Abstract
To identify sporulation-specific proteins that might serve as targets of developmental regulatory factors in Streptomyces, we examined total proteins of Streptomyces griseus by two-dimensional gel electrophoresis. Among five proteins that were present at high levels during sporulation but absent from vegetative cells, two of the proteins, P3 and P4, were absent from developmental mutants that undergo aberrant morphogenesis. The deduced amino acid sequence of the gene that encodes P3 (EshA) showed extensive similarity to proteins from mycobacteria and a cyanobacterium, Synechococcus, that are abundant during nutritional stress but whose functions are unknown. Uniquely among these proteins, EshA contains a cyclic nucleotide-binding domain, suggesting that the activity of EshA may be modulated by a cyclic nucleotide. The eshA gene was strongly expressed from a single transcription start site only during sporulation, and accumulation of the eshA transcript depended on a developmental gene, bldA. During submerged sporulation, a null mutant strain that produced no EshA could not extend sporogenic hyphae from new branch points but instead accelerated septation and spore maturation at the preexisting vegetative filaments. These results indicated that EshA is required for the growth of sporogenic hyphae and localization of septation and spore maturation but not for spore viability.
Streptomyces is a gram-positive bacterium that undergoes morphological differentiation. In a nutritionally favorable condition, Streptomyces spores germinate to give rise to vegetative hyphae, which are characterized by filamentous, multigenomic cells called mycelia. Sporulation begins by the growth of aerial hyphae (or sporogenic hyphae when the sporulation process is induced in liquid culture; 34), which subsequently undergo septation to form chains of unicellular spores. We previously identified two types of sporogenic hyphae during sporulation of Streptomyces griseus (22): we suggest the term “distal” sporogenic hyphae for those that grow from the tips of preexisting vegetative filaments and the term “proximal” sporogenic hyphae for those that grow de novo within the vegetative hyphae at the onset of sporulation. Since both vegetative growth and spore formation are inherently polar processes similar to those observed in yeast and filamentous fungi (18), formation of sporogenic hyphae in Streptomyces is likely to require proteins that direct the location of sporogenic hyphae, establishment of polarity, and emergence of the sporogenic hyphae.
To understand the molecular details of Streptomyces sporulation, researchers have identified developmental genes primarily by complementation of nonsporulating mutants. Most of the sporulation-specific genes that have been characterized appear to encode regulatory functions. Some, such as bldA, which encodes tRNA (38), are required for the production of aerial hyphae (35, 43) and antibiotics (3, 34, 43). Genes that specifically regulate spore formation include the whi genes, so named because mutations in these genes commonly prevent formation of the spore compartments and the spore-associated pigment that is acquired late in development (11). Included among these proteins are WhiG, a ς subunit of RNA polymerase (12), and WhiH, a putative transcription regulator of the GntR family (52). A second sporulation-specific ς factor is encoded by sigF. WhiG is required for the intermediate event of sporulation septation, whereas SigF is required for subsequent maturation of the spores (49).
Complete understanding of the role of developmentally controlled regulatory factors requires the characterization of the target genes they regulate. A few candidates for the targets have recently emerged. The whiH promoter is recognized by WhiG, a sporulation-specific ς factor (52). The whiE gene cluster encoding grey spore pigment is controlled by several whi genes in Streptomyces coelicolor (29). Sporulating cultures undergo cycles of synthesis and degradation of glycogen, and some of the enzymes of glycogen synthesis are active in aerial hyphae (7). The ftsZ gene, which is required for septation in S. coelicolor (41) and is differentially regulated during sporulation in S. griseus (J. Kwak, unpublished data), would therefore be a likely target of a factor that regulates development.
Here we describe results from attempts to identify additional sporulation-specific genes encoding enzymes or structural proteins that might depend on the Whi proteins or other regulatory factors for their expression during development. For these studies, we have exploited the ability of S. griseus to undergo sporulation while submerged in liquid culture during nutritional downshift (33) or phosphate starvation (31). Under these conditions sporulation is relatively rapid and synchronous; sporogenic hyphae are evident at 4 h and continue to elongate for the next 6 h, septation occurs at approximately 10 h, and spores mature during the subsequent 10 to 12 h (34). Our objectives were to use two-dimensional gel electrophoresis to identify sporulation-specific proteins, to characterize the genes encoding these proteins, and to establish the mechanisms of their developmental regulation. Here we report on one such protein, designated P3, that contains a cyclic nucleotide-binding domain required for growth of sporogenic hyphae at an early stage of streptomycete morphogenesis. On the basis of its role in sporulation, we rename P3 as EshA (extension of sporogenic hyphae).
MATERIALS AND METHODS
Bacterial strains and plasmids.
S. griseus NRRL B-2682 was used as the wild-type strain. SKK2025, -2026, and -2027 were three independent isolates containing the eshA null allele. SKK1015 was used as the class IIIA mutant, SKK1008 was used as the class IIIB mutant, and SKK1003 was used as the class IIIC mutant, as described previously (34). Escherichia coli DH5α was used for routine plasmid construction and preparation. E. coli ET12567 (dam, dcm, and hsd; 39) was used to demethylate plasmid DNA prior to its methylation in vitro and its introduction into S. griseus (J. Kwak, unpublished). We used E. coli Top10 (Invitrogen, Carlsbad, Calif.) to express His-tagged EshA for making antibodies.
pKK842 was constructed by ligating a 2.4-kb SalI fragment of genomic DNA from S. griseus NRRL B-2682 to pGEM-4Z (Promega). The EcoRI-HindIII fragment containing this 2.4-kb SalI fragment was ligated to pIJ2925 (27) to generate pKK847. pKK856 was constructed by ligating the 2.4-kb fragment from pKK847 as a BglII fragment into the BamHI site of the low-copy vector pXE4 (26). pKK1416 contained eshA on an approximately 8.0-kb BamHI-HindIII fragment in pXE4. pKK1417 contained the same 8.0-kb fragment in pKK1400 in which a 1.8-kb Tsr cassette (60) was inserted as a BamHI fragment into the BglII site of pIJ2920 (27).
To fuse EshA to a hexahistidine tag, we used the vector pTrcHis-A (Invitrogen). Two oligonucleotides (oligonucleotide 164, 5′-AGTCGGATCCGCTAGCATGACTGTTGACTCGACCTCGGA-3′, corresponding to nucleotides [nt] 329 to 352 of the 2.4-kb SalI fragment and containing adjacent BamHI and NheI sites, and oligonucleotide 165, 5′-AGAACGTCCAGGGTGACCTCGTC-3′, corresponding to nt 801 to 779) were used to amplify the N-terminal coding region of eshA from pKK842, to introduce NheI and BamHI sites upstream of and in frame with the eshA start codon. This PCR product was ligated to BamHI-SacI-digested pUC18 to generate pKK852. The 1.6-kb SacI-HindIII fragment from pKK842, containing the C-terminal coding region of eshA, was ligated to pTrcHis-A that had been digested similarly, to yield pKK853. The 0.4-kb NheI-SacI fragment from pKK852 was ligated to similarly digested pKK853 to form pKK855, which contained the hexahistidine coding sequence fused to the N-terminal coding sequence of eshA.
To construct the null allele of eshA, a PCR-amplified fragment was generated by using an M13 reverse sequencing primer (5′-TCACACAGGAAACAGCTATGA-3′) as the upstream primer and an internal primer containing a BclI site (5′-CGGGAGGTGATCACCTGCATCTGCG-3′, corresponding to nt 456 to 432 of the 2.4-kb SalI fragment) as the downstream primer. The amplification product was digested with EcoRI and BclI and ligated to pKK847 that had been similarly digested. This ligation generated pKK2011, which contained a deleted version of eshA comprising the first 116 nt and the last 515 nt of the eshA coding sequence. This plasmid was digested with BclI and ligated to a 1.3-kb apramycin resistance cassette (obtained from P. Solenberg and R. Baltz in pCZA263) from pKK974. This ligation generated pKK2012. The Apr-disrupted eshA allele was transferred as a BglII fragment from pKK2012 to pKK1400, which contains the 1.8-kb thiostrepton resistance cassette (60) in pIJ2920 (27). This yielded pKK2014, which was used as described below to construct the eshA null mutant.
Growth and induction of sporulation.
E. coli cultures were grown in Luria broth (2) supplemented with ampicillin (100 μg/ml) or apramycin (100 μg/ml) as needed. Starter cultures of S. griseus were grown in SpM (31) for 2 to 5 days to generate spore suspensions that were then used as inoculum for induction of sporulation. Cultures of bald mutants were treated similarly except that the SpM culture was 36 to 48 h old at the time of subculture. SpM agar, supplemented as needed with apramycin (20 μg/ml) or thiostrepton (5 μg/ml), was used for maintenance of streptomycete strains. SpMR (3) was used for protoplast transformation. Trypticase soy broth (BBL Microbiology Systems), 2XYT (53), or Luria broth was used for isolation of genomic and plasmid DNA from S. griseus (24).
Two different methods were used to obtain sporulating submerged cultures of S. griseus. To induce sporulation by phosphate starvation or nutritional downshift, 50 ml of glucose-ammonia minimal medium supplemented with 1% casein hydrolysate (U. S. Biochemicals; salt-free) was inoculated with 0.05 to 0.5 ml of an SpM starter culture and was incubated at 30°C on a shaker (250 rpm) until the absorbance at 500 nm reached 3.0. Then the culture was harvested by centrifugation at 22°C, was washed once with prewarmed medium lacking casein hydrolysate (nutritional downshift) and phosphate (phosphate starvation), and was transferred to 50 ml of identical prewarmed medium in a 250-ml flask. The time of transfer was considered 0 h (equivalent to vegetative growth). To obtain sporulation as a consequence of nutrient exhaustion, 0.5 ml of SpM starter culture was inoculated into 50 ml of SpM, and incubation at 30°C and 250 rpm was continued. Typically the culture ceased exponential growth at an A500 of 9, and free spores and spore chains were first evident 6 h later.
Protein analysis.
To prepare crude extracts, vegetative or sporulating cultures were harvested by centrifugation at 14,000 × g for 15 min at 4°C. The cells were washed once with 1 M KCl and were suspended in 50 mM Tris-HCl, pH 7.5. Cells were disrupted in a French press (14,000 lb/in2), and the lysate was centrifuged at 14,000 × g for 5 min. The supernatant was used as the crude extract. Protein concentration was determined by the ultraviolet absorbance method of Ehresmann et al. (19). Equal amounts of protein in sodium dodecyl sulfate (SDS) sample buffer were applied, and the gels were run and stained according to standard methods (2, 36).
To examine total proteins by two-dimensional gel electrophoresis (48), we extracted proteins by using modifications of published methods (20, 21). Cells harvested from a 50-ml culture were suspended in 2 ml of prechilled extraction buffer (50 mM Tris-HCl, pH 7.5, 5 mM MgCl2). After disruption in a French press (14,000 lb/in2), the broken cells were centrifuged at 12,000 × g for 5 min and the supernatant was saved. Deoxyribonuclease I (Sigma; 100 μg/ml) and ribonuclease A (Sigma; 50 μg/ml) were added to the supernatant, and the mixture was incubated at 4°C for 20 min. The digested extract was combined with an equal volume of 20% (wt/vol) trichloroacetic acid in acetone and kept at −20°C for 30 min. The proteins were collected by centrifugation as above, were washed twice with cold acetone, and were dried in vacuo for 5 min. The residue was dissolved in 1 ml of solubilization buffer containing 9.8 M urea, 4% Nonidet P-40 (U. S. Biochemicals), 1% Ampholine (pH 3.5 to 10; Pharmacia), 1% Pharmalyte (pH 2.5 to 5; Pharmacia), and 2 mM dithiothreitol.
The isoelectric focusing gel for the first dimension was prepared according to the procedure of O'Farrell (48) with minor modifications (20). The gel was prerun in a GT2 tube gel unit (Hoefer Scientific Instruments, San Francisco, Calif.) at 400 V for 30 min and at 800 V for 30 min. Protein (25 μl) was loaded and was focused at 800 V for 12 h. The gel either was equilibrated for 15 min in running buffer (36) or was frozen at −20°C and equilibrated immediately before electrophoresis in the second dimension. To determine the pI, a tube gel was cut in 5-mm slices, and each slice was macerated in 0.5 ml of water. The pH of each suspension was then measured with a pH meter. After electrophoresis, the gel either was fixed in 50% methanol and 10% acetic acid and stained with Coomassie blue R-250 or was immediately prepared for electrophoretic transfer (Idea Scientific Co., Minneapolis, Minn.) onto polyvinylidene difluoride membrane (Bio-Rad). CAPS-NaOH (pH 11.0; 10 mM)–10% methanol was used as a transfer buffer. The membrane was stained with a 0.1% (wt/vol) solution of Ponceau S in 1% acetic acid. Protein spots were excised from multiple membranes and were sent to the Harvard Microchemistry Facility for amino acid analysis and sequence determination.
Anti-EshA antibodies were made by purification of soluble EshA from the His tag system (Invitrogen) according to the manufacturer's instructions. EshA was the only protein evident in the preparation by polyacrylamide gel electrophoresis (PAGE) after elution with 500 mM imidazole in 50 mM Tris-HCl, pH 7.5, and 0.3 M NaCl. A homogenate of the pure protein in polyacrylamide was used to immunize chickens (Cocalico Biologicals, Reamstown, Pa.). Rabbit anti-chicken antibodies conjugated to alkaline phosphatase were from Jackson Immunoresearch and were detected by using the chromogenic method (Boehringer Mannheim).
To isolate total proteins for immunoblot hybridization, equal biomasses of cells (0.25 to 1.0 ml of liquid cultures) were harvested by centrifugation from different growth stages (during sporulation by phosphate starvation, nutritional downshift, and SpM). The cell pellets were resuspended in 0.5 ml of 50 mM Tris-HCl (pH 7.5)–10 mM MgCl2, 2 mg of lysozyme was added, and the suspensions were incubated for 37°C for 10 min. The extracts were combined with 2× SDS sample buffer (2, 36), and 30 μl of each extract was loaded onto the wells for SDS-PAGE. To isolate membrane proteins from cytoplasmic and ribosomal fractions, we used a modification of the procedure of Mikulik and Janda (44). One milliliter of the starter culture (4-day culture in SpM) was inoculated into 50 ml of SpM. The culture was harvested at 6 h after the culture reached an optical density at 500 mm of 9.0. This time point is when the culture enters stationary phase and starts to form sporogenic hyphae. The cells were harvested by centrifugation at 14,000 × g for 10 min. The cells were washed once and resuspended in 1.5 ml of buffer A (20 mM Tris-HCl, pH 7.6, 10 mM MgCl2, 0.5 mM phenylmethylsulfonyl fluoride). The cells were disrupted by a French press at 14,000 lb/in2. The cell lysate was centrifuged at 14,000 × g for 10 min at 4°C in a Beckman JA-20 rotor to remove the cell wall and other debris. The crude lysate was centrifuged at 44,000 × g for 30 min at 4°C to remove some larger membrane fragments. The pellet (P44) was resuspended in 1.5 ml of buffer A. The supernatant (S44) was carefully removed and was centrifuged at 150,000 × g for 14 h. After centrifugation, the supernatant (S150) was removed and the pellet (P150) was resuspended in 1.5 ml of buffer B (buffer A + 1 M NH4Cl) at 4°C for 1 h and was layered over 10 ml of a 50% sucrose cushion (10 mM Tris-HCl, pH 7.4, 500 mM NH4Cl, 10 mM MgCl2, 6 mM 2-mercaptoethanol). The cushion was centrifuged at 150,000 × g for 10 h at 4°C. Four fractions (upper cushion, brown layer, lower cushion, and pellet) were isolated after the centrifugation. The pellets were dissolved in 1.5 ml of buffer B. Thirty microliters of each protein fraction was loaded onto a slab gel for SDS-PAGE and Western hybridization.
DNA manipulation and analysis.
Standard (2, 24, 53) or previously published (35) methods were used for analysis and manipulation of DNA fragments for colony hybridization, plasmid DNA minipreparations, and transformations. For Southern analysis we used the Genius II system after transfer of the DNA to a positively charged membrane (Boehringer Mannheim). We generated nested deletions by using exonuclease III digestion (23) of pKK842 to determine the nucleotide sequence of both strands of the 2.4-kb SalI fragment according to the dideoxynucleotide method (53). We used Deep Vent polymerase (New England Biolabs) for PCR amplifications according to the manufacturer's recommendations.
The single-stranded DNA used in the S1 nuclease protection experiments was produced by using Deep Vent polymerase with a single oligonucleotide that was complementary to the coding sequence. The downstream primers oligonucleotide 166 (5′-TGGACTGCCGGGGCACTTCCAG-3′, corresponding to nt 380 to 359 of the SalI fragment) and oligonucleotide 167 (5′-TCCTGCATCTGCGGGGCGGACTT-3′, corresponding to nt 444 to 422) were used to produce runoff polymerization. The plasmid pKK842 digested with EcoRI was used as a template. The reaction conditions were similar to those recommended by the manufacturer for amplification of double-stranded DNA, except that 2 μl of dimethyl sulfoxide and 0.5 μg of linearized template DNA were combined in reaction buffer containing 3 mM MgCl2, and the reaction proceeded through 60 cycles. The single-stranded fragment was purified by using diatomaceous earth resin (9) after electrophoresis on a 1% agarose gel.
RNA studies.
RNA was purified from vegetative and sporulating cultures of S. griseus NRRL B-2682, SKK1003, SKK1008, and SKK1015 by a standard method (24) with some modifications as follows. Ten microliters of vegetatively growing cells (A500, 3.0) was harvested by centrifugation at 4°C and then was suspended in 10 ml of modified Kirby mixture (24). The cell suspension was passed through a French pressure cell (14,000 lb/in2), and the lysate was collected in 12-ml polypropylene tubes containing 5 ml of Tris-buffered phenol-chloroform (1:1, pH 8.0). After vigorous vortexing for 1 min, the mixture was centrifuged at 7,700 × g for 10 min. The upper phase was reextracted, vortexed, and centrifuged. The aqueous phase was recovered, and the RNA was precipitated, was digested with DNase (RNase-free; Boehringer Mannheim) for 2 h at 37°C, and then was extracted and precipitated as described above and dissolved in water.
To determine the transcription 5′ end point, we used the S1 nuclease protection assay (24) by combining 50 μg of RNA with 100,000 cpm of end-labeled, single-stranded DNA probe. The sequencing reactions were prepared with the same primer that had been used to make the probe.
Gene disruption.
pKK2014 was passed through E. coli ET12567 to remove methyl groups from adenine and cytosine residues and subsequently was methylated with a mixture of commercially purchased methyltransferases, mAluI and mSssI, and endogenous methyltransferases from S. griseus NRRL B-2682 (40). After overnight incubation at 30°C, the reaction mixture was extracted once with phenol-chloroform and twice with chloroform and then was precipitated with ethanol. The methylated DNA was dissolved in water and was introduced into protoplasts of S. griseus NRRL B-2682 suspended in P buffer (24; J. Kwak, unpublished). Apramycin-resistant transformants were selected and were screened for thiostrepton sensitivity; this phenotype was indicative of a double-crossover event. Of 150 apramycin-resistant transformants, 3 were sensitive to thiostrepton, indicating that recombination had occurred on both sides of the apramycin cassette. Three such strains, SKK2025, -2026, and -2027, were identified.
Microscopy.
Phase contrast microscopy was performed with a Zeiss D-7082 microscope equipped with a 35-mm camera. Since streptomycete filaments grow in all directions, we improved the viewing quality by applying 1-μl samples to the slides and spreading them as thinly as possible with coverslips prior to viewing at ×1,000 total magnification. TMax 400 film was used for the photographs. Either the prints or the negatives were scanned and were imported as TIFF files into Corel PhotoPaint 8 for cropping and then into CorelDraw 8 for labeling and assembly of composite photographs. The appearance of each TIFF file was adjusted with Corel PhotoPaint 8 to resemble the microscopic view as closely as possible.
RESULTS
Identification of sporulation-specific proteins.
To identify proteins that accumulate to high levels under sporulation conditions (phosphate starvation and nutritional downshift) but not during vegetative growth and that may therefore depend on developmental genes, we compared total proteins of sporulating cells with those of vegetative cells by two-dimensional PAGE after staining with Coomassie brilliant blue. By comparing the protein populations from both sporulation conditions, we sought to exclude proteins that might have been induced as a result of the phosphate regulatory network. Five sporulation-specific proteins (named P1, P2, P3, P4, and P5) were present up to at least 12 h after induction (Fig. 1); after this time, the thick walls made the maturing spores refractory to breakage at high pressure in a French press (34). The molecular weights and isoelectric points measured on two-dimensional PAGE are shown in Table 1. These proteins became visible at different times during the first 12 h of sporulation (Table 1).
FIG. 1.
Identification of sporulation-specific proteins by two-dimensional PAGE in the wild-type strain of S. griseus. Total protein profiles from vegetatively growing cells (A) and from cells after 12 h of phosphate starvation (B) are shown. Arrowheads mark five sporulation-specific proteins (P1 through P5). The numbers indicate molecular mass standards in kDa. The proteins were stained with Coomassie brilliant blue R-250. The proteins are more horizontally spread in panel B than in panel A due to longer running during isoelectric focusing.
TABLE 1.
Accumulation of developmentally regulated proteins in the wild-type strain of S. griseus during the first 12 h of sporulationa
| Protein | Mol mass (kDa) | pl | Protein accumulation at sporulation time (h):b
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 (veg) | 2 | 4 | 6 | 8 | 10 | 12 | |||
| P1 | 82 | 5.2 | − | − | + | + | + | + | + |
| P2 | 55 | 5.3 | − | − | ± | ± | ± | + | + |
| P3 (EshA) | 52 | 5.5 | − | − | + | + | + | + | + |
| P4 | 52 | 5.5 | − | − | ± | + | + | + | + |
| P5 | 36 | 5.2 | − | − | − | − | + | + | + |
Sporulation was induced by both phosphate starvation and nutritional downshift in separate experiments.
+, protein present at a high level; ±, protein present at a low level; −, protein absent; veg, equivalent to vegetative growth.
Since our hypothesis that proteins P1 through P5 were sporulation specific implied that developmental mutants might lack some or all of these proteins, we looked for their production in class III nonsporulating mutants of S. griseus (3). The class III mutants, which include bldA mutants (34, 35) as well as those with mutations in other uncharacterized genes, have complex phenotypes. The colonies appear bald on an agar medium that ordinarily supports luxuriant sporulation. When induced to sporulate in liquid culture by phosphate starvation or nutritional downshift, the class III mutants prematurely undergo nucleoid segregation, septation, and spore maturation throughout the preexisting vegetative filaments (34). Ectopic development of septation and spore formation in the mutants is accompanied by premature fragmentation of the nascent spore chains. Thick spore walls form in these mutants by 6 h (class IIIA and C) to 8 h (bldA [class IIIB]) of sporulation, fully 4 to 6 h earlier than in the wild-type strain (34). Two-dimensional gel electrophoresis revealed that extracts prepared from all class III mutant strains during the first 6 h of sporulation lacked P3 (EshA) and P4, whereas P1 and P2 were present in the extracts. P5 was produced at high levels by 4 h of phosphate starvation in the bldA mutant (Table 2), which is 4 h earlier than in the wild-type strain. We chose P3 (EshA) for further study.
TABLE 2.
Accumulation of developmentally regulated proteins in the class III bald mutantsa
| Protein | Protein accumulation at sporulation time (h):b
|
|||
|---|---|---|---|---|
| 0 (veg), for strains SKK1015, −1008, and −1003 | 4, for the indicated strain
|
|||
| SKK1015 | SKK1008 | SKK1003 | ||
| P1 | − | + | + | + |
| P2 | − | ± | ± | ± |
| P3 (EshA) | − | − | − | − |
| P4 | − | − | − | − |
| P5 | − | − | + | − |
Sporulation was induced by both phosphate starvation and nutritional downshift in separate experiments.
+, protein present at a high level; ±, protein present at a low level; −, protein absent; veg, equivalent to vegetative growth.
The eshA gene encodes a protein related to stress-induced proteins of other bacteria but with a likely cyclic nucleotide-binding domain.
The N-terminal amino acid sequence of EshA was identified as TVDSTSEARLEVPRQ. From this sequence a degenerate oligonucleotide (5′-GARGCSMGBCTSGARGTSCC-3′, corresponding to the seventh through thirteenth amino acid of the sequence) was synthesized and was used to identify the eshA gene by Southern hybridization. A 2.4-kb SalI fragment of genomic DNA from wild-type S. griseus hybridized to the oligonucleotide. Two streptomycete open reading frames (ORFs) were found in the DNA fragment by the Frame computer program (67). The first ORF encoded a polypeptide of 470 amino acids with a molecular mass of 52 kDa and an isoelectric point of 5.3. The second one was a partial ORF. The complete ORF (GenBank accession no. L76204) corresponded to the eshA structural gene because (i) the N-terminal sequence of the mature protein was identical to that deduced from the nucleotide sequence (less the fMet); (ii) the calculated isoelectric point of the deduced protein (5.3) was close to that of EshA (5.5) on the basis of its migration in the first dimension of two-dimensional gel electrophoresis; and (iii) the calculated and observed molecular masses were identical (52 kDa). The translation initiation codon of eshA occurred 11 nt downstream of a strong ribosome-binding site (AGGAG; 32). Two alternative, secondary structures could be formed from the sequence immediately downstream of the TAG stop codon: one contained two adjacent, imperfect inverted repeats (nt 1743 to 1766 and nt 1772 to 1799; ΔG = −31 kcal/mol) and the other contained a single, imperfect inverted repeat (nt 1743 to 1796; ΔG = −35 kcal/mol) resembling a factor-independent transcription terminator (15).
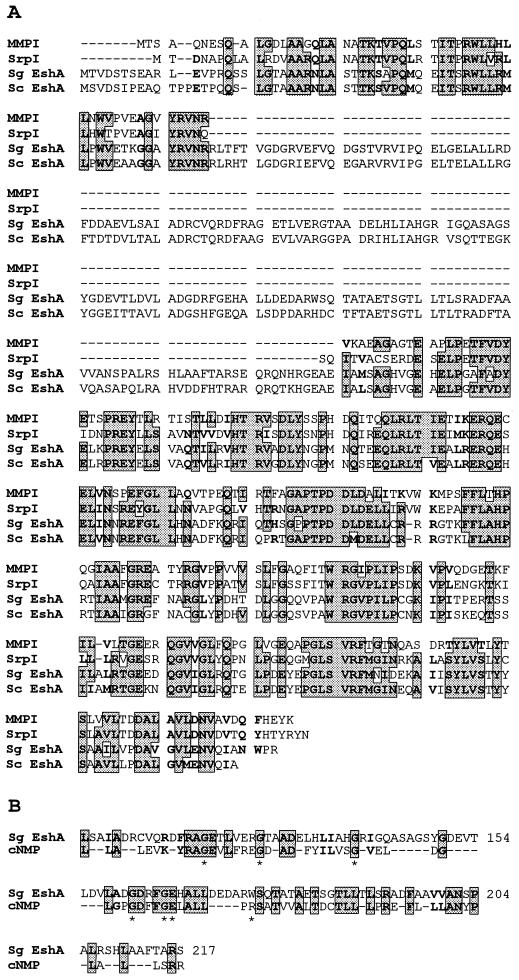
If EshA is an important protein in streptomycete sporulation, then we would expect it to be present in diverse species. We therefore examined S. coelicolor and its close relative Streptomyces lividans for the occurrence of eshA-related DNA sequences by Southern hybridization using a 1.5-kb SphI fragment that contained the eshA coding sequence, 31 nt of upstream DNA, and 93 nt of downstream DNA. Both species showed identical patterns: a 4.3-kb BamHI fragment, a 7.5-kb PstI fragment, and a 2.4-kb SalI fragment hybridized at moderate stringency (≥ 75% identity) to the probe (data not shown). Additionally, a BLAST search of the partial genome sequence of S. coelicolor (www.sanger.ac.uk/Projects/S_coelicolor/blast_server.shtml) revealed the presence of an ORF that is 76% identical (85% similar) to the deduced amino acid sequence of S. griseus eshA (Fig. 2A).
FIG. 2.
Alignment of the amino acid sequence of S. griseus EshA (Sg EshA) with homologous proteins and the cNMP-binding domain. (A) Alignment with MMP-1 (SwissProt accession no. P46841), SrpI (SwissProt accession no. Q55032), and S. coelicolor EshA (Sc EshA; www.sanger.ac.uk/Projects/S_coelicolor/blast_server.shtml). (B) Alignment of the cNMP-binding domain. The cNMP-binding domain sequence given is the most probable amino acid at each position for the cNMP-binding superfamily described by McCue et al. (42). The shaded amino acids in rectangles represent amino acid identities, and bold fonts show similar amino acids (A and G; M, I, L, and V; F, W, and Y; D and E; N and Q; K, R, and H; S and T) that are present in at least three of the four proteins. The seven amino acids shown to be involved in cNMP binding are marked with asterisks.
A BLAST 2.0 search of the nonredundant database at the National Center for BioTechnology Information (NCBI) (Fig. 2A; 1) revealed that the deduced amino acid sequence of the eshA gene shared significant similarity with a mycobacterial protein (MMP-1 from Mycobacterium leprae [SwissProt accession no. P46841] and a 35-kDa protein from Mycobacterium avium complex [SwissProt accession no. Q48899]) and with SrpI from the cyanobacterium Synechococcus PCC7942 (SwissProt accession no. Q55032) (61). Although the function of neither orthologous protein is known, MMP-1 is immunodominant in infected hosts (65) and SrpI is the deduced product of the third gene of a three-gene operon that is strongly expressed during sulfur starvation (47). Alignment of these proteins showed that all three shared extensive similarity at the N terminus and within the C-terminal half (Fig. 2A). The C terminus of each may contain a 14- to 17-amino-acid transmembrane anchor (PHDhtm program; 51).
Noteworthy in this alignment was a domain in EshA that was absent from MMP-1 and SrpI (Fig. 2A). Multiple sequence alignment (42) and domain searches (4) revealed significant homology of this domain with cyclic nucleotide (cNMP)-binding domains from both procaryotic and eucaryotic cNMP-binding proteins (Fig. 2B). This family of proteins contains those in which the activity is modulated by a cNMP, such as E. coli Crp, cNMP-dependent protein kinases, and gated ion channels (42, 56). These cNMP-binding proteins have five glycine residues believed to be important for formation of the cNMP-binding pocket and two conserved residues that interact with the cNMP (Fig. 2B; 42). Dot plot analyses (The University of Wisconsin Genetics Computer Group Program; 16) also showed strong similarity to the cNMP-binding domains of the catabolite activator protein from Haemophilus influenzae and the cGMP-dependent protein kinase from Drosophila melanogaster (data not shown). Computer analyses using PROBE (46) and Classifier (50) also demonstrated that EshA shared homology with procaryotic and eucaryotic proteins containing cNMP-binding domains.
EshA copurifies with the membrane.
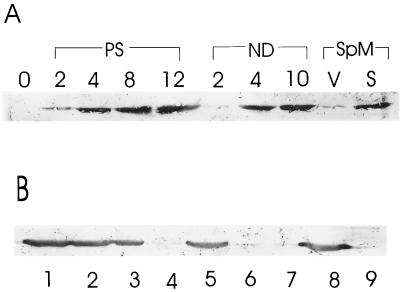
To confirm the expression of EshA during sporulation, we performed Western hybridization experiments using a chicken antibody raised against recombinant EshA. We induced submerged sporulation by using the complex medium SpM (31), as well as by phosphate starvation and nutritional downshift. As expected, EshA was detected in cell extracts of all sporulated cultures (Fig. 3A). EshA was absent in extracts prepared from all class III nonsporulating mutants that had been sporulated by the same induction methods (data not shown).
FIG. 3.
Detection of EshA by immunoblotting. (A) The accumulation of EshA during phosphate starvation (PS), nutritional downshift (ND), and SpM culture. The numbers indicate the hours of culture after shift into the sporulation induction medium. 0 shows the vegetatively growing cell immediately before the shift. V and S represent the vegetative cells and sporulating cells in liquid SpM culture (see Materials and Methods). (B) Detection of EshA using immunoblotting from crude extract (lane 1), S44 (lane 2), P44 (large membrane fragments; lane 3), S150 (lane 4), P150 (lane 5), upper cushion (lane 6), brown layer (lane 7), lower cushion (lane 8), and the pellet (lane 9; ribosomal fraction; 44).
MMP-1 copurified with membrane fractions (65), and the program PHDhtm (51) showed that EshA, MMP-1 from mycobacteria, and SrpI from Synechococcus contained putative transmembrane anchor domains at their C termini. To test whether EshA is a membrane protein, we separated the membrane fraction from cytoplasmic and ribosomal fractions by using differential centrifugation and detected EshA by using Western hybridization. EshA was much more abundant in the protein extracts containing membrane fraction and large membrane fragments (Fig. 3B, lanes 1, 2, 3, 5, and 8) than in the cytoplasmic or ribosomal fractions (Fig. 3B, lanes 4, 6, 7, and 9).
eshA is developmentally regulated and depends on class III developmental genes.
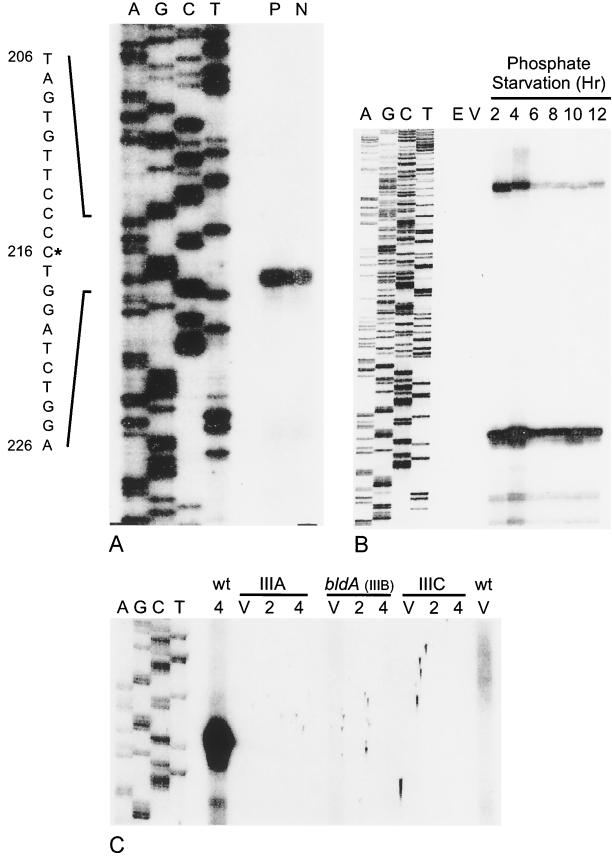
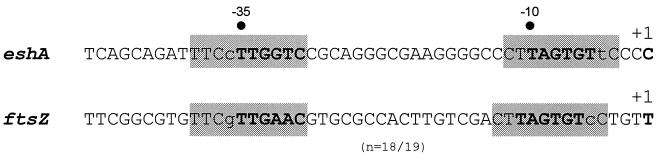
Two eshA transcripts were identified by a high-resolution S1 nuclease protection assay using a single-stranded probe synthesized from oligonucleotide 167 (Fig. 4B). The 5′ endpoint of the major transcript mapped 113 nt upstream and the longer one mapped approximately 300 nt upstream of the translation start codon (Fig. 4A and B). However, the longer signal was not evident when the probe synthesized from oligonucleotide 166 was used, suggesting that the longer signal could be attributed to an artifact. The same site was identified for the major transcript by using both probes (from oligonucleotides 166 and 167), and the endpoint was identical for cultures that were induced to sporulate by either phosphate starvation or nutritional downshift. The eshA transcript was undetectable in RNA from vegetative hyphae but was abundant in RNA extracted at 2 h of sporulation, the earliest time that was examined. A time course analysis showed that the level of eshA transcript remained high through 12 h of sporulation (Fig. 4B). The appearance of the eshA transcript by 2 h of sporulation correlated well with the abundance of the protein at 4 h. PCR amplification and nuclease protection analysis confirmed that the class III mutants contained the eshA gene but lacked the transcript (Fig. 4C). There was a putative promoter, including −10 (TAGTGT) and −35 (TTGGTC) regions measured from the 5′ endpoint of the eshA transcript, resembling the consensus sequence recognized by ςhrdB in Streptomyces (28, 57). The nucleotide sequence in the regions was identical in 16 of 20 positions to the sporulation-specific promoter (Pspo) of ftsZ in S. griseus (Fig. 5), which is transcribed at high levels only during sporulation (J. Kwak, unpublished).
FIG. 4.
Analysis of eshA transcripts by S1 nuclease protection. A single-stranded 400-nt DNA probe (including 20 nt of pGEM-4Z polylinker and 328 nt upstream and 52 nt downstream of the translation start site of the eshA structural gene) was prepared by using oligonucleotide 166 for a primer extension in panel A. Oligonucleotide 167, which extended 116 nt downstream of the initiation codon, was used for the experiments shown in panels B and C. In each case the sequence ladder (A, G, C, T) was extended from the same primer and used as the size marker. (A) Identification of the 5′ end of the eshA transcript in the wild-type strain of S. griseus. P, RNA from cells that had been starved for phosphate for 4 h; N, RNA from cells that had been subjected to nutritional downshift for 4 h. The nucleotide sequence shown to the left of the autoradiogram is that of the sense strand. The asterisk marks the 5′ end of the transcript, and the nucleotide numbers refer to positions in the 2.4-kb SalI fragment (GenBank accession no. L76204). (B) Time course of eshA transcription during phosphate starvation. Total RNA was prepared from vegetative cells (V) and cells that had been starved for phosphate for 2, 4, 6, 8, 10, or 12 h. E. coli tRNA (E) served as the negative control. (C) Analysis of the eshA transcript in the class III mutants during phosphate starvation. The sequence ladder and probe were prepared as described for panel B. Total RNA was prepared from the wild-type strain of S. griseus and class III developmental mutants SKK1015 (class IIIA), SKK1008 (bldA; class IIIB), and SKK1003 (class IIIC) that were growing vegetatively (V) or starved for 2 or 4 h. The film was deliberately overexposed to demonstrate the absence of signal in the mutants.
FIG. 5.
The nucleotide sequence homology of the promoter regions between eshA and ftsZ. The shaded sequences mark the similar regions. The bold letters inside the shaded boxes show the putative −10 and −35 regions. n indicates the spacings between the putative −10 and −35 regions. +1 indicates the 5′ end of transcripts from both genes detected by high-resolution S1 nuclease mapping.
An eshA mutant is defective in sporulation-specific growth.
We constructed an eshA null allele by replacing the coding region from Glu39 to Leu300 with an apramycin resistance cassette. The disrupted structure of the eshA gene was confirmed by Southern hybridization analysis; all three isolates contained the 2.9-kb SalI fragment expected for the disrupted gene and lacked the 2.4-kb SalI fragment characteristic of the intact eshA gene (data not shown). As expected, both fragments were present in a transformant resistant to both apramycin and thiostrepton. An immunoblot showed that the wild-type strain but not the null mutants produced EshA within 6 h after the end of exponential growth in nutrient exhaustion medium (data not shown).
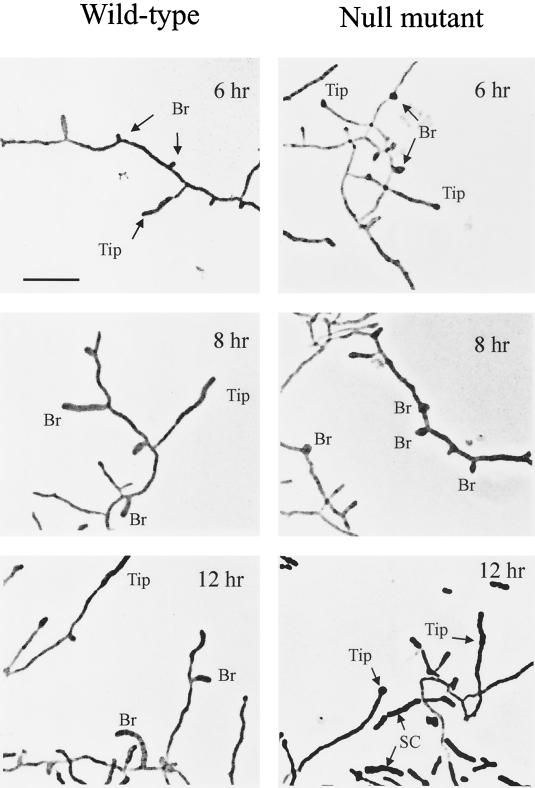
We were not able to detect any difference in growth and sporulation between the null mutant and the wild-type strain when sporulation occurred on solid media. Like the wild-type strain, the disrupted mutant produced streptomycin and grew at a normal rate (μ = 0.47 ± 0.03 h−1) in the complex medium. On buffered SpM agar, which permits abundant sporulation of the wild-type strain, the mutant sporulated to the same extent as the wild-type strain: the colony was as hairy as the wild-type strain and the mutant produced abundant spores when observed with a microscope. Therefore, we turned to the submerged sporulation system (nutritional downshift) for more detailed analysis. Under this condition, the wild-type strain follows a reproducible time course of sporulation (22, 34). In liquid culture, the sporulation yield (sonication resistance units) of the eshA mutant was the same as that of the wild-type strain (5 × 109 to 7 × 109 sr/ml; 34). The spores from these mutants did not have any defects in sonication resistance, lysozyme tolerance, or germination (31, 34) when compared to the wild-type strain. The dramatic aspect of the mutant phenotype during submerged sporulation was the aborted growth of branch sporogenic hyphae (Fig. 6). Phase contrast microscopy showed that the defective branch sporogenic hyphae initiated growth but failed to extend beyond a length of about 1 to 2 μm, even after prolonged incubation. Instead, septation and spore maturation were accelerated at the tips of preexisting vegetative hyphae or de novo synthesized distal sporogenic hyphae (Fig. 6). Frequently these stunted branch sporogenic hyphae became swollen at their bases, perhaps indicative of isotropic rather than polar growth. By 12 h, almost all of the deformed branch sporogenic hyphae had detached from the vegetative hyphae (Fig. 6).
FIG. 6.
Phase-contrast photomicroscopy of the eshA null mutant (SKK2025) and the wild-type strain of S. griseus after 6, 8, and 12 h of sporulation induced by nutritional downshift. Sporogenic hyphae emerging from new branches (Br) and from preexisting vegetative hyphae (Tip) are marked. SC marks the spore chains. The bar represents 6.0 μm. No differences were apparent in vegetative cultures of the mutant and wild-type strains.
The eshA null mutant formed septate spore chains at the vegetative hyphal tips at 8 to 10 h. After 12 h of induction the nascent spore chains of the mutant were fragmented and released. In comparison, the wild-type strain requires 12 h to undergo septation and an additional 10 h to form fragmented, mature spore chains (Fig. 6; 34). The spore chains that formed in the null mutant were abnormal, however, because the distal “spore” in the chain generally appeared larger when compared to other spores in the chain (Fig. 6). The septated region was noticeably longer than the “distal” sporogenic hyphae of the wild-type strain.
To demonstrate that the phenotype was caused by the deletion of the eshA gene, we used two different strategies to complement the mutant. In the first case, we introduced the wild-type allele contained in either the 2.4-kb SalI fragment on pXE4 (pKK856) or the 8-kb BamHI-HindIII fragment containing eshA plus approximately 3.5 kb of upstream and 2.5 kb of downstream sequences (pKK1416). Then we compared sporulation of these transformants with those that contained the vector only. In the second approach, we constructed the same 8-kb eshA fragment in the nonreplicative vector pKK1400 at BamHI and EcoRI sites (pKK1417) and generated a single crossover strain by integrating into the chromosome by homologous recombination at the eshA locus. Because the presence of thiostrepton in the medium (to select for the maintenance of the plasmid) lengthened the time required for development of both the wild-type strain and the mutant, we excluded thiostrepton from the growth and nutritional downshift media. At the end of the experiment, the viable count of spores (measured as sonication-resistant units per ml; 34) showed that thiostrepton resistance was maintained in more than 90% of the spores. This indicated that there was little if any loss of the plasmid during the course of the experiment. The null mutant was complemented by the eshA gene in trans; branch sporogenic hyphae formed to the same extent as in the wild-type strain (data not shown). Since the complementing fragment lacked a complete downstream ORF, we conclude that the mutant phenotype was caused by the lack of EshA.
DISCUSSION
EshA is a stress-response protein.
EshA is an abundant protein that accumulates during sporulation induced by phosphate starvation and nutritional downshift in S. griseus. Similar proteins are present in a cyanobacterium and two species of mycobacteria. Although the functions of these homologous proteins are not known, they are produced abundantly when the cells experience nutritional stress: SrpI when Synechococcus encounters sulfur stress and MMP-1 during infection by M. leprae, which multiplies in phagosomes of the infected host (65). Since EshA is also made during nutritional stress, we have proposed that EshA, SrpI, and MMP-1 define a new family of bacterial stress-response proteins (61). MMP-1 is believed to be a membrane protein and is routinely isolated as multimers in excess of 20 subunits (65; W. J. Britton, personal communication). Our results also showed that EshA localizes to the cell membrane (Fig. 3B) and possibly exists as large multimers (J. Kwak, unpublished). A putative transmembrane anchor in EshA, MMP-1, and SrpI was found by using the program PHDhtm (51). In light of its abundance and and localization to the membrane, we speculate that EshA may be part of a structural element. There is no EshA ortholog in E. coli, Bacillus subtilis, or other procaryotes whose genomes have been completely sequenced. A gene apparently homologous to EshA was also present in S. coelicolor and S. lividans, two species that are not closely related to S. griseus (32).
EshA is a determinant of growth of sporogenic hyphae.
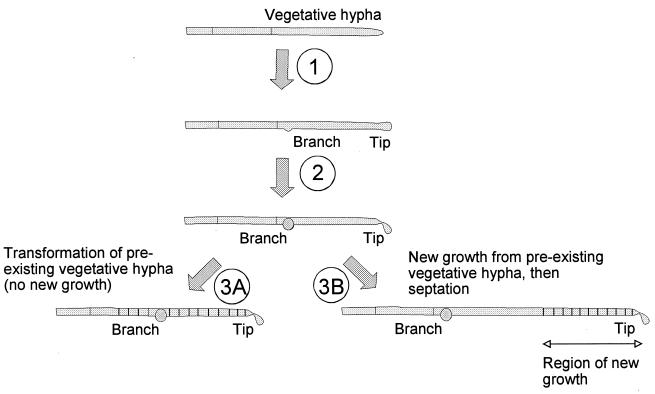
This study demonstrated that EshA is required for the maintenance of growth of sporogenic hyphae from de novo branches and for proper formation of distal sporogenic hyphae, since the absence of EshA resulted in abortive growth of branch sporogenic hyphae. We do not exclude the possibility that EshA may function as an inhibitory factor of septum formation in the preexisting vegetative hyphae until sporogenic hyphae are fully mature. However, EshA does not seem to be required for vegetative growth and branching, sporulation septum formation, or spore maturation. Despite the absence of full-length sporogenic hyphae in the null mutants, spore chains and mature spores formed from the preexisting vegetative hyphae. Consistently, the spores from these mutants did not have any defects in sonication resistance, lysozyme tolerance, or germination (31, 34) when compared to the wild-type strain. The premature septation and fragmentation demonstrated that these spore chains form ectopically by transformation of the vegetative filaments (Fig. 6 and 7). The ectopic septation and spore formation from the preexisting vegetative or distal sporogenic mycelia without mature growth of proximal sporogenic hyphae (34) are also observed in the class III nonsporulating mutants that do not accumulate EshA during sporulation. Taking into consideration its possible existence as a structural component, and the phenotypes observed in the eshA null mutants and the class III mutants, it is conceivable that this aspect of the null mutant phenotype could be caused by a high local concentration of factors required for septation and spore maturation that might mislocalize to the vegetative hyphae in the absence of sporogenic hyphal growth (Fig. 6 and 7). For this reason, we named P3 as EshA (extension of sporogenic hyphae). We speculate that the aberrant phenotype of the mutant observed in submerged culture also occurs on solid culture. The delicate morphological changes may not have been detected, since the cells harvested from solid cultures were more heterogeneous and less synchronous.
FIG. 7.
Diagram of alternative ways in which spores could be made in the null mutant. Initial formation of the sporogenic hyphae is unaltered (step 1). Subsequently, in the absence of EshA the proximal sporogenic hyphae form a bulbous structure characteristic of apolar growth. Simultaneously the apex of the distal sporogenic hypha becomes deformed (step 2). The spore structures arise either by ectopic septation and maturation in the preexisting vegetative hyphae (step 3A) or by proper localization in the distal sporogenic hypha following a period of new growth (step 3B).
Our assignment of EshA as a protein required for growth of sporogenic hyphae in Streptomyces raises the question of the roles of the orthologous proteins in mycobacteria and Synechococcus. Limitation of Synechococcus for sulfur leads to the global activation of a number of genes whose products are responsible for the utilization of alternative sources of sulfur (37), degradation of light harvesting centers (17, 55), and formation of a quiescent structure that requires extensive remodeling of the cell membrane and wall (A. R. Grossman, personal communication). In view of the eshA null phenotype, we hypothesize that SrpI may be necessary for the morphological changes that occur under this condition. Although mycobacteria are members of the Actinomycetales that grow with rudimentary branching, whether M. leprae displays morphological changes during infection coincident with synthesis of MMP-1 is not known (P. J. Brennan and W. J. Britton, personal communication).
Developmentally regulated expression of EshA.
Because the eshA transcript was evident only during sporulation, expression of eshA is regulated at least in part at the transcriptional level. There was a single transcript 5′ endpoint, regardless of whether sporulation was induced by phosphate starvation or nutritional downshift. This endpoint could alternatively correspond to a site at which a longer transcript was processed, but our results suggest that the 5′ endpoint of the eshA transcript marks the transcription start site since there was a putative promoter including −10 and −35 regions from the 5′ endpoint and there was no evidence of a transcript 5′ end further upstream. Although either inverted repeat downstream of eshA may act as a factor-independent transcription terminator, we do not yet know whether eshA and the downstream ORF are cotranscribed.
The bald colony morphology of the class III mutants suggests that none of these mutations is allelic to eshA. Indeed, we know that this is true for the class IIIB (bldA) mutants. Moreover, the eshA null mutant produced streptomycin, unlike the class III mutants. The absence of the eshA transcript in the class III mutants was a consequence of the lack of gene expression. This result shows that the class III mutants are defective in regulatory factors that mediate synthesis of EshA and other proteins necessary for sporulation and antibiotic production. One of these factors is a tRNA, the product of bldA. The requirement of the class III developmental genes, including bldA, for transcription of eshA prompted us to reexamine the morphology of a bldA mutant of S. griseus. We confirmed the previous results (34) indicating that these mutants did not make branch sporogenic hyphae, that the formation of spore chains in this strain occurred ectopically, and that this mutant underwent premature fragmentation of developing spore chains. The observation indicates that the aberrant phenotype of class III mutants is caused to a considerable extent by the absence of EshA.
We speculate that the known sporulation-specific sigma factors are not responsible for transcription of eshA. We can readily rule out recognition by WhiG; whiG mutants are blocked at a later stage of sporulation because whiG mutants form lengthy aerial hyphae but not spore compartments (11, 30). Likewise, ςF is not a candidate because it is required for the relatively late events of spore maturation (49). However, the putative promoter for eshA including the −10 and −35 regions is highly homologous to the consensus sequence recognized by ςhrdB (28, 57), which is the essential sigma factor in Streptomyces (8). These results suggest that class III developmental genes, including bldA, regulate the transcription of eshA during sporulation via a regulatory factor other than a sigma factor.
The role of cyclic nucleotides in Streptomyces.
EshA contains a cNMP-binding domain which is absent from the corresponding proteins from Synechococcus and Mycobacterium. Such a domain is present in proteins whose activity is modulated by a cNMP but not in proteins that hydrolyze cNMPs (56). The eucaryotic cGMP-dependent phosphodiesterases and cAMP receptor proteins of Dictyostelium also contain distinctly different cNMP-binding domains. EshA is a member of the cNMP-binding protein superfamily identified using PROBE (46) and Classifier (50). In E. coli Crp (62) and the regulatory unit of bovine cAMP-dependent kinase (58), for which the three-dimensional structure has been solved, seven invariant amino acid residues are required for interaction with the cNMP: the stability of an eight-stranded β-barrel likely depends on the five Gly residues; the ribose 2′OH of cAMP is hydrogen bonded to Glu72; and Arg82 interacts with one of the exocyclic oxygens of the phosphate moiety (42). These seven residues are all conserved in EshA (42).
Cyclic nucleotides play widespread regulatory roles in eucaryotes, where they govern processes such as polarized cell growth in filamentous fungi (6) and cation conductance in retinal photoreceptors (66). The known role of cyclic nucleotides in procaryotes is, up to now, much more limited. The only procaryotic proteins known to bind cAMP are Crp and DnaA (25). In proteobacteria, which include enteric bacteria and members of phylogenetically closely related gram-negative genera, Crp controls a variety of regulons, including those involved in catabolism (5), virulence (14, 64), and development of competence (10). With the exception of Crp, however, many of the other members of the Crp-like protein family, such as Fnr and FixK, have lost one or more of the amino acids thought to play central roles in binding to cAMP (42) while retaining their DNA-binding domains. Cyclic AMP has not been identified as an effector in the low-GC gram-positive bacteria. B. subtilis does not produce cAMP (P. Setlow, personal communication) and lacks a gene resembling those encoding either adenylate or guanylate cyclase. Cyclic diguanylic acid functions as a reversible allosteric activator of cellulose biosynthesis in Acetobacter xylinum (63).
Streptomycetes accumulate cNMPs both intracellularly and extracellularly. Recent studies have shown that cAMP accumulates to a high extracellular level during the second exponential growth phase of S. griseus (45) and S. coelicolor (59). The gene encoding adenylate cyclase has been isolated from S. coelicolor (13). In addition to its inability to synthesize cAMP, a cya null mutant shows a complex phenotype that includes impaired initiation of growth during spore germination and morphogenesis; the latter defect is brought about by the inability to overcome the acidic environment generated by the extracellular accumulation of organic acids from glucose at the end of the first exponential growth period (59).
ACKNOWLEDGMENTS
We thank Keith Chater, Chuck Daniels, and Tina Henkin for critically commenting on the manuscript. We also thank Tom Thompson for helpful discussion, R. Baltz and P. Solenberg for providing the apramycin-resistance cassette, D. MacNeil for providing the E. coli ET12567 strain, Bill Lane of the Harvard Microbiochemistry Lab for amino acid sequence determination, Tim Vojt and Don Ordaz for photoimaging processing, and Minho Kim for computer drawing.
This work was supported by grant MCB-9724038 from the National Science Foundation.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent A, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1990. [Google Scholar]
- 3.Babcock M J, Kendrick K E. Cloning of DNA involved in sporulation of Streptomyces griseus. J Bacteriol. 1988;170:2802–2808. doi: 10.1128/jb.170.6.2802-2808.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman A, Birney E, Durbin R, Eddy S R, Howe K L, Sonnhammer E L. The Pfam protein families database. Nucleic Acids Res. 2000;28:263–266. doi: 10.1093/nar/28.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botsford J L, Harman J G. Cyclic AMP in prokaryotes. Microbiol Rev. 1992;56:100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruno K S, Aramayo R, Minke P F, Metzenberg R L, Plamann M. Loss of growth polarity and mislocalization of septa in a Neurospora mutant altered in the regulatory subunit of cAMP-dependent protein kinase. EMBO J. 1996;15:5772–5782. [PMC free article] [PubMed] [Google Scholar]
- 7.Bruton C J, Plaskitt K A, Chater K F. Tissue-specific glycogen branching isoenzymes in a multicellular prokaryote, Streptomyces coelicolor A3(2) Mol Microbiol. 1995;18:89–99. doi: 10.1111/j.1365-2958.1995.mmi_18010089.x. [DOI] [PubMed] [Google Scholar]
- 8.Buttner M J, Chater K F, Bibb M J. Cloning, disruption, and transcriptional analysis of three RNA polymerase sigma factor genes of Streptomyces coelicolor A3(2) J Bacteriol. 1990;172:3367–3378. doi: 10.1128/jb.172.6.3367-3378.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter M J, Milton I D. A simple method for DNA purification on silica particles. Nucleic Acids Res. 1993;21:1044. doi: 10.1093/nar/21.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandler M S. The gene encoding cAMP receptor protein is required for competence development in Haemophilus influenzae. Proc Natl Acad Sci USA. 1992;89:1626–1630. doi: 10.1073/pnas.89.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chater K F. A morphological and genetic mapping study of white colony mutants of Streptomyces coelicolor. J Gen Microbiol. 1972;72:9–28. doi: 10.1099/00221287-72-1-9. [DOI] [PubMed] [Google Scholar]
- 12.Chater K F, Bruton C J, Plaskitt K A, Buttner M J, Mendez C, Helmann J D. The developmental fate of Streptomyces coelicolor hyphae depends upon a gene product homologous with the motility sigma factor of Bacillus subtilis. Cell. 1989;59:133–143. doi: 10.1016/0092-8674(89)90876-3. [DOI] [PubMed] [Google Scholar]
- 13.Danchin A, Pidoux J, Krin E, Thompson C J, Ullmann A. The adenylate cyclase catalytic domain of Streptomyces coelicolor is carboxy-terminal. FEMS Microbiol Lett. 1993;114:145–152. doi: 10.1111/j.1574-6968.1993.tb06565.x. [DOI] [PubMed] [Google Scholar]
- 14.de Crecy-Lagard V, Glaser P, Lejeune P, Sismeiro O, Barber C E, Daniels M J, Danchin A. A Xanthomonas campestris pv. campestris protein similar to catabolite activation factor is involved in regulation of phytopathogenicity. J Bacteriol. 1990;172:5877–5883. doi: 10.1128/jb.172.10.5877-5883.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng Z, Kieser T, Hopwood D A. Activity of a Streptomyces transcription terminator in Escherichia coli. Nucleic Acids Res. 1987;15:2665–2675. doi: 10.1093/nar/15.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolganov N, Grossman A R. A polypeptide with similarity to phycocyanin alpha-subunit phycocyanobilin lyase involved in degradation of phycobilisomes. J Bacteriol. 1999;181:610–617. doi: 10.1128/jb.181.2.610-617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drubin D G, Nelson W J. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 19.Ehresmann B, Imbault P, Weil J H. Spectrophotometric determination of protein concentration in cell extracts containing tRNA's and rRNA's. Anal Biochem. 1973;54:454–463. doi: 10.1016/0003-2697(73)90374-6. [DOI] [PubMed] [Google Scholar]
- 20.Garrels J I. Quantitative two-dimensional gel electrophoresis of proteins. Methods Enzymol. 1983;100:411–423. doi: 10.1016/0076-6879(83)00070-1. [DOI] [PubMed] [Google Scholar]
- 21.Granier F. Extraction of plant proteins for two-dimensional electrophoresis. Electrophoresis. 1988;9:712–718. doi: 10.1002/elps.1150091106. [DOI] [PubMed] [Google Scholar]
- 22.Hao J, Kendrick K E. Visualization of penicillin-binding proteins during sporulation of Streptomyces griseus. J Bacteriol. 1998;180:2125–2132. doi: 10.1128/jb.180.8.2125-2132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984;28:351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- 24.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces. A laboratory manual. Norwich, Great Britain: The John Innes Foundation; 1985. [Google Scholar]
- 25.Hughes P, Landoulsi A, Kohiyama M. A novel role for cAMP in the control of the activity of the E. coli chromosome replication initiator protein, DnaA. Cell. 1988;55:343–350. doi: 10.1016/0092-8674(88)90057-8. [DOI] [PubMed] [Google Scholar]
- 26.Ingram C, Brawner M, Youngman P, Westpheling J. xylE functions as an efficient reporter gene in Streptomyces spp.: use for the study of galP1, a catabolite-controlled promoter. J Bacteriol. 1989;171:6617–6624. doi: 10.1128/jb.171.12.6617-6624.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen G R, Bibb M J. Derivatives of pUC18 that have BglII sites flanking a modified multiple cloning site and that retain the ability to identify recombinant clones by visual screening of Escherichia coli colonies. Gene. 1993;124:133–134. doi: 10.1016/0378-1119(93)90774-w. [DOI] [PubMed] [Google Scholar]
- 28.Kang J G, Hahn M Y, Ishihama A, Roe J H. Identification of sigma factors for growth phase-related promoter selectivity of RNA polymerases from Streptomyces coelicolor A3(2) Nucleic Acids Res. 1997;25:2566–2573. doi: 10.1093/nar/25.13.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelemen G H, Brian P, Flardh K, Chamberlin L, Chater K F, Buttner M J. Developmental regulation of transcription of whiE, a locus specifying the polyketide spore pigment in Streptomyces coelicolor A3(2) J Bacteriol. 1998;180:2515–2521. doi: 10.1128/jb.180.9.2515-2521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelemen G H, Brown G L, Kormanec J, Potuckova L, Chater K F, Buttner M J. The positions of the sigma-factor genes, whiG and sigF, in the hierarchy controlling the development of spore chains in the aerial hyphae of Streptomyces coelicolor A3(2) Mol Microbiol. 1996;21:593–603. doi: 10.1111/j.1365-2958.1996.tb02567.x. [DOI] [PubMed] [Google Scholar]
- 31.Kendrick K E, Ensign J C. Sporulation of Streptomyces griseus in submerged culture. J Bacteriol. 1983;155:357–366. doi: 10.1128/jb.155.1.357-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim E, Kim H, Kang K H, Kho Y H, Park Y-H. Complete nucleotide sequence of a 16S ribosomal RNA gene from Streptomyces griseus subsp. griseus. Nucleic Acids Res. 1991;19:1149. doi: 10.1093/nar/19.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroening T A, Kendrick K E. In vivo regulation of histidine ammonia-lyase activity from Streptomyces griseus. J Bacteriol. 1987;169:823–829. doi: 10.1128/jb.169.2.823-829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwak J, Kendrick K E. Bald mutants of Streptomyces griseus that prematurely undergo key events of sporulation. J Bacteriol. 1996;178:4643–4650. doi: 10.1128/jb.178.15.4643-4650.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwak J, McCue L A, Kendrick K E. Identification of bldA mutants of Streptomyces griseus. Gene. 1996;171:75–78. doi: 10.1016/0378-1119(96)00066-2. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Laudenbach D E, Grossman A R. Characterization and mutagenesis of sulfur-regulated genes in a cyanobacterium: evidence for function in sulfate transport. J Bacteriol. 1991;173:2739–2750. doi: 10.1128/jb.173.9.2739-2750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawlor E J, Baylis H A, Chater K F. Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a tRNA-like product in Streptomyces coelicolor A3(2) Genes Dev. 1987;1:1305–1310. doi: 10.1101/gad.1.10.1305. [DOI] [PubMed] [Google Scholar]
- 39.MacNeil D J, Gewain K M, Ruby C L, Dezeny G, Gibbons P H, MacNeil T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene. 1992;111:61–68. doi: 10.1016/0378-1119(92)90603-m. [DOI] [PubMed] [Google Scholar]
- 40.Matsushima P, Baltz R H. Transformation of Saccharopolyspora spinosa protoplasts with plasmid DNA modified in vitro to avoid host restriction. Microbiology. 1994;140:139–143. [Google Scholar]
- 41.McCormick J R, Su E P, Driks A, Losick R. Growth and viability of Streptomyces coelicolor mutant for the cell division gene ftsZ. Mol Microbiol. 1994;14:243–254. doi: 10.1111/j.1365-2958.1994.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 42.McCue L A, McDonough K A, Lawrence C E. Functional classification of cNMP-binding proteins and nucleotide cyclases with implications for novel regulatory pathways in Mycobacterium tuberculosis. Genome Res. 2000;10:204–219. doi: 10.1101/gr.10.2.204. [DOI] [PubMed] [Google Scholar]
- 43.Merrick M J. A morphological and genetic mapping study of bald colony mutants of Streptomyces coelicolor. J Gen Microbiol. 1976;96:299–315. doi: 10.1099/00221287-96-2-299. [DOI] [PubMed] [Google Scholar]
- 44.Mikulik K, Janda I. Protein kinase associated with ribosomes phosphorylates ribosomal proteins of Streptomyces collinus. Biochem Biophys Res Commun. 1997;238:370–376. doi: 10.1006/bbrc.1997.7297. [DOI] [PubMed] [Google Scholar]
- 45.Neumann T, Piepersberg W, Distler J. Decision phase regulation of streptomycin production in Streptomyces griseus. Microbiology. 1996;142:1953–1963. [Google Scholar]
- 46.Neuwald A F, Liu J S, Lipman D J, Lawrence C E. Extracting protein alignment models from the sequence database. Nucleic Acids Res. 1997;25:1665–1677. doi: 10.1093/nar/25.9.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicholson M L, Gaasenbeek M, Laudenbach D E. Two enzymes together capable of cysteine biosynthesis are encoded on a cyanobacterial plasmid. Mol Gen Genet. 1995;247:623–632. doi: 10.1007/BF00290354. [DOI] [PubMed] [Google Scholar]
- 48.O'Farrell P H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 49.Potúcková L, Kelemen G H, Findlay K C, Lonetto M A, Buttner M J, Kormanec J. A new RNA polymerase sigma factor, ςF, is required for the late stages of morphological differentiation in Streptomyces spp. Mol Microbiol. 1995;17:37–48. doi: 10.1111/j.1365-2958.1995.mmi_17010037.x. [DOI] [PubMed] [Google Scholar]
- 50.Qu K, McCue L A, Lawrence C E. Bayesian protein family classifier. Proc Int Conf Intell Syst Mol Biol. 1998;6:131–139. [PubMed] [Google Scholar]
- 51.Rost B, Fariselli P, Casadio R. Topology prediction for helical transmembrane proteins at 86% accuracy. Protein Sci. 1996;5:1704–1718. doi: 10.1002/pro.5560050824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryding N J, Kelemen G H, Whatling C A, Flardh K, Buttner M J, Chater K F. A developmentally regulated gene encoding a repressor-like protein is essential for sporulation in Streptomyces coelicolor A3(2) Mol Microbiol. 1998;29:343–357. doi: 10.1046/j.1365-2958.1998.00939.x. [DOI] [PubMed] [Google Scholar]
- 53.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 54.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwarz R, Grossman A R. A response regulator of cyanobacteria integrates diverse environmental signals and is critical for survival under extreme conditions. Proc Natl Acad Sci USA. 1998;95:11008–11013. doi: 10.1073/pnas.95.18.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shabb J B, Corbin J D. Cyclic nucleotide-binding domains in proteins having diverse functions. J Biol Chem. 1992;267:5723–5726. [PubMed] [Google Scholar]
- 57.Strohl W R. Compilation and analysis of DNA sequences associated with apparent streptomycete promoters. Nucleic Acids Res. 1992;20:961–974. doi: 10.1093/nar/20.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su Y, Dostmann W R, Herberg F W, Durick K, Xuong N H, Ten Eyck L, Taylor S S, Varughese K I. Regulatory subunit of protein kinase A: structure of deletion mutant with cAMP binding domains. Science. 1995;269:807–813. doi: 10.1126/science.7638597. [DOI] [PubMed] [Google Scholar]
- 59.Süsstrunk U, Pidoux J, Taubert S, Ullmann A, Thompson C J. Pleiotropic effects of cAMP on germination, antibiotic biosynthesis and morphological development in Streptomyces coelicolor. Mol Microbiol. 1998;30:33–46. doi: 10.1046/j.1365-2958.1998.01033.x. [DOI] [PubMed] [Google Scholar]
- 60.Thompson C J, Kieser T, Ward J M, Hopwood D A. Physical analysis of antibiotic-resistance genes from Streptomyces and their use in vector construction. Gene. 1982;20:51–62. doi: 10.1016/0378-1119(82)90086-5. [DOI] [PubMed] [Google Scholar]
- 61.Triccas J A, Winter N, Roche P W, Gilpin A, Kendrick K E, Britton W J. Molecular and immunological analyses of the Mycobacterium avium homolog of the immunodominant Mycobacterium leprae 35-kilodalton protein. Infect Immun. 1998;66:2684–2690. doi: 10.1128/iai.66.6.2684-2690.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber I T, Steitz T A. Structure of a complex of catabolite gene activator protein and cyclic AMP refined at 2.5 Å resolution. J Mol Biol. 1987;198:311–326. doi: 10.1016/0022-2836(87)90315-9. [DOI] [PubMed] [Google Scholar]
- 63.Weinhouse H, Sapir S, Amikam D, Shilo Y, Volman G, Ohana P, Benziman M. c-di-GMP-binding protein, a new factor regulating cellulose synthesis in Acetobacter xylinum. FEBS Lett. 1997;416:207–211. doi: 10.1016/s0014-5793(97)01202-7. [DOI] [PubMed] [Google Scholar]
- 64.West S E H, Sample A K, Runyen-Janecky L J. The vfr gene product, required for Pseudomonas aeruginosa exotoxin A and protease production, belongs to the cyclic AMP receptor protein family. J Bacteriol. 1994;176:7532–7542. doi: 10.1128/jb.176.24.7532-7542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winter N, Triccas J A, Rivoire B, Pessolani M C, Eiglmeier K, Lin E M, Hunter S W, Brennan P J, Britton W J. Characterization of the gene encoding the immunodominant 35 kDa protein of Mycobacterium leprae. Mol Microbiol. 1995;16:865–876. doi: 10.1111/j.1365-2958.1995.tb02314.x. [DOI] [PubMed] [Google Scholar]
- 66.Wolbring G, Schnetkamp P P. Modulation of the calcium sensitivity of bovine retinal rod outer segment guanylyl cyclase by sodium ions and protein kinase A. Biochemistry. 1996;35:11013–11018. doi: 10.1021/bi960699e. [DOI] [PubMed] [Google Scholar]
- 67.Wright F, Bibb M J. Codon usage in the G+C−rich Streptomyces genome. Gene. 1992;113:55–65. doi: 10.1016/0378-1119(92)90669-g. [DOI] [PubMed] [Google Scholar]