1. Introduction
Migraine is a complex neurovascular disorder that afflicts approximately 15% of the population with an almost three times higher prevalence in women over men [7]. Migraine presents as moderate to severe headaches that are characterized as either with or without a visual aura consisting of reversible visual, sensory, or motor symptoms that usually precedes the headache phase [2; 10; 13]. Migraine is considered to be a strongly heritable disorder and the genetic bases for familial hemiplegic migraine (FHM) are established and well-studied, but these rare monogenetic mutations are present in a very small fraction of migraineurs [2; 52]. Conversely, little is known of the underlying genetic basis for common forms of migraine. To this end, over the last decade, multiple genome-wide association studies (GWAS) have been conducted and identified single-nucleotide polymorphisms (SNPs) linked to either protection from migraine or associated with increased migraine susceptibility [13; 14; 23; 25; 32]. While these studies have identified many genes that may underlie common migraine, subsequent functional analyses are needed to determine how these factors contribute to migraine pathogenesis.
Some of the most the common mutations associated with migraine risk genome-wide reside in or near the genetic locus of the transient receptor potential melastatin 8 (TRPM8) channel [13; 14; 23; 25; 32; 38]. TRPM8 is a cold-sensitive, non-selective cation channel expressed in primary afferent neurons of the trigeminal (TG) or dorsal root ganglia (DRG) that mediates both innocuous and noxious cold sensations, as well as is necessary for cold allodynia and hyperalgesia in various pathological settings [40; 48; 63]. While its role in general somatosensation and nociception has been well established, whether the channel mediates migraine-related pain is unclear [19]. Of the migraine-related TRPM8 SNPs identified, all are localized either upstream of the transcription start site or are intronic, thus are likely to not alter channel function but appear to effect channel expression [13; 14; 23; 25; 29]. Preclinical studies examining the role of TRPM8 in migraine are limited and contradictory [8; 56]. For example, the TRPM8 agonist menthol was found to inhibit pain behaviors in mice induced by inflammation of the dura [56], whereas another study found that stimulation of the rat dura with a different agonist produced allodynia in both the face and hind paw, sensitization that was attenuated with known migraine therapeutics [8]. Thus, while TRPM8 is genetically linked to migraine prevalence, it is unclear if activation or inhibition of TRPM8 afferents can be an effective avenue for migraine treatment.
To address the role of TRPM8 in this disorder we used mouse genetics and TRPM8 antagonism to determine if TRPM8 channels or neurons are required for migraine-like pain in inducible rodent migraine models. Here, we report that TRPM8 is required for both evoked and spontaneous pain observed in wildtype animals induced by two migraine-related pain triggers. Moreover, inhibition of TRPM8 channels during the migraine induction phase was effective in preventing migraine-related pain both acutely and chronically, but did not alter pain that occurs when chronic migraine is established. These data demonstrate that TRPM8 is required for the induction of migraine-like pain and is worthy of critical attention as a potential therapeutic target to treat this devastating disorder.
2. Experimental procedures
2.1. Animals
All experiments were approved by the University of Southern California Institutional Animal Care and Use Committee. Mice greater than 8 weeks of age were used in all experiments and housed under a 12-hour light-dark cycle in a temperature-controlled environment with ad lib access to food and water. Unless otherwise note, equal numbers of male and female mice were used. Mice were lightly anesthetized with 5% isoflurane prior to each injection. Wildtype and TRPM8−/− mice [6] were purchased from Jackson Laboratories and were on the C57Bl/6 background. TRPM8DTR mice were generated in-house and TRPM8 afferents were selectively ablated as described previously [40]. Specifically, TRPM8DTR mice and their wildtype littermates aged ≥8 weeks were given two intraperitoneal (i.p.) injections of diphtheria toxin (DTx; 50μg/kg in normal saline; Sigma) administered three days apart. Migraine induction and behavioral tests were performed at least 14 days post the initial DTx injection and the effectiveness of TRPM8 neuron ablation was confirmed by cold behavior testing prior to the experiments described herein (mean cold plantar latency DTx-treated wildtype mice 14.1±0.69 sec versus 26.9 ± 0.56sec for TRPM8DTR DTx-treated; 30sec cut-off time used [40]). The selective TRPM8 antagonist 1-phenylethyl-4-(benzyloxy)-3-methoxybenzyl(2-aminoethyl)carbamate (PBMC; Focus Biomolecules) [39] was dissolved in DMSO then diluted to a final concentration of 1mg/ml in 0.9% saline. PBMC (10mg/kg) or vehicle solutions (5% DMSO in saline) were administered subcutaneously as described [39], followed by migraine-related pain induction with the timelines described in Results.
2.2. Nitroglycerin model
Nitroglycerin (NTG) was freshly prepared from a 1% solution in propylene glycol (VWR, CAT# 101095-632) then diluted in 0.9% saline. Mice were given an intraperitoneal injection of 10mg/kg NTG [54] with control mice injected with a vehicle solution of 0.9% saline plus equal amounts of propylene glycol as in the NTG injections. Injected mice were placed in the test chamber for recovery prior behavioral testing. To model chronic migraine-associated pain, five NTG injections were performed intraperitoneally every other day for 9 days as described [54].
2.3. Calcitonin gene-related peptide (CGRP) model
Rat α-CGRP (Sigma-Aldrich, CAT#C0292) was handled following the supplier’s instructions prior to being diluted in 0.9% saline and injected intraperitoneally at 0.1mg/kg. Controls mice were injected with an equal volume of 0.9% saline [45].
2.4. Hind paw von Frey Assay
Mechanical sensitivity (50% withdrawal threshold) was determined 2 hours (NTG) or 30 minutes (CGRP) post-injection with calibrated von Frey (Semmes-Weinstein) monofilaments using the up and down method as previously described [12; 49; 51; 67]. Briefly, filaments (starting with the 0.4g filament; flexible force range of 0.008 to 2 g) were applied to the plantar surface of the hind paw for up to 5–6 seconds until the filament bent or a positive response was noted (withdrawal, licking or shaking of the hind paw). Depending of the response of the animal the next heaviest (no response) or lightest (response) filament was used in sequential order for a total of four additional filaments using the up-down method, with the 50% withdrawal threshold calculated as described [12]. Following testing, animals were returned to their home cages until the next test/treatment day.
2.5. Mouse Grimace Scale
Mice were individually placed unrestrained in a 4-ounce horizontal paper cup (First Street) placed 20–30cm above a layer of soft mouse bedding, and separated by 10cm horizontally and 10cm vertically to avoid interaction between animals. All mice were habituated to the testing environment for 2 hours the day before testing and then an additional 90 minutes prior to each experiment. Eight-minute videos were acquired prior and then 2 hours (NTG) or 30 minutes (CGRP) post-treatment using a high-resolution digital video camera (Nikon D7500). Five images were randomly selected from each video such that there was at least 1 minute between each image, and there was no exploration, grooming or sleeping behavior observed during or 2 seconds before or after the selected images. The Mouse Grimace Scale was determined using a three-point scoring system as previously described [42]. Briefly, five distinct facial features (orbital tightening, nose bulge, cheek bulge, ear position, and whisker change) were coded in a three-point scale (0 = not present, 1 = moderately present, 2 = obviously present), and each animal was independently scored by at least two individuals.
2.6. Statistical Analysis
All data represented as mean and +/− SEM error bars. Power analysis was performed to determine an appropriate sample size. Statistical analysis was performed using Prism Graphpad 9 software. For tests within subjects pre- versus post-treatment a paired t-test with Wilcoxon matched-pairs signed rank test was used. For tests between subjects, two-way analyses of variance (ANOVAs) were performed. A two-way repeated measures ANOVA, with a Holm-Sidak post hoc test, were performed for chronic pain measurements across treatments times and conditions. All experimenters were blinded to genotype, pharmacological treatments, and the various migraine induction procedures used.
3. RESULTS
3.1. Functional expression of TRPM8 channels is required for nitroglycerin induced acute and chronic mechanical allodynia.
Activation of the nitric oxide (NO) signaling pathway via the NO donor nitroglycerin (NTG) induces a delayed migraine-like headache in human migraineurs [64]. In mice, systemic NTG induces both acute and chronic cutaneous mechanical allodynia that is attenuated by sumatriptan and topiramate, respectively [4; 49; 54; 65]. NTG is proposed to induce migraine-like pain via sensitization of the trigeminovascular system which leads to vasodilation of cranial blood vessels and activation of pathways involved in nociception [4; 54].
Using this established rodent migraine model, we sought to determine if TRPM8 plays a functional role in these pain behaviors in mice, specifically testing cutaneous mechanical allodynia. Wildtype and TRPM8-null mice (TRPM8−/−) [6], of both sexes, were given a single intraperitoneal (i.p.) injection of either vehicle or NTG (10mg/kg) with their hind paw mechanical sensitivity tested two hours post-injection [35; 54; 67]. As previously reported, at all days tested we observed a significant decrease in the 50% mechanical withdrawal threshold in wildtype mice injected with NTG compared to baseline sensitivity, as well as compared to vehicle-injected mice (Figure 1A). Remarkably, this phenotype was absent in TRPM8−/− mice who exhibited no significant differences in mechanical sensitivity after NTG treatment compared to vehicle injected animals or baseline at all days tested (Figure 1B). Conversely, NTG-injected TRPM8−/− mice were significantly different than wildtype animals post-NTG injection (p<0.01 wildtype versus TRPM8−/− post-NTG at Day 1; n=8 each genotype; two-way ANOVA). Moreover, as we and others have shown, naïve TRPM8−/− mice exhibit no deficiencies in acute mechanical sensitivity (p>0.05 wildtype versus TRPM8−/− baseline (BL); n=8 each genotype; two-way ANOVA) [6; 18; 40; 43], important as this demonstrates that this lack of a phenotype in TRPM8−/− mice is not due to differences in basal mechanosensation between the two genotypes.
Figure 1. Mechanical allodynia induced by repeated NTG requires TRPM8 as does the development chronic migraine-like pain.

(Top) Schematic illustrates the repeated injection protocol of either vehicle (saline plus PG) or NTG over the course of 5 injections. (A) At all days tested, wildtype mice receiving repeated intermittent NTG injections show enhanced mechanical allodynia two hours post-injection compared to vehicle injected controls (**p<0.01, ***p<0.001, two-way repeated measures ANOVA, with a Holm-Sidak post hoc test, n=8 of each genotype). (B) In contrast, mechanical sensitivity is unaffected in TRPM8−/− mice with repeated NTG injections at each day tested (nsp>0.05 compared to vehicle, two-way repeated measures ANOVA, with a Holm-Sidak post hoc test, n=6–8 of each genotype). (C) Basal mechanical allodynia developed with repeated NTG injections in wildtypes, becoming significant by day 5 compared to vehicle injected animals (*p<0.05, **p<0.01, two-way repeated measures ANOVA, with a Holm-Sidak post hoc test, n=8–10 for each genotype). (D) Chronic basal mechanical allodynia was not produced by repeated NTG in TRPM8−/− mice (nsp>0.05 compared to vehicle at all days tested, two-way repeated measures ANOVA, with a Holm-Sidak post hoc test, n=6–8 for each genotype).
In addition to inducing acute effects, repeated, intermittent treatment with nitroglycerin produces enhanced mechanical allodynia and basal sensitization, the latter proposed to represent a model of chronic migraine [54]. Thus, we repeated the injections of vehicle or NTG every two days for a total of five injections to determine if TRPM8−/− mice mount a pain phenotype after each injection over this span [4; 54]. Mice were tested prior to and two hours post-injection on each day and consistent with previous studies wildtype mice show enhanced mechanical allodynia after each injection of NTG that was significantly different than vehicle injected mice (Figure 1A). In contrast, the post-injection mechanical sensitivity of TRPM8−/− mice was unaffected with repeated injections and not different than vehicle injected mice (Figure 1B). Furthermore, to assess chronic pain, we examined basal mechanical sensitivity in the mice given repeated NTG injections, observing a significant increase in mechanical allodynia in wildtype mice starting at Day 5, compared to Day 1 or animals injected with vehicle (Figure 1C). In contrast, TRPM8−/− mice injected with NTG did not exhibit any changes in basal mechanical sensitivity compared to before the NTG injections and were indistinguishable from vehicle controls (Figure 1D). Thus, in addition to acute mechanical allodynia, TRPM8 is required for the development of chronic pain in the NTG mouse model.
3.2. The absence of NTG-induced pain in mice lacking TRPM8 is not sexually dimorphic.
Migraine is a sexually dimorphic disease that affects women more than men [62]. Moreover, we have found that TRPM8-mediated cold allodynia induced by neurogenic inflammation is also sexually dimorphic [67]. Thus, to determine if the lack of a phenotype observed in TRPM8-nulls is due to sex differences we increased the number of mice of each sex tested in Figure 1 and examined mechanical sensitivity in wildtype and TRPM8−/− mice pre- and post-NTG injection. When separated by sex, we observed that a single injection of NTG induced mechanical allodynia equally in naive male (Fig. 2A) and female (Fig. 2B) wildtype mice. We did not find a statistically significant difference in the levels of mechanical sensitization between males and females after NTG injection (p>0.05, n=10–12, two-way ANOVA). Consistent with our ensemble results, TRPM8−/− mice of either sex did not exhibit acute mechanical sensitization after a single NTG injection as we observed no difference in mechanical sensitivity pre- versus post-NTG injection in TRPM8−/− mice of either sex. (Figs 2A, B).
Figure 2. Lack of acute and chronic mechanical allodynia in both male and female TRPM8−/− mice.

The absence of mechanical allodynia in TRPM8−/− mice after a single NTG injection was consistent in both male (A) and female (B) mice (**p<0.01, ***p<0.001 pre- versus post-NTG wildtype mice; nsp>0.05 pre- versus post-NTG TRPM8−/− mice; paired t-test with Wilcoxon matched-pairs signed rank test, n=10–12 for each genotype). Basal mechanical sensitivity (pre-NTG) was not significantly different between the two genotypes of either sex (p>0.05, two-way ANOVA), but was significantly different post-NTG between the genotypes of both sexes (**p<0.01, ***p<0.001; two-way ANOVA). Similar results were obtained in both male (C) and female (D) mice with chronic migraine-like pain (Day 9) before and after NTG injection (**p<0.01, ***p<0.001, ****p<0.0001 pre- versus post-NTG wildtype mice; nsp>0.05 pre- versus post-NTG TRPM8−/− mice; paired t-test with Wilcoxon matched-pairs signed rank test, n=9 wildtype males, n=7 TRPM8−/− males, n=12 wildtype females, n=8 TRPM8−/− females). In addition, there still were significant differences post-NTG between the genotypes of both sexes (***p<0.001, ****p<0.0001; two-way ANOVA).
Next, we asked if there were sex differences when chronic mechanical allodynia has developed. Mice of both genotypes were examined before and after the fifth NTG injection (see Figure 1A) and, as with acute mechanical allodynia, we did not observe a significant difference between male and female wildtype mice after injection of NTG (p>0.05, n=6–10 for each genotype, two-way ANOVA). Moreover, wildtype mice of both sexes developed equivalent levels of basal mechanical allodynia with repeated NTG injections (pre-injected, p>0.05, n=6–10, two-way ANOVA). Lastly, as in the acute setting withdrawal thresholds in male (Figure 2C) and female (Figure 2D) TRPM8−/− mice injected with NTG were significantly different than similarly treated wildtype mice, but did not exhibit differences in basal mechanical sensitivity. Thus, these data show that TRPM8 is required for peripheral tactile allodynia in the NTG model of acute and chronic migraine irrespective of sex.
3.3. TRPM8 afferents are required for NTG-induced mechanical allodynia.
Genetic compensation in response to gene deletion is a well-known occurrence that can influence interpretation of the phenotypic analyses of gene-null mice [22]. To address this potential confound in our assessment of the physiological role of TRPM8, we and others previously developed a conditional and selective TRPM8-neuron ablation strategy using transgenic expression of the simian receptor for diphtheria toxin in TRPM8 afferents (TRPM8DTR) [40; 53]. TRPM8DTR mice are allowed to develop normally into adulthood after which TRPM8 neurons are selectively ablated with systemic injections of Diphtheria Toxin (DTx), allowing for a comparison of the molecular versus cellular role for TRPM8.
Using this established approach, we asked if mice lacking TRPM8 afferents (DTx-injected TRPM8DTR mice) exhibit either acute or chronic mechanical allodynia in the hind paw after systemic treatment with NTG, similar to our analysis of TRPM8−/− mice. TRPM8DTR (ablated) and wildtype (control) littermates were given two consecutive intraperitoneal injections of DTx (50 g/kg in saline) administered 3 days apart [40]. Greater than two-weeks later, these animals were tested for a lack of cold sensitivity using the cold plantar assay to validate ablation of TRPM8-positive afferents (see Methods [40]). Mice were then given repeated injections of NTG or vehicle, and mechanical sensitivity was tested as described above. Similar to our previous results, DTx-injected wildtype mice exhibited profound mechanical allodynia after each injection of NTG compared to vehicle injected animals (Figure 3A), whereas there was no effect in TRPM8 afferent ablated mice (Figure 3B). Similarly, repeated NTG injections produced chronic mechanical allodynia in DTx-treated wildtypes (Figure 3C), a phenotype absent in mice lacking TRPM8 neurons (Figure 3D). Thus, NTG-induced acute and chronic cutaneous mechanical allodynia requires both TRPM8 channels and neurons.
Figure 3. Mice lacking TRPM8 afferents do not develop mechanical allodynia after NTG treatment.
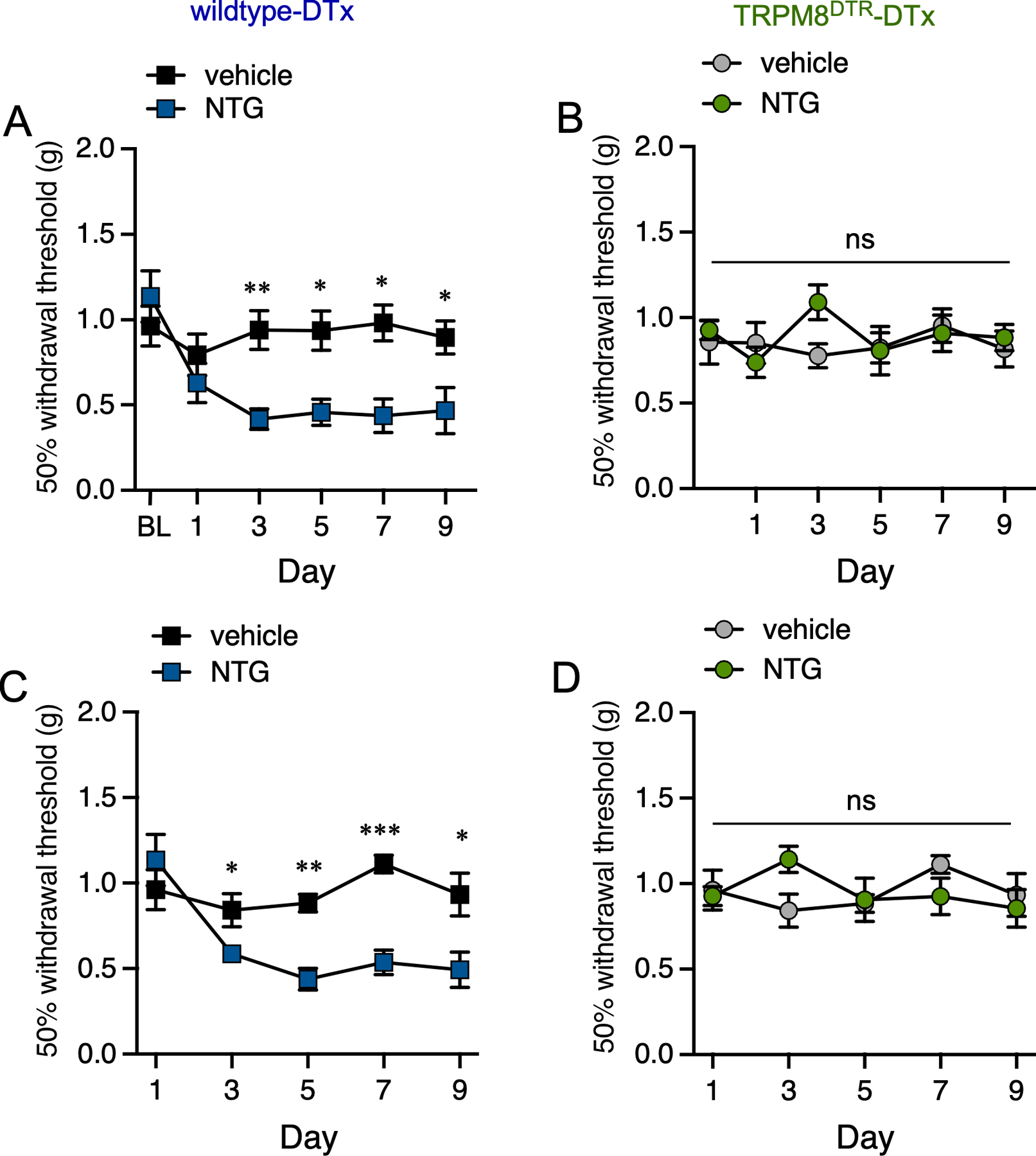
(A) DTx-treated wildtype mice exhibit mechanical allodynia two hours post-injection of NTG compared to vehicle injected controls at all days tested (*p<0.05, **p<0.01, two-way repeated measures ANOVA, with a Holm-Sidak post hoc test, n=8 for both conditions). (B) Mechanical sensitivity is unaffected in DTx-injected TRPM8DTR mice with repeated NTG injections at each day tested (nsp>0.05 compared to vehicle, two-way repeated measures ANOVA, with a Holm-Sidak post hoc test, n=6–7 for both conditions). (C) Basal mechanical allodynia was observed in DTx-treated wildtype mice given repeated NTG injections when compared to mice treated with vehicle (*p<0.05, **p<0.01, ***p<0.001, two-way repeated measures ANOVA, with a Holm-Sidak post hoc test, n=8 for each condition). (D) A similar phenotype was absent in TRPM8DTR mice treated with DTx (nsp>0.05, two-way repeated measures ANOVA, with a Holm-Sidak post hoc test, n=6–7 for both conditions).
3.4. Spontaneous pain induced by NTG requires TRPM8 channels and neurons.
A caveat for using cutaneous mechanical allodynia as a read-out for migraine-related pain is that it is a measure of evoked and not spontaneous pain and, while a traditional pre-clinical model of migraine, may not be truly relatable to headaches. Thus, we employed the Mouse Grimace Scale (MGS), an established approach to assess pain behaviors in mice [9; 42], to further test the necessity of TRPM8 channels and afferents in this pain phenotype. Specifically, wildtype and TRPM8−/− were given repeated injections of NTG to induce chronic pain (Figure 4, see schematic, 3 injections of NTG or vehicle every 2 days) then we assessed their MGS responses at baseline (BL) and post-treatment on Day 7 (Figure 4A, 4th injection). As expected, NTG-treated wildtype mice exhibited significant changes in the MGS score compared to baseline and vehicle injected animals (Fig. 4B). Furthermore, as this protocol produces chronic migraine like pain, we also observed a significant difference in MGS between vehicle and NTG injected mice at baseline on Day 7. Conversely, we did not observe any differences in MGS in TRPM8−/− mice pre- and post-NTG injection, or between vehicle and NTG injected mice (Fig. 4C).
Figure 4. Spontaneous NTG-induced migraine-like pain requires TRPM8.

The Mouse Grimace Scale was used to assess spontaneous pain in wildtype and TRPM8−/− mice before and after injection of either vehicle or NTG on Day 7 (top). In wildtype mice (A), NTG injection induced clear discomfort compared to BL (*p<0.05, paired t-test with Wilcoxon matched-pairs signed rank test, n=14) and vehicle injected mice (*p<0.05, two-way ANOVA, n=10). There was no difference pre and post vehicle injection (p>0.05, paired t-test with Wilcoxon matched-pairs signed rank test). (B) In contrast, neither vehicle (n=14) or NTG (n=12) injection induced pain in TRPM8−/− mice post injection (nsp>0.5, BL versus post-injection, paired t-test with Wilcoxon matched-pairs signed rank test), nor was there as a difference in animals injected with vehicle versus NTG (nsp>0.05, two-way ANOVA). (C) Similarly, MGS ratings were significantly different in DTx injected wildtype mice given NTG compared to BL (*p<0.05, paired t-test with Wilcoxon matched-pairs signed rank test, n=9) and vehicle injected mice (*p<0.05, two-way ANOVA, n=5–9). As with TRPM8−/− mice, no difference was observed in mice injected with vehicle (nsp>0.05, BL versus post-injection, paired t-test with Wilcoxon matched-pairs signed rank test). (D) DTx-injected TRPM8DTR mice exhibited no signs of discomfort with either vehicle or NTG injections (nsp>0.5, BL versus post-injection, paired t-test with Wilcoxon matched-pairs signed rank test, n=11), and there was no difference in animals injected with vehicle versus NTG injection (nsp>0.05, two-way ANOVA, n=8–11).
We repeated this experimental protocol in mice lacking TRPM8 afferents, observing an identical phenotype in that control mice exhibited an increased MGS score after injection of NTG (Figure 4C, BL versus post-injection), as well as showed chronic pain (BL MGS of vehicle versus NTG injected animals). As with mice lacking TRPM8 channels, DTx-treated TRPM8DTR mice exhibited no change in their MGS scores either pre- or post-NTG injections or compared to vehicle injected mice (Figure 4D). Thus, as with mechanical allodynia, mice lacking either TRPM8 channels or neurons are insensitive to the effects of either acute or chronic NTG in terms of spontaneous pain.
3.5. TRPM8 is required for calcitonin gene-related peptide (CGRP) induced migraine-like pain.
CGRP plays a central role in the pathophysiology of migraine as it induces migraine-like headache in migraineurs and inhibiting its function can alleviate migraines [36]. NTG activation of the trigeminovascular system increases calcitonin gene-related peptide (CGRP) in both the peripheral and central nervous system [11], and CGRP receptor antagonism reduces pain evoked by NTG in rodents [33; 34]. Thus, to further establish the role of TRPM8 in migraine-like pain we asked in CGRP-induced nociceptive behaviors were dependent on TRPM8. Intraperitoneal injections of 0.1mg/kg α-CGRP were performed in wildtype and TRPM8−/− mice and we assessed pain using the Mouse Grimace Scale 30 minutes post-treatment [33]. CGRP produced an increased MGS score in wildtype mice that was significantly different than prior to the injection (BL), and to vehicle injected mice (Figure 5A). In contrast, and consistent with the NTG model, we observed no difference in pain responses in TRPM8−/− mice with systemic CGRP compared to baseline or control mice injected with vehicle (Figure 5B). We also examined hind paw mechanical allodynia in mice of both genotypes given a single i.p. injection of CGRP [3; 16], observing a similar phenotype as we find with NTG. Wildtype mice showed increased mechanical sensitivity 30 minutes post-injection compared to baseline, whereas TRPM8−/− animals were unaffected (Figure 5C). Thus, TRPM8 channels are required to CGRP-induced pain in mice.
Figure 5. Migraine-like pain induced by systemic CGRP requires TRPM8.

(A) Spontaneous pain (Mouse Grimace Scale) was measured 30min post-systemic CGRP in naïve wildtype mice (**p<0.01 BL versus post-injection, paired t-test with Wilcoxon matched-pairs signed rank test, n=6) but not after vehicle (nsp>0.05, paired t-test with Wilcoxon matched-pairs signed rank test, n=8). There was a significant difference in the grimace score between mice injected with either vehicle or CGRP (***p<0.001, two-way ANOVA). (B) In contrast, TRPM8−/− mice injected with either vehicle or CGRP exhibited no discomfort post injection (nsp>0.5, paired t-test with Wilcoxon matched-pairs signed rank test, n=6–8). Consistently, there was no difference in animals injected with vehicle versus CGRP (nsp>0.05, two-way ANOVA). (C) A single injection of CGRP induced mechanical allodynia 30min post-injection in wildtype but not in TRPM8−/− mice (*p<0.05, pre- versus post-CGRP wildtype mice; nsp>0.05 pre- versus post-CGRP TRPM8−/− mice; paired t-test with Wilcoxon matched-pairs signed rank test, n=6–8). In addition, there were significant differences between wildtype and TRPM8−/− mice after the CGRP injections (**p<0.01, two-way ANOVA).
3.6. Inhibition of TRPM8 prevents NTG-induced acute mechanical allodynia and the development of chronic migraine-like pain.
While our results with TRPM8−/− and TRPM8DTR mice are intriguing, they do not provide any insights into the possible relevance of TRPM8 as a potential target for migraine-like pain treatment. We and others have previously characterized a potent and selective TRPM8 antagonist 1-phenylethyl-4-(benzyloxy)-3-methoxybenzyl(2-aminoethyl)carbamate (PBMC) in the inhibition of acute cold sensation and pathological cold allodynia [28; 31; 39; 51; 67; 68]. Specifically, a systemic injection of 10mg/kg PBMC inhibits cold responses without affecting thermoregulatory mechanisms [39]. Moreover, PBMC inhibits cold behaviors selectively with no known alterations in mechanosensation when administered either locally or systemically. Therefore, we asked if pharmacological inhibition of TRPM8 channels in vivo with PBMC could affect hind paw mechanical allodynia in wildtype mice treated with NTG acutely and chronically.
To determine if pre-treatment with PBMC altered acute mechanical allodynia induced by NTG we first tested wildtype mice for basal mechanical sensitivity then these animals were injected with either PBMC (10mg/kg) or vehicle 30 minutes prior to i.p. injections of NTG as above (see schematic in Figure 6A). Mechanical sensitivity was then reassessed 2 hours post NTG in these animals and, as expected, we found that mechanical allodynia was present in mice treated with the PBMC vehicle prior to NTG injection (Figure 6A, BL versus NTG). However, in mice given PBMC prior to NTG, we observed no difference in mechanosensation, as well as these mice were significantly different than mice pretreated with vehicle (Figure 6A). Thus, inhibition to TRPM8 channels prevents NTG induced acute mechanical allodynia, a phenotype consistent with our analyses of TRPM8−/− and afferent ablated mice.
Figure 6. TRPM8 antagonism prevents NTG-induced acute mechanical allodynia and development of chronic migraine-like pain.

(A) Wildtype mice pretreated with PBMC 30mins prior to injection with NTG (see schematic) exhibited normal mechanical sensitivity (nsp>0.05, BL versus NTG, paired t-test with Wilcoxon matched-pairs signed rank test, n=7). Vehicle treated mice were allodynic after injection with NTG (*p<0.05, paired t-test with Wilcoxon matched-pairs signed rank test, n=8) and significantly different than animals pre-treated with PBMC (***p<0.001, two-way ANOVA). (B) After baseline testing (Day 1) wildtype mice were given four repeated injections of NTG every two days followed by behavioral reassessments before and 30mins after injection with either PBMC or vehicle on Day 9 (see schematic). Animals from both groups developed basal mechanical allodynia by Day 9 (*p<0.05, **p<0.01 BL Day 1 versus Day 9, paired t-test with Wilcoxon matched-pairs signed rank test, n=8 each condition) which was unaffected by treatment with either PBMC or vehicle (nsp>0.05 Day 9 BL versus post-injection; *p<0.05, **p<0.01 BL Day 1 versus Day 9 post-injection). (C) Mice given PBMC prior to each intermittent NTG injection were significantly different from mice receiving NTG alone starting at Day 5 (*p<0.05, **p<0.01, ***p<0.001, two-way repeated measures ANOVA, with a Holm-Sidak post hoc test, NTG versus PBMC-NTG, n=7–8 for each condition). However, mice that received PBMC prior to the first 4 NTG injections were still allodynic when a 5th NTG (no PBMC pre-treatment) was injected on Day 9 (*p<0.05 PBMC-NTG: basal versus post-NTG, paired t-test with Wilcoxon matched-pairs signed rank test). The magnitude of the withdrawal latencies was similar to mice untreated with PBMC (nsp>0.05 post-NTG: NTG versus PBMC-NTG, two-way ANOVA, n=5–8 each condition).
Next, we asked if PBMC had a similar effect on established NTG-mediated chronic mechanical allodynia in mice given repeated injections of NTG every 2 days as described previously (see schematic Figure 6B). Two days after the last NTG treatment (4th injection), these mice were tested for basal mechanical allodynia, injected with PBMC or vehicle then mechanical sensitivity was tested 30 minutes later. As shown previously, these mice developed basal mechanical allodynia with repeated NTG treatments (Figure 6B, BL on Day 1 versus Day 9), but unlike acute NTG nociception, this phenotype was not ameliorated by inhibition of TRPM8 with PBMC as mechanical allodynia was still present after treatment with PBMC (Figure 6B, BL on Day 9 versus post-PBMC). Moreover, there was not difference in the mechanical sensitivity of the mice injected with either PBMC or vehicle (p>0.05, two-way ANOVA), thereby demonstrating that while TRPM8 inhibition can prevent the acute induction of migraine-like pain in mice, it does not alter established pain in the chronic state.
Lastly, as inhibition of TRPM8 prevents acute NTG-induced mechanical allodynia, we reasoned that the chronic nociceptive behaviors induced by repeated NTG treatments would also require active TRPM8 channels. To test this, we performed repeated NTG injections (4 total) in wildtypes to produce chronic mechanical allodynia, but one cohort of mice were pre-treated with PBMC prior to NTG and basal mechanical sensitivity was measured prior to each injection. As expected, mice injected with NTG alone developed chronic mechanical allodynia after the second injection (Figure 6C). Strikingly, mice pretreated with PBMC exhibited no changes in mechanical sensitivity at all days tested and were significantly different from the animals treated with NTG starting after the second set of injections (Figure 6C). To determine if PBMC-mediated inhibition of NTG-induced allodynia was long lasting we performed a fifth NTG injection on Day 9 after the measurement of basal sensitivity, finding that NTG still induced mechanical allodynia 2 hours post-injection (Figure 6C, PBMC-NTG, basal versus post-NTG). Of note, these mice did not receive PBMC before this final NTG treatment and the magnitude of mechanical sensitization was similar to mice that received repeated NTG injection without PBMC (post-NTG: NTG versus PBMC-NTG). Thus, while inhibition of TRPM8 channels is unable to ameliorate chronic mechanical allodynia produced by NTG, our results demonstrate that functioning TRPM8 channels are required for the formation of NTG-induced acute and chronic mechanical allodynia.
4. Discussion
TRPM8 has been identified as a migraine susceptibility gene in multiple genome-wide association studies (GWAS), with a series of mutations that reside either in cis or within the TRPM8 gene, but only in noncoding regions, making it likely that they do not alter channel function [1; 2; 15; 19; 23–26; 30; 37; 57; 60]. Indeed, the rs10166942 allele, located 5′ of the start codon is one of the most robustly migraine-associated SNPs genome-wide [14; 25; 38]. Individuals harboring the rs10166942[C] mutation, which is associated with reduced migraine risk, have lower TRPM8 expression and reduced sensitivity to cold, results suggesting a correlation between TRPM8 expression and migraine [29]. In addition to the ancestral rs10166942[C] variant, the T mutation at this locus is associated with increased migraine risk [21; 38]. The T variant is common in higher latitudes compared to individuals near the equator (88% in Finland vs 5% in Nigeria) and proposed to have been selected to give an evolutionary advantage to those living in colder climates [21]. Moreover, it is speculated that this positive selection for a mutation that may confer increased migraine partially accounts for the geographical differences in migraine susceptibility, higher prevalence in higher latitudes, lower prevalence near the equator [66]. Such findings are consistent with a meta-analysis of quantitative sensory testing (QST) in humans which found that migraine patients have higher pain ratings to a suprathreshold cold stimulus compared to non-migraineurs [50]. In addition, episodic migraineurs were observed to be more sensitive to cold stimuli compared to control patients [58]. Regardless of the validity of this hypothesis, these data indicate that TRPM8 likely plays some role in migraine.
Prior to our study, the role of TRPM8 in the context of migraine was limited and unclear [8; 56]. For example, the cold-mimetic menthol was shown to inhibit pain behaviors in mice induced by inflammation of the dura [56], whereas another study found that stimulation of meningeal TRPM8 channels with a different agonist produced mechanical allodynia in both the face and hind paw, sensitization that was attenuated with known migraine therapeutics [8]. Thus, it was unclear if activation or inhibition of TRPM8 afferents is an effective avenue for migraine treatment. Here, we now show that TRPM8 channels and afferents are required for cutaneous allodynia and spontaneous pain induced by NTG and CGRP. For the former, the production of nitric oxide (NO−) leads to a cascade of events, including production of inflammatory mediators, release of CGRP, mast cell degranulation, oxidative stress, among others [17]. Additionally, around one-third of TRPM8 trigeminal ganglion neurons express CGRP [60], yet there is no direct evidence of CGRP expression in TRPM8 dural afferents. Thus, where TRPM8 participates in this complex milieu of events remains to be determined, but its known role in pain may provide some insights [47]. For example, TRPM8 is required for cold allodynia and hyperalgesia induced by both inflammation and a number of distinct neuropathic conditions. We have shown cold allodynia stimulated by neurogenic inflammation via activation of the nociceptive ion channel TRPA1 is mediated by TRPM8 [67], consistent with the known role of TRPA1 as a gatekeeper of inflammation [5], and it’s involvement in the sensitization of meningeal afferents in headache pain [20; 44]. Furthermore, TRPA1 activation induces cold pain upstream of TRPM8 in a process that is blocked by inhibition of CGRP signaling [67]. As we find that TRPM8 is required for CGRP-induced mechanical allodynia and increased MGS scores, this suggests a potential mechanism whereby CGRP produced by NO− works upstream of TRPM8 to promote this specific pathology. However, Burgos-Vega et al. showed that the hind paw and facial allodynia induced by application of a TRPM8-specific agonist to the dura could be abrogated by inhibition of NO− production [8], suggesting that TRPM8 functions upstream of this pathway. Thus, where this channel sits in the transduction mechanisms of NTG- and CGRP-induced migraine-like remains to be elucidated.
Cold itself can induce facial pain, termed Headache after Ingestion of a Cold Stimulus (HICS) and colloquially referred to as “brain freeze” or an “ice cream headache” [61]. The Headache Classification Committee of the International Headache Society (IHS) recently described this as a “short-lasting frontal or temporal pain, which may be intense, induced in susceptible people by passage of cold material (solid, liquid, or gaseous) over the palate and/or posterior pharyngeal wall”. A number of studies have attempted to correlate the occurrence and severity of HICS in those who experience migraine or tension-type headaches. For example, an early survey found that adult migraineurs experienced HICS approximately 3 times higher than non-migraineurs [55]. Similarly, in an examination of adolescents with migraine it was noted they had a higher frequency of HICS than those without migraine [27]. In an experimental model of an ‘ice-cream headache’, cold-induced headaches occurred with a more than 2-fold greater prevalence in those with migraine versus tension-type headaches [59]. Likewise, women experiencing active migraines are twice as likely to experience a headache after ingesting cold water [46]. Lastly, a recent report found that while there was not a greater risk of migraineurs getting HICS, pain intensity was higher when tension-type headaches and migraines were present [41]. While the underlying mechanisms of HICS are unclear, it is likely to involve TRPM8, and the above observations suggest there may be a corollary between cold and migraine pain.
Genetic deletion of TRPM8 and ablation or electrically silencing TRPM8 afferents has not been shown to alter normal or pathological mechanical sensitivity [40; 51]. Thus, the novel results herein are the first indication that TRPM8 channels are involved in mechanical allodynia, albeit by a mechanism that is not yet understood. Based on this evidence, it is doubtful that TRPM8 is acting as a mechanically-gated receptor in this model, but is more likely to be playing a pro-nociceptive role coupled with other transduction pathways that lead to migraine pain. Indeed, the lack of a nociceptive phenotype in our assessment of mouse facial features shows that TRPM8 is involved in not just mechanical allodynia but also spontaneous migraine-like pain. Furthermore, our finding that inhibition of TRPM8 channels prevents acute but not established chronic mechanical allodynia suggests that TRPM8 is involved in the proalgesic sensitization, but not the direct detection, of mechanical stimuli. Thus, while it is clear that TRPM8 is the predominant cold sensor in mammals, mediating innocuous cool and noxious cold sensation, our results and those of others in both rodents and humans indicates that it now can be considered as an important protein mediating migraine- and headache-related pain.
Acknowledgements
The authors wish to thank current and former members of the McKemy Laboratory for their assistance in the completion of this work. The present study was funded by grant NS118852 from the National Institutes of Health (NIH). The authors declare no financial or conflicts of interest.
REFERENCES
- [1].An XK, Ma QL, Lin Q, Zhang XR, Lu CX, Qu HL. PRDM16 rs2651899 variant is a risk factor for Chinese common migraine patients. Headache 2013;53(10):1595–1601. [DOI] [PubMed] [Google Scholar]
- [2].Anttila V, Wessman M, Kallela M, Palotie A. Genetics of migraine. Handb Clin Neurol 2018;148:493–503. [DOI] [PubMed] [Google Scholar]
- [3].Avona A, Burgos-Vega C, Burton MD, Akopian AN, Price TJ, Dussor G. Dural Calcitonin Gene-Related Peptide Produces Female-Specific Responses in Rodent Migraine Models. J Neurosci 2019;39(22):4323–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, Ptacek LJ, Ahn AH. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia 2010;30(2):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: A gatekeeper for inflammation. Annu Rev Physiol 2013;75:181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007;448(7150):204–208. [DOI] [PubMed] [Google Scholar]
- [7].Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache 2015;55(1):21–34. [DOI] [PubMed] [Google Scholar]
- [8].Burgos-Vega CC, Ahn DD, Bischoff C, Wang W, Horne D, Wang J, Gavva N, Dussor G. Meningeal transient receptor potential channel M8 activation causes cutaneous facial and hindpaw allodynia in a preclinical rodent model of headache. Cephalalgia 2016;36(2):185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Burgos-Vega CC, Quigley LD, Trevisan Dos Santos G, Yan F, Asiedu M, Jacobs B, Motina M, Safdar N, Yousuf H, Avona A, Price TJ, Dussor G. Non-invasive dural stimulation in mice: A novel preclinical model of migraine. Cephalalgia 2019;39(1):123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol 2000;47(5):614–624. [PubMed] [Google Scholar]
- [11].Capuano A, Greco MC, Navarra P, Tringali G. Correlation between algogenic effects of calcitonin-gene-related peptide (CGRP) and activation of trigeminal vascular system, in an in vivo experimental model of nitroglycerin-induced sensitization. Eur J Pharmacol 2014;740:97–102. [DOI] [PubMed] [Google Scholar]
- [12].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- [13].Chasman DI, Anttila V, Buring JE, Ridker PM, Schurks M, Kurth T, International Headache Genetics C. Selectivity in genetic association with sub-classified migraine in women. PLoS Genet 2014;10(5):e1004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chasman DI, Schurks M, Anttila V, de Vries B, Schminke U, Launer LJ, Terwindt GM, van den Maagdenberg AM, Fendrich K, Volzke H, Ernst F, Griffiths LR, Buring JE, Kallela M, Freilinger T, Kubisch C, Ridker PM, Palotie A, Ferrari MD, Hoffmann W, Zee RY, Kurth T. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet 2011;43(7):695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen SP, Fuh JL, Chung MY, Lin YC, Liao YC, Wang YF, Hsu CL, Yang UC, Lin MW, Chiou JJ, Wang PJ, Chen PK, Fan PC, Wu JY, Chen YT, Kao LS, Shen-Jang Fann C, Wang SJ. Genome-wide association study identifies novel susceptibility loci for migraine in Han Chinese resided in Taiwan. Cephalalgia 2018;38(3):466–475. [DOI] [PubMed] [Google Scholar]
- [16].De Logu F, Nassini R, Landini L, Geppetti P. Pathways of CGRP Release from Primary Sensory Neurons. Handb Exp Pharmacol 2019;255:65–84. [DOI] [PubMed] [Google Scholar]
- [17].Demartini C, Greco R, Zanaboni AM, Sances G, De Icco R, Borsook D, Tassorelli C. Nitroglycerin as a comparative experimental model of migraine pain: From animal to human and back. Prog Neurobiol 2019;177:15–32. [DOI] [PubMed] [Google Scholar]
- [18].Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron 2007;54(3):371–378. [DOI] [PubMed] [Google Scholar]
- [19].Dussor G, Cao YQ. TRPM8 and Migraine. Headache 2016;56(9):1406–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, Preti D, Appendino G, Geppetti P, Dodick DW, Vanderah TW, Porreca F, Dussor G. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain 2012;153(9):1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Edvinsson L. Role of CGRP in Migraine. Handb Exp Pharmacol 2019;255:121–130. [DOI] [PubMed] [Google Scholar]
- [22].El-Brolosy MA, Stainier DYR. Genetic compensation: A phenomenon in search of mechanisms. PLoS Genet 2017;13(7):e1006780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Esserlind AL, Christensen AF, Le H, Kirchmann M, Hauge AW, Toyserkani NM, Hansen T, Grarup N, Werge T, Steinberg S, Bettella F, Stefansson H, Olesen J. Replication and meta-analysis of common variants identifies a genome-wide significant locus in migraine. Eur J Neurol 2013;20(5):765–772. [DOI] [PubMed] [Google Scholar]
- [24].Fan X, Wang J, Fan W, Chen L, Gui B, Tan G, Zhou J. Replication of migraine GWAS susceptibility loci in Chinese Han population. Headache 2014;54(4):709–715. [DOI] [PubMed] [Google Scholar]
- [25].Freilinger T, Anttila V, de Vries B, Malik R, Kallela M, Terwindt GM, Pozo-Rosich P, Winsvold B, Nyholt DR, van Oosterhout WP, Artto V, Todt U, Hamalainen E, Fernandez-Morales J, Louter MA, Kaunisto MA, Schoenen J, Raitakari O, Lehtimaki T, Vila-Pueyo M, Gobel H, Wichmann E, Sintas C, Uitterlinden AG, Hofman A, Rivadeneira F, Heinze A, Tronvik E, van Duijn CM, Kaprio J, Cormand B, Wessman M, Frants RR, Meitinger T, Muller-Myhsok B, Zwart JA, Farkkila M, Macaya A, Ferrari MD, Kubisch C, Palotie A, Dichgans M, van den Maagdenberg AM, International Headache Genetics C. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat Genet 2012;44(7):777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fu X, Yang J, Wu X, Lin Q, Zeng Y, Xia Q, Cao L, Huang B, Huang G. Association between PRDM16, MEF2D, TRPM8, LRP1 gene polymorphisms and migraine susceptibility in the She ethnic population in China. Clin Invest Med 2019;42(1):E21–E30. [DOI] [PubMed] [Google Scholar]
- [27].Fuh JL, Wang SJ, Lu SR, Juang KD. Ice-cream headache--a large survey of 8359 adolescents. Cephalalgia 2003;23(10):977–981. [DOI] [PubMed] [Google Scholar]
- [28].Gardiner JC, Kirkup AJ, Curry J, Humphreys S, O’Regan P, Postlethwaite M, Young KC, Kitching L, Ethell BT, Winpenny D, McMurray G. The role of TRPM8 in the Guinea-pig bladder-cooling reflex investigated using a novel TRPM8 antagonist. Eur J Pharmacol 2014;740:398–409. [DOI] [PubMed] [Google Scholar]
- [29].Gavva NR, Sandrock R, Arnold GE, Davis M, Lamas E, Lindvay C, Li CM, Smith B, Backonja M, Gabriel K, Vargas G. Reduced TRPM8 expression underpins reduced migraine risk and attenuated cold pain sensation in humans. Sci Rep 2019;9(1):19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ghosh J, Pradhan S, Mittal B. Genome-wide-associated variants in migraine susceptibility: a replication study from North India. Headache 2013;53(10):1583–1594. [DOI] [PubMed] [Google Scholar]
- [31].Gonzalez A, Ugarte G, Restrepo C, Herrera G, Pina R, Gomez-Sanchez JA, Pertusa M, Orio P, Madrid R. Role of the Excitability Brake Potassium Current IKD in Cold Allodynia Induced by Chronic Peripheral Nerve Injury. J Neurosci 2017;37(12):3109–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gormley P, Anttila V, Winsvold BS, Palta P, Esko T, Pers TH, Farh KH, Cuenca-Leon E, Muona M, Furlotte NA, Kurth T, Ingason A, McMahon G, Ligthart L, Terwindt GM, Kallela M, Freilinger TM, Ran C, Gordon SG, Stam AH, Steinberg S, Borck G, Koiranen M, Quaye L, Adams HH, Lehtimaki T, Sarin AP, Wedenoja J, Hinds DA, Buring JE, Schurks M, Ridker PM, Hrafnsdottir MG, Stefansson H, Ring SM, Hottenga JJ, Penninx BW, Farkkila M, Artto V, Kaunisto M, Vepsalainen S, Malik R, Heath AC, Madden PA, Martin NG, Montgomery GW, Kurki MI, Kals M, Magi R, Parn K, Hamalainen E, Huang H, Byrnes AE, Franke L, Huang J, Stergiakouli E, Lee PH, Sandor C, Webber C, Cader Z, Muller-Myhsok B, Schreiber S, Meitinger T, Eriksson JG, Salomaa V, Heikkila K, Loehrer E, Uitterlinden AG, Hofman A, van Duijn CM, Cherkas L, Pedersen LM, Stubhaug A, Nielsen CS, Mannikko M, Mihailov E, Milani L, Gobel H, Esserlind AL, Christensen AF, Hansen TF, Werge T, International Headache Genetics C, Kaprio J, Aromaa AJ, Raitakari O, Ikram MA, Spector T, Jarvelin MR, Metspalu A, Kubisch C, Strachan DP, Ferrari MD, Belin AC, Dichgans M, Wessman M, van den Maagdenberg AM, Zwart JA, Boomsma DI, Smith GD, Stefansson K, Eriksson N, Daly MJ, Neale BM, Olesen J, Chasman DI, Nyholt DR, Palotie A. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet 2016;48(8):856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Greco R, Demartini C, Zanaboni AM, Tassorelli C. Chronic and intermittent administration of systemic nitroglycerin in the rat induces an increase in the gene expression of CGRP in central areas: potential contribution to pain processing. J Headache Pain 2018;19(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Greco R, Mangione AS, Siani F, Blandini F, Vairetti M, Nappi G, Sandrini G, Buzzi MG, Tassorelli C. Effects of CGRP receptor antagonism in nitroglycerin-induced hyperalgesia. Cephalalgia 2014;34(8):594–604. [DOI] [PubMed] [Google Scholar]
- [35].Guo Z, Czerpaniak K, Zhang J, Cao YQ. Increase in trigeminal ganglion neurons that respond to both calcitonin gene-related peptide and pituitary adenylate cyclase-activating polypeptide in mouse models of chronic migraine and posttraumatic headache. Pain 2021;162(5):1483–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the Trigeminal System in Migraine. Headache 2019;59(5):659–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kaur S, Ali A, Ahmad U, Pandey AK, Singh B. rs2651899 variant is associated with risk for migraine without aura from North Indian population. Mol Biol Rep 2019;46(1):1247–1255. [DOI] [PubMed] [Google Scholar]
- [38].Key FM, Abdul-Aziz MA, Mundry R, Peter BM, Sekar A, D’Amato M, Dennis MY, Schmidt JM, Andres AM. Human local adaptation of the TRPM8 cold receptor along a latitudinal cline. PLoS Genet 2018;14(5):e1007298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One 2011;6(9):e25894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci 2013;33(7):2837–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kraya T, Schulz-Ehlbeck M, Burow P, Watzke S, Zierz S. Prevalence and characteristics of headache attributed to ingestion or inhalation of a cold stimulus (HICS): A cross-sectional study. Cephalalgia 2020;40(3):299–306. [DOI] [PubMed] [Google Scholar]
- [42].Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 2010;7(6):447–449. [DOI] [PubMed] [Google Scholar]
- [43].Lippoldt EK, Elmes RR, McCoy DD, Knowlton WM, McKemy DD. Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J Neurosci 2013;33(30):12543–12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Marone IM, De Logu F, Nassini R, De Carvalho Goncalves M, Benemei S, Ferreira J, Jain P, Li Puma S, Bunnett NW, Geppetti P, Materazzi S. TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain 2018;141(8):2312–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mason BN, Kaiser EA, Kuburas A, Loomis MM, Latham JA, Garcia-Martinez LF, Russo AF. Induction of Migraine-Like Photophobic Behavior in Mice by Both Peripheral and Central CGRP Mechanisms. J Neurosci 2017;37(1):204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mattsson P. Headache caused by drinking cold water is common and related to active migraine. Cephalalgia 2001;21(3):230–235. [DOI] [PubMed] [Google Scholar]
- [47].McKemy DD. Molecular basis of peripheral innocuous cold sensitivity. Handb Clin Neurol 2018;156:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002;416(6876):52–58. [DOI] [PubMed] [Google Scholar]
- [49].Moye LS, Pradhan AAA. Animal Model of Chronic Migraine-Associated Pain. Curr Protoc Neurosci 2017;80:9 60 61–69 60 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nahman-Averbuch H, Shefi T, Schneider VJ 2nd, Li D, Ding L, King CD, Coghill RC. Quantitative sensory testing in patients with migraine: a systematic review and meta-analysis. Pain 2018;159(7):1202–1223. [DOI] [PubMed] [Google Scholar]
- [51].Ongun S, Sarkisian A, McKemy DD. Selective cold pain inhibition by targeted block of TRPM8-expressing neurons with quaternary lidocaine derivative QX-314. Commun Biol 2018;1:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Peck KR, Johnson YL, Smitherman TA. Migraine. Handb Clin Neurol 2016;138:283–293. [DOI] [PubMed] [Google Scholar]
- [53].Pogorzala LA, Mishra SK, Hoon MA. The cellular code for mammalian thermosensation. J Neurosci 2013;33(13):5533–5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain 2014;155(2):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Raskin NH, Knittle SC. Ice cream headache and orthostatic symptoms in patients with migraine. Headache 1976;16(5):222–225. [DOI] [PubMed] [Google Scholar]
- [56].Ren L, Dhaka A, Cao YQ. Function and postnatal changes of dural afferent fibers expressing TRPM8 channels. Mol Pain 2015;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Schurks M. Genetics of migraine in the age of genome-wide association studies. J Headache Pain 2012;13(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schwedt TJ, Krauss MJ, Frey K, Gereau RWt. Episodic and chronic migraineurs are hypersensitive to thermal stimuli between migraine attacks. Cephalalgia 2011;31(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Selekler HM, Erdogan MS, Budak F. Prevalence and clinical characteristics of an experimental model of ‘ice-cream headache’ in migraine and episodic tension-type headache patients. Cephalalgia 2004;24(4):293–297. [DOI] [PubMed] [Google Scholar]
- [60].Sintas C, Fernandez-Morales J, Vila-Pueyo M, Narberhaus B, Arenas C, Pozo-Rosich P, Macaya A, Cormand B. Replication study of previous migraine genome-wide association study findings in a Spanish sample of migraine with aura. Cephalalgia 2015;35(9):776–782. [DOI] [PubMed] [Google Scholar]
- [61].Starling AJ. Unusual Headache Disorders. Continuum (Minneap Minn) 2018;24(4, Headache):1192–1208. [DOI] [PubMed] [Google Scholar]
- [62].Stewart WF, Shechter A, Rasmussen BK. Migraine prevalence. A review of population-based studies. Neurology 1994;44(6 Suppl 4):S17–23. [PubMed] [Google Scholar]
- [63].Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci 2007;27(51):14147–14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Thomsen LL, Kruuse C, Iversen HK, Olesen J. A nitric oxide donor (nitroglycerin) triggers genuine migraine attacks. Eur J Neurol 1994;1(1):73–80. [DOI] [PubMed] [Google Scholar]
- [65].Tipton AF, Tarash I, McGuire B, Charles A, Pradhan AA. The effects of acute and preventive migraine therapies in a mouse model of chronic migraine. Cephalalgia 2016;36(11):1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Vigano A, Manica A, Di Piero V, Leonardi M. Did Going North Give Us Migraine? An Evolutionary Approach on Understanding Latitudinal Differences in Migraine Epidemiology. Headache 2019;59(4):632–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yamaki S, Chau A, Gonzales L, McKemy DD. Nociceptive afferent phenotyping reveals that transient receptor potential ankyrin 1 promotes cold pain through neurogenic inflammation upstream of the neurotrophic factor receptor GFRalpha3 and the menthol receptor transient receptor potential melastatin 8. Pain 2021;162(2):609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yudin Y, Lutz B, Tao YX, Rohacs T. Phospholipase C delta4 regulates cold sensitivity in mice. J Physiol 2016;594(13):3609–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]


