Abstract
The Bacillus subtilis genome encodes two members of the Lon family of prokaryotic ATP-dependent proteases. One, LonA, is produced in response to temperature, osmotic, and oxidative stress and has also been implicated in preventing ςG activity under nonsporulation conditions. The second is encoded by the lonB gene, which resides immediately upstream from lonA. Here we report that transcription of lonB occurs during sporulation under ςF control and thus is restricted to the prespore compartment of sporulating cells. First, expression of a lonB-lacZ transcriptional fusion was abolished in strains unable to produce ςF but remained unaffected upon disruption of the genes encoding the early and late mother cell regulators ςE and ςK or the late forespore regulator ςG. Second, the fluorescence of strains harboring a lonB-gfp fusion was confined to the prespore compartment and depended on ςF production. Last, primer extension analysis of the lonB transcript revealed −10 and −35 sequences resembling the consensus sequence recognized by ςF-containing RNA polymerase. We further show that the lonB message accumulated as a single monocistronic transcript during sporulation, synthesis of which required ςF activity. Disruption of the lonB gene did not confer any discernible sporulation phenotype to otherwise wild-type cells, nor did expression of lonB from a multicopy plasmid. In contrast, expression of a fusion of the lonB promoter to the lonA gene severely reduced expression of the ςG-dependent sspE gene and the frequency of sporulation. In confirmation of earlier observations, we found elevated levels of ςF-dependent activity in a spoIIIE47 mutant, in which the lonB region of the chromosome is not translocated into the prespore. Expression of either lonB or the PlonB-lonA fusion from a plasmid in the spoIIIE47 mutant reduced ςF -dependent activity to wild-type levels. The results suggest that both LonA and LonB can prevent abnormally high ςF activity but that only LonA can negatively regulate ςG.
Sporulation in the rod-shaped bacterium Bacillus subtilis is initiated by an asymmetric division that produces a smaller prespore and a larger mother cell (11, 36, 49). Progress through the morphological stages of sporulation is governed by a cascade of four compartment-specific RNA polymerase sigma factors that appear in the order ςF, ςE, ςG, and ςK (11, 28, 49). The first compartment-specific sigma factor, ςF, initiates the prespore-specific program of gene expression and is replaced by ςG in this sporangial chamber at later stages of development (16, 19, 24, 29, 34, 49). Conversely, the mother cell-specific line of gene expression is initiated by the activation of ςE, which is later replaced by ςK (3, 4, 6, 60). ςF is synthesized prior to the formation of the sporulation septum, together with three other proteins, SpoIIAA, SpoIIAB, and SpoIIE, required for its prespore-specific activation (13, 14, 57). SpoIIAB is an anti-sigma factor that binds to ςF and holds it inactive in the predivisional cell and in the mother cell compartment of the sporulating cell (9, 31). SpoIIAA is an anti-anti-sigma factor, which can bind to and counteract SpoIIAB, releasing active ςF (1, 5, 8, 31). SpoIIAB is also a serine protein kinase that can phosphorylate SpoIIAA, and phosphorylated SpoIIAA is unable to bind to SpoIIAB (1, 5, 9, 31). The third protein, SpoIIE, is a membrane-bound serine phosphatase that can dephosphorylate SpoIIAA (7, 12). Dephosphorylation of SpoIIAA by the SpoIIE phosphatase occurs preferentially in the prespore chamber, promoting the binding of SpoIIAA to SpoIIAB and the prespore-specific activation of ςF (20, 25), which in turn leads to the synthesis of ςG in the prespore. However, ςG is kept in an inactive form until the engulfment stage of sporulation (stage III), presumably as the result of direct binding by the SpoIIAB anti-sigma factor (19, 21). Activation of ςG seems to require the proteolysis of SpoIIAB (19, 21). Once active, ςG transcribes its own gene, allowing a rapid increase in the cellular concentration of ςG. Because of its positive autoregulatory nature, ςG synthesis and activity are subject to multiple levels of control that prevent the expression of genes unnecessary or even deleterious for nonsporulating cells as well as the premature expression of the ςG regulon during development (19, 30, 38, 42, 43). For example, mutations in either the lonA gene, encoding a member of the Lon family of prokaryotic ATP-dependent serine proteases, or in spoIIAB permit inappropriate expression of ςG -dependent genes under conditions that do not promote sporulation (38, 42). The lonA gene is induced in response to several stresses, such as salt, ethanol, and oxidative stress or heat shock, but its precise role in stress management has not been determined (39). B. subtilis also possesses a second Lon-like protease that has been implicated in posttranslational regulation of ςH.
Since Lon proteases have already been shown to play a role in differentiation processes in other microorganisms (47, 52, 56), we decided to investigate their possible role in the regulation of compartment-specific gene expression during endospore development. We found lonB transcription itself to be compartmentalized during sporulation, dependent on ςF, and hence restricted to the forespore compartment. lonB did not seem to interfere with the activities of either ςF or ςG in a wild-type strain. In contrast, in confirmation and extension of earlier results, we show that lonA can act specifically to reduce ςG activity (but not that of ςF) when expressed in the forespore in an otherwise wild-type strain.
MATERIALS AND METHODS
Bacterial strains, media, and general methods.
Escherichia coli DH5α was used for routine cloning experiments. The B. subtilis strains used in this work are listed in Table 1. The wild-type strain MB24 (trpC2 metC3) and congenic derivatives bearing different spo alleles (Table 1) were used for the analysis of β-galactosidase production driven by various lacZ fusions. The efficiency of sporulation was determined 18 h after the onset of sporulation as described previously (18). Sporulation of B. subtilis was induced by growth and exhaustion in Difco sporulation medium (DSM) or by resuspension (2, 33). Antibiotics and 5-bromo-4-chloro-3-indolyl-β-galactosidase-d-galactopyranoside (X-Gal) were used as previously described (17, 18).
TABLE 1.
B. subtilis strains used
| Strain | Genotype and phenotype | Origin |
|---|---|---|
| MB24 | trpC2 metC3 | Laboratory stock |
| AH38 | leuA8 spoIIGB55 Spo− | Laboratory stock |
| AH45 | trpC2 metC3 spoIIIGΔ1 Spo− | Laboratory stock |
| AH77 | trpC2 metC3 ΔsigK::erm Spo− | Laboratory stock |
| AH392 | trpC2 metC3 ΔamyE::spoIID-lacZ Cmr | Laboratory stock |
| AH524 | trpC2 metC3 SPβsspE -lacZ Cmr | Laboratory stock |
| AH969 | trpC2 metC3 ΔamyE::spoIIIG-lacZ Cmr | Laboratory stock |
| AH2350 | trpC2 metC3 pMK3 Nmr | This work |
| AH2351 | trpC2 metC pMS56 Nmr | This work |
| AH2356 | trpC2 metC3 ΔamyE::lonB-lacZ Cmr | This work |
| AH2357 | trpC2 metC3 spoIIAC::erm Ermr | Laboratory stock |
| AH2358 | AH2357 ΔamyE::lonB-lacZ Cmr Ermr | This work |
| AH2359 | AH38 ΔamyE::lonB-lacZ Cmr | This work |
| AH2360 | AH45 ΔamyE::lonB-lacZ Cmr | This work |
| AH2361 | AH77 ΔamyE::lonB-lacZ Cmr | This work |
| AH2368 | trpC2 metC3 pMS72 Nmr | This work |
| AH2369 | AH2368 ΔamyE::spoIID-lacZ Nmr Cmr | This work |
| AH2370 | AH2368 SPβsspE-lacZ Nmr Cmr MLSr | This work |
| AH2372 | AH2350 ΔamyE::spoIID-lacZ Nmr Cmr | This work |
| AH2373 | AH2350 SPβsspE-lacZ Nmr Cmr MLSr | This work |
| AH2382 | spoIIIE47 ΔspoIIIG::spec ΔamyE::lonB- lacZ Spcr Cmr | This work |
| AH2383 | spoIIIE47 ΔspoIIIG::spec ΔamyE::spoIIIG- lacZ Spcr Cmr | This work |
| AH2385 | AH2382 pMK3 Spcr Cmr Nmr | This work |
| AH2386 | AH2382 pMS56 Spc Cmr Nmr | This work |
| AH2387 | AH2383 pMK3 Spcr Cmr Nmr | This work |
| AH2388 | AH2383 pMS56 Spcr Cmr Nmr | This work |
| AH2389 | AH2383 pMS72 Spcr Cmr Nmr | This work |
| AH2421 | AH2356 pMK3 Cmr Nmr | This work |
| AH2422 | AH2356 pMS72 Cmr Nmr | This work |
| AH2423 | AH969 pMK3 Cmr Nmr | This work |
| AH2424 | AH969 pMS72 Cmr Nmr | This work |
| AH2425 | AH2382 pMS72 Spcr Cmr Nmr | This work |
| AH2426 | AH2383 pMS76/ Spcr Cmr Nmr | This work |
| AH2427 | trpC2 metC3 lonB::spc pMS72 Spcr Nmr | This work |
| AH2433 | trpC2 metC3 pMS76 Nmr | This work |
| AH2434 | trpC2 metC3 pMS94 Nmr | This work |
| AH2435 | trpC2 metC3 lonB::lonB-lacZ Cmr | This work |
| AH2436 | trpC2 metC3 spoIIAC::erm lonB::lonB-lacZ Ermr Cmr | This work |
| BSM105 | trpC pheA lonB::spec Spcr | This work |
| BSM110 | trpC pheA lonB::pSH4 Cmr | This work |
| BSM111 | trpC pheA spoIIAC::erm lonB::pSH4 Cmr Ermr | This work |
| JH642 | trpC pheA | Laboratory stock |
| MO512 | trpC pheA spoIIGB::erm Ermr | P. Stragier |
| MO1073 | trpC pheA spoIIAC::erm Ermr | P. Stragier |
Construction of a lonB insertional mutation.
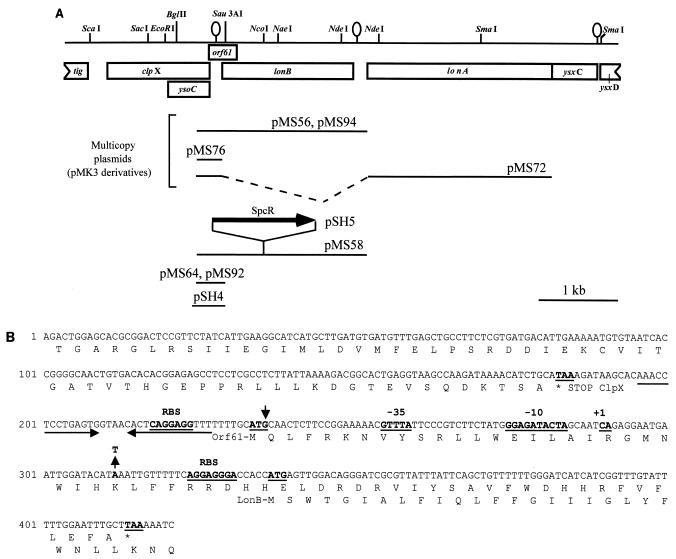
A 2,090-bp DNA fragment containing the entire lonB coding sequence as well as 336 bp upstream of its start codon was generated by high-fidelity PCR with oligonucleotides lonB-61D (5′-CGCAAGACTGCAGCACGCGGACTCCG-3′) and lonB-2151R (5′-TAAAACAGTCTCCTGCAGTAGTATACCC-3′). The amplified product was purified, doubly digested with XhoI and BglII, and cloned between the SalI and BamHI sites of pLITMUS 38 (New England Biolabs), yielding plasmid pMS58 (Fig. 1). Next, a spectinomycin resistance (Spcr) determinant was obtained by PCR with pAH256 (17) as the template and the primers pAH256-sf2 (5′-CGAATCCATGGCGCGCACCGTACGTC-3′) and pAH256-sr2 (5′-GAGACGTCACCATGGGAAGC-3′). After digestion with NcoI, the Spcr cassette was inserted at the unique NcoI site within the lonB gene of pMS58, a step that produced pSH5. Competent cells of strain JH642 were transformed with ScaI-linearized pSH5, with selection for Spcr cells. This cross-generated the lonB insertional mutant BSM105, which was shown by Southern blot hybridization to result from the integration of the plasmid into the chromosomal lonB region by a double-crossover (marker replacement) event.
FIG. 1.
lonB region of the B. subtilis chromosome and sequence of the lonB promoter region. (A) Partial restriction map and genetic organization of the lonB locus. The boxes below the restriction map indicate coding regions of the different genes in the region, as deduced from the analysis of the B. subtilis genome sequence (22). Note that orf61 was not considered in the annotation of the B. subtilis genome sequence but has previously been suggested by Liu et al. (26). The stem-loop structures downstream of clpX and downstream of lonB and of lonA indicate the positions of possible transcription terminators. Lines below the restriction map depict DNA fragments cloned into the indicated plasmids. In pMS72, the dashed region was deleted to fuse the lonB promoter to the start codon of the lonA gene. Plasmid pMS94 carries the same insert as pMS56 but has a nonsense mutation at codon 27 of orf61. Plasmid pMS64 was used to transfer a lonB-lacZ fusion to the amyE locus, whereas pMS92 (which carries an identical insert) was used to transfer the fusion to the lonB locus by a single reciprocal crossover. pSH5 contains the same insert as pMS58, but the lon gene is disrupted by the insertion of an spcr cassette. In plasmid pSH4, the regulatory region of lonB from −342 to +68 with respect to the ATG start codon was fused to a gfp gene lacking its own promoter.(B) Sequence of the lonB promoter region as well as the first 29 codons of the gene and the complete orf61. The sequence is the same as in plasmid pMS76, a multicopy plasmid that carries the lonB promoter region. Potential ribosome binding sites (RBS) as well as start and stop codons (bold and underlined) are indicated. The region of dyad symmetry downstream of clpX that may act as a transcriptional terminator is indicated by horizontal arrows (see above). The lonB transcriptional start site determined by primer extension analysis is underlined and labeled “+1” just downstream of −10 and −35 sequences that may be utilized by E ςF (see text). The second weak but ςF-independent signal observed in the primer extension analysis is indicated by the arrowhead above the G in the starting codon of orf61. The site of an A-to-T transversion that creates a nonsense mutation at codon 27 of orf61 is also indicated.
Construction of transcriptional fusions of lonB to lacZ and gfp.
The 2,090-bp DNA fragment generated by high-fidelity PCR with oligonucleotides lonB-61D and lonB-2151R (see above) was digested with EcoRI and Sau3AI, and a 353-bp fragment was isolated. This fragment was inserted into the amyE integrational plasmid pSN32 that had been cut with EcoRI and BamHI (32), producing pMS64, which carries a lonB-lacZ transcriptional fusion (Fig. 1). Integration of ScaI-linearized pMS64 into the amyE locus of strain MB24 produced the chloramphenicol-resistant (Cmr) AmyE− strain AH2356 (Table 1). Chromosomal DNA was prepared from this strain and used to transfer the lonB-lacZ fusion into various Spo− recipients by DNA-mediated transformation with selection for chloramphenicol resistance (Table 1). Plasmid pMS64 was then digested with EcoRI and SacI to release a 2,356-bp fragment that was purified and inserted into EcoRI- and SacI-digested pJM783 (35), creating pMS92. Plasmid pMS92 was used to transfer a lonB-lacZ transcriptional fusion to the lonB locus of a wild-type and a sigF host by a single reciprocal crossover (Campbell-type recombination) that created strains AH2435 and AH2436, respectively (Table 1). For construction of a transcriptional fusion of lonB to the gfp gene, a 423-bp PCR fragment containing the regulatory region of lonB was generated with the primers lonB-SH3 (5′-GAGAGCGGCCGCAACGGATTCTTTATTGATTTCG-3′) and lonB-SH2 (5′-GAGACCCGGGCAAGACTGGAGCACGC-3′). After digestion with NotI and SmaI, this PCR fragment was inserted into pFSB79, an amyE integrational plasmid with a promoterless gfp gene (44; F. Spiegelhalter and E. Bremer, unpublished data) that had been cut with the same enzymes. The resulting plasmid, pSH4, was introduced into the wild-type strain JH642 and its corresponding sigF mutant MO1073 by transformation followed by selection for chloramphenicol resistance (Table 1). Cmr colonies of this transformation that had retained amylase activity were screened by PCR. Derivatives of the wild-type strain JH642 and the sigF mutant MO1073 that had pSH4 integrated into the lonB region by a Campbell-type integration were named BSM110 and BSM111, respectively.
Multicopy plasmids bearing different fragments from the lonB region.
To produce a version of the lonB gene in a multicopy plasmid, the same PCR fragment used in the construction of pMS58 was cloned between the BamHI and SalI sites of pMK3 (50) to create pMS56 (Fig. 1A). The Quickchange protocol (Stratagene) was used to convert the lysine-encoding 27th codon of orf61 (Fig. 1B) to the nonsense codon TAA, creating the pMS56 derivative pMS94. The whole BamHI-SalI insert in pMS94 was sequenced to ensure that no other mutations were fortuitously introduced by the mutagenesis protocol. A plasmid containing just the lonB regulatory region in pMK3 (pMS76 [Fig. 1A]) was constructed by inserting a 354-bp XhoI-digested PCR product obtained with the oligonucleotide primers lonB-61D (see above) and lonB-415R (5′-CCCTGTCCAACCATGGTGGTCCC-3), between the SmaI and SalI sites of pMK3. A version of lonA fused to the lonB promoter was constructed as follows. The lonA gene was PCR amplified with primers lonA-286D (5′-GGAGGTGTCAGTCCATGGCAGAAG-3′) and lonA-2798R (5′-GGCCAATGCGAATTCCGGAAGCCC-3′). The PCR fragment was digested with EcoRI and inserted into pUC18 that had been cut with SmaI and EcoRI, creating pMS65, in which an NcoI site overlaps the lonA initiation codon. The lonB promoter fragment was generated by PCR with primers lonB-61D and lonB-415R (see above), digested with NcoI (an NcoI site was introduced that overlapped the lonB start codon), and inserted between the NcoI and SalI sites of pMS65. This ligation created pMS70. A fragment carrying the lonB promoter fused to the lonA gene was obtained from pMS70 by digestion with PstI and EcoRI and inserted between the same sites of pMK3 (50), yielding pMS72.
RNA primer extension and Northern blot analysis.
RNA was prepared by a modified acid phenol method (54) from cultures of a wild-type strain (JH642), a sigF mutant (MO1073), and a sigE mutant (MO512) at different times after resuspension in sporulation inducing medium as well as from a wild-type strain, JH642, which expressed either sigB or sigF under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter Pspac from plasmids pDG1481 and pSDA4, respectively (27, 48). The RNA (10 μg) was subject to Northern blot or primer extension analysis essentially as described before (41, 55). The probe for the Northern blot analysis was a 1,605-bp-long digoxygenin-labeled antisense RNA produced in vitro with T7 RNA polymerase from a PCR fragment internal to the lonB gene, generated with oligonucleotides lonB-SH1 (5′-ACAACGTTGAGCTTGAGTTTG-3′) and lonB-SH5 (5′-GAGATAATACGACTCACTATAGGGAGGTTCTTCAGCTATTCCT GTG-3′, which incorporates a T7 promoter at its 5′-end). For primer extension experiments, the oligonucleotide lonB-SH6 (5′-ATACAAACCGATGATGATCCCA-3′) was labeled at its 5′ end with [γ-32P]ATP (3,000 mCi/mmol). A sequencing ladder was generated with the same oligonucleotide using plasmid pSH4 as the template.
Enzyme assays.
β-Galactosidase activity was measured using the substrate o-nitrophenyl-β-d-galactoside as previously described (18, 45). The data reported in figures 2, 6, and 7 are derived from representative experiments that were repeated at least two times.
Fluorescence microscopy.
Samples of cultures of a wild-type strain (BSM110) and its isogenic sigF mutant (BSM111) bearing a transcriptional lonB-gfp fusion integrated into the lonB region were collected about 2.5 h after the onset of sporulation in DSM. The samples were observed in a Zeiss fluorescence microscope using a 450-490/FT510/LP520 filter set. Images were recorded and processed for publication using Adobe Photoshop.
RESULTS
Transcription of lonB is under the control of ςF.
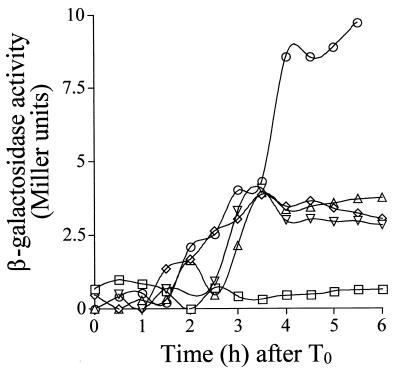
A wild-type strain (AH2356) and congenic derivatives with deletions of the genes encoding the four sporulation-specific sigma factors (Table 1) were induced to sporulate by growth and resuspension in a minimal sporulation medium (2, 33), and the formation of β-galactosidase from a lonB-lacZ transcriptional fusion inserted into the amyE locus was monitored during the course of sporulation. In a wild-type strain, lonB-lacZ-driven β-galactosidase production showed only background levels at the onset of sporulation. However, the enzyme levels increased sharply around 120 min after the initiation of sporulation, reaching a maximum level around hour 3 of sporulation (Fig. 2). Induction of lonB-lacZ transcription was prevented by deletion of the sigF gene but not by mutation of the gene coding for ςE, ςG, or ςK (Fig. 2). A similar lonB-lacZ induction pattern was observed when the strains were induced to sporulate by growth and exhaustion in DSM (data not shown). Thus, during sporulation the ςF form of RNA polymerase controls transcription of lonB. lonB did not seem to be transcribed by RNA polymerase carrying the other forespore-specific sigma factor, ςG (Fig. 2), nor did its transcription require a functional copy of spoIIGB, which encodes the mother cell regulator ςE. In both DSM and resuspension medium, lonB-lacZ transcription not only appeared to be independent of ςE production but also showed a twofold increase in a sigE mutant (Fig. 2). This increase is likely to reflect the disporic phenotype of sigE mutants, in which ςF is active in both prespore compartments of the sporangium (24).
FIG. 2.
Dependency of lonB-lacZ expression, illustrated by time courses of lonB-lacZ-driven β-galactosidase production in a wild-type strain (AH2356; inverted triangles) and in strains bearing deletion mutations of the following loci: sigF (AH2358; squares), sigE (AH2359; circles), sigG (AH2360; diamonds), and sigK (AH2361; triangles). Sporulation was induced by the resuspension method. Samples were collected every 30 min after the resuspension and onset of sporulation (T0) and assayed for β-galactosidase activity. Enzyme activity is indicated in Miller units (see Materials and Methods). Background levels of enzyme activity in the wild-type strain MB24 were subtracted in all cases.
lonB is transcribed during sporulation from a ςF -dependent promoter.
The results described above suggested the location of a ςF-dependent promoter, within the 336 bp preceding the lonB translational start site (Fig. 1A and B). A primer extension analysis with RNA prepared from wild-type cells at different times after the onset of sporulation revealed two major extension products, which corresponded to the adjacent nucleotides CA, 45 bp upstream of the lonB start codon (Fig. 1B and 3). Upstream from this position, we found sequences that strongly resembled the −10 and −35 regions recognized by ςF in several other promoters (15). ςF specificity of this promoter gained additional support when RNA isolated from sigF and sigE mutants at 90 min after resuspension was subject to primer extension analysis. Whereas no signal was observed when RNA from a sigF mutant was used, the utilization of RNA from the sigE mutant produced the same two major extension products observed in the wild type (Fig. 3). These extension products were also observed when ςF was artificially produced during exponential growth in rich medium from the IPTG-inducible promoter Pspac in JH642 harboring the plasmid pSDA4 (Fig. 3). Thus, the CA dinucleotide at positions 333 and 334 (Fig. 1B and 3) is likely to represent the transcriptional start site for the ςF-recognized promoter of lonB. ςF -dependent expression of lonB during sporulation has independently been discovered by Piggot and coworkers (O. Amaya and P. Piggot, personal communication).
FIG. 3.
Mapping of the 5′ end of the lonB transcript. Total RNA was isolated from wild-type strain JH642 (lanes 1 to 4) as well as from mutants lacking SigF (MO1073; lane 5) and SigE (MO512; lane 6) at different times after initiation of sporulation in resuspension medium. Additional RNA samples were prepared during exponential growth in rich medium (2×YT) from strain JH642 carrying plasmid pSDA4 or pDG1481, which allow production of active SigF (lanes 7 and 8) or SigB (lanes 9 and 10), respectively. Samples for lanes 7 and 9 were collected from cultures grown in the absence of the inducer IPTG, and samples were analyzed in lanes 8 and 10 were collected 30 min after IPTG addition to 1 mM. Primer extension was performed as described in Materials and Methods. The 5′ ends of the transcripts were determined by comparison with a DNA-sequencing ladder generated with the same primer and run in parallel on the same gel (lanes A, C, G, and T) and were labeled with arrowheads.
Liu et al. (26) reported the existence of a short open reading frame (orf61) which starts 103 bp upstream from the lonB start codon and overlaps by 25 codons the 5′ end of the lonB coding region (Fig. 1). However, the primer extension analysis presented above indicates that ςF-dependent transcription during early sporulation did not include the complete orf61 coding region.
A second, much weaker extension product that was present prior to the onset of ςF activity was found to correspond to a G 101 bp upstream of the lonB translational start site. This position is just downstream of a region of dyad symmetry that could represent a transcription termination signal of clpX. Thus, the possibility exists that some lonB transcripts originate from a promoter upstream of orf61, perhaps in the tig-clpX region (Fig. 1A). In support of this view, we note that the β-galactosidase activity of a lonB-lacZ fusion observed prior to activation of ςF was slightly higher when the fusion was integrated into the lonB region (strain AH2435 [data not shown]) instead of the amyE locus (Fig. 2). The suggestion that clpX and lonB are cotranscribed prior to the asymmetric division of sporulation is compatible with previous work implicating LonB in the regulation of ςH activity at the entry into the stationary phase of growth (26).
The ςF-dependent lonB transcript is monocistronic and lonB-gfp expression is confined to the prespore compartment.
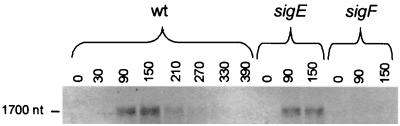
Analysis of the 5′ ends of the lonB transcript showed that during sporulation lonB is transcribed mainly from a single promoter that is utilized by the ςF form of RNA polymerase and that orf61 is not part of that transcript (Fig. 1 and 3; see above). It further indicated that transcripts originating upstream from lonB could accumulate in the predivisional cell, prior to the activation of ςF. Northern blot analysis was performed to establish whether lonB was cotranscribed with its flanking genes (clpX and lonA) during sporulation. Probing of blots of RNA samples prepared from sporulating cells of the wild-type strain JH642 and of the sigE (MO512) and sigF (MO1073) mutants with a lonB-specific probe revealed a single specific signal that corresponded to a message size of about 1,700 nucleotides (Fig. 4). The size of the transcript is in agreement with the distance between the lonB transcriptional start site and the stop codon of the gene (1699 bp). In consonance with the analysis of lonB-lacZ expression in resuspension medium (Fig. 2), synthesis of the 1,700-nucleotide-long message could be detected 30 min after the initiation of sporulation and reached a maximum some 2 h later. Moreover, its synthesis was completely dependent on sigF but not on sigE expression (Fig. 4). The results of the Northern blot analysis were corroborated by DNA array experiments with RNA from sporulating wild-type bacteria and sigF or sigE mutants. In these DNA array analyses, lonB was independently discovered as a ςF -dependent gene (L. Steil et al., unpublished data). While lonB displayed a strong sigF-dependent increase in expression, the genes flanking lonB (clpX and lonA) did not reveal such an induction during sporulation (Steil et al., unpublished).
FIG. 4.
Northern blot analysis of lonB transcription during sporulation. Sporulation of wild-type (wt) strain JH642 and the sigE and sigF mutants MO512 and MO1073, respectively, was induced by the resuspension method (see Materials and Methods). Total RNA was prepared from 10-ml samples collected immediately after resuspension and at the indicated times (in minutes) thereafter. The RNA samples were electrophoretically resolved on agarose-formaldehyde gels, vacuum transferred to neutral nylon membranes, and immobilized by UV cross-linking. The RNA blot was then probed with a 1,605-bp fragment internal to the lonB coding sequence, which was digoxigenin labeled. The position of the lonB transcript is indicated, as well as size estimation, based on the relative position of appropriate size markers. nt, nucleotides.
Heat, ethanol, and other stresses induce the synthesis of the lonA mRNA from a ςA-dependent promoter located between the two NdeI sites in front of the lonA gene (Fig. 1A and reference 39). To determine whether lonB might also respond to stress, RNA was isolated from the wild-type strain JH642 before and after exposure to 4% ethanol and subjected to slot blot analysis. These experiments failed to reveal any induction of lonB by ethanol stress (data not shown). Furthermore, production of active ςB during exponential growth of JH642(pDG1481) by induction of the Pspac promoter with IPTG did not result in any significant induction of lonB (Fig. 3). Our results indicate that during sporulation lonB is transcribed mainly as a monocistronic message that includes neither clpX-orf61 nor lonA. Together with those of Riethdorf et al. (39), our results further suggest that lonA and lonB respond to entirely different environmental cues.
To directly localize the cellular compartment of lonB transcription, we fused lonB to the gfp gene of Aequorea victoria, encoding the green fluorescent protein (GFP) (44, 53), and integrated the fusion into the lonB region of a wild-type strain and its isogenic sigF mutant. Samples of both strains were collected at various times after the onset of sporulation following nutrient exhaustion in DSM and analyzed by fluorescence microscopy. Whereas the sigF mutant strain (BSM111) did not display any signal (not shown), the green fluorescence that resulted from GFP accumulation was restricted to the forespore in the wild-type strain, BSM110 (Fig. 5). This fluorescence peaked about 3 hs after the onset of sporulation, consistent with the analysis of lonB-lacZ transcription in resuspension medium (Fig. 2) and in DSM (not shown). The failure of observing fluorescence in the mother cell compartment of wild-type cells or in sigF mutants proved that transcriptional readthrough into lonB from clpX contributed to a very minor extent to the overall expression of lonB during sporulation. We conclude that expression driven by the promoter upstream of lonB is restricted to the prespore compartment of sporulating cells.
FIG. 5.
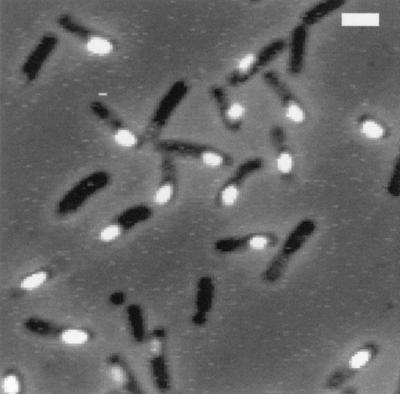
Localization of lonB-gfp expression. A lonB-gfp transcriptional fusion was introduced into the lonB region of wild-type strain JH642. Bacteria were induced to sporulate in DSM, and samples were collected around 150 min after the onset of sporulation (defined as the end of the exponential phase of growth). The cells were mounted on a microscope slide without fixation and observed in a Zeiss fluorescence microscope in either phase-contrast or fluorescence mode. An overlay of the phase-contrast and fluorescence pictures is displayed. Bar = 2 μm.
Expression of lonA but not of lonB from a multicopy plasmid arrests sporulation at the engulfment stage.
A comparison of wild-type strain JH642 and its isogenic lonB insertion mutant strain BSM105 revealed that the lonB::spc mutation did not impair the frequency of formation of heat-resistant spores at 37 or 50°C, nor did it significantly affect the expression of genes belonging to the different sporulation-specific regulons (data not show). The lonB::spc mutation is not expected to have a polar effect on lonA expression, since unlike a lonA mutant allele (42), it did not enhance ςG-dependent gene expression in nonsporulating cells (data not shown).
Even though we failed to observe a sporulation phenotype upon its disruption, the lonB gene could still play a role in controlling the level of regulatory factors or proteins otherwise involved in morphogenesis. To explore these possibilities, we decided to overexpress the lonB gene, as well as several other lon alleles, from a multicopy plasmid (Fig. 1A and Table 2). We introduced the lonB gene (pMS56), its promoter region (pMS76), or the lonB promoter fused to the lonA gene (pMS72) into the multicopy plasmid pMK3 (50). To determine whether orf61 had a role in the control of lonB activity, we also constructed a pMK3 derivative bearing a copy of the lonB gene in which a nonsense mutation was created at codon 27 of orf61 (pMS94). These plasmids were introduced into competent cells of the wild-type host MB24. The strains bearing a multicopy lonB gene with or without a nonsense mutation in orf61 or bearing the lonB promoter region were shown to sporulate with the same efficiency as the wild-type parental strain or the strain (AH2350) carrying the pMK3 vector (Table 2). However, cells harboring pMS72 (PlonB-lonA; strain AH2368) were severely impaired in the ability to sporulate (Table 2), suggesting that lonA expression from the lonB promoter was interfering with a function essential for sporulation. Because of the previously characterized effect of lonA in preventing inappropriate ςG-directed transcriptional activity (42), we hypothesized that expression of lonA in the prespore was similarly diminishing the ability of ςG to utilize its cognate promoters. To test this hypothesis, we monitored the expression of reporter genes for ςF, ςE, ςG, and ςK activity in cells harboring pMS72 (PlonB-lonA) or the parental plasmid pMK3. The presence of plasmid pMK3 had no noticeable effect on the expression of the different sporulation regulons (Fig. 6). In contrast, the forespore-specific expression of the PlonB-lonA allele (pMS72) severely interfered with the ability of ςG to utilize the sspE promoter (Fig. 6D). The decrease in sspE-lacZ transcription caused by pMS72 was not due to impaired transcription of spoIIIG in the presence of the multicopy PlonB-lonA allele (Fig. 6B). Thus, it is likely that the forespore-specific expression of lonA from the lonB promoter interfered with the activity of ςG. In addition, because ςG is required for the activation of ςK at late stages of development, expression of the ςK-dependent gerE-lacZ fusion was also severely curtailed (not shown). The observation that the expression of lonA in the forespore interfered with ςG activity suggested that one role of lonB could be to prevent the accumulation or activity of LonA in the forespore. However, this did not seem to be the case because expression of the PlonB-lonA allele in the lonB::spc mutant (strain AH2427 [Table 2]) did not aggravate the sporulation phenotype of strain AH2368 (in which the multicopy PlonB-lonA allele is propagated in a lonB+ background [Table 2]). Moreover, a c-Myc epitope-tagged allele of lonA allowed the prompt detection of the epitope during growth and early stationary phase in 2xYT but not during sporulation in DSM, suggesting that the levels of LonA may be extremely low (data not shown). The results suggest that even in a multicopy situation, lonB could not interfere significantly with sporulation (Table 2), whereas lonA could reduce ςG- but not ςF-dependent activity when expressed in the forespore compartment (Table 2 and Fig. 6).
TABLE 2.
Effect of multicopy plasmids bearing lon alleles on sporulation
| Strain | Plasmid | lon allelea | Sporulation (CFU/ml)
|
% Sporulationb | |
|---|---|---|---|---|---|
| Viable cells | Spores | ||||
| MB24 | wt | 7.01 × 108 | 8.09 × 108 | 100 | |
| AH2350 | pMK3 | wt | 4.53 × 108 | 7.96 × 108 | 100 |
| AH2351 | pMS56 | lonB | 2.60 × 108 | 2.30 × 108 | 88 |
| AH2433 | pMS76 | PlonB | 4.26 × 108 | 4.21 × 108 | 100 |
| AH2368 | pMS72 | PlonB-lonA | 1.11 × 108 | 3.74 × 106 | 3 |
| AH2427 | pMS72 | PlonB-lonA (lonB::spc) | 3.80 × 108 | 7.60 × 105 | 0.2 |
| AH2434 | pMS94c | orf61(K27Stop) lonB | 1.7 × 108 | 1.65 × 108 | 97 |
In all strains except AH2427, the chromosome contains the wild-type allele of the lonB gene.
Defined as the ratio between the heat-resistant spore count and the total (viable) cell count.
Similar to pMS56 but carrying a nonsense mutation in orf61 (codon 27).
FIG. 6.
Effect of expression of a PlonB-lonA fusion (see text) in a multicopy plasmid on expression of lonB-lacZ (A), spoIIIG-lacZ (B), spoIID-lacZ (C), and sspE-lacZ (D) fusions during sporulation in DSM. Strains bearing the indicated fusions were transformed with the pMK3 vector (open circles) or with its derivative pMS72 (in which the lonB promoter was fused to the lonA gene; squares). Close circles represent expression of the different fusions in a wild-type strain without plasmids. Samples were collected every 30 min after the end of the exponential phase of growth (defined as the onset of sporulation) and assayed for β-galactosidase activity. Enzyme activity is expressed in Miller units (see Materials and Methods). Background levels of enzyme activity in the wild-type strain MB24 were subtracted in all cases.
Both lonA and lonB can reduce ςF-dependent activity in a spoIIIE mutant.
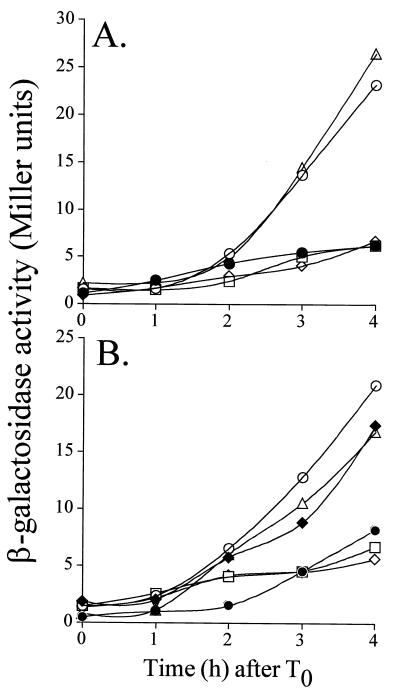
In wild-type cells, both ςF and SpoIIAB disappear at approximately equal rates from the two-sporangium compartments (23). However, in class I spoIIIE mutants, ςF and SpoIIAB disappear at a much greater rate from the mother cell than from the prespore, causing the prevalence of cells with increased ςF- and SpoIIAB-specific immunofluorescence signals in the prespore (25). Moreover, the transcription of several ςF-dependent transcriptional fusions to lacZ has been shown to be higher in spoIIIE mutants than in wild-type cells (59). In those mutants, only about 30% of the chromosome centered around oriC is enclosed in the prespore, but ςF activity correctly localizes to this compartment (59). The lonB locus is located at about 246° on the genetic map and is therefore outside the region of the chromosome present in the prespore compartment of a spoIIIE mutant (22). In agreement with this observation, we found no ςF-dependent increase in the activity of a lonB-lacZ fusion integrated at the lonB locus in a spoIIIE47 mutant (data not shown). In contrast, the integration of a similar fusion at the amyE locus of a spoIIIE47 recipient (close to oriC at 8° on the genetic map) resulted in its ςF-dependent induction (Fig. 7A; see also below). We also monitored expression of the ςF-dependent spoIIIG-lacZ fusion (integrated at the amyE locus) in cells of a spoIIIE47 spoIIIG::spc double mutant. The spoIIIG::spc mutation was introduced to eliminate the contribution of ςG (whose promoter specificity partially overlaps that of ςF) to the transcription of certain ςF-dependent promoters (51). The results in Fig. 7B confirm that as in the case of the lonB-lacZ fusion, spoIIIG-lacZ was overexpressed in the mutant compared to a congenic wild-type strain. To test the idea that the increased activity of ςF in the prespore of spoIIIE47 cells could be caused by the absence of the lonB gene product, we introduced pMS56 (which carries the lonB gene) in cells of the mutant. The results show that reintroduction of lonB into the prespore via a replicative plasmid restored expression of lonB-lacZ and spoIIIG-lacZ to wild-type levels (Fig. 7). Neither the pMK3 vector nor its derivative carrying the lonB promoter region (pMS76, which was analyzed only in the case of spoIIIG-lacZ), caused a similar effect (Fig. 7). Interestingly, the introduction of pMS72 (carrying the PlonB-lonA allele) also restored wild-type levels of expression of the indicated fusions (Fig. 7). It should be noted that expression of lonA from the lonB promoter in a wild-type strain did not reduce expression of the ςF-dependent lonB- and spoIIIG-lacZ fusions (Fig. 6A and B). Moreover, the introduction of pMS56 (multicopy lonB gene) in a wild-type strain did not significantly interfere with spoIIIG-lacZ expression (not shown). Therefore, it is unlikely that the reduction of lonB-lacZ and spoIIIG-lacZ activity caused by the introduction of multicopy alleles of lonB or lonA in the spoIIIE47 spoIIIG::spc double mutant was due to degradation of the reporter enzyme. The results suggest that in the spoIIIE47 mutant both LonA and LonB can act to reduce the levels of ςF-directed gene expression, even though neither LonA nor LonB appeared to interfere with ςF-dependent transcriptional activity in otherwise wild-type cells.
FIG. 7.
Both lonB and lonA can negatively regulate ςF activity in a class I spoIIIE mutant. Expression of lonB-lacZ (A) and spoIIIG-lacZ (B) was determined in a wild-type strain (closed circles), a spoIIIE47 ΔspoIIIG::spc double mutant (open circles), or derivatives bearing plasmids pMK3 (triangles), pMS56 (squares), and pMS72 (diamonds). Expression of spoIIIG-lacZ in the spoIIIE47 ΔspoIIIG::spc strain was also monitored in the presence of pMS76, which carries only the lonB promoter (B, close diamonds). Sporulation was induced in DSM, and samples were collected every 60 min after its onset (defined as the end of the exponential phase of growth) to assay for β-galactosidase activity. Enzyme activity is expressed in Miller units (see Materials and Methods). Background levels of enzyme activity in the wild-type strain MB24 were subtracted in all cases.
DISCUSSION
The results described herein show that the lonB gene of B. subtilis is transcribed during sporulation exclusively in the forespore compartment of the sporulating cell. Transcription of lonB is dependent on the expression of the sigF gene and likely occurs from a promoter directly recognized by ςF, located just upstream from the lonB coding region. Transcription from this promoter results in the production of a monocistronic message which does not include the downstream lonA gene. This is of importance, as expression of lonA in the forespore compartment interfered with the activity (but not with the production) of ςG and strongly impaired sporulation (Fig. 6 and Table 2). lonB belongs to a first temporal class of ςF-dependent genes, which does not require the additional activity of ςE in the mother cell for transcription. Moreover, transcription of lonB seems to be exclusively under ςF control, with only a negligible contribution of ςG (Fig. 2). Thus the lonB-encoded product is expected to be present in the forespore since its synthesis required the production and subsequent activation of ςF (16, 24, 29, 34, 49). The early prespore-specific transcription of lonB suggests that it may function early in the prespore developmental program. The observation that LonB can, under certain genetic conditions, act to reduce ςF-dependent activity implies that it may be part of a feedback mechanism designed to keep ςF activity within certain limits. If such a mechanism exists and is relevant for sporulation, then lonB must be redundant, because we failed to detect a phenotype associated with loss of LonB in wild-type cells. Alternatively, LonB could also contribute to the removal of ςH and/or ςE from the prespore compartment. An influence of LonB on restricting ςE levels in the prespore seems unlikely since pro- ςE did not accumulate in the prespore compartment of a lonB mutant (W. G. Haldenwang, personal communication). Moreover, ςE activity correctly localizes to the mother cell compartment in a strain able to activate processing of pro- ςE to its active form in the absence of ςF (27, 61).
Two other proteins that appear to be specifically removed from the prespore compartment are the SpoIIE phosphatase and the SpoIIAB kinase, both by mechanisms that at least in part depend on spoIIIE function (25, 37). We did not specifically address this question here, but our results suggest that at least in the spoIIIE47 strain, both LonB and LonA can reduce ςF-dependent transcriptional activity. Interestingly, this reduction is only to a point where the levels of ςF-dependent gene expression are comparable to those observed in a wild-type strain. Expression of lonB of the PlonB-lonA allele in wild-type cells did not interfere with the levels of ςF-directed gene expression, again suggesting that the capacity of LonA and LonB to act on ςF is somehow regulated. Schmidt et al. (42) have shown that LonA can contribute to prevent inappropriate expression of ςF activity under nutritional conditions that do not support efficient sporulation. In extension of these studies, we found that transcription of lonA in the forespore can significantly reduce ςG-directed gene expression and the frequency of sporulation. Surprisingly, even though both proteases reduced ςF-directed gene expression in the spoIIIE47 mutant, only LonA appeared to be capable of interfering with ςG activity during sporulation. Either LonB is not active at the time during sporulation when ςG accumulates in the prespore or LonB differs from LonA in its substrate specificity at least toward ςG. In this respect it is interesting that LonA and LonB particularly differ in their N-terminal regions, which consist of 250 and 78 residues, respectively. Recent reports have implicated residues in this N-terminal region of Lon in the discrimination between substrates. For example, a single amino acid change, of aspartate 240 to a lysine in LonA from E. coli, prevents it from interacting with its specific substrate RcsA but does not impair its ability to interact with and degrade the cell division inhibitor SulA (10). Mutant forms of the Lon protease from Mycobacterium smegmatis lacking its 277 N-terminal residues showed neither peptidase nor ATPase activity despite the fact that the deleted region included neither the catalytic serine residue (at residue 675) nor the ATP binding motifs (40). Shorter deletions (of 90 and 225 residues) resulted in proteins with peptidase activity against small unstructured peptides but severely impaired in their ability to degrade the protein substrates alpha-casein in vitro or RcsA in vivo (40). A different study has revealed a second region involved in substrate recognition in Lon and the Clp proteins (46). These proteins share a common design (see reference 46 and references therein) and have a homologous sensor and substrate discrimination domain of about 100 amino acids, located downstream of the ATPase domain. LonB, which lacks an extended N-terminal domain as well as the sensor and substrate discrimination domain, may need to interact with a second protein to form a functional protease; alternatively, the recognition of specific substrates by LonB may follow different rules. If LonB needs to associate with a second protein to form an active protease, then synthesis of this putative subunit may be under ςF control. This putative polypeptide should be encoded by a gene located close to the oriC marker (58), because LonB was able to reduce ςF-dependent gene expression in a spoIIIE47 mutant. The substrate specificities of LonA and LonB have not been studied in detail and so far in B. subtilis include only ςG and ςH, respectively (26, 42; this work). More detailed studies will be necessary to extend the range of in vivo substrates for both proteases and to unravel their role in B. subtilis.
ACKNOWLEDGMENTS
M.S. and S.H. contributed equally to this work.
We are grateful to E. Bremer, P. Piggot, F. Spiegelhalter, P. Stragier, and P. Zuber for gifts of strains and plasmids. Furthermore we thank M. Niederweis for the gift of the GFP variant with increased fluorescence and P. Piggot for communicating results prior to publication.
This work was supported by grants from the Deutsche Forschungsgemeinschaft and the Max-Planck-Gesellschaft to U.V., Praxis XXI/PCNA/C/BI0/13201/98 and PCTI/1999/BME/35109 to A.O.H. from the Fundação para a Ciência e a Tecnologia (FCT), and by grant GM54395 from the National Institutes of Health to C.P.M. M.S. is the recipient of a Ph.D fellowship from the FCT.
REFERENCES
- 1.Alper S, Duncan L, Losick R. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis. Cell. 1994;77:195–205. doi: 10.1016/0092-8674(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 2.Boschwitz H, Yudkin M. The pattern of protein synthesis in spoIVC mutants of Bacillus subtilis resuspended in sporulation medium. J Gen Microbiol. 1983;129:3211–3214. doi: 10.1099/00221287-129-10-3211. [DOI] [PubMed] [Google Scholar]
- 3.Cutting S, Drinks A, Schmidt R, Kunkel B, Losick R. Forespore-specific transcription of a gene in the signal transduction pathway that governs Pro-ςK processing in Bacillus subtilis. Genes Dev. 1991;5:456–466. doi: 10.1101/gad.5.3.456. [DOI] [PubMed] [Google Scholar]
- 4.Cutting S, Oke V, Driks A, Losick R, Lu S, Kroos L. A forespore checkpoint for mother cell gene expression during development in Bacillus subtilis. Cell. 1990;62:239–250. doi: 10.1016/0092-8674(90)90362-i. [DOI] [PubMed] [Google Scholar]
- 5.Diederich B, Wilkinson J F, Magnin T, Najafi M, Erringston J, Yudkin M D. Role of interactions between SpoIIAA and SpoIIAB in regulating cell-specific transcription factor ςF of Bacillus subtilis. Genes Dev. 1994;8:2653–2663. doi: 10.1101/gad.8.21.2653. [DOI] [PubMed] [Google Scholar]
- 6.Driks A, Losick R. Compartmentalized expression of a gene under the control of sporulation transcription factor ςE in Bacillus subtilis. Proc Natl Acad Sci USA. 1991;88:9934–9938. doi: 10.1073/pnas.88.22.9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan L, Alper S, Arigoni F, Losick R, Stragier P. Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division. Science. 1995;270:641–644. doi: 10.1126/science.270.5236.641. [DOI] [PubMed] [Google Scholar]
- 8.Duncan L, Alper S, Losick R. SpoIIAA governs the release of the cell-type specific transcription factor ςF from its anti-sigma factor SpoIIAB. J Mol Biol. 1996;260:147–164. doi: 10.1006/jmbi.1996.0389. [DOI] [PubMed] [Google Scholar]
- 9.Duncan L, Losick R. SpoIIAB is an anti-ς factor that binds to and inhibits transcription by regulatory protein ςF from Bacillus subtilis. Proc Natl Acad Sci USA. 1993;90:2325–2329. doi: 10.1073/pnas.90.6.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebel W, Skinner M M, Dierksen K P, Scott J M, Trempy J E. A conserved domain in Escherichia coli Lon protease is involved in substrate discriminator activity. J Bacteriol. 1999;181:2236–2243. doi: 10.1128/jb.181.7.2236-2243.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feucht A, Magnin T, Yudkin M D, Errington J. Bifunctional protein required for asymmetric cell division and cell-specific transcription in Bacillus subtilis. Genes Dev. 1996;10:794–803. doi: 10.1101/gad.10.7.794. [DOI] [PubMed] [Google Scholar]
- 13.Gholamhoseinian A, Piggot P J. Timing of spoII gene expression relative to septum formation during sporulation of Bacillus subtilis. J Bacteriol. 1989;171:5747–5749. doi: 10.1128/jb.171.10.5747-5749.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzman P, Westpheling J, Youngman P. Characterization of the promoter region of the Bacillus subtilis spoIIE operon. J Bacteriol. 1988;170:1598–1609. doi: 10.1128/jb.170.4.1598-1609.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harry E J, Pogliano K, Losick R. Use of immunofluorescence to visualize cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1995;177:3386–3393. doi: 10.1128/jb.177.12.3386-3393.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henriques A O, Beall B W, Moran C P., Jr CotM of Bacillus subtilis, a member of the alpha-crystallin family of stress proteins, is induced during development and participates in spore outer coat formation. J Bacteriol. 1997;179:1887–1897. doi: 10.1128/jb.179.6.1887-1897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henriques A O, Beall B W, Roland K, Moran C P., Jr Characterization of cotJ, a ςE-controlled operon affecting the polypeptide composition of the coat of Bacillus subtilis spores. J Bacteriol. 1995;177:3394–3406. doi: 10.1128/jb.177.12.3394-3406.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellner E M, Decatur A, Moran C P. Two-stage regulation of an anti-sigma factor determines developmental fate during bacterial endospore formation. Mol Microbiol. 1996;21:913–924. doi: 10.1046/j.1365-2958.1996.461408.x. [DOI] [PubMed] [Google Scholar]
- 20.King N, Dreesen O, Stragier P, Pogliano K, Losick R. Septation, dephosphorylation and the activation of ςF during sporulation in Bacillus subtilis. Genes Dev. 1999;13:1156–1167. doi: 10.1101/gad.13.9.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchman P A, DeGrazia H, Kellner E M, Moran C P., Jr Forespore-specific disappearance of the sigma-factor antagonist SpoIIAB: implications for its role in determination of cell fate in Bacillus subtilis. Mol Microbiol. 1993;8:663–671. doi: 10.1111/j.1365-2958.1993.tb01610.x. [DOI] [PubMed] [Google Scholar]
- 22.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 23.Lewis P J, Magnin T, Errington J. Compartmentalized distribution of the proteins controlling the prespore-specific transcription factor ςF of Bacillus subtilis. Genes Cells. 1996;1:881–894. doi: 10.1046/j.1365-2443.1996.750275.x. [DOI] [PubMed] [Google Scholar]
- 24.Lewis P J, Partridge S R, Errington J. ς factors, asymmetry, and the determination of cell fate in Bacillus subtilis. Proc Natl Acad Sci USA. 1994;91:3849–3853. doi: 10.1073/pnas.91.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis P J, Wu L J, Errington J. Establishment of prespore-specific gene expression in Bacillus subtilis: localization of SpoIIE phosphatase and initiation of compartment-specific proteolysis. J Bacteriol. 1998;180:3276–3284. doi: 10.1128/jb.180.13.3276-3284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J J, Cosby W M, Zuber P. Role of Lon and ClpX in the post-translational regulation of a sigma subunit of RNA polymerase required for cellular differentiation in Bacillus subtilis. Mol Microbiol. 1999;33:415–428. doi: 10.1046/j.1365-2958.1999.01489.x. [DOI] [PubMed] [Google Scholar]
- 27.Londono-Vallejo J A, Stragier P. Cell-cell signaling pathway activating a developmental transcription factor in Bacillus subtilis. Genes Dev. 1995;9:503–508. doi: 10.1101/gad.9.4.503. [DOI] [PubMed] [Google Scholar]
- 28.Losick R, Stragier P. Crisscross regulation of cell-type-specific gene expression during development in Bacillus subtilis. Nature. 1992;355:601–604. doi: 10.1038/355601a0. [DOI] [PubMed] [Google Scholar]
- 29.Margolis P, Driks A, Losick R. Establishment of cell type by compartmentalized activation of a transcription factor. Science. 1991;254:562–565. doi: 10.1126/science.1948031. [DOI] [PubMed] [Google Scholar]
- 30.Mason J M, Hackett R H, Setlow P. Regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores: studies using lacZ gene fusions. J Bacteriol. 1988;170:239–244. doi: 10.1128/jb.170.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Min K T, Hilditch C M, Diederich B, Errington J, Yudkin M D. ςF, the first compartment-specific transcription factor of B. subtilis, is regulated by an anti-sigma factor that is also a protein kinase. Cell. 1993;74:735–742. doi: 10.1016/0092-8674(93)90520-z. [DOI] [PubMed] [Google Scholar]
- 32.Mota L J, Tavares P, Sa-Nogueira I. Mode of action of AraR, the key regulator of l-arabinose metabolism in Bacillus subtilis. Mol Microbiol. 1999;33:476–489. doi: 10.1046/j.1365-2958.1999.01484.x. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson W L, Setlow P. Sporulation, germination and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biology methods for Bacillus. Chichester, England: John Wiley & Sons Ltd.; 1990. pp. 391–450. [Google Scholar]
- 34.Partridge S R, Foulger D, Errington J. The role of ςF in prespore-specific transcription in Bacillus subtilis. Mol Microbiol. 1991;5:757–767. doi: 10.1111/j.1365-2958.1991.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 35.Perego M, Hoch J A. Isolation and sequence of the spo0E gene: its role in initiation of sporulation in Bacillus subtilis. Mol Microbiol. 1987;1:125–132. doi: 10.1111/j.1365-2958.1987.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 36.Piggot P J, Coote J G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976;40:908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pogliano K, Hofmeister A E M, Losick R. Disappearance of the ςE transcription factor from the forespore and the SpoIIE phosphatase from the mother cell contributes to establishment of cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1997;179:3331–3341. doi: 10.1128/jb.179.10.3331-3341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rather P N, Coppolecchia R, DeGrazia H, Moran C P., Jr Negative regulator of ςG-controlled gene expression in stationary-phase Bacillus subtilis. J Bacteriol. 1990;172:709–715. doi: 10.1128/jb.172.2.709-715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riethdorf S, Völker U, Gerth U, Winkler A, Engelmann S, Hecker M. Cloning, nucleotide sequence, and expression of the Bacillus subtilis lon gene. J Bacteriol. 1994;176:6518–6527. doi: 10.1128/jb.176.21.6518-6527.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roudiak S G, Shrader T E. Functional role of the N-terminal region of the Lon protease from Mycobacterium smegmatis. Biochemistry. 1998;37:11255–11263. doi: 10.1021/bi980945h. [DOI] [PubMed] [Google Scholar]
- 41.Scharf C, Riethdorf R, Ernst H, Engelmann S, Völker U, Hecker M. Thioredoxin is an essential protein induced by multiple stresses in Bacillus subtilis. J Bacteriol. 1998;180:1869–1877. doi: 10.1128/jb.180.7.1869-1877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt R, Decatur A L, Rather P N, Moran C P, Jr, Losick R. Bacillus subtilis Lon protease prevents inappropriate transcription of genes under the control of the sporulation transcription factor ςG. J Bacteriol. 1994;176:6528–6537. doi: 10.1128/jb.176.21.6528-6537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt R, Margolis P, Duncan L, Coppolecchia R, Moran C P, Jr, Losick R. Control of developmental transcription factor ςF by sporulation regulatory proteins SpoIIAA and SpoIIAB in Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:9221–9225. doi: 10.1073/pnas.87.23.9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scholz Q, Thiel A, Hillen W, Niederweis M. Quantitative analysis of gene expression with an improved green fluorescent protein. Eur J Biochem. 2000;267:1565–1570. doi: 10.1046/j.1432-1327.2000.01170.x. [DOI] [PubMed] [Google Scholar]
- 45.Serrano M, Zilhao R, Ricca E, Ozin A J, Moran C P, Jr, Henriques A O. A Bacillus subtilis secreted protein with a role in endospore coat assembly and function. J Bacteriol. 1999;181:3632–3643. doi: 10.1128/jb.181.12.3632-3643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith C K, Baker T A, Sauer R T. Lon and Clp family proteases and chaperones share homologous substrate-recognition domains. Proc Natl Acad Sci USA. 1999;96:6678–6682. doi: 10.1073/pnas.96.12.6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart B J, Enosberlage J L, McCarter L L. The lonS gene regulates swarmer cell differentiation of Vibrio parahaemolyticus. J Bacteriol. 1997;179:107–114. doi: 10.1128/jb.179.1.107-114.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stragier P, Bonamy C, Karmazyn-Campelli C. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell. 1988;52:697–704. doi: 10.1016/0092-8674(88)90407-2. [DOI] [PubMed] [Google Scholar]
- 49.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan M A, Yasbin R E, Young F E. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene. 1984;29:21–26. doi: 10.1016/0378-1119(84)90161-6. [DOI] [PubMed] [Google Scholar]
- 51.Sun D X, Cabrera-Martinez R M, Setlow P. Control of transcription of the Bacillus subtilis spoIIIG gene, which codes for the forespore-specific transcription factor ςG. J Bacteriol. 1991;173:2977–2984. doi: 10.1128/jb.173.9.2977-2984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tojo N, Inouye S, Komanq T. The lonD gene is homologous to the lon gene encoding an ATP-dependent protease and is essential for the development of Myxococcus xanthus. J Bacteriol. 1993;175:4545–4549. doi: 10.1128/jb.175.14.4545-4549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsien R Y. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 54.Völker U, Engelmann S, Maul B, Riethdorf S, Völker A, Schmid R, Mach H, Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994;140:741–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
- 55.Wetzstein M, Völker U, Dedio J, Lobau S, Zuber U, Schiesswohl M, Herget C, Hecker M, Schumann W. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J Bacteriol. 1992;174:3300–3310. doi: 10.1128/jb.174.10.3300-3310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright R, Stephens C, Zweiger G, Shapiro L, Alley M R. Caulobacter Lon protease has a critical role in cell-cycle control of DNA methylation. Genes Dev. 1996;10:1532–1542. doi: 10.1101/gad.10.12.1532. [DOI] [PubMed] [Google Scholar]
- 57.Wu J J, Piggot P J, Tatti K M, Moran C P., Jr Transcription of the Bacillus subtilis spoIIA locus. Gene. 1991;101:113–116. doi: 10.1016/0378-1119(91)90231-y. [DOI] [PubMed] [Google Scholar]
- 58.Wu L J, Errington J. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science. 1994;264:572–575. doi: 10.1126/science.8160014. [DOI] [PubMed] [Google Scholar]
- 59.Wu L J, Errington J. Use of asymmetric cell division and spoIIIE mutants to probe chromosome orientation and organization in Bacillus subtilis. Mol Microbiol. 1998;27:777–786. doi: 10.1046/j.1365-2958.1998.00724.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhang B, Kroos L. A feedback loop regulates the switch from one sigma factor to the next in the cascade controlling Bacillus subtilis mother cell gene expression. J Bacteriol. 1997;179:6138–6144. doi: 10.1128/jb.179.19.6138-6144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang L, Higgins M L, Piggot P J, Karow M L. Analysis of the role of prespore gene expression in the compartmentalization of mother cell-specific gene expression during sporulation of Bacillus subtilis. J Bacteriol. 1996;178:2813–2817. doi: 10.1128/jb.178.10.2813-2817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]