Abstract
Testicular germ cell tumors, including seminomas, originate mainly from the testicles and rarely from extragonadal locations, often retroperitoneum and mediastinum. Moreover, primary seminal vesicle tumors are extremely rare, and the most described histology is adenocarcinoma. We report, as far as we know, the second case of primary seminoma of the seminal vesicle.
Keywords: Seminal vesicle, Seminoma
1. Introduction
Seminomas constitute 50% of germ cell tumors. 95–98% originate in the testicles, and 2–5% arise in the midline of the body (from pineal gland to coccyx - most commonly the mediastinum, retroperitoneum, and cranium).1,2 They typically present as slowly growing, asymptomatic masses, have bad prognosis, and do not respond to conventional treatment. Extragonadal origin can only be established after excluding the primary testicular.2,3
Seminal vesicle tumors usually constitute contiguous invasion from malignancies in the prostate, bladder, or rectum. Primary malignancies of the seminal vesicles are rare: 100 cases of adenocarcinomas, mesenchymal tumors, and mixed epithelial tumors have been reported.4 Strict diagnostic criteria to identify tumors as primary seminal vesicle malignancies were set by Dalgaard and Gierston in 1856: “a microscopically verified carcinoma, exclusively or mainly located in the seminal vesicle; another primary simultaneous carcinoma has to be excluded; and tumor should resemble the architecture of the normal seminal vesicle”.5
This is the second case of a primary seminal vesicle seminoma reported in the literature. The first one was described by Adachi et al., in 1991.3
2. Case
We report the case of a 43-year-old Colombian man, without biological offspring, diagnosed with hypogonadism, receiving testosterone replacement since 27 years old. He complained of 10 years of paresthesias on his right leg, a burning sensation in his right testicle, and painful ejaculation. Digital rectal examination revealed a normal prostate, and a bilaterally palpable petrous irregular mass on the base of it. Both testicles were descended, small, and painful.
Transrectal ultrasound revealed a 42-cc heterogeneous, irregular mass on the posterior right face of the prostate, with vascular activity, originating from the right seminal vesicle. Specific Prostate Antigen was 0,4 ng/dL. Abdominopelvic contrast enhanced magnetic resonance showed a mass originating from the seminal vesicles, and a metastatic adenopathy in the right hemi-pelvis (Fig. 1A). There were no retroperitoneal lymph nodes enlarged. Thorax scan was normal.
Fig. 1.
A, B: A. Abdominopelvic magnetic resonance. Thick arrows signal tumor. Thin arrow signals pelvic lymphadenopathy. B. Surgical Specimen. Foley catheter through the prostatic urethra. Nelaton catheter repairing the right ureter. Arrow signaling the tumor.
A trans-perineal biopsy showed normal prostatic tissue, and fragments of a poorly differentiated carcinoma: solid nests of cells, high nucleus/cytoplasm ratio, pleomorphic, atypic, and hyperchromatic nucleus, without clear formation of ducts.
A radical prostatectomy plus extended pelvic lymphadenectomy plus right hemitrigone resection plus right ureteral reimplantation, were undertaken 2 months after his first visit. During surgery, a macroscopically normal prostate was found. Both seminal vesicles were petrous, completely infiltrated by a neoplastic mass. The right one measured 7 cm in the greatest diameter, the left one 4 cm. There was a pelvic mass adjacent to the bladder, of 10cm in the greatest diameter, comprising hypogastric and obturator ipsilateral lymph nodes, and the right obturator nerve. The right hemi-trigone and a right distal ureteral segment were compromised by the tumor and were resected in bloc. Right ureter was reimplanted. The patient recovered without immediate complications and was discharged on the third postoperative day.
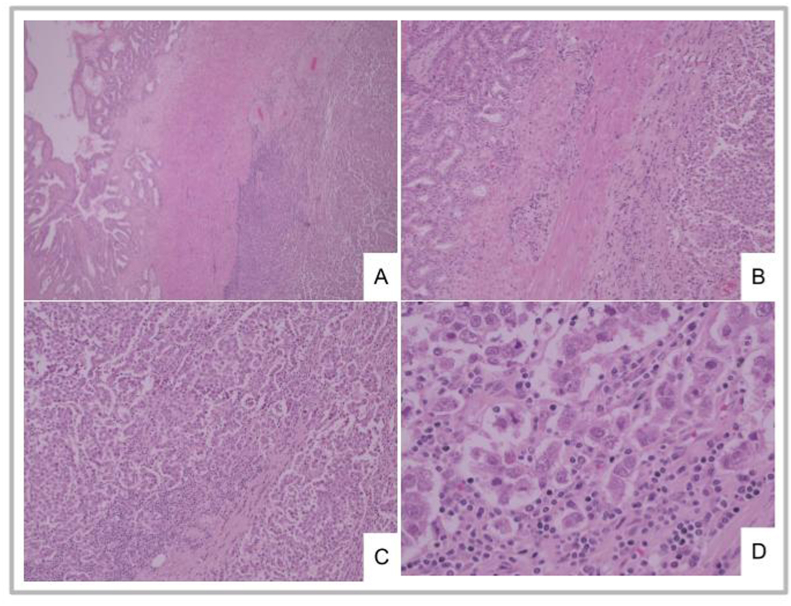
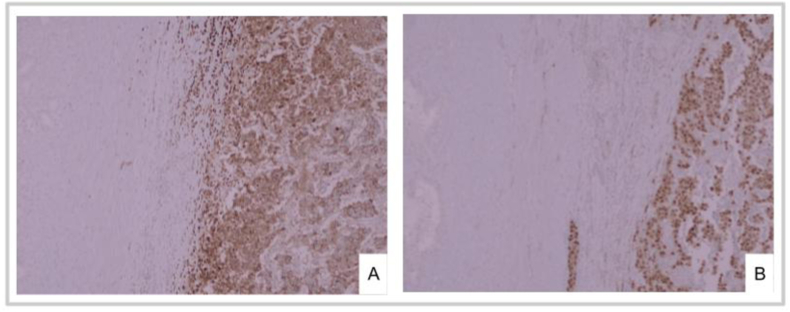
The pathologist received a nodular, irregular, whitish, 8x5x4 cm mass replacing seminal vesicles (Fig. 1 B). Microscopic analysis reported a seminoma compromising seminal vesicles, primarily the right one, with lympho-vascular invasion. (Fig. 2 A, B, C, D). Tumoral cells were positive for D2-40 and OCT4 in the immunohistochemical study (Fig. 3 A, B). There was extensive ureteral wall and peri-ureteral soft tissue involvement. Surgical margins were negative. 3/41 lymph nodes were infiltrated (1 left obturator, and 2 right lymph nodes included in the pelvic mass.)
Fig. 2.
A, B, C, D: A y B. Seminal vesicle: tumoral infiltration of the right half (A x40, B x100). C y D. Tumoral cell cords separated by connective tissue with lymphocytic infiltration (C x100, D x400).
Fig. 3.
A, B. A. Immunohistochemical stain for D2-40. B. Immunohistochemical stain for OCT4.
A testicular doppler ultrasound reported right epididymitis and small, hypoechoic testicles, with echogenic bilateral pinpoint focus. Postoperative germinal tumor markers were normal. Given the diagnosis of seminoma, the bilateral testicular atrophy, and the hormonal replacement the patient was chronically receiving, 4 months after the first surgery a radical bilateral orchiectomy was undertaken. Microscopic analysis reported atrophic testicular parenchyma, and dystrophic calcifications on both testicles. Both were negative for malignancy. Spermatic chords and epididymis were normal.
He received 3 cycles of adjuvant chemotherapy with BEP (Bleomycin Etoposide Cisplatin). His tumor markers are normal after one year observation.
3. Discussion
We described the second case of a primary seminal vesicle seminoma. Adachi's case was a 48 year old man, without any relevant background, with hematuria and left flank pain. Our case debuted with neurological symptoms. Both had a mass above the prostate on the digital examination. The first had normal testis at physical and sonographic assessment. Tumor markers were negative for both.
Even though diagnostic approaches differed slightly, both had seminal vesicle masses confirmed by contrast enhanced images, without metastatic disease.
First man was taken to an abdominal exploration and a rapid frozen section biopsy of the mass indicated seminoma. He underwent a radical cysto-prostatectomy and bilateral cutaneous ureterostomies, as the tumor infiltrated the left wall of the bladder and the left ureter. His seminal vesicles were replaced by a 6 cm grayish lobulated homogeneous mass. Microscopic analysis reported large, pleomorphic tumoral cells, with clear cytoplasmic margins, large central nucleus, big nucleolus, and small lymphoid interstitial cells. Immunohistochemical analysis reported positive leukocyte common antigen. We performed an open radical prostatectomy with bilateral extended pelvic lymphadenectomy and right ureteral reimplantation. Tumoral cells were positive for D240 and OCT-4. Because of the use of different tumor markers, we cannot directly compare results.
Our diagnostic approach was done ambulatorily, with a 3-day postoperative hospital stay. He developed symptoms because of obturator nerve injury, and urinary incontinence, as a complication of radical prostatectomy. Adachi's man's diagnostic approaches were done during an 8-month hospital stay, and post-operative recuperation took 4 months.
The first man received adjuvant radiotherapy, including para-aortic lymph nodes, and left sacral region. Our case received adjuvant chemotherapy. Surgical resection was not considered enough for anyone.
4. Conclusions
Primary seminoma of seminal vesicles has only been reported once before. Both were similar ages, but clinical backgrounds, diagnostic and therapeutic approaches were different. There is no way of determining the best approach for these cases, given they are the only ones described, with no report of long term follow up.
Author contributions
Daniela Franco-Buenaventura: Conceptualization; Data curation; Formal analysis; Investigation; Visualization; Writing - original draft; Writing - editing; Ruiz Londoño, David: Project administration; Supervision; Writing - review; Validation; J Forero: Surgeon; Validation. Varela R, Rodolfo: Validation.
References
- 1.Xu J., Zhao J., Geng S., et al. Primary seminoma arising in the middle mediastinum: a case report. Oncol Lett. 2016 Jul 1;12(1):348–350. doi: 10.3892/ol.2016.4575. [Internet] [cited 2021 Apr 5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malde S. Primary retroperitoneal seminoma: an unusual cause of testicular pain. JRSM Short Rep. 2010;1(7):1–3. doi: 10.1258/shorts.2010.010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adachi Y., Rokujyo M., Kojima H., Nagashima K. Primary seminoma of the seminal vesicle: report of a case. J Urol. 1991 Sep;146(3):857–859. doi: 10.1016/s0022-5347(17)37944-2. PMID: 1875512. [DOI] [PubMed] [Google Scholar]
- 4.Ramamurthy R., Periasamy S., Mettupalayam V. Primary malignancy of seminal vesicle: a rare entity. Indian J Urol. 2011 Jan;27(1) doi: 10.4103/0970-1591.78417. [cited 2021 Apr 5] [Internet] 137–9. Available from:/ pmc/articles/PMC3114576/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daalgard J.B., Giersten J.C. Primary carcinoma of the seminal vesicle: case and Survey. Acta Pathol Microbiol Scand. 1956;39(4):255–267. doi: 10.1111/j.1699-0463.1956.tb03400.x. [Internet] [cited 2021 Apr 5] [DOI] [PubMed] [Google Scholar]