Graphical abstract
Keywords: Nanocellulose, Ultrasound, Green technology, Cavitation, Process intensification
Highlights
-
•
Acoustic wave induces microstreaming, bubble oscillation, microjetting, and shockwave.
-
•
Ultrasound assists in improving nanocellulose properties and production efficiency.
-
•
Effects of ultrasonic parameters on nanocellulose extraction and surface modification.
-
•
Barriers to ultrasound application and avenues for future developments.
Abstract
With rising consumer demand for natural products, a greener and cleaner technology, i.e., ultrasound-assisted extraction, has received immense attention given its effective and rapid isolation for nanocellulose compared to conventional methods. Nevertheless, the application of ultrasound on a commercial scale is limited due to the challenges associated with process optimization, high energy requirement, difficulty in equipment design and process scale-up, safety and regulatory issues. This review aims to narrow the research gap by placing the current research activities into perspectives and highlighting the diversified applications, significant roles, and potentials of ultrasound to ease future developments. In recent years, enhancements have been reported with ultrasound assistance, including a reduction in extraction duration, minimization of the reliance on harmful chemicals, and, most importantly, improved yield and properties of nanocellulose. An extensive review of the strengths and weaknesses of ultrasound-assisted treatments has also been considered. Essentially, the cavitation phenomena enhance the extraction efficiency through an increased mass transfer rate between the substrate and solvent due to the implosion of microbubbles. Optimization of process parameters such as ultrasonic intensity, duration, and frequency have indicated their significance for improved efficiency.
1. Introduction
Massive industrialisation and natural resource exploitation have underpinned the human flourishing of today, yet at the expense of environmental quality on the ground of the overuse of non-sustainable materials such as plastics. A shift towards utilizing renewable starting materials and adopting a more environmentally benign processing approach is crucial for sustainable development. Among the green materials, cellulose is the most abundant natural biopolymer on earth, with a global annual production of approximately 7.5 million tons [1], [2]. It constitutes nearly 35–50 % of the plant cell wall, posing great biodegradability, renewability, and inherent biocompatibility. Fascinatingly, cellulose materials in the nanoscale dimensions offer exceptional, propitious functionalities like high strength, large aspect ratio, high reinforcing potential, and tailorable surface functionalization. Termed ‘nanocellulose’, this bionanomaterial is a revolutionary green substrate for diversified advanced applications, mainly to replace non-sustainable materials. The two main categories of plant-derived nanocellulose are cellulose nanocrystal (CNC) and cellulose nanofiber (CNF). CNCs are characterized by a short, rod-like structure with maximum crystallinity that engenders rigidity and stiffness. In contrast, CNFs have a longer, thread-like structure and exhibit greater flexibility due to more amorphous regions within these nanofibers. Although both can be drafted from plant sources, they exhibit distinguished physicochemical properties due to their structural disparity, making them useful in various applications [3], [4], [5], [6], [7], [8], [9]. For instance, CNCs are good building materials and reinforcement in polymer matrices due to their high mechanical strength [10]. At the same time, CNFs can serve as an additive in papermaking, tissue scaffold, and substrate for flexible electronics [3], [4], [5], [6], [7], [8], [9].
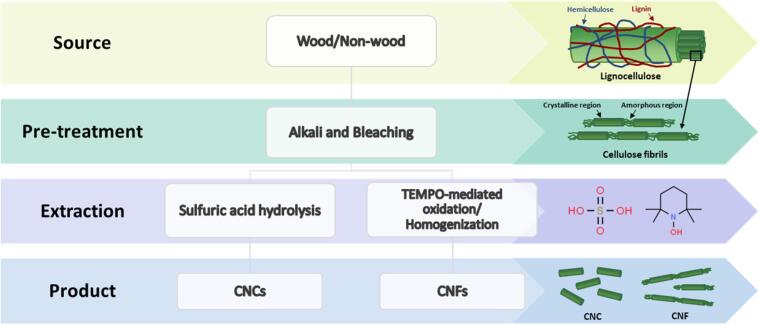
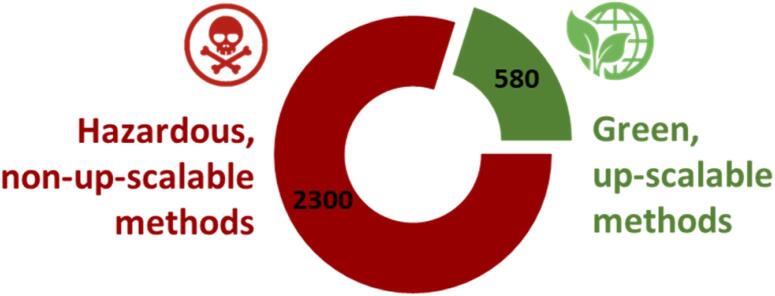
The ubiquitous and obsolete lignocellulosic biomass waste is an excellent source of nanocellulose, which helps dwindle production costs and ameliorate the environmental burden arising from waste generation. The effective extraction of nanocellulose from lignocellulosic biomass can be achieved via a top-down approach by overcoming the interfibrillar hydrogen bonding energy of lignocellulose to separate the convoluted structure into individual entities of nanocellulose [11]. Over the past few decades, various techniques have been extensively employed to pretreat different lignocellulosic sources and extract nanocellulose from them (Fig. 1). Sulfuric acid hydrolysis and 2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO)-mediated oxidation have been the mainstays for CNC and CNF production, respectively, owing to their effectiveness in tackling the recalcitrant biomass structure. Sulfuric acid catalyzes the degradation of amorphous regions in fibers with its active protons, thereby allowing depolymerization of fibers into CNCs [12]. On the other hand, TEMPO-mediated oxidation deconstructs fibers to give CNFs through regioselective oxidation of C6 primary hydroxyl groups of cellulose [13]. The chief principle for these conventional techniques lies in the harsh reaction environment to disrupt the bonding in cellulose for the final liberation of nanocellulose. They involve hazardous chemicals (i.e., concentrated sulfuric acid and strong oxidizing agents) that are difficult to recover or treat and results in high maintenance and operational costs [14], [15], [16]. Besides, mechanical treatment such as high-pressure homogenization can produce CNFs directly by comminuting the fibers under high pressure (50–2000 MPa) with repeated treatments [17]. Nevertheless, the downside is the intensive energy required and a low production yield due to the non-selective nature of mechanical shredding. Because of these challenges, the conventional techniques for nanocellulose extraction are impeded for their commercial-scale applicability to economic and environmental concerns, which otherwise would overthrow the purpose of utilizing nanocellulose by creating more harm than good. As depicted in Fig. 2, less than one-third of nanocellulose production strategies are green and up-scalable, suggesting that development of a more sustainable alternative is indispensably needed.
Fig. 1.
Conventional strategies for the extraction of nanocellulose.
Fig. 2.
The distribution of hazardous, non-up-scalable, and green up-scalable methods for nanocellulose production [18].
Among the novel methods developed, ultrasound has recently emerged as a technology for extracting nanocellulose under milder reaction conditions. The intense physical forces such as shear forces, shock waves, turbulence, and microjets induced by acoustic cavitation contribute to the cleavage of the lignocellulose structure and the effective release of the deep-seated nanocellulose. Coupling ultrasound with other chemical strategies manifested enhanced extraction yield, accelerated chemical reaction, and better colloidal dispersion that are beneficial for fully expressing the nanocellulose properties (e.g., specific surface area). Compared to conventional mechanical disintegration, ultrasonic effects are less vigorous and thus, resulting in less degradation of the crystalline parts of nanocellulose. By carefully adjusting the ultrasonic parameters such as intensity, duration, and frequency, the cavitation effects reflect on process rates and efficacy in the extraction of nanocellulose, which are not easily attainable under conventional treatments. In addition, this technique is duly recognized as non-hazardous since ultrasound radiation does not interact with molecular species directly but through the cavitation collapse, producing local extremity of temperature and pressure conditions [19]. Ultrasound technology also has a simple setup and low-cost capital investment. Despite its potential for sustainable nanocellulose extraction, there remain further avenues for research to put ultrasound at the industrial level, given the limited understanding of the optimization strategies to revamp the intrinsic features of sonochemistry to maximize benefits of ultrasound in nanocellulose. Several drawbacks, such as deterioration of nanocellulose properties and production efficiency, inability for standalone implementation, high energy consumption, difficulty in equipment design and process scale-up, and safety and regulatory concerns remain major challenges in implementing the technology. While the research concerning sonochemistry in nanocellulose extraction has been proliferating over the years, there is absence of a comprehensive summary to elucidate the current state of knowledge. Intrigued by this, the present review provides a well-informed panorama of types of generators and fundamental mechanisms of sonochemistry. Besides, the various applications of ultrasound in the extraction and modification of nanocellulose are recounted, emphasizing the influences of ultrasonic parameters on the nanocellulose properties, such as size and morphology, degree of crystallinity, physicochemical properties, and thermal stability. Lastly, it is concluded with an overview of the challenges based on the available studies, along with suggestions to proffer exemplary guidance towards the commercialization of this potential technique.
2. Ultrasound
Ultrasound is, by definition, the sound waves with frequencies beyond 20 kHz, higher than the human auditory threshold [20], [21]. It is employed in many fields, including detection, ranging, non-destructive testing, cleaning, mixing, and chemical processing. Generally, two ultrasound categories, namely low-intensity and high-intensity ultrasound, are classified corresponding to their frequency and intensity [22], [23], [24], [25], [26], [27]. The low-intensity ultrasound, also known as diagnostic ultrasound, is characterized by high frequency (5–10 MHz) and low power intensity (<1 W/cm2). It is non-destructive and has a weak to zero cavitation impact in the propagation medium, making it suitable for detecting and characterizing materials [24], [26]. Contrarily, the high-intensity ultrasound, also described as power ultrasound, features low frequency (20–100 kHz) and high power intensity (10–1000 W/cm2). It is disruptive and, thus, instigates physical and chemical interactions by generating microbubble streams and the implosion of bubbles [27]. With the capability to change the physicochemical properties of the medium and material subjected to the treatment, high-intensity ultrasound takes an imperative place in extraction, cell disruption, emulsification, and crystal modification [28]. Based on this, it is the focal point in nanocellulose extraction.
2.1. Mechanism of action
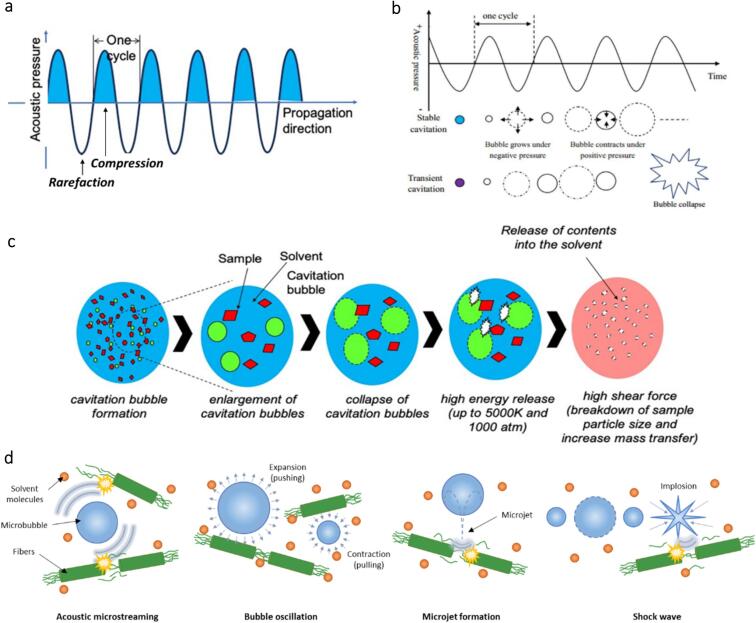
High-intensity ultrasound propagates through a medium via induction of a series of waves comprising compression (high pressure) and rarefaction (low pressure) cycles (Fig. 3(a)) [20], [22], [23]. The high crests and low troughs represent wave amplitudes corresponding to peak compressional and rarefactional values [29]. Acoustic cavitation from ultrasound influences chemical reactivity, otherwise termed ‘sonochemistry’ [30]. Sonochemistry assists physical and chemical processes and effectively leads to controlled shapes and sizes of nanomaterials [31]. The flow of the ultrasonic mechanism that leads to sono-physical effects is depicted in Fig. 3(c). Two basic stages are involved in the ultrasonic mechanism: nucleation and bubble dynamics involving expansion, oscillation, and collapse [32]. Nucleation begins with the formation of cavitation bubbles, also known as microbubbles. They are formed when the negative pressure exerted during rarefaction exceeds the intermolecular van der Waals forces that keep the liquid intact.
Fig. 3.
(a) Propagation of ultrasound waves [27]. (b) Illustration of stable and transient cavitation. (c) The mechanism of acoustic cavitation induced by ultrasonic waves [22], [27]. (d) Phenomena resulting from the acoustic oscillation of ultrasound waves and their effects on nanocellulose extraction.
Typically, acoustic cavitation leads to two situations, which are transient cavitation and stable cavitation (Fig. 3(b)) [33]. Transient cavitation occurs when bubbles collapse upon exceeding the equilibrium size [34]. In contrast, stable cavitation occurs when bubbles do not reach the critical size for collapse and continually oscillate in the medium [34]. In transient cavitation, the implosion of bubbles forms shear forces and shock waves carrying a large quantity of energy. For instance, the collapse of a cavitation bubble at an ultrasound frequency of 20 kHz release extreme temperatures and pressures of 5000 K and 1000 atm at the molecular level [23]. This huge sonolytic energy leads to the formation of high-velocity microjets (Fig. 3(d)) capable of disrupting the surface of solids and exposing new solid surfaces. With an enlarged surface area of solids being exposed, ultrasound then promotes the penetration of reagents into solids through sonophoresis and sonoporation. Furthermore, the sonolytic energy generated is sufficient to break hydrogen–oxygen bonds in water molecules and release hydrogen and hydroxyl radicals that could initiate secondary chemical reactions without any external reagents [35].
On the other hand, in stable cavitation, acoustic microstreaming occurs, wherein the microbubble volume pulsates around their equilibrium size (Fig. 3(d)). The generated velocities stemming from the microbubble pulsation engender shear stresses on the neighbouring particles, promoting physical and chemical interactions in the medium [25], [36]. Bubble oscillation also occurs during stable cavitation, in which microbubbles increase and decrease in size repeatedly in the absence of the collapse phenomenon (Fig. 3(d)). They are stable and isolated, allowing neighbouring air to diffuse into or out of them relentlessly, aiding the mixing effects of air bubbles in the medium [37], [38], [39]. As a result, continuous turbulence is generated amidst the propagation of acoustic oscillations, contributing to the improvement of heat and mass transfer [40]. Enhanced mass transfer further engenders more efficient reaction kinetics by increasing the number of collisions at microscopic levels [20], [41]. To summarize the sonochemical effects of acoustic cavitation, the oscillations of ultrasound waves prompt the occurrence of shock waves, microjets, acoustic microstreaming, and bubble oscillation.
2.2. Ultrasound generators
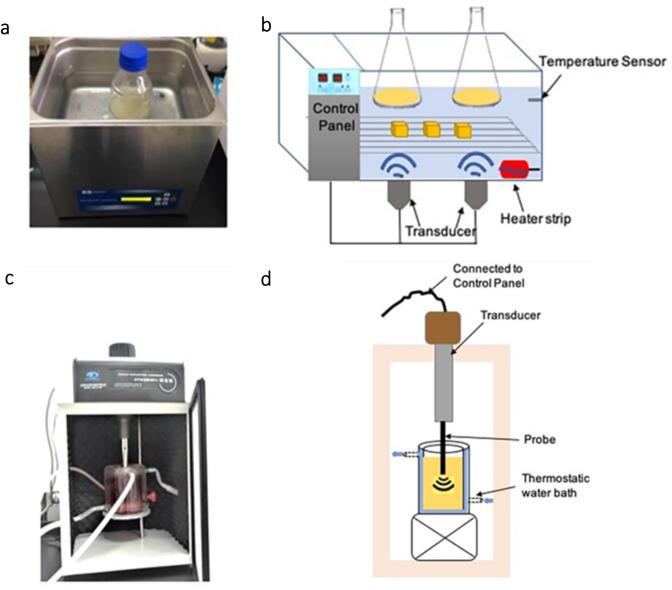
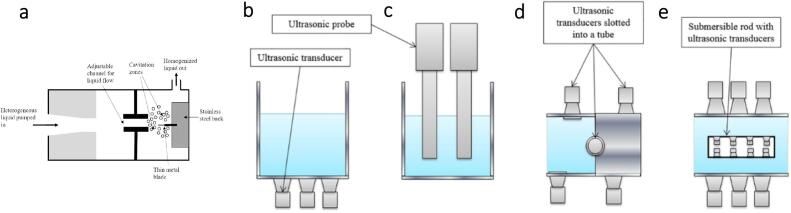
In nanocellulose extraction and surface modification, ultrasound is mostly applied to supplement the chemical treatment using two equipment types: ultrasonic bath and ultrasonic probe. The heart of ultrasonic equipment is its transducer [12], which converts electronic signals to ultrasonic waves. An ultrasonic bath is an inexpensive system normally used to sonicate liquid samples in vessels immersed in the bath (Fig. 4(a)). The sound waves are indirectly transmitted via the medium in an ultrasonic bath (Fig. 4(b)) [42]. It usually operates at a lower energy level with a lesser concentration of ultrasound irradiation emitting from a comparably large irradiation surface [43]. Hence, it is widely accepted for deagglomerating and homogenizing nanocellulose suspension and degassing nanocellulose-based hydrogel.
Fig. 4.
(a, b) Lab-based ultrasonic bath and (c, d) ultrasonic probe [27].
In contrast, the ultrasonic probe irradiates highly concentrated ultrasound waves from the tip of a small area [28]. This device is immersed directly into the vessel sample (Fig. 4(c)). The ultrasonic horn is bonded to the transducer to amplify the irradiation intensity, permitting the direct transfer of ultrasonic waves into the medium (Fig. 4(d)) [44]. The huge localized energy diminishes exponentially due to the excessive bubble cloud formation adjacent to the tip [43]. Consequently, the cavitation activity of the probe sonicator is usually restricted in the irradiation area around the tip [45], [46]. Realistically, the ultrasonic probe is more commonly used in industrial applications due to the greater acoustic power generated, which can be 100 times greater than that of an ultrasonic bath. It is ideal for particle size reduction in nanocellulose extraction, where small particle sizes can be attained within a short processing time. Nevertheless, the corresponding energy is only available close to the probe tip.
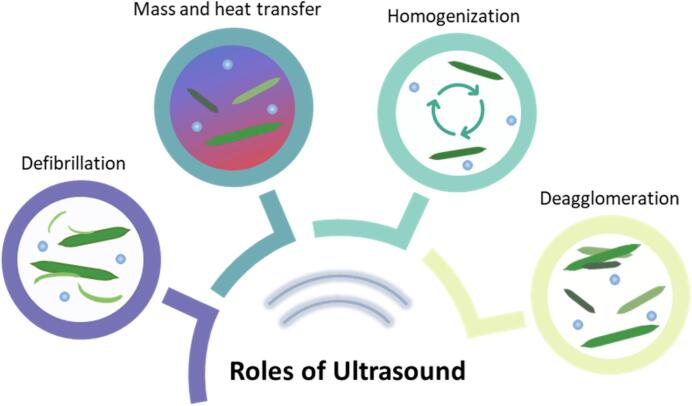
3. Roles of ultrasound assistance in nanocellulose extraction and surface modification
Defibrillation, mass and heat transfer, homogenization, and deagglomeration are the main roles ultrasound plays in nanocellulose extraction and surface modification (Fig. 5). Under the mechanical impacts of acoustic cavitation, ultrasonication can improve the efficiency of the defibrillation process [47], [48], [49]. The implosion of bubbles generates ultrasonic energy (10–100 kJ/mol) within the hydrogen bond's energy, accelerating the disruption of hydrogen bonding within the cellulose network [50]. The hydrodynamic forces also break down the weak boundaries between micron-sized cellulose fibers. As a result, the microfibrils split gradually across the axial direction, facilitating the disintegration into nanofibers.
Fig. 5.
Role of ultrasound assistance in nanocellulose extraction and surface modification.
In chemical treatments, ultrasonication plays a “process booster” role [26], [27], [51], [52], [53], [54]. It intensifies the mass and heat transfer rate in the chemical extraction process. The implosion of microbubbles generates localized shear forces, turbulence, and mixing that enhance mass and heat transfer. The shear forces disrupt the surface of nanocellulose, exposing new solid surfaces. Consequently, diffusion boundary layers decrease, facilitating solvent penetration into the unreachable internal solid matrix by chemical treatments. This increases the productivity of the extraction process, which is desired in large-scale production.
Additionally, ultrasonication portrays the effect of homogenization in chemical treatments [47], [55], [56], [57], [58]. The microstreaming derived from microbubble implosion and nonlinear bubbles oscillations suggests a uniform mixing in the liquid that allows very efficient agitation. This allows cellulosic materials to disperse uniformly in the reaction medium for effective contact with reactants, which facilitates the reaction kinetics and production of nanocellulose with desirable particle size distribution [59].
After the completion of chemical treatments, nanocellulose is dried through freeze-drying, oven-drying, or spray-drying for convenient storage and transportation. Nevertheless, nanocellulose particles tend to agglomerate upon drying due to the attractive hydrogen bonding forces towards the neighbouring particles. This phenomenon is known as hornification, and the redispersion of hornified nanocellulose requires substantial energy. The clusters of nanocellulose particles affect the stability of a suspension, which cripples its final application. In addressing the hornification of nanocellulose, ultrasonication is the most widely employed method for nanocellulose redispersion. The formation of high-speed microjets due to acoustic cavitation could press high-pressure liquid between the aggregates and separate nanoparticles effectively. This is particularly important to slow Ostwald ripening effect or agglomeration among the nanoparticles during storage [60], [61], [62], [63].
4. Application of ultrasound in nanocellulose extraction
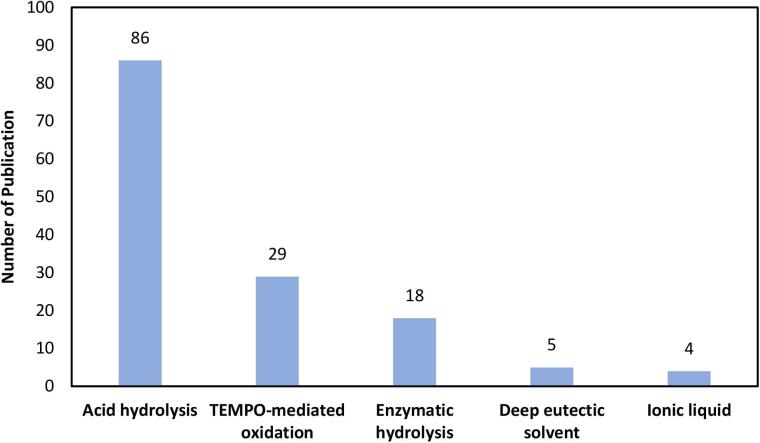
Ultrasound is frequently brought up alongside nanocellulose extraction methods, particularly chemical treatments. This is due to the phenomena caused by ultrasonic waves that are beneficial for intensifying chemical reactions, including shock waves, bubble oscillation, high-speed microjets, and microstreaming [50], [64], [65], [66], [67]. The increasing number of publications concerning the application of ultrasound in nanocellulose extraction is envisioned given its reported favorable effects in enhancing the efficiency, expediting the process, and producing nanocellulose with desirable properties (Fig. 6). Another motivation behind combining ultrasound with chemical treatments is to resolve the high energy consumption of ultrasound-alone treatment [68]. Due to their effectiveness and controllability, acid hydrolysis, TEMPO-mediated oxidation, and enzymatic hydrolysis are the most widely employed chemical treatments for nanocellulose extraction. The fundamental mechanisms of these extraction methods are explicated in the following sections to elucidate the possible improvement with ultrasound assistance. Multifaceted ultrasound parametric effects, and connection between sonochemical mechanisms and nanocellulose properties are also deciphered based on the latest research works.
Fig. 6.
The number of publications on ultrasonic-assisted nanocellulose extraction. Data analysis was completed using the Web of Science search system on 1st June 2022, using the keywords of “ultrasound”, “nanocellulose”, “cellulose nanocrystal”, “cellulose nanofiber”, “nanocrystalline cellulose”, “nanofibrillated cellulose”, “acid hydrolysis”, “TEMPO”, “enzymatic”, “deep eutectic solvent”, and “ionic liquid”.
4.1. Ultrasound-assisted acid hydrolysis
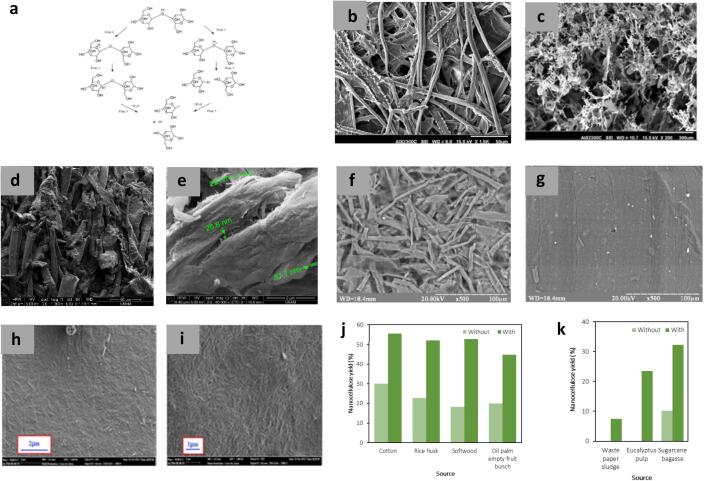
Traditionally, the acid hydrolysis process dominates CNC isolation and has been well employed in the industry, such as CelluForce, the leading CNC manufacturer [69], [70], [71], [72], [73]. Sulfuric acid is commonly employed as the hydrolyzing agent; other acids such as hydrochloric acid, hydrobromic acid, and phosphoric acid were occasionally considered. Unlike sulfuric acid, which induces negative charges upon hydrolysis, providing substantial repulsion among the extracted particles, CNC extracted using hydrochloric acid, and hydrobromic acid usually poses a high tendency to agglomerate. Apart from inorganic acids, organic acids such as formic acid, oxalic acid, citric acid, and maleic acid were also successfully applied as hydrolyzing agents [74], [75], [76], [77], [78], [79]. In general, hydrogen ions generated through the ionization of acid mediums during hydrolysis destroy the glycosidic bonds of cellulose molecular chains. This leads to fibrillation with the loss of non-crystalline or amorphous regions. The mechanism of acid hydrolysis is shown in Fig. 7(a). With ultrasonication, the formation, growth, and collapse of microbubbles followed by liquid microjets on the fibre surface significantly impact the cellulose particles suspended in an acid medium. This leads to breaking the interfibrillar hydrogen networks, exposing more hydroxyl groups on the surface of cellulose molecules [80]. The microjets formed also assist in reducing the particle size distribution from the large micron-sized aggregates [81]. Fig. 7(b-i) shows the scanning electron microscopy (SEM) images of CNC with and without ultrasound assistance in various sources. Moreover, the additional mechanical treatment of ultrasonication reduced agglomeration among the nanocellulose [14].
Fig. 7.
(a) Mechanism of acid hydrolysis [82]. SEM images of (b, c) rice straw after chemical pretreatment and nanocellulose after high-speed homogenization and ultrasonication [47] (d, e) non-sonicated and sonicated CNC from cotton [83] (f, g) organosolv straw pulp after hydrolysis and after ultrasonic-assisted hydrolysis [84] (h, i) CNC after hydrolysis and after ultrasound-assisted hydrolysis [85]. Enhancement in nanocellulose yield from various cellulose sources after ultrasound assistance using (j) conventional acid hydrolysis [86], [87], [88], [89], [90], [91], [92] and (k) weak acid hydrolysis [93], [94], [95].
The process duration could be significantly reduced due to the remarkable enhancement in mass and heat transfer by ultrasonication [96], [97]. Conventional acid hydrolysis commonly takes between 60 and 120 min [98], [99]. Guo et al. concluded that ultrasonication could achieve CNC production at a short hydrolysis time (45 min) with significantly increased yield [97]. No significant yield variations were noted when hydrolysis was extended to 90 min and 120 min [97]. On some occasions, fast hydrolysis promotes the extraction of smaller particles, whereas slow hydrolysis leads to the degradation of the crystalline region and the formation of larger nanoparticles [100]. As previously stated, the mechanical impact of acoustic cavitation led to a higher grinding degree of the cellulose fibers that enhanced the rapid transportation of acid molecules into the inner amorphous surface, indirectly increasing the contact area for the acid catalyst. Due to ultrasonication, acid diffuses faster compared to the conventional process. This is particularly important for commercial production as it significantly reduces production costs.
Despite the successful extraction of CNC, acid hydrolysis is limited due to its low production yield [97], [101]. Under typical hydrolysis conditions, CNC yield always lies within 10–30 %. On the other hand, with ultrasound assistance, Guo et al. increased the yield from 18.3 % to 52.8 % for a hydrolysis period of 45 min [97]. Previous works using various sources demonstrated that the nanocellulose yield doubled for ultrasound-assisted acid hydrolysis (Fig. 7(j)). Mazela et al. observed a reduction in dimension of approximately 49 % when ultrasonication was performed after acid hydrolysis [82]. Ultrasonication also breaks down the microfibrils into uniformly dispersed nano-sized fibrils. For instance, CNC’s polydispersity (PDI) decreased after ultrasonication, indicating greater uniformity of nano-sized cellulose crystals [102]. This is important to promote monomodal particle distribution from the initial bimodal particle size distribution [103], [104]. Besides, ultrasonication also increased the suspension stability against agglomeration. The zeta potential of CNC decreased from −24 mV to –33 mV, suggesting better stability [105], [106], [107].
The largest challenge in acid hydrolysis is the high sulfuric acid concentration [56], [108], [109], [110]. Generally, the optimal acid concentration of greater than 60 wt% is required to break down the amorphous regions of fibers for a reasonable yield of CNC [111], [112]. For instance, CelluForce produces CNC from bleached Kraft pulp using 64 wt% sulfuric acid [113], [114], [115]. Nevertheless, the capital cost is high, accounting for solvent corrosiveness. Also, a high operational cost is inevitable for intensive effluent treatment. As a countermeasure, mild hydrolysis was proposed with a lower acid concentration. The advantages include simpler waste processing, lower environmental impact, and cheaper raw material cost. Nevertheless, the mild process requires high pressure and temperature [116]. This is possible through ultrasonication. Ismail et al. extracted CNC with an average diameter between 38 and 70 nm through hydrolysis using 40 wt% sulfuric acid combined with 120 min ultrasonication. Following this, Maimunah et al. further reduced the concentration of sulfuric acid to 6 wt% at an elevated temperature of 80 ˚C, with the assistance of ultrasonication for 2 h [117]. This treatment produced nanocellulose with a lower crystallinity, i.e., 42 %, as many amorphous regions remained intact within the fibrils network.
Despite the enhancement, ultrasound-assisted acid hydrolysis was found to limit practical utilization. The concern lies in the washing process, where tenfold dilution (with water) was required to cease the hydrolysis reaction or neutralization with sodium hydroxide that complicated salt removal [118], [119]. Besides, chilled water was often needed to quench the reaction as conventional hydrolysis occurs at 40–70 ˚C [101]. More efforts are required to achieve sustainable economic manufacturing while obtaining a viable nanocellulose yield. Weak acid hydrolysis coupled with ultrasonication was proposed [120], [121]. The benefits of weak acid are easy recovery, reusability, less corrosivity, and significantly reduced capital and operation costs [122], [123]. Wang et al. observed a 97 % improvement in efficiency due to the removal of time-consuming downstream processes such as dialysis in strong organic acid hydrolysis [16]. Nonetheless, weak acid coupled with ultrasonication could only produce CNF. As for CNC, the yield from weak acid hydrolysis with ultrasound was relatively low, as shown in Fig. 7(k).
Before extraction, the pretreatment of cellulose is also crucial as it affects acid accessibility, particularly the lignin content [124], [125], [126]. A strong bleaching agent (e.g., sodium hydroxide) is conventionally employed to disintegrate lignin's ester linkages and solubilization [125], [127], [128], [129]. According to Ismail et al., the increase in ultrasonic treatment time suggests complete lignin removal, as observed from the FTIR spectrum at 1280 cm−1. The peak corresponding to the aryl group's C—O stretching in lignin disappeared [130], [131]. Luo et al. conducted the extraction of lignin-containing nanofibrils with oxalic acid hydrolysis assisted by ultrasonication led to the lowest lignin content [120]. The sono-assisted treatment accelerated lignin dissolution due to ultrasonic cavitation's hydrodynamic shear force. However, extending the ultrasonic irradiation beyond the optimal conditions gave no benefit in lignin removal.
Additionally, ultrasound assistance was found to enhance the water-holding capacity of nanocellulose, which is crucial for utilizing nanocellulose as a drug delivery carrier [132], [133]. Balquinta et al. concluded that the combination of acid hydrolysis and ultrasonication resulted in a highly entangled network with increased water holding capacity [98]. The shearing and cavitation effect increased the entanglement network where water molecules were entrapped within the fibers. It permitted a higher drug loading onto the carrier, although the diffusion coefficient of the drug molecules must also be considered. This enhancement could make nanocellulose a promising candidate for applications as a drug delivery carrier and requires a further in-depth investigation.
In contrast, ultrasonication could also negatively impact the extraction of nanocellulose in terms of its properties, particularly the degree of crystallinity [87], [134], [135]. The degree of crystallinity is essential as it contributes to the mechanical strength of nanocellulose. It was found that ultrasonication did not alter the main crystal structure of CNC from X-ray diffraction spectra (XRD) [136], [137], [138], [139]. However, the crystallinity of nanocellulose depends strongly on the ultrasonic period. In the work of Li et al., a declining curve of crystallinity was reported with increasing duration of sonication treatments [134]. Maisa et al. observed a similar result of reducing the crystallite size and crystallinity utilizing 64 wt% sulfuric acid hydrolysis combined with 60 min ultrasonication [87]. This decline might be attributed to the prolonged ultrasonic treatment, which slightly degraded the CNC. Hence, it is important to utilize optimum conditions as ultrasonication has shown potential to enhance the selective degradation of amorphous regions and the negative effects of over- degradation and agglomeration of nanocellulose. Thus, ultrasound is a highly upcoming technology that can be applied in acid hydrolysis to subdue the practical limitations of conventional hydrolysis. Table 1 summarizes the process conditions and the nanocellulose sizes obtained using ultrasound-assisted acid hydrolysis.
Table 1.
Representative summary of previous works on ultrasound-assisted acid hydrolysis for nanocellulose extraction.
| Raw material | Acid | Ultrasound system | Sonication conditions | Nanocellulose size (nm) | Ref. |
|---|---|---|---|---|---|
| Strong acid | |||||
| Cotton | Sulfuric acid (40 wt%) |
Probe | 24 kHz, 320 W, 120 min (Post-treatment) |
W = 18–29 L = 200–800 |
[116] |
| Oil palm empty fruit bunch | Sulfuric acid (64 wt%) |
Bath | 37 kHz, 320 W, 120 min (Simultaneous) 37 kHz, 320 W, 30 min (Post-treatment) |
W = 30–40 | [140] |
| Banana pseudo-stems | Sulfuric acid (64 wt%) |
Bath | 25 kHz, 750 W | W = 2.24 ± 0.57 L = 125 ± 28 |
[141] |
| Sawdust | Sulfuric acid (65 wt%) |
Bath | 45 kHz, 300 W, 60 min (Post-treatment) |
W = 35 ± 7.40 L = 238.7 ± 81.2 |
[86] |
| Microcrystalline cellulose (MCC) | Sulfuric acid (65 wt%) |
Bath | 45 kHz, 300 W, 6 h (Post-treatment) |
W = 8–120 | [82] |
| Cotton | Sulfuric acid (50 wt%) |
Probe | 20 kHz, 120 W, 60 min (Post-treatment) |
W = 20–100 | [142] |
| Ginkgo seed shells | Sulfuric acid (62 wt%) |
Probe | 20 kHz, 600 W, 60 min (Post-treatment) |
W = 24 ± 1.7 L = 310 ± 15.9 |
[143] |
| Hemp | Sulfuric acid (60 wt%) | Probe | 22 kHz, 1 h (Post-treatment) |
W = 8–36 | [144] |
| Weak acid | |||||
| Hardwood kraft pulp | Maleic acid (60 wt%) |
NA | 400 W, 45 min (Post-treatment) |
W = <1 µm | [121] |
| Celery | Oxalic acid (30 wt%) |
Probe | 15 kHz, 500 W, 18 min (Post-treatment) |
W = 5.50–39.30 | [120] |
| Eucalyptus pulp | Citric acid (65 wt%) |
Probe | 50 kHz, 45 W, 3 min (Post-treatment) |
W = 9 ± 3 L = 215 ± 89 |
[93] |
| W, width; L, length. | |||||
4.2. Ultrasound-assisted TEMPO-mediated oxidation
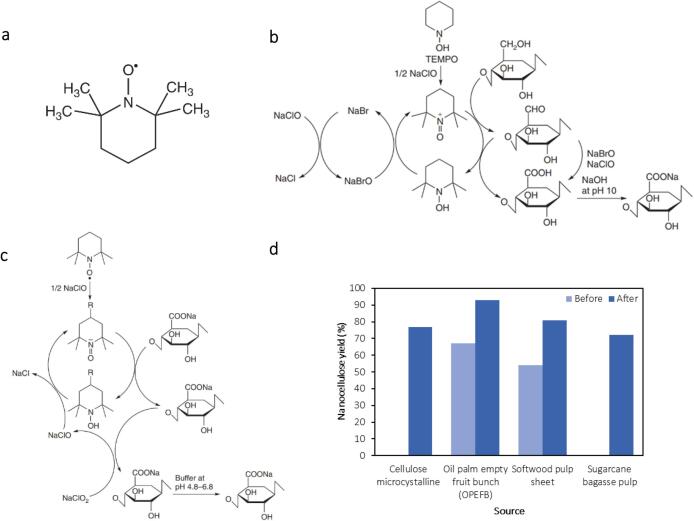
TEMPO-mediated oxidation causes depolymerization of cellulose polymer chains in three steps [145]. Firstly, it involves the initiation of β-elimination of glycosidic bonds in the presence of intermediate aldehyde groups (C6) in the cellulose molecules under alkaline conditions. This occurs when NaOCl converts one mole of C6 primary hydroxyl groups of cellulose into carboxylate groups via the C6 aldehyde group [146]. The TEMPO reagent structure and the general steps during the oxidation process in alkaline and acidic mediums are depicted in Fig. 8(a-c). When TEMPO-mediated oxidation is performed under an alkaline environment (pH 10), the reaction occurs readily at room temperature and forms aldehyde groups, resulting in reduced thermal stability, discoloured product after drying, and hindered microflibrillation [82]. On the contrary, improvement in thermal stability and controlled cellulose depolymerization originating from the absence of aldehyde group formation, which can be achieved by conducting the reaction in an acidic medium (pH 6–6.5) at a slightly higher temperature (50–60 °C) [147], [148].
Fig. 8.
(a) Structure of TEMPO catalyst [147]. Mechanism of TEMPO-mediated oxidation in (b) alkaline and (c) acidic medium [147]. (d) Enhancement of nanocellulose yield observed from various cellulose sources after ultrasound assistance in enzymatic hydrolysis [160], [165], [166], [167].
Ultrasonication is enforced in TEMPO-mediated oxidation, where the cavitational bubbles collapse in the medium with oxidants that could assist the substitution of reactants onto C6 hydroxyl groups. TEMPO-mediated oxidation is a mild reaction, providing a shorter nanocellulose production duration [149]. However, it usually yields a low degree of oxidation [48]. TEMPO oxidation is ineffective in removing or breaking down the amorphous regions as it solely focuses on oxidizing the hydroxyl groups within the cellulose structure [150], [151]. Hence, ultrasonication could be of significant assistance [152], [153]. One important feature is mechanically disintegrating the oxidized cellulose fibers [154], [155], [156].
Combining an effective mechanical treatment with TEMPO-mediated oxidation can facilitate cellulose fiber disintegration into smaller fragments. Liu et al. observed that corncob-derived oxidized cellulose fibers have lengths and widths in micrometers [157]. A similar observation is reported for cellulose extracted from jute, hemp, and flax via TEMPO-mediated oxidation, in which the particle size was observed in the micron size [158]. Concerning the role of ultrasound, the cavitation effect creates a localized region of high temperature and pressure, promoting the isolation of nano-sized fibers. The microfibrils are disintegrated into submicron fibrils and completely broken down into nanofibrils. Zhou’s group demonstrated that ultrasound reduced the average length of CNC prepared via TEMPO-mediated oxidation from 292 nm to 190 nm [159]. Similarly, Rohaizu and Wanrosli concluded that mechanical defibrillation using an ultrasonic probe is necessary as the particles’ length and width were reduced from 140 nm and 15 nm to 122 nm and 4 nm, respectively [160]. Ultrasound-assisted TEMPO-mediated oxidation also increases the nanocellulose yield, as depicted in Fig. 8(d). Moreover, the cavitation effect produces a more homogenous size distribution of nanofibers [161], [162], [163], [164]. This renders its feasibility in the industrial application as it can control product quality and consistency within the desired range, e.g., fiber morphology.
The essence of TEMPO-mediated oxidation lies in the key catalysts: TEMPO and NaBr/NaOCl. However, the main drawbacks of TEMPO-mediated oxidation are always associated with its high operational cost, recovery, and negative environmental impact. Therefore, it is of utmost importance to minimize the consumption of chemicals in the oxidation process. Loranger et al. observed no changes in the end product of nanocellulose with reduced dosages of TEMPO and NaBr through TEMPO oxidation in a sonoreactor [168]. It was observed that the reduction in NaBr could be up to 60 % with a good influence on the carboxylate content compared to the reduction using TEMPO. Typically, the TEMPO-mediated oxidation reaction produces a comparatively low carboxylate content due to the intact structure of cellulose fibres. The highly ordered cellulose structure with a high degree of crystallinity that keeps the core intact could instigate oxidation on the fibre surface but impedes the TEMPO accessibility to the inner cellulose crystals.
On the other hand, ultrasound-assisted TEMPO-mediated oxidation impacts remarkably on the carboxylate content [146], [159], [160], [169], [170], [171], [172]. Ultrasonication significantly enhances oxidation efficiency, with approximately a 15 to 30 % increase in the carboxylate functional groups. The shear forces and microjets strike at the fiber surface result in enhanced chemical reactions due to better TEMPO penetration into the inner fiber regions. Besides, the increase in nanocellulose yield from enhanced fibrillation renders greater exposure of hydroxyl groups to be substituted by the carboxylate groups, thereby increasing carboxylate content. Rohaizu and Wanrosli observed a more than 100 % increase in the carboxylate content from 0.58 mmol/g to 1.31 mmol/g in CNC extraction via ultrasound-assisted TEMPO-mediated oxidation [160]. Compared to non-assisted oxidation, Qin et al. emphasized that CNC with high carboxylate content was directly produced from ultrasound-assisted TEMPO-mediated oxidation [170]. Correspondingly, Serrano et al. observed an increase in the oxidation degree from 0.17 to 0.27 in ultrasonic irradiation coupled with TEMPO-mediated oxidation [141].
Considering the high carboxylate content, introducing carboxylic groups results in electrostatic repulsion that decouples the interfibrillar hydrogen bonds [157], [169], [173]. Simultaneously, it increases the colloidal stability of the suspension where the fibers do not aggregate. For example, Serrano et al. observed changes in the zeta potential (ζ) from −45 mV to −53 mV when ultrasonic waves were applied under the same oxidation conditions [141]. Wicaksono et al. suggested that suspensions with zeta potential greater than ±40 mV are stable over time [174]. The change in zeta potential indicates an increase in the carboxylate groups in the suspension during ultrasound-assisted TEMPO-mediated oxidation. Additionally, Wu et al. prepared CNF from coconut coir fibres and noticed that the suspension remained stable without sedimentation after exposing it to ultrasonic irradiation [175]. An increase in the carboxylate content could subject the fibres to a stronger cavitation effect [176], which is beneficial for cellulose defibrillation at lower energy requirements.
Despite the enhancement in ultrasound-assisted TEMPO oxidation, the difficulties in extracting CNC remain challenging. As opposed to hydrolysis processes, the combination of TEMPO-mediated oxidation and ultrasonication does not selectively break down the amorphous region only. Thus, ultrasound-assisted TEMPO oxidation was often a pretreatment for the hydrolysis reaction [165]. The degree of crystallinity reduced remarkably during ultrasonication [160], [164], [167], [169]. The crystallinity decrease is due to the partial destruction of hydrogen bonds and the ordered cellulose structure under ultrasonic irradiation, resulting in crystalline structure changes and subsequent crystallinity loss. Regarding fibres separation, the formation of completely individualized nanocelluloses from ultrasound-assisted oxidation cannot recover in high yields as solids. Thus, similar to acid hydrolysis, the nanocelluloses dispersed in the oxidation mixture must be purified and isolated using dialysis to separate them from the catalyst and other water-soluble compounds.
Overall, ultrasound-mediated TEMPO oxidation is one of the most effective chemical treatments that appear to be a potential alternative to traditional extraction methods. The mild reaction with high yield and high carboxylate content is appealing. Further research on reducing oxidation time and reusing the reaction medium is needed to improve the effectiveness. A greener route of TEMPO-mediated oxidation with ultrasound assistance provides a more viable option than conventional TEMPO-mediated oxidation and the potential to upscale a sustainable process. Table 2 summarizes the process conditions and the nanocellulose sizes obtained using ultrasound-assisted TEMPO-mediated oxidation.
Table 2.
Representative summary of previous works on TEMPO-mediated oxidation with ultrasound assistance.
| Raw material | Oxidant concentrations (per g fiber) |
Sonication conditions | Nanocellulose size (nm) | Ref. | |||
|---|---|---|---|---|---|---|---|
| TEMPO | NaBr | NaClO2 | NaClO | ||||
| Sugarcane bagasse pulp | 16 mg | 100 mg | – | 2.0–11.0 mmol | 25 kHz, 450 W, 30 min (Post-treatment) |
W = 15 ± 8 L = 264 ± 69 |
[167] |
| Microcrystalline cellulose | 16 mg | 100 mg | – | 20 mmol | 20 kHz, 270 W, 120 min (Post-treatment) |
W = 5.1–6.4 L = 190 |
[159] |
| Softwood kraft pulp | 16 mg | 100 mg | – | 20 mmol | 20 kHz, 270 W, 120 min (Post-treatment) |
W = 3.5–3.6 L = 185 |
[159] |
| Banana pseudo-stem | 16 mg | – | 100 mg | 10 mg | 20 kHz, 1000 W, 60 min (Post-treatment) |
W = 2.24 ± 0.57 L = 125 ± 28 |
[141] |
| Fique fibers | 16 mg | 100 mg | – | 37 mmol | 40 kHz, 130 W, 120 min (Simultaneous) 20 kHz, 750 W, 10 min (Post-treatment) |
W = 2 L = 200–438 |
[169] |
| Coconut waste husk | 0.50 mmol | – | 15 mmol | 11 mmol | 25 kHz, 1000 W, 60 min (Post-treatment) |
W = 5.6 ± 1.5 L = 150–300 |
[177] |
| W, width; L, length. | |||||||
4.3. Ultrasound-assisted enzymatic hydrolysis
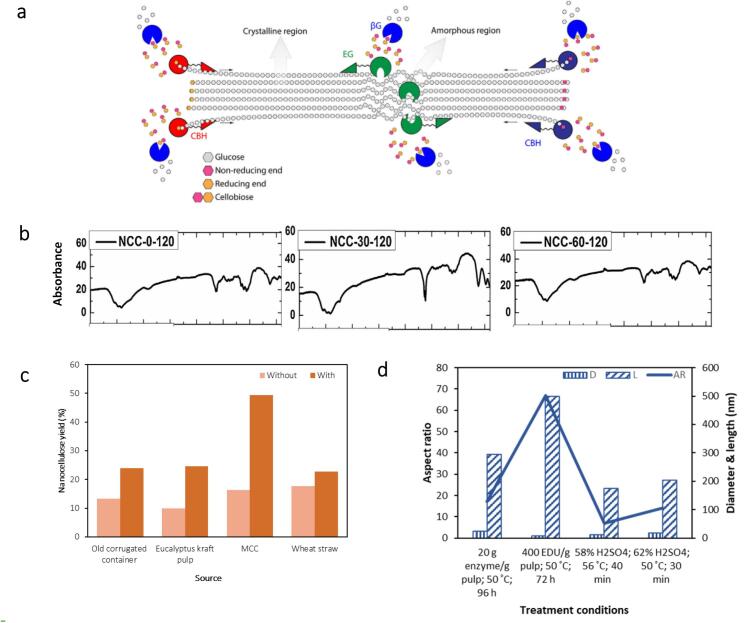
Enzymatic hydrolysis is gaining much interest as it employs mild extraction conditions and requires no chemical reagents or peculiar solvents. It also requires lower energy and capital cost. This method utilises an exclusive enzyme called endoglucanase, which can destroy the intramolecular hydrogen bond of cellulose fibres. Endoglucanase is extracted from one of the fungi-secreted modular enzymes – cellulase, that is mostly separated from other cellulase components and applied alone as the monocomponent enzyme. This is because other cellulase components, including cellobiohydrolase (CBH) and β-glucosidase are not in favor of nanocellulose extraction as they are more prone to converting cellulose into soluble sugars, ascribing to their ability to hydrolyse β-1,4-glycosidic linkage of the celluboise (Fig. 9(a)) [178]. Satyamurthy et al. employed commercial cellulase from Aspergillus niger to obtain CNC, which led to a relatively low yield of 22 %, owing to a higher conversion of glucose [179].
Fig. 9.
(a) Cellulose hydrolysis using T. reesei cellulase, depicting cellobiohydrolase (CBH), -glucosidases ( G), and endoglucase (EG) [193]. (b) FTIR spectra of CNC were obtained from wheat straw under various ultrasonic durations (0, 30, 60 min) [137]. (c) Enhancement of nanocellulose yield from utilising various cellulose sources after ultrasound assistance in enzymatic hydrolysis [137], [192], [194], [195]. (d) Comparison of eucalyptus kraft pulp aspect ratio from ultrasound-assisted enzyme hydrolysis and acid hydrolysis [192], [196], [197], [198].
On the other hand, endoglucanase is characterized by its selective catalytic action on amorphous regions, which can preserve the crystalline regions. Enzymatic hydrolysis employing monocomponent endoglucanase performs well under mild reactions (pH 4–7; 45–50 °C) [180]; this is particularly important to reduce energy consumption and avoid over-depolymerization [181]. Nonetheless, the high cost of enzymes hinders its application [182], [183], [184], [185]. In addition, enzymatic hydrolysis often suggests a lower yield for nanocellulose due to the limitation in enzyme reactivity. Lignin forms a barrier against hydrolysis of amorphous regions making the enzymes unable to penetrate through the lignin ‘shield’ easily. It requires mechanical treatment to increase the fibres accessibility for better enzyme action [186], [187], [188], [189], [190], [191]. Ultrasonication could significantly enhance the enzymatic hydrolysis of cellulose, in which the cavitation effect disrupts more disordered or amorphous regions leading to an increase in the crystalline regions. Squinca’s group increased the crystallinity index from 57.5 % to 78.3 % with 1 min ultrasonication for every 10 min of hydrolysis [192]. However, the extension of ultrasonic treatment time would also negatively impact the crystallinity due to the non-selective removal of both amorphous and crystalline regions by ultrasonication. Cui et al. achieved a crystallinity of 87.5 % at 30 min ultrasonication but decreased to 82.3 % at 60 min ultrasonication [137]. Fourier transform infrared (FTIR) spectra shows that the band with the stretching vibration of OH groups (3400 cm−1) became narrower after ultrasonic-assisted enzymatic hydrolysis [137] (Fig. 9(b)), confirming the damage to the intramolecular hydrogen bond of cellulose that suggests the fibrillation effect. Therefore, ultrasonication facilitates the disintegration of nanocellulose agglomerates, resulting in decreased width and length and a shifted particle size distribution to smaller distribution [137].
Another drawback in enzymatic hydrolysis is the slow diffusion of enzyme macromolecules in the liquid phase across the boundary layer of the solid cellulose surface, which leads to a longer hydrolysis period that ranges from 24 h to 120 h, depending on the cellulose source and enzyme loading [181], [199], [200]. Conventionally, agitation is used to heighten the hydrolysis process. In recent years, ultrasonication is proven to be an efficient method to increase the mass transfer rate of enzymes to solid surfaces. The implosion of cavitation bubbles generates an intense micro-convection that contributes to the subsequent occurrences – greater reaction velocity and better enzyme-substrate affinity, which remarkably improves the kinetics of the hydrolysis process. Besides, ultrasound can break weak hydrogen bonds and Van der Waals forces in enzymes, altering the spatial conformation of enzymes to become more flexible to link with the substrate [201]. This supports the enzyme binding towards the insoluble cellulose, facilitating the formation of the enzyme-substrate complex and driving greater hydrolytic activity [201]. Nguyen and Le demonstrated a significant improvement of up to 39.4 % in cellulase activity under optimum ultrasonic conditions. Likewise, Borah and colleagues concluded that ultrasonication increases hydrolysis kinetics by nearly 10-fold, ascribing to enhanced enzyme diffusion and desirably modified enzyme structure [186]. With a promising increment of enzymatic kinetics in the sonicated environment, Cui et al. reported an improvement in nanocellulose yield by 30 % [137]. This enhancement in enzymatic hydrolysis rate can also significantly reduce the requirement of high enzyme dosage, thereby decreasing the production cost. The yield improvement achieved by ultrasound-assisted enzyme hydrolysis is illustrated in Fig. 9(c).
Interestingly, it could be noted that ultrasonic-assisted enzyme hydrolysis resulted in nanocellulose with high aspect ratios (Fig. 9(d)). This is because the cavitation effect of ultrasound exhibits a weaker shear force along the axial direction, which tends to give rise to longer fibrils [202], [203]. A high aspect ratio is desirable when the extracted nanocellulose is geared towards developing reinforcement materials due to the high interfacial area of longer fibrils for more efficient load transfer into the reinforcing crystal phase. Despite these advantages of ultrasound assistance, the nanocellulose yields are still much lower than the traditional extraction with sulfuric acid. In addition, ultrasonication at higher amplitudes and extended treatment time can adversely affect enzyme activity. Vigorous shock waves and high shear generated under extreme ultrasonic conditions disrupt polypeptide chains in enzymes, leading to far-reaching modification of the protein’s three-dimensional structure that can ultimately result in loss of biological activity [201], [204]. The formation of free radicals induced by cavitation may also suppress enzyme activity when they react with amino acids that essentially participate in enzyme stability, substrate binding, and/or catalytic activity [204]. These disadvantageous effects of ultrasound on enzymes incur a higher production cost to reactivate or replace the deactivated enzymes. Due to these challenges, ultrasound-assisted enzyme hydrolysis has yet to mature as an industrial-scale production technology. More efforts to overcome low hydrolysis yield and enzyme sensitivity towards ultrasound are needed. Table 3 summarizes the process conditions and the nanocellulose sizes obtained using ultrasound-assisted enzymatic hydrolysis.
Table 3.
Representative summary of previous works on enzymatic hydrolysis with ultrasound assistance.
| Raw material | Enzyme | Enzyme dosage | Sonication conditions | Nanocellulose size (nm) | Ref. |
|---|---|---|---|---|---|
| Cotton | Celluclast 1.5 L | 44 U/g fiber |
20 kHz, 300 W, 90 min (Simultaneous) |
NA | [205] |
| Cotton linter | Cellulase (Cerena sp.) |
20 U/g fiber |
30 kHz, 100 W, 20 min (Post-treatment) |
Z-avg = 204.6 ± 8.0 | [206] |
| Eucalyptus kraft pulp | Monocomponent endoglucanase | 400 U/g pulp |
20 kHz, 315 W, 10 min (Post-treatment) |
W = 6–10 L = 400.0 – 600.0 |
[196] |
| Cotton | Cellulase (Aspergillus Niger) |
<100 µg/mL | NA (Post-treatment) |
W = 30–45 L = 250–900 |
[207] |
| Microcrystalline cellulose | Endoglucanase (Penicillium oxalicum M12) |
15 mg/g |
40 kHz, 100 W, 30 min (Post-treatment) |
W = 17.9–33.8 Z-avg = 357–863 |
[208] |
| Poplar wood | Celulase (Novozyme) |
10 U/g wood |
25 kHz, 200 W, 20 min (Post-treatment) |
Z-avg = 310.0 ± 5.0 | [209] |
| W, width; L, length; Z-avg, Z-average. | |||||
4.4. Effects of ultrasonic parameters
Nanocellulose production efficiency and efficacy through ultrasonic-mediated chemical treatments are greatly influenced by the intensity of acoustic cavitation, which can be fine-tuned by the ultrasonic conditions applied. The key ultrasonic parameters include frequency, power, the medium used, temperature, time, and solid loading. Low frequency of below 50 kHz and high power ratings of 3 to 10 W/mL [210] are the prerequisites to break the interfibrillar bonding and confer nanofibrillation of biomass. Excessive sonication conditions are detrimental to product properties and production efficiency. Dilute suspension with a low solid loading of <1 % to ultrasound treatment is often the requirement due to the tendency of cellulosic materials to agglomerate and form a viscous suspension that is difficult to process. Table 4 illustrates their effects on nanocellulose extraction.
Table 4.
Ultrasonic parameters and their effects on nanocellulose production.
| Parameters | Effects | Ref. |
|---|---|---|
| Frequency | Implementation of lower frequency:- Induces higher acoustic intensity, resulting in higher yield at a shorter duration |
[211] |
| Power | Implementation of a higher power:- Increases degree of surface modification, as evidenced by increased carboxylate content, benefitting the product functionalities and improving the dispersion- Improves yield and reduces operating time- Increases degree of fibrillation with insignificant effect on DP- Increases medium temperature at a faster rate, promoting fibrillation- Results in nanocellulose with higher WRV due to greater exposed surface area where hydroxyl groups rest on |
[14], [212], [53] |
| Solvent | Utilization of reactive medium (e.g., acid vs water) and/or higher concentration of the reactive medium:- Results in uniform shape and smaller size- Improves yield- Imparts functional groups on the surface |
[14] |
| Temperature | Implementation of higher sonication temperature:- Improves reaction kinetics, resulting in smaller fiber and larger exposed surface area- Increases yield and carboxylate content until achieving the optimum point, and then they decrease throughout- DP remains unaffected |
[64], [211] |
| Time | Prolongation of sonication time:- Improves yield and crystallinity with increased cavitation effect, but only to an optimum point, and then they decrease throughout- Reduces fiber size- Increases WRV due to larger exposed surface- Excessively long operating duration is harmful to the ultrasonic probe tip |
[64], [14], [212], [53], [213] |
| Solid loading | Utilization of higher solid loading:- Causes irregular distribution of cavitation effect- Increases the number and size of microbubbles, which can coalesce into vapor clusters, perturbing the ultrasonic wave’s flow in the medium- Decreases degree of fibrillation and WRV of nanocellulose |
[64] |
| DP, degree of polymerization; WRV, water retention value | ||
5. Application of ultrasound in nanocellulose surface modification
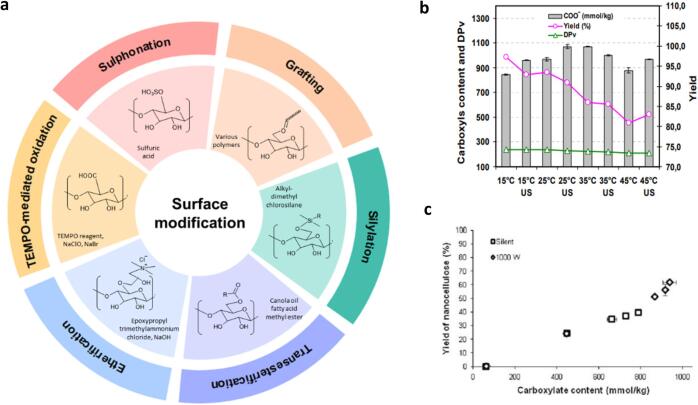
A large number of hydroxyl groups on the nanocellulose surface offers a unique platform for incorporating functional moieties such as sulfate groups, carboxyl groups, and macromolecules using various modification techniques. Engineering the surface chemistry of nanocellulose is an imperative approach to attain enhanced targeted properties that, in turn, increase its usability towards a broader application spectrum. The surface modification of nanocellulose can be conducted simultaneously or following the extraction step, depending on the strategies employed. The surface modification route is generally considered via physical, chemical and biological approaches [214]. However, ultrasound applications are more prominent in chemical modification through non-covalent interactions such as those featured in Fig. 10(a). The roles of ultrasound and the effects of ultrasonic parameters for the two categories of modification, specifically synchronous modification and post-modification, are discussed in the following sections. Table 5 summarises the ultrasonic conditions for some of the representative nanocellulose surface modification techniques.
Fig. 10.
(a) General chemical approach for the surface modification of nanocellulose [215]. (b) Carboxyl content, degree of depolymerization (DP), and yield against the ultrasonication temperature [145]. (c) Correlation between the yield of nanocellulose and carboxylate content [146].
Table 5.
Ultrasonic conditions in the surface modification of nanocellulose.
| Modification | Nanocellulose type | Ultrasonic Equipment | Ultrasonic Conditions |
Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Purpose | Power (W) | Time (min) | Freq (kHz) | Temp (˚C) | ||||
| Sulphonation | CNC | Probe | Pre-treatment | 750 | 15 | 25 | – | [141] |
| CNC | Probe | Post-treatment | 800 | 5 | – | 45 | [218] | |
| CNC | Probe | Post-treatment | 400 | 120 | 24 | 10 | [83] | |
| CNC | Probe | Post-treatment | – | 60 | 22 | – | [119] | |
| CNC | Bath | Post-treatment | 350 | 15 | 53 | – | [219] | |
| TEMPO-mediated oxidation | CNF | Bath | Synchronous | 1000 | 60 | 170 | 14 | [146] |
| CNF | Probe | Post-treatment | 300 | 20 | – | – | [175] | |
| CNC | Probe | Post-treatment | 400 | 30 | 20 | – | [160] | |
| Etherification | CNF | Bath | Pre-treatment | – | 240 | – | – | [232] |
| Bath | Pre-treatment Post-treatment |
600 600 |
240 30 |
40 20 |
65 – |
[233] | ||
| Transesterification | CNC | Probe | Pre-treatment | – | 1 | 20 | – | [229] |
| Silylation | CNC | Bath | Pre-treatment | – | 20 | – | – | [231] |
| Grafting | CNC | Probe | Pre-treatment | 400 | 2 | 20 | – | [234] |
5.1. Ultrasound-assisted synchronous modification
The synchronous modification technique for nanocellulose includes sulphonation and TEMPO-mediated oxidation, to which ultrasound can be applied to enhance both the yield and the degree of modification. Sulfonation is a process of esterifying the hydroxyl groups of nanocellulose; it can occur during nanocellulose extraction via acid hydrolysis using sulfuric acid [216]. The anchored sulfate moieties on the nanocellulose surface carry anionic charges, which are vital to impart electrostatic repulsion for stabilizing the colloids and reducing the interfibrillar hydrogen bonding that can help prevent hornification of nanocellulose upon drying [217]. To ensure particle size homogeneity, ultrasound assistance is usually employed at the post-reaction stage to disintegrate the incompletely hydrolysed fibres as well as disperse some agglomerated nanocellulose particles [83], [119], [218], [219]. The improved particle size uniformity and colloidal stability conferred by ultrasound assistance are critical for permitting reproducible properties in the end applications. Notably, as the acid hydrolysis process needs precise control over the reaction conditions to avoid over-depolymerization, there are more crucial concerns with monitoring the extraction efficacy, particularly the particle size, yield, and crystallinity, over controlling the extent of sulphonation [220]. Accordingly, sulphonation has limited flexibility for charge density manipulation, and the as-produced sulfated nanocellulose is most often re-modified in a separate process after the extraction step for attaining the desirable functionalities [221], [222], [223], [224].
TEMPO-mediated oxidation also allows the simultaneous extraction of nanocellulose and regioselective conversion of the hydroxyl groups at C6 of cellulose to carboxylate groups. As reported by Rattaz et al., ultrasound improves the degree of functionalization remarkably, evidenced by the increased carboxylate content of the ensuing nanocellulose with increasing ultrasonic intensity. A 30 % significant rise in the carboxylate content was also found for ultrasound-assisted modification compared to without ultrasound [146]. This higher degree of functionalization is attributed to ultrasound-induced shock waves and micro-jets that destroy the particles’ structure, exposing more functionalizable surfaces on which the carboxyl groups can be decorated. When investigating the effects of varying ultrasonic temperatures, Mishra et al. concluded an optimum temperature of 25 ˚C for maximizing the extent of carboxylation via TEMPO-mediated oxidation [145]. However, the highest possible yield was attained at a relatively lower ultrasonic temperature (15 ˚C) (Fig. 10(b)) [145]. This suggests a trade-off between the yield and degree of carboxylation. Rattaz et al. further confirmed that the nanocellulose yield and the carboxylate content are interdependent for such a synchronous modification process, suggesting that neither of them can be tailored separately regardless of the ultrasonic intensity applied (Fig. 10(c)) [146]. Therefore, ultrasound application in synchronous modification of nanocellulose focuses on dispersion and disintegration; adjustment of ultrasonic parameters to optimize either the extraction or modification performance is challenging.
5.2. Ultrasound-assisted post-modification
Post-modification of nanocellulose can be achieved through various chemistries, but those generally involve ultrasound assistance are TEMPO-mediated oxidation, etherification, transesterification, silylation, and grafting. Except for TEMPO-mediated oxidation primarily used for CNF extraction, these modification techniques accentuate solely by inducing hydrophobicity. Considering the intrinsic hydrophilic nature of nanocellulose, tuning the charge density via post-modification strategies to strike a desirable balance between hydrophilicity and hydrophobicity is critical to improving the interfacial compatibility between nanocellulose and hydrophobic or nonpolar matrices in nanocomposites [223], [225]. Ultrasound is an exceptional mechanical treatment that can facilitate post-functionalization of nanocellulose surfaces while minimizing alteration of structural dimensions and crystallinity owing to the mild mechanistic effects of cavitation.
The post-TEMPO-mediated oxidation offers a higher degree of oxidation than the synchronous approach due to the absence of impurities in the starting material that can compete for reaction, which thus consumes the oxidants [226]. Unlike TEMPO-mediated oxidation that decorates nanocellulose with anionic moieties, etherification is a cationization process entailing nucleophilic substitution of the alkali-activated hydroxyl groups of nanocellulose with epoxidated molecules. This modification agent is susceptible to degradation via hydrolysis, which can deteriorate its cationizing capacity; therefore, strict control of water content in the reaction medium is essential [227]. Besides, nanocellulose can be modified through transesterification with esters to substantially increase hydrophobicity for use as coatings or reinforcing agents [228]. Adding acids or bases is necessary to catalyse this reaction [228], [229]. Silylation is another promising technique for synthesizing superhydrophobic nanocellulose due to the high level of substitution it can achieve, which is attributed to the strong affinity of the silane coupling agents towards hydroxyl groups of cellulose even at room temperature [230]. Nevertheless, concentrated silane coupling agents can solubilize cellulose. Thus, a trade-off exists between the extent of silylation and the preservation of morphology that needs to be carefully addressed [230]. Grafting of nanocellulose can be performed by “grafting onto”, which covalently links polymers with nanocellulose and “grafting from”, which functionalizes nanocellulose with an initiator, after which monomers can be polymerized directly from the surface.
The implementation of ultrasonic treatment can be seen at stages before, during, and after the surface modification step (Table 5). Unless modification is performed using the suspension form of nanocellulose, applying ultrasound before and/or during the modification process is a requisite for uniformly dispersing nanocellulose in the reaction mixture. This maximizes the accessibility of chemically active sites and facilitates an equally modified surface chemistry without causing degradation in the nanocellulose [220], [222]. Besides, ultrasound can also expose more hydroxyl groups on the cellulose surface by cavitation disintegration, making them readily available for modification [220]. On the other hand, post-ultrasonication merely disintegrates the surface modified-nanocellulose to desirable particle sizes and aspect ratios, of which tailoring to meet specific requirements.
In summary, ultrasonication enhances the surface modification of nanocellulose by exposing more hydroxyl groups for reaction through dispersion and disintegration by cavitation without compromising the crystalline structure of nanocellulose. Eloquent evidence demonstrated that the degree of modification is closely associated with the concentration of the modification agents [146], [229], [231]. At the same time, the effects of various sonication conditions are rarely investigated, and more relevant studies are needed to exploit the advantageous effects of cavitation maximally.
6. Challenges and recommendations
Ultrasound irradiation has been acknowledged as a process intensifier for nanocellulose extraction via chemical or enzymatic treatments [235], [236], [237]. Owing to the intensive cavitation effects, ultrasound proficiently promotes defibrillation of fibers, penetration of reagents into fibers, homogenization of suspension, and agglomerate break-up; thus, resulting in overall better product properties (i.e., higher colloidal stability, higher water holding capacity, higher degree of crystallinity and higher aspect ratio), yield enhancement, process time reduction, and reduction of reliance on harmful solvents. Despite holding substantial promises, several major hurdles need further developments to surmount before ultrasound technology can progress to conducive industrial-scale implementation. These challenges of ultrasound application, along with the corresponding future recommendations, are discussed in the following sections.
6.1. Deterioration in nanocellulose properties and production efficiency
Ultrasound can adversely impinge on the final nanocellulose properties, such as reducing the degree of crystallinity [82], [238], [239], [240], [241]. Due to the non-selective mechanistic nature of the ultrasonic wave, the ordered cellulose structure could be deconstructed by excessive cavitation upon prolonged treatment and/or under high sonic intensity. The deterioration in crystallinity is crucial in nanocellulose production as it is strongly associated with the mechanical strength and thermal stability of nanocellulose, affecting its applicability, such as reinforcement in polymer matrix composites, electronics, etc. Mapping operating conditions between chemical treatment and ultrasonication are thus indispensable to minimize physical damage to the product.
Aside from the possible deterioration of product properties, nanocellulose production efficiency can be negatively affected by ultrasound as well. The tendency of ultrasound-induced active free radicals towards interfering with and/or initiating secondary reactions is speculated, given the knowledge of their capability of oxidizing chemicals [210] and homolytically cleaving lignin-carbohydrate bonds in biomass [242]. Nonetheless, the exact mechanisms and magnitude of the influences on nanocellulose extraction and surface modification in chemical systems remain insufficiently understood compared to enzymatic systems. Future related studies may begin with quantifying the amount of free radicals generated [243] under different sonication conditions and monitoring the molecular and compositional changes of the used solvent and the ensuing nanocellulose throughout the reaction.
On the contrary, sono-effects on the enzymatic system are well elucidated in many previous studies. Localized high temperatures and mechanical stresses generated by ultrasound potentially disrupt the secondary and tertiary structures of enzymes, leading to enzyme denaturation and inactivation [244]. Beyond that, ultrasound-induced free radicals, which possess unpaired electrons and high reactivity, can alter the charge distribution on the enzyme surface, consequently damaging the active site geometries and causing loss of enzyme-substrate affinity [245]. Thus, enzyme stability under ultrasound fields becomes a limiting factor in their combined application for nanocellulose production. To improve the enzyme stability against ultrasound-induced high temperatures and shear stresses, Martins et al. reported packing enzymes onto the interface of oil/water micro-emulsions. They realised that the enzyme's half-life was doubled when used to pretreat cotton, rendering substantially viable industrial implementation [246].
On the other hand, adding free radical scavengers like polyethylene glycol and polyvinyl alcohol is a direct strategy to capture free radicals from the medium and eliminate their pernicious effects on enzymes [247]. As each enzyme exhibits different tolerance levels to ultrasound fields [248], [249], [250], it is also possible to discern the most promising enzymes with high tolerance to harsh environments and high productivity. Moreover, researchers can also engineer enzyme stability through strategies such as entropic stabilization by introducing disulfide bridges into native enzyme structures and others, which were thoroughly reviewed by Eijsink et al. [251]. In summary, optimal sonochemical or enzymatic conditions and configurations are vital to prevent undesirable product degradation and efficiency reduction, ultimately leveraging the ultrasound’s advantageous cavitation effects.
6.2. Inability for standalone implementation
Beyond the negative effects on nanocellulose properties and production efficiency, ultrasound application is limited to merely serving a supplementary role in nanocellulose extraction and surface modification. Previous works employed ultrasound simultaneously or as a post-treatment succeeding chemical or enzymatic treatments [82], [239], [252], [253], [254], [255], [256], [257], [258]. Despite the promise of improving production efficiency as well as curtailing chemical and energy consumption, the implementation of ultrasound-only treatment is rarely reported. It was advocated for the inability to stand alone in nanocellulose extraction from raw lignocellulosic materials. This is attributed to the recalcitrance of the lignin matrix, wherein at least 80 % of its removal is required to enable the liberation of cellulose fiber for subsequent nanofibrillation. This can only be effectively attained by chemical pretreatment [259].
On the other hand, in several attempts to use purer cellulosic sources, ultrasound-only treatment was reported with successful isolation of CNF. Nonetheless, energy-intensive application of long sonication time (1 h) and high temperature (60 °C) were needed, in addition to the need for frequent replacement of the horn due to its proneness to damage towards heavy usage [260]. In terms of performance, Wu et al. found that the implementation of ultrasonication at 600 W for 30 min was slightly more robust than both high-pressure homogenization (HPH) at 100 MPa for three passes and 140 MPa for one pass [261]. The former resulted in smaller mean particle size, a lower polydispersity index, and a higher swelling ratio [261]. Hu et al. scrutinized the particle dimensions and found that CNF obtained from ultrasonication-only treatment generally manifested longer lengths than HPH [202]. Longer CNFs are favourable for their reinforcing properties, but this result also suggests the limitation of ultrasound-only treatment for CNC production. The reason is that the ultrasonic transducer generates a relatively weaker axial shear force that can barely fragmentize cellulose fibers [202], [203].
The initial concentration subjected to the ultrasound process significantly affects the efficacy when using ultrasound as the standalone treatment. Particle size reduction of CNF was improved by increasing the initial suspension concentration to 1 %, and a further increase of the initial concentration indicated an opposite effect [202]. As an inference, cellulose suspension becomes more viscous at higher initial concentrations, tending to exert greater resistance to the flow and leading to poorer defibrillation efficiency [202], [262]. Higher output power is thus required for such high loads to maintain the desired conditions of amplitude and intensity [263]. On the other hand, ultrasound applications generally generate more heat than other mechanical treatments, requiring an ice bath for thermolabile samples like lignin-containing nanocellulose, which is susceptible to condensation at high temperatures. Overall, ultrasonication presents inherent inadequacy for standalone implementation, considering the recalcitrance of biomass, increased power requirement, and the consistency range effective for ultrasonic treatment. Thus, it is more suitable to assist with other treatments to achieve nanocellulose production.
6.3. High energy consumption
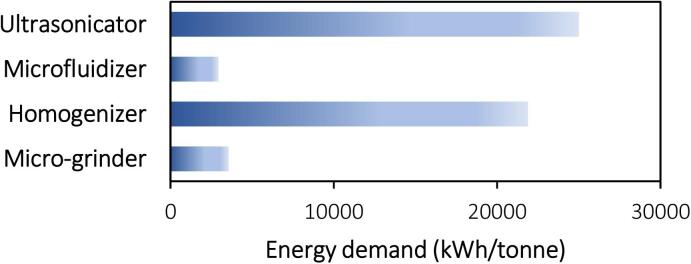
Another major impediment to the commercial success of ultrasound applications is the higher energy requirements than the existing mechanical treatments to achieve effective nanofibrillation. The average energy required to produce each tonne of nanocellulose through ultrasound was reported as the highest among the existing mechanical treatments employed (Fig. 11). Although comparable to homogenization, several multiples of the energy of microfluidization and grinding render ultrasound unattractive for industrialization. This high energy demand is aroused by the low energy conversion efficiency from electrical to acoustic cavitation, which is capped below 30 % for conventional generators [264]. Substantial energy is dissipated as heat, pressure, and noise that go into waste without contributing to cavitation and microstreaming [264]. Gratifyingly, recent advances in piezoelectric transducers reduce energy loss and achieve a remarkably higher energy conversion efficiency of approximately 80 % [264]. This makes the commercial application of ultrasound more energy-efficient and, thus, cost-effective. High- and low-intensity ultrasonication have thus been commonly exercised in the food industry for emulsification, crystallization, cleaning, etc. [265]. Nonetheless, for nanocellulose extraction, large-scale ultrasonication (20,000–20,000 kWh/tonne) consumes much greater energy than the laboratory scale (1760–1943 kWh/tonne) [252], [266], [267], unlike other mechanical treatments that normally show improved energy efficiency at large-scale [268]. This opposing phenomenon of ultrasound technology is possibly arising from poor scale-up design. The small active zone of ultrasonic equipment concentrating at the transducer tips requires thoughtful equipment design with adequate reactor geometry and transducer orientation to maximize effective sonication. More relevant discussions are included in the following section.
Fig. 11.
Energy demand for the production of nanocellulose from various mechanical treatments [252], [266], [269].
Besides, current research on sonochemistry accentuates the implementation of pulsing irradiation rather than classical continuous irradiation to improve energy efficiency further. Several researchers reported a remarkable energy saving of more than 50 % using pulsed ultrasound-assisted extraction without significant difference in the product quality in food applications [270], [271], [272]. While pulsation demonstrates potential as a viable industrial option, considerable studies on its application in nanocellulose extraction are still needed to chalk its effects on product properties and production efficiency. Besides, pulsed ultrasonication's disintegration and mixing effects, especially in a continuous system, are still in question [273]. Innovative ultrasonication equipment design with sufficiently lesser energy loss continues to be the pursuit of researchers to debottleneck ultrasound-assisted nanocellulose production and make the production economically feasible.
6.4. Difficulty in equipment design and process scale-up
Nanocellulose is produced industrially by less than 20 companies around the globe, most of which either implement harsh chemical or mechanochemical treatments using the standard disintegration equipment (e.g., refiner) (Table 6). Strikingly, Blue Goose Biorefineries and America Process are distinctive. They reported adopting ultrasonication in the post-treatment step recently to supplement their respective chemical processes for the production of CNC and CNF. Their initiatives ascertain the viability of implementing large-scale ultrasonication for nanocellulose extraction. Nevertheless, further, development is still needed as many market analyses highlighted the high capital investment for current technology and lack of technical expertise for process optimization and scale-up as the major challenges to nanocellulose production industry [274], [275], [276], [277]. Concern that higher costly capital will become apparent for ultrasound technology since large-scale ultrasonic equipment is unavailable. This is because it remains difficult to find clear protocols and design formulas to achieve consistent and anticipated results due to the hard-to-predict geometric and volume effects that bring about unevenly distributed acoustic fields and complex flow patterns within ultrasonication reactors [273], [278]. Notwithstanding that several ground-level research on cavitation behaviours, theoretical links between geometric and operating conditions, and field distribution have contributed to well-followed ultrasonication reactor design approaches, a high level of uncertainty still exists when transferring the laboratory-scale results to an industrial scale, requiring painstaking studies using numerical modelling to minimize the design errors [279], [280], [281]. Therefore, customizing ultrasonic equipment for specific applications remains the common practice of implementing this technology in industries.
Table 6.
Summary of companies involved in nanocellulose industrial production.
| Type | Company | Production capacity (tonne per year) | Process involved | Ref.a, b |
|---|---|---|---|---|
| CNC | CelluForce, Canada | 260 | H2SO4 hydrolysis, membrane filtration, spray dryer | [286] |
| Alberta Innovates, Canada | 5 | H2SO4 hydrolysis, centrifuge, membrane filtration, spray drying | [286] | |
| U.S. Forest Products Lab, US | 3 | H2SO4 hydrolysis, dilution, neutralization, ultrafiltration, reverse osmosis filtration, diafiltration | [287] | |
| Anomera, Canada | 1 | Dilute H2O2 oxidation, heating or UV radiation, centrifugation or diafiltration, spray drying | [288] | |
| Blue Goose Biorefineries, Canada | 2 | Transition metal-catalysed oxidation, ultrasonication | [289] | |
| CNF | University of Maine, US | 260 | Mechanical disc refiner | [287] |
| American Process, US | 130 | Fractionation with SO2 and ethanol, mechanical treatment (refining, sonication, or microfluidization) | [290] | |
| US Forest Products Lab, US | <1 | TEMPO oxidation, neutralization, disc refining, centrifugation, ultrafiltration, diafiltration, freeze-drying |
[286], [287] |
Commercial sonication bath and probe are only suitable for laboratories and pilot operations due to the inherently inhomogeneous acoustic field distribution and the low energy efficiency. Both effects are more controllable when using small working volumes (Fig. 12(b, c)). A small active zone of ultrasonic equipment is a long-known challenge for its equipment design. Ultrasonic intensity vanishes exponentially as the waves move away from the sources [245], creating distinguished active and passive zones. Such uneven acoustic intensities distributed across the medium dwindle effective cavitation, leading to inconsistent product quality and energy wastage. In light of this, large-scale ultrasonic reactor designs must consider reactor geometry and transducer configuration to ensure a well-distributed acoustic field with a broader range of active zone, in addition to accommodating high acoustic intensities and handling large throughput [210], [282]. Whistle, flow cell, and tube reactors are more pragmatic configurations that have easier and greater scalability [210] (Fig. 12(a, d, e)), while flow cell and tube reactors offer greater effective acoustic field coverage with their multiple, arrayed transducers. The difference between flow cell and tube reactor is the installation site of transducers [283], where the former has slot-in transducers that confer high sono-energy output that can effectively intensify chemical reactions in nanocellulose production, but with compromise on probe tip erosion. The latter with a wall-mounted transducer works on the contrary and may be more suitable for producing nanocellulose in an enzymatic system, considering the enzyme stability, as well as for dispersing and homogenizing nanocellulose suspension at the post-manufacturing stage.
Fig. 12.
(a) Whistle reactor, (b) bath reactor, (c) probe reactor, (d) flow cell reactor, and (e) tube reactor [284], [285].
Besides, problems regarding temperature control, energy transfer efficiency, and probe tip erosion shall be thoroughly addressed during process design. A better understanding of the complex relationship among the operating conditions, i.e., ultrasonic time, intensity, frequency, solids loading, etc., is also essential to fuel development towards optimal isolation of nanocellulose with the desired properties. Besides, to tackle the lack of technical expertise in sonochemistry, a close alliance between academic researchers and industries may benefit the industries by nurturing a more industry-specific skilled workforce and catalysing technological innovation and growth, which guarantee enhanced competitiveness.
6.5. Safety and regulatory concerns
Safety is one important aspect concerning the commercial development of nanocellulose. Safety concerns can be divided into occupational safety, product safety, and international safety standards. Firstly, occupational safety is crucial in ultrasonication due to acoustic cavitation’s large heat and noise generation [293], [294]. Cavitation threshold should exist to ensure safety during irradiation; however, only limited studies are available on this aspect. For product safety, a report on CNC indicates that it could induce inflammatory effects and cytotoxicity on human lungs, particularly upon inhalation [295]. Nevertheless, an exposure limit to nanocellulose is not established yet due to insufficient experimental data on nanocellulose exposure simulated in environmental conditions that closely mimic realistic industrial practice. Lastly, the international safety standards for nanocellulose are still under development. A few bodies are involved in developing standards for nanocellulose, such as Working Party on Manufactured Nanomaterials (WPMN) established by the Organisation for Economic Co-operation and Development (OECD), ISO TC 229 Nanotechnologies, etc. The standards are coordinated under four main areas: terminology and nomenclature, measurement and characterization, environment, health and safety, and material specification [296].
7. Conclusion and future outlook
Sustainable exploitation of nanocellulose has always been vilified, given that most established extraction methods are based on heavy use of corrosive and toxic chemicals. Ultrasound is an emerging technology that can act as a process booster for effective environmentally-friendlier extraction and surface modification of nanocellulose at reduced chemical usage, shortened process time, and improved yield, owing to the accelerated reaction and enzyme kinetics driven by acoustic streaming and cavitation. Beyond that, microjetting provides sufficient energy to cause nanofibrillation, indicating the capability of ultrasound to substitute mechanical treatments commonly employed in the post-nanocellulose isolation step. Moreover, acoustic effects disintegrate, homogenize, and deagglomerate nanocellulose suspension less vigorously, lowering the chances of degrading the crystalline domains. The ensuing nanocellulose thus demonstrated enhanced properties, including higher water holding capacity, higher surface group content, a higher degree of crystallinity, and higher aspect ratio. The overall enhancement of ultrasound is far-reaching by the classical technologies employing chemical, enzymatic, mechanical, or any combination thereof.
Nevertheless, most current research centred on using ultrasound for dispersion, deagglomeration, and only minimal disintegration. To unleash the full potential of ultrasound and leverage its benefits, future research should focus more on extending ultrasound usage towards greater roles, for instance, mediating chemical extraction. Furthermore, ultrasound would take a primary position in facilitating the various greener nanocellulose production methods developed lately, which focus on utilizing less hazardous reactants such as dilute mineral acid, weak organic acid, deep eutectic solvent, etc. These greener methods share the common drawback of lacking robustness, by which ultrasound intensification can invigorate the process and contribute to a greater impact on sustainability. Combining ultrasound with other mechanical (i.e., high-pressure homogenization) or heat treatments (i.e., microwave irradiation) presents an upcoming trend to improve production efficiency further and reduce chemical reliance.
Apart from that, the successful implementation of ultrasound for nanocellulose production at the industrial level still requires considerable effort in tackling its multifaceted challenges. The way forward is to first delve into the mechanism of ultrasonic intensification, free radicals’ effects, and pulsed sonication’s effects in different chemical and enzymatic systems to hone better fundamental understandings to buttress effective process design and optimization. On top of that, the parametric window and cause-effect relation between operating conditions and production efficiency need to be elucidated to allow the systematic development of well-rounded equipment and process scale-up guidelines that enable energy efficiency improvement, ultrasound efficacy maximization, and cost-effectiveness enhancement. Besides, benefitting from the meteoric evolution of digital technologies, nanocellulose production with ultrasound assistance can be fused with the Internet of Things and artificial intelligence to alleviate real-time process monitoring and control, thereby streamlining the production, alleviating efficiency and safety, as well as reducing off-spec production. Considering that energy constitutes the backbone of ultrasound implementation, opportunities also arise with integrating heat and utilizing bioenergy (i.e., biogas and heat) obtained from incinerating the unreacted cellulosic materials to help reduce the carbon footprint of the inevitable energy requirement. By and large, ultrasound is a promising technology. With meticulous research and development, addressing the shortcomings highlighted can pave the way toward meeting the challenge of extracting bionanomaterial efficiently with innocuous mechanisms.
CRediT authorship contribution statement
Do Yee Hoo: Writing – original draft. Zhen Li Low: Writing – original draft. Darren Yi Sern Low: Writing – review & editing. Siah Ying Tang: Writing – review & editing. Sivakumar Manickam: Conceptualization, Writing – review & editing. Khang Wei Tan: Supervision. Zhen Hong Ban: Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are thankful to Xiamen University Malaysia Campus for the financial support through the Xiamen University Malaysia Research Fund (XMUMRF/2022-C10/IENG/0048 and XMUMRF/IENG/0002).
Contributor Information
Khang Wei Tan, Email: khangwei.tan@xmu.edu.my.
Zhen Hong Ban, Email: bzhong@xmu.edu.my.
Data availability
The authors are unable or have chosen not to specify which data has been used.
References
- 1.Wu W., Yu Q., You L., Chen K., Tang H., Liu J. Global cropping intensity gaps: Increasing food production without cropland expansion. Land Use Policy. 2018;76:515–525. [Google Scholar]
- 2.Thomas P., Duolikun T., Rumjit N.P., Moosavi S., Lai C.W., Bin Johan M.R., Fen L. Comprehensive review on nanocellulose: recent developments, challenges and future prospects. J. Mech. Behav. Biomed. Mater. 2020;110 doi: 10.1016/j.jmbbm.2020.103884. [DOI] [PubMed] [Google Scholar]
- 3.Phanthong P., Reubroycharoen P., Hao X., Xu G., Abudula A., Guan G. Nanocellulose: extraction and application. Carbon Resour. Convers. 2018;1:32–43. [Google Scholar]
- 4.Ferreira F.V., Otoni C.G., De France K.J., Barud H.S., Lona L.M.F., Cranston E.D., Rojas O.J. Porous nanocellulose gels and foams: breakthrough status in the development of scaffolds for tissue engineering. Mater. Today. 2020;37:126–141. [Google Scholar]
- 5.Wang J., Tavakoli J., Tang Y. Bacterial cellulose production, properties and applications with different culture methods – A review. Carbohydr. Polym. 2019;219:63–76. doi: 10.1016/j.carbpol.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 6.S. Agwuncha, C. Anusionwu, S. Owonubi, E. Sadiku, U. Busuguma, I. Ibrahim, Extraction of cellulose nanofibers and their eco/friendly polymer composites, in, 2019, pp. 37–64.
- 7.Trache D. Nanocellulose as a promising sustainable material for biomedical applications. AIMS Mater. Sci. (2372–0484) 2018;5:201–205. [Google Scholar]
- 8.Du C., Li H., Li B., Liu M., Zhan H. Characteristics and properties of cellulose nanofibers prepared by TEMPO oxidation of corn husk. BioResources. 2016;11:5276–5284. [Google Scholar]
- 9.Abol-Fotouh D., Hassan M.A., Shokry H., Roig A., Azab M.S., Kashyout A.-E.-H.-B. Bacterial nanocellulose from agro-industrial wastes: low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020;10:3491. doi: 10.1038/s41598-020-60315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zinge C., Kandasubramanian B. Nanocellulose based biodegradable polymers. Eur. Polym. J. 2020;133 [Google Scholar]
- 11.Janardhnan S., Sain M.M. Isolation of cellulose microfibrils - an enzymatic approach. BioResources. 2006;1:176–188. [Google Scholar]
- 12.Fan J.-S., Li Y.-H. Maximizing the yield of nanocrystalline cellulose from cotton pulp fiber. Carbohydr. Polym. 2012;88:1184–1188. [Google Scholar]
- 13.Liimatainen H., Sirviö J., Visanko M., Hormi O., Niinimäki J. Sulfonated cellulose nanofibrils obtained from wood pulp through regioselective oxidative bisulfite pre-treatment. Cellulose. 2013;20 [Google Scholar]
- 14.Filson P.B., Dawson-Andoh B.E. Sono-chemical preparation of cellulose nanocrystals from lignocellulose derived materials. Bioresour. Technol. 2009;100:2259–2264. doi: 10.1016/j.biortech.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 15.Jiang F., Esker A.R., Roman M. Acid-catalyzed and solvolytic desulfation of H2SO4-hydrolyzed cellulose nanocrystals. Langmuir. 2010;26:17919–17925. doi: 10.1021/la1028405. [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Xu J., Zhu S., Wu Q., Li J., Gao Y., Wang B., Li J., Gao W., Zeng J., Chen K. Preparation of nanocellulose in high yield via chemi-mechanical synergy. Carbohydr. Polym. 2021;251 doi: 10.1016/j.carbpol.2020.117094. [DOI] [PubMed] [Google Scholar]
- 17.Methods for extraction of nanocellulose from various sources, in: Handbook of Nanocellulose and Cellulose Nanocomposites, 2017, pp. 1–49.
- 18.Gicquel E. Grenoble Institute of Technology; 2017. Development of stimuli-responsive CNC hydrogels for smart development; p. 330. [Google Scholar]
- 19.Suresh C.A., Rakshit A., Garima A. In: Sonochemistry: An Emerging Green Technology. Suresh C.A., Rakshit A., Garima A., editors. Apple Academic Press Oakville; Canada: 2018. Sonochemistry: an emerging green technology; p. 402. [Google Scholar]
- 20.Mason T.J. Blackie Academic and Professional; London, UK: 1998. Power Ultrasound in Food Processing – the Way Forward. [Google Scholar]
- 21.Maadi M. The Graduate School of Natural and Applied Sciences. Middle East Technical University; 2013. Integrated circuit design for flip-chip bonded capacitive micromachined ultrasonic transducers; p. 205. [Google Scholar]
- 22.Zhang P., Zhu Z., Sun D.W. Using power ultrasound to accelerate food freezing processes: Effects on freezing efficiency and food microstructure. Crit. Rev. Food Sci. Nutr. 2018;58:2842–2853. doi: 10.1080/10408398.2018.1482528. [DOI] [PubMed] [Google Scholar]
- 23.Jambrak A.R., Lelas V., Mason T.J., Krešić G., Badanjak M. Physical properties of ultrasound treated soy proteins. J. Food Eng. 2009;93:386–393. [Google Scholar]
- 24.Tao Y., Sun D.W. Enhancement of food processes by ultrasound: a review. Crit. Rev. Food Sci. Nutr. 2015;55:570–594. doi: 10.1080/10408398.2012.667849. [DOI] [PubMed] [Google Scholar]
- 25.Zheng L., Sun D.-W. Innovative applications of power ultrasound during food freezing processes—a review. Trends Food Sci. Technol. 2006;17:16–23. [Google Scholar]
- 26.Zhang Z., Sun D.-W., Zhu Z., Cheng L. Enhancement of crystallization processes by power ultrasound: current state-of-the-art and research advances. Compr. Rev. Food Sci. Food Saf. 2015;14:303–316. [Google Scholar]
- 27.Fu X., Belwal T., Cravotto G., Luo Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochem. 2020;60 doi: 10.1016/j.ultsonch.2019.104726. [DOI] [PubMed] [Google Scholar]
- 28.Keven Silva E., Zabot G.L., Toledo Hijo A.A.C., Meireles M.A.A. Encapsulation of bioactive compounds using ultrasonic technology. Ultrasound: Adv. Food Process. Preserv. 2017:323–350. [Google Scholar]
- 29.O'Brien W.D. Ultrasound-biophysics mechanisms. Prog. Biophys. Mol. Biol. 2007;93:212–255. doi: 10.1016/j.pbiomolbio.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson L.H., Doraiswamy L.K. Sonochemistry: science and engineering. Ind. Eng. Chem. Res. 1999;38:1215–1249. [Google Scholar]
- 31.Pollet B.G. The use of power ultrasound and sonochemistry for the production of energy materials. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2019.104851. [DOI] [PubMed] [Google Scholar]
- 32.Mason T.J., Lorimer J.P., Saleem S., Paniwnyk L. Controlling emissions from electroplating by the application of ultrasound. Environ. Sci. Technol. 2001;35:3375–3377. doi: 10.1021/es001944r. [DOI] [PubMed] [Google Scholar]
- 33.Patist A., Bates D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008;9:147–154. [Google Scholar]
- 34.Suslick K.S. 4th ed. John Wiley & Sons; New York: 1998. Kirk-Othmer Encyclopedia of Chemical Technology. [Google Scholar]
- 35.Ashokkumar M., Lee J., Kentish S., Grieser F. Bubbles in an acoustic field: an overview. Ultrason. Sonochem. 2007;14:470–475. doi: 10.1016/j.ultsonch.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Simal S., Benedito J., Sánchez E.S., Rosselló C. Use of ultrasound to increase mass transport rates during osmotic dehydration. J. Food Eng. 1998;36:323–336. [Google Scholar]
- 37.Ashokkumar M. The characterization of acoustic cavitation bubbles - an overview. Ultrason. Sonochem. 2011;18:864–872. doi: 10.1016/j.ultsonch.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Chow R., Blindt R., Kamp A., Grocutt P., Chivers R. The microscopic visualisation of the sonocrystallisation of ice using a novel ultrasonic cold stage. Ultrason. Sonochem. 2004;11:245–250. doi: 10.1016/j.ultsonch.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Kiani H., Zhang Z., Delgado A., Sun D.-W. Ultrasound assisted nucleation of some liquid and solid model foods during freezing. Food Res. Int. 2011;44:2915–2921. [Google Scholar]
- 40.Kim H., Suslick K. The effects of ultrasound on crystals: sonocrystallization and sonofragmentation. Crystals. 2018;8 [Google Scholar]
- 41.Yao Y., Pan Y., Liu S. Power ultrasound and its applications: a state-of-the-art review. Ultrason. Sonochem. 2020;62 doi: 10.1016/j.ultsonch.2019.104722. [DOI] [PubMed] [Google Scholar]
- 42.Taurozzi J.S., Hackley V.A., Wiesner M.R. Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment–issues and recommendations. Nanotoxicology. 2011;5:711–729. doi: 10.3109/17435390.2010.528846. [DOI] [PubMed] [Google Scholar]
- 43.Son Y., No Y., Kim J. Geometric and operational optimization of 20-kHz probe-type sonoreactor for enhancing sonochemical activity. Ultrason. Sonochem. 2020;65 doi: 10.1016/j.ultsonch.2020.105065. [DOI] [PubMed] [Google Scholar]
- 44.Son Y., Lim M., Song J., Khim J. Liquid height effect on sonochemical reactions in a 35 kHz sonoreactor. Jpn. J. Appl. Phys. 2009;48 [Google Scholar]
- 45.Price G.J., Harris N.K., Stewart A.J. Direct observation of cavitation fields at 23 and 515 kHz. Ultrason. Sonochem. 2010;17:30–33. doi: 10.1016/j.ultsonch.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Petrier C., Lamy M., Benachcene A., David B., Renaoudin V., Gondrexon N. Sonochemical degradation of phenol in dilute aqueous solutions: comparison of the reaction rates at 20 and 487 kHz. J. Phys. Chem. C. 1994;98:10514–10520. [Google Scholar]
- 47.Dilamian M., Noroozi B. A combined homogenization-high intensity ultrasonication process for individualizaion of cellulose micro-nano fibers from rice straw. Cellulose. 2019;26:5831–5849. [Google Scholar]
- 48.Aimin T., Hongwei Z., Gang C., Guohui X., Wenzhi L. Influence of ultrasound treatment on accessibility and regioselective oxidation reactivity of cellulose. Ultrason. Sonochem. 2005;12:467–472. doi: 10.1016/j.ultsonch.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Wang X., Wu Y., Huang J. Investigation of morphology of Vetier (Vetiveria zizanioides) cellulose micro/nano fibrils isolated by high intensity ultrasonication. Adv. Mater. Res. 2011;284–286:796–800. [Google Scholar]
- 50.Tischer P.C., Sierakowski M.R., Westfahl H., Jr., Tischer C.A. Nanostructural reorganization of bacterial cellulose by ultrasonic treatment. Biomacromolecules. 2010;11:1217–1224. doi: 10.1021/bm901383a. [DOI] [PubMed] [Google Scholar]
- 51.Lu Q., Ran L., Chen S., Lu L., Li L., Li Y. Facile one-step fabrication of functional cellulose nanocrystals with high efficiency under microwave-ultrasound synergy. J. Phys. Conf. Ser. 2020;1622 [Google Scholar]
- 52.Lu Z., Wu Z., Fan L., Zhang H., Liao Y., Zheng D., Wang S. Rapid and solvent-saving liquefaction of woody biomass using microwave-ultrasonic assisted technology. Bioresour. Technol. 2016;199:423–426. doi: 10.1016/j.biortech.2015.09.048. [DOI] [PubMed] [Google Scholar]
- 53.Lu Z., Fan L., Zheng H., Lu Q., Liao Y., Huang B. Preparation, characterization and optimization of nanocellulose whiskers by simultaneously ultrasonic wave and microwave assisted. Bioresour. Technol. 2013;146:82–88. doi: 10.1016/j.biortech.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 54.Lu Q., Tang L., Lin F., Wang S., Chen Y., Chen X., Huang B. Preparation and characterization of cellulose nanocrystals via ultrasonication-assisted FeCl3-catalyzed hydrolysis. Cellulose. 2014;21:3497–3506. [Google Scholar]
- 55.Tonoli G.H., Teixeira E.M., Correa A.C., Marconcini J.M., Caixeta L.A., Pereira-da-Silva M.A., Mattoso L.H. Cellulose micro/nanofibres from Eucalyptus kraft pulp: preparation and properties. Carbohydr. Polym. 2012;89:80–88. doi: 10.1016/j.carbpol.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 56.Brinchi L., Cotana F., Fortunati E., Kenny J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: technology and applications. Carbohydr. Polym. 2013;94:154–169. doi: 10.1016/j.carbpol.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 57.Mahardika M., Abral H., Kasim A., Arief S., Asrofi M. Production of nanocellulose from pineapple leaf fibers via high-shear homogenization and ultrasonication. Fibers. 2018;6 [Google Scholar]
- 58.McCarthy N.A., Kennedy D., Hogan S.A., Kelly P.M., Thapa K., Murphy K.M., Fenelon M.A. Emulsification properties of pea protein isolate using homogenization, microfluidization and ultrasonication. Food Res. Int. 2016;89:415–421. doi: 10.1016/j.foodres.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 59.L. Neway-Keane, S. Carrington, Controlling the Stability of Medicinal Suspensions, in, 2016.
- 60.Gielen B., Jordens J., Thomassen L., Braeken L., Van Gerven T. Agglomeration control during ultrasonic crystallization of an active pharmaceutical ingredient. Crystals. 2017;7 [Google Scholar]
- 61.Kusters K.A., Pratsinis S.E., Thoma S.G., Smith D.M. Ultrasonic fragmentation of agglomerate powders. Chem. Eng. Sci. 1993;48:4119–4127. [Google Scholar]
- 62.Vikash V. Kumar, Ultrasonic-assisted de-agglomeration and power draw characterization of silica nanoparticles. Ultrason. Sonochem. 2020;65 doi: 10.1016/j.ultsonch.2020.105061. [DOI] [PubMed] [Google Scholar]
- 63.O. Kudryashova, S. Vorozhtsov, A. Khrustalyov, M. Stepkina, Ultrasonic dispersion of agglomerated particles in metal melt, 2016.
- 64.Wang S., Cheng Q. A novel process to isolate fibrils from cellulose fibers by high-intensity ultrasonication, Part 1: Process optimization. J. Appl. Polym. Sci. 2009;113:1270–1275. [Google Scholar]
- 65.Nogi M., Yano H. Transparent nanocomposites based on cellulose produced by bacteria offer potential innovation in the electronics device industry. Adv. Mater. 2008;20:1849–1852. [Google Scholar]
- 66.Chen W., Yu H., Liu Y., Chen P., Zhang M., Hai Y. Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr. Polym. 2011;83:1804–1811. [Google Scholar]
- 67.Li Q., Renneckar S. Supramolecular structure characterization of molecularly thin cellulose I nanoparticles. Biomacromolecules. 2011;12:650–659. doi: 10.1021/bm101315y. [DOI] [PubMed] [Google Scholar]
- 68.Velmurugan R., Muthukumar K. Sono-assisted enzymatic saccharification of sugarcane bagasse for bioethanol production. Biochem. Eng. J. 2012;63:1–9. [Google Scholar]
- 69.Bondeson D., Mathew A., Oksman K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose. 2006;13:171–180. [Google Scholar]
- 70.Dong X.M., Revol J.-F., Gray D.G. Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose. 1998;5:19–32. [Google Scholar]
- 71.Das K., Ray D., Bandyopadhyay N.R., Ghosh T., Mohanty A.K., Misra M. A study of the mechanical, thermal and morphological properties of microcrystalline cellulose particles prepared from cotton slivers using different acid concentrations. Cellulose. 2009;16:783–793. [Google Scholar]
- 72.Lu P., Hsieh Y.-L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010;82:329–336. [Google Scholar]
- 73.Rajinipriya M., Nagalakshmaiah M., Robert M., Elkoun S. Importance of agricultural and industrial waste in the field of nanocellulose and recent industrial developments of wood based nanocellulose: A review. ACS Sustainable Chem. Eng. 2018;6:2807–2828. [Google Scholar]
- 74.Du H., Parit M., Wu M., Che X., Wang Y., Zhang M., Wang R., Zhang X., Jiang Z., Li B. Sustainable valorization of paper mill sludge into cellulose nanofibrils and cellulose nanopaper. J. Hazard. Mater. 2020;400 doi: 10.1016/j.jhazmat.2020.123106. [DOI] [PubMed] [Google Scholar]
- 75.Yu H., Abdalkarim S.Y.H., Zhang H., Wang C., Tam K.C. Simple process to produce high-yield cellulose nanocrystals using recyclable citric/hydrochloric acids. ACS Sustainable Chem. Eng. 2019;7:4912–4923. [Google Scholar]
- 76.Li T., Chen C., Brozena A.H., Zhu J.Y., Xu L., Driemeier C., Dai J., Rojas O.J., Isogai A., Wagberg L., Hu L. Developing fibrillated cellulose as a sustainable technological material. Nature. 2021;590:47–56. doi: 10.1038/s41586-020-03167-7. [DOI] [PubMed] [Google Scholar]
- 77.Li D., Henschen J., Ek M. Esterification and hydrolysis of cellulose using oxalic acid dihydrate in a solvent-free reaction suitable for preparation of surface-functionalised cellulose nanocrystals with high yield. Green Chem. 2017;19:5564–5567. [Google Scholar]
- 78.Bian H., Chen L., Dai H., Zhu J.Y. Integrated production of lignin containing cellulose nanocrystals (LCNC) and nanofibrils (LCNF) using an easily recyclable di-carboxylic acid. Carbohydr. Polym. 2017;167:167–176. doi: 10.1016/j.carbpol.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 79.Dorgan J.R., Braun B. Single-step method for the isolation and surface functionalization of cellulosic nanowhiskers. Biomacromolecules. 2009;10:334–341. doi: 10.1021/bm8011117. [DOI] [PubMed] [Google Scholar]
- 80.Niu F., Li M., Huang Q., Zhang X., Pan W., Yang J., Li J. The characteristic and dispersion stability of nanocellulose produced by mixed acid hydrolysis and ultrasonic assistance. Carbohydr. Polym. 2017;165:197–204. doi: 10.1016/j.carbpol.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 81.Csiszar E., Kalic P., Kobol A., Ferreira Ede P. The effect of low frequency ultrasound on the production and properties of nanocrystalline cellulose suspensions and films. Ultrason. Sonochem. 2016;31:473–480. doi: 10.1016/j.ultsonch.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 82.Mazela B., Perdoch W., Peplinska B., Zielinski M. Influence of chemical pre-treatments and ultrasonication on the dimensions and appearance of cellulose fibers. Materials (Basel) 2020;13 doi: 10.3390/ma13225274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ibrahim I.K., Hussin S.M., Al-Obaidi Y.M. Extraction of cellulose nano crystalline from cotton by ultrasonic and its morphological and structural characterization, International Journal of. Mater. Chem. Phys. 2015;1:99–109. [Google Scholar]
- 84.Barbash V.A., Yaschenko O.V., Shniruk O.M. Preparation and Properties of Nanocellulose from Organosolv Straw Pulp. Nanoscale Res. Lett. 2017;12:241. doi: 10.1186/s11671-017-2001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.I. Risnasari, E. Herawati, Yunasfi, S.D. Pandiangan, P. Adelina, Preparation and characterization of nanocrystalline cellulose using ultrasonic assisted autohydrolysis, IOP Conf. Series: Earth Environ. Sci., 374 (2019).
- 86.Shaheen T.I., Emam H.E. Sono-chemical synthesis of cellulose nanocrystals from wood sawdust using Acid hydrolysis. Int. J. Biol. Macromol. 2018;107:1599–1606. doi: 10.1016/j.ijbiomac.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 87.Maciel M., Benini K., Voorwald H.J.C., Cioffi M.O.H. Obtainment and characterization of nanocellulose from an unwoven industrial textile cotton waste: Effect of acid hydrolysis conditions. Int. J. Biol. Macromol. 2019;126:496–506. doi: 10.1016/j.ijbiomac.2018.12.202. [DOI] [PubMed] [Google Scholar]
- 88.Jordan J.H., Easson M.W., Dien B., Thompson S., Condon B.D. Extraction and characterization of nanocellulose crystals from cotton gin motes and cotton gin waste. Cellulose. 2019;26:5959–5979. [Google Scholar]
- 89.Zhou Y.M., Fu S.Y., Zheng L.M., Zhan H.Y. Effect of nanocellulose isolation techniques on the formation of reinforced poly(vinyl alcohol) nanocomposite films, Express. Polymer Letters. 2012;6:794–804. [Google Scholar]
- 90.Rashid S., Dutta H. Characterization of nanocellulose extracted from short, medium and long grain rice husks. Ind. Crops Prod. 2020;154 [Google Scholar]
- 91.Padzil F.N.M., Lee S.H., Ainun Z.M.A., Lee C.H., Abdullah L.C. Potential of oil palm empty fruit bunch resources in nanocellulose hydrogel production for versatile applications: a review. Materials (Basel) 2020;13 doi: 10.3390/ma13051245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Andalia R., Rahmi R., Julinawati J., Helwati H. Isolation and characterization of cellulose from rice husk waste and sawdust with chemical method. J. Nat. 2020;20:6–9. [Google Scholar]
- 93.Bondancia T.J., de Aguiar J., Batista G., Cruz A.J.G., Marconcini J.M., Mattoso L.H.C., Farinas C.S. Production of nanocellulose using citric acid in a biorefinery concept: effect of the hydrolysis reaction time and techno-economic analysis. Ind. Eng. Chem. Res. 2020;59:11505–11516. [Google Scholar]
- 94.Peretz R., Sterenzon E., Gerchman Y., Kumar Vadivel V., Luxbacher T., Mamane H. Nanocellulose production from recycled paper mill sludge using ozonation pretreatment followed by recyclable maleic acid hydrolysis. Carbohydr. Polym. 2019;216:343–351. doi: 10.1016/j.carbpol.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 95.Budhi Y.W., Fakhrudin M., Culsum N.T.U., Suendo V., Iskandar F. Preparation of cellulose nanocrystals from empty fruit bunch of palm oil by using phosphotungstic acid. IOP Conf. Series: Earth Environ. Sci. 2018;105:1–6. [Google Scholar]
- 96.Sasongko A., Legahati N. Effect of citric acid concentration and ultrasonic power on oligosaccharide production from salacca zalacca seeds using ultrasonic assisted acid hydrolysis (UAAH) method. SNITT - Politeknik Negeri Balikpapan. 2020;2021:395–400. [Google Scholar]
- 97.Guo J., Guo X., Wang S., Yin Y. Effects of ultrasonic treatment during acid hydrolysis on the yield, particle size and structure of cellulose nanocrystals. Carbohydr. Polym. 2016;135:248–255. doi: 10.1016/j.carbpol.2015.08.068. [DOI] [PubMed] [Google Scholar]
- 98.Balquinta M.L., Andrés S.C., Cerrutti P., Califano A.N., Lorenzo G. Effect of bacterial nanocellulose post-synthetic processing on powders and rehydrated suspensions characteristics. J. Food Eng. 2020;280 [Google Scholar]
- 99.Ching T.W., Haritos V., Tanksale A. Ultrasound-assisted conversion of cellulose into hydrogel and functional carbon material. Cellulose. 2018;25:2629–2645. [Google Scholar]
- 100.Agi A., Junin R., Gbadamosi A., Abbas A., Azli N.B., Oseh J. Influence of nanoprecipitation on crystalline starch nanoparticle formed by ultrasonic assisted weak-acid hydrolysis of cassava starch and the rheology of their solutions. Chem. Eng. Process. - Process Intensif. 2019;142 [Google Scholar]
- 101.Beltramino F., Roncero M.B., Torres A.L., Vidal T., Valls C. Optimization of sulfuric acid hydrolysis conditions for preparation of nanocrystalline cellulose from enzymatically pretreated fibers. Cellulose. 2016;23:1777–1789. [Google Scholar]
- 102.Meirelles A.A.D., Costa A.L.R., Cunha R.L. Cellulose nanocrystals from ultrasound process stabilizing O/W Pickering emulsion. Int. J. Biol. Macromol. 2020;158:75–84. doi: 10.1016/j.ijbiomac.2020.04.185. [DOI] [PubMed] [Google Scholar]
- 103.Meirelles A.A.D., Costa A.L.R., Cunha R.L. The stabilizing effect of cellulose crystals in O/W emulsions obtained by ultrasound process. Food Res. Int. 2020;128 doi: 10.1016/j.foodres.2019.108746. [DOI] [PubMed] [Google Scholar]
- 104.Li C., Sun P., Yang C. Emulsion stabilized by starch nanocrystals. Starch - Stärke. 2012;64:497–502. [Google Scholar]
- 105.Mohaiyiddin M.S., Lin O.H., Owi W.T., Chan C.H., Chia C.H., Zakaria S., Villagracia A.R., Akil H.M. Characterization of nanocellulose recovery from Elaeis guineensis frond for sustainable development. Clean Technol. Environ. Policy. 2016;18:2503–2512. [Google Scholar]
- 106.] S. Naduparambath, V.J. T, S. V, P.S. M, A.K. Balan, P. E, Isolation and characterisation of cellulose nanocrystals from sago seed shells, Carbohydr Polym, 180 (2018) 13–20. [DOI] [PubMed]
- 107.Morais J.P., Rosa Mde F., de Souza Filho S., Mde L.D., Nascimento D.M., Cassales A.R. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr. Polym. 2013;91:229–235. doi: 10.1016/j.carbpol.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 108.Klemm D., Kramer F., Moritz S., Lindstrom T., Ankerfors M., Gray D., Dorris A. Nanocelluloses: a new family of nature-based materials. Angew. Chem. Int. Ed. Engl. 2011;50:5438–5466. doi: 10.1002/anie.201001273. [DOI] [PubMed] [Google Scholar]
- 109.Mat Zain N.F. Preparation and characterization of cellulose and nanocellulose from pomelo (citrus grandis) albedo. J. Nutrit. Food Sci. 2014;05 [Google Scholar]
- 110.Camargo L.A., Pereira S.C., Correa A.C., Farinas C.S., Marconcini J.M., Mattoso L.H.C. Feasibility of manufacturing cellulose nanocrystals from the solid residues of second-generation ethanol production from sugarcane bagasse. Bioenergy Res. 2016;9:894–906. [Google Scholar]
- 111.Wen Z. Bioprocessing for value-added products from renewable resources: value-added products from animal manure. New Technol. Appl. 2007:629–651. [Google Scholar]
- 112.Ye S., Cheng J.Y. Hydrolysis of lignocellulosic materials for ethanol production_a review. Bioresour. Technol. 2002;83:1–11. doi: 10.1016/s0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- 113.CelluForce, Cellulose Nanocrystals, in, 2016.
- 114.Park C.-W., Han S.-Y., Namgung H.-W., et al. Overview of the preparation methods of nano-scale cellulose. J. Korea Tech. Assoc. Pulp Paper Ind. 2017;49:9–17. [Google Scholar]
- 115.K. Nelson, D. Turpin, Advancing commercialization of nanocellulose: critical challenges workshop report, (2020).
- 116.Al-Khateeb I.K., Hussin S., Alobaidi Y.M. Extraction of cellulose nano crystalline from cotton by ultrasonic and its morphological and structural characterization. Int. J. Mater. Chem. Phys. 2015;1:99–109. [Google Scholar]
- 117.Asem M., Jimat D.N., Jafri N.H.S., Wan Nawawi W.M.F., Azmin N.F.M., Abd Wahab M.F. Entangled cellulose nanofibers produced from sugarcane bagasse via alkaline treatment, mild acid hydrolysis assisted with ultrasonication. J. King Saud Univ. – Eng. Sci. 2021 [Google Scholar]
- 118.Wulandari W.T., Rochliadi A., Arcana I.M. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf. Series: Mater. Sci. Eng. 2016;107:1–7. [Google Scholar]
- 119.Barbash V.A., Yashchenko O.V., Vasylieva O.A. Preparation and properties of nanocellulose from miscanthus x giganteus. J. Nanomater. 2019;2019:1–8. [Google Scholar]
- 120.Luo J., Huang K., Xu Y., Fan Y. A comparative study of lignocellulosic nanofibrils isolated from celery using oxalic acid hydrolysis followed by sonication and mechanical fibrillation. Cellulose. 2019;26:5237–5246. [Google Scholar]
- 121.Kusumaningrum W.B., Amanda P., Suryanegara L., Masruchin N. The effectivity of one-pot concentrated maleic anhydride hydrolysis for betung bamboo pulp (Dendrocalamus asper sp) IOP Conf. Series: Earth Environ. Sci. 2020;572 [Google Scholar]
- 122.Chimentao R.J., Lorente E., Gispert-Guirado F., Medina F., Lopez F. Hydrolysis of dilute acid-pretreated cellulose under mild hydrothermal conditions. Carbohydr. Polym. 2014;111:116–124. doi: 10.1016/j.carbpol.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 123.Kootstra A.M.J., Beeftink H.H., Scott E.L., Sanders J.P.M. Comparison of dilute mineral and organic acid pretreatment for enzymatic hydrolysis of wheat straw. Biochem. Eng. J. 2009;46:126–131. [Google Scholar]
- 124.Zheng D., Zhang Y., Guo Y., Yue J. Isolation and characterization of nanocellulose with a novel shape from walnut (Juglans Regia L.) shell agricultural waste. Polymers (Basel) 2019;11 doi: 10.3390/polym11071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Watkins D., Nuruddin M., Hosur M., Tcherbi-Narteh A., Jeelani S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015;4:26–32. [Google Scholar]
- 126.Ditzel F.I., Prestes E., Carvalho B.M., Demiate I.M., Pinheiro L.A. Nanocrystalline cellulose extracted from pine wood and corncob. Carbohydr. Polym. 2017;157:1577–1585. doi: 10.1016/j.carbpol.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 127.Sabaruddin F.A., Paridah M.T., Sapuan S.M., Ilyas R.A., Lee S.H., Abdan K., Mazlan N., Roseley A.S.M., Abdul Khalil H.P.S. The effects of unbleached and bleached nanocellulose on the thermal and flammability of polypropylene-reinforced kenaf core hybrid polymer bionanocomposites. Polymers (Basel) 2020;13 doi: 10.3390/polym13010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trilokesh C., Uppuluri K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019;9:16709. doi: 10.1038/s41598-019-53412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harini S., Mu'minul M., Purwanto P., Wijayanti D.A., Kuwat T. Proceedings of the 2014 International Conference on Physics. Atlantis Press; 2014. Nanocrystalline Cellulose Studied With a Conventional SEM; pp. 12–15. [Google Scholar]
- 130.Ismail K.I., Sabri M.H., Yusra M.A. Extraction of cellulose nano crystalline from cotton by ultrasonic and its morphological and structural characterization. Int. J. Mater. Chem. Phys. 2015;1:99–109. [Google Scholar]
- 131.Lukmanul H.Z., Mehdi J., Paridah M.T., Sameneh K. Isolation and characterization of cellulose whiskers from kenaf (Hibiscus cannabinus L.) bast fibers. J. Biomater. Nanobiotechnol. 2013;4:37–44. [Google Scholar]
- 132.Bhavana V.M., Rahul K.S., Satish V.P. Study on the drug loading and release potential of bacterial cellulose. Cellul. Chem. Technol. 2016;50:219–223. [Google Scholar]
- 133.Jacob S., Nair A.B., Shah J., Sreeharsha N., Gupta S., Shinu P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li J., Zhang X., Zhang M., Xiu H., He H. Ultrasonic enhance acid hydrolysis selectivity of cellulose with HCl-FeCl3 as catalyst. Carbohydr. Polym. 2015;117:917–922. doi: 10.1016/j.carbpol.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 135.Louis A.C.F., Venkatachalam S. Energy efficient process for valorization of corn cob as a source for nanocrystalline cellulose and hemicellulose production. Int. J. Biol. Macromol. 2020;163:260–269. doi: 10.1016/j.ijbiomac.2020.06.276. [DOI] [PubMed] [Google Scholar]
- 136.Tang Y., Yang S., Zhang N., Zhang J. Preparation and characterization of nanocrystalline cellulose via low-intensity ultrasonic-assisted sulfuric acid hydrolysis. Cellulose. 2013;21:335–346. [Google Scholar]
- 137.Cui S., Zhang S., Ge S., Xiong L., Sun Q. Green preparation and characterization of size-controlled nanocrystalline cellulose via ultrasonic-assisted enzymatic hydrolysis. Ind. Crops Prod. 2016;83:346–352. [Google Scholar]
- 138.Sharifah B.A.H., Chowdhury Z.Z., Karim M.Z., Ali M.E. Catalytic isolation and physicochemical properties of nanocrystalline cellulose (NCC) using HCl-FeCl3 system combined with ultrasonication. BioResources. 2016;11:3840–3855. [Google Scholar]
- 139.Shahabi-Ghahafarrokhi I., Khodaiyan F., Mousavi M., Yousefi H. Preparation and characterization of nanocellulose from beer industrial residues using acid hydrolysis/ultrasound. Fibers Polym. 2015;16:529–536. [Google Scholar]
- 140.Zianor Azrina Z.A., Beg M.D.H., Rosli M.Y., Ramli R., Junadi N., Alam A. Spherical nanocrystalline cellulose (NCC) from oil palm empty fruit bunch pulp via ultrasound assisted hydrolysis. Carbohydr. Polym. 2017;162:115–120. doi: 10.1016/j.carbpol.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 141.Meng F., Wang G., Du X., Wang Z., Xu S., Zhang Y. Extraction and characterization of cellulose nanofibers and nanocrystals from liquefied banana pseudo-stem residue. Compos. B Eng. 2019;160:341–347. [Google Scholar]
- 142.Pandi N., Sonawane S.H., Anand Kishore K. Synthesis of cellulose nanocrystals (CNCs) from cotton using ultrasound-assisted acid hydrolysis. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ni Y., Li J., Fan L. Effects of ultrasonic conditions on the interfacial property and emulsifying property of cellulose nanoparticles from ginkgo seed shells. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barbash V.A., Yashchenko O.V., Yakymenko O.S., Zakharko R.M. Extraction of organosolv pulp and production of nanocellulose from hemp fibers. KPI Science News. 2021:83–90. [Google Scholar]
- 145.Mishra S.P., Manent A.-S., Chabot B., Daneault C. Production of nanocellulose from native cellulose - various options utilizing ultrasound. BioResources. 2012;7:422–436. [Google Scholar]
- 146.Rattaz A., Mishra S.P., Chabot B., Daneault C. Cellulose nanofibres by sonocatalysed-TEMPO-oxidation. Cellulose. 2011;18:585–593. [Google Scholar]
- 147.H. Kargarzadeh, M. Loelovich, I. Ahmad, S.G. Thoma, A. Dufresne, Methods for Extraction of Nanocellulose from Various Sources, in: H. Kargarzadeh, I. Ahmad, S.G. Thoma, A. Dufresne (Eds.) Handbook of Nanocellulose and Cellulose Nanocomposites, 2017, pp. 1–49.
- 148.Isogai A., Saito T., Fukuzumi H. TEMPO-oxidized cellulose nanofibers. Nanoscale. 2011;3:71–85. doi: 10.1039/c0nr00583e. [DOI] [PubMed] [Google Scholar]
- 149.Isogai A. Development of completely dispersed cellulose nanofibers. Proc Jpn Acad Ser B Phys Biol Sci. 2018;94:161–179. doi: 10.2183/pjab.94.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sang X., Qin C., Tong Z., Kong S., Jia Z., Wan G., Liu X. Mechanism and kinetics studies of carboxyl group formation on the surface of cellulose fiber in a TEMPO-mediated system. Cellulose. 2017;24:2415–2425. [Google Scholar]
- 151.Tang Z., Li W., Lin X., Xiao H., Miao Q., Huang L., Chen L., Wu H. TEMPO-oxidized cellulose with high degree of oxidation. Polymers (Basel) 2017;9 doi: 10.3390/polym9090421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Johnson R.K., Zink-Sharp A., Renneckar S.H., Glasser W.G. A new bio-based nanocomposite: fibrillated TEMPO-oxidized celluloses in hydroxypropylcellulose matrix. Cellulose. 2008;16:227–238. [Google Scholar]
- 153.Saito T., Kimura S., Nishiyama Y., Isogai A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules. 2007;8:2485–2491. doi: 10.1021/bm0703970. [DOI] [PubMed] [Google Scholar]
- 154.Mandal A., Chakrabarty D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011;86:1291–1299. [Google Scholar]
- 155.Suslick K.S., Choe S.-B., Cichowlas A.A., Grinstaff M.W. Sonochemical synthesis of amorphous iron. Nature. 1991;353:414–416. [Google Scholar]
- 156.Suslick K.S., Price G.J. Applications of ultrasound to materials chemistry. Annu. Rev. Mater. Sci. 1999;29:295–326. [Google Scholar]
- 157.Liu C., Li B., Du H., Lv D., Zhang Y., Yu G., Mu X., Peng H. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr. Polym. 2016;151:716–724. doi: 10.1016/j.carbpol.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 158.Alila S., Besbes I., Vilar M.R., Mutjé P., Boufi S. Non-woody plants as raw materials for production of microfibrillated cellulose (MFC): A comparative study. Ind. Crops Prod. 2013;41:250–259. [Google Scholar]
- 159.Zhou Y., Saito T., Bergstrom L., Isogai A. Acid-free preparation of cellulose nanocrystals by TEMPO oxidation and subsequent cavitation. Biomacromolecules. 2018;19:633–639. doi: 10.1021/acs.biomac.7b01730. [DOI] [PubMed] [Google Scholar]
- 160.Rohaizu R., Wanrosli W.D. Sono-assisted TEMPO oxidation of oil palm lignocellulosic biomass for isolation of nanocrystalline cellulose. Ultrason. Sonochem. 2017;34:631–639. doi: 10.1016/j.ultsonch.2016.06.040. [DOI] [PubMed] [Google Scholar]
- 161.Abdul Khalil H.P., Davoudpour Y., Islam M.N., Mustapha A., Sudesh K., Dungani R., Jawaid M. Production and modification of nanofibrillated cellulose using various mechanical processes: a review. Carbohydr. Polym. 2014;99:649–665. doi: 10.1016/j.carbpol.2013.08.069. [DOI] [PubMed] [Google Scholar]
- 162.Huerta R.R., Silva E.K., Ekaette I., El-Bialy T., Saldana M.D.A. High-intensity ultrasound-assisted formation of cellulose nanofiber scaffold with low and high lignin content and their cytocompatibility with gingival fibroblast cells. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2019.104759. [DOI] [PubMed] [Google Scholar]
- 163.Wang Q.Q., Zhu J.Y., Gleisner R., Kuster T.A., Baxa U., McNeil S.E. Morphological development of cellulose fibrils of a bleached eucalyptus pulp by mechanical fibrillation. Cellulose. 2012;19:1631–1643. [Google Scholar]
- 164.Barbash V.A., Yashchenko O.V., Gondovska A.S., Deykun I.M. Preparation and characterization of nanocellulose obtained by TEMPO-mediated oxidation of organosolv pulp from reed stalks. Appl. Nanosci. 2021 [Google Scholar]
- 165.Gong J., Mo L., Li J. A comparative study on the preparation and characterization of cellulose nanocrystals with various polymorphs. Carbohydr. Polym. 2018;195:18–28. doi: 10.1016/j.carbpol.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 166.Koshani R., van de Ven T.G.M., Madadlou A. Characterization of carboxylated cellulose nanocrytals isolated through catalyst-assisted H2O2 oxidation in a one-step procedure. J. Agric. Food Chem. 2018;66:7692–7700. doi: 10.1021/acs.jafc.8b00080. [DOI] [PubMed] [Google Scholar]
- 167.Zhang K., Sun P., Liu H., Shang S., Song J., Wang D. Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr. Polym. 2016;138:237–243. doi: 10.1016/j.carbpol.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 168.Loranger E., Jradi K., Daneault C. IEEE International Ultrasonics Symposium. IEEE; Dresden, Germany: 2013. Nanocellulose production by ultrasound-assisted TEMPO oxidation of kraft pulp on laboratory and pilot scales. [Google Scholar]
- 169.Ovalle-Serrano S.A., Gomez F.N., Blanco-Tirado C., Combariza M.Y. Isolation and characterization of cellulose nanofibrils from Colombian Fique decortication by-products. Carbohydr. Polym. 2018;189:169–177. doi: 10.1016/j.carbpol.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 170.Cheng M., Qin Z., Chen Y., Liu J., Ren Z. Facile one-step extraction and oxidative carboxylation of cellulose nanocrystals through hydrothermal reaction by using mixed inorganic acids. Cellulose. 2017;24:3243–3254. [Google Scholar]
- 171.Mishra S.P., Manent A.-S., Chabot B., Daneault C. The use of sodium chlorite in post-oxidation of TEMPO-oxidized pulp: effect on pulp characteristics and nanocellulose yield. J. Wood Chem. Technol. 2012;32:137–148. [Google Scholar]
- 172.Mishra S.P., Manent A.-S., Chabot B., Daneault C. Production of nanocellulose from native cellulose – various options utilizing ultrasound. BioResources. 2012;7:422–436. [Google Scholar]
- 173.Fujisawa S., Okita Y., Fukuzumi H., Saito T., Isogai A. Preparation and characterization of TEMPO-oxidized cellulose nanofibril films with free carboxyl groups. Carbohydr. Polym. 2011;84:579–583. [Google Scholar]
- 174.Wicksono R., Syamsu K., Yuliasih I., Nasir M. Cellulose nanofibers from cassava bagasse characterization and application on tapioca-film. Chem. Mater. Res. 2013;3:79–87. [Google Scholar]
- 175.Czaikoski A., da Cunha R.L., Menegalli F.C. Rheological behavior of cellulose nanofibers from cassava peel obtained by combination of chemical and physical processes. Carbohydr. Polym. 2020;248 doi: 10.1016/j.carbpol.2020.116744. [DOI] [PubMed] [Google Scholar]
- 176.Besbes I., Alila S., Boufi S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011;84:975–983. [Google Scholar]
- 177.Wu J., Du X., Yin Z., Xu S., Xu S., Zhang Y. Preparation and characterization of cellulose nanofibrils from coconut coir fibers and their reinforcements in biodegradable composite films. Carbohydr. Polym. 2019;211:49–56. doi: 10.1016/j.carbpol.2019.01.093. [DOI] [PubMed] [Google Scholar]
- 178.Nechyporchuk O., Pignon F., Belgacem M.N. Morphological properties of nanofibrillated cellulose produced using wet grinding as an ultimate fibrillation process. J. Mater. Sci. 2014;50:531–541. [Google Scholar]
- 179.Satyamurthy P., Jain P., Balasubramanya R.H., Vigneshwaran N. Preparation and characterization of cellulose nanowhiskers from cotton fibres by controlled microbial hydrolysis. Carbohydr. Polym. 2011;83:122–129. [Google Scholar]
- 180.Ribeiro R.S.A., Pohlmann B.C., Calado V., Bojorge N., Pereira N., Jr. Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng. Life Sci. 2019;19:279–291. doi: 10.1002/elsc.201800158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dai J., Chae M., Beyene D., Danumah C., Tosto F., Bressler D.C. Co-Production of cellulose nanocrystals and fermentable sugars assisted by endoglucanase treatment of wood pulp. Materials (Basel) 2018;11 doi: 10.3390/ma11091645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Luo X., Liu J., Zheng P., Li M., Zhou Y., Huang L., Chen L., Shuai L. Promoting enzymatic hydrolysis of lignocellulosic biomass by inexpensive soy protein. Biotechnol. Biofuels. 2019;12:51. doi: 10.1186/s13068-019-1387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kong-Win Chang J., Duret X., Berberi V., Zahedi-Niaki H., Lavoie J.M. Two-step thermochemical cellulose hydrolysis with partial neutralization for glucose production. Front. Chem. 2018;6:117. doi: 10.3389/fchem.2018.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Olkiewicz M., Tylkowski B., Montornés J.M., Garcia-Valls R., Gulaczyk I. Modelling of enzyme kinetics: cellulose enzymatic hydrolysis case. Physical Sciences Reviews. 2020:1–39. [Google Scholar]
- 185.A.d.A. Guilherme, P.V.F. Dantas, J.C.J. Soares, E.S.d. Santos, F.A.N. Fernandes, G.R.d. Macedo, Pretreatments and enzymatic hydrolysis of sugarcane bagasse aiming at the enhancement of the yield of glucose and xylose, Brazilian Journal of Chemical Engineering, 34 (2017) 937-947.
- 186.Borah A.J., Agarwal M., Poudyal M., Goyal A., Moholkar V.S. Mechanistic investigation in ultrasound induced enhancement of enzymatic hydrolysis of invasive biomass species. Bioresour. Technol. 2016;213:342–349. doi: 10.1016/j.biortech.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 187.Berlin A., Gilkes N., Kilburn D., Bura R., Markov A., Skomarovsky A., Okunev O., Gusakov A., Maximenko V., Gregg D., Sinitsyn A., Saddler J. Evaluation of novel fungal cellulase preparations for ability to hydrolyze softwood substrates – evidence for the role of accessory enzymes. Enzyme Microb. Technol. 2005;37:175–184. [Google Scholar]
- 188.Harun R., Danquah M.K. Enzymatic hydrolysis of microalgal biomass for bioethanol production. Chem. Eng. J. 2011;168:1079–1084. [Google Scholar]
- 189.Shutova V.V., Yusipovich A.I., Parshina E.Y., Zakharkin D.O., Revin V.V. Effect of particle size on the enzymatic hydrolysis of polysaccharides from ultrafine lignocellulose particles. Appl. Biochem. Microbiol. 2012;48:312–317. [PubMed] [Google Scholar]
- 190.Inoue H., Yano S., Endo T., Sakaki T., Sawayama S. Combining hot-compressed water and ball milling pretreatments to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Biotechnol. Biofuels. 2008;1:2. doi: 10.1186/1754-6834-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Menon V., Rao M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012;38:522–550. [Google Scholar]
- 192.Squinca P., Bilatto S., Badino A.C., Farinas C.S. Nanocellulose Production in Future Biorefineries: An Integrated Approach Using Tailor-Made Enzymes. ACS Sustainable Chem. Eng. 2020;8:2277–2286. [Google Scholar]
- 193.Pirich C.L., Picheth G.F., Fontes A.M., Delgado-Aguilar M., Ramos L.P. Disruptive enzyme-based strategies to isolate nanocelluloses: a review. Cellulose. 2020;27:5457–5475. [Google Scholar]
- 194.Tang Y., Shen X., Zhang J., Guo D., Kong F., Zhang N. Extraction of cellulose nano-crystals from old corrugated container fiber using phosphoric acid and enzymatic hydrolysis followed by sonication. Carbohydr. Polym. 2015;125:360–366. doi: 10.1016/j.carbpol.2015.02.063. [DOI] [PubMed] [Google Scholar]
- 195.Zhang Q., Lu Z., Su C., Feng Z., Wang H., Yu J., Su W. High yielding, one-step mechano-enzymatic hydrolysis of cellulose to cellulose nanocrystals without bulk solvent. Bioresour. Technol. 2021;331 doi: 10.1016/j.biortech.2021.125015. [DOI] [PubMed] [Google Scholar]
- 196.Siqueira G.A., Dias I.K.R., Arantes V. Exploring the action of endoglucanases on bleached eucalyptus kraft pulp as potential catalyst for isolation of cellulose nanocrystals. Int. J. Biol. Macromol. 2019;133:1249–1259. doi: 10.1016/j.ijbiomac.2019.04.162. [DOI] [PubMed] [Google Scholar]
- 197.Beck-Candanedo S., Roman M., Gray D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules. 2005;6:1048–1054. doi: 10.1021/bm049300p. [DOI] [PubMed] [Google Scholar]
- 198.Wang Q.Q., Zhu J.Y., Reiner R.S., Verrill S.P., Baxa U., McNeil S.E. Approaching zero cellulose loss in cellulose nanocrystal (CNC) production: recovery and characterization of cellulosic solid residues (CSR) and CNC. Cellulose. 2012;19:2033–2047. [Google Scholar]
- 199.Paakko M., Ankerfors M., Kosonen H., Nykanen A., Ahola S., Osterberg M., Ruokolainen J., Laine J., Larsson P.T., Ikkala O., Lindstrom T. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules. 2007;8:1934–1941. doi: 10.1021/bm061215p. [DOI] [PubMed] [Google Scholar]
- 200.de Aguiar J., Bondancia T.J., Claro P.I.C., Mattoso L.H.C., Farinas C.S., Marconcini J.M. Enzymatic deconstruction of sugarcane bagasse and straw to obtain cellulose nanomaterials. ACS Sustainable Chem. Eng. 2020;8:2287–2299. [Google Scholar]
- 201.Nguyen T., Le V. Effects of ultrasound on cellulolytic activity of cellulase complex. Int. Food Res. J. 2013;20:557–563. [Google Scholar]
- 202.Hu R., Min Z., Adhikari B., Liu Y. Effect of homogenization and ultrasonication on the physical properties of insoluble wheat bran fibres. Int. Agrophys. 2015;29:423–432. [Google Scholar]
- 203.Chen W., Li Q., Wang Y., Yi X., Zeng J., Yu H., Liu Y., Li J. Comparative study of aerogels obtained from differently prepared nanocellulose fibers. ChemSusChem. 2014;7:154–161. doi: 10.1002/cssc.201300950. [DOI] [PubMed] [Google Scholar]
- 204.Kentish S., Feng H. Applications of power ultrasound in food processing. Annual Rev. Food Sci. Technol. 2014;5:263–284. doi: 10.1146/annurev-food-030212-182537. [DOI] [PubMed] [Google Scholar]
- 205.Szabo O.E., Csiszar E. Some factors affecting efficiency of the ultrasound-aided enzymatic hydrolysis of cotton cellulose. Carbohydr. Polym. 2017;156:357–363. doi: 10.1016/j.carbpol.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 206.Beltramino F., Blanca Roncero M., Vidal T., Valls C. A novel enzymatic approach to nanocrystalline cellulose preparation. Carbohydr. Polym. 2018;189:39–47. doi: 10.1016/j.carbpol.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 207.Chen X.Q., Pang G.X., Shen W.H., Tong X., Jia M.Y. Preparation and characterization of the ribbon-like cellulose nanocrystals by the cellulase enzymolysis of cotton pulp fibers. Carbohydr. Polym. 2019;207:713–719. doi: 10.1016/j.carbpol.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 208.Yang T., Guo Y., Gao N., Li X., Zhao J. Modification of a cellulase system by engineering Penicillium oxalicum to produce cellulose nanocrystal. Carbohydr. Polym. 2020;234 doi: 10.1016/j.carbpol.2020.115862. [DOI] [PubMed] [Google Scholar]
- 209.Zhang Y., Chen J., Zhang L., Zhan P., Liu N., Wu Z. Preparation of nanocellulose from steam exploded poplar wood by enzymolysis assisted sonication. Mater. Res. Express. 2020;7 [Google Scholar]
- 210.Luo J., Fang Z., Smith R.L. Ultrasound-enhanced conversion of biomass to biofuels. Prog. Energy Combust. Sci. 2014;41:56–93. [Google Scholar]
- 211.S.P. Mishra, A.-S. Manent, B. Chabot, C. Daneault, Production of nanocellulose from native cellulose - various option utilizing ultrasound, 2011, 7 (2011) 15.
- 212.Hamid S.B.A., Zain S.K., Das R., Centi G. Synergic effect of tungstophosphoric acid and sonication for rapid synthesis of crystalline nanocellulose. Carbohydr. Polym. 2016;138:349–355. doi: 10.1016/j.carbpol.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 213.Csiszar E., Kalic P., Kobol A., Ferreira E.d.P. The effect of low frequency ultrasound on the production and properties of nanocrystalline cellulose suspensions and films. Ultrason. Sonochem. 2016;31:473–480. doi: 10.1016/j.ultsonch.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 214.J. Lewandowska-Łańcucka, A. Karewicz, K. Wolski, S. Zapotoczny, Surface Functionalization of Nanocellulose-Based Hydrogels, in: Cellulose-Based Superabsorbent Hydrogels, 2018, pp. 1-29.
- 215.Trache D., Tarchoun A.F., Derradji M., Hamidon T.S., Masruchin N., Brosse N., Hussin M.H. Nanocellulose: from fundamentals to advanced applications. Front. Chem. 2020;8:392. doi: 10.3389/fchem.2020.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Habibi Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014;43:1519–1542. doi: 10.1039/c3cs60204d. [DOI] [PubMed] [Google Scholar]
- 217.Islam M.T., Alam M.M., Zoccola M. Review on modification of nanocellulose for application in composites. Int. J. Innov. Res. Sci. Eng. Technol. 2013;2:9. [Google Scholar]
- 218.Al-Dulaimi A.A., Wanrosli W.D. Isolation and characterization of nanocrystalline cellulose from totally chlorine free oil palm empty fruit bunch pulp. J. Polym. Environ. 2016;25:192–202. [Google Scholar]
- 219.Abdul Amir H.F., Lim Y.H., Chew I.M.L., Choong T.S.Y., Tan M.C., Tan K.W., Korsunsky A.M., Guo Z. NanoCrystalline cellulose isolated from oil palm empty fruit bunch and its potential in cadmium metal removal. MATEC Web Conf. 2016;59 [Google Scholar]
- 220.Foo M., Chien Wei O., Tan K.W., Chew I. A step closer to sustainable industrial production: tailor the properties of nanocrystalline cellulose from oil palm empty fruit bunch. J. Environ. Chem. Eng. 2020;8 [Google Scholar]
- 221.Foo M., Tan C., Lim P., Ooi E., Tan K., Chew I. Surface-modified nanocrystalline cellulose from oil palm empty fruit bunch for effective binding of curcumin. Int. J. Biol. Macromol. 2019;138 doi: 10.1016/j.ijbiomac.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 222.Banerjee M., Saraswatula S., Willows L.G., Woods H., Brettmann B. Pharmaceutical crystallization in surface-modified nanocellulose organogels. J. Mater. Chem. B. 2018;6:7317–7328. doi: 10.1039/c8tb01554f. [DOI] [PubMed] [Google Scholar]
- 223.Robles E., Urruzola I., Labidi J., Serrano L. Surface-modified nano-cellulose as reinforcement in poly(lactic acid) to conform new composites. Ind. Crops Prod. 2015;71:44–53. [Google Scholar]
- 224.Singh S., Dhakar G.L., Kapgate B.P., Maji P.K., Verma C., Chhajed M., Rajkumar K., Das C. Synthesis and chemical modification of crystalline nanocellulose to reinforce natural rubber composites. Polym. Adv. Technol. 2020;31:3059–3069. [Google Scholar]
- 225.Salajková M. Department of Fibre and Polymer Technology, Royal Institute of Technology; KTH, Stockholm, Sweden: 2012. Nanocelluloses – surface modification and use in functional materials. [Google Scholar]
- 226.Balea A., Merayo N., De La Fuente E., Negro C., Blanco Á. Assessing the influence of refining, bleaching and TEMPO-mediated oxidation on the production of more sustainable cellulose nanofibers and their application as paper additives. Ind. Crops Prod. 2017;97:374–387. [Google Scholar]
- 227.Zaman M., Xiao H., Chibante F., Ni Y. Synthesis and characterization of cationically modified nanocrystalline cellulose. Carbohydr. Polym. 2012;89:163–170. doi: 10.1016/j.carbpol.2012.02.066. [DOI] [PubMed] [Google Scholar]
- 228.Wei L., Agarwal U., Hirth K., Matuana L., Sabo R., Stark N. Chemical modification of nanocellulose with canola oil fatty acid methyl ester. Carbohydr. Polym. 2017;169 doi: 10.1016/j.carbpol.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 229.Dhuiège B., Pecastaings G., Sèbe G. Sustainable approach for the direct functionalization of cellulose nanocrystals dispersed in water by transesterification of vinyl acetate. ACS Sustainable Chem. Eng. 2019;7:187–196. [Google Scholar]
- 230.Goussé C., Chanzy H., Excoffier G., Soubeyrand L., Fleury E. Stable suspensions of partially silylated cellulose whiskers dispersed in organic solvents. Polymer. 2002;43:2645–2651. [Google Scholar]
- 231.Yu H.-Y., Chen R., Chen G.-Y., Liu L., Yang X.-G., Yao J.-M. Silylation of cellulose nanocrystals and their reinforcement of commercial silicone rubber. J. Nanopart. Res. 2015;17:361. [Google Scholar]
- 232.Frank B.P., Smith C., Caudill E.R., Lankone R.S., Carlin K., Benware S., Pedersen J.A., Fairbrother D.H. Biodegradation of functionalized nanocellulose. Environ. Sci. Technol. 2021;55:10744–10757. doi: 10.1021/acs.est.0c07253. [DOI] [PubMed] [Google Scholar]
- 233.Gu H., Gao X., Zhang H., Chen K., Peng L. Fabrication and characterization of cellulose nanoparticles from maize stalk pith via ultrasonic-mediated cationic etherification. Ultrason. Sonochem. 2020;66 doi: 10.1016/j.ultsonch.2019.104932. [DOI] [PubMed] [Google Scholar]
- 234.Xu Q., Poggi G., Resta C., Baglioni M., Baglioni P. Grafted nanocellulose and alkaline nanoparticles for the strengthening and deacidification of cellulosic artworks. J. Colloid Interface Sci. 2020;576:147–157. doi: 10.1016/j.jcis.2020.05.018. [DOI] [PubMed] [Google Scholar]
- 235.Chatterjee R., Sajjadi B., Mattern D.L., Chen W.-Y., Zubatiuk T., Leszczynska D., Leszczynski J., Egiebor N.O., Hammer N. Ultrasound cavitation intensified amine functionalization: A feasible strategy for enhancing CO2 capture capacity of biochar. Fuel. 2018;225:287–298. [Google Scholar]
- 236.Abral H., Lawrensius V., Handayani D., Sugiarti E. Preparation of nano-sized particles from bacterial cellulose using ultrasonication and their characterization. Carbohydr. Polym. 2018;191:161–167. doi: 10.1016/j.carbpol.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 237.Frone A.N., Panaitescu D.M., Donescu D., Spataru C.I., Radovici C., Trusca R., Somoghi R. Preparation and characterization of PVA composites with cellulose nanofibers obtained by ultrasonication. BioResources. 2010;6:487–512. [Google Scholar]
- 238.Vizireanu S., Panaitescu D.M., Nicolae C.A., Frone A.N., Chiulan I., Ionita M.D., Satulu V., Carpen L.G., Petrescu S., Birjega R., Dinescu G. Cellulose defibrillation and functionalization by plasma in liquid treatment. Sci. Rep. 2018;8:15473. doi: 10.1038/s41598-018-33687-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sumari S., Surnarno S., Roesyadi A. Effects of ultrasound on the morphology, particle size, crystallinity and crystallite size of cellulose. Sci. Study Res.: Chem. Chem. Eng. 2013;14:229–239. [Google Scholar]
- 240.Hu Z., Zhai R., Li J., Zhang Y., Lin J. Preparation and characterization of nanofibrillated cellulose from bamboo fiber via ultrasonication assisted by repulsive effect. Int. J. Polymer Sci. 2017;2017:1–9. [Google Scholar]
- 241.Li W., Yue J., Liu S. Preparation of nanocrystalline cellulose via ultrasound and its reinforcement capability for poly(vinyl alcohol) composites. Ultrason. Sonochem. 2012;19:479–485. doi: 10.1016/j.ultsonch.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 242.Subhedar P.B., Gogate P.R. Alkaline and ultrasound assisted alkaline pretreatment for intensification of delignification process from sustainable raw-material. Ultrason. Sonochem. 2014;21:216–225. doi: 10.1016/j.ultsonch.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 243.Sutkar V.S., Gogate P.R. Design aspects of sonochemical reactors: Techniques for understanding cavitational activity distribution and effect of operating parameters. Chem. Eng. J. 2009;155:26–36. [Google Scholar]
- 244.Szabo O.E., Csiszar E. The effect of low-frequency ultrasound on the activity and efficiency of a commercial cellulase enzyme. Carbohydr. Polym. 2013;98:1483–1489. doi: 10.1016/j.carbpol.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 245.Yaldagard M., Mortazavi S.A., Tabatabaie F. The effect of ultrasound in combination with thermal treatment on the germinated barley’s alpha-amylase activity. Korean J. Chem. Eng. 2008;25:517–523. [Google Scholar]
- 246.Martins M., Azoia N., Silva C., Cavaco-Paulo A. Stabilization of enzymes in micro-emulsions for ultrasound processes. Biochem. Eng. J. 2014;93 [Google Scholar]
- 247.Basto C., Tzanov T., Cavaco-Paulo A. Combined ultrasound-laccase assisted bleaching of cotton. Ultrason. Sonochem. 2007;14:350–354. doi: 10.1016/j.ultsonch.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 248.Huang G., Chen S., Dai C., Sun L., Sun W., Tang Y., Xiong F., He R., Ma H. Effects of ultrasound on microbial growth and enzyme activity. Ultrason. Sonochem. 2017;37:144–149. doi: 10.1016/j.ultsonch.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 249.Pan Y., Chen L., Pang L., Chen X., Jia X., Li X. Ultrasound treatment inhibits browning and improves antioxidant capacity of fresh-cut sweet potato during cold storage. RSC Adv. 2020;10:9193–9202. doi: 10.1039/c9ra06418d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Potapovich M.V., Eremin A.N., Metelitsa D.I. Ultrasonic and thermal inactivation of catalases from the bovine liver, the methylotrophic yeast Pichia pastoris, and the fungus Penicillium piceum. Prikl. Biokhim. Mikrobiol. 2005;41:603–611. [PubMed] [Google Scholar]
- 251.Eijsink V.G., Bjørk A., Gåseidnes S., Sirevåg R., Synstad B., van den Burg B., Vriend G. Rational engineering of enzyme stability. J. Biotechnol. 2004;113:105–120. doi: 10.1016/j.jbiotec.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 252.Li Q., McGinnis S., Sydnor C., Wong A., Renneckar S. Nanocellulose life cycle assessment. ACS Sustainable Chem. Eng. 2013;1:919–928. [Google Scholar]
- 253.Soontornchaiboon W., Kim S.M., Pawongrat R. Effects of alkaline combined with ultrasonic pretreatment and enzymatic hydrolysis of algricultural wastes for high reducing sugar production. Sains Malaysiana. 2016;45:955–962. [Google Scholar]
- 254.Vanderfleet O.M., Osorio D.A., Cranston E.D. Optimization of cellulose nanocrystal length and surface charge density through phosphoric acid hydrolysis. Philos Trans A Math Phys Eng Sci. 2018;376 doi: 10.1098/rsta.2017.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sharma P.R., Chattopadhyay A., Sharma S.K., Geng L., Amiralian N., Martin D., Hsiao B.S. Nanocellulose from spinifex as an effective adsorbent to remove cadmium(II) from water. ACS Sustainable Chem. Eng. 2018;6:3279–3290. [Google Scholar]
- 256.Onyianta A.J., Dorris M., Williams R.L. Aqueous morpholine pre-treatment in cellulose nanofibril (CNF) production: comparison with carboxymethylation and TEMPO oxidisation pre-treatment methods. Cellulose. 2017;25:1047–1064. [Google Scholar]
- 257.Rocha I., Ferraz N., Mihranyan A., Strømme M., Lindh J. Sulfonated nanocellulose beads as potential immunosorbents. Cellulose. 2018;25:1899–1910. [Google Scholar]
- 258.Laitinen O., Suopajarvi T., Osterberg M., Liimatainen H. Hydrophobic, superabsorbing aerogels from choline chloride-based deep eutectic solvent pretreated and silylated cellulose nanofibrils for selective oil removal. ACS Appl. Mater. Interfaces. 2017;9:25029–25037. doi: 10.1021/acsami.7b06304. [DOI] [PubMed] [Google Scholar]
- 259.E. Brännvall, The Limits of Delignification in Kraft Cooking, 2017, 12 (2017) 27.
- 260.Mahardika M., Abral H., Kasim A., Arief S., Asrofi M. Production of nanocellulose from pineapple leaf fibers via high-shear homogenization and ultrasonication. Fibers. 2018;6:28. [Google Scholar]
- 261.Wu C., McClements D.J., He M., Zheng L., Tian T., Teng F., Li Y. Preparation and characterization of okara nanocellulose fabricated using sonication or high-pressure homogenization treatments. Carbohydr. Polym. 2021;255 doi: 10.1016/j.carbpol.2020.117364. [DOI] [PubMed] [Google Scholar]
- 262.Chen P., Yu H., Liu Y., Chen W., Wang X., Ouyang M. Concentration effects on the isolation and dynamic rheological behavior of cellulose nanofibers via ultrasonic processing. Cellulose. 2012;20 [Google Scholar]
- 263.P. Dhankhar, Homogenization Fundamentals, in, 2014.
- 264.Casadonte D.J., Flores M., Petrier C. The use of pulsed ultrasound technology to improve environmental remediation: a comparative study. Environ. Technol. 2005;26:1411–1418. doi: 10.1080/09593332608618615. [DOI] [PubMed] [Google Scholar]
- 265.A. Patist, D. Bates, Industrial Applications of High Power Ultrasonics, in, 2010, pp. 599–616.
- 266.Siró I., Plackett D. Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose. 2010;17:459–494. [Google Scholar]
- 267.Bharimalla A.K., Deshmukh S.P., Patil P.G., Vigneshwaran N. Energy efficient manufacturing of nanocellulose by chemo- and bio-mechanical processes: a review. World J. Nano Sci. Eng. 2015;05:204–212. [Google Scholar]
- 268.Li W., Leong T.S.H., Ashokkumar M., Martin G.J.O. A study of the effectiveness and energy efficiency of ultrasonic emulsification. PCCP. 2018;20:86–96. doi: 10.1039/c7cp07133g. [DOI] [PubMed] [Google Scholar]
- 269.Spence K.L., Venditti R.A., Rojas O.J., Habibi Y., Pawlak J.J. A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose. 2011;18:1097–1111. [Google Scholar]
- 270.Pan Z., Qu W., Ma H., Atungulu G.G., McHugh T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2012;19:365–372. doi: 10.1016/j.ultsonch.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 271.Patience N.A., Schieppati D., Boffito D.C. Continuous and pulsed ultrasound pectin extraction from navel orange peels. Ultrason. Sonochem. 2021;73 doi: 10.1016/j.ultsonch.2021.105480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kobus Z., Krzywicka M., Pecyna A., Buczaj A. Process efficiency and energy consumption during the ultrasound-assisted extraction of bioactive substances from hawthorn berries. Energies. 2021;14:7638. [Google Scholar]
- 273.Kiss A., Geertman R., Wierschem M., Skiborowski M., Gielen B., Jordens J., John J., Van Gerven T. Ultrasound-assisted emerging technologies for chemical processes. J. Chem. Technol. Biotechnol. 2017;93 doi: 10.1002/jctb.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Transparency Market Research, Nanocrystalline cellulose market, in, 2018.
- 275.Fortune Business Insights, Market Research Report, in, 2021.
- 276.Industry ARC, Nanocellulose market - Forecast (2022-2027), in, Industry ARC, 2021.
- 277.Markets and Markets, Nanocellulose market, in, 2022.
- 278.Girard M., Vidal D., Bertrand F., Tavares J.R., Heuzey M.-C. Evidence-based guidelines for the ultrasonic dispersion of cellulose nanocrystals. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Gogate P.R., Pandit A.B. Sonochemical reactors: scale up aspects. Ultrason. Sonochem. 2004;11:105–117. doi: 10.1016/j.ultsonch.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 280.Dong Z., Delacour C., Mc Carogher K., Udepurkar A.P., Kuhn S. Continuous ultrasonic reactors: design, mechanism and application. Materials (Basel) 2020;13:344. doi: 10.3390/ma13020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Gogate P.R., Tatake P.A., Kanthale P.M., Pandit A.B. Mapping of sonochemical reactors: review, analysis, and experimental verification. AIChE J. 2002;48:1542–1560. [Google Scholar]
- 282.Asgharzadehahmadi S., Abdul Raman A.A., Parthasarathy R., Sajjadi B. Sonochemical reactors: Review on features, advantages and limitations. Renew. Sustain. Energy Rev. 2016;63:302–314. [Google Scholar]
- 283.G. Andaluri, Ultrasound induced destruction of emerging contaminants, in, 2011.
- 284.Thambi N., Singha P., Dwivedi M., Singh S. Hydrodynamic cavitation and its application in food and beverage industry: A review. J. Food Process Eng. 2019:e13144. [Google Scholar]
- 285.Mat-Shayuti M.S., Tuan Ya T.M.Y.S., Abdullah M.Z., Megat Khamaruddin P.N.F., Othman N.H. Progress in ultrasonic oil-contaminated sand cleaning: a fundamental review. Environ. Sci. Pollut. Res. 2019;26:26419–26438. doi: 10.1007/s11356-019-05954-w. [DOI] [PubMed] [Google Scholar]
- 286.A. Rudie, Commercialization of cellulose nanofibril (CNF) and cellulose nanocrystal (CNC): Pathway and challenges, in, 2017, pp. 761-797.
- 287.Nelson K., Retsina T., Iakovlev M., van Heiningen A., Deng Y., Shatkin J.A., Mulyadi A. In: Materials Research for MAnufacturing: An Industrial Perspective of Turning Materials Into New Products. Madsen L.D., Svedberg E.B., editors. Springer International Publishing; Cham: 2016. American process: production of low cost nanocellulose for renewable, advanced materials applications; pp. 267–302. [Google Scholar]
- 288.M.P. Andrews, T. Morse, Method for producing functionalized nanocrystalline cellulose and functionalized nanocrystalline cellulose thereby produced, in, Anomera Inc, 2016.
- 289.S. MCalpine, J. Koneshny, Production of crystalline cellulose, in, Nano-green Biorefineries Inc, 2017.
- 290.K. Nelson, T. Retsina, V. Pylkkanen, R.O. Connor, Processes and apparatus for producing nanocellulose, and compositions and products produced thereform, in, API Intellectual Property Holdings, 2015.
- 291.Miller J. TAPPI; 2018. Cellulose nanomaterials production update. [Google Scholar]
- 292.Nishihata J. McGrill; 2021. Anomera: a Spinoff in Full Flight. [Google Scholar]
- 293.F.M. Al-Oqla, M. Rababah, Challenges in design of nanocellulose and its composites for different applications, in: Cellulose-Reinforced Nanofibre Composites, 2017, pp. 113-127.
- 294.Topaz M. Possible long-term complications in ultrasound-assisted lipoplasty induced by sonoluminescence, sonochemistry, and thermal effect. Aesthet Surg J. 1998;18:19–24. doi: 10.1016/s1090-820x(98)80019-8. [DOI] [PubMed] [Google Scholar]
- 295.Clift M.J., Foster E.J., Vanhecke D., Studer D., Wick P., Gehr P., Rothen-Rutishauser B., Weder C. Investigating the interaction of cellulose nanofibers derived from cotton with a sophisticated 3D human lung cell coculture. Biomacromolecules. 2011;12:3666–3673. doi: 10.1021/bm200865j. [DOI] [PubMed] [Google Scholar]
- 296.H. Kargarzadeh, I. Ahmad, I.A. Thomas, A. Dufresne, Environmental Health and Safety of Cellulose Nanomaterials and Composites, Handbook of Nanocellulose and Cellulose Nanocomposites, (2017) 683–729.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors are unable or have chosen not to specify which data has been used.