Abstract
In the past two decades, work on the microbiota-gut-brain axis has led to a renewed appreciation for the interconnectedness between body systems in both clinical and scientific circles. In the USA alone, millions of adults are burdened with non-communicable chronic diseases whose putative etiologies were previously thought to be restricted to either the gut or brain, such as inflammatory bowel disease, irritable bowel syndrome, Parkinson’s and Alzheimer’s disease, and autism spectrum disorder. However, the recent explosion of research into the impacts of the gut microbiome on diverse aspects of human health has revealed the potentially critical importance of reciprocal interactions between the gut microbiota, the immune system, and the brain in diverse diseases and disorders. In this review, we revisit the history of gut-brain interactions in science and medicine, which dates back to at least the eighteenth century, and outline how concepts in this field have shifted and evolved across eras. Next, we highlight the modern resurgence of gut-brain axis research, focusing on neuro-immune-microbiota interactions and recent progress towards a mechanistic understanding of the diverse impacts of the microbiome on human health. Finally, we offer a forward-looking perspective on the future of microbiota-gut-brain research, which may eventually reveal new paths towards the treatment of diverse diseases influenced by the complex connections between the microbiota and the brain.
Keywords: Neuroinflammation, Gut-brain axis, Microbiota, Microbial metabolites, Neurodegenerative diseases
Introduction
The recent explosion of interest in the role of the gut microbiota in human health has led to a growing interest in the so-called microbiota-gut-brain axis. However, the first studies of the gut-brain axis date back more than three centuries. In this review, we explore the saga of the gut-brain axis over the centuries, with a focus on microbiota-neuroimmune communication. We begin by outlining the clinical and scientific conceptualization of the gut-brain axis in the 1700s before reviewing modern investigations of the underlying mechanisms governing gut-brain communication. Finally, we speculate on how our understanding of the myriad links between the gut and brain may shift in the future.
Gut-brain axis: a historical perspective
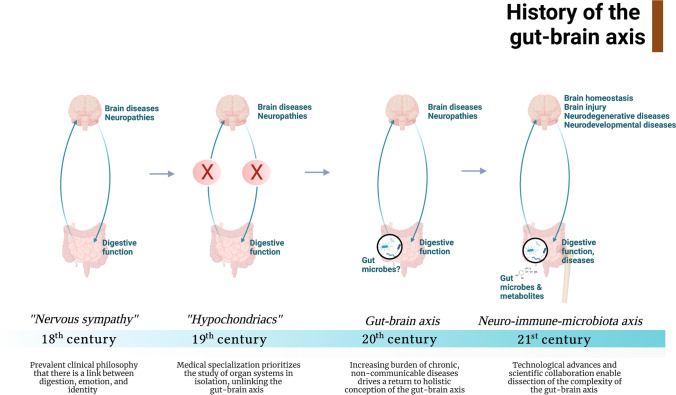
Scientific and clinical perspectives on the gut-brain axis have historically cycled between holistic and individualized approaches. Early descriptions of the gut-brain connection can be traced back at least three centuries. In the eighteenth century, physicians’ conception of the connection between the gut and brain was primarily holistic. It centered around the idea that digestion, emotions, and identity are linked and that individuals’ digestive functions influence both mind and mood [1–3]. Furthermore, this connection was bidirectional: the mind affects digestive function, and digestive function influences the mind (Fig. 1). In 1765, Robert Whytt, a Scottish physician, introduced the concept of nervous sympathy in which all internal body organs, including the gut and the brain, are interconnected by a single communication network [4]. Nervous sympathy thus reflects how eighteenth and nineteenth century scientists and physicians conceptualized the reciprocal nature of the gut-brain axis—that is, an unhealthy digestive system causes an abnormal mind.
Fig. 1.
History of the gut-brain axis: trends in clinical and scientific understanding of the gut-brain axis from the eighteenth to twenty-first centuries. In the eighteenth century, the gut-brain axis was conceptualized by most clinicians and scientists as two organs that constantly communicate. However, this view shifted in the nineteenth century as medicine became increasingly specialized. In the twentieth century, a resurgence of interest in gut-brain communication emerged as the USA faced an increasing burden of chronic, non-communicable diseases. The modern twenty-first century understanding of the gut-brain axis is characterized by an appreciation for its complexity, the emerging fields of the gut microbiome and neuroimmunology, and the increasing promise of gut-brain interventions as novel therapeutic approaches to treat neurological disease
Beginning in the late nineteenth century and continuing into the twentieth century, increased specialization in medicine ushered in a Golden Age of medical discovery [5]. This enabled rigorous and focused dissection of specific health problems, leading to more in-depth clinical science and medical breakthroughs such as the polio vaccine in 1955 [6]. However, partitioning different organs to various clinical specialties set back the clock on the holistic approach to understanding the gut-brain connection that was dominant in the eighteenth century. For example, nineteenth-century physicians often dismissed patients with gastrointestinal issues of unclear etiology as hypochondriacs. And, even in the 1970s, physicians would often diagnose patients that exhibited gastrointestinal symptoms with no apparent organic cause with psychiatric rather than gastrointestinal illness [7].
The development of the germ theory of disease by Louis Pasteur and Robert Koch ushered in a new era of microbe-focused research and innovation [8]. The burden of communicable diseases decreased dramatically beginning in the mid-nineteenth century due to improvements in sanitation hygiene as well as the development of vaccines and antibiotics. At the same time, the burden of non-communicable diseases rapidly increased [9]. Unlike infectious diseases, non-communicable conditions were more lifestyle-driven and often chronic [5, 9]. Faced with this new challenge, clinicians and scientists once again began to approach the body holistically. This transition was marked by a steady progression of research seeking to dissect how the gut affects central nervous system functions and vice versa. For example, surgeons observed that post-operative jejunoileostomy patients experienced episodic central nervous system symptoms, such as slurred speech and confusion, which sometimes recurred months after their operation [10]. These observations challenged previous conceptions that the small intestine and the colon were merely tubes for waste materials [7].
Indigenous microbial inhabitants of the gut, now known as the microbiota, also came into focus as a potential key to understanding the gut-brain connection. One of the first proponents of this concept was Russian embryologist Elie Metchnikoff, who proposed that probiotic bacteria found in yogurt could promote health and delay senility more than a century ago [11]. This idea remained largely dormant for many decades, before reemerging in the mid to late twentieth century when multiple research groups began to explore the impact of alterations in the gut microbiota on mammalian phenotypes in rodent models. Ian Rowland in the UK found that toxic concentrations of mercury and associative neurotoxic symptoms were more pronounced in rats treated with antibiotics [12]. Antibiotic administration in rats also altered biogenic amine concentrations in plasma [13, 14]. The generation of germ-free rats that lack all indigenous microbes led to further targeted studies of the gut-brain axis and numerous studies in the late-twentieth century revealed that diverse CNS phenotypes differed between GF and conventional animals. Linda Hegstrand and R. Jean Hine from the William S. Middleton Memorial Veterans Hospital in Wisconsin found that conventional rats had higher hypothalamic histamine levels than germ-free (GF) rats [15]. Furthermore, 1,3-dinitrobenzene administration induced ataxia in GF but not conventional rats [16]. These studies laid the foundation for a growing consensus that gut-brain connections are critical for human health. At the turn of the century, scientists began to examine whether specific commensal bacteria might even prevent or reverse neuropathology. In 1965, following on earlier studies of the probiotic Lactobacillus by Minoru Shirota and Elie Metchnikoff in intestinal health [11], William Macbeth of Harvard Medical School performed one of the first experiments testing the impact of probiotics on the brain when he successfully treated two patients with hepatic encephalopathy with Lactobacillus acidophilus [17].
In the late twentieth century, advances in understanding the connection between the gut and the brain were spurred on by the establishment of the new field of neuroimmunology, which began to challenge the traditional assumption that the brain is segregated from the immune system (Fig. 1). A series of pioneering studies by early proponents of this field demonstrated a critical role for T lymphocytes in maintaining brain homeostasis, injury repair, and resolution of neuroinflammation [18–24]. The shift of clinical research towards a more team-based and multidisciplinary approach in the late twentieth century [25] led to mechanistic insights into the gut-brain axis and the immune system’s role in these interactions. Patrick Dougherty’s lab in the 1980s found that the bacterial product 6–0-stearoyl-muramyl dipeptide (MDP) could attenuate opiate withdrawal severity in a dose-dependent fashion when injected directly into the brain [26, 27]. Sylvain Nadeau and Serge Rivest at Laval University found that myeloid-derived cells in the brain express the LPS receptor CD14, indicating that brain-resident myeloid cells may sense peripheral bacterial products [28]. At the same time, a few studies began to indicate a critical role for the immune system in mediating the bidirectional communication between the gut and the brain. Specifically, Baciu et al. found that tuberomammillary lesions dramatically reduced the phagocytic activity of circulating immune cells [29]. These early indications of neuro-immune-microbiota connections paved the way for the gut-brain-microbiome boom that would occur in the twenty-first century. During this same era, advances in next-generation sequencing enabled facile assessments of microbiota composition independent of microbial culture via 16S rRNA gene sequencing. Many early research efforts using these technologies focused on identifying individual causative agents of non-communicable human diseases; however, after failing to find individual pathogens responsible for these diseases, the field largely converged on the modern holistic view of microbiota-mediated impacts on host health [30].
Modern understanding of neuro-immune-microbiota connections
Modern clinical conceptions of the neuro-immune-microbiota axis are, in essence, an homage to the eighteenth- and nineteenth-century holistic approaches to understanding the gut-brain connection. A preponderance of clinical and pre-clinical data underscore the consistent comorbidity between neuropsychiatric diseases and intestinal pathologies [31, 32]. Correspondingly, the gut microbiota has been found to modulate psychological outcomes, such as behavioral abnormalities in neurodevelopment and the anti-seizure effects of ketogenic diets [33, 34]. An explosion of recent studies has also provided insights into the role of the neuro-immune-microbiota axis in complex diseases such as ulcerative colitis, inflammatory bowel disease, Parkinson’s disease, and multiple sclerosis [35, 36]. Indeed, in the past 21 years, there have been 2.5 times as many publications in this field as compared to the past century altogether. Most recently, spurred on by the rapid growth of the microbiome field, mechanistic insights into the role of the immune system in the gut-brain axis have started to come into focus. Below, we offer a brief review of the current understanding of the neuroimmune-microbiota connection with a focus on the influence of bacteria and their metabolites on neurodegenerative disease.
The classical role of microglia in neuroinflammation
Considered endogenous macrophages of the central nervous system, microglia are essential for tissue homeostasis in the brain. Microglial activation is central to the macrophage theory of depression, where neuroinflammation is a core contributor to abnormal depressive behaviors [37]. Microglia dysfunction is also prominent in other neuropathologies, including schizophrenia (increased microglial activity and density, elevated expression of proinflammatory cytokines) [38, 39], Parkinson’s disease (increased activation in the substantia nigra by alpha-synuclein, proinflammatory profile) [40, 41], Alzheimer’s disease (increased activation, synaptic remodeling) [42–44], and multiple sclerosis (increased activation profile and oxidative stress) [45, 46].
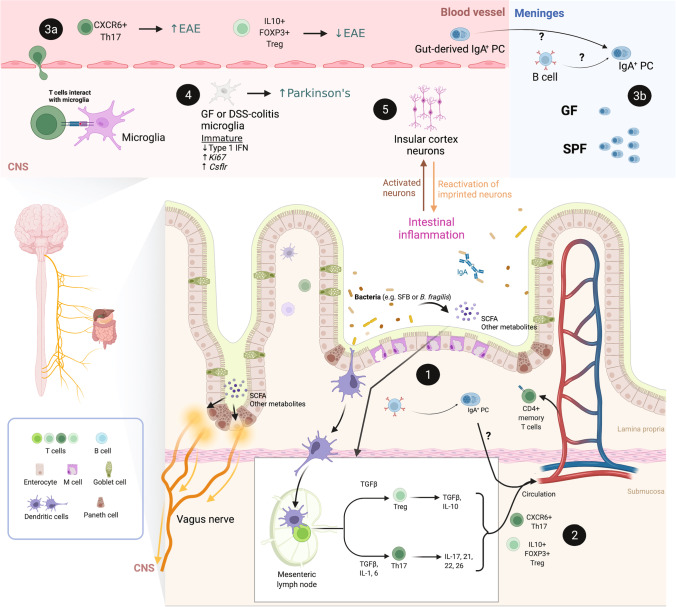
Building upon earlier experiments from the Rivest lab [28], multiple research groups found that GF mice and mice with a dysbiotic gut microbiota have abnormal microglia populations in the hippocampus, cortex, and cerebellum [47–49]. Microglia from GF mice are immature as compared to microglia from conventionalized mice [48], as characterized by higher Ki67 and Csflr expressions and diminished capacity to produce a variety of chemokines and cytokines upon infection [48]. This “dysbiotic” microglial population ultimately leads to a reduced ability to fight against both systemic and local bacterial and viral infections [48]. Microglial morphology is also altered in GF mice and mice colonized with simple bacterial communities, with increased branching [48].
Altered microglial profiles in GF versus conventional mice can be at least partially explained by the effects of microbial-derived metabolites on microglia (Fig. 2). The gut microbiota produces thousands of unique small-molecule metabolites, some of which can accumulate systemically and reach extra-intestinal tissues, including the brain [35, 50]. Short-chain fatty acids (SCFAs), which are produced during microbial fermentation of dietary fiber, are among the best-studied microbial metabolites [50]. Reduced SCFA concentrations have been associated with multiple CNS pathologies, such as brain amyloidosis in Alzheimer’s disease [51]. Furthermore, SCFA administration restored microglial activation profiles and functions in GF mice [48]. Conversely, microbial-derived SCFA promoted microglial activation and enhanced motor dysfunction in a mouse model of Parkinson’s disease [52]. SCFAs also induced microglial production of neuroprotective IL-10 [53]. Beyond SCFA, other microbial metabolites such as indole and its derivatives have also been shown to influence microglial activation and neurotoxicity [54–57]. Microglial activation is also thought to be an initial step in the chemical-induced neurotoxic cascade [58]. Gut microbes that synthesize AhR agonists from dietary tryptophan (e.g. Peptostreptococcus russellii), also influence microglial activation by promoting TGFα production and modulating astrocyte activation and neuroinflammation [54–56].
Fig. 2.
Mechanistic insights into the neuro-immune-microbiota axis. The role of microglia, T and B lymphocytes, and neurons in mediating interactions between the gut and the brain in homeostasis and disease. Modern techniques in microbial manipulation and sterilization (e.g., germ-free [GF] mice) and immunological advances enable precise dissection of the role of specific gut microbes and immune cells in modulating central nervous system (CNS) diseases. Gut microbes and their metabolites influence intestinal T and B cell activation and differentiation (1). A subset of intestinal T and B cells can circulate from the gut (2) to the meninges where they influence the local neuro-immune microenvironment by releasing cytokines (e.g., IL-17a, IL-10) and antibodies (e.g., IgA) that act on central neurons and microglia and protect against meningeal infection (3a: T cells; 3b: B cells). Dysbiotic and GF mice have altered microglia, which are immature and hyperproliferative (4). Lastly, accumulating evidence suggests that vagal and insular cortex neurons are critical in mediating the bi-directional communication between the gut and the brain and that insula neurons can retrieve and reactivate past inflammatory events (5). Abbreviations: EAE: experimental autoimmune encephalomyelitis; GF: germ-free; SPF: specific-pathogen-free; PC: plasma cells; CNS: central nervous system; SCFA: short-chain fatty acids; SFB: segmented filamentous bacteria
Emerging evidence also suggests that microglial neuroinflammation in the central nervous system may result from microbiota-mediated priming via the peripheral immune system (Fig. 2). In mice treated with dextran sulfate sodium (DSS) to induce colitis, researchers observed parallel and synergistic inflammatory responses in the gut mucosa and the cerebral cortex, marked by increased expression of IL6 and iNOS [59]. DSS-colitis ultimately led to microglial alterations characterized by increased activation and elevated cytokine levels, which mirrors the microglial phenotype in germ-free mice [48]. Potential priming of microglia by peripheral immunity is also supported by data from Parkinson’s disease mouse models. For example, DSS–induced intestinal inflammation led to accelerated brain neuropathology and motor dysfunction in a Parkinson’s disease model [60]. The age of onset of motor dysfunction was also significantly earlier in DSS-treated mice compared to mice that were not challenged with DSS. Although the authors did not profile microglia in these studies, these findings are consistent with the microglia hypothesis of Parkinson’s disease progression. However, it is unknown precisely how peripheral immune responses reprogram microglia. Illuminating the nature of this neuromodulation will be vital to develop strategies to protect the central nervous system from the pathological impacts of peripheral immune activation by the microbiome.
Adaptive immunity: T lymphocytes in neuro-immune-microbiota communication
Immune cells in the meningeal compartments are essential for maintaining neurological homeostasis, including regulating behavior and resolution of neuroinflammation after injury [61]. T lymphocytes are critical coordinators and effectors of both mucosal and systemic immunity. Intestinal dendritic cells constantly sample the luminal contents of the gut, resulting in persistent priming of T cells by gut bacteria that primarily results in regulatory T cell (Treg) expansion [62, 63]. Thus, commensal microbes profoundly impact intestinal T cell activation and differentiation. Microbial metabolites, such as SCFAs, maintain immune homeostasis by inducing Treg differentiation [64, 65] (Fig. 2). Conversely, butyrate can activate antigen-specific CD8 + T cell populations, promoting anti-pathogen immunity in the gut [66]. Other metabolites that can alter T lymphocyte functionality include ascorbate (induction of T cell apoptosis) [67], mevalonate and dimethylglycine (inhibit the development of IFNγ + CD8 T cells) [68], and poly-γ-glutamic acid (induction of regulatory T cells), to name just a few [69]. Specific gut bacteria can also induce defined T cell subsets, such as Th17 induction by segmented filamentous bacteria (SFB) and Treg induction by Bacteroides fragilis [70–73].
Microbiota-induced peripheral T cell dysregulation can potentially lead to alterations in T cell populations in the CNS. For example, microbiota-induced increases in Th17 differentiation contribute to maternal immune activation–induced behavioral abnormalities [74] and exacerbate the mouse model of multiple sclerosis experimental autoimmune encephalomyelitis (EAE) [75] [76]. By combining single-cell RNA-sequencing and repertoire sequencing of Th17 T cells across peripheral and central tissues, a recent study solidified the connections between peripheral and CNS T cells in EAE. This study identified two subsets of Th17 cells in the CNS: a homeostatic SLAMF6 + population and a pathogenic CXCR6 + population that migrates to the central nervous system in EAE [77] (Fig. 2). Homeostatic SLAMF6 + Th17 cell populations in intestinal tissues gave rise to pathogenic CXCR6 + Th17 cells, which were significantly reduced in mice treated with antibiotics. These data suggest that microbial composition and density play a critical role in maintaining SLAMF6 + Th17 cells [77]. The conversion of SLAMF6 + to CXCR6 + Th17 cells was also reduced in antibiotic-treated mice [77], providing an attractive mechanistic explanation for the resistance of germ-free mice to EAE.
While SFB-induced Th17 cells can promote EAE, B. fragilis–induced Tregs can ameliorate EAE [78] (Fig. 2). The B. fragilis zwitterionic capsular polysaccharide A protects against severe inflammation in EAE by inducing the conversion of naive T cells to IL-10 + FoxP3+ regulatory T cells [79]. Although the exact mechanism of protection is unclear, it is postulated that these Tregs directly combat IL-17a induction. Recent studies describe a new role for the gut microbiota in the cross talk between peripheral and CNS immunity. Using paired single-cell and TCR repertoire sequencing, Papparlardo et al. found that the majority of T cells in human cerebrospinal fluid exhibit features characteristic of peripheral T cells, suggesting that peripheral T cell populations play a significant role in CNS immunity [80]. Furthermore, Benakis et al. found that bacterial dysbiosis suppressed effector T cell trafficking from the gut to the leptomeninges after acute ischemic brain injury [81]. Finally, the recent discovery that T cells inhabit the meninges both at homeostasis and during inflammation raises a myriad of new questions about the nature of this surveillance and its implication for central nervous system health and disease [82].
In addition to classical T cells, innate-like lymphocytes may also influence neuronal functions. For example, the release of IL-17a by γδ T cells in the meninges can directly activate cortical glutaminergic neurons and induce anxiety-like behaviors [61]. The gut microbiome also has a profound impact on the selection and function of invariant natural killer T (iNKT) cells [84]; thus, NKT cells may also form an additional link between the gut and the brain [85].
Adaptive immunity: B lymphocytes in neuro-immune-microbiota communication
Accumulating data indicate that B cells also play a critical role in regulating CNS immunity. As with T lymphocytes, microbial metabolites can shape B lymphocyte differentiation and function (Fig. 2). Lactobacillus-derived 3-idoleacetic acid and lipopolysaccharide (LPS) enhance the production of IL-35 by Bregs [86]. SCFAs influence plasma cell differentiation and antibody production [87, 88], where acetate induces IgA production and butyrate suppresses IgA production [89, 90]. In the intestine, IgA+ plasma cells critically regulate microbiota composition and barrier function [91], and IgA production by intestinal plasma cells is rapidly induced following microbial colonization [92]. IgA levels in the meninges also depend on microbial colonization status. Like T cells, gut-derived plasma cells can also traffic to the brain [93]. Although IgA+ plasma cells are abundant in the dural venous sinuses of conventional mice, only very low levels of IgA+ plasma cells are found in the dural sinuses of GF mice. Colonization of ex-GF mice with human fecal microbes restored IgA+ plasma cell populations in the dural venous sinuses; notably, dural B cell repertoires overlapped with intestinal plasma cells, suggesting that dural venous sinus plasma cells originated in the gut [93]. IgA-producing plasma cells in the dural venous sinuses provide critical protection against infection as depletion of meningeal IgA+ plasma cells resulted in infiltration of fungi to the brain after intravascular injection [93].
Finally, several recent studies have highlighted immunoregulatory roles for gut-derived IgA+ plasma cells in the central nervous system. Early evidence of immune suppression by B cells emerged in 2010 when Lloyd Casper’s group found that antibiotic treatment of EAE mice induced expansion of CD5+ B cell populations in lymphoid organs and adoptive transfer of these CD5+ B cells conferred protection against EAE pathology [94]. More recently, microbiota-specific IgA+ plasma cells were shown to protect against EAE by trafficking to the central nervous system [95], presumably as part of a homeostatic response to reduce inflammation. This protection is mediated by gut-derived IgA+ plasma cells, which can traffic to the central nervous system and reduce disease severity in an IL-10-dependent manner [96]. Together, these studies show that the impacts of microbiota-mediated education of the immune system extend beyond the intestine to the CNS in both health and disease.
The potential role of vagal and central neurons in neuro-immune-microbiota connections
The vagus nerve can serve as a physical conduit that directly relays signals from the gut microbiota to the central nervous system (Fig. 2). The vagus nerve comprises 80% afferent fibers and 20% efferent fibers [97], and vagal nerve endings in the gastrointestinal tract can sense luminal inputs [98, 99]. Numerous microbial-derived metabolites have been shown to impact vagal activation. The microbiota-derived SCFA oleate activates the vagus nerve via the CCK-A receptor [100], and butyrate can directly activate vagal afferent terminals in the gut [100]. Furthermore, vagal fibers express pattern recognition receptors such as Toll-like receptor 4, enabling direct detection of and activation by bacterial products [101]. Interestingly, targeted vagal stimulation can suppress LPS-induced pro-inflammatory cytokine production by microglia [102, 103]. The vagus nerve’s role in the gut-brain axis is commonly studied using vagotomized humans and mice. For example, vagotomized humans had a significantly lower risk of developing Parkinson’s disease [104, 105]. In mice, Lactobacillus rhamnosus–induced amelioration of anxiety- and depression-related behaviors was diminished after vagotomy [106, 107].
The vagus nerve is also thought to serve as a physical transporter of protein aggregates in Alzheimer’s and Parkinson’s disease via a mechanism analogous to prion disease. The accumulation of α-synuclein aggregates in gut vagal endings often precedes CNS symptoms in progressive Parkinson’s disease, and recent studies have shown that α-synuclein aggregates injected into the gut can transit to the brain via the vagus nerve [108] (Fig. 2). Gut bacteria can also produce amyloid proteins, such as curli or CsgA, which is important for biofilm formation and epithelial adhesion [109, 110]. Colonization with E. coli–producing curli exacerbated motor deficit in a mouse model of Parkinson’s disease [111], suggesting that microbial influences on α-synuclein aggregation in the gut can seed or enhance disease progression via the trafficking of peripheral protein aggregates to the central nervous system.
Recent evidence also suggests that microbial metabolites can directly influence the CNS (Fig. 2). For example, the circulation of microbial metabolites to the brain can affect thalamic axonogenesis in early life [112]. Also, specific microbial-derived metabolites associated with neuropsychiatric disorders, such as 3-(3-hydroxyphenyl)-3-hydroxypropanoic acid (HPHPA), are more abundant in cerebrospinal fluid from conventional mice as compared to GF mice [113]. Finally, recent pioneering studies underscore the bidirectional nature of the gut-brain axis (Fig. 2). Koren et al. demonstrated that a subset of neurons in the insular cortex were activated by DSS-induced intestinal inflammation [114]. Remarkably, reactivation of these neurons after removal of DSS led to the re-induction of intestinal inflammation, suggesting that these neurons can store and retrieve past immunological activation. Prior observations of psychosomatic immune responses, including conditional allergic responses, indicate the potential generalizability of CNS-mediated recall of past immune responses. Future exploration of the memory and recall capacity of the brain in neuro-immune-microbiota cross talk promises to further solidify this fascinating concept.
Conclusion
Scientific and clinical endeavors to understand the gut-brain axis over the past three centuries have evolved in fits and starts toward the modern holistic approach that underlies the explosion of new interest in this area. The emergence of neuroimmunology as a core discipline that links the study of the nervous and immune systems has critically enabled our current understanding of the gut-brain connection. Furthermore, the technology-fueled explosion in microbiome research over the past two decades has ushered in a new era of exploration of the microbiota-gut-brain axis. Going forward, holistic approaches and close collaborations between normally disparate disciplines will be critical to expose the core mechanistic principles that underlie the complex and multi-dimensional interactions between these systems.
Acknowledgements
Figures were created with BioRender.com.
Funding
This work was supported by a grant from the Aligning Science Across Parkinson’s ASAP-000529 through the Michael J. Fox Foundation for Parkinson’s Research (MJFF). MN gratefully acknowledges support from the National Institutes of Health NIGMS (T32GM136651) and NIAID (F30AI157227). NWP gratefully acknowledges support from the Leona M. and Henry B. Helmsley Charitable Trust (3083), the Common Fund of the National Institutes of Health (DP2DK125119), Chan Zuckerberg Initiative, the Ludwig Family Foundation, the Mathers Foundation, the Pew Charitable Trust, NIGMS (RM1GM141649), NIA (R01AG068863), and F. Hoffmann-La Roche. For the purpose of open access, the author has applied a CC BY 4.0 public copyright license to all Author Accepted Manuscripts arising from this submission.
Declarations
Ethics approval
An ethics statement is not applicable because this study is based exclusively on published literature.
Competing interests
N.W.P. is a co-founder of and consultant for Artizan Biosciences and Design Pharmaceuticals, and has received research funding from Artizan Biosciences and F. Hoffmann-La Roche Ltd.
Footnotes
This article is a contribution to the special issue on: Neuroimmune Interactions in Health and Disease - Guest Editors: David Hafler & Lauren Sansing
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moore AM, Mathias M, Valeur J. Contextualising the microbiota-gut-brain axis in history and culture. Microbial Ecol Health Dis. 2018;30:1546267. doi: 10.1080/16512235.2019.1546267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller I (2015) A modern history of the stomach: gastric illness, medicine and British society, 1800–1950: Routledge
- 3.Forth CE, Carden-Coyne A (2005) Cultures of the abdomen: diet, digestion, and fat in the modern world: Springer
- 4.King LS (1970) Robert Whytt, the Soul, and Medicine. JAMA 211: 303-
- 5.Weisz G. The emergence of medical specialization in the nineteenth century. Bull Hist Med. 2003;77:536–575. doi: 10.1353/bhm.2003.0150. [DOI] [PubMed] [Google Scholar]
- 6.Baicus A. History of polio vaccination. World J Virol. 2012;1:108–114. doi: 10.5501/wjv.v1.i4.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotton P (2014) A history of luminal gastroenterology in Britain: the inside guide. BMJ Publishing Group
- 8.Koch R. An address on bacteriological research. Br Med J. 1890;2:380–383. doi: 10.1136/bmj.2.1546.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakovljevic MB, Milovanovic O (2015) Growing burden of non-communicable diseases in the emerging health markets: the case of BRICS. Front Publ Health 3: 65 [DOI] [PMC free article] [PubMed]
- 10.Ayub A, Faloon WW, Heinig RE. Encephalopathy following jejunoileostomy. Jama. 1981;246:970–973. doi: 10.1001/jama.1981.03320090032024. [DOI] [PubMed] [Google Scholar]
- 11.Mackowiak PA. Recycling metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front Public Health. 2013;1:52. doi: 10.3389/fpubh.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowland IR, Davies MJ, Evans JG. Tissue content of mercury in rats given methylmercuric chloride orally: influence of intestinal flora. Arch Environ Health. 1980;35:155–160. doi: 10.1080/00039896.1980.10667485. [DOI] [PubMed] [Google Scholar]
- 13.Sandler M, Karoum F, Ruthven CR, Calne DB. m-Hydroxyphenylacetic acid formation from L-dopa in man: suppression by neomycin. Science. 1969;166:1417–1418. doi: 10.1126/science.166.3911.1417. [DOI] [PubMed] [Google Scholar]
- 14.Fischer JE, James JH, Baldessarini R. Changes in brain amines following portal flow diversion and acute hepatic coma: effects of levodopa (L-dopa) and intestinal sterilization. Surg Forum. 1972;23:348–350. [PubMed] [Google Scholar]
- 15.Hegstrand LR, Hine RJ. Variations of brain histamine levels in germ-free and nephrectomized rats. Neurochem Res. 1986;11:185–191. doi: 10.1007/BF00967967. [DOI] [PubMed] [Google Scholar]
- 16.Philbert MA, Gray AJ, Connors TA. Preliminary investigations into the involvement of the intestinal microflora in CNS toxicity induced by 1,3-dinitrobenzene in male F-344 rats. Toxicol Lett. 1987;38:307–314. doi: 10.1016/0378-4274(87)90013-0. [DOI] [PubMed] [Google Scholar]
- 17.Macbeth WA, Kass EH, McDermott WV., Jr Treatment of hepatic encephalopathy by alteration of intestinal flora with Lactobacillus acidophilus. Lancet. 1965;1:399–403. doi: 10.1016/S0140-6736(65)90002-4. [DOI] [PubMed] [Google Scholar]
- 18.Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz M, Peralta Ramos JM, Ben-Yehuda H. A 20-year journey from axonal injury to neurodegenerative diseases and the prospect of immunotherapy for combating Alzheimer’s disease. J Immunol. 2020;204:243. doi: 10.4049/jimmunol.1900844. [DOI] [PubMed] [Google Scholar]
- 21.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Nun A, Cohen IR. Vaccination against autoimmune encephalomyelitis (EAE): attenuated autoimmune T lymphocytes confer resistance to induction of active EAE but not to EAE mediated by the intact T lymphocyte line. Eur J Immunol. 1981;11:949–952. doi: 10.1002/eji.1830111119. [DOI] [PubMed] [Google Scholar]
- 23.Vandenbark AA, Hashim G, Offner H. Immunization with a synthetic T-cell receptor V-region peptide protects against experimental autoimmune encephalomyelitis. Nature. 1989;341:541–544. doi: 10.1038/341541a0. [DOI] [PubMed] [Google Scholar]
- 24.Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nutma E, Willison H, Martino G, Amor S. Neuroimmunology - the past, present and future. Clin Exp Immunol. 2019;197:278–293. doi: 10.1111/cei.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dougherty PM, Dafny N. Neuroimmune intercommunication, central opioids, and the immune response to bacterial endotoxin. J Neurosci Res. 1988;19:140–148. doi: 10.1002/jnr.490190119. [DOI] [PubMed] [Google Scholar]
- 27.Dougherty PM, Drath DB, Dafny N. Evidence of an immune system to brain communication axis that affects central opioid functions: muramyl peptides attenuate opiate withdrawal. Eur J Pharmacol. 1987;141:253–260. doi: 10.1016/0014-2999(87)90270-6. [DOI] [PubMed] [Google Scholar]
- 28.Nadeau S, Rivest S. Regulation of the gene encoding tumor necrosis factor alpha (TNF-alpha) in the rat brain and pituitary in response in different models of systemic immune challenge. J Neuropathol Exp Neurol. 1999;58:61–77. doi: 10.1097/00005072-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Baciu I, Olteanu A, Prodan T, Băiescu M, Vaida A. Changes of phagocytic biological rhythm by reduction of circadian times and by influences upon hypothalamus. Int J Neurosci. 1988;41:143–153. doi: 10.3109/00207458808985750. [DOI] [PubMed] [Google Scholar]
- 30.Byndloss MX, Bäumler AJ. The germ-organ theory of non-communicable diseases. Nat Rev Microbiol. 2018;16:103–110. doi: 10.1038/nrmicro.2017.158. [DOI] [PubMed] [Google Scholar]
- 31.Taché Y, Bernstein CN. Evidence for the role of the brain-gut axis in inflammatory bowel disease: depression as cause and effect? Gastroenterology. 2009;136:2058. doi: 10.1053/j.gastro.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wouters MM, Van Wanrooy S, Nguyen A, Dooley J, Aguilera-Lizarraga J, Van Brabant W, Garcia-Perez JE, Van Oudenhove L, Van Ranst M, Verhaegen J. Psychological comorbidity increases the risk for postinfectious IBS partly by enhanced susceptibility to develop infectious gastroenteritis. Gut. 2016;65:1279–1288. doi: 10.1136/gutjnl-2015-309460. [DOI] [PubMed] [Google Scholar]
- 33.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173:1728–41.e13. doi: 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson A, Yang D, Vella M, Chiu IM. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021;14:555–565. doi: 10.1038/s41385-020-00368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-M. [DOI] [PubMed] [Google Scholar]
- 38.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M, Yamauchi Y, Yamada S, Kanba S. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115–121. doi: 10.1016/j.pnpbp.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. Faseb j. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 41.Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Exp Mol Med. 2006;38:333–347. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- 42.Koenigsknecht-Talboo J, Meyer-Luehmann M, Parsadanian M, Garcia-Alloza M, Finn MB, Hyman BT, Bacskai BJ, Holtzman DM. Rapid microglial response around amyloid pathology after systemic anti-Abeta antibody administration in PDAPP mice. J Neurosci. 2008;28:14156–14164. doi: 10.1523/JNEUROSCI.4147-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiang Z, Haroutunian V, Ho L, Purohit D, Pasinetti GM. Microglia activation in the brain as inflammatory biomarker of Alzheimer's disease neuropathology and clinical dementia. Dis Markers. 2006;22:95–102. doi: 10.1155/2006/276239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer's disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother. 2018;18:83–90. doi: 10.1080/14737175.2018.1400909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P, Amor S, Teunissen CE, van Horssen J, Dijkstra CD. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation. 2013;10:35. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(599–609):e1–3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 48.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mezö C, Dokalis N, Mossad O, Staszewski O, Neuber J, Yilmaz B, Schnepf D, de Agüero MG, Ganal-Vonarburg SC, Macpherson AJ, Meyer-Luehmann M, Staeheli P, Blank T, Prinz M, Erny D. Different effects of constitutive and induced microbiota modulation on microglia in a mouse model of Alzheimer's disease. Acta Neuropathol Commun. 2020;8:119. doi: 10.1186/s40478-020-00988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Postler TS, Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 2017;26:110–130. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marizzoni M, Cattaneo A, Mirabelli P, Festari C, Lopizzo N, Nicolosi V, Mombelli E, Mazzelli M, Luongo D, Naviglio D, Coppola L, Salvatore M, Frisoni GB. Short-chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in Alzheimer's disease. J Alzheimers Dis. 2020;78:683–697. doi: 10.3233/JAD-200306. [DOI] [PubMed] [Google Scholar]
- 52.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet M-F, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–80.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park J, Wang Q, Wu Q, Mao-Draayer Y, Kim CH. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci Rep. 2019;9:8837. doi: 10.1038/s41598-019-45311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao C-C, Ardura-Fabregat A, de Lima KA, Gutiérrez-Vázquez C, Hewson P, Staszewski O. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557:724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, Alvarez JI, Kébir H, Anandasabapathy N, Izquierdo G, Jung S, Obholzer N, Pochet N, Clish CB, Prinz M, Prat A, Antel J, Quintana FJ. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22:586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ntranos A, Park H-J, Wentling M, Tolstikov V, Amatruda M, Inbar B, Kim-Schulze S, Frazier C, Button J, Kiebish MA, Lublin F, Edwards K, Casaccia P (2021) Bacterial neurotoxic metabolites in multiple sclerosis cerebrospinal fluid and plasma. Brain [DOI] [PMC free article] [PubMed]
- 58.Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- 59.Dempsey E, Abautret-Daly Á, Docherty NG, Medina C, Harkin A. Persistent central inflammation and region specific cellular activation accompany depression- and anxiety-like behaviours during the resolution phase of experimental colitis. Brain Behav Immun. 2019;80:616–632. doi: 10.1016/j.bbi.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 60.Kishimoto Y, Zhu W, Hosoda W, Sen JM, Mattson MP (2019) Chronic mild gut inflammation accelerates brain neuropathology and motor dysfunction in α-Synuclein mutant mice. Neuromolecular Med 21(3):239–249. https://doi.org/10.1007/s12017-019-08539-5. Epub 2019 May 11. [DOI] [PMC free article] [PubMed]
- 61.Alves de Lima K, Rustenhoven J, Da Mesquita S, Wall M, Salvador AF, Smirnov I, Martelossi Cebinelli G, Mamuladze T, Baker W, Papadopoulos Z, Lopes MB, Cao WS, Xie XS, Herz J, Kipnis J. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol. 2020;21:1421–1429. doi: 10.1038/s41590-020-0776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bakdash G, Vogelpoel LT, van Capel TM, Kapsenberg ML, de Jong EC. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015;8:265–278. doi: 10.1038/mi.2014.64. [DOI] [PubMed] [Google Scholar]
- 64.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science (New York, N.Y.) 2013;341:569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bachem A, Makhlouf C, Binger KJ, de Souza DP, Tull D, Hochheiser K, Whitney PG, Fernandez-Ruiz D, Dähling S, Kastenmüller W, Jönsson J, Gressier E, Lew AM, Perdomo C, Kupz A, Figgett W, Mackay F, Oleshansky M, Russ BE, Parish IA, Kallies A, McConville MJ, Turner SJ, Gebhardt T, Bedoui S. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity. 2019;51:285–97.e5. doi: 10.1016/j.immuni.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 67.Chang Y-L, Rossetti M, Vlamakis H, Casero D, Sunga G, Harre N, Miller S, Humphries R, Stappenbeck T, Simpson KW. A screen of Crohn’s disease-associated microbial metabolites identifies ascorbate as a novel metabolic inhibitor of activated human T cells. Mucosal Immunol. 2019;12:457–467. doi: 10.1038/s41385-018-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, Shiota A, Takeshita K, Yasuma-Mitobe K, Riethmacher D, Kaisho T, Norman JM, Mucida D, Suematsu M, Yaguchi T, Bucci V, Inoue T, Kawakami Y, Olle B, Roberts B, Hattori M, Xavier RJ, Atarashi K, Honda K. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565:600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 69.Lee K, Hwang S, Paik DJ, Kim WK, Kim JM, Youn J. Bacillus-derived poly-γ-glutamic acid reciprocally regulates the differentiation of T helper 17 and regulatory T cells and attenuates experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2012;170:66–76. doi: 10.1111/j.1365-2249.2012.04637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan TG, Sefik E, Geva-Zatorsky N, Kua L, Naskar D, Teng F, Pasman L, Ortiz-Lopez A, Jupp R, Wu H-JJ, Kasper DL, Benoist C, Mathis D. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci. 2016;113:E8141–E8150. doi: 10.1073/pnas.1617460113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, Ishikawa E, Shima T, Hara T, Kado S, Jinnohara T, Ohno H, Kondo T, Toyooka K, Watanabe E, Yokoyama S-i, Tokoro S, Mori H, Noguchi Y, Morita H, Ivanov Ivaylo I, Sugiyama T, Nuñez G, Camp JG, Hattori M, Umesaki Y, Honda K. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell. 2015;163:367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, Huh JR. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549:528–532. doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci. 2011;108:4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Korn T, Anderson AC, Bettelli E, Oukka M. The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis. J Neuroimmunol. 2007;191:51–60. doi: 10.1016/j.jneuroim.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schnell A, Huang L, Singer M, Singaraju A, Barilla RM, Regan BML, Bollhagen A, Thakore PI, Dionne D, Delorey TM, Pawlak M, Meyer zuHorste G, Rozenblatt-Rosen O, Irizarry RA, Regev A, Kuchroo VK. Stem-like intestinal Th17 cells give rise to pathogenic effector T cells during autoimmunity. Cell. 2021;184:6281–98.e23. doi: 10.1016/j.cell.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haghikia A, Jörg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, Balogh A, Ostermann AI, Schebb NH, Akkad DA, Grohme DA, Kleinewietfeld M, Kempa S, Thöne J, Demir S, Müller DN, Gold R, Linker RA. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43:817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 79.Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 80.Pappalardo JL, Zhang L, Pecsok MK, Perlman K, Zografou C, Raddassi K, Abulaban A, Krishnaswamy S, Antel J, van Dijk D, Hafler DA (2020) Transcriptomic and clonal characterization of T cells in the human central nervous system. Sci Immunol 5 [DOI] [PMC free article] [PubMed]
- 81.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mundt S, Greter M, Flügel A, Becher B. The CNS immune landscape from the viewpoint of a T cell. Trends Neurosci. 2019;42:667–679. doi: 10.1016/j.tins.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Earls RH, Menees KB, Chung J, Gutekunst C-A, Lee HJ, Hazim MG, Rada B, Wood LB, Lee J-K. NK cells clear & #x3b1;-synuclein and the depletion of NK cells exacerbates synuclein pathology in a mouse model of & #x3b1;-synucleinopathy. Proc Natl Acad Sci. 2020;117:1762–1771. doi: 10.1073/pnas.1909110117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zeissig S, Blumberg RS. Commensal microbiota and NKT cells in the control of inflammatory diseases at mucosal surfaces. Curr Opin Immunol. 2013;25:690–696. doi: 10.1016/j.coi.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cui Y, Wan Q (2019) NKT cells in neurological diseases. Front Cell Neurosci 13 [DOI] [PMC free article] [PubMed]
- 86.Su X, Zhang M, Qi H, Gao Y, Yang Y, Yun H, Zhang Q, Yang X, Zhang Y, He J, Fan Y, Wang Y, Guo P, Zhang C, Yang R. Gut microbiota–derived metabolite 3-idoleacetic acid together with LPS induces IL-35+ B cell generation. Microbiome. 2022;10:13. doi: 10.1186/s40168-021-01205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim M, Qie Y, Park J, Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 2016;20:202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanchez HN, Moroney JB, Gan H, Shen T, Im JL, Li T, Taylor JR, Zan H, Casali P. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat Commun. 2020;11:60. doi: 10.1038/s41467-019-13603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y, Huang X, Xiao Y, Yao S, Zhao Q. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017;10:946–956. doi: 10.1038/mi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.White CA, Pone EJ, Lam T, Tat C, Hayama KL, Li G, Zan H, Casali P. Histone deacetylase inhibitors upregulate B cell microRNAs that silence AID and Blimp-1 expression for epigenetic modulation of antibody and autoantibody responses. J Immunol. 2014;193:5933–5950. doi: 10.4049/jimmunol.1401702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pabst O, Slack E. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol. 2020;13:12–21. doi: 10.1038/s41385-019-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seikrit C, Pabst O. The immune landscape of IgA induction in the gut. Semin Immunopathol. 2021;43:627–637. doi: 10.1007/s00281-021-00879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fitzpatrick Z, Frazer G, Ferro A, Clare S, Bouladoux N, Ferdinand J, Tuong ZK, Negro-Demontel ML, Kumar N, Suchanek O, Tajsic T, Harcourt K, Scott K, Bashford-Rogers R, Helmy A, Reich DS, Belkaid Y, Lawley TD, McGavern DB, Clatworthy MR. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature. 2020;587:472–476. doi: 10.1038/s41586-020-2886-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ochoa-Repáraz J, Mielcarz DW, Haque-Begum S, Kasper LH. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes. 2010;1:103–108. doi: 10.4161/gmic.1.2.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pröbstel AK, Zhou X, Baumann R, Wischnewski S, Kutza M, Rojas OL, Sellrie K, Bischof A, Kim K, Ramesh A, Dandekar R, Greenfield AL, Schubert RD, Bisanz JE, Vistnes S, Khaleghi K, Landefeld J, Kirkish G, Liesche-Starnecker F, Ramaglia V, Singh S, Tran EB, Barba P, Zorn K, Oechtering J, Forsberg K, Shiow LR, Henry RG, Graves J, Cree BAC, Hauser SL, Kuhle J, Gelfand JM, Andersen PM, Schlegel J, Turnbaugh PJ, Seeberger PH, Gommerman JL, Wilson MR, Schirmer L, Baranzini SE (2020) Gut microbiota-specific IgA(+) B cells traffic to the CNS in active multiple sclerosis. Sci Immunol 5 [DOI] [PMC free article] [PubMed]
- 96.Rojas OL, Pröbstel A-K, Porfilio EA, Wang AA, Charabati M, Sun T, Lee DSW, Galicia G, Ramaglia V, Ward LA, Leung LYT, Najafi G, Khaleghi K, Garcillán B, Li A, Besla R, Naouar I, Cao EY, Chiaranunt P, Burrows K, Robinson HG, Allanach JR, Yam J, Luck H, Campbell DJ, Allman D, Brooks DG, Tomura M, Baumann R, Zamvil SS, Bar-Or A, Horwitz MS, Winer DA, Mortha A, Mackay F, Prat A, Osborne LC, Robbins C, Baranzini SE, Gommerman JL. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell. 2019;176:610–24.e18. doi: 10.1016/j.cell.2018.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bonaz B, Bazin T, Pellissier S. The Vagus Nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166:209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Powley TL, Spaulding RA, Haglof SA. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol. 2011;519:644–660. doi: 10.1002/cne.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;281:G907–G915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- 101.Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, Watkins LR. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meneses G, Bautista M, Florentino A, Díaz G, Acero G, Besedovsky H, Meneses D, Fleury A, Del Rey A, Gevorkian G, Fragoso G, Sciutto E. Electric stimulation of the vagus nerve reduced mouse neuroinflammation induced by lipopolysaccharide. J Inflamm (Lond) 2016;13:33. doi: 10.1186/s12950-016-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang J, Ma L, Chang L, Pu Y, Qu Y, Hashimoto K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psychiatry. 2020;10:186. doi: 10.1038/s41398-020-00878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, Svenningsson P, Chen H, Wirdefeldt K. Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology. 2017;88:1996–2002. doi: 10.1212/WNL.0000000000003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sørensen HT. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol. 2015;78:522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 106.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bharwani A, West C, Champagne-Jorgensen K, McVey Neufeld KA, Ruberto J, Kunze WA, Bienenstock J, Forsythe P. The vagus nerve is necessary for the rapid and widespread neuronal activation in the brain following oral administration of psychoactive bacteria. Neuropharmacology. 2020;170:108067. doi: 10.1016/j.neuropharm.2020.108067. [DOI] [PubMed] [Google Scholar]
- 108.Friedland RP. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J Alzheimers Dis. 2015;45:349–362. doi: 10.3233/JAD-142841. [DOI] [PubMed] [Google Scholar]
- 109.Tursi SA, Tükel Ç. Curli-containing enteric biofilms inside and out: matrix composition, immune recognition, and disease implications. Microbiol Mol Biol Rev. 2018;82:e00028–e118. doi: 10.1128/MMBR.00028-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vidakovic L, Singh PK, Hartmann R, Nadell CD, Drescher K. Dynamic biofilm architecture confers individual and collective mechanisms of viral protection. Nat Microbiol. 2018;3:26–31. doi: 10.1038/s41564-017-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sampson TR, Challis C, Jain N, Moiseyenko A, Ladinsky MS, Shastri GG, Thron T, Needham BD, Horvath I, Debelius JW, Janssen S, Knight R, Wittung-Stafshede P, Gradinaru V, Chapman M, Mazmanian SK (2020) A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. eLife 9: e53111 [DOI] [PMC free article] [PubMed]
- 112.Vuong HE, Pronovost GN, Williams DW, Coley EJL, Siegler EL, Qiu A, Kazantsev M, Wilson CJ, Rendon T, Hsiao EY. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. 2020;586:281–286. doi: 10.1038/s41586-020-2745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chu C, Murdock MH, Jing D, Won TH, Chung H, Kressel AM, Tsaava T, Addorisio ME, Putzel GG, Zhou L, Bessman NJ, Yang R, Moriyama S, Parkhurst CN, Li A, Meyer HC, Teng F, Chavan SS, Tracey KJ, Regev A, Schroeder FC, Lee FS, Liston C, Artis D. The microbiota regulate neuronal function and fear extinction learning. Nature. 2019;574:543–548. doi: 10.1038/s41586-019-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koren T, Re Y, Amer M, Krot M, Boshnak N, Ben-Shaanan TL, Azulay-Debby H, Zalayat I, Avishai E, Hajjo H, Schiller M, Haykin H, Korin B, Farfara D, Hakim F, Kobiler O, Rosenblum K, Rolls A. Insular cortex neurons encode and retrieve specific immune responses. Cell. 2021;184:5902–15.e17. doi: 10.1016/j.cell.2021.10.013. [DOI] [PubMed] [Google Scholar]