Abstract
Background:
Providing care over telehealth grew slowly until the COVID-19 pandemic. Since the onset of the COVID-19 pandemic, providing mental health care was readily adapted to virtual means; however, clinical trial research is nascent in adapting methods and procedures to the virtual world.
Methods:
We present protocol modifications to pivot a multisite randomized controlled trial study, conducted at Southeastern and Pacific Northwestern Veterans Affairs Health Care Systems, from being conducted in-person to virtually, following the onset of the COVID-19 pandemic. We measured outcomes of posttraumatic stress disorder (PTSD) symptoms and psychophysiological markers of stress among female Veterans with PTSD secondary to military sexual trauma. We collected qualitative data about provider and participant experiences with telehealth.
Results:
Across sites, 200 participants were consented (48 virtually), 132 were randomized (28 to virtual groups), and 117 completed data collection and treatment (69 completed all or some data collection or treatment virtually).
Conclusions:
The pivots made for this study were in response to the COVID-19 pandemic and offer innovative procedures leveraging technology and contributing to the broader landscape of conducting research virtually.
Clinical Trials Number:
Keywords: synchronous telehealth, group therapy, protocol, COVID adaptation, Veteran, telemedicine
Background and Introduction
Telehealth broadly describes remote health care without physical contact through telecommunication technologies (e.g., phone, video teleconferencing, and so on).1-3 Historically, telehealth has been used to improve access to clinical care and has been deployed in the military, prisons, and rural areas.4,5 Convenience and cost saving models for telehealth emerged supported by advancements in technology and internet connectivity facilitating implementation in clinical settings.1,6,7
To expand the application of telehealth, clinical research has been tasked with evolving so that the process of conducting research through telehealth is as accessible as provision of clinical telehealth. The success of clinical telehealth is a positive prognosis for the evolution of conducting clinical research through telehealth as well. Relatively few studies have been contributed with regard to the “how” of conducting telehealth research. Some of those have target cultural sensitivity in conducting research with Indigenous peoples and suggesting study design strategies for improving the efficiency and quality of research conducted using telecommunications.8,9
TELEHEALTH IN THE VETERANS AFFAIRS
The Department of Veterans affairs (VA) first introduced telehealth in 1959 with the use of two-way televisions for mental health treatment between facilities.10 Three phases of telehealth took place between 1994 and 2014 focusing on innovation, national implementation, and expansion, respectively.11 Supporting this effort, the Veterans Access Choice and Accountability Act in 2014 directed the expansion of VA health care access within 40 miles of all enrolled Veterans.12
BARRIERS
The implementation of telehealth has been limited by technological, financial, regulatory, and legal barriers.13 Two salient technological limitations are data security to protect personal health information and broadband connectivity.14 Financial barriers for health care organizations implementing telehealth are complicated by insurance reimbursement and costs associated with equipment, training, and communications.13 The legal framework in telehealth is complicated by state medical licensing, credentialing, and vague or underdeveloped policies and laws governing legal liabilities and practicing guidelines.15 Mental health providers have expressed concerns with telemedicine related to assessment, lack of control, and triaging, specifically for patients at risk for suicide.16
TELEHEALTH INCREASE DURING DISASTER AND CRISIS
Responses to national disasters and times of crises have been aided by utilization of telehealth which in turn has increased through those phenomena. Telehealth visits increased from 9% to 51% in the early months of the COVID-19 pandemic-related “lockdown” in March 2020.17 Utilization of telehealth services occurred during the H1N1 pandemic and hurricane Katrina; however, the COVID-19 global pandemic rapidly propelled telehealth adaptations for providers and patients.18 Despite significant strides in telehealth before the COVID-19 pandemic, providers reported that patients experienced difficulty with internet connectivity and navigation.19 Nevertheless, telehealth used during H1N1 and COVID-19 pandemics increased access to care while minimizing the transmission risk of infectious diseases.20
Concurrently, there has been advancement and increased utilization of biotechnologies such as wearable sensing devices in telehealth services.21 These advances demonstrate the evolving landscape of health care, alongside the evolution of technology and during times of disaster and crisis.
TELECOMMUNICATIONS AND RESEARCH
While telecommunications have been utilized in consumer and marketing research for many years, research involving patient interaction and clinical trials has been slower to advance.22,23 A salient barrier in recruitment for clinical trials conducted in person is access, particularly among underrepresented communities.24 Feasibly, increasing access to telehealth will increase access among minority populations to participate in clinical trial research, as has been observed in clinical settings.25
PRESENT STUDY
In response to the COVID-19 pandemic, VA clinical research services were rapidly restricted to protect patients and staff from viral transmission. We present modifications made to pivot a multisite randomized controlled trial (RCT) at a Southeastern and Pacific Northwestern VA Health Care Systems from in-person to virtual study implementation. The following sections highlight study methods and changes, contrasting the in-person methods with virtual methods, noting challenges, and solutions to them.
Methods
DESIGN
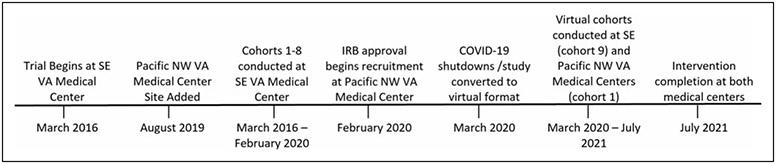
This RCT measured outcomes of post-traumatic stress disorder (PTSD) symptoms and psychophysiological markers of stress among women Veterans with PTSD secondary to military sexual trauma. This study began at a VA in the Southeastern region of the United States (U.S.) and, after seven cohorts, was expanded to include the Pacific Northwestern VA site. See Study Timeline, Figure 1. At the time of becoming a multisite study, three additional cohorts were anticipated for each site. The first cohort of those three for each site were impacted by changes made to research at the onset of COVID-19. The two remaining cohorts were not conducted. The research was approved by the VA and Emory University Institutional Review Boards (IRB) and VA research oversight subcommittees.
Fig. 1.
Timeline of events for the multisite RCT study conducted at SE and Pacific NW and VA Health Care Systems. Trials began in March 2016 at the SE site. Eight cohorts were conducted between March 2016 and February 2020 when the Pacific NW site was approved by the IRB to begin recruitment. In February 2020, recruitment for cohort one at the Pacific NW site coincided with completing cohort eight and enrolling cohort nine at the SE site. In March 2020, COVID-19 shutdowns occurred and study was converted to virtual format. In July 2021, interventions were completed at both sites. IRB, Institutional Review Boards; NW, Northwestern; RCT, randomized controlled trial; SE, Southeastern; VA, Veterans affairs.
Participants and interventions.
Participants at the Pacific Northwestern site were recruited through three means: (1) self-referral, (2) provider referral, and (3) medical chart reviews. Potential participants were educated about the study, consented and, if enrolled, were randomly assigned to an intervention of either 12 sessions of group Cognitive Processing Therapy (CPT) or 10 sessions of group Trauma Center Trauma Sensitive Yoga (TCTSY) facilitated by certified providers (therapists and TCTSY facilitators, respectively). Intervention descriptions and the rationale for using these approaches for the purpose of this study are presented elsewhere.26 Table 1 presents participant demographic characteristics, organized by site.
Table 1.
Demographics Characteristics by Site
| PACIFIC NORTHWEST (n = 28) |
SOUTHEAST (n = 103) |
|
|---|---|---|
| Age, mean (SD) | 47.5 (11.7) | 48.43 (11.2) |
| Education, n (%) | ||
| 12 years (high school) | 2 (7.1) | 16 (15.5) |
| 13–16 years (college) | 19 (67.9) | 80 (77.7) |
| 17–20 years (college) | 7 (25) | 7 (6.8) |
| Race, n (%) | ||
| Black/African American | 2 (7.1) | 93 (90.3) |
| Asian | – | 1 (1.0) |
| White | 24 (85.8) | 1 (1.0) |
| Mixed | 1 (3.6) | 7 (6.8) |
| American Indian/Alaska Native | 1 (3.6) | |
| Relationship status, n (%) | ||
| Nonpartnered | 15 (53.6) | 72 (69.9) |
| Married/partnered | 13 (46.4) | 31 (30.1) |
| Household monthly income, n (%) | ||
| Less than $2K/month | 5 (17.9) | 44 (43.1) |
| $2K/month or more | 23 (82.1) | 58 (56.9) |
| Employment, n (%) | ||
| Less than full-time | 21 (75) | 71 (68.9) |
| Full-time | 7 (25) | 32 (31.1) |
SD, standard deviation.
Measures.
To assess improvements to PTSD, the Clinician Administered PTSD Scale for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (CAPS-5), self-report measures of health, and psychophysiological markers of stress were measured at four timepoints (baseline/T1, mid-intervention/T2, 2-weeks postintervention/T3, and 3-months postintervention/T4). Qualitative data were collected through interviews with facilitators and participants.
INFORMED CONSENT AND ENROLLMENT
For participants enrolled using in-person methods, informed consent and in-depth screening to determine eligibility occurred during one 3-h visit. Following a brief phone screen and the review of informed consent documents (ICDs), signatures were collected, and a copy was made for eligible participants. Once consented, an in-depth screening was conducted to determine final eligibility. If enrolled, participants were randomized after completing baseline data collection. In-person methods are described elsewhere.26
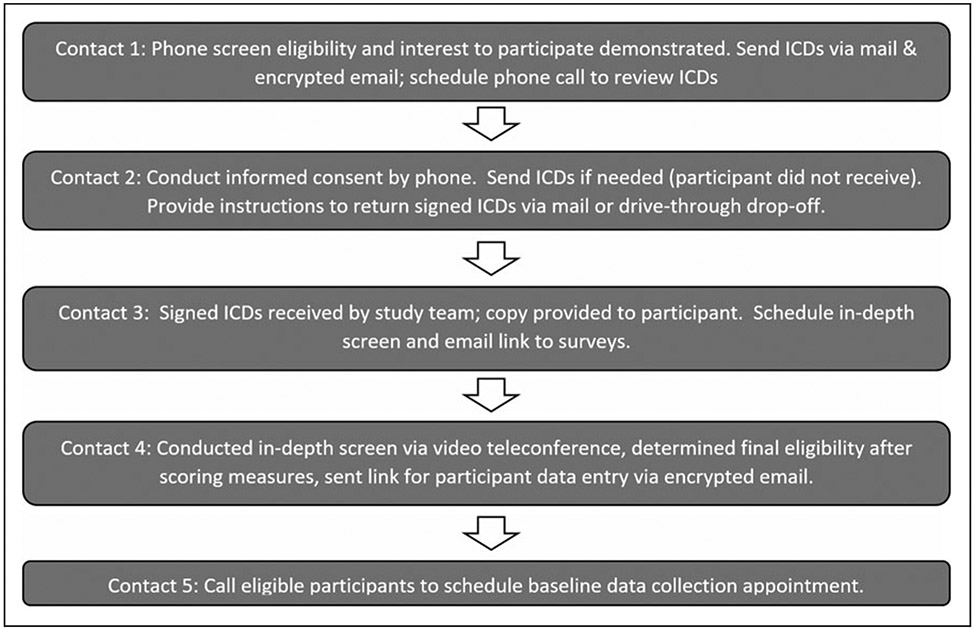
Virtual adaptations for processes of consent, in-depth screening, and enrollment included five contacts and combined the use of phone, secure email, mail, fax, drive-through/drop-off, and video teleconference; the process is illustrated in Figure 2.
Fig. 2.
Virtual informed consent and enrollment procedures for virtual study implementation in the present study. Contact 1 included determining phone screen eligibility and interest in participating, sending ICDs through mail and encrypted email, and scheduling a phone call to review the ICDs. Contact 2 included a phone call for informed consent, sending additional ICDs as necessary, and providing instructions for returning signed ICDs through mail or drive-through drop-off. In contact 3, the signed ICDs were received by the study team, a copy was provided to the participant, the in-depth screen was scheduled, and a link to surveys was sent through email. Contact 4 included the in-depth screening process using video teleconference, determining final eligibility following the scoring of screening measures, and sending an additional link to participants for data entry through email. In contact 5, eligible participants were scheduled for their baseline data collection appointment. ICDs, informed consent documents.
VIRTUAL DATA COLLECTION
When conducted in person, data collection was completed in one 3- to 5-h visit. Virtual adaptations for data collection processes spanned two to three contacts lasting 3- to 5-h total and combined the use of the same virtual means described as Informed Consent and Enrollment.
Following baseline data collection, participants were randomized. A phone call was made to inform the participant of their group assignment. Group materials were emailed or mailed to participants; alternatively, they were offered to drive-through/pickup their materials.
Diagnostic interviews and self-report measures.
Before the COVID-19 related study pivots, a research team member administered hard copy outcome measures in-person, later entering responses into the REDCap database. Following the virtual modifications, data were collected through video teleconferencing. Before these appointments, participants were sent an encrypted email with a link unique to them for one-time use so that they could enter their responses to self-report measures that would be recorded and stored in real time using REDCap. Study staff would review responses for completion and clarify discrepancies in responses. Following survey completion, participants were interviewed using a structured diagnostic interview for PTSD.
Occasionally, participants would complete surveys before the video teleconference appointment. In addition, a portion of participants encountered difficulty when attempting to access the encrypted emails that included the link to complete the study surveys. When connectivity problems arose, phone was used instead of video teleconference or the data collection was rescheduled for a later date.
Heart rate variability.
Heart Rate Variability (HRV) was measured at T1, T3, and T4 using the Firstbeat Bodyguard 2 (©Firstbeat Technologies Oy, Jyväskylä, Finland). The device is attached to the body using electrodes and can be worn during various activities but not in water. Recording automatically begins once attached to the body. Twenty-four-hour recordings are recommended to improve the quality of data collection over longer periods of time. Participants were instructed to wear the device for 48 h before returning to research staff. Data were downloaded using USB port and stored for analysis.
Participants were required to verbalize understanding of safety precautions regarding the monitor’s use. Occasionally, participants had difficulty with the devices and would wear and return the device, which had not been active and had not collected data. Under these circumstances, a replacement device would be provided for a second attempt to collect HRV data.
Electrocardiogram.
Twelve-lead electrocardiograms (ECGs) were obtained at T1 and T4 to evaluate changes in heart rhythm using portable ©iMedrix KardioScreen ECG (Milpitas, CA) devices. The portable device wirelessly captured ECG signals from leads placed on the participant and transmitted them to tablets using Bluetooth communication. Once collected and analyzed by the tablet, the ECG recordings were remotely transmitted by encrypted email to research staff to ensure quality before storing data.
Each participant needed to demonstrate a working understanding of the Android and Bluetooth technology used by this method. In addition, participants were responsible for applying ECG leads at the appropriate locations on their torsos. Supplemented by diagrams, the research assistant verbalized instructions for proper lead placement to ensure high-quality readings were obtained. Occasionally, poor electrode placement made for poor readings. Troubleshooting was made in real-time and required the participant to email the ECG file to the research assistant for analysis before moving to the next step of the psychophysiological data collection. If technological issues with Bluetooth interfered with completing ECG, iMedrix offered technology support. On two occasions, challenges attaching electrodes while managing unfamiliar technology caused participants to forgo collection of ECG data.
Before the COVID-19 study pivots, a research team member demonstrated the use of the HRV device in-person and participants left that appointment to use the devices, returning them once they had collected their data; ECGs were administered in real-time by a study team member. Following the protocol modifications, participants were provided these devices through mail or drive-through pickup and were returned by the same means following data collection.
Dried blood spot collection.
Blood samples were obtained at T1, T3, and T4 visits. In the original protocol, a research team member performed blood draws in-person; following the COVID-19 protocol modifications, participants collected their own samples, guided by a study staff member via video teleconference or phone. Participants were provided a kit with materials and instructions to be used to assist with the collection of dried blood spots (DBSs), as well as materials for packaging and shipping specimens. Blood drops were collected by study participants by applying a sterile, single-use lancet (BD Microtainer™; Contact-Activated Lancet, Franklin Lakes, NJ) to a finger. Blood from the finger was absorbed onto filter paper (Whatman no. 903; GE Healthcare, Piscataway, NJ) and allowed to dry for at least 4 h before being packed for shipping or drive through/drop-off return.
Occasionally, lancets would fail to activate resulting in no puncture to the finger; for this reason, up to three lancets were provided in each data collection kit. On other occasions, blood flow would not be stimulated and, in those cases, tips for collecting blood were attempted with participants.27 Rarely, participants would complete the blood collection procedure incorrectly, before receiving instructions from study personnel who had planned to guide them through it. In those cases, new materials were sent to the participants.
VIRTUAL DELIVERY OF INTERVENTION SESSIONS
Participants were randomized to CPT or TCTSY. Before the COVID-19 related study pivots, groups of 8–12 women were led through 60-75-min yoga classes, conducted by two facilitators (those methods are published elsewhere).26 Having two facilitators allowed for assisting participants with modifications that might be required to accommodate physical limitations and to address any distress that might arise during yoga practice. Following the protocol modifications, participants and facilitators convened for yoga classes using video teleconferencing over VA-approved platforms to adhere to Centers for Disease Control and Prevention (CDC) social distancing guidelines. Participants in the CPT group engaged in group intervention sessions utilizing video, audio, and chat functions to interact.
Contrastingly, the TCTSY participants disabled their video and audio functions for the entirety of the group yoga practice then utilized the chat function at the conclusion of each session to debrief. Participants disabled their video and audio to adhere to confidentiality practices. The TCTSY sessions were recorded to evaluate the effectiveness of virtual delivery and for utilization in TCTSY supervision to ensure adherence to the framework. In contrast, there is an established precedent for virtual delivery of CPT; therefore, the necessity for recording sessions was negated. In addition, due to the nature of the intervention, participants required the use of their video and audio functions, and therefore, recording would not comply with confidentiality practices.
A study staff member was designated as technology support for the intervention sessions. This provided a point of contact for participants to assist in resolving connectivity issues, reducing burden and disruption for group facilitators and participants. In addition, participants were provided reminder calls and emails. These processes mitigated technology issues and provided a source of support for resolving challenges when they arose.
DATA MANAGEMENT
Managing the collection and security of hard copy and electronic data employed password protected spreadsheets, shared calendars, coding systems, and tracking of the date of each outgoing and incoming contact with participants. Tracking systems were organized by progress in the study and included updated contact information, dates of outgoing and incoming correspondence, and contact efforts, as well as the initials of the staff member who attempted contact. Once enrolled, a separate similar but coded spreadsheet was used, which replaced identifiers with codes.
Results
Before COVID-19 restrictions, we enrolled 152 participants from the Southeastern VA site and seven participants from the Pacific Northwestern VA site. In total, at the Southeastern VA site, nine cohorts were enrolled. The first eight cohorts (March 2016–February 2020) completed all study procedures and interventions in-person at VA settings. The final cohort at that site (Cohort 9) had completed consent, baseline data collection, and 4 weeks of in-person intervention sessions when COVID-19 restrictions were implemented in March 2020. After in-person visits were restricted, the Pacific Northwestern VA site initiated virtual adaptations to enroll 48 participants (Table 2). In total, across the sites, 69 participants completed all or some portion of the research study virtually. Table 2 presents the frequency of participants engaged in the virtual adaptation of the study by cohort and study site.
Table 2.
Participant Engagement by Cohort and Site
| COHORTS 1–8 | COHORT 9 | COHORT 10 | TOTAL | |
|---|---|---|---|---|
| Site | Southeastern U.S. | Southeastern U.S. | Pacific northwest | |
| Design | In-person | Hybrid | Virtual | |
| Consented (n) | 131 | 21 | 48 | 200 |
| Randomized (n) | 91 | 12 | 28 | 131 |
| Completed data collection (n) | 91 | 6 | 19 | 116 |
| Completed some virtual data collection or intervention | 0 | 7 | 48 | 69 |
Discussion
CONTEXT IS KEY
The crisis that the COVID-19 pandemic created for all health care systems is well-documented and requires flexibility, innovation, and acceptance of some insurmountable limitations, all while attempting to provide the best care possible. Health care facilities and clinics have the guidance of CDC, as well as state and federal guidelines, to assist in modifications to be made to allow health care processes to continue. The context in which the adaptions of the research procedures in this multisite RCT is essential to understanding the adaptations themselves, the challenges and limitations, and the successes encountered in the process allowed this study to proceed during the COVID-19 pandemic. The modifications and circumstances described here are limited to the sites that were involved in this study and may inform but may not be generalizable to other health care settings or VA facilities.
TIMING AND NOTIFICATION OF THE RESTRICTIONS
The first COVID-19-related shutdowns occurred mid-March 2020, at a time when policies, information, and guidelines were changing rapidly. At the Pacific Northwest VA site, the study team convened virtually and requested consultation and guidance from VA privacy officers, information security officers, and IRB personnel. The suggested adaptations that were written into the modified protocol concerned approved technology and telehealth platforms, methods for communicating with research participants, and data collection and storage platforms. Overcoming challenges to telehealth, including technology access, technology competency, and data security, required innovation in the context of rapid and frequent changes in COVID-19 protocols.
INFORMED CONSENT DOCUMENTS
Collecting ICDs before collecting data was met with significant challenges disrupting the flow of enrollment. Due to time constraints, reviewing consent was often done with participants using electronic versions of consent documents, which could be delivered in minutes where hard copy document delivery took days to weeks. Errors made by participants on the ICDs meant having to send/receive new hard copy documents resulting in time lost and increased attrition. Complications to completing the consent process reflect a limitation that inadvertently affected our pool of participants, resulting in a participant pool with more technology fluency and access.
INTERVENTION DELIVERY CHALLENGES AND BENEFITS
Telehealth platforms eliminated the necessity for participants to travel to access treatment, which reduced burden and increased access. In addition, the ability to access treatment from their homes reportedly reduced childcare constraints for some participants. Similar to the findings of other studies, the use of in-home telehealth platforms reportedly reduced burden as many participants indicated that visiting VA medical centers and interacting with male Veterans cause an increase in PTSD symptoms.28 In addition to eliminating scenarios of stress, participants were provided with an increased sense of privacy and security while participating in their home environment. Participants reported an appreciation for the ability to have their video and audio off to take a moment for themselves during sessions, a form of privacy that would not have been feasible in person.
Despite the many benefits of telehealth, it also presents additional costs and burden. There was additional burden with utilizing the telehealth platforms during intervention sessions. Difficulties with connectivity were heightened in a group setting, and individual challenges occasionally provided distractions for the group as a whole.29 While this was mitigated by designating a staff member to be on call during interventions for technological support, it still proved to be a barrier. While participating from home resolved travel and childcare issues, it also produced challenges. Participants reported having a harder time focusing due to distractions and background noise in their homes and the homes of others in the session.29 A few participants reported having difficulty finding private locations in their homes for the duration of the sessions. These challenges were unique to virtual delivery and would not have been present in person.
VIRTUAL ASSESSMENT CHALLENGES AND BENEFITS
Virtually conducted research relies heavily on participants’ technology literacy which, indirectly, contributes to barriers in securing and retaining participants. There is more work performed by the participant which increases the burden on the participant and may require the participant to engage in activities traditionally completed by technicians. However, the benefit of a virtual study such as this is that it saved participants’ transportation costs. Similarly, they were able to participate in the comfort of their own home while reducing risk of exposure to COVID-19.
CONSIDERATIONS FOR DBSs
Collection of blood samples during the pandemic required modifications to ensure the safety of participants and study team members. To continue our investigation of the biological mechanisms involved in PTSD and intervention response, we obtained DBS samples from participants. Recently, a variety of new DBS applications have been developed, including methods for cytokine analysis.30-32
These innovations are timely for our research, but changes were needed to introduce DBS methods to study protocols. For example, adaptations for the use of DBS methods required that all study team members who handled, shipped, or received supplies related to DBS collection were required to have previously completed the Dangerous Goods & Psychophysiological Materials Shipper training. Additional supplies had to be purchased for safety. However, efforts needed to comply with these requirements did not reduce the potential benefits of collecting biological samples at home, such as expanding participation opportunities to those who had transportation barriers or felt uncomfortable in a hospital setting.
MANAGING AND ADDRESSING RISK
Individuals with PTSD are at a higher risk for suicidal ideation.30 Managing imminent suicidal ideation when using virtual means with individuals in remote areas requires a protocol involving local police and a procedure for assessing risk and hospitalizing participants if necessary. On one occasion during data collection, a participant disclosed a level of risk which prompted the assessor to involve the responsible clinician who completed a more in-depth screen. Hospitalization was not required, but had it been, the study team was equipped with a protocol to contact local police to assist those with imminent risk to find safety and hospitalization.
Conclusions
This RCT provided clinical care to Veterans that due to safety precautions of the early pandemic was threatened to be abruptly ceased. As such, it was essential that adaptations be made to enable continuation of the trial. It was incumbent upon the investigators to be innovative and flexible to continue to provide and evaluate the interventions, assuring their alignment with local and national guidance. The changes made for this virtual pivot required prospective participants to have a working proficiency of mobile technology and be able to access internet connectivity for the duration of the study.
The modified, virtual study protocol described here may be applied to research aimed toward populations that are geographically remote or have other factors limiting access to health care facilities. While the impetus for adapting this clinical trial was clearly unfortunate, the successful adaptation acknowledged the many applications and new opportunities for telehealth. It is incumbent that eResearch/tele-research advances alongside clinical practice in having clearly defined processes and ethics for its conduct. A major benefit of doing so is that marginalized, underrepresented populations who have previously not had access to study participation will be able to do so. The inclusion of diverse populations in research is critical for our ability to obtain study findings that can have clinical benefit for all people.
It is likely that the transition to a virtual world during the COVID-19 pandemic will provide enhanced appreciation of the benefits of telehealth and tele-research and it is our hope and expectation that it will continue to be offered to patients and participants when social distancing guidelines are relaxed. The publication of this article lands at the beginning of the third year of the global COVID-19 pandemic. At the time of publication, social distancing guidelines prevail and restrictions and closures of public spaces exist in some parts of the country, and gatherings (especially indoors) are cautioned against by the CDC. While the pandemic wages on, the adaptation of all facets of life continues, health care and treatment outcome research included.
It is our hope that this publication will assist in the ongoing adaptations made to allow as many facets and domains of life to continue, although in a new and safe way. The benefits of the successful modification to deliver CPT and TCTSY interventions through virtual means for the purposes of research may now provide others conducting virtual studies with potential methods for doing so alongside evolving technologies to assist with virtually conducted research.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at VA Portland Health Care System, Portland, OR, Atlanta VA Health Care System, Decatur, GA, Oregon Health & Science University, Emory University and the Agency for Healthcare Research and Quality. The authors acknowledge the Veterans who participated in this study for their military service and study participation. The authors thank the additional staff and consultants who contributed to this project. The authors also thank the clinicians and yoga facilitators who treated study participants.
Funding Information
This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, grant no. 5I01HX001087-02 and Agency for Healthcare Research and Quality, grant no. This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, grant no. 5I01HX001087-02. This work was also supported, in part, by Merit Review award no. 5 I01BX002061-06 from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development.
Footnotes
Disclaimer
The contents of this article do not represent the views of the U.S. Department of Veterans Affairs, the U.S. Government, or the official views of the Agency for Healthcare Research and Quality.
Disclosure Statement
No competing financial interests exist.
REFERENCES
- 1.Daschle T, Dorsey R. The return of the house call. Ann Intern Med 2015;162:587–588. [DOI] [PubMed] [Google Scholar]
- 2.Dorsey ER, Topol EJ. State of telehealth. N Engl J Med 2016;375:154–161. [DOI] [PubMed] [Google Scholar]
- 3.Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc, 2020;27:957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bashsur R, Shannon GW. History of telemedicine: Evolution, context, and transformation, Vol. 2009. New Rochelle, NY: Mary Ann Liebert, 2009. [Google Scholar]
- 5.Brown EM. The Ontario telemedicine network: A case report. Telemed J E Health 2013;19:373–376. [DOI] [PubMed] [Google Scholar]
- 6.American Medical Association. AMA digital health care 2016 & 2019 study findings. 2020. Available at https://www.ama-assn.org/system/files/2020-02/ama-digital-health-study.pdf (last accessed January 27, 2022).
- 7.Ornstein KA, Leff B, Covinsky KE, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med 2015;175:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maar MA, Beaudin V, Yeates K, et al. Wise practices for cultural safety in electronic health research and clinical trials with indigenous people: Secondary analysis of a randomized clinical trial. J Med Internet Res 2019;16:e14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker TB, Gustafson DH, Shah D. How can research keep up with ehealth? Ten strategies for increasing the timeliness and usefulness of ehealth research. J Med Internet Res 2014;16:10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wittson CL, Affleck DC, Johnson V. Two-way television in group therapy. Ment Hosp 1961;12:22–23. [DOI] [PubMed] [Google Scholar]
- 11.Darkins A. The growth of telehealth services in the Veterans Health Administration between 1994 and 2014: A study in the diffusion of innovation. Telemed J E Health 2014;20:761–768. [DOI] [PubMed] [Google Scholar]
- 12.Landow SM, Oh DH, Weinstock MA. Teledermatology within the veterans health administration, 2002–2014. Telemed J E Health 2015;21:769–773. [DOI] [PubMed] [Google Scholar]
- 13.LeRouge C, Garfield MJ. Crossing the telemedicine chasm: Have the US barriers to widespread adoption of telemedicine been significantly reduced? Int J Environ Res Public Health 2013;10:6472–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alverson DC, Shannon S, Sullivan E, et al. Telehealth in the trenches: Reporting back from the frontlines in Rural America. Telemed J E Health 2004;10:S-95–S-109. [PubMed] [Google Scholar]
- 15.Jacobson PD, Selvin E. Licensing telemedicine: The need fora national system. Telemed J E Health 2000;6:429–439. [DOI] [PubMed] [Google Scholar]
- 16.Gilmore AK, Ward-Ciesielski EF. Perceived risks and use of psychotherapy via telemedicine for patients at risk for suicide. J Telemed Telecare 2019;25:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen O, Fox B, Mills N et al. COVID-19 and commercial pharma: Navigating an uneven recovery. 2020. Available at https://www.mckinsey.com/industries/life-sciences/our-insights/covid-19-and-commercial-pharma-navigating-an-uneven-recovery (last accessed November 12, 2021). [Google Scholar]
- 18.Scott A, Woodhouse LD, Watson D, et al. The Southeast Telehealth Network: Using technology to overcome the barriers to rural public health practice. J Public Health Manag Prac 2011;17:164–166. [DOI] [PubMed] [Google Scholar]
- 19.Triana AJ, Gusdorf RE, Shah KP, et al. Technology literacy as a barrier to telehealth during COVID-19. Telemed J E Health 2020;26:1118–1119. [DOI] [PubMed] [Google Scholar]
- 20.Monaghesh E, Hajizadeh A. The role of telehealth during COVID-19 outbreak: A systematic review based on current evidence. BMC Public Health 2020:20:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding X, Clifton D, Ji N, et al. Wearable sensing and telehealth technology with potential applications in the coronavirus pandemic. IEEE Rev Biomed Eng 2020;14:48–70. [DOI] [PubMed] [Google Scholar]
- 22.Fong B, Fong ACM, Li CK. Telemedicine technologies: Information technologies in medicine and telehealth. Chichester, West Sussex, UK: John Wiley & Sons, 2011. [Google Scholar]
- 23.Wijesooriya NR, Mishra V, Brand P, et al. COVID-19 and telehealth, education, and research adaptations. Paediatr Resp Rev 2020;35:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmotzer GL. Barriers and facilitators to participation of minorities in clinical trials. Ethn Dis 2012;22:226–230. [PubMed] [Google Scholar]
- 25.Anderson A, O'Connell SS, Thomas C, et al. Telehealth interventions to improve diabetes management among Black and Hispanic patients: A systematic review and meta-analysis. J Racial Ethn Health Disparities 2022;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly U, Haywood T, Segell E, et al. Trauma-sensitive yoga for posttraumatic stress disorder in women veterans who experienced military sexual trauma: Interim results from a randomized controlled trial. J Altern Complement Med 2021;27:S45–S59. [DOI] [PubMed] [Google Scholar]
- 27.McDade TW. Development and validation of assay protocols for use with dried blood spot samples. Am J Hum Biol 2014;26:1–9. [DOI] [PubMed] [Google Scholar]
- 28.Marshall V, Stryczek KC, Haverhals L, et al. The focus they deserve: Improving women Veterans' health care access. Womens Health Issues 2021;31:399–407. [DOI] [PubMed] [Google Scholar]
- 29.Kneeland ET, Hilton BT, Fitzgerald HE, et al. Providing cognitive behavioral group therapy via videoconferencing: Lessons learned from a rapid scale-up of telehealth services. Pract Innov 2021;6:221–235. [Google Scholar]
- 30.Stevens D, Wilcox HC, MacKinnon DF, et al. Posttraumatic stress disorder increases risk for suicide attempt in adults with recurrent major depression. Depress Anxiety 2013;30:940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller EM, McDade TW. A highly sensitive immunoassay for interleukin-6 in dried blood spots. Am J Hum Biol 2012;24:863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villar-Hernández R, Latorre I, De Souza-Galvão ML, et al. Use of IP-10 detection in dried plasma spots for latent tuberculosis infection diagnosis in contacts via mail. Sci Rep 2019;9:3943. [DOI] [PMC free article] [PubMed] [Google Scholar]