geometry shows that calcium and aluminum atoms make 4 bonds with neighboring atoms in almost all of the optimized molecules, while in those molecules that involve the zinc atom, 2 bonds with neighboring atoms are observed.
The entropy values for each molecule show that in most of them (approximately 87%), the lowest value is for the molecules that have the Al atom, and in 80% of the molecules, the highest molecular entropy value occurs when they contain the Ca atom.
The results of the molecular entropy are in agreement with the data obtained from the bond distances. The data indicate that the aluminum atom generates a molecular contraction around it, that is, it promotes greater and stronger interactions, evidenced by lower bond distances around it, a greater number of interactions and a lower value of molecular entropy, when the same molecules with a different metal atom are compared.
Materials with the Al atom would be expected to be more compact and therefore would correlate with materials of higher density, higher hardness, and higher strength.
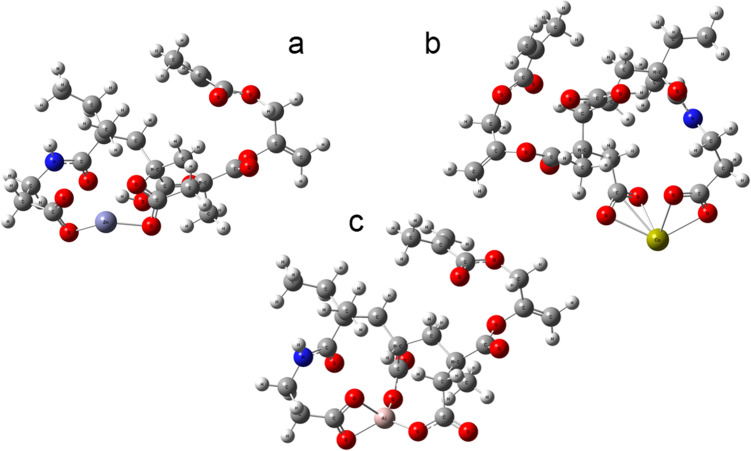
Figure 5 provides a representation of the interactions that occur with the different metals and the branched polyacid.
Fig. 5.
Molecules optimized to M06/6-311G(d,p) of MBA-1-GM1 with cations. a Zn, b Ca, c Al
We have also determined the interaction energy of the metal with the polymer, in order to quantify the possible stabilization or not that occurs in the molecule.
It was calculated as Gibb energy difference of the GIC molecules and the modified polymers and respective metals, that is:
| 4 |
where , interaction energy; , glass ionomer conventional energy; , modified polymer energy; and , metal energy (Ca, Zn, or Al).
The results are compiled in Table 3. Each energy component used in Eq. (4) can be found in Table S3.
Table 3.
: Interaction energy (Hartree) between modified polymer and metal atom. Calculated to M06/6-311G(d,p)
| Molecule | Metal | Molecule | Metal | Molecule | Metal | |||
|---|---|---|---|---|---|---|---|---|
| AACA-1-GM3 | Ca | 0.965729 | AGA-2-GM2 | Ca | 0.944139 | MGA-1-GM2 | Ca | 0.987918 |
| Zn | 1.125804 | Zn | 1.120595 | Zn | 1.140861 | |||
| Al | 1.558237 | Al | 1.550672 | Al | 1.566133 | |||
| AADH-1-GM3 | Ca | 0.964736 | AH-2-GM2 | Ca | 0.95241 | MH-1-GM3 | Ca | 0.963901 |
| Zn | 1.161891 | Zn | 1.139624 | Zn | 1.140749 | |||
| Al | 1.613027 | Al | 1.588899 | Al | 1.539402 | |||
| AADOH-3-GM3 | Ca | 0.966217 | EU-2-GM3 | Ca | 0.944463 | NMP-1-GM2 | Ca | 0.979992 |
| Zn | 1.168629 | Zn | 1.139819 | Zn | 1.146478 | |||
| Al | 1.721253 | Al | 1.220413 | Al | 1.696044 | |||
| ABA-1-GM2 | Ca | 0.962179 | MBA-1-GM2 | Ca | 0.963159 | NVC-1-GM2 | Ca | 1.001583 |
| Zn | 1.134088 | Zn | 1.131577 | Zn | 1.146076 | |||
| Al | 1.576354 | Al | 1.567417 | Al | 1.291851 | |||
| AG-1-GM1 | Ca | 0.972577 | MG-1-GM1 | Ca | 0.969037 | NVP-1-GM2 | Ca | 0.967644 |
| Zn | 1.147477 | Zn | 1.149828 | Zn | 1.136887 | |||
| Al | 1.597945 | Al | 1.654396 | Al | 1.145604 |
From Table 3, in principle, it can be seen that the interaction energy is positive for all the polymers modified with GM and with each of the Ca, Zn, and Al metals.
That is, GICs are less stable than the respective polymers without undergoing acid–base reaction. It was also found that the greatest molecular destabilization occurs in the interaction of each of the polymers with aluminum and that the Ca atom generates the most stable molecules.
For example, average interaction energy values of 1.5, 1.1, and 0.97 Hartree are observed when the metal is Al, Zn, and Ca, respectively.
The energetic results obtained are in agreement with the compactness of the structures, previously analyzed with the bond distances and the entropic component.
Conclusions
A series of promising molecules were computationally evaluated to be used as glass-ionomer-type materials, built in 3 successive stages. This article presented the results obtained from the last 2 stages of the study. The results of the addition of glycidyl methacrylate to the polyacid indicate that the most stable molecules are those generated by the addition to the carboxylic groups of the fraction of the molecule corresponding to itaconic acid; there, the enthalpy component prevails over the entropic unfavorability.
The basic structures of the glass-ionomers, composed of the most stable molecules obtained from the addition of GM to the studied polyacids and individual Ca (2+), Zn (2+), and Al (3+) ions, were optimized.
The results from the molecular optimization allow us to conclude that the aluminum atom allows a greater compaction of the structure, evidenced in the number of atomic bonds it forms, the distances of the bonds, and the total entropy of the molecule. Although, they are also structured with less relative stability.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful for the support received by the Universidad Nacional de Colombia — Medellín during the research stage. S.Q thanks the General Research Directorate (DGI) of the Universidad Santiago de Cali, Santiago de Cali. P.R. also thanks the Instituto Tecnológico Metropolitano (ITM).
Author contribution
All authors contributed to the study conception and design. Jair Gaviria, Silvia Quijano and Pablo Ruiz, made a substantial contribution to data acquisition, material preparation, analysis and interpretation of data. The first draft of the manuscript was written by Jair Gaviria and Pablo Ruiz. Jairo Quijano critically reviewed the intellectual content. All authors read and approved the final manuscript.
Funding
Open Access funding provided by Colombia Consortium
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information file).
Code availability
Not applicable.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith DC. Development of glass-ionomer cement systems. Biomaterials. 1998;19:467–478. doi: 10.1016/S0142-9612(97)00126-9. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson J. Chemistry of glass-ionomer cements: a review. Biomaterials. 1998;19:485–494. doi: 10.1016/S0142-9612(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 3.Mount GJ (2002) An atlas of glass-ionomer cements. A clinician´s guide. 3rd edn. Martin Dunitz, London
- 4.Kent B, Wilson A. The properties of a glass-ionomer cement. Br Dent J. 1973;1350:322–326. doi: 10.1038/sj.bdj.4803083. [DOI] [PubMed] [Google Scholar]
- 5.Wilson AD. Glass-ionomer cement origins, development and future. Clin Mater. 1991;7:275–282. doi: 10.1016/0267-6605(91)90070-V. [DOI] [PubMed] [Google Scholar]
- 6.Wilson A, Nicholson J. Polyalkenoate cements. In: Wilson A, Nicholson J, editors. Acid–base cements: their biomedical and industrial applications. Cambridge: University Press; 1993. pp. 90–196. [Google Scholar]
- 7.Crisp S, Wilson A (1977) Cements comprising acrylic and itaconic acid copolymers and fluoroaluminosilicate glass powder. US Patent No. 4016124. United States
- 8.Mount G, Makinson O. Glass-ionomer restorative cements: clinical implications of the setting reaction. Oper Dent. 1982;7:134–141. [PubMed] [Google Scholar]
- 9.Crisp S, Wilson A. Reactions in glass ionomer cements: I. Decomposition of the powder. J Dent Res. 1974;53:1408–1413. doi: 10.1177/00220345740530061901. [DOI] [PubMed] [Google Scholar]
- 10.Crisp S, Wilson A. Reactions in glass ionomer cements: III. The precipitation reaction. J Dent Res. 1974;53:1420–1424. doi: 10.1177/00220345740530062101. [DOI] [PubMed] [Google Scholar]
- 11.Wei W, Xie D, Puckett A. Synthesis of amino acid-containing polyacids and their application in self-cured glass-ionomer cement. Eur Polym J. 2003;39:959–968. doi: 10.1016/S0014-3057(02)00308-7. [DOI] [Google Scholar]
- 12.Sidhu S, Watson T. Resin-modified glass-ionomer materials: a status report for the American Journal of Dentistry. Am J Dent. 1995;8:59–67. [PubMed] [Google Scholar]
- 13.Wilson A. Resin-modified glass-ionomer cement. Int J Prosthodont. 1990;3:425–429. [PubMed] [Google Scholar]
- 14.Gaviria J, Quijano J, Ruiz P. Computational study of the formation reaction of polyacid precursors of glass-ionomer materials. J Mater Sci Chem Eng. 2021;9:1–9. [Google Scholar]
- 15.Gaviria J, García CG, Velez E, Quijano J. The mechanism of formation of glass-ionomer cement: a theoretical study. Model Numer Simul Mater Sci. 2013;3:149–154. [Google Scholar]
- 16.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, FukudaR Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, VothGA Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09. Revision B01. Wallingford CT: Gaussian Inc.; 2010. [Google Scholar]
- 17.Zhao Y, Truhlar DG. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor Chem Acc. 2008;120:215–241. doi: 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]
- 18.Ditchfield R, Hehre WJ, Pople JA. Self-consistent molecular-orbital methods. IX. An extended gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys. 1971;54:724–728. doi: 10.1063/1.1674902. [DOI] [Google Scholar]
- 19.Hirshfeld FL. Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta. 1977;44:129–138. doi: 10.1007/BF00549096. [DOI] [Google Scholar]
- 20.Oláh J, Van Alsenoy C, Sannigrahi AB. Condensed Fukui functions derived from stockholder charges: assessment of their performance as local reactivity descriptors. J Phys Chem A. 2002;106:3885–3890. doi: 10.1021/jp014039h. [DOI] [Google Scholar]
- 21.Korchowiec J, Uchimaru T. The charge transfer Fukui function: extension of the finite-difference approach to reactive systems. J Phys Chem A. 1988;102:10167–10172. doi: 10.1021/jp9829290. [DOI] [Google Scholar]
- 22.Lu T, Chen F. Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem. 2012;33:580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Rong C, Chattaraj PK, Liu S. A comparative study to predict regioselectivity, electrophilicity and nucleophilicity with Fukui function and Hirshfeld charge. Theor Chem Acc. 2019;138:1–9. doi: 10.1007/s00214-019-2515-1. [DOI] [Google Scholar]
- 24.McQuarrie D, Simon J. Molecular thermodynamics. Sausalito: University Science Books; 1999. [Google Scholar]
- 25.Tomasi J, Mennucci B, Cammi R. Quantum mechanical continuum solvation models. Chem Rev. 2005;105:2999–3094. doi: 10.1021/cr9904009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information file).
Not applicable.