Abstract
Rationale
In adults, personalised asthma treatment targets patients with type 2 (T2)-high and eosinophilic asthma phenotypes. It is unclear whether such classification is achievable in children.
Objectives
To define T2-high asthma with easily accessible biomarkers and compare resulting phenotypes across all ages.
Methods
In the multicentre clinical All Age Asthma Cohort (ALLIANCE), 1125 participants (n=776 asthmatics, n=349 controls) were recruited and followed for 2 years (1 year in adults). Extensive clinical characterisation (questionnaires, blood differential count, allergy testing, lung function and sputum induction (in adults)) was performed at baseline and follow-ups. Interleukin (IL)-4, IL-5 and IL-13 were measured after stimulation of whole blood with lipopolysaccharide (LPS) or anti-CD3/CD28.
Measurements and main results
Based on blood eosinophil counts and allergen-specific serum IgE antibodies, patients were categorised into four mutually exclusive phenotypes: “atopy-only”, “eosinophils-only”, “T2-high” (eosinophilia + atopy) and “T2-low” (neither eosinophilia nor atopy). The T2-high phenotype was found across all ages, even in very young children in whom it persisted to a large degree even after 2 years of follow-up. T2-high asthma in adults was associated with childhood onset, suggesting early origins of this asthma phenotype. In both children and adults, the T2-high phenotype was characterised by excessive production of specific IgE to allergens (p<0.0001) and, from school age onwards, by increased production of IL-5 after anti-CD3/CD28 stimulation of whole blood.
Conclusions
Using easily accessible biomarkers, patients with T2-high asthma can be identified across all ages delineating a distinct phenotype. These patients may benefit from therapy with biologicals even at a younger age.
Short abstract
T2-high asthma defined by blood eosinophilia and atopy occurs across all ages and is associated with high levels of allergen-specific IgE, increased propensity for IL-5 production of leukocytes and persistence of asthma into adulthood https://bit.ly/35X11EF
Introduction
Asthma is a heterogeneous disease comprising several endotypes and clinical phenotypes. Adult studies have identified type 2 (T2) inflammation as key immune response in asthma pathobiology, resulting in the broad classification of T2-high and T2-low asthma [1]. T2 inflammation is characterised by increased secretion of interleukin (IL)-4, IL-5 and IL-13 by T-cells or innate cells, associated with clinical features such as allergic sensitisation and bronchoconstriction, eosinophilic airway inflammation and airway mucus production. While T2-high asthma was initially described by molecular signatures of T2 cytokines in affected airway samples [2], simpler and clinically available biomarkers such as fractional exhaled nitric oxide (FeNO), total or specific immunoglobulin E (sIgE), blood or sputum eosinophils are now being used [3]. These biomarkers in combination with T2-targeting monoclonal antibodies have opened up new personalised treatment strategies, especially for severe asthma [4, 5].
Asthma affects patients from all age groups ranging from preschool children to senior adults, but comparative studies regarding T2 inflammation across all age groups are scarce [5–7]. This could ultimately lead to adoption of adult definitions of T2-high asthma for children. However, children often need age-adjusted cut-offs for biomarkers which has been recognised with regard to FeNO, but might also hold true for blood eosinophils [8]. Many biomarkers used in adults are difficult to assess in children of younger age groups, i.e. sputum or even FeNO, which limits the possibilities of age-spanning comparisons. Furthermore, the lack of knowledge about age-appropriate definitions of T2 inflammation impedes research on long-term asthma trajectories from childhood to adulthood and comparative investigations into clinical phenotypes and therapeutic success. Although T2-targeting drugs are currently primarily used in severe asthma, future applications in moderate asthma to influence long-term outcome are potentially conceivable, but age-specific and cross-age research into biomarkers for T2-high asthma will be prerequisite.
The aim of this study was to investigate in the multicentre All Age Asthma Cohort (ALLIANCE) cohort whether such classification is feasible in children of all age groups and adults using routine biomarkers such as blood eosinophil counts and allergen-specific IgE antibodies.
We assessed whether the classification results in comparable phenotypes in children and in adults and assessed stability over time. Furthermore, we characterised patient categories by clinical features and immune response profiling across a broad age range from infancy to old age. These analyses reveal age-specific and age-spanning characteristics of T2-high asthma and facilitate future research into personalised therapeutic and preventative strategies.
Methods
Study design
The ALLIANCE cohort of the German Center for Lung Research (DZL) is a prospective multicentre asthma cohort recruiting in five paediatric specialist centres (Hannover, Lubeck, Munich, Marburg and Cologne) and two adult specialist centres (LungenClinic Grosshansdorf and Research Centre Borstel). All local ethics committees approved the study protocol. Parents of study participants aged <18 years and study participants aged ≥18 years gave written informed consent. The study was registered at ClinicalTrials.gov (paediatric arm NCT02496468, adult arm NCT02419274).
Inclusion criteria were age-adapted: children aged 6 months to 5 years were eligible for inclusion if they had at least two episodes of wheeze during the past 12 months based on parental report (“preschool wheezer”). Children aged ≥6 years and adults were included based on a history of doctor-diagnosed asthma according to the Global Initiative for Asthma (GINA) guidelines [9] and German guidelines [10]. Current or former smoking was not an exclusion criterion. Age- and sex-matched healthy controls were recruited into both arms if they were never diagnosed with asthma or preschool wheeze, but irrespective of other allergic diseases. Spirometry and FeNO were measured in all participants aged ≥6 years. Laboratory tests included differential blood count (all participants), sputum cytology (only adults) and sIgE against 36 allergens in all patients measured by Euroline (Euroimmun, Germany). For cytokine measurements, 1 mL of whole blood was stimulated with lipopolysaccharide (LPS) or anti-CD3/CD28 (TruCulture; Myriad Rbm, USA) for 48 h at 37°C. Supernatant was collected and stored at −80°C. T2 cytokines were measured centrally using Bio-Plex assays (Bio-Rad, USA). Details regarding methods, study design and definition of clinical variables are specified in the supplementary material and published elsewhere [11].
Statistical analysis
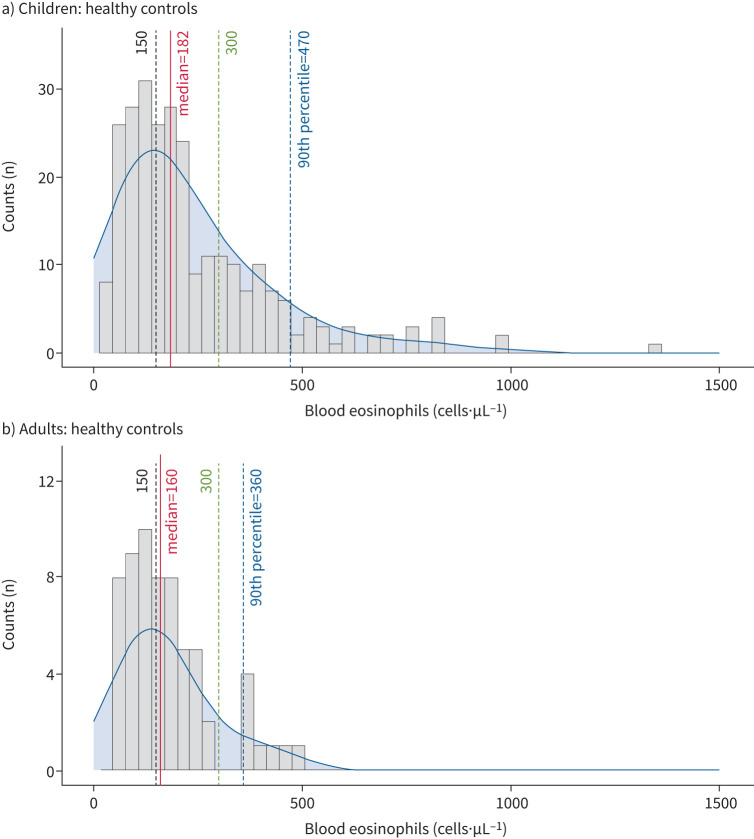
Blood eosinophil (b-Eos) counts from healthy subjects in the ALLIANCE cohort were used to define increased blood eosinophils as counts above the 90th percentile (figure 1 and supplementary figure E2). This resulted in a cut-off of ≥470 cells·µL−1 in children of all age groups and ≥360 cells·µL−1 in adults. Eosinophil counts did not differ significantly between healthy children aged <6 years and ≥6 years (supplementary figure E3). Atopy was defined as sIgE ≥0.7 kU·L−1 against at least one of 36 aero- or food allergens or by summing up all allergen-specific IgEs to all allergens to reflect the degree of sensitisation [12, 13]. Details on the allergen panels can be found in the supplementary methods. Asthma phenotypes in children were defined as atopy-only (b-Eos <470 cells·µL−1 and any serum sIgE ≥0.7 kU·L−1); Eos-only (b-Eos ≥470 cells·µL−1, all sIgE <0.7 kU·L−1); T2-high (b-Eos ≥470 cells·µL−1, any sIgE ≥0.7 kU·L−1); and T2-low (b-Eos <470 cells·µL−1, all sIgE <0.7 kU·L−1). The same phenotype definitions were applied in adults, but with a b-Eos cut-off of ≥360 cells·µL−1.
FIGURE 1.
Distribution of blood eosinophils counts of healthy children and adults. Distribution of blood eosinophil levels among a) healthy children (n=275) and b) healthy adults (n=64) in the All Age Asthma Cohort (ALLIANCE) cohort. Lines indicate median, 90th percentile and literature-based cut-offs (150 cells·µL−1 and 300 cells·µL−1).
Demographics and clinical categorical variables were compared across phenotypes (Chi-squared test). Means between groups were compared using the unpaired t-test. Kruskal–Wallis and Wilcoxon testing was used to compare continuous variables across phenotypes. To investigate the independent contribution of atopy (discrete variable) and blood eosinophils (continuous variable) to asthma, we used multivariable logistic regression models, adjusting for age, gender, atopic comorbidities, parental history of asthma, active and passive smoking, siblings and daycare, including two-way interaction between covariates using 95% confidence interval and Wald-test p-value. The best models were selected using the Akaike information criterion (backward variable selection, using p<0.3 in univariable model). Model fit and predictive accuracy were assessed using the receiver operating area under the curve (AUC) [14]. Statistical significance was set at p<0.05 and descriptive statistics were summarised as mean±sd, interquartile range (IQR) and n (%). Data were analysed using R version 4.0.2 [15]. In children and adults, standard curve-derived cytokine values were analysed. Spirometry values were analysed as z-scores [16].
Results
Subject characteristics
Demographic and clinical information at baseline was collected for 1125 subjects from three age groups: 282 children aged 6–18 years with asthma (“children and adolescents”), 218 adults with asthma (“adults”) and 276 children aged <6 years with preschool wheeze in addition to healthy controls in all age groups (supplementary figure E1 and supplementary table E3). As asthma cannot be confidently diagnosed in children aged <6 years, the term “preschool wheeze” was chosen for this age group, although some children with recurrent wheeze at preschool age will develop early-onset asthma.
Blood eosinophils and FeNO were higher and atopy was more prevalent in paediatric and adult asthma patients compared to healthy control subjects. Forced expiratory volume in 1 s (FEV1), FEV1/forced vital capacity (FVC) and forced expiratory flow at 25–75% of FVC were significantly lower in adult and paediatric asthma patients than in healthy controls (supplementary table E3). In preschool wheezers, atopy was less prevalent compared to healthy controls, while blood eosinophils were increased.
Classification of phenotypes
Using the 90th percentile as a cut-off for blood eosinophilia and a clinically relevant cut-off for atopy (≥0.7 kU·L−1) [13], subjects were categorised into four mutually exclusive groups: atopy-only; Eos-only; T2-high with both eosinophilia and atopy; and T2-low with neither atopy nor eosinophilia (table 1). Comparing the distribution of phenotypes within each age group, the T2-high phenotype was most prevalent in children and adolescents (40.2%), followed by adults (24.6%) and preschool children (16.9%). FeNO was significantly increased in the T2-high group compared to T2-low and atopy-only group in all age groups, while no difference was seen between the T2-high group and the Eos-only group (supplementary table E4).
TABLE 1.
Distribution of type 2 (T2) asthma phenotypes across age groups
| Participants | Atopy-only | Eos-only | T2-high | T2-low | |
| Children | |||||
| Wheeze (age <6 years) | 219 | 40 (18.26) | 31 (14.16) | 37 (16.89) | 111 (50.68) |
| Asthma (age ≥6 years) | 254 | 105 (41.34) | 6 (2.36) | 102 (40.16) | 41 (16.14) |
| Adults: asthma | 211 | 83 (39.34) | 36 (17.06) | 52 (24.64) | 40 (18.96) |
Data are presented as n or n (%). Frequencies of the four T2 phenotypes are shown for children with preschool wheeze or asthma and adults with asthma. Eos: eosinophils.
Next, we compared our classification to other proposed biomarkers for T2 inflammation as FeNO and sputum eosinophils [17, 18]. A combination of FeNO ≥35 ppb and sputum eosinophils ≥3% resulted in a similar prevalence (26.7%) (supplementary table E5) of the T2-high phenotype compared to our definition based on increased b-Eos and atopy (24.6%) (table 1). However, overlap of patients with a T2-high asthma definition based on FeNO and sputum eosinophils and a T2-high asthma definition based on atopy and blood eosinophils was only 20% in adult patients (supplementary figure E4). Among the cases classified as T2-high by FeNO and sputum eosinophils (n=31, 37%), 19 subjects were classified as b-Eos-only; seven as atopy-only; and only two subjects were classified as T2-low (three cases had missing information on atopy). By increasing the cut-off to FeNO ≥50 ppb and sputum Eos ≥3%, the proportion of patients classified as T2-high phenotype reduced as one would expect, given that patients with values below this cut-off would belong to the T2-low category (supplementary table E5). For phenotype definition in adults and children, we also ran a sensitivity analysis using b-Eos cut-off values of ≥150 and ≥300 cells·µL−1, often used for prescribing biologicals [4]. Results regarding markers of airway inflammation as FeNO and sputum eosinophils remained similar in adults (supplementary tables E4 and E6), but changed considerably in children, especially using the lowest eosinophil cut-off (supplementary table E7), again pointing to the need for higher cut-off values in children.
Clinical characteristics and associated features of the T2-high phenotype
The most defining feature of the T2-high phenotype across all age groups was a high degree of atopy as assessed by the sum of all allergen-specific IgEs (table 2). In contrast, allergic comorbidities (eczema, hay fever) were similarly present in the T2-high and atopy-only group in subjects aged >6 years and adults. In preschool children, eczema was specifically elevated within the T2-high group. Furthermore, FeNO was increased in both children and adults in the T2-high phenotype compared to atopy-only and T2-low, but similar compared to Eos-only (table 3).
TABLE 2.
Association between type 2 (T2) asthma phenotypes and clinical features
| Participants | Atopy-only | Eos-only | T2-high | T2-low | p-value | |
| Children: wheeze (age <6 years) | 219 | 40 | 31 | 37 | 111 | |
| Female | 12 (30.0) | 11 (35.5) | 10 (27.0) | 38 (34.2) | 0.8260 | |
| BMI (kg·m−2) | 16.32±1.75 | 16.68±1.65 | 16.16±1.77 | 16.44±1.53 | 0.2987 | |
| Passive smoker | 1 (2.6) | 1 (3.2) | 2 (5.4) | 4 (3.6) | 0.9267 | |
| Maternal history of asthma | 3 (7.9) | 3 (10.3) | 11 (29.7) | 27 (24.8) | 0.0345 | |
| Paternal history of asthma | 7 (18.4) | 6 (20.7) | 9 (24.3) | 18 (16.5) | 0.7565 | |
| Eczema | 13 (32.5) | 2 (6.5) | 21 (56.8) | 16 (14.4) | <0.0001 | |
| Hay fever | 8 (20.0) | 0 (0.0) | 11 (29.7) | 1 (0.9) | <0.0001 | |
| Sum of sIgE | 102.93±157.99c,d,d | 6.57±0.18d,d | 199.57±166.49c,d,d | 6.63±0.39d,d | <0.0001 | |
| Siblings | 29 (72.5) | 21 (67.7) | 28 (75.7) | 65 (58.6) | 0.1732 | |
| Daycare | 31 (81.6) | 19 (61.3) | 33 (89.2) | 80 (72.1) | 0.0369 | |
| Eosinophil counts (cells·µL−1) | 262.4±138.8a,d,d | 821.9±498.4d,d | 893.4±355.0d,d | 208.6±124.3a,d,d | <0.0001 | |
| Neutrophil counts (cells·µL−1) | 3164.5±1193.4 | 3622.9±1187.7 | 3750.7±1640.4 | 3408.7±1488.8 | 0.3147 | |
| Children: asthma (age ≥6 years) | 254 | 105 | 6 | 102 | 41 | |
| Female | 42 (40.0) | 2 (33.3) | 29 (28.4) | 17 (41.5) | 0.2832 | |
| BMI (kg·m−2) | 21.24±6.22b | 18.67±3.00 | 18.90±4.16b | 20.20±5.37 | 0.0116 | |
| Passive smoker | 20 (19.6) | 0 (0.0) | 12 (11.8) | 4 (10.0) | 0.2162 | |
| Maternal history of asthma | 25 (24.8) | 0 (0.0) | 28 (27.7) | 12 (29.3) | 0.4636 | |
| Paternal history of asthma | 22 (21.8) | 0 (0.0) | 25 (24.8) | 6 (14.6) | 0.3280 | |
| Eczema | 45 (42.9) | 1 (16.7) | 49 (48.0) | 8 (19.5) | 0.0079 | |
| Hay fever | 58 (55.2) | 1 (16.7) | 59 (57.8) | 3 (7.3) | <0.0001 | |
| Sum of sIgE | 148.11±126.58a,c,d | 6.84±0.43c,c | 194.67±156.64a,c,d | 6.59±0.27d,d | <0.0001 | |
| Siblings | 79 (75.2) | 6 (100.0) | 80 (78.4) | 30 (73.2) | 0.4919 | |
| Eosinophil counts (cells·µL−1) | 256.6±124.4d | 591.0±145.5a,d | 804.5±330.4a,d,d | 225.4±112.6d,d | <0.0001 | |
| Neutrophil counts (cells·µL−1) | 3795.0±2335.9 | 3644.8±1520.8 | 3636.0±1799.3 | 3341.5±953.5 | 0.9943 | |
| Adults: asthma | 211 | 83 | 36 | 52 | 40 | |
| Female | 51 (61.4) | 22 (61.1) | 20 (38.5) | 26 (65.0) | 0.0268 | |
| BMI (kg·m−2) | 28.11±5.62 | 27.95±7.38 | 27.66±4.90 | 27.39±5.38 | 0.9355 | |
| Active smoker | 6 (7.2) | 0 (0.0) | 3 (6.0) | 4 (10.0) | 0.3369 | |
| Ex-smoker | 37 (44.6) | 18 (50.0) | 18 (34.6) | 20 (50.0) | ||
| Never-smoker | 40 (48.2) | 28 (50.0) | 31 (59.6) | 16 (40.0) | ||
| Maternal history of asthma | 12 (14.5) | 2 (5.6) | 3 (5.8) | 7 (17.5) | 0.1645 | |
| Paternal history of asthma | 10 (12.0) | 4 (11.1) | 5 (9.6) | 2 (5.0) | 0.6680 | |
| Eczema | 3 (3.6) | 0 (0.0) | 6 (11.5) | 0 (0.0) | 0.0169 | |
| Hay fever | 62 (74.7) | 10 (27.8) | 46 (88.5) | 8 (20.0) | <0.0001 | |
| Sum of sIgE | 88.50±95.65a,d,d | 12.98±0.06d,d | 170.31±215.69a,d,d | 13.01±0.14d,d | <0.0001 | |
| Eosinophil counts (cells·µL−1) | 182.6±96.4d,d | 660.9±418.7d,d | 607.4±285.4d,d | 161.6±94.2d,d | <0.0001 | |
| Neutrophil counts (cells·µL−1) | 4650.4±2294.8 | 5272.1±2609.5 | 4636.1±1705.3 | 5259.6±2874.9 | 0.2791 |
Data are presented as n, n (%) or mean±sd, unless otherwise stated. Eos: eosinophils; BMI: body mass index; sIgE: specific immunoglobulin E. p-values are based on Chi-squared and Kruskal–Wallis tests; the symbols indicate for which phenotypes the continuous variables significantly differ. a: p<0.05, b: p<0.01, c: p<0.001, d: p<0.0001 for contrasts (Wilcoxon test). Plain, underlined, bold and italic symbols indicate for which phenotypes the continuous variables significantly differ.
TABLE 3.
Association between type 2 (T2) asthma phenotypes and clinical features
| Participants | Atopy-only | Eos-only | T2-high | T2-low | p-value | |
| Children: wheeze (age <6 years) | 219 | 40 | 31 | 37 | 111 | |
| Age (years) | 3.99±1.42d,d | 2.39±1.08d,d | 4.03±1.43d,d | 2.66±1.21d,d | <0.0001 | |
| ICS use# | 22 (55.0) | 7 (22.6) | 21 (56.8) | 33 (30.0) | 0.0009 | |
| ICS dose#,¶ | ||||||
| Low | 13 (68.4) | 5 (71.4) | 16 (80.0) | 19 (67.9) | 0.1835 | |
| Medium | 6 (31.6) | 1 (14.3) | 2 (10.0) | 9 (32.1) | ||
| High | 0 (0.0) | 1 (14.3) | 2 (10.0) | 0 (0.0) | ||
| GINA control+ | ||||||
| Uncontrolled | 9 (22.5) | 14 (45.2) | 17 (45.9) | 29 (26.6) | 0.1605 | |
| Partly controlled | 14 (35.0) | 9 (29.0) | 9 (24.3) | 39 (35.8) | ||
| Controlled | 17 (42.5) | 8 (25.8) | 11 (29.7) | 41 (37.6) | ||
| Exacerbations§ per person per year | 1.0±2.23 | 1.27±2.63 | 0.89±1.20 | 0.58±1.04 | 0.3754 | |
| Children: asthma (age ≥6 years) | 254 | 105 | 6 | 102 | 41 | |
| Age (years) | 11.72±3.16a,c | 10.74±2.18 | 10.06±2.81c | 10.27±3.28a | 0.0009 | |
| FEV1 (z-score) | −0.41±1.09 | 0.11±1.45 | −0.34±1.67 | −0.52±0.96 | 0.4803 | |
| FVC (z-score) | 0.00±1.00 | −0.02±0.90 | 0.19±1.61 | 0.01±0.90 | 0.9740 | |
| FEV1/FVC (z-score) | −0.68±1.22 | 0.07±1.00a | −0.81±1.09a | −0.85±1.07 | 0.2163 | |
| FEF25–75 (z-score) | −0.79±1.25 | −0.26±1.29 | −0.96±1.30 | −0.95±1.06 | 0.4121 | |
| ΔFEV1ƒ (%) | 8.18±7.45 | 14.75±2.81 | 12.19±13.20 | 7.15±7.15 | 0.2365 | |
| FeNO (ppb) | 22.47±17.67a,c | 39.47±52.46 | 42.33±57.69a,d | 13.11±14.96c,d | <0.0001 | |
| ICS use# | 79 (76.7) | 4 (66.7) | 74 (73.3) | 25 (61.0) | 0.2868 | |
| ICS dose#,¶ | ||||||
| Low | 44 (58.7) | 1 (25.0) | 37 (56.1) | 13 (54.2) | 0.2805 | |
| Medium | 27 (36.0) | 3 (75.0) | 19 (28.8) | 9 (37.5) | ||
| High | 4 (5.3) | 0 (0.0) | 10 (15.2) | 2 (8.3) | ||
| GINA control+ | ||||||
| Uncontrolled | 16 (15.4) | 1 (16.7) | 12 (11.9) | 6 (14.6) | 0.5980 | |
| Partly controlled | 50 (48.1) | 2 (33.3) | 40 (39.6) | 14 (34.1) | ||
| Controlled | 38 (36.5) | 3 (50.0) | 49 (48.5) | 21 (51.2) | ||
| Exacerbations§ per person per year | 0.52±1.47 | 0.17±0.41 | 0.48±1.07 | 0.24±0.94 | 0.2867 | |
| Adults: asthma | 211 | 83 | 36 | 52 | 40 | |
| Age (years) | 50.00±12.73a,b | 57.17±11.12b,b | 48.52±14.13b,b | 56.20±14.51a,b | 0.0036 | |
| FEV1 (z-score) | −1.58±1.55 | −1.81±1.36 | −1.88±1.33 | −1.89±1.46 | 0.5609 | |
| FVC (z-score) | −0.54±1.25 | −0.39±1.05 | −0.50±1.12 | −0.55±0.95 | 0.6960 | |
| FEV1/FVC (z-score) | −1.81±1.32 | −2.33±1.27 | −2.19±1.38 | −1.82±1.42 | 0.1136 | |
| FEF25–75 (z-score) | −1.68±1.29a,a | −2.18±1.21a | −2.26±1.26a | −1.78±1.38 | 0.0692 | |
| ΔFEV1 (%) | 8.73±9.96 | 7.17±8.87a | 10.13±8.31a,a | 6.52±7.99a | 0.0572 | |
| FeNO (ppb) | 30.43±23.81a,b,c | 50.50±35.67c,d | 48.83±53.92b,d | 20.33±12.38a,d,d | <0.0001 | |
| ICS use# | 69 (83.1) | 36 (100.0) | 46 (88.5) | 37 (92.5) | 0.0471 | |
| ICS dose#,¶ | ||||||
| Low | 26 (37.7) | 1 (2.8) | 9 (19.6) | 11 (29.7) | 0.0058 | |
| Medium | 28 (40.6) | 20 (55.6) | 20 (43.5) | 13 (35.1) | ||
| High | 15 (21.7) | 15 (41.7) | 17 (37.0) | 13 (32.1) | ||
| OCS use | 10 (12.0) | 17 (47.2) | 8 (15.4) | 12 (30.0) | 0.0001 | |
| Paediatric asthma onset | 33 (39.8) | 4 (11.1) | 29 (56.9) | 6 (15.0) | <0.0001 | |
| Severity## | ||||||
| Mild | 32 (38.6) | 0 (0.0) | 12 (23.1) | 5 (12.5) | <0.0001 | |
| Moderate | 28 (33.7) | 9 (25.0) | 17 (32.7) | 14 (35.0) | ||
| Severe | 23 (27.7) | 27 (75.0) | 23 (44.2) | 21 (52.5) | ||
| GINA control+ | ||||||
| Uncontrolled | 26 (31.3) | 25 (69.4) | 21 (40.4) | 14 (35.0) | <0.0001 | |
| Partly controlled | 25 (30.1) | 7 (19.4) | 21 (40.4) | 21 (52.5) | ||
| Controlled | 32 (38.6) | 4 (11.1) | 10 (19.2) | 5 (12.5) | ||
| Exacerbations§ per person per year | 1.21±2.12b | 2.83±2.91b | 2.27±3.16 | 2.75±3.62 | 0.0025 |
Data are presented as n, n (%), mean±sd, unless otherwise stated. Eos: eosinophils; ICS: inhaled corticosteroid; GINA: Global Initiative for Asthma; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; FEF25–75: forced expiratory flow at 25–75% of FVC; ΔFEV1: percentage increase of FEV1 after albuterol administration (bronchodilator response); FeNO: exhaled nitric oxide fraction; OCS: oral corticosteroid. #: medication taken at the time of the study visit in children and adults; ¶: categorised into mild, moderate and high according to GINA guidelines; +: assessed according to GINA control status; §: exacerbations requiring any systemic steroid treatment (children) or systemic steroids for ≥3 days (adults) or uptitration of regular OCS per person in the past 12 months; ƒ: only available for 94 children; ##: assessed according to GINA treatment steps. p-values are based on Chi-squared and Kruskal–Wallis tests; the symbols indicate for which phenotypes the continuous variables significantly differ. a: p<0.05, b: p<0.01, c: p<0.001, d: p<0.0001 for contrasts (Wilcoxon test). Plain, underlined, bold and italic symbols indicate for which phenotypes the continuous variables significantly differ.
Some characteristics of the T2-high phenotype were only seen in certain age groups (table 3). In adults, patients with T2-high asthma were younger and more often had childhood-onset asthma. Asthma severity and asthma control differed between all phenotypes: T2-high asthma patients showed higher severity and higher exacerbation rate per person per year than atopy-only, but were overall less severely affected than patients from T2-low and eos-only groups.
Phenotypes were less contrasting in children. In children and adolescents, no differences occurred between the four phenotypes regarding lung function, exacerbations, asthma control or inhaled corticosteroid (ICS) use. Similar results were seen for preschool wheezers, with the exemption of more ICS use in both atopy-only and T2-high groups. We saw no association of active and passive smoking, neutrophils and number of siblings with any of the phenotypes in any age group. Daycare attendance as proxy for increased exposure to paediatric infections was less likely in wheezers with Eos-only and T2-low phenotype; however, children with these phenotypes were also younger (table 2).
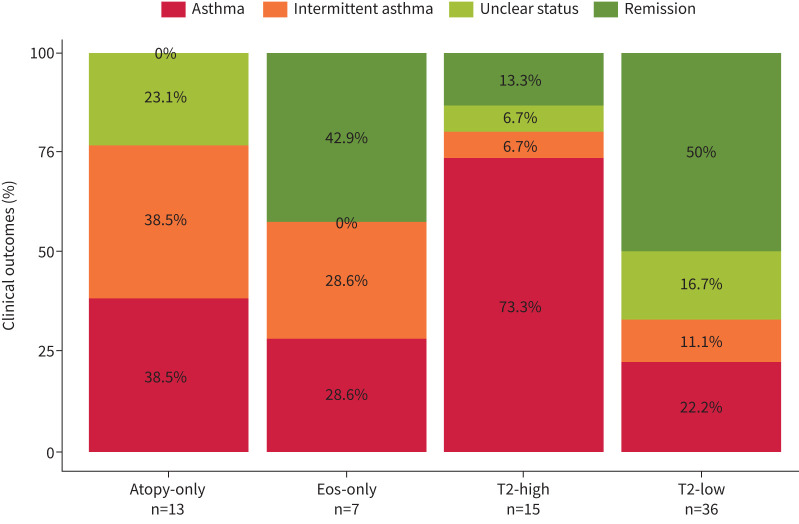
In 71 children included with preschool wheeze we assessed the outcome at the first follow-up visit at age ≥6 years based on questionnaire data (supplementary table E2). Persistence of symptoms consistent with an asthma diagnosis was highest in the T2-high group (n=15, 73.3%), followed by atopy-only (n=13, 38.5%). The atopy-only group showed a high proportion of patients with “intermittent asthma symptoms” (n=5, 38.5%). Remission of symptoms was highest among T2-low subjects (n=36, 50.0%) (figure 2).
FIGURE 2.
Asthma outcome of children included as preschool wheezers. Clinical outcomes of children included with preschool wheeze were assessed at the first visit aged ≥6 years and classified as remission, asthma, intermittent asthma or unclear status. Mean±sd age was 6.7±0.65 years. Children were grouped according to T2 phenotypes at baseline.
Cytokine levels across phenotypes
High levels of interleukin (IL)-5 production after anti-CD3/CD28 stimulation of whole blood were observed in school-aged children with the eosinophilic phenotypes T2-high and Eos-only (supplementary table E8). A trend for higher IL-5 secretion was also seen in adults, in both atopic phenotypes, T2-high and Atopy-only, especially in patients aged ≥45 years (supplementary tables E9 and E10). Furthermore, adults showed increased IL-5 levels after LPS stimulation, but only in the Eos-only phenotype and with lower levels than after anti-CD3/CD28 stimulation. No significant differences were seen for IL-5 in preschool children. IL-4 did not differ between the four phenotypes in adults. Equally, no phenotype-specific changes were seen for IL-13 in all age groups.
Prevalence of phenotypes across age groups
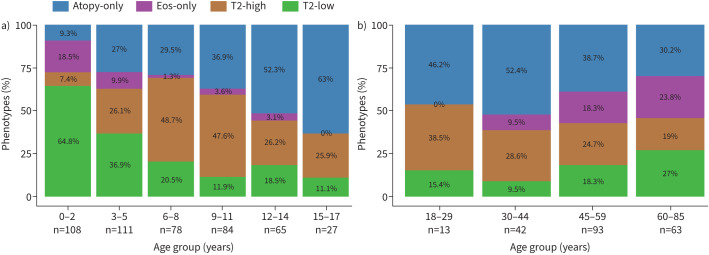
T2-high groups were not uniformly distributed across ages, but showed lower proportions in younger children (aged <6 years) and older patients (aged ≥45 years) (figure 3). The phenotype Eos-only was seen almost exclusively in very young children aged <3 years and adults aged >45 years, with increasing prevalence particularly in adults aged >60 years.
FIGURE 3.
Prevalence of type 2 (T2) asthma phenotypes across all age groups. T2 phenotypes in a) children with preschool wheeze (aged <6 years) or asthma (aged ≥6 years), and b) adults with asthma. Phenotypes were defined as atopy-only (children: blood eosinophils (b-Eos) <470 cells·µL−1, adults: b-Eos <360 cells·µL−1, any specific immunoglobulin E (sIgE) ≥0.7 kU·L−1); Eos-only (children: b-Eos ≥470 cells·µL−1, adults: b-Eos ≥360 cells·µL−1, all sIgE <0.7 kU·L−1); T2-high (children: b-Eos ≥470 cells·µL−1, adults: b-Eos ≥360 cells·µL−1, any sIgE ≥0.7 kU·L−1); and T2-low (children: b-Eos <470 cells·µL−1, adults: b-Eos <360 cells·µL−1, all sIgE <0.7 kU·L−1).
Persistence of phenotypes
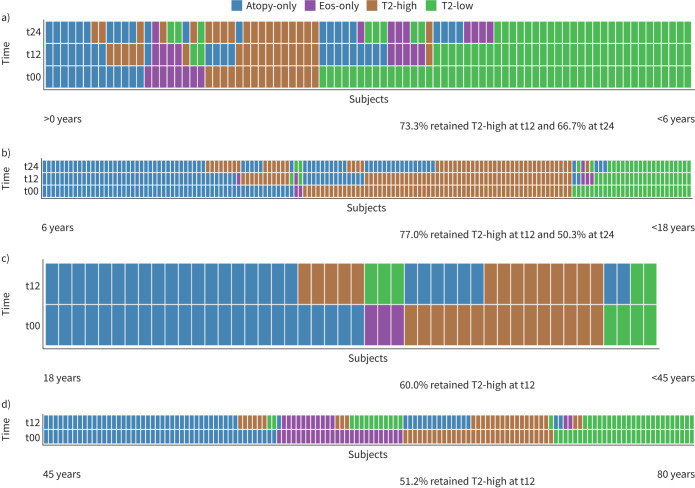
Longitudinal stability of phenotypes was assessed after 1 and 2 years in children, and after 1 year in adults. Overall, stability of the T2-high phenotype was slightly higher in children than in adults. After 1 year of follow-up, 73.3% (preschool children) and 77.0% (children and adolescents) retained the T2-high phenotype in contrast to 60.0% (18–45 years) and 51.2% (≥45 years) in adults. Furthermore, a high stability of the T2-high phenotype was found for preschool children, with 66.7% retaining their phenotype for 2 years, while the proportion of children and adolescents with persistent T2-high asthma reduced from 77.0% to 50.8% after 2 years, respectively (figure 4a–d).
FIGURE 4.
Longitudinal stability of type 2 (T2) asthma phenotypes. Asthma phenotypes are shown at baseline and at two follow-ups after 12 (t12) and 24 months (t24) in children. Adults had one follow-up after 12 months (t12). Clustering was done according to baseline phenotype. a) Children with preschool wheeze (aged <6 years), n=85; b) children with asthma (aged ≥6 years), n=147; c) adults with asthma (aged 18–45 years), n=46; d) adults with asthma (aged ≥45 years), n=134. Eos: eosinophils.
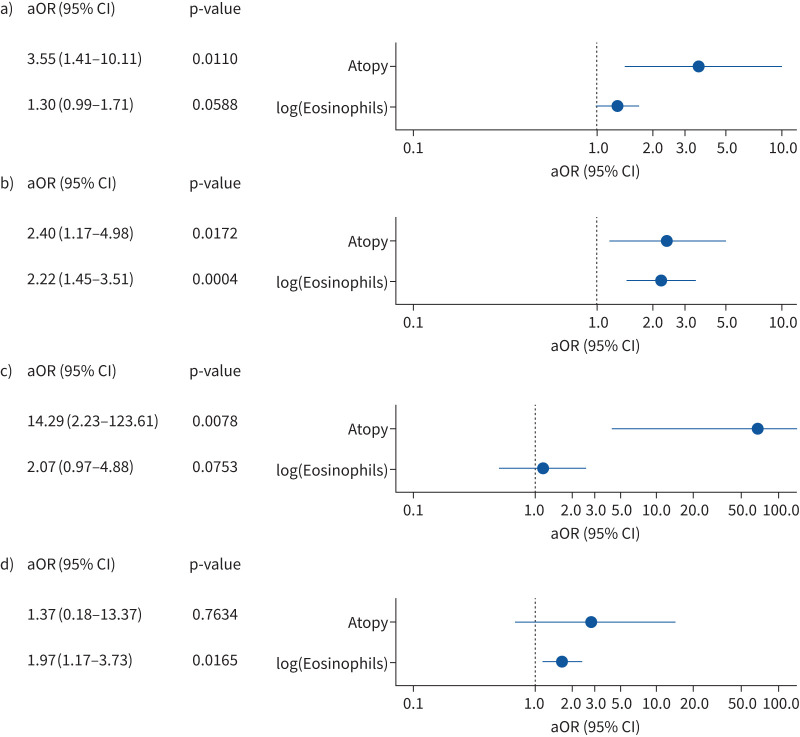
Individual contributions of atopy and blood eosinophils to disease risk across age groups
Our T2 phenotype definition was based on clinical experience. We were interested in understanding the individual contribution of eosinophils and sIgE levels to “asthma risk” after adjusting for potential confounders like atopic comorbidities, parental history, active and passive smoking, siblings, daycare and sex (figure 5). All models had a good performance fit (AUC 0.760–0.969). The findings support the predominant role of atopy to preschool wheeze and asthma risk until mid-adulthood. In addition to atopy, eosinophils clearly influenced asthma risk in children and adolescents (figure 5). Conversely, blood eosinophil levels, but not atopy, were a significant contributor to asthma risk in adults aged ≥45 years, coinciding with increasing frequencies of asthma patients with the Eos-only phenotype and late asthma onset (table 3).
FIGURE 5.
Multivariable logistic regression models: association between atopy and blood eosinophils with preschool wheeze and asthma. Contribution of atopy and blood eosinophils is shown for risk of preschool wheeze and asthma after adjustment for confounders. a) Children with wheeze versus healthy children (aged <6 years); b) children with asthma versus healthy children (aged ≥6 years); c) adult asthma patients versus healthy adults (aged ≥18 to <45 years); and d) adult asthma patients versus healthy adults (aged ≥45 years). Atopy was defined as at least one allergen with specific immunoglobulin E ≥0.7 kU·L−1. aOR: adjusted odds ratio.
Discussion
In the ALLIANCE cohort, patients of all age groups displayed a T2-high phenotype defined by the presence of eosinophilia and atopy, with highest prevalence of T2-high asthma in school-aged children and young adults. Moreover, adults with T2-high asthma were significantly more likely to have childhood-onset asthma and children with preschool wheeze and T2-high phenotype were more likely to develop asthma at the age of 6 years. Across all ages, T2-high asthma was consistently and strongly (p<0.0001) associated with a high degree of atopy as assessed by the sum of all allergen-specific IgEs. Specifically, in children with T2-high asthma, we also found an augmented IL-5 response after T-cell stimulation, and both atopy and eosinophils contributed to disease risk particularly in this age group. T2-high asthma defined by atopy and blood eosinophilia thus outlines a phenotype linked to onset in childhood, which tracks to or re-occurs in adulthood. There is increasing evidence that asthma is not one disease, but rather a syndrome consisting of many phenotypes and possibly distinct underlying endotypes [19]. In adult patients, molecular phenotyping of lower airway samples has revealed a T2 signature in a significant proportion of subjects [2, 20]. The term was first used to describe a subgroup of mild–moderate adult asthmatics with an IL-13 inducible gene signature of the airway epithelium, which coincided with increased airway and blood eosinophils, higher sensitisation levels and good response to ICS [2]. Afterwards, several cohorts confirmed similar T2 cytokine-driven molecular signatures in either mucosal biopsies or sputum cells, mainly in adults with severe asthma [21, 22] but also children [21]. Increased FeNO, blood or sputum eosinophils and sensitisation against allergens were associated in most studies with a T2-high airway gene signature with sensitisation being specifically important in younger adults and children [2, 23].
There is still no consensus about a definition of a T2-high phenotype across studies and age groups. In clinical routine, invasive procedures assessing sputum or airway samples are difficult to perform, particularly in young children, advocating for more easily accessible proxies of a T2-high signature like blood eosinophils and measures of atopy. For blood eosinophilia, we used the 90th percentile cut-off for b-Eos of our healthy control population, which amounted to ≥470 cells·µL−1 in children and ≥360 cells·µL−1 in adults. Atopy was defined as at least one allergen-specific IgE ≥0.7 kU·L−1 based on previously established clinical relevant cut-offs [13]. This phenotype definition was also reflective of T2 airway inflammation: FeNO levels (children and adults) and sputum eosinophils (adults) were increased in both the T2-high and eosinophil phenotype. In addition, children and adolescents with T2-high asthma showed increased IL-5 production after T-cell stimulation with anti-CD3/CD28 in comparison with the atopy-only group. This observation might indicate an augmented propensity of T-cells to respond to activation, which is not solely dependent on atopy, suggesting that our definition is clinically useful for identifying patients with an underlying T2 endotype.
The most distinct clinical feature in the T2-high phenotype across all age groups of the ALLIANCE cohort was a high degree of sensitisation against allergens compared to all other phenotypes, particularly atopy-only. While atopy was defined as binary variable for the phenotype definitions using sIgE ≥0.7 kU·L−1 as a cut-off, this does not reflect the degree of sensitisation, for which the sum of all allergen-specific IgEs is a better measure. Accordingly, the sum of all allergen-specific IgEs was significantly higher in the T2-high than in the atopy-only group, even though both groups were defined as “atopic” (preschool wheeze (p=0.013), asthmatic children (p=0.032) and asthmatic adults (p=0.012)). Atopy is a complex trait that does not necessarily result in allergic disease. Recently, subgroups of atopy with varying clinical relevance were identified by latent class analyses showing that early and multiple sensitisations are not only the strongest predictor of asthma, hospital admissions and lung function deficits, but also of increased production of IgE towards aeroallergens [24, 25]. These findings suggest that early and augmented production of sIgEs resulting in an increased sum of sIgE reflects the early-life origins of T2-high across all age groups. This notion is supported by the high percentage of adults with the T2-high phenotype reporting childhood onset of asthma and the association between T2-high phenotype and asthma outcome at school age in the children with preschool wheeze. Additionally, the T2-high phenotype showed a high percentage of children retaining the T2-high phenotype at the second follow-up, particularly in the preschool group. Due to the broad inclusion criteria for children aged <6 years, our cohort comprises all phenotypes of preschool wheeze, ranging from children with transient symptoms to children with a high risk of developing childhood asthma according to the GINA definition. However, our definition of T2-high seems useful to identify preschool children at increased risk of asthma persistence. Additive effects of blood eosinophils and atopy for prediction of asthma at school age has been shown by others as well [26, 27]. Confirmation by longer follow-up of the ALLIANCE cohort is needed.
Intriguingly, an increasing proportion of the study population had an Eos-only phenotype at both ends of the age spectrum. However, further analysis showed marked age-dependent differences. In adults aged ≥45 years, eosinophils, but not atopy were associated with asthma diagnosis. Clinically, the Eos-only phenotype was characterised by increased severity, oral corticosteroid use and high exacerbation rates in parallel with less GINA control. Interestingly, the Eos-only group in adults also showed an increase in IL-5 after stimulation with LPS, pointing towards additional and distinct pathways of IL-5 production apart from specific T-cell receptor stimulation. In preschool children, the Eos-only group did not markedly differ from other phenotypes, apart from less ICS usage. No association with IL-5 production was seen after either stimulation. Eosinophils may thus adopt different roles in disease pathology in both age groups. They may confer tissue damage and bronchoconstriction in asthmatic patients, but might also promote antiviral innate host defence in viral-induced wheeze [28, 29]. This is of particular relevance should T2-targeting biologicals be licensed for use in that age group in the future.
Several studies have shown that patients with severe, T2-high or eosinophilic asthma benefit from biological therapies targeting eosinophils or related inflammatory pathways, which have become standard treatment of severe asthmatics. Our blood eosinophil cut-off of ≥360 cells·µL−1 in adults is approximately in line with recommendations for prescribing T2-targeting antibodies [4, 10]. However, we and others [8] have identified higher eosinophil levels in healthy children, raising the need for more research into clinically relevant cut-offs for prescribing T2-targeting biologicals in children.
The early origins of the T2-high phenotype seen in our study population in addition to the specific association of increased IL-5 secretion upon T-cell stimulation raises the question of future therapeutic use of T2-targeting biologicals for children and adolescents with nonsevere asthma, for example to mitigate asthma exacerbations. Furthermore, it is conceivable that such therapies might also be used for secondary prevention, as currently investigated for omalizumab [30].
We acknowledge some limitations of our study. Our definition of the four phenotypes was based on biomarkers available in all age groups, but other definitions of T2-high asthma exist, especially in adult clinical practice [4]. Overlap between distinct definitions and biomarkers of T2 inflammation is often only moderate, as we have seen in the adult arm of our cohort and which has been reported by other studies [31–33]. FeNO and sputum are difficult to obtain in young children in a standard clinical setting, and we were therefore not able to include these biomarkers in our age-spanning analysis. Since the collection of sputum and FeNO data in preschool children is not easily available, new approaches of assessing airway inflammation should be part of future research.
While most categories had balanced numbers of study participants, the Eos-only group in school-aged children was too small for reasonable comparisons. The same applies to paediatric patients with severe asthma or frequent exacerbations. Furthermore, the majority of ALLIANCE asthma patients were under long-term therapy with inhaled corticosteroids and 22.3% of the adult asthmatics were on regular oral corticosteroids, which both influence blood eosinophil numbers [34] and may, therefore, have biased the phenotype categorisation. Our population is Caucasian and results may not be generalisable to other ethnic groups. Lastly, we only considered T2 cytokines IL-13, IL-4 and IL-5 in our analysis and not ratios between T2 cytokines and counter-balancing cytokines such as IL-10, as previous authors have done [35]. Our work shows the importance of including patients from childhood to adulthood in studies investigating asthma phenotypes. While many authors still restrict research into asthma phenotypes to either children or adults, a more inclusive approach as utilised by the ALLIANCE cohort reveals similarities and differences between age groups with better precision and will improve uncovering of age-spanning trajectories. To our knowledge, this is the first study on T2-high asthma phenotypes across all ages. We found a high age-dependent occurrence of this phenotype in the ALLIANCE patient population and identified it already in early childhood using easily available biomarkers. Future studies need to confirm the trajectories described in this cross-sectional analysis. Confirmation of the early T2-high phenotype in childhood might ultimately facilitate personalised preventative or therapeutic strategies in the future.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02288-2021.Supplement (730.9KB, pdf)
Shareable PDF
Acknowledgements
We are thankful to our patients and healthy participants for their invaluable contribution to this work. We are indebted to our study participants and their families for participating in the study and the staff of the participating hospitals and primary care practices and our cooperation partners within the DZL for the support and recruitment. We also thank the study nurses Nicola Korherr, Arzu Yilmazsubasi (Dr von Hauner Children’s Hospital, Ludwig Maximilians University, Munich, Germany), Nicole Rahmanian (Dept of Pediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, Germany), Petra Hundack-Winter, Susann Prange and Corinna Derworth (LungenClinic Grosshansdorf, Grosshansdorf, Germany), Johanna Döhling, Romina Pritzkow and Eva Wittmer (Forschungszentrum Borstel), Nadine Weissheimer and Elvira Ehlers-Jeske (Division of Pediatric Pneumology and Allergology, University Medical Center Schleswig-Holstein, Luebeck, Germany) and Nasanin Schröder (Dept of Pediatric Pneumology, Allergology and Neonatology, Hannover Medical School); the data managers Edith Riess (Dr von Hauner Children’s Hospital, Ludwig Maximilians University) and Julia Kontsendorn (Dept of Pediatric Pneumology, Allergology and Neonatology, Hannover Medical School); the lung function technicians Elvira Kolling (Dr von Hauner Children’s Hospital, Ludwig Maximilians University), Andrea Suender (Forschungszentrum Borstel), Annegret Telsemeyer, Dunja Tennhardt and Christiane Staabs (Division of Pediatric Pneumology and Allergology, University Medical Center Schleswig-Holstein) and Cornelia Stolpe and Ines Krömer (Dept of Pediatric Pneumology, Allergology and Neonatology, Hannover Medical School); as well as the lab technicians Isolde Schleich and Tatjana Netz (Dr von Hauner Children’s Hospital, Ludwig Maximilians University) and Jana Bergmann, Anika Dreier and Christin Albrecht (Dept of Pediatric Pneumology, Allergology and Neonatology, Hannover Medical School) for their invaluable assistance and support. The BioMaterial Bank Nord is supported by the German Center for Lung Research. The BioMaterialBank Nord is member of popgen 2.0 network (P2N) which is supported by a grant from the German Ministry for Education and Research (01EY1103).
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.01031-2022
Study group: Oliver Fuchs (Department of Paediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, Germany, and Comprehensive Pneumology Center, Munich (CPC-M), Germany; German Center for Lung Research (DZL); Department of Paediatric Respiratory Medicine, Inselspital, University Children's Hospital of Bern, University of Bern, Bern, Switzerland), Barbara Roesler (Department of Paediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, Germany, and Comprehensive Pneumology Center, Munich (CPC-M), Germany; German Center for Lung Research (DZL)), Nils Welchering (Department of Paediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, Germany, and Comprehensive Pneumology Center, Munich (CPC-M), Germany; German Center for Lung Research (DZL)), Naschla Kohistani-Greif (Department of Paediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, Germany, and Comprehensive Pneumology Center, Munich (CPC-M), Germany; German Center for Lung Research (DZL)), Johanna Kurz (Department of Paediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, Germany, and Comprehensive Pneumology Center, Munich (CPC-M), Germany; German Center for Lung Research (DZL); Department of Paediatric Respiratory Medicine, Inselspital, University Children's Hospital of Bern, University of Bern, Bern, Switzerland), Katja Landgraf-Rauf (Department of Paediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, Germany, and Comprehensive Pneumology Center, Munich (CPC-M), Germany; German Center for Lung Research (DZL)), Kristina Laubhahn (Department of Paediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, Germany, and Comprehensive Pneumology Center, Munich (CPC-M), Germany; German Center for Lung Research (DZL)), Claudia Liebl (Department of Paediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, Germany, and Comprehensive Pneumology Center, Munich (CPC-M), Germany; German Center for Lung Research (DZL)), Markus Ege (Department of Paediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, Germany, and Comprehensive Pneumology Center, Munich (CPC-M), Germany; German Center for Lung Research (DZL)), Alexander Hose (Department of Paediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, Germany, and Comprehensive Pneumology Center, Munich (CPC-M), Germany; German Center for Lung Research (DZL)), Esther Zeitlmann (Department of Paediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, Germany, and Comprehensive Pneumology Center, Munich (CPC-M), Germany; German Center for Lung Research (DZL)), Mira Berbig (Department of Paediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, Germany, and Comprehensive Pneumology Center, Munich (CPC-M), Germany; German Center for Lung Research (DZL)), Carola Marzi (Institut für Asthma- und Allergieprävention (IAP), Helmholtz Zentrum Munich, Deutsches Forschungszentrum für Gesundheit und Umwelt (GmbH), Munich, Germany), Christina Schauberger (Department of Paediatric Allergology, Dr von Hauner Children's Hospital, Ludwig Maximilians University, Munich, Germany, and Comprehensive Pneumology Center, Munich (CPC-M), Germany; German Center for Lung Research (DZL)), Ulrich Zissler (Center of Allergy & Environment (ZAUM), Technical University of Munich and Helmholtz Center Munich, German Research Center for Environmental Health, Munich, Germany; German Center for Lung Research (DZL), Munich, Germany), Carsten Schmidt-Weber (Center of Allergy & Environment (ZAUM), Technical University of Munich and Helmholtz Center Munich, German Research Center for Environmental Health, Munich, Germany; German Center for Lung Research (DZL), Munich, Germany), Isabell Ricklefs (University Children's Hospital, Luebeck, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Gesa Diekmann (University Children's Hospital, Luebeck, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Lena Liboschik (University Children's Hospital, Luebeck, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Gesche Voigt (University Children's Hospital, Luebeck, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Laila Sultansei (University Children's Hospital, Luebeck, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Gyde Nissen (University Children's Hospital, Luebeck, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Inke R. König (Institute for Medical Biometry and Statistics, University Luebeck, University Medical Centre Schleswig-Holstein, Campus Luebeck, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Anne-Marie Kirsten (Pulmonary Research Institute at LungenClinic Grosshansdorf, Grosshansdorf, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Frauke Pedersen (LungenClinic Grosshansdorf GmbH, Grosshansdorf, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Henrik Watz (Pulmonary Research Institute at LungenClinic Grosshansdorf, Grosshansdorf, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Benjamin Waschki (LungenClinic Grosshansdorf GmbH, Grosshansdorf, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Christian Herzmann (Research Center Borstel – Medical Clinic, Borstel, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Mustafa Abdo (LungenClinic Grosshansdorf GmbH, Grosshansdorf, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Heike Biller (LungenClinic Grosshansdorf GmbH, Grosshansdorf, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Karoline I. Gaede (Research Center Borstel – Medical Clinic, Borstel, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Xenia Bovermann (University Children's Hospital, Luebeck, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Alena Steinmetz (University Children's Hospital, Luebeck, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Berrit Liselotte Husstedt (University Children's Hospital, Luebeck, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Catharina Nitsche (University Children's Hospital, Luebeck, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Vera Veith (LungenClinic Grosshansdorf GmbH, Grosshansdorf, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Marlen Szewczyk (LungenClinic Grosshansdorf GmbH, Grosshansdorf, Germany, and Airway Research Center North (ARCN), Germany; German Center for Lung Research (DZL)), Folke Brinkmann (Department of Paediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, Germany, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Germany; German Center for Lung Research (DZL); Department of Paediatric Pneumology, University Children's Hospital, Ruhr-University Bochum, Bochum, Germany), Aydin Malik (Department of Paediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, Germany, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Germany; German Center for Lung Research (DZL)), Nicolaus Schwerk (Department of Paediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, Germany, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Germany; German Center for Lung Research (DZL)), Christian Dopfer (Department of Paediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, Germany, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Germany; German Center for Lung Research (DZL)), Mareike Price (Department of Paediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, Germany, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Germany; German Center for Lung Research (DZL)), Adan Chari Jirmo (Department of Paediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, Germany, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Germany; German Center for Lung Research (DZL)), Anika Habener (Department of Paediatric Pneumology, Allergology and Neonatology, Hannover Medical School, Hannover, Germany, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Germany; German Center for Lung Research (DZL)), David S. DeLuca (Hannover Medical School, Hannover, Germany, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Germany; German Center for Lung Research (DZL)), Svenja Gaedcke (Hannover Medical School, Hannover, Germany, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Germany; German Center for Lung Research (DZL)), Bin Liu (Hannover Medical School, Hannover, Germany, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Germany; German Center for Lung Research (DZL)), Mifflin-Rae Calveron (Hannover Medical School, Hannover, Germany, and Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Germany; German Center for Lung Research (DZL)), Stefanie Weber (University Children's Hospital Marburg, University of Marburg, Germany, and University of Giessen Marburg Lung Center (UGMLC); Member of the German Center for Lung Research), Tom Schildberg (University of Cologne, Faculty of Medicine and University Hospital Cologne, Department of Pediatrics, Cologne, Germany), Silke van Koningsbruggen-Rietschel (University of Cologne, Faculty of Medicine and University Hospital Cologne, Department of Pediatrics, Cologne, Germany), Miguel Alcazar (University of Cologne, Faculty of Medicine and University Hospital Cologne, Translational Experimental Pediatrics – Experimental Pulmonology, Department of Pediatric and Adolescent Medicine, Germany; University of Cologne, Faculty of Medicine and University Hospital Cologne, Center for Molecular Medicine Cologne (CMMC), Germany; Excellence Cluster on Stress Responses in Aging-associated Diseases (CECAD), University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany; Institute for Lung Health, University of Giessen and Marburg Lung Centre (UGMLC), Member of the German Centre for Lung Research (DZL), Gießen, Germany).
Author contributions: N. Maison, J. Omony, E. von Mutius and R. Grychtol contributed to conceptualisation, methodology, data analysis and presentation of the published work and wrote the manuscript. S. Illi and T. Bahmer contributed to conceptualisation, methodology, provided scientific advice, specifically critical review, commentary or revision of the manuscript. J. Omony and S. Illi performed data curation and statistical analysis. D. Thiele conducted management activities to annotate, scrub and maintain research data. C. Skevaki, A-M. Dittrich, M. Weckmann, C. Happle and B. Schaub planned and supervised cytokine measurement and data curation, provided scientific advice, specifically critical review, commentary or revision of the manuscript. M. Meyer and S. Foth contributed to patient recruitment. E. Rietschel, H. Renz, M.V. Kopp, K.F. Rabe and G. Hansen provided scientific advice, specifically critical review, commentary or revision of the manuscript. K.F. Rabe, M.V. Kopp, G. Hansen and E. von Mutius are principal investigators of the DZL ALL Age Asthma Cohort and secured funding and conceived ideas, formulation and evolution of overarching research goals and aims.
Conflict of interest: N. Maison, J. Omony, S. Illi, D. Thiele, A.M. Dittrich, C. Happle, M. Meyer, S. Foth and R. Grychtol have nothing to disclose. C. Skevaki reports grants and personal fees from Hycor Biomedical, Bencard Allergie, Thermo Fisher Scientific as well as grants from Mead Johnson Nutrition (MJN), Universities Giessen and Marburg Lung Centre, the German Centre for Lung Research (DZL), University Hospital Giessen and Marburg, Deutsche Forschungsgemeinschaft (DFG). T. Bahmer reports grants from the Federal Ministry for Education and Research (BMBF) for the German Center for Lung Research (DZL) and personal fees from AstraZeneca, GlaxoSmithKline, Novartis, Roche and Chiesi. M. Weckmann reports grants from Federal Ministry for Education and Research (BMBF), University of Luebeck and German Academic Exchange Service. B. Schaub reports grants from DFG, BMBF, the EU as well from GlaxoSmithKline, Sanofi and Novartis. H. Renz reports grants from German Center for Lung Disease (DZL) and Universities Giessen Marburg Lung Center. M.V. Kopp reports grants and personal fees from Allergopharma GmbH and Vertex GmbH; additional, personal fees from Sanofi GmbH, Infectopharm GmbH and Leti GmbH. E. Rietschel reports personal lecture payments for Nutricia Milupa GmbH and Novartis Pharma, and honoraria for participation in advisory boards for MICE-Mylan, Novartis Pharma GmbH and Boehringer Ingelheim GmbH. K.F. Rabe recieved personal payments or honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi Pharmaceuticals, Novartis, Sanofi & Regeneron, GlaxoSmithKline, Berlin Chemie and Roche; K.F. Rabe also discloses participation on data safety monitoring boards/advisory boards for AstraZeneca and Sanofi Regeneron, and leadership or fiduciary role in the German Center for Lung Research (DZL), German Chest Society (DGP) and American Thoracic Society (ATS). G. Hansen reports grants from German Federal Ministry of Education and Research (BMBF) and German Research Foundation (DFG) as well as personal fees from Sanofi GmbH, MedUpdate, and Abbvie. E. von Mutius reports grants from the German Center for Lung Research (DZL) as well as royalties/licenses held by Elsevier GmbH, Gerog Thieme Verlag, Springer Verlag GmbH, Elsevier Ltd; furthermore, consultation fees were received from the Chinese University of Hong Kong, European Commission, HiPP GmbH and AstraZeneca; E. von Mutius also received payments and/or support for meetings/travel from the Massachusetts Medical Society, Springer-Verlag GmbH, Elsevier Ltd, Böhringer Ingelheim International GmbH, European Respiratory Society (ERS), University Utrecht, Salzburg, Colorado and Imperial College London, Springer Medizin Verlag GmbH, Japanese Society of Pediatric Allergy and Clinical Immunology, Klinkum Rechts der Isar, Paul-Martini-Stiftung; further support for meetings/travel was granted by Verein zur Förderung der Pneumologie am Krankenhaus Groshansdorf, Pneumologie Development Mondial Congress & Events GmbH, American Academy of Allergy, Asthma & Immunology, Margaux Orange, Volkswagen Stiftung, Österreichische Gesellschaft für Allergologie & Immunologie, OM Pharma SA, Hanson Wade Ltd, iKOMM GmbH, DSI Dansk Bornestma Center, American Thoracic Society, HiPP GmbH; E. von Mutius has patent EP2361632, EP1411977, EP1637147 and EP 1964570 (licensed to Protectimmun), furthermore patent LU101064 is pending; E. von Mutius participates in the following data monitoring or advisory boards: EXPANSE, BEAMS External Scientific Advisory Board, Journal of Allergy and Clinical Immunology: in Practice, Children's Respiratory and Environmental Workgroup (CREW), International Scientific & Societal Advisory Board of Utrecht Life Sciences, External Review Panel of the Faculty of Veterinary Science (University of Utrecht), Gottfried Wilhelm Leibniz Programme, Asthma UK for Applied Research, Advisory Board of The Lancet Respiratory Medicine, CHILD (Canadian Healthy Infant Longitudinal Development Study).
Support statement: This research was supported by the German Center for Lung Research (Federal Ministry of Education and Research) and the Wilsing Stiftung, Cologne. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Hammad H, Lambrecht BN. The basic immunology of asthma. Cell 2021; 184: 2521–2522. doi: 10.1016/j.cell.2021.04.019 [DOI] [PubMed] [Google Scholar]
- 2.Woodruff PG, Modrek B, Choy DF, et al. . T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009; 180: 388–395. doi: 10.1164/rccm.200903-0392OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamant Z, Vijverberg S, Alving K, et al. . Toward clinically applicable biomarkers for asthma: an EAACI position paper. Allergy 2019; 74: 1835–1851. doi: 10.1111/all.13806 [DOI] [PubMed] [Google Scholar]
- 4.Agache I, Beltran J, Akdis C, et al. . Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines – recommendations on the use of biologicals in severe asthma. Allergy 2020; 75: 1023–1042. doi: 10.1111/all.14221 [DOI] [PubMed] [Google Scholar]
- 5.Pavord I, Bahmer T, Braido F, et al. . Severe T2-high asthma in the biologics era: European experts’ opinion. Eur Respir Rev 2019; 28: 190054. doi: 10.1183/16000617.0054-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teague WG, Phillips BR, Fahy JV, et al. . Baseline features of the Severe Asthma Research Program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract 2018; 6: 545–554. doi: 10.1016/j.jaip.2017.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming L, Heaney L. Severe asthma – perspectives from adult and pediatric pulmonology. Front Pediatr 2019; 7: 389. doi: 10.3389/fped.2019.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartl S, Breyer MK, Burghuber OC, et al. . Blood eosinophil count in the general population: typical values and potential confounders. Eur Respir J 2020; 55: 1901874. doi: 10.1183/13993003.01874-2019 [DOI] [PubMed] [Google Scholar]
- 9.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2011. Available from: http://ginasthma.org/
- 10.German Medical Association (BÄK), National Association of Statutory Health Insurance Physicians (KBV), Working Group of Scientific Medical Societies (AWMF) . Nationale VersorgungsLeitlinie Asthma – Langfassung, 4. Auflage. [National Asthma Care Guideline – Long Version]. Version 1. 2020. Date last accessed: 1 December 2020. www.versorgungsleitlinien.de/themen/asthma/ DOI: 10.6101/AZQ/000469
- 11.Fuchs O, Bahmer T, Weckmann M, et al. . The all age asthma cohort (ALLIANCE) – from early beginnings to chronic disease: a longitudinal cohort study. BMC Pulm Med 2018; 18: 140. doi: 10.1186/s12890-018-0705-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoos AM, Jelding-Dannemand E, Stokholm J, et al. . Single and multiple time-point allergic sensitization during childhood and risk of asthma by age 13. Pediatr Allergy Immunol 2019; 30: 716–723. doi: 10.1111/pai.13109 [DOI] [PubMed] [Google Scholar]
- 13.Skevaki C, Tafo P, Eiringhaus K, et al. . Allergen extract- and component-based diagnostics in children of the ALLIANCE asthma cohort. Clin Exp Allergy 2021; 51: 1331–1345. doi: 10.1111/cea.13964 [DOI] [PubMed] [Google Scholar]
- 14.Olivetti E, Greiner S, Avesani P. Statistical independence for the evaluation of classifier-based diagnosis. Brain Inform 2015; 2: 13–19. doi: 10.1007/s40708-014-0007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R Project for Statistical Computing . R: A Language and Environment for Statistical Computing. Vienna, R Foundation for Statistical Computing, 2020. www.R-project.org/ [Google Scholar]
- 16.Quanjer PH, Stanojevic S, Cole TJ, et al. . Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silkoff PE, Strambu I, Laviolette M, et al. . Asthma characteristics and biomarkers from the Airways Disease Endotyping for Personalized Therapeutics (ADEPT) longitudinal profiling study. Respir Res 2015; 16: 142. doi: 10.1186/s12931-015-0299-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossios C, Pavlidis S, Hoda U, et al. . Sputum transcriptomics reveal upregulation of IL-1 receptor family members in patients with severe asthma. J Allergy Clin Immunol 2018; 141: 560–570. doi: 10.1016/j.jaci.2017.02.045 [DOI] [PubMed] [Google Scholar]
- 19.Pavord ID, Beasley R, Agusti A, et al. . After asthma: redefining airways diseases. Lancet 2018; 391: 350–400. doi: 10.1016/S0140-6736(17)30879-6 [DOI] [PubMed] [Google Scholar]
- 20.Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: moving toward precision medicine. J Allergy Clin Immunol 2019; 144: 1–12. doi: 10.1016/j.jaci.2019.05.031 [DOI] [PubMed] [Google Scholar]
- 21.Silkoff PE, Laviolette M, Singh D, et al. . Identification of airway mucosal type 2 inflammation by using clinical biomarkers in asthmatic patients. J Allergy Clin Immunol 2017; 140: 710–719. doi: 10.1016/j.jaci.2016.11.038 [DOI] [PubMed] [Google Scholar]
- 22.Peters MC, Kerr S, Dunican EM, et al. . Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J Allergy Clin Immunol 2019; 143: 104–113. doi: 10.1016/j.jaci.2017.12.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poole A, Urbanek C, Eng C, et al. . Dissecting childhood asthma with nasal transcriptomics distinguishes subphenotypes of disease. J Allergy Clin Immunol 2014; 133: 670–678. doi: 10.1016/j.jaci.2013.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belgrave DCM, Granell R, Turner SW, et al. . Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med 2018; 6: 526–534. doi: 10.1016/S2213-2600(18)30099-7 [DOI] [PubMed] [Google Scholar]
- 25.Hose AJ, Depner M, Illi S, et al. . Latent class analysis reveals clinically relevant atopy phenotypes in 2 birth cohorts. J Allergy Clin Immunol 2017; 139: 1935–1945. doi: 10.1016/j.jaci.2016.08.046 [DOI] [PubMed] [Google Scholar]
- 26.Castro-Rodríguez JA, Holberg CJ, Wright AL, et al. . A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000; 162: 1403–1406. doi: 10.1164/ajrccm.162.4.9912111 [DOI] [PubMed] [Google Scholar]
- 27.Anderson HM, Lemanske RF Jr, Arron JR, et al. . Relationships among aeroallergen sensitization, peripheral blood eosinophils, and periostin in pediatric asthma development. J Allergy Clin Immunol 2017; 139: 790–796. doi: 10.1016/j.jaci.2016.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg HF, Dyer KD, Domachowske JB. Eosinophils and their interactions with respiratory virus pathogens. Immunol Res 2009; 43: 128–137. doi: 10.1007/s12026-008-8058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabogal Piñeros YS, Bal SM, Dijkhuis A, et al. . Eosinophils capture viruses, a capacity that is defective in asthma. Allergy 2019; 74: 1898–1909. doi: 10.1111/all.13802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phipatanakul W, Mauger DT, Guilbert TW, et al. . Preventing asthma in high risk kids (PARK) with omalizumab: design, rationale, methods, lessons learned and adaptation. Contemp Clin Trials 2021; 100: 106228. doi: 10.1016/j.cct.2020.106228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Couillard S, Shrimanker R, Chaudhuri R, et al. . Fractional exhaled nitric oxide nonsuppression identifies corticosteroid-resistant type 2 signaling in severe asthma. Am J Respir Crit Care Med 2021; 204: 731–734. doi: 10.1164/rccm.202104-1040LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malinovschi A, Fonseca JA, Jacinto T, et al. . Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in National Health and Nutrition Examination Survey subjects. J Allergy Clin Immunol 2013; 132: 821–827. doi: 10.1016/j.jaci.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 33.Frøssing L, Silberbrandt A, Von Bülow A, et al. . The prevalence of subtypes of type 2 inflammation in an unselected population of patients with severe asthma. J Allergy Clin Immunol Pract 2021; 9: 1267–1275. doi: 10.1016/j.jaip.2020.09.051 [DOI] [PubMed] [Google Scholar]
- 34.Evans PM, O'Connor BJ, Fuller RW, et al. . Effect of inhaled corticosteroids on peripheral blood eosinophil counts and density profiles in asthma. J Allergy Clin Immunol 1993; 91: 643–650. doi: 10.1016/0091-6749(93)90270-P [DOI] [PubMed] [Google Scholar]
- 35.Holt PG, Strickland D, Bosco A, et al. . Distinguishing benign from pathologic TH2 immunity in atopic children. J Allergy Clin Immunol 2016; 137: 379–387. doi: 10.1016/j.jaci.2015.08.044 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02288-2021.Supplement (730.9KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02288-2021.Shareable (587.7KB, pdf)