Abstract
Main conclusion
This manuscript identifies cherry orthologues of genes implicated in the development of pericarpic fruit and pinpoints potential options and restrictions in the use of these targets for commercial exploitation of parthenocarpic cherry fruit.
Abstract
Cherry fruit contain a large stone and seed, making processing of the fruit laborious and consumption by the consumer challenging, inconvenient to eat ‘on the move’ and potentially dangerous for children. Availability of fruit lacking the stone and seed would be potentially transformative for the cherry industry, since such fruit would be easier to process and would increase consumer demand because of the potential reduction in costs. This review will explore the background of seedless fruit, in the context of the ambition to produce the first seedless cherry, carry out an in-depth analysis of the current literature around parthenocarpy in fruit, and discuss the available technology and potential for producing seedless cherry fruit as an ‘ultimate snacking product’ for the twenty-first century.
Keywords: Parthenocarpy, Fruit, Cherry, Seed, June drop
Introduction
Cherry (Prunus avium) trees are believed to be native to Europe, south Asia and to an isolated region of the western Himalaya (Faust and Surányi 1997). Commonly known as sweet cherry, it is a deciduous tree belonging to the Rosaceae family (https://sweetbiomics.com/) (Ganopoulou et al. 2022), which also includes apple (Malus x domestica), peach (Prunus persica), and strawberry (Fragaria vesca and F. ananassa). Young cherry trees show strong apical dominance with a straight trunk which grows to between 15 and 32 m in height. The species has become naturalized in North America and Australia, since it is largely cultivated in these regions due to its commercial importance.
The sweet cherry fruit is a drupe, 1–2 cm in diameter with an attractive appearance, due to its colour (bright red to dark purple) and pleasant intense flavour. The fruit are also nutrient dense, high in fibre and contain a large range of bioactive compounds including polyphenols, anthocyanins, carotenoids, and vitamins (Kelley et al. 2018; McCune et al. 2011; Vignati et al. 2022). Cherries also contain high levels of minerals including magnesium (10 mg/100 g), potassium (200 mg/100 g), phosphorous (20 mg/100 g), and calcium (14 mg/100 g) (Ferretti et al. 2010). Cherries are also a good source of tryptophan and serotonin (Cubero et al. 2010; Garrido et al. 2012). These data suggest that increasing cherry consumption could be beneficial to human health and quality of life of consumers (Alba et al. 2019; Bell et al. 2014; Coelho Rabello Lima et al. 2015; Ferretti et al. 2010; Kelley et al. 2018; McCune et al. 2011;). Some of the most critical attributes desired by consumers include colour, firmness, sweetness, and flavour intensity (Cliff et al. 1996; Guyer et al. 1993; Kappel et al. 1996; Zheng et al. 2016); however, consumers from different regions set different requirements for what is considered a good cherry. For example, in Norwegian, American, and UK markets, consumers prefer larger dark cherries (Crisosto et al. 2003; Lyngstad and Sekse 1995; Sekse and Lyngstad 1996; Wermund and Fearne 2000). Growers and supermarkets often have a different set of criteria for evaluating cherry quality. Specifically, growers want cherries that are firm enough to resist damage during picking, processing, and transporting and easy to harvest (long peduncles), while supermarkets insist on a long shelf life.
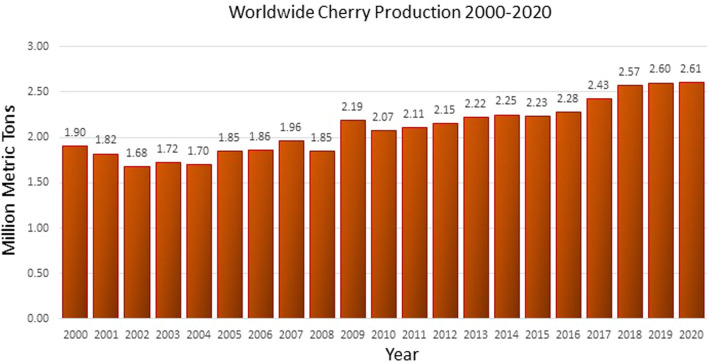
Worldwide, cherry production has increased by almost 40% over the last 20 years as the fruit becomes more popular with consumers (Fig. 1). However, cherry fruit contains a large stone and seed, the presence of which makes it a potential choking hazard for small children and inconvenient to eat ‘on the move’. Identifying a means of generating a seedless, stoneless cherry has the potential of making the fruit even more attractive to the consumer. Previous data have shown that seedless cultivars of fruit such as sweet orange (Citrus sinensis), grape (Vitis vinifera), and watermelon (Citrullus lanatus) have significantly increased consumer consumption (Pollack 2001). In the case of cherry, the absence of the seeds/stone would likewise increase its potential as a snacking product and would also reduce processing costs, thus improving profit margins. An interesting model for stoneless fruit has been identified in P. domestica (Plum).
Fig. 1.
Graphical representation of worldwide cherry production between 2000 and 2020 (Statista.org). Cherry production has increased by 37% between 2000 and 2020 with the lowest production in 2002
Stoneless plum, a retrospective
The role of fruit is to protect the embryo and seed during development and facilitate their dispersal within the environment. Normally, after fertilization of the ovules, fruits develop from the ovary, a process that is coordinated by signals from the developing embryo (Gillaspy et al. 1993). This process of seed, stone, and fruit development is controlled and synchronized by phytohormones (Gillaspy et al. 1993). Previous work, however, has demonstrated that fruit set can be uncoupled from fertilization, resulting in the formation of seedless crops (Gorguet et al. 2005; Hu et al. 2018; Varoquaux et al. 2000). A fruit is considered seedless if it is devoid of seeds, has a significant reduction in seed number, or contains a small number of aborted seeds (Varoquaux et al. 2000). Seedless fruits can be obtained by two different routes, first, parthenocarpy, where the fruit develops without fertilization (Joldersma and Liu 2018; Picarella and Mazzucato 2019) and second, stenospermocarpy, where the seeds abort following fertilization, resulting in traces of aborted seed within the fruit (Varoquaux et al. 2000). Seedlessness has been reported in many species, including grape, citrus, tomato, pear, and banana (Ding et al. 2013; Kim et al. 1992; Klap et al. 2017; Royo et al. 2018; Simmonds 1953; Talon et al. 1990; Wang et al. 2021).
An interesting model for stoneless fruit is seen in a naturally occurring plum (P. domestica) cultivar called “Stoneless” (or “Pitless”), a name given on the basis that it has an underdeveloped stone that makes the fruit partially stoneless. The “Stoneless” ancestor is the variety “Sans-Noyau”, which is also stoneless (its name in French means “without pit”). “Sans-Noyau” has been known in France for several hundred years and in the 1890s was transported to the U.S., where it was crossed with varieties of commercial interest to transmit the stoneless trait. However, the results were not commercially successful (Callahan et al. 2015). Compared to a stoned plum, the expression pattern of lignin biosynthetic genes is similar (Galimba et al. 2020). However, the number of cells in the endocarp has been shown to be reduced compared to the stoned control used in the study (Callahan et al. 2009, 2015). An RNA expression comparison between “Stoneless” and normal stoned cultivars showed that more than 2000 genes are differentially expressed during fruit development, although the authors did not report the identity of these genes and confirmed the necessity of further analysis to find the gene mutation responsible of the stoneless phenotype (Galimba et al. 2020). Segregation analysis suggested that the “Stoneless” trait is dominant and linked to a single gene (Callahan et al. 2015).
Physiological changes during cherry seed development
In the cherry ovary, there are two ovules that arise from the opposite placentae of the single carpel, while in some cultivars, one of the two fertilized arrests its growth before full bloom and collapses; in other cultivars, both ovules develop, but one of the two embryos aborts.
During fertilization, one of the two sperm cells fuses with the egg cell to form the diploid zygote, 2n, and the other one fuses with the central cell, which is 2n, to form the triploid tissue called the endosperm, the function of which is to accumulate nutrients that will be used by the embryo during the seed development. At the time of fertilization, there is a rapid elongation of the megasporocyte, which is enclosed by the megaspore membrane, while after fertilization occurs, both the embryo and the endosperm develop very slowly for a considerable time. In the first stages of the endosperm development after mitosis, there is no cell wall formation, leading to the formation of a non-cellularized syncytium. Cellularization starts at the micropylar pole of the megagametophyte and proceeds progressively to the chalazal end and from the periphery inwards to the centre. The delayed development period of the endosperm is followed by a period of very rapid enlargement (Tukey 1933).
The first zygotic division is transverse and asymmetric, leading to the formation of two cells, a smaller one above a larger cell, the suspensor cell, which forms the suspensor, the function of which is to connect the embryo to the mother plant, allowing the exchanges of nutrients and signal molecules. The suspensor is small, and it persists through to maturity of the embryo. The development of the embryo is even slower than that of the endosperm at the beginning, and then, this phase is followed by a sudden rapid development. At maturity, the embryo is made by two well-formed developed cotyledons, surrounded by a thin layer of endosperm and a fine line of nucellar tissue, and everything is enclosed by the seed coat, a completely maternal tissue, since it is derived from the ovule integuments. As noted above, two embryos can be formed in some cultivars, but one of the two suddenly arrests in development with the nucellar tissue that collapses and the integuments that shrivel. The aborted embryo can either disintegrate 5 days after arrest or can remain intact for up to 42 days.
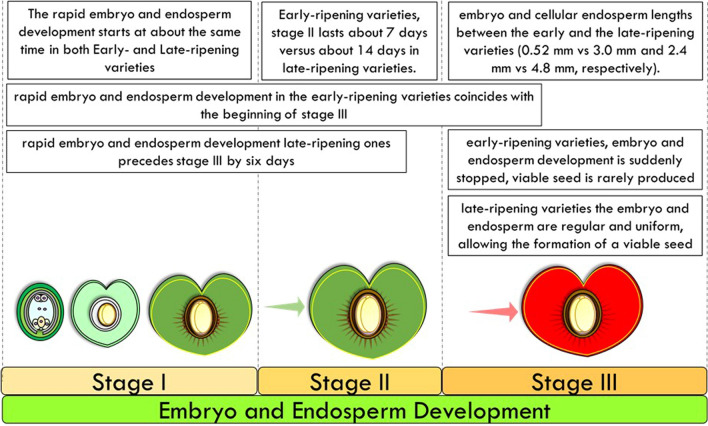
There is a wide variation in the stage of the fruit and embryo development at which abortion can occur. Seed abortion is usually associated with fruit drop (see section June drop), but it could also be a feature of mature fruits in certain varieties. For example, early ripening varieties produce only about 15% of viable seeds, while the seeds of the late-ripening varieties are nearly 100% viable (Fig. 2).
Fig. 2.
Graphical representation of embryo and endosperm development in the cultivated cherry during the three stages of fruit development
During the pre-bloom phase, both the early ripening and the late-ripening varieties develop similarly in all parts, with the only difference being that the early ripening varieties develop slightly in advance and at a slightly greater rate than the late-ripening cherries (Stage I). In early ripening varieties, stage II lasts about half the time compared to late-ripening varieties (about 7 days versus about 14 days when comparing the Early Purple Guigne and the Downer cultivars). The rapid embryo and endosperm development starts at about the same time in both types, and while in the early ripening varieties, this also coincides with the beginning of stage III; in the late-ripening ones, this precedes stage III by 6 days (Tukey 1933).
At the beginning of stage III, there is a big difference in embryo and cellular endosperm lengths between the early and the late-ripening varieties (0.52 mm vs 3.0 mm and 2.4 mm vs 4.8 mm, respectively). In early-ripening varieties, embryo and endosperm development is halted at varying stages, so a viable seed is produced only rarely, while in the late-ripening varieties, they are regular and uniform, allowing the formation of a viable seed. Indeed, the seeds formed by early-ripening varieties are shrivelled, because both the nucellus and the integuments collapse, while the seed of the late-ripening varieties are plump and well filled. Seed abortion occurs at an earlier date in the early ripening varieties, producing smaller aborted embryos, compared with those ripening a few days later. Moreover, seed abortion seems to be not necessarily associated with poor pollination, sterility, incompatibilities, or even nutrition (Tukey 1933, 1934).
The role of hormones in seed development
Gibberellin is sufficient to initiate fruit set
Hormones play an essential role during all the developmental processes and it has long been known that the application of phytohormones to emasculated flowers induces the formation of parthenocarpic fruit (Sjut and Bangerth 1982). Crane et al. (1960) showed that gibberellin (GA) treatment of different plants belonging to the Prunus genus is sufficient to start fruit set in some of them. Indeed, while P. domestica (plum) and P. avium (sweet cherry) did not produce any parthenocarpic fruit, P. dulcis (almond), P. armeniaca (apricot), and P. persica (peach) developed parthenocarpic fruits with a success rate of 11.8%, 15.4% (500 ppm GA treatment), and 73.4%, respectively (50 ppm GA treatment applied twice). Although the highest fruit set was recorded in peach with the lowest concentration of GA, the highest concentration (500 ppm) led to a higher level of stone development and degree of sclerification, which is absent in the fruits treated with 50 ppm solution. The parthenocarpic peaches reached maturation about 10 days before the seeded fruit (Crane et al. 1960). It is likely that the failure to induce parthenocarpy in cherry was due to an incorrect concentration or type of GA, because Wen et al. (2019) showed that treating emasculated P. pseudocerasus (Chinese cherry) pistils with 300 mg/L GA3 solution was enough to initiate the development of parthenocarpic fruit. At 7 days after treatment (DAT), there is an upregulation of the Gibberellin 2-beta-dioxygenase gene (PaGA2ox), which encodes a GA biosynthetic enzyme. Moreover, a list of differentially expressed genes has been reported, and this could be used to identify those involved in the fruit set process in cherry (Wen et al. 2019). Furthermore, Galimba et al. showed that the application of GA3 to Honeycrisp apple flowers triggers the development of parthenocarpic apple fruit (Galimba et al. 2019).
As regards mode of action, gibberellins seem to be involved in the activation of the cell cycle in the ovary-wall cells. In fact, Mesejo et al. (2016) compared the GA levels and the gene expression in two different species of Citrus, Satsuma (Citrus unshiu) and the hybid clementine (Citrus x clementina), which are obligate and facultative parthenocarpic, respectively. In Satsuma, the expression levels of CYCA1.1, which encodes for a cyclin expressed during the G2 phase and controls the G2/M phases of the cell cycle (Fabian et al. 2000), are higher than in clementine. Interestingly, in Satsuma, there is a peak of GA20 oxidase 2 (GA20ox2) expression just before the peak of CYCA1.1 expression, while in clementine, this is delayed. Another GA biosynthesis-related gene, GA3 oxidase 2, has a higher expression level in Satsuma than clementine, during fruit development. Treatments with GA3 caused an increase of GA20 and GA1, which is the most important GA precursor in Satsuma ovaries. More importantly, a 132% increase of CYCA1.1 expression has been observed 6 h after treatment, and this effect was lost after 2 days. The treatment with paclobutrazol (PBZ), an inhibitor of GA biosynthesis, caused a decrease of CYCA1.1 expression after 6 h and this effect was maintained up to 2 days after, proving once again that GAs influence the transcription rate of CYCA1.1. GA3 enhances cell division, increasing the number of cell layers in the pericarp (effect lost at 15 DAT), while the opposite and expected effect has been observed after PBZ treatment. These effects on cell division and parthenocarpic fruit set upon GA3 application have also been observed in clementine (Mesejo et al. 2016).
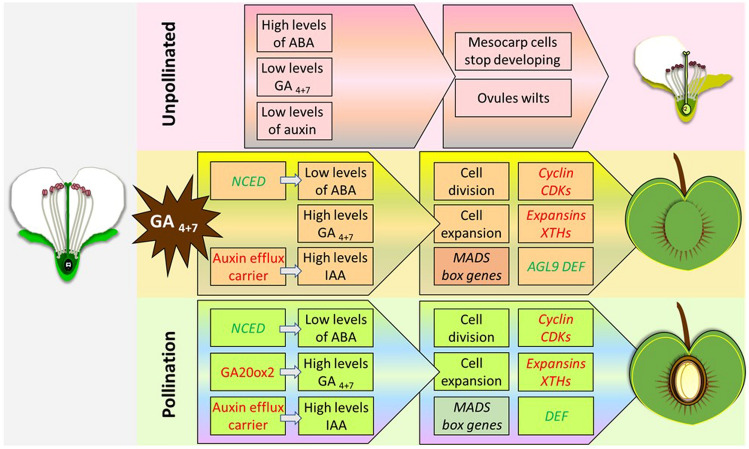
GAs have also been shown to promote the transcriptional activation of many other genes that are involved in fruit development. For example, transcriptome analysis of the “Dangshansuli” pear treated with GA4+7, which induces parthenocarpic fruits, showed that 31 cyclin and 2 CDK genes were up-regulated at 3 DAT, while in the pollinated control, this occurred at 9 DAT. Also, genes encoding expansins, enzymes involved in the modification of the cell wall, have been shown be up-regulated in both the GA4+7-treated and pollinated ovaries. Briefly, 103 cell wall-related DEGs have been identified between unpollinated and GA4+7-treated ovaries and 72 DEGs between unpollinated and pollinated ones at 3 DAT. Moreover, the number of differentially expressed transcription factor genes with similar expression patterns between GA4+7 and pollinated ovaries increases with time (32 at 3DAT, 139 at 9 DAT and 329 at 14 DAT). These transcription factors belong mostly to the bHLH, MYB, NAC, and WRKY families. The treatment with GA also influences the other hormonal pathways, since the treated ovaries showed a small but significant increase of IAA at 3 and 14 DAT, and a significant drop of ABA. Indeed, PbARF5, PbARF6, PbARF18, PbARF19-like, and Pb9-cis-epoxycarotenoid dioxygenase (NCED), which encode key enzymes in the steps for the synthesis of ABA (Schwartz et al. 1997, 2003; Simkin 2021), have been found to be down-regulated both in the GA4+7-treated and pollinated ovaries. Therefore, the model proposed by Liu and collaborators suggests that GA4+7 mimics the effect that pollination has in changing the hormones levels, causing a downstream change of expression of gene involved in different aspects of the transition from ovary to fruit (Liu et al. 2018) (Fig. 3).
Fig. 3.
Proposed model of the crosstalk between auxin and GA signalling pathways during fruit development. In this model, three different scenarios are represented: unpollinated, GA4+7-treated, and following pollination. Red font represents genes up-regulated and green font genes down-regulated
Auxins can act as an alternative signal replacing pollination and fertilization
Auxins not only plays a central role in embryogenesis, but also during the development of the other two seed structures, the endosperm and the seed coat. This class of compounds seems to be the driving force behind the development of all the seed components, and to be involved in their growth synchronization. The main type of auxin in higher plants is indole-3-acetic acid (IAA) the biosynthesis of which starts from tryptophan through a process that comprises two steps and is well conserved among higher plants (Mano and Nemoto 2012; Zhao 2014).
It has been proposed that auxin(s) can act as an alternative signal replacing pollination and fertilization to initiate the fruit growth (Serrani et al. 2008). Indeed, exogenous application of auxin to unfertilized ovules or ectopic production in the central cell is enough to activate mitosis in this cell and starts endosperm development (Figueiredo et al. 2015). Interestingly, the “Dangshansuli” pear emasculated pistils treated with the synthetic auxin 2,4-D have been shown to produce parthenocarpic fruits, which are significantly smaller than the seeded fruits. Furthermore, ovaries treated simultaneously with 2,4-D and Paclobutrazol (PBZ) show a significant reduction of cortex thickness, cells’ division, and cell expansion. PBZ is a triazole plant growth regulator that inhibits cell elongation and internode extension by inhibiting gibberellin biosynthesis (Davis et al. 1991). Moreover, in the ovaries treated with 2,4-D, two pear cyclin-dependent genes, Pbcyclin-dependent kinase B2-2 and, Pbcyclin-dependent kinase B2-2-like, and one expansin gene, Pbexpansine-A10, were up-regulated. On the contrary, they demonstrated down-regulation when co-application with PZB occurred.
Auxin acts upstream of gibberellin
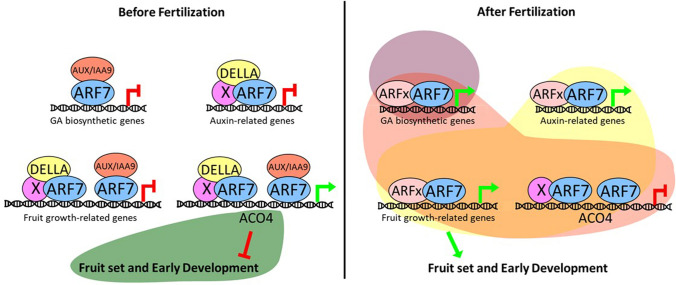
Auxin seems to act upstream of GA in the induction of fruit set, since auxin can trigger GA biosynthesis but not vice versa (Dorcey et al. 2009). 2,4-D mostly induces the production of bioactive GA4 (fourfold increase) but not GA3, in the treated ovaries and a decrease when PBZ was also applied. The final evidence consists of the upregulation of PbGA20ox2-like and PbGA3ox-1, involved in the production of GA4, and the down-regulation of PbGA2ox1-like and PbGA2ox2-like, which converts the GA4 precursors into an inactive form (Cong et al. 2019). Furthermore, in tomato, auxin-induced parthenocarpic fruit development is mediated partially by GA, since co-application of auxin and PBZ causes a significant reduction in parthenocarpy (Serrani et al. 2008). Moreover, bioactive GAs levels are high in the auxin-induced and entire parthenocarpic fruits because of the increased expression of GA biosynthetic genes and the repression of GA catabolism genes (Mignolli et al. 2014; Serrani et al. 2008). However, while IAA application promotes the formation of a parthenocarpic fruit by increasing the pericarp cell layers and enlarging the placenta, GA-treated fruits have fewer cell layers, but cells are larger. Parthenocarpic fruits induced by simultaneous application of IAA and GA display a final size and cellular structure that are similar to the seeded fruit. Through different types of experiments, such as Yeast two Hybrid (Y2H), Y3H, and co-immunoprecipitation, it has been shown that SlARF7, which is an activator of ARF, interacts both with SlIAA and SlDELLA, through different regions (Hu et al. 2018) (Fig. 4).
Fig. 4.
Proposed model of the crosstalk between auxin and GA signalling pathways during tomato fruit development. Before fertilization, SlARF7 (Auxin Response Factor 7) interacts with SlDELLA and SlAUX/IAA9, repressing the transcription of GA biosynthetic genes and the auxin-related genes. They also activate the transcription of ACO4, enhancing ethylene (green area) levels in the ovary which stays frozen. After fertilization, auxin signal (purple circle) comes from the fertilized ovule, promoting the degradation of SlAUX/IAA9. Gibberellins (yellow area) are synthetised and promote the degradation of SlDELLA and a further accumulation of auxin (pink area). SlARF7 can interact with other SlARF proteins and promote fruit set
In addition, ARF7 can form homodimers and heterodimers, but while the homodimerization is not affected by the interactions with SlDELLA or SlIAA9, the heterodimerization is slightly affected. A luciferase assay demonstrated that while GA20ox1, GA3ox1, and GH3.2, which encode an auxin-amino acid conjugating enzyme that converts auxin to an inactive form, are repressed by SlARF7–SLIAA9 complex, they are induced by the SlDELLA-SlARF7 complex. Interestingly, SlDELLA seems to antagonize the SlARF7–SLIAA9 repressive effect on the target genes, such as Expansin5 (EXP5) and ACC oxidase4 (ACO4), which is an ethylene biosynthetic gene. As mentioned above, simultaneous application of auxin and GAs produces the formation of a fruit with a structure similar to the seeded fruit, and this has been observed also in the e pro and SlARF7i pro double mutants, where both the pathways are affected. This can be explained by the fact that in tomato, the crosstalk between GAs and IAA, during the fruit set, occurs through DELLA, ARFs, and IAA9 (Hu et al. 2018). These data and those highlighted by Sharif et al. (Sharif et al. 2022) demonstrate that synergistic and antagonistic crosstalk between hormones is essential for determining the fate of fruit set.
Genetic control of seed development
Seed development is a very elaborate process where three different components develop, each fundamental for reproductive success. Although seed development still has many unknown elements that have to be understood, some important discoveries have been made, especially in Arabidopsis thaliana, and one aspect that is clear is that not only is there synchronization of growth of the embryo, endosperm, and seed coat, but also it seems that there is a crosstalk between them, a transfer of signals and information that regulates and coordinates this elaborate process. The TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS (TAA/TAR) gene family encodes enzymes that conduct auxin biosynthesis during the first step, while the YUCCA (YUC) family works during the second step. There are also alternative biosynthetic pathways, but the TAA/TAR-YUCCA one is the major pathway (Mashiguchi et al. 2011). Mutations in either of the two steps of auxin biosynthesis led to aberrant embryo phenotypes: the yuc1 yuc4 yuc10 quadruple mutant and the taa1 tar1 tar2 triple mutant have similar phenotypes, with abnormal division of the hypophysis, rootless seedlings, very short or no hypocotyl and the majority of them has only one cotyledon (Cheng et al. 2007; Stepanova et al. 2008). Notably, mutants in auxin biosynthesis show aberrant embryo phenotypes of the same type as those present in mutants in which the transport of auxin is affected. More precisely, loss of PIN functionality leads to severe effects on embryo development, i.e., the level of aberration increases with the higher loss of PINs. According to their phosphorylation state, PIN proteins change their intracellular localization. If they are phosphorylated, they are targeted to the apical part of the plasma membrane, while if dephosphorylated, they are targeted to the basal part of the plasma membrane. The phosphorylation and dephosphorylation is effected by PID and PROTEIN PHOSPHATASE 2A (PP2A), respectively, which are serine–threonine protein kinases; pid and pp2a mutants exhibit similar phenotypes to the one reported for the pin mutants, where the auxin distribution is altered (Bennett et al. 1995; Benjamins et al. 2001; Friml et al. 2004; Michniewicz et al. 2007). In addition, the correct perception of auxin, together with AUX/IAA degradation, is fundamental as well during embryogenesis, since mutants display the same phenotypes as those observed when either biosynthesis or transport are impaired. Mutation of the F-box protein, TIR1, which is involved, upon auxin binding, in the degradation of AUX/IAA proteins, is not enough to affect embryo development, since redundancy has been observed with the closely related AUXIN SIGNALLING F-BOX PROTEIN 1 (AFB1), AFB2, and AFB3 (Dharmasiri et al. 2005a, b; Kepinski and Leyser 2005; Tan et al. 2007). Indeed, the quadruple mutant shows defects in embryogenesis, because it often fails to produce the hypocotyl and the root, and has only one cotyledon (Parry et al. 2009). AUXIN RESPONSE FACTOR (ARF) proteins are fundamental players in auxin signalling, and for this reason, there is a quite high degree of redundancy, so it is unlikely that a mutation in only one of these genes leads to a strong phenotype. The only factor that does not follow this is MONOPTEROS/ARF5 (MP/ARF5) whose mutant is rootless, because it exhibits an aberrant division pattern in the hypophysis (Berleth and Jurgens 1993; Hardtke and Berleth 1998; Weijers et al. 2006). Moreover, most of the defects observed and reported in mutants where auxin biosynthesis, transport, or signalling is affected can be explained by the alteration in the activity of MP/ARF5. Indeed, some of the targets of MP are essential transcription factors involved in embryogenesis; these include the WUSCHEL RELATED HOMEOBOX 9 (WOX9) transcription factor gene, which is differentially expressed during the early embryo development. Mutations in MP, WOX2, WOX8, and WOX9 have synergistic effects, meaning that both regulatory pathways converge in controlling the same embryo patterning processes (Haecker et al. 2004); mp, pin1, and pid mutants all fail to demonstrate separate cotyledons and to establish the bilateral symmetry of the cotyledons. Two NAC transcription factor genes, CUP SHAPED COTYLEDON1 (CUC1) and CUC2, are responsible for the formation of the boundaries between different developing areas and work redundantly to regulate the initial formation of the SAM and the separation of the cotyledons, together with SHOOT MERISTEMLESS (STM). While CUC1 and CUC2 are restricted to the cotyledon margins, STM is localized only in the SAM (Aida et al. 1997, 1999). MP, PIN1, and PID have been observed to be required for the activation of CUC2 in the boundaries and the repression of CUC1 in the cotyledons (Aida et al. 2002; Furutani et al. 2004). Mutation of CUC1 partially restores the correct cotyledon development in the double mutant pin1pid, meaning that probably the defects observed are partially caused by an ectopic expression of CUC1 in the cotyledons (Furutani et al. 2004). However, it is still unclear if MP directly regulates the expression of CUC genes or just promotes PIN1 expression and this has a downstream effect on CUC1 and CUC2.
YUC10 and TAR1 have been demonstrated to be imprinted in the endosperm and paternally expressed. This epigenetic regulatory mechanism is conserved among distantly related species, such as Oryza sativa, Capsella. rubella, and A. lyrata (Du et al. 2014; Hatorangan et al. 2016; Klosinska et al. 2016; Luo et al. 2011). Another example is strawberry, where the homologues of YUC10 and TAR1 are specifically expressed in the endosperm, although their imprinting has not yet been determined (Kang et al. 2013). An interesting hypothesis is that there is a sort of auxin transport from the endosperm to the embryo, and since YUC10 and YUC11 are specifically expressed in the endosperm, this could explain the defects observed in the embryos of those mutants (Figueiredo et al. 2015; Figueiredo and Köhler 2018; Robert et al. 2013). To support this hypothesis, it is known that the auxin importer gene LAX1 is specifically expressed in the tip of the cotyledons, but to date, the import of auxin from the endosperm has not yet been demonstrated (Robert et al. 2015). In Arabidopsis, in particular, it has been demonstrated that if auxin biosynthesis or signalling is altered, there is a dramatic effect on the endosperm proliferation rate. Another example of the crucial role of auxin in endosperm development can be found in the woody plant Jatropha curca, and in particular, JcARF9 has been proven to activate cell cycle regulators in the endosperm, such as JcCKA1, JcCYCD2, and JcCYCD5 (Sun et al. 2017). Endosperm development is also highly sensitive to auxin in both raspberry and blackberry seeds (Jennings et al. 1967; Jennings 1971). However, although this crucial role of auxin during endosperm development has been observed, very little is known about its dynamics and transport during the process.
Seed coat development has been proven to be triggered only after fertilization of the central cell and not of the egg cell, meaning that the endosperm is involved in sending signals to the ovule integuments to initiate their conversion to seed coat (Roszak and Köhler 2011; Weijers et al. 2003). However, no cytoplasmic connections have been found between the endosperm and the seed coat, and thus, the communication between these two structures has to rely on small molecules such as peptides and hormones that can cross the plasma membrane (Figueiredo and Köhler 2016; Ingram 2010). Further experimental evidence that auxin produced in the endosperm affects seed coat development has been found in plants where auxin biosynthesis is down-regulated in the endosperm, resuting in seed coat development being significantly altered. Both the transport of auxin from the endosperm to the embryo, and the transport from the triploid tissue to the seed coat remain to be investigated. MADS-box transcription factor AGAMOUS-LIKE62 (AGL62) mutant has been shown to lack the ability to export auxin to the seed coat, and as a consequence, to form it, although the reason why is still unclear. It also appears that epigenetics has a role in the initiation of the seed coat development, blocking it in the unfertilized ovule, and that auxin plays a role in the regulation of this epigenetic mechanism (Figueiredo and Köhler 2016; Roszak and Köhler 2011). Epigenetics is also involved in endosperm development, since a large group of the polycomb repressive complex (PcG) proteins have been identified in the endosperm (Pien and Grossniklaus 2007).
Furthermore, auxin is not the only hormone that has a role during seed development. Gibberellins (GAs) are tetracyclic diterpenoids and they are required for the correct development of the seed, since the GA-deficient mutants display an altered seed development and a significant level of abortions. In tomato, the GA-deficient mutant ga-1 displays altered development, not only of the seed, but also of the flower and the fruit (Groot et al. 1987), while in pea, the lh mutant, which has a significant reduction in the synthesis of GA, has an clear decrease in the seed weight and reduction in seed number in each pod (Swain et al. 1993, 1997). Another example comes from a study involving the constitutive overexpression of the pea GA2 oxidase2 (GA2ox2) gene, which encodes for an enzyme that irreversibly converts active GAs and their precursors into the inactive forms. In Arabidopsis, this causes seed abortion at different stages of the developmental process, since only 3% of the viable seeds carry the GA2ox2 transgene. Thus, the effect of the over expression of GA2ox2 reduces the seed number, due to seed abortion, and the fruit size. However, it has not been possible to measure the GA levels in the aborted seeds because of their small size, so although it is likely that the abortion of the seeds was caused by a low level of active GAs, it has not been demonstrated in this study (Singh et al. 2002). The target of the GA signalling pathway is the Gibberellic Acid Stimulated Arabidopsis (GASA) gene family whose expression is activated and stimulated in response to GAs (Herzog et al. 1995). GASA4, for example, is activated in the shoot apex, in the developing flowers and embryos, and its overexpression leads to a significant increase in seed size and seed weight, while the gasa4 mutant has smaller seeds (Roxrud et al. 2007). Gibberellins act downstream of auxin, since exogenous application of the latter to unfertilized ovules is enough to activate the transcription of the GA biosynthetic genes (Dorcey et al. 2009). Finally, a predominant role of cytokinins has been observed during the early endosperm development, influencing the final dimension of the seed (Day et al. 2008). Arabidopsis histidine kinases (AHKs) are used by the plant cells as cytokinin receptors, and they target the Arabidopsis histidine phosphotransfer proteins (AHPs), which are involved in cytokinin signal transduction. Both ahk2ahk3ahk4 and ahp2ahp3ahp5 triple mutants produce seeds that are twice the size of those in the wild type (Hutchison et al. 2006; Müller and Sheen 2007; Riefler et al. 2006).
As mentioned above, MADS-box transcription factor genes are also involved in ovule seed development, and among these SEEDSTICK (STK), SHATTERPROOF 1 (SHP1) and SHP2 are included. Indeed, the stk shp1 shp2 triple mutant has a visible funiculus, but the integuments are converted to carpel-like structures, showing that these three genes together are indispensable for ovule identity determination (Brambilla et al. 2007; Pinyopich et al. 2003). Moreover, STK has been shown to work in association with the MADS-box transcription factor ARABIDOPSIS Bsister (ABS) in the development of the endothelium, which is the innermost layer of the seed coat, directly in contact with the endosperm. The stk abs double mutant, which lacks the endothelium presence in the seed coat, shows a great reduction of seed production due to abortions during both ovule and seed development (Mizzotti et al. 2012). Moreover, STK is responsible for the correct formation of the cell wall in the seed coat, since it directly regulates factors that take part in the biosynthesis of the cellulose–pectin matrix of the cell wall (Ezquer et al. 2016). In addition, the stk mutant not only shows cells in the seed coat with an altered morphology, but also an altered accumulation of proanthocyanidins. RNA-seq and in-situ hybridization and ChIP data have shown that STK binds the promoter of BANYULS/ANTHOCYANIDIN REDUCTASE (BAN/ANR), which is involved in the proanthocyanidins biosynthetic pathway. Therefore, STK is also involved in the control of secondary metabolism in the seed coat (Mizzotti et al. 2014). Finally, STK seems to be essential for correct seed development, and its mutation can lead to the production of a stenospermocarpic fruit.
Seedless fruit has been identified in multiple species: can this information provide a route to the development of a seedless cherry?
Parthenocarpy and stenospermocarpy in fruiting plants
In some horticultural crops, fruit set and development can occur without the fertilization of the ovules in a process known as parthenocarpy (from Greek “virgin fruit”) (Picarella and Mazzucato 2019; Sharif et al. 2022). Natural parthenocarpy can be obligated in sterile species, such as banana and pineapple, or facultative as in some tomato mutants.
Parthenocarpy has been reported in 96 Angiosperm taxa (Gustafson 1942; Picarella and Mazzucato 2019). The group that presents the highest number of parthenocarpic fruits is the Rosidae (49.8%), where the Rosaceae contribute with six species (the second bigger contribution after the Anacardiaceae and the Rutaceae with eight species). Interestingly, about a half of the parthenocarpic species are trees and between the monospermic fruit-type species, the ones that develop a drupe-type fruit make up the majority. In particular, Prunus persica (L.) Batsch and Prunus cerasifera Ehrh are the two examples of woody plants with a drupe-type fruit that belong to the Rosaceae, in which parthenocarpy had been reported (Gustafson 1939, 1942; Picarella and Mazzucato 2019). Parthenocarpy is an important agricultural trait that can mitigate poor fruit setting caused by unfavorable pollination conditions (Gou et al. 2022). Similarly, seedless fruit can be formed by a preocess termed stenospermocarpy, which is the formation of fruit with spontaneous or induced abortion of fertilized seeds (watermelon and grapes). However, this process, unlike parthenocarpy, requires pollination.
Genetic insights of seedlessness
The MADS-box transcription factors involved in the identity determination of the floral whorls, according to the ABCDE model (Theißen 2001), have been observed to be involved in the formation of a seedless fruit. Indeed, the apple mutant Rae Ime has been reported to be parthenocarpic if not pollinated, while if it is pollinated, it produces seeds with smaller embryos. Its flowers have no petals and stamens, while an apple wild-type flower has five petals and between 9 and 20 stamens, Rae Ime also posesses two whorls of five sepals and an increased number, up to 15, styles and carpels, while the wild-type flower typically has five (Pratt 1988; Yao et al. 2001).
There are other apple mutants, Spencer Seedless and Wallington Bloomless for example, which produce flowers with a similar phenotype of Rae Ime, and are parthenocarpic. This phenotype is similar to that observed in the Arabidopsis mutants pi and ap3. PISTILLATA (PI) and APETALA3 (AP3) belong to the B-class and are involved in the identity determination of the petals, and stamens, with the A-class and C-class MADS-box transcription factors, respectively (Goto and Meyerowitz 1994; Jack et al. 1992; Theißen and Saedler 2001). It has been observed that the apple gene MdPI, which encodes a protein with 64% identity to that encoded by AtPI and which has a similar expression pattern compared to Arabidopsis, in Rae Ime has a 9332 bp LRT-retrotransposon insertion that affects the function of this gene. The mutation of MdPI has also been found in Spencer Seedless and Wallington Bloomless (Table 1) (Yao et al. 2001). Furthermore, co-suppression of MdPI in transgenic apple was also shown to result in parthenocarpy [see Tanaka and Wade (2022) for review].
Table 1.
Summary of the genes involved in parthenocarpic fruit development and potential targets to induce parthenocarpy in cherry
| Target | Pathway | Plant | Phenotype | Reference |
|---|---|---|---|---|
|
Auxin Response Factor 7 (ARF7) |
Auxin | Arabidopsis | Parthenocarpy, seedless/pseudoembryos, size, and shape similar to WT | Goetz et al. (2007) |
| Tomato | Down-regulation of ARF7 resulted in the formation of parthenocarpic fruit and altered shape. SlARF7 RNAi lines also display a down-regulation of SlARF5 and SlARF8B, suggesting that ARF7 cannot promote parthenocarpy unless ARF5 levels are also reduced | de Jong et al. (2009, 2011); Hu et al. (2018) | ||
|
Auxin Response Factor 8 (ARF8) |
Auxin | Eggplant | Natural parthenocarpic mutant showed that ARF8, is down-regulated in buds compared to wild-type plants. Transgenic RNAi lines of ARF8 exhibited parthenocarpy in unfertilized flowers. ARF8 negatively regulates fruit initiation | Du et al. (2016) |
| Arabidopsis | Mutations in ARF8 uncouple fruit initiation from fertilization, resulting in the formation of seedless, parthenocarpic fruit/pseudoembryos, size, and shape similar to wt | Goetz et al. (2007) | ||
| Tomato | Expression of an aberrant ARF8 mutant transcript from Arabidopsis in tomato results in parthenocarpy | Goetz et al. (2007) | ||
|
Indole-3-Acetic Acid Inducible 9 (IAA/9) |
Auxin | Tomato | down-regulation of IAA9 resulted in parthenocarpic fruit and auxin-related alterations in the leaf morphology | Zhang et al. (2007) |
| Parthenocarpy, seedless, early fruit growth, normal size, and shape | Wang et al. (2005) | |||
| AUCSIA | Auxin | Tomato | Aucsia gene silencing causes parthenocarpic fruit development in tomato. Aucsia silenced tomato plants are characterized by facultative (seedless fruits when flowers are emasculated) and rarely, obligate parthenocarpy (i.e., seedless fruit from pollinated flowers). The facultative parthenocarpic fruits were similar in shape to wild-type fruit | Molesini et al. (2009, 2020) |
| PIN4 | Auxin-transport | Tomato | The mutation of the auxin efflux carrier encoding gene PIN4 in tomato has been reported to trigger the development of parthenocarpic fruit | Mounet et al. (2012) |
| DELLA | GA | Arabidopsis | Parthenocarpy observed in DELLA mutants is directly attributed to the constitutive activation of GA signalling | Fuentes et al. (2012) |
|
DELLA Pistillata (PI) |
GA MADS-Box |
Arabidopsis | DELLA mutants have impaired fertilization (seed set) | Dorcey et al. (2009) |
| Tomato | Silencing of DELLA induces facultative parthenocarpy, seedless fruit with reduced size, altered morphology | Martí et al. (2007); Livne et al. (2015); Carrera et al. (2012) | ||
| Apple | Abolishing the normal expression of the Pistillata gene in apple confers parthenocarpic fruit development | Yao et al. (2001) | ||
| Sepallata (SEP) | MADS-Box | Tomato | Antisense and co-suppression of Sepallata in the transgenic ovary developed into parthenocarpic fruit without pollination. The transgenic fruit are bigger than the wild type, although it remains green for a longer before starting the maturation process | Ampomah-Dwamena et al. (2002) |
| SEEDSTICK (STK) | MADS-Box | Arabidopsis | Controls structural and mechanical properties of the Arabidopsis seed coat | Ezquer et al. (2016) |
| AGAMOUS like-6 (AGL6) | MADS-Box | Tomato | Seedless fruits are of normal weight and shape. Down-regulation in natural mutant causes parthenocarpy and low seed | Klap et al. (2017); Takisawa et al. (2018) |
| AGAMOUS like-11 (AGL11) | MADS-Box | Tomato | Gene silencing of AGL11 in tomato produces seedless fruits. Seedlessness is proportional to transcript accumulation levels | Ocarez and Mejía (2016) |
| Tomato | Gene silencing of both AGL11 and a second MADS-Box gene, MBP3, produces all flesh seedless fruit exhibit enhanced firmness and improved post-harvest storage | Huang et al. (2021) | ||
| Grape | Seed abortion caused by a single amino acid substitution in VviAGL11 is the major cause of seedlessness | Royo et al. (2018) | ||
| Grape | Dominant mutation in VviAGL11, homologue of STK, are seedless Seeded fruit is bigger than seedless fruit due to higher expression of VviAGL11 | Mejía et al. (2011) |
SEPALLATA (SEP) genes encode for E-class MADS-box transcription factors, which are necessary for the function of all the other classes. Indeed, the sep1sep2sep3 triple mutant displays an indeterminate flower growth where all the whorls are composed of sepals (Ditta et al. 2004; Pelaz et al. 2000; Theißen 2001).
Tomato MADS-box 29 (TM29) is a single copy gene and has been shown to be the SEPALLATA homolog in tomato, since the encoded protein shares a 68%, 63%, and 58% amino acid sequence identity with AtSEP1, AtSEP2, and AtSEP3, respectively, and also, the expression pattern is conserved. In addition, TM29 has been found in the vegetative and inflorescence meristems, so it seems to have an additional function compared to SEP1. Down-regulation of TM29 through RNAi in tomato leads the production of a mutant with some flower defects, such as green and sterile anthers, and a pistil that develops a parthenocarpic fruit without pollination and fertilization (Table 1). The transgenic fruit is bigger than the wild type, although they remains green for a longer time before starting the maturation process. Moreover, ectopic shoot formation has been observed in the transgenic fruit, which becomes swollen and misshapen. The shoot produces in turn a new fruit from which a new ectopic shoot arises, and this cycle can be repeated three or four times (Ampomah-Dwamena et al. 2002). This severe phenotype can be explained by the fact the SEPALLATA genes are very basal factors (E-class) that work in the determination of all the whorls.
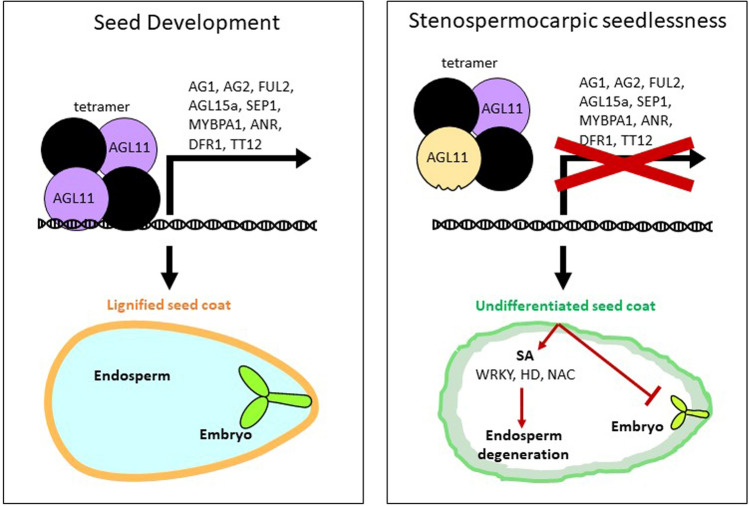
The opposite scenario can be observed in tomato and grapevine mutants of TOMATO AGAMOUS-LIKE 11 (AGL11/TAG11) and Vitis vinifera AGAMOUS-LIKE 11 (VviAGL11) respectively (Table 1). TAGL11 is the STK homolog in tomato, it shares 81% similarity at the nucleotide level with the Arabidopsis gene, and it controls fruit and seed development, being expressed in the inner integuments of the ovule. TAG11 has been silenced using an antisense construct and the lines produced do not exhibit any significant difference in fruit number, date of maturity, final ripening colour, and fruit size. Most of these lines are seedless, and some have few seeds, smaller seeds, or ovule traces. The number of seeds is positively correlated with the expression level of TAG11 in these lines (Ocarez and Mejía 2016). Further work by Huang et al (2021) used crispr/cas9 to silence AGL11 and a second MADS-Box gene, MBP3 in the same lines. This work resulted in the production of all-flesh, seedless transgenic lines with enhanced firmness and improved post-harvest storage; however, average fruit size was severely reduced. Similar results have been reported in pepper, with parthenocarpic fruit reported to be 30% smaller than seeded fruit (Heuvelink and Körner 2001). These data suggest that multi-target approaches may be necessary to achieve seedless cherry and maintain fruit size. VviAGL11 is the homolog of STK in grapevine and it starts to be highly expressed when the seed begins to develop, immediately after berry set (Mejía et al. 2011). Comparing different seeded and seedless genotypes of grapevine has shown that the seeded ones have bigger fruit and a higher expression of VviAGL11 than the seedless ones. Seedless Ruby is a stenospermocarpic mutant that has a dominant mutation in VviAGL11, since the heterozygous plants are seedless. The dominant phenotype is due to a single missense mutation (Arg197 to Leu197), which does not allow the formation of the correct transcriptional complex that is involved in activating all the genes necessary for the normal seed coat development and lignification, which, as mentioned in the previous paragraphs, is crucial for the correct endosperm and embryo development (Ocarez and Mejía 2016; Royo et al. 2018) (Fig. 5).
Fig. 5.
Model of seedlessness proposed by Royo et al. (Royo et al. 2018) to explain the seedless phenotype in grape. The panel on the left shows the initiation of seed morphogenesis that occurs under normal development. The wild-type VviAGL11 (AGAMOUS-LIKE11) protein complex (shown in purple) either directly or indirectly initiates the expression of genes involved in seed coat sclerification, permitting embryo development. The panel on the right shows a heterozygous individual with a dominant phenotype due to a single missense mutation in the VviAGL11 protein (Arg197 to Leu197; shown in yellow). This mutation prevents the assembly of the multiprotein complex halting seed coat differentiation, leading to degeneration of the embryo and endosperm
Two members of the Auxin Response Factor (ARF) family, ARF7 and ARF8, have recently been implicated in fruit initiation (Table 1). The transcript levels of both genes are highly expressed in non-pollinated flowers and are down-regulated after pollination. Down-regulation of ARF7 in tomato resulted in the formation of parthenocarpic fruit and a significant reduction in the accumulation of GAs compared to seeding fruit (de Jong et al. 2009, 2011). Hu et al. (2018) reported that SlARF7 RNAi lines also display a down-regulation of SlARF5 and SlARF8B. These results clearly demonstrate that ARF7 acts to modulate both auxin and gibberellin during fruit set and mediates crosstalk between auxin and gibberellin signalling during tomato fruit development; however, the co-down-regulation of SlARF5 suggests that SlARF7 cannot promote parthenocarpy unless SlARF5 levels are also reduced.
Similar results have been found with AUXIN RESPONSE FACTOR8 (ARF8) in tomato, eggplant, and Arabidopsis. In Arabidopsis, ARF8 mutants display parthenocarpic siliques and the expression of the Arabidopsis mutant allele in tomato results in the production of seedless fruits (Goetz et al. 2006, 2007; Du et al. 2016). The introduction of the mutant ARF8 allele into Arabidopsis did not result in the suppression of the endogenous ARF8 transcript; however, the aberrant transcripts compromised the function of endogenous ARF8 (Goetz et al. 2007). It has been proposed that in both Arabidopsis and tomato, ARF proteins can bind Aux/IAA proteins to form a protein complex that inhibits or activates auxin responsive genes (Hardtke et al. 2004; Tatematsu et al. 2004; Ulmasov et al. 1999). The accumulation of the mutant transcript is thought to destabilize the formation and/or function of the ARF-IAA complex, permitting fruit set in the absence of pollination (Goetz et al. 2006; Swain and Koltunow 2006). Furthermore, the down-regulation of Indole-3-acetic acid inducible 9 (IAA9) (Table 1), the second member of this complex, using an antisense approach in tomato, resulted in parthenocarpic fruit development and auxin-related alterations in leaf morphology (Wang et al. 2005). Kim et al (2020) reported that the silencing of AUX/IAA9 in tomato mimics an increase in auxin levels in the unpollinated ovaries and promotes the expression of auxin and gibberellins biosynthetic genes, which are activated during the fruit set. Auxin and GA crosstalk has been better investigated by Hu et al. (2018). Indeed, the tomato entire (SlIAA9 loss of function), SlARF7 RNAi, and procera (SlDELLA loss of function) mutants show strong parthenocarpy.
June drop
Fruit trees produce a large quantity of flowers, more than are necessary to produce a healthy crop of fruit. In the case of pears and apples, the trees thin themselves, dropping small fruit so as to not overburden the plant, and thereby reducing the chance that branches will break under excessive weight. This process, often referred to as ‘June drop’ or ‘cherry run-off’, begins in late June and continues until mid-July. If just one in 20 flowers produces a fruit, the tree is carrying a healthy crop. These remaining fruits develop normally, becoming bigger at the expense of the fruitlets lost earlier. However, cherries, and other stone fruit, often do not need thinning and do not drop excess flowers requiring extra manual resources to protect crop yield. However, in some years, sweet cherry trees lose a significant proportion of their fruit before ripening. This loss varies from year to year and, in some seasons, can result in a total loss of the crop. In the UK in 2000, for example, cherry trees lost as much as 90% of the fruit set before harvest.
At the start of fruit drop in cherry, when fruits with aborted seeds are abscising, there is significantly more ethylene in the pedicels than in the flesh of the fruits. In both sweet and tart cherry, ethylene was shown to be elevated (fivefold) in fruit flesh and pedicles of abscising fruit compared to adhering fruits (Blanpied 1972). Furthermore, since the application of endogenous ethylene promote the abscission of sweet and tart cherries (Edgerton and Hatch 1969), the combined data suggest that ethylene may play a role in early fruit drop. Furthermore, polar auxin transport inhibitors increased fruit abscission by 30%, demonstrating that maintaining auxin (indole-3-acetic acid, IAA) transport and concentrations in the abscission zone plays a role in fruit retention (Blanusa et al. 2005; Guinn and Brummett 1987). It has been reported that IAA delays abscission by lowering the cells sensitivity to ethylene (Sexton et al. 1985), and yet also stimulates ethylene production, while ethylene is a potent inhibitor of auxin transport (An et al. 2020). This interplay between these two hormones demonstrates the extreme complexity between the regulation of fruit set and fruit abscission (for review see (An et al. 2020; Taylor and Whitelaw 2001). As previously noted, during fruit development, communication between the seed/embryo and the fruit, necessary for the developmental synchronization during fruit formation, involves phytohormones (auxin and gibberellins) produced either in the developing embryo, endosperm, or in the seed coat (Figueiredo and Köhler 2016; Gillaspy et al. 1993; Ingram 2010; Varoquaux et al. 2000).
The complex inter-hormonal signalling mechanism that identifies an aborting seed to the tree and results in fruit abscission is poorly understood; however, it is probable that either a signal travels from the aborting seed to the tree to induce fruit abscission or there is a loss of signal from the seed coat, embryo, or endosperm, that would normally be present during successful seed development.
Options for the generation of seedless cherry and intellectual property rights in the domain of seedless fruit
Seed, stone, and fruit development is a tightly controlled process involving key phytohormones, such as gibberellins, auxins, and environmental cues. The absence of the seeds/stone would be advantageous for producers, reducing processing costs and increasing profits and for the consumer. The presence of the stone and seed makes it both inconvenient to eat ‘on the move’ and potentially dangerous for children. Removal of this stone through genetic means could be potentially transformative for the cherry industry and facilitate rapid market expansion in the UK and beyond. Furthermore, parthenocarpic fruits are firmer and fleshier than pollinated fruits due to the absence of the seeds (Gou et al. 2022; Varoquaux et al. 2000).
Exogenous application of plant hormones has been shown to result in the development of parthenocarpic fruit; however, large-scale introduction of such treatments would prove costly to implement; thus, genetic engineering and genome editing strategies aimed at altering hormone biosynthesis or signalling to obtain parthenocarpic fruits seems a preferable approach. This review highlights some of the potential gene targets for the generation of seedless fruit. We have identified, in the genome databases, orthologues of many of these genes in cherry (Table 2). Manipulation of the listed genes have been shown to induce, directly or indirectly, the development of seedless fruit in the listed species. The table highlights the action required in cherry to potentially obtain a seedless cherry. Previous results in tomato have shown that the down-regulation of ARF7 promotes parthenocarpy; however, this induction requires the co-down-regulation of ARF5 in these plants (Hu et al. 2018). Orthologues of both ARF7 and ARF5 have been identified in the Cherry (Table 2). To obtain parthenocarpic cherry fruit, it may be necessary to knock-down both ARF7 and ARF5 simultaneously.
Table 2.
A list of cherry homologs of the genes involved the development and parthenocarpic fruit in tested species
| Gene | Species | Ref | Putative cherry orthologue | Action |
|---|---|---|---|---|
| ARF8 | Arabidopsis, Tomato, Eggplant | Goetz et al. (2007; Du et al. (2016) | Pav_sc0001314.1_g050.1.mk | Knock-out |
| ARF7 | Tomato | de Jong et al. (2009) | Pav_sc0000129.1_g1480.1.mk | Knock-out |
| ARF5 | Tomato | Hu et al. (2018) | Pav_sc0002080.1_g180.1.mk | Knock-out |
| ARF5-putative | Tomato | Hu et al. (2018) |
Pav_sc0011314.1_g020.1.br Pav_sc0003104.1_g030.1.br Pav_sc0002826.1_g100.1.br Pav_sc0001759.1_g010.1.br |
Knock-out |
| AUX/IAA9 | Tomato, Eggplant | Zhang et al. (2007); Chen et al. (2017) |
Pav_sc0002393.1_g030.1.mk Pav_sc0002327.1_g560.1.mk |
Knock-out |
| AUCSIA | Tomato | Molesini et al. (2020) | Pav_sc0000175.1_g020.1.mk | Knock-out |
| PIN4 | Tomato | Mounet et al. (2012) | Pav_sc0000103.1_g670.1.mk | Knock-out |
| DELLA | Arabidopsis. Tomato | Martí et al. (Fuentes et al. (2012; Livne et al. (2015); 2007; | Pav_sc0000464.1_g350.1.mk | Knock-out |
| GA20ox | Arabidopsis, Tomato, Citrus | García-Hurtado et al. (2012); Mesejo et al. (2016) | Pav_sc0000072.1_g640.1.mk | Up-regulation |
| SEPETALLA (TM29) | Tomato | Ampomah-Dwamena et al. (2002) |
Pav_sc0000176.1_g060.1.mk Pav_sc0000091.1_g150.1.mk Pav_sc0000661.1_g410.1.mk Pav_sc0001080.1_g900.1.mk |
Knock-out |
| PISTILLATA | Apple, Grape | Yao et al. (2001); Fernandez et al. (2013) | Pav_sc0000195.1_g010.1.mk | Knock-out |
| HYDRA | Tomato | Rojas-Gracia et al. (2017) |
Pav_sc0000124.1_g160.1.mk Pav_sc0002264.1_g290.1.mk |
Knock-out |
| AGL6 | Tomato | Klap et al. (2017); Takisawa et al. (2018) | Pav_sc0000072.1_g670.1.mk | Knock-out |
| AGL11 | Grape, Tomato | Ocarez and Mejía (2016); Royo et al. (2018) | Pav_sc0002136.1_g280.1.mk | Knock-out |
Cherry genes were identifed in the Genome Database Rosaceae: https://www.rosaceae.org/species/prunus_avium/genome_v1.0.a1 and AUCSIA and HYDRA were identified by blast of tomato sequences against the cherry genome on EnsemblPlants. Manipulation of the listed genes has been shown to induce, directly or indirectly, the development of parthenocarpic fruit in the listed species
As previously noted, down-regulation of DELLA in tomato causes parthenocarpy (Carrera et al. 2012; Martí et al. 2007; Livne et al. 2015). A single copy of DELLA has been identified in tomato. Here, we have identified a single copy of DELLA in sweet cherry making it an ideal target for generating seedless fruit. In tomato, we should note that the fruit is smaller than wild type. However, in tomato, a mutation in DELLA (procera) includes elongated internode length, thinner leaves, and reduced lobing of the main leaflets, which could influence fruit development and fruit size. One option in sweet cherry would be to use a targeted approach, down-regulating DELLA at early fruit development and in the fruit leaving wild-type levels in the vegetative tissues. The identified orthologue of DELLA gene in the cherry database, Pav_sc0000464.1_g350.1.mk (Table 2), is highly expressed in the unpollinated ovaries at anthesis and decreases only after pollination.
Another option, the down-regulation of the SEPETALLA gene in tomato resulted in parthenocarpic fruit that is bigger than the wild type (Ampomah-Dwamena et al. 2002), suggesting that this may be a more viable target in sweet cherry; however, in this instance, four copies of the PISTILLATA gene have been identified in sweet cherry genome; therefore, identifying the correct target may be more complex.
Over the last few decades, agricultural research has adopted technologies such as genetic engineering and more recently ‘genome editing’ to improve traits in key crops (Aglawe et al. 2018; Georges and Ray 2017; Simkin 2019; Wilson et al. 2019). There have been recent advances in the tools available to carry out this work, including vectors for multiple gene insertion (Engler et al. 2008, 2009, 2014; Exposito-Rodriguez et al. 2017; Marillonnet and Werner 2015) and tissue-specific promoters (Alotaibi et al. 2018, 2019; Kuntz et al. 1998; Mukherjee et al. 2015; Simkin et al. 2006, 2007).
It has previously been demonstrated that the CRISPR-Cas9 genome editing system can be used to breed parthenocarpic tomato plants (Huang et al. 2021). To use site-directed mutagenesis to obtain knock-out mutants of the cherry orthologues, two factors need to be taken into account. First, fruit trees are included in the group of the most reluctant species for in vitro tissue culture. In vitro cherry regeneration and transformation are remarkably challenging, because it has a low success rate and is time-consuming (Vergara et al. 2021). There are, however, a few regeneration and transformation protocols developed for commercially important cherry varieties; these involve the use of leaves, shoots, cotyledons, and epicotyls as initial explants (Bhagwat and Lane 2004; Blando et al. 2007; Canli and Tian 2008; Feeney et al. 2007; Matt and Jehle 2005; Zong et al. 2019; Vergara et al. 2021). Second, the interest is in confirming the phenotype at the flower and fruit level, and sweet cherry has a long juvenile phase (from 4 to 10 years), since it is a woody plant. This obstacle could be overcome by overexpressing a florigen gene such as FLOWERING LOCUS-T (FT). In fact, in plum (P. domestica), the overexpression of the poplar ortholog of FT1 has significantly reduced the juvenile phase, allowing to fruits set just 1 year after seed germination (Srinivasan et al. 2012).
The development of a commercially viable stoneless and seedless cherry must take into account current intellectual property rights (Table 3) that may affect where and how a new variety can be marketed and whether licensing agreements are required before a products can be commercialised. PIN4, for example, a copy of which has been identifed in sweet cherry (Table 2), known to be involved in the development of parthenocarpic tomato fruit (Mounet et al. 2012), is the subject of an active patent until at least 2036 (Van Dun et al. 2021). Other potatial targets, including the manipulation of the Auxin Response Factor (Bouzayen et al. 2013), and DELLA (Ariizumi et al. 2021), are also covered by patent applications (Table 3). One of the most promising targets, down-regulation of AGL6 (Table 1) has been shown to result in seedless tomato fruit, and importantly, these fruits were described as being of nornal weight and shape (Klap et al. 2017; Takisawa et al. 2018), which is not always the case; however, use of AGL6 is the subject of a patent application (Barg et al. 2021).
Table 3.
Summary of the relevant patent (legal status denoted as active) and patent applications (denoted as pending) for targets identified in Tables 1 and 2 pertaining to the development of seedless and stoneless fruit
| Patent number | Gene | Title | Applicants | Pub | Earliest priority | Legal status | Ref |
|---|---|---|---|---|---|---|---|
| US 10941411 B2 | PIN4 | Modified gene resulting in parthenocarpic fruit set | Rijk Zwaan Zaadteelt en Zaadhandel Bv | Mar 9, 2021 | Jan 30, 2015 |
Active until 2036 |
Van Dun et al. (2021) |
|
WO 2013 034722 A1 |
ARF | New parthenocarpic plants with modified expression of auxin response factors and the microRNAs inducing said modified expression | Institut National Polytechnique de Toulouse | Mar 14, 2013 | Sep 7, 2011 | Pending | Bouzayen et al. (2013) |
|
US 2021 0037779 A1 |
AGL6 | Parthenocarpic plants and methods of producing same | The State of Israel Ministry of Agriculture | Feb 11, 2021 | Jan 21, 2016 | Pending | Barg et al. (2021) |
|
WO 2020 252167 A1 |
STK | Methods of producing plants with altered fruit development and plants derived therefrom | Pairwise Plants Services Inc | Dec 17, 2020 | Jun 11, 2019 | Pending | Crawford and Poorten (2020) |
|
WO 2021 040011 A1 |
DELLA | Fruit-bearing plant exhibiting high temperature resistance, high yield, and parthenocarpy | Univ Tsukuba, Ibaraki, Japan | Mar 4, 2021 | Aug 30, 2018 | Pending | Ariizumi et al. (2021) |
The earliest priority date is the date at which the patent application claims priority over any other applications filed after that date
When granted, patents may potentially restrict the use of specific technologies for the development of parthenocarpic cherry, restrict gene targets to induce parthenocpary using genome editing, or restrict access to the market in specific countries or regions. It should be stressed that patent applications often contain speculative or unsubstantiated claims and there is no certainty that the applications in this summary will be approved and thereby achieve the status of a granted patent.
Conclusions
Seedless/stoneless cherry fruit would be highly advantageous to the cherry industry. First, it is well known that sweet cherry pollination is mediated by insects, which is important for the production of a viable crop (Eeraerts et al. 2020). Wild pollinators, including solitary bees, are essential to ensure sweet cherry yields (Eeraerts et al. 2019); however, growers rely on commercial domesticated honeybees (Apis mellifera) for pollination at a cost up to 1000 euro per hectare, a considerable investment for commercial cherry producers. The absence of the need for pollination also beings the potential for higher fruit yields and the absence of the seed/stone an increase in flesh content. With no need for pollination, the significant costs of providing commercial domesticated honeybees to carry out this function would no longer be required, thus not only reducing production costs but also the reliance on the presence of wild bee populations and solitary bees, which also play a considerable role in fruit set. Furthermore, reliance on honeybees also comes with the drawback that they remain inactive under unfavorable weather conditions, such as at temperatures below 12 °C or heavy rainfall, which can provide additional drawbacks to cherry production. Second, one of the possible causes of ‘June drop’ is poor or inadequate pollination, possibly due to a reduction in insect activity. Fruit set without pollination has the potential to mitigate June drop and increase and protect yields. Finally, there is also the suggestion that June drop occurs due to signals received by the plant from the aborted seed. In the absence of the seed, that signal is removed, and fruit previously dropped may develop to full maturity, thereby increasing cherry yield for growers and potentially reducing cost to the consumer. The absence of the seed has been reported to increase fruit quality (seed can have a hard texture and/or unpleasant flavour). Furthermore, seeds can release compounds that result in fruit spoilage, and therefore, the absence of the seed can increase shelf life. In such instances, a seedless fruit may still be advantageous to growers even if the stone remains; removal of the need for pollination increasing yields and the inhibition of June drop reducing yield loss. Seedless fruit has also been reported to reduce yield fluctuations in pepper (Heuvelink and Körner 2001).
In conclusion, there are a large number of factors involved in fruit set and the pathways are not linear, but there are regulation checkpoints at different levels, redundancy and crosstalk, which together make this process extremely complicated. There is still a lot that has to be learnt and constantly new proteins, pathways, and hormones are being found to have a function in this already complex scenario; these variables include the role of jasmonic acid and its derivatives (Schubert et al. 2019), SPOROCYELESS/NOZZLE (Rojas-Gracia et al. 2017), and also microRNAs (Wang et al. 2018). It should be noted that we have addressed the removal of the seed from cherry fruit in this review; however, the seed is contained within a lignified stone, which remains in the absence of the seed. The removal of the stone would be the next step in the breeding of a truly seedless/stoneless fruit fit for industrial exploitation.
Author contribution statement
EV and AJS: wrote the first draft of the manuscript; ML, MC, and JMD: contributed to the revised version of the manuscript.
Acknowledgements
E.V. was supported by ‘Understanding Fruit Development in the Cultivated Cherry’ project funded by Berry Gardens Growers Ltd awarded (https://www.berrygardens.co.uk/) to A.J.S. A.J.S. is supported by the Growing Kent and Medway Programme, UK; Ref 107139.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors report no conflicts of interest in this work and have nothing to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aglawe SB, Barbadikar KM, Mangrauthia SK, Madhav MS. New breeding technique "genome editing" for crop improvement: applications, potentials and challenges. 3 Biotech. 2018;8(8):336. doi: 10.1007/s13205-018-1355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M. Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell. 1997;9(6):841–857. doi: 10.1105/tpc.9.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida M, Ishida T, Tasaka M. Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development. 1999;126(8):1563–1570. doi: 10.1242/dev.126.8.1563. [DOI] [PubMed] [Google Scholar]
- Aida M, Vernoux T, Furutani M, Traas J, Tasaka M. Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development. 2002;129(17):3965–3974. doi: 10.1242/dev.129.17.3965. [DOI] [PubMed] [Google Scholar]
- Alba CM-A, Daya M, Franck C. Tart Cherries and health: current knowledge and need for a better understanding of the fate of phytochemicals in the human gastrointestinal tract. Crit Rev Food Sci Nutr. 2019;59(4):626–638. doi: 10.1080/10408398.2017.1384918. [DOI] [PubMed] [Google Scholar]
- Alotaibi SS, Sparks CA, Parry MAJ, Simkin AJ, Raines CA. Identification of leaf promoters for use in transgenic wheat. Plants. 2018;7(2):27. doi: 10.3390/plants7020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alotaibi SS, Alyassi H, Alshehawi A, Gaber A, Hassan MM, Aljuaid BS, Simkin AJ, Raines CA. Functional analysis of SBPase gene promoter in transgenic wheat under different growth conditions. Biotechnology. 2019;1:15–23. [Google Scholar]
- Ampomah-Dwamena C, Morris BA, Sutherland P, Veit B, Yao JL. Down-regulation of TM29, a tomato SEPALLATA homolog, causes parthenocarpic fruit development and floral reversion. Plant Physiol. 2002;130(2):605–617. doi: 10.1104/pp.005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Althiab Almasaud R, Bouzayen M, Zouine M, Chervin C. Auxin and ethylene regulation of fruit set. Plant Sci. 2020;292:110381. doi: 10.1016/j.plantsci.2019.110381. [DOI] [PubMed] [Google Scholar]
- Ariizumi T, Ezura H, Harada K, Shinozaki Y, Yano R, Riku U (2021) Fruit-bearing plant exhibiting high temperature resistance, high yield, and parthenocarpy. Patent WO 2021/040011 A1
- Barg R, Salts Y, Klap C, Arazi I, Yeshayahou E, Bolger A, Shabtai S (2021) Parthenocarpic plants and methods of producing same. Patent US 2021/0037779 A1
- Bell PG, McHugh MP, Stevenson E, Howatson G. The role of cherries in exercise and health. Scand J Med Sci Sports. 2014;24(3):477–490. doi: 10.1111/sms.12085. [DOI] [PubMed] [Google Scholar]
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R. The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development. 2001;128(20):4057–4067. doi: 10.1242/dev.128.20.4057. [DOI] [PubMed] [Google Scholar]
- Bennett SR, Alvarez J, Bossinger G, Smyth DR. Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J. 1995;8(4):505–520. doi: 10.1046/j.1365-313X.1995.8040505.x. [DOI] [Google Scholar]
- Berleth T, Jurgens G. The role of the MONOPTEROS gene in organising the basal body region of the Arabidopsis embryo. Development. 1993;118(2):575–587. doi: 10.1242/dev.118.2.575. [DOI] [Google Scholar]
- Bhagwat B, Lane WD. In vitro shoot regeneration from leaves of sweet cherry (Prunus avium) ‘Lapins’ and ‘Sweetheart. Plant Cell Tissue Organ Cult. 2004;78(2):173–178. doi: 10.1023/B:TICU.0000022552.12449.71. [DOI] [Google Scholar]
- Blando F, Chiriaco L, Gerardi C, Lucchesini M, Rampino P. Sweet cherry (Prunus avium L.) 'Giorgia', adventitious regeneration from leaves of microplants. Eur J Hortic Sci. 2007;72:138–143. [Google Scholar]
- Blanpied GD. A study of ethylene in apple, red raspberry, and cherry. Plant Physiol. 1972;49(4):627–630. doi: 10.1104/pp.49.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanusa T, Else MA, Atkinson CJ, Davies WJ. The regulation of sweet cherry fruit abscission by polar auxin transport. Plant Growth Regul. 2005;45(3):189–198. doi: 10.1007/s10725-005-3568-9. [DOI] [Google Scholar]
- Bouzayen M, Zouine M, Pech J-C, Latche A (2013) New parthenocarpic plants with modified expression of auxin response factors and the micrornas inducing said modified expression. Patent WO 2013/034722 A1
- Brambilla V, Battaglia R, Colombo M, Masiero S, Bencivenga S, Kater MM, Colombo L. Genetic and molecular interactions between BELL1 and MADS box factors support ovule development in Arabidopsis. Plant Cell. 2007;19(8):2544–2556. doi: 10.1105/tpc.107.051797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan AM, Dardick C, Scorza R. Characterization of ‘Stoneless’: a naturally occurring, partially stoneless plum cultivar. J Am Soc Hortic Sci. 2009;134(1):120–125. doi: 10.21273/JASHS.134.1.120. [DOI] [Google Scholar]
- Callahan A, Dardick C, Tosetti R, Lalli D, Scorza R. 21st century approach to improving Burbank’s ‘Stoneless’ plum. J Am Soc Hortic Sci. 2015;50(2):195–200. [Google Scholar]
- Canli FA, Tian L. In vitro shoot regeneration from stored mature cotyledons of sweet cherry (Prunus avium L.) cultivars. Sci Hortic. 2008;116(1):34–40. doi: 10.1016/j.scienta.2007.10.023. [DOI] [Google Scholar]
- Carrera E, Ruiz-Rivero O, Peres LE, Atares A, Garcia-Martinez JL. Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol. 2012;160(3):1581–1596. doi: 10.1104/pp.112.204552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang M, Tan J, Huang S, Wang C, Zhang H, Tan T. Comparative transcriptome analysis provides insights into molecular mechanisms for parthenocarpic fruit development in eggplant (Solanum melongena L.) Plos one. 2017;12(6):e0179491. doi: 10.1371/journal.pone.0179491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell. 2007;19(8):2430–2439. doi: 10.1105/tpc.107.053009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff MA, Dever MC, Hall JW, Giraud B. Development and evaluation of multiple regression models for predicting sweet cherry liking. Food Res Int. 1996;28(6):583–589. doi: 10.1016/0963-9969(95)00041-0. [DOI] [Google Scholar]
- Coelho Rabello Lima L, Oliveira Assumpção C, Prestes J, Sérgio Denadai B. Consumption of cherries as a strategy to attenuate exercise-induced muscle damage and inflamation in humans. Nutr Hosp. 2015;32(5):1885–1893. doi: 10.3305/nh.2015.32.5.9709. [DOI] [PubMed] [Google Scholar]
- Cong L, Yue R, Wang H, Liu J, Zhai R, Yang J, Wu M, Si M, Zhang H, Yang C, Xu L, Wang Z. 2,4-D-induced parthenocarpy in pear is mediated by enhancement of GA4 biosynthesis. Physiol Plant. 2019;166(3):812–820. doi: 10.1111/ppl.12835. [DOI] [PubMed] [Google Scholar]
- Crane JC, Primer PE, Campbell RC. Gibberellin induced parthenocarpy in Prunus. Proc Am Soc Hortic Sci. 1960;75:129–137. [Google Scholar]
- Crawford BCW, Poorten TJ (2020) Methods of producing plants with altered fruit development and plants derived therefrom. Patent WO 2020/252167 A1
- Crisosto CH, Crisosto GM, Metheney P. Consumer acceptance of ‘Brooks’ and ‘Bing’ cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol Technol. 2003;28(1):159–167. doi: 10.1016/S0925-5214(02)00173-4. [DOI] [Google Scholar]
- Cubero J, Toribio F, Garrido M, Hernández MT, Maynar J, Barriga C, Rodríguez AB. Assays of the amino acid tryptophan in cherries by HPLC-fluorescence. Food Anal Methods. 2010;3(1):36–39. doi: 10.1007/s12161-009-9084-1. [DOI] [Google Scholar]
- Davis TD, Curry EA, Steffens GL. Chemical regulation of vegetative growth. Crit Rev Plant Sci. 1991;10(2):151–188. doi: 10.1080/07352689109382310. [DOI] [Google Scholar]
- Day RC, Herridge RP, Ambrose BA, Macknight RC. Transcriptome analysis of proliferating Arabidopsis endosperm reveals biological implications for the control of syncytial division, cytokinin signaling, and gene expression regulation. Plant Physiol. 2008;148(4):1964–1984. doi: 10.1104/pp.108.128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong M, Wolters-Arts M, Feron R, Mariani C, Vriezen WH. The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J. 2009;57(1):160–170. doi: 10.1111/j.1365-313X.2008.03671.x. [DOI] [PubMed] [Google Scholar]
- de Jong M, Wolters-Arts M, Garcia-Martinez JL, Mariani C, Vriezen WH. The Solanum lycopersicum AUXIN RESPONSE FACTOR 7 (SlARF7) mediates cross-talk between auxin and gibberellin signalling during tomato fruit set and development. J Exp Bot. 2011;62(2):617–626. doi: 10.1093/jxb/erq293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435(7041):441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M. Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell. 2005;9(1):109–119. doi: 10.1016/j.devcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Ding J, Chen B, Xia X, Mao W, Shi K, Zhou Y, Yu J. Cytokinin-induced parthenocarpic fruit development in tomato is partly dependent on enhanced gibberellin and auxin biosynthesis. PLoS One. 2013;8(7):e70080. doi: 10.1371/journal.pone.0070080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky MF. The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr Biol. 2004;14(21):1935–1940. doi: 10.1016/j.cub.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Dorcey E, Urbez C, Blázquez MA, Carbonell J, Perez-Amador MA. Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J. 2009;58(2):318–332. doi: 10.1111/j.1365-313X.2008.03781.x. [DOI] [PubMed] [Google Scholar]
- Du M, Luo M, Zhang R, Finnegan EJ, Koltunow AM. Imprinting in rice: the role of DNA and histone methylation in modulating parent-of-origin specific expression and determining transcript start sites. Plant J. 2014;79(2):232–242. doi: 10.1111/tpj.12553. [DOI] [PubMed] [Google Scholar]
- Du L, Bao C, Hu T, Zhu Q, Hu H, He Q, Mao W. SmARF8, a transcription factor involved in parthenocarpy in eggplant. Mol Genet Genom. 2016;291(1):93–105. doi: 10.1007/s00438-015-1088-5. [DOI] [PubMed] [Google Scholar]
- Edgerton LJ, Hatch AH. Promoting abscission of cherries and apples for mechanical harvesting. Proc N Y State Hortic Soc. 1969;114:109–113. [Google Scholar]
- Eeraerts M, Smagghe G, Meeus I. Pollinator diversity, floral resources and semi-natural habitat, instead of honey bees and intensive agriculture, enhance pollination service to sweet cherry. Agr Ecosyst Environ. 2019;284:106586. doi: 10.1016/j.agee.2019.106586. [DOI] [Google Scholar]
- Eeraerts M, Borremans L, Smagghe G, Meeus I. A growers’ perspective on crop pollination and measures to manage the pollination service of wild pollinators in sweet cherry cultivation. Insects. 2020;11(6):372. doi: 10.3390/insects11060372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C, Kandzia R, Marillonnet S. A one pot, one step, precision cloning method with high throughput capability. PLoS One. 2008;3(11):e3647. doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C, Gruetzner R, Kandzia R, Marillonnet S. Golden gate shuffling: a one-pot DNA shuffling method based on type IIs restriction enzymes. PLoS One. 2009;4(5):e5553. doi: 10.1371/journal.pone.0005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C, Youles M, Gruetzner R, Ehnert TM, Werner S, Jones JD, Patron NJ, Marillonnet S. A golden gate modular cloning toolbox for plants. ACS Synth Biol. 2014;3(11):839–843. doi: 10.1021/sb4001504. [DOI] [PubMed] [Google Scholar]
- Exposito-Rodriguez M, Laissue PP, Lopez-Calcagno PE, Mullineaux PM, Raines CA, Simkin AJ. Development of pGEMINI, a plant gateway destination vector allowing the simultaneous integration of two cDNA via a single LR-clonase reaction. Plants (basel) 2017;6(4):55. doi: 10.3390/plants6040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezquer I, Mizzotti C, Nguema-Ona E, Gotté M, Beauzamy L, Viana VE, Dubrulle N, Costa de Oliveira A, Caporali E, Koroney AS, Boudaoud A, Driouich A, Colombo L. The developmental regulator SEEDSTICK controls structural and mechanical properties of the Arabidopsis seed coat. Plant Cell. 2016;28(10):2478–2492. doi: 10.1105/tpc.16.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian T, Lorbiecke R, Umeda M, Sauter M. The cell cycle genes cycA1;1 and cdc2Os-3 are coordinately regulated by gibberellin in planta. Planta. 2000;211(3):376–383. doi: 10.1007/s004250000295. [DOI] [PubMed] [Google Scholar]
- Faust M, Surányi D. Origin and dissemination of cherry. Hortic Rev. 1997;19:263–317. [Google Scholar]
- Feeney M, Bhagwat B, Mitchell JS, Lane WD. Shoot regeneration from organogenic callus of sweet cherry (Prunus avium L.) Plant Cell Tissue Organ Cult. 2007;90(2):201–214. doi: 10.1007/s11240-007-9252-1. [DOI] [Google Scholar]
- Fernandez L, Chaïb J, Martinez-Zapater JM, Thomas MR, Torregrosa L. Mis-expression of a PISTILLATA-like MADS box gene prevents fruit development in grapevine. Plant J. 2013;73(6):918–928. doi: 10.1111/tpj.12083. [DOI] [PubMed] [Google Scholar]
- Ferretti G, Bacchetti T, Belleggia A, Neri D. Cherry antioxidants: from farm to table. Molecules. 2010;15(10):6993–7005. doi: 10.3390/molecules15106993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo DD, Köhler C. Bridging the generation gap: communication between maternal sporophyte, female gametophyte and fertilization products. Curr Opin Plant Biol. 2016;29:16–20. doi: 10.1016/j.pbi.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Figueiredo DD, Köhler C. Auxin: a molecular trigger of seed development. Genes Dev. 2018;32(7–8):479–490. doi: 10.1101/gad.312546.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo DD, Batista RA, Roszak PJ, Köhler C. Auxin production couples endosperm development to fertilization. Nature Plants. 2015;1:15184. doi: 10.1038/nplants.2015.184. [DOI] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PB, Ljung K, Sandberg G, Hooykaas PJ, Palme K, Offringa R. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science. 2004;306(5697):862–865. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- Fuentes S, Ljung K, Sorefan K, Alvey E, Harberd NP, Østergaard L. Fruit growth in Arabidopsis occurs via DELLA-dependent and DELLA-independent gibberellin responses. Plant Cell. 2012;24(10):3982–3996. doi: 10.1105/tpc.112.103192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani M, Vernoux T, Traas J, Kato T, Tasaka M, Aida M. PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development. 2004;131(20):5021–5030. doi: 10.1242/dev.01388. [DOI] [PubMed] [Google Scholar]
- Galimba KD, Bullock DG, Dardick C, Liu Z, Callahan AM. Gibberellic acid induced parthenocarpic ‘Honeycrisp’ apples (Malus domestica) exhibit reduced ovary width and lower acidity. Hortic Res. 2019;6:41. doi: 10.1038/s41438-019-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimba K, Tosetti R, Loerich K, Michael L, Pabhakar S, Dove C, Dardick C, Callahan A. Identification of early fruit development reference genes in plum. PLoS One. 2020;15(4):e0230920. doi: 10.1371/journal.pone.0230920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganopoulou M, Michailidis M, Angelis L, Ganopoulos I, Molassiotis A, Xanthopoulou A, Moysiadis T. Could causal discovery in proteogenomics assist in understanding gene-protein relations? A perennial fruit tree case study using sweet cherry as a model. Cells. 2022;11(1):92. doi: 10.3390/cells11010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Hurtado N, Carrera E, Ruiz-Rivero O, López-Gresa MP, Hedden P, Gong F, García-Martínez JL. The characterization of transgenic tomato overexpressing gibberellin 20-oxidase reveals induction of parthenocarpic fruit growth, higher yield, and alteration of the gibberellin biosynthetic pathway. J Exp Bot. 2012;63(16):5803–5813. doi: 10.1093/jxb/ers229. [DOI] [PubMed] [Google Scholar]
- Garrido M, Espino J, Toribio-Delgado AF, Cubero J, Maynar-Mariño JI, Barriga C, Paredes SD, Rodríguez AB. A jerte valley cherry-based product as a supply of tryptophan. Int J Tryptophan Res. 2012;5:IJTR.S 9394. doi: 10.4137/ijtr.S9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Ray H. Genome editing of crops: a renewed opportunity for food security. GM Crops and Food. 2017;8(1):1–12. doi: 10.1080/21645698.2016.1270489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W. Fruits: a developmental perspective. Plant Cell. 1993;5(10):1439–1451. doi: 10.1105/tpc.5.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz M, Vivian-Smith A, Johnson SD, Koltunow AM. AUXIN RESPONSE FACTOR8 is a negative regulator of fruit initiation in Arabidopsis. Plant Cell. 2006;18(8):1873–1886. doi: 10.1105/tpc.105.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz M, Hooper LC, Johnson SD, Rodrigues JC, Vivian-Smith A, Koltunow AM. Expression of aberrant forms of AUXIN RESPONSE FACTOR8 stimulates parthenocarpy in Arabidopsis and tomato. Plant Physiol. 2007;145(2):351–366. doi: 10.1104/pp.107.104174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorguet B, van Heusden AW, Lindhout P. Parthenocarpic fruit development in tomato. Plant Biol. 2005;7(2):131–139. doi: 10.1055/s-2005-837494. [DOI] [PubMed] [Google Scholar]
- Goto K, Meyerowitz EM. Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev. 1994;8(13):1548–1560. doi: 10.1101/gad.8.13.1548. [DOI] [PubMed] [Google Scholar]
- Gou C, Zhu P, Meng Y, Yang F, Xu Y, Xia P, Chen J, Li J. Evaluation and genetic analysis of parthenocarpic germplasms in cucumber. Genes. 2022;13(2):225. doi: 10.3390/genes13020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot SP, Bruinsma J, Karssen CM. The role of endogenous gibberellin in seed and fruit development of tomato: studies with a gibberellin-deficient mutant. Physiol Plant. 1987;71(2):184–190. doi: 10.1111/j.1399-3054.1987.tb02865.x. [DOI] [Google Scholar]
- Guinn G, Brummett DL. Concentrations of abscisic acid and indoleacetic acid in cotton fruits and their abscission zones in relation to fruit retention. Plant Physiol. 1987;83(1):199–202. doi: 10.1104/pp.83.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson FG. Auxin distribution in fruits and its significance in fruit development. Am J Bot. 1939;26(4):189–194. doi: 10.1002/j.1537-2197.1939.tb12888.x. [DOI] [Google Scholar]
- Gustafson FG. Parthenocarpy: natural and artificial. Bot Rev. 1942;8(9):599–654. doi: 10.1007/BF02881046. [DOI] [Google Scholar]
- Guyer DE, Sinha NK, Chang TS, Cash JN. Physiochemical and sensory characteristics of selected Michigan sweet cherry (Prunus avium L.) cultivars. J Food Qual. 1993;16(5):355–370. doi: 10.1111/j.1745-4557.1993.tb00121.x. [DOI] [Google Scholar]
- Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development. 2004;131(3):657–668. doi: 10.1242/dev.00963. [DOI] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17(5):1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Ckurshumova W, Vidaurre DP, Singh SA, Stamatiou G, Tiwari SB, Hagen G, Guilfoyle TJ, Berleth T. Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL 4. Development. 2004;131(5):1089–1100. doi: 10.1242/dev.00925. [DOI] [PubMed] [Google Scholar]
- Hatorangan MR, Laenen B, Steige KA, Slotte T, Köhler C. Rapid evolution of genomic Imprinting in two species of the Brassicaceae. Plant Cell. 2016;28(8):1815–1827. doi: 10.1105/tpc.16.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog M, Dorne AM, Grellet F. GASA, a gibberellin-regulated gene family from Arabidopsis thaliana related to the tomato GAST1 gene. Plant Mol Biol. 1995;27(4):743–752. doi: 10.1007/bf00020227. [DOI] [PubMed] [Google Scholar]
- Heuvelink E, Körner O. Parthenocarpic fruit growth reduces yield fluctuation and blossom-end rot in sweet pepper. Ann Bot. 2001;88(1):69–74. doi: 10.1006/anbo.2001.1427. [DOI] [Google Scholar]
- Hu J, Israeli A, Ori N, Sun T-p. The interaction between DELLA and ARF/IAA mediates crosstalk between gibberellin and auxin signaling to control fruit initiation in tomato. Plant Cell. 2018;30(8):1710–1728. doi: 10.1105/tpc.18.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Hu G, Wang K, Frasse P, Maza E, Djari A, Deng W, Pirrello J, Burlat V, Pons C, Granell A, Li Z, van der Rest B, Bouzayen M. Interaction of two MADS-box genes leads to growth phenotype divergence of all-flesh type of tomatoes. Nat Commun. 2021;12(1):6892. doi: 10.1038/s41467-021-27117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison CE, Li J, Argueso C, Gonzalez M, Lee E, Lewis MW, Maxwell BB, Perdue TD, Schaller GE, Alonso JM, Ecker JR, Kieber JJ. The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell. 2006;18(11):3073–3087. doi: 10.1105/tpc.106.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram GC. Family life at close quarters: communication and constraint in angiosperm seed development. Protoplasma. 2010;247(3–4):195–214. doi: 10.1007/s00709-010-0184-y. [DOI] [PubMed] [Google Scholar]
- Jack T, Brockman LL, Meyerowitz EM. The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell. 1992;68(4):683–697. doi: 10.1016/0092-8674(92)90144-2. [DOI] [PubMed] [Google Scholar]
- Jennings DL. Some genetic factors affecting the development of endocarp, endosperm and embryo in raspberries. New Phytol. 1971;70(5):885–895. doi: 10.1111/j.1469-8137.1971.tb02589.x. [DOI] [Google Scholar]
- Jennings DL, Craig DL, Topham PB. The role of the male parent in the reproduction of Rubus. Heredity. 1967;22(1):43–55. doi: 10.1038/hdy.1967.4. [DOI] [Google Scholar]
- Joldersma D, Liu Z. The making of virgin fruit: the molecular and genetic basis of parthenocarpy. J Exp Bot. 2018;69(5):955–962. doi: 10.1093/jxb/erx446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Darwish O, Geretz A, Shahan R, Alkharouf N, Liu Z. Genome-scale transcriptomic insights into early-stage fruit development in woodland strawberry Fragaria vesca. Plant Cell. 2013;25(6):1960–1978. doi: 10.1105/tpc.113.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappel F, Fisher-Fleming B, Hogue E. Fruit characteristics and sensory attributes of an ideal sweet cherry. HortScience. 1996;31(3):443–446. doi: 10.21273/hortsci.31.3.443. [DOI] [Google Scholar]
- Kelley DS, Adkins Y, Laugero KD. A review of the health benefits of cherries. Nutrients. 2018;10(3):368. doi: 10.3390/nu10030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435(7041):446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- Kim IS, Okubo H, Fujieda K. Endogenous levels of IAA in relation to parthenocarpy in cucumber (Cucumis sativus L.) Sci Hortic. 1992;52(1):1–8. doi: 10.1016/0304-4238(92)90002-T. [DOI] [Google Scholar]
- Kim J-S, Ezura K, Lee J, Kojima M, Takebayashi Y, Sakakibara H, Ariizumi T, Ezura H. The inhibition of SlIAA9 mimics an increase in endogenous auxin and mediates changes in auxin and gibberellin signalling during parthenocarpic fruit development in tomato. J Plant Physiol. 2020;252:153238. doi: 10.1016/j.jplph.2020.153238. [DOI] [PubMed] [Google Scholar]
- Klap C, Yeshayahou E, Bolger AM, Arazi T, Gupta SK, Shabtai S, Usadel B, Salts Y, Barg R. Tomato facultative parthenocarpy results from SlAGAMOUS-LIKE 6 loss of function. Plant Biotechnol J. 2017;15(5):634–647. doi: 10.1111/pbi.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosinska M, Picard CL, Gehring M. Conserved imprinting associated with unique epigenetic signatures in the Arabidopsis genus. Nature Plants. 2016;2:16145. doi: 10.1038/nplants.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz M, Chen HC, Simkin AJ, Römer S, Shipton CA, Drake R, Schuch W, Bramley PM. Upregulation of two ripening-related genes from a non-climacteric plant (pepper) in a transgenic climacteric plant (tomato) Plant J. 1998;13(3):351–361. doi: 10.1046/j.1365-313X.1998.00032.x. [DOI] [Google Scholar]
- Liu L, Wang Z, Liu J, Liu F, Zhai R, Zhu C, Wang H, Ma F, Xu L. Histological, hormonal and transcriptomic reveal the changes upon gibberellin-induced parthenocarpy in pear fruit. Horticul Res. 2018;5:1. doi: 10.1038/s41438-017-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livne S, Lor VS, Nir I, Eliaz N, Aharoni A, Olszewski NE, Eshed Y, Weiss D. Uncovering DELLA-independent gibberellin responses by characterizing new tomato procera mutants. Plant Cell. 2015;27(6):1579–1594. doi: 10.1105/tpc.114.132795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Taylor JM, Spriggs A, Zhang H, Wu X, Russell S, Singh M, Koltunow A. A genome-wide survey of imprinted genes in rice seeds reveals imprinting primarily occurs in the endosperm. PLoS Genet. 2011;7(6):e1002125. doi: 10.1371/journal.pgen.1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyngstad L, Sekse L. Economic aspects of developing a high sweet cherry product in Norway. Acta Hortic. 1995;379(39):313–320. doi: 10.17660/ActaHortic.1995.379.39. [DOI] [Google Scholar]
- Mano Y, Nemoto K. The pathway of auxin biosynthesis in plants. J Exp Bot. 2012;63(8):2853–2872. doi: 10.1093/jxb/ers091. [DOI] [PubMed] [Google Scholar]
- Marillonnet S, Werner S. Assembly of multigene constructs using golden gate cloning. In: Castilho A, editor. Glyco-engineering: methods and protocols. New York: Springer; 2015. pp. 269–284. [DOI] [PubMed] [Google Scholar]
- Martí C, Orzáez D, Ellul P, Moreno V, Carbonell J, Granell A. Silencing of DELLA induces facultative parthenocarpy in tomato fruits. Plant J. 2007;52(5):865–876. doi: 10.1111/j.1365-313X.2007.03282.x. [DOI] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, McSteen P, Zhao Y, Hayashi K, Kamiya Y, Kasahara H. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA. 2011;108(45):18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt A, Jehle JA. In vitro plant regeneration from leaves and internode sections of sweet cherry cultivars (Prunus avium L.) Plant Cell Rep. 2005;24(8):468–476. doi: 10.1007/s00299-005-0964-6. [DOI] [PubMed] [Google Scholar]
- McCune LM, Kubota C, Stendell-Hollis NR, Thomson CA. Cherries and health: a review. Crit Rev Food Sci Nutr. 2011;51(1):1–12. doi: 10.1080/10408390903001719. [DOI] [PubMed] [Google Scholar]
- Mejía N, Soto B, Guerrero M, Casanueva X, Houel C, de los Ángeles Miccono M, Ramos R, Le Cunff L, Boursiquot J-M, Hinrichsen P, Adam-Blondon A-F. Molecular, genetic and transcriptional evidence for a role of VvAGL11 in stenospermocarpic seedlessness in grapevine. BMC Plant Biol. 2011;11(1):57. doi: 10.1186/1471-2229-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesejo C, Yuste R, Reig C, Martínez-Fuentes A, Iglesias DJ, Muñoz-Fambuena N, Bermejo A, Germanà MA, Primo-Millo E, Agustí M. Gibberellin reactivates and maintains ovary-wall cell division causing fruit set in parthenocarpic Citrus species. Plant Sci. 2016;247:13–24. doi: 10.1016/j.plantsci.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, Schwab R, Weigel D, Meyerowitz EM, Luschnig C, Offringa R, Friml J. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell. 2007;130(6):1044–1056. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Mignolli F, Vidoz ML, Mariotti L, Lombardi L, Picciarelli P. Induction of gibberellin 20-oxidases and repression of gibberellin 22-oxidases in unfertilized ovaries of entire tomato mutant, leads to accumulation of active gibberellins and parthenocarpic fruit formation. Plant Growth Regul. 2014;75:415–425. doi: 10.1007/s10725-014-0002-1. [DOI] [Google Scholar]
- Mizzotti C, Mendes MA, Caporali E, Schnittger A, Kater MM, Battaglia R, Colombo L. The MADS box genes SEEDSTICK and ARABIDOPSIS Bsister play a maternal role in fertilization and seed development. Plant J. 2012;70(3):409–420. doi: 10.1111/j.1365-313X.2011.04878.x. [DOI] [PubMed] [Google Scholar]
- Mizzotti C, Ezquer I, Paolo D, Rueda-Romero P, Guerra RF, Battaglia R, Rogachev I, Aharoni A, Kater MM, Caporali E, Colombo L. SEEDSTICK is a master regulator of development and metabolism in the Arabidopsis seed coat. PLoS Genet. 2014;10(12):e1004856. doi: 10.1371/journal.pgen.1004856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molesini B, Pandolfini T, Rotino GL, Dani V, Spena A. Aucsia gene silencing causes parthenocarpic fruit development in tomato. Plant Physiol. 2009;149(1):534–548. doi: 10.1104/pp.108.131367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molesini B, Dusi V, Pennisi F, Pandolfini T. How hormones and MADS-Box transcription factors are involved in controlling fruit set and parthenocarpy in tomato. Genes (basel) 2020;11(12):1441. doi: 10.3390/genes11121441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounet F, Moing A, Kowalczyk M, Rohrmann J, Petit J, Garcia V, Maucourt M, Yano K, Deborde C, Aoki K, Bergès H, Granell A, Fernie AR, Bellini C, Rothan C, Lemaire-Chamley M. Down-regulation of a single auxin efflux transport protein in tomato induces precocious fruit development. J Exp Bot. 2012;63(13):4901–4917. doi: 10.1093/jxb/ers167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Stasolla C, Brule-Babel A, Ayele BT. Isolation and characterization of rubisco small subunit gene promoter from common wheat (Triticum aestivum L.) Plant Signal Behav. 2015;10(2):e989033. doi: 10.4161/15592324.2014.989033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B, Sheen J. Arabidopsis cytokinin signaling pathway. Sci STKE. 2007;2007(407):cm5. doi: 10.1126/stke.4072007cm5. [DOI] [PubMed] [Google Scholar]
- Ocarez N, Mejía N. Suppression of the D-class MADS-box AGL11 gene triggers seedlessness in fleshy fruits. Plant Cell Rep. 2016;35(1):239–254. doi: 10.1007/s00299-015-1882-x. [DOI] [PubMed] [Google Scholar]
- Parry G, Calderon-Villalobos LI, Prigge M, Peret B, Dharmasiri S, Itoh H, Lechner E, Gray WM, Bennett M, Estelle M. Complex regulation of the TIR1/AFB family of auxin receptors. Proc Natl Acad Sci USA. 2009;106(52):22540–22545. doi: 10.1073/pnas.0911967106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature. 2000;405(6783):200–203. doi: 10.1038/35012103. [DOI] [PubMed] [Google Scholar]
- Picarella ME, Mazzucato A. The occurrence of seedlessness in higher plants; Insights on roles and mechanisms of parthenocarpy. Front Plant Sci. 2019;9:1997. doi: 10.3389/fpls.2018.01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pien S, Grossniklaus U. Polycomb group and trithorax group proteins in Arabidopsis. Biochem Biophys Acta. 2007;1769(5–6):375–382. doi: 10.1016/j.bbaexp.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Pinyopich A, Ditta GS, Savidge B, Liljegren SJ, Baumann E, Wisman E, Yanofsky MF. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature. 2003;424(6944):85–88. doi: 10.1038/nature01741. [DOI] [PubMed] [Google Scholar]
- Pollack S (2001) Consumer demand for fruits and vegetables: The U.S. example. Changing structure of global food consumption and trade. Economic Research Service Publication WR-S-01-1. U.S. Department of Agriculture, Washington, DC
- Pratt C. Apple flower and fruit: morphology and anatomy. Hortic Rev. 1988;10:273–308. [Google Scholar]
- Riefler M, Novak O, Strnad M, Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell. 2006;18(1):40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert HS, Grones P, Stepanova AN, Robles LM, Lokerse AS, Alonso JM, Weijers D, Friml J. Local auxin sources orient the apical-basal axis in Arabidopsis embryos. Curr Biol. 2013;23(24):2506–2512. doi: 10.1016/j.cub.2013.09.039. [DOI] [PubMed] [Google Scholar]
- Robert HS, Grunewald W, Sauer M, Cannoot B, Soriano M, Swarup R, Weijers D, Bennett M, Boutilier K, Friml J. Plant embryogenesis requires AUX/LAX-mediated auxin influx. Development. 2015;142(4):702–711. doi: 10.1242/dev.115832. [DOI] [PubMed] [Google Scholar]
- Rojas-Gracia P, Roque E, Medina M, Rochina M, Hamza R, Angarita-Díaz MP, Moreno V, Pérez-Martín F, Lozano R, Cañas L, Beltrán JP, Gómez-Mena C. The parthenocarpic hydra mutant reveals a new function for a SPOROCYTELESS-like gene in the control of fruit set in tomato. New Phytol. 2017;214(3):1198–1212. doi: 10.1111/nph.14433. [DOI] [PubMed] [Google Scholar]
- Roszak P, Köhler C. Polycomb group proteins are required to couple seed coat initiation to fertilization. Proc Natl Acad Sci. 2011;108(51):20826–20831. doi: 10.1073/pnas.1117111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxrud I, Lid SE, Fletcher JC, Schmidt ED, Opsahl-Sorteberg HG. GASA4, one of the 14-member Arabidopsis GASA family of small polypeptides, regulates flowering and seed development. Plant Cell Physiol. 2007;48(3):471–483. doi: 10.1093/pcp/pcm016. [DOI] [PubMed] [Google Scholar]
- Royo C, Torres-Pérez R, Mauri N, Diestro N, Cabezas JA, Marchal C, Lacombe T, Ibáñez J, Tornel M, Carreño J, Martínez-Zapater JM, Carbonell-Bejerano P. The major origin of seedless grapes Is associated with a missense mutation in the MADS-Box gene VviAGL11. Plant Physiol. 2018;177(3):1234–1253. doi: 10.1104/pp.18.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert R, Dobritzsch S, Gruber C, Hause G, Athmer B, Schreiber T, Marillonnet S, Okabe Y, Ezura H, Acosta IF, Tarkowska D, Hause B. Tomato MYB21 acts in ovules to mediate jasmonate-regulated fertility. Plant Cell. 2019;31(5):1043–1062. doi: 10.1105/tpc.18.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR. Specific oxidative cleavage of carotenoids by VP14 of maize. Science. 1997;276(5320):1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, McCarty DR, Welch W, Zeevaart JA. Substrate specificity and kinetics for VP14, a carotenoid cleavage dioxygenase in the ABA biosynthetic pathway. Biochem Biophys Acta. 2003;1619(1):9–14. doi: 10.1016/s0304-4165(02)00422-1. [DOI] [PubMed] [Google Scholar]
- Sekse L, Lyngstad L. Strategies for maintaining high quality in sweet cherries during harvesting, handling and marketing. Acta Hortic. 1996;410:351–355. doi: 10.17660/ActaHortic.1996.410.55. [DOI] [Google Scholar]
- Serrani JC, Ruiz-Rivero O, Fos M, García-Martínez JL. Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J. 2008;56(6):922–934. doi: 10.1111/j.1365-313X.2008.03654.x. [DOI] [PubMed] [Google Scholar]
- Sexton R, Lewis LN, Trewavas AJ, Kelly P. Ethylene and abscission. In: Roberts JA, T GA, editors. Ethylene and plant development. London, UK: Butterworths; 1985. pp. 173–196. [Google Scholar]
- Sharif R, Su L, Chen X, Qi X. Hormonal interactions underlying parthenocarpic fruit formation in horticultural crops. Hortic Res. 2022 doi: 10.1093/hr/uhab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin AJ. Genetic engineering for global food security: photosynthesis and biofortification. Plants. 2019;8(12):586. doi: 10.3390/plants8120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin AJ. Carotenoids and apocarotenoids in planta: Their role in plant development, contribution to the flavour and aroma of fruits and flowers, and their nutraceutical benefits. Plants. 2021;10(11):2321. doi: 10.3390/plants10112321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin AJ, Qian T, Caillet V, Michoux F, Ben Amor M, Lin C, Tanksley S, McCarthy J. Oleosin gene family of Coffea canephora: quantitative expression analysis of five oleosin genes in developing and germinating coffee grain. J Plant Physiol. 2006;163(7):691–708. doi: 10.1016/j.jplph.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Simkin AJ, McCarthy J, Petiard V, Tanksley S, Lin C (2007) Oleosin genes and promoters from coffee. Patent WO 2007/005928 A2
- Simmonds NW. The development of the banana fruit. J Exp Bot. 1953;4(1):87–105. doi: 10.1093/jxb/4.1.87. [DOI] [Google Scholar]
- Singh DP, Jermakow AM, Swain SM. Gibberellins are required for seed development and pollen tube growth in Arabidopsis. Plant Cell. 2002;14(12):3133–3147. doi: 10.1105/tpc.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjut V, Bangerth F. Induced parthenocarpy—a way of changing the levels of endogenous hormones in tomato fruits (Lycopersicon esculentum Mill.) 1. Extractable hormones. Plant Growth Regul. 1982;1(4):243–251. doi: 10.1007/BF00024718. [DOI] [Google Scholar]
- Srinivasan C, Dardick C, Callahan A, Scorza R. Plum (Prunus domestica) trees transformed with poplar FT1 result in altered architecture, dormancy requirement, and continuous flowering. PLoS One. 2012;7(7):e40715. doi: 10.1371/journal.pone.0040715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jürgens G, Alonso JM. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133(1):177–191. doi: 10.1016/j.cell.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang C, Wang N, Jiang X, Mao H, Zhu C, Wen F, Wang X, Lu Z, Yue G, Xu Z, Ye J. Manipulation of auxin response factor 19 affects seed size in the woody perennial Jatropha curcas. Sci Rep. 2017;7(1):40844. doi: 10.1038/srep40844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Koltunow AM. Auxin and fruit initiation plant Physiology. Sunderland, MA: Sinauer Associates Inc; 2006. [Google Scholar]
- Swain SM, Reid JB, Ross JJ. Seed development in Pisum. Planta. 1993;191(4):482–488. doi: 10.1007/BF00195749. [DOI] [Google Scholar]
- Swain SM, Reid JB, Kamiya Y. Gibberellins are required for embryo growth and seed development in pea. Plant J. 1997;12(6):1329–1338. doi: 10.1046/j.1365-313x.1997.12061329.x. [DOI] [Google Scholar]
- Takisawa R, Nakazaki T, Nunome T, Fukuoka H, Kataoka K, Saito H, Habu T, Kitajima A. The parthenocarpic gene Pat-k is generated by a natural mutation of SlAGL6 affecting fruit development in tomato (Solanum lycopersicum L.) BMC Plant Biol. 2018;18(1):72. doi: 10.1186/s12870-018-1285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon M, Zacarias L, Primo-Millo E. Hormonal changes associated with fruit set and development in mandarins differing in their parthenocarpic ability. Physiol Plant. 1990;79(2):400–406. doi: 10.1111/j.1399-3054.1990.tb06759.x. [DOI] [Google Scholar]
- Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446(7136):640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Wada W. Apple MADS genes are involved in parthenocarpy and floral organ formation. Hortic J. 2022 doi: 10.2503/hortj.UTD-R018. [DOI] [Google Scholar]
- Tatematsu K, Kumagai S, Muto H, Sato A, Watahiki MK, Harper RM, Liscum E, Yamamoto KT. MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. Plant Cell. 2004;16(2):379–393. doi: 10.1105/tpc.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JE, Whitelaw CA. Signals in abscission. New Phytol. 2001;151:323–340. doi: 10.1046/j.0028-646x.2001.00194.x. [DOI] [Google Scholar]
- Theißen G. Development of floral organ identity: stories from the MADS house. Curr Opin Plant Biol. 2001;4(1):75–85. doi: 10.1016/s1369-5266(00)00139-4. [DOI] [PubMed] [Google Scholar]
- Theißen G, Saedler H. Floral quartets. Nature. Communications. 2001;409(6819):469–471. doi: 10.1038/35054172. [DOI] [PubMed] [Google Scholar]
- Tukey HB. Embryo abortion in early ripening varieties of Prunus avium. Bot Gaz. 1933;94(3):433–468. doi: 10.1086/334322. [DOI] [Google Scholar]
- Tukey HB. Growth of the embryo, seed, and pericarp of the sour cherry (Prunus cerasus) in relation to season of fruit ripening. Proc Am Soc Hortic Sci. 1934;31:125–144. [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. Dimerization and DNA binding of auxin response factors. Plant J. 1999;19(3):309–319. doi: 10.1046/j.1365-313x.1999.00538.x. [DOI] [PubMed] [Google Scholar]
- Van Dun CMP, Lastdrager MB, Huijbregts-Doorduin LJ. Modified gene resulting in parthenocarpic fruit set. Patent US. 2021;10941411:B2. [Google Scholar]
- Varoquaux F, Blanvillain R, Delseny M, Gallois P. Less is better: new approaches for seedless fruit production. Trends Biotechnol. 2000;18(6):233–242. doi: 10.1016/s0167-7799(00)01448-7. [DOI] [PubMed] [Google Scholar]
- Vergara R, Olivares F, Olmedo B, Toro C, Muñoz M, Zúñiga C, Mora R, Plantat P, Miccono M, Loyola R, Aguirre C, Prieto H. Gene editing in Prunus Spp. The challenge of adapting regular gene transfer procedures for precision breeding. In: Küden AB, Küden A, editors. Prunus - Recent Advances. London: IntechOpen; 2021. [Google Scholar]
- Vignati E, Lipska M, Dunwell JM, Caccamo M, Simkin AJ. Fruit development in sweet cherry. Plants. 2022;11(12):1531. doi: 10.3390/plants11121531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, Chaabouni S, Latché A, Pech JC, Bouzayen M. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell. 2005;17(10):2676–2692. doi: 10.1105/tpc.105.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Jogaiah S, Zhang W, Abdelrahman M, Fang JG. Spatio-temporal expression of miRNA159 family members and their GAMYB target gene during the modulation of gibberellin-induced grapevine parthenocarpy. J Exp Bot. 2018;69(15):3639–3650. doi: 10.1093/jxb/ery172. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang H, Liang F, Cong L, Song L, Li X, Zhai R, Yang C, Wang Z, Ma F, Xu L. PbEIL1 acts upstream of PbCysp1 to regulate ovule senescence in seedless pear. Hortic Res. 2021 doi: 10.1038/s41438-021-00491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D, Van Hamburg JP, Van Rijn E, Hooykaas PJ, Offringa R. Diphtheria toxin-mediated cell ablation reveals interregional communication during Arabidopsis seed development. Plant Physiol. 2003;133(4):1882–1892. doi: 10.1104/pp.103.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D, Schlereth A, Ehrismann JS, Schwank G, Kientz M, Jürgens G. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Dev Cell. 2006;10(2):265–270. doi: 10.1016/j.devcel.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Wen B, Song W, Sun M, Chen M, Mu Q, Zhang X, Wu Q, Chen X, Gao D, Wu H. Identification and characterization of cherry (Cerasus pseudocerasus G. Don) genes responding to parthenocarpy induced by GA3 through transcriptome analysis. BMC Genet. 2019;20(1):65. doi: 10.1186/s12863-019-0746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermund U, Fearne A. Key challenges facing the cherry supply chain in the UK. Acta Hortic. 2000;536:613–624. doi: 10.17660/ActaHortic.2000.536.74. [DOI] [Google Scholar]
- Wilson F, Harrison K, Armitage AD, Simkin AJ, Harrison RJ. CRISPR/Cas9-mediated mutagenesis of phytoene desaturase in diploid and octoploid Strawberry. BMC Plant Methods. 2019;15:45. doi: 10.1186/s13007-019-0428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J-L, Dong Y-H, Morris BAM. Parthenocarpic apple fruit production conferred by transposon insertion mutations in a MADS-box transcription factor. Proc Natl Acad Sci USA. 2001;98(3):1306–1311. doi: 10.1073/pnas.98.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen R, Xiao J, Qian C, Wang T, Li H, Ouyang B, Ye Z. A single-base deletion mutation in SlIAA9 gene causes tomato (Solanum lycopersicum) entire mutant. J Plant Res. 2007;120(6):671–678. doi: 10.1007/s10265-007-0109-9. [DOI] [PubMed] [Google Scholar]
- Zhao Y. Auxin biosynthesis. Arabidopsis Book. 2014;12:e0173. doi: 10.1199/tab.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Yue C, Gallardo K, McCracken V, Luby J, McFerson J. What attributes are consumers looking for in sweet cherries? Evidence from choice experiments. Agric Resour Econ Rev. 2016;45(1):124–142. doi: 10.1017/age.2016.13. [DOI] [Google Scholar]
- Zong X, Denler BJ, Danial GH, Chang Y, Song G-Q. Adventitious shoot regeneration and Agrobacterium tumefaciens-mediated transient transformation of almond × peach hybrid rootstock ‘Hansen 536’. HortScience. 2019;54(5):936–940. doi: 10.21273/hortsci13930-19. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.