Abstract
The global coronavirus disease 2019 (COVID-19) pandemic spurred an urgent need for vaccination and herd immunity. Recently, mRNA vaccines for COVID-19 have been used widely despite reports of several adverse events. Most adverse effects are mild, although a few are associated with neurological complications. Unfortunately, there is a scarcity of information on peripheral nerve complications after COVID-19 mRNA vaccination. We report the case of an immunocompetent young male patient who suffered from ipsilateral wrist drop with multiple lymphadenopathy in the cervical and axillary region after Pfizer–BioNTech vaccination. He experienced unilateral wrist drop, which significantly improved with corticosteroid treatment. Based on knowledge of this adverse effect, careful surveillance and increased awareness are needed for early diagnosis. To the best of our knowledge, this is the first reported case in the English literature of radial neuropathy resulting in wrist drop in a recently vaccinated and young immunocompetent patient.
Keywords: COVID-19, vaccination, lymphadenopathy, BNT162b2 vaccine, radial neuropathy
INTRODUCTION
With the World Health Organization (WHO) declaring coronavirus disease 2019 (COVID-19) a global pandemic, vaccination became of paramount importance to minimize the effects of the pandemic. The COVID-19 mRNA vaccines contain mRNA that stimulates the human’s immune response. The mRNA recognizes a spike protein, which is characteristic of COVID-19, as an antigen, thereby eliciting an immune reaction to instruct the defense mechanism.1 However, careful observation is still needed due to the lack of information regarding the safety of these vaccines. Recently, several vaccine-related adverse events involving peripheral nerves have been reported.2,3 An excessive immune reaction can provoke these adverse effects. Herein, we present the case of an adult male patient who suffered from ipsilateral wrist drop after Pfizer–BioNTech COVID-19 vaccination.
CASE REPORT
A 21-year-old man was hospitalized for acute pain and weakness in his left arm. He was an immunocompetent young patient with no previous medical history prior to inoculation. Two days prior to admission, he had received a second dose of BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine in the deltoid area of his left arm. He gradually noticed a severe burning sensation and weakness of the left arm. Wrist drop was observed in half a day after the vaccination. In contrast, he experienced mild pain/tenderness after the first dose; this resolved spontaneously in 3 days.
On neurologic examination, there were prominent wrist and finger drop on the left side, which was near paralysis [Medical Research Council (MRC) grade 1–2/5] of wrist and finger extension. In addition, there was weakness in arm extension and flexion (MRC grade 3–4/5) on the left, but shoulder abduction and wrist extension were normal. On sensory examination, there was dysesthesia and numbness over the lateral dorsum of the left hand extending into the posterior forearm and upper arm. Reflexes in the left brachioradialis and triceps were hypoactive, whereas those of the right were normoactive.
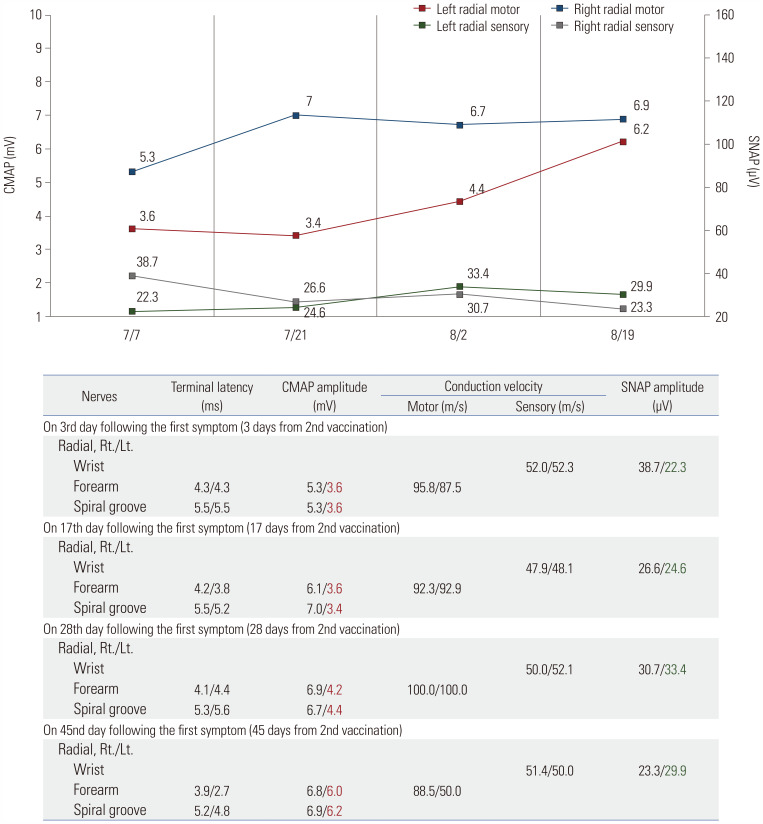
Nerve conduction studies (NCSs) were performed 3 days after the second inoculation. These studies revealed that left radial compound muscle action potential (CMAP) was markedly reduced, compared to the right side. Sensory nerve action potential (SNAP) was also relatively reduced in the left radial nerve (Fig. 1 and Supplementary Table 1, only online). Follow-up NCS also showed reduced CMAP, and needle electromyography showed acute denervation of the left extensor carpi radialis longus, extensor digitorum communis, and anconeus muscles 17 days after symptom initiation (Fig. 1 and Supplementary Tables 1 and 2, only online). Based on these results, left radial neuropathy in the axilla with demyelination and associated mild axonal injury were highly suspected.
Fig. 1. Serial NCS results of the radial nerves. CMAP, compound muscle action potential; Lt, left; NCS, nerve conduction study; Rt, right; SNAP, sensory nerve action potential.
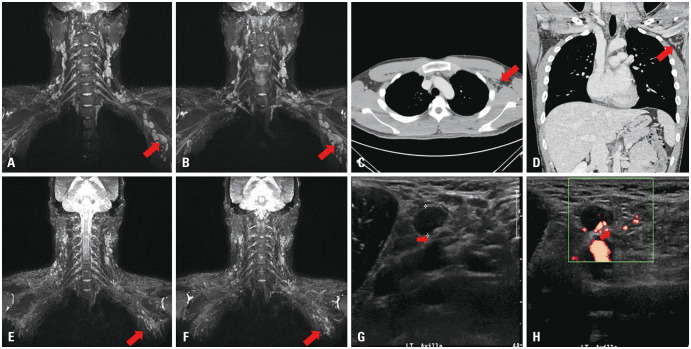
Initial brachial plexus MRI revealed multiple enlarged benign lymph nodes (LN) along the neck (level IV), posterolateral area of axilla and axillary vessels adjacent to the brachial plexus, with the largest measuring 2.5×1.6 cm. There were no other enhanced lesions in the brachial plexus or peripheral nerves (Fig. 2). In addition, an enlarged LN with a size of 10.7×7.5 mm compressing the radial nerve was discovered on left axillary ultrasound performed 4 days after high-dose steroid treatment. LNs with thickened cortex were hypoechoic, well-circumscribed, ovoid, and firm, and tenderness was elicited by LN compression using an ultrasound probe, suggestive of compressive neuropathy. Ultrasonography-guided fine needle aspiration confirmed reactive hyperplasia with negative results for malignancy and tuberculosis (Fig. 2 and Table 1). Laboratory findings for vasculitis, autoimmune disorders, malignancy, and infectious diseases were unremarkable (Table 1).
Fig. 2. Imaging findings of the patient. (A and B) Brachial plexus MRI on the fifth day after the onset of symptoms shows multiple enlarged LNs on the left axilla (arrow). There was no evidence of direct invasion to the brachial plexus. The largest LN was approximately 2.5–3.0 cm in length. (C and D) Chest CT performed on the seventh day after the onset of symptoms also reveals multiple enlarged LNs, suggesting the possibility of reactive lymphadenopathy. The size of the largest LN was reduced to 6–7 mm after intravenous steroid loading treatment (arrow). (E and F) MRI for brachial plexus on the 28th day after the onset of symptoms. Compared to a previous study, the size and number of enlarged benign LNs along the carotid and axillary vessels (left>right) have almost normalized (arrow). (G and H) Ultrasonography (USG) on the left axilla level I. USG on the ninth day after the onset of symptoms shows that the biggest LN measures approximately 10.7×7.5 mm in size. Hypoechoic radial nerve is seen compressed by enlarged LN within mixed echogenic soft tissue in the longitudinal plane (arrow). The results of fine needle aspiration suggested benign reactive hyperplasia of the LN. LN, lymph node.
Table 1. Laboratory Findings of the Patient.
| Laboratory analysis | Result | Unit | Reference values | Laboratory analysis | Result | Unit | Reference values | ||
|---|---|---|---|---|---|---|---|---|---|
| Infection | Malignancy | ||||||||
| Lymphocytes | 21.5 | % | 19–48 | CEA | 0.55 | ng/mL | 0–5 | ||
| monocytes | 5.4 | % | 3.4–9 | CA 19-9 | 4.8 | U/mL | 0–35 | ||
| CRP | 0.1 | mg/dL | 0–0.5 | CA 15-3 | 3.8 | U/mL | 0–31.3 | ||
| Anti-CMV IgM Antibody | 5.8, negative | Index | CA 125 | 4.3 | U/mL | 0–35 | |||
| Anti-CMV IgG Antibody | 66.1, positive | U/mL | |||||||
| Epstein-Barr VCA IgM | <10.0, negative | Index | |||||||
| Epstein-Barr VCA IgG | 44.6, positive | U/mL | Autoimmune | ||||||
| EBNA IgG | 75.2, positive | U/mL | Lupus anticoagulant | Negative | Negative | ||||
| Anti-SARS-CoV-2 IgG antibody | Positive | Index | Protein C activity | 90 | % | 70–130 | |||
| HSV IgG | Negative (<0.5) | Index | Protein S activity | 83 | % | 65–140 | |||
| HSV IgM | Negative (0.6) | Index | Complement C3 | 106 | mg/dL | 90–180 | |||
| Anti-HIV | Negative | Index | Complement C4 | 26 | mg/dL | 10–40 | |||
| RPR-VDRL | <0.1 | R.U | 0–0.9 | Anti-B2 Glycoprotein Ab (IgM) | Negative (<1.1) | Index | |||
| HBs Ag | Negative | Anti-B2 Glycoprotein Ab (IgG) | Negative (<6.4) | Index | |||||
| Anti-HBs | Negative (<3.10) | Antinuclear Ab(quan) | Negative (<1:40) | ||||||
| Anti-HCV | Negative (<0.02) | Anti-DNA(quan) | Negative (<1:40) | ||||||
| Anti-HAV(IgM) | Negative | ||||||||
| Anti-HAV(IgG) | Positive | ||||||||
| Toxoplasma Ab IgG | Negative (<3.0) | Index | PBS | ||||||
| Toxoplasma Ab IgM | Negative (<3.0) | Index | No specific abnormal findings | ||||||
| Cryptococcus | Negative | ||||||||
Ag, antigen; CA, cancer antigen; CEA, carcinoembryonic antigen; CMV, cytomegalovirus; CRP, C-reactive protein; DNA, deoxyribonucleic acid; EBNA, Epstein-Barr virus nuclear antigen; HAV, hepatitis A virus; HBs Ag, hepatitis B virus surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; IgM, immunoglobulin M; IgG, immunoglobulin G; PBS, peripheral blood smear; R.U, RPR Unit; quan, quantitative; RPR-VDRL, rapid plasma reagin venereal disease research laboratory; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Non-enhanced chest computed tomography performed on the third day of steroid intravenous treatment also showed multiple enlarged homogenous LNs (approximately 6–7 mm in size) along the axillary vessels on the left side (Fig. 2). Based on these results, the patient was diagnosed with radial neuropathy associated with ipsilateral axillary lymphadenopathy (LAP) following COVID-19 vaccination. He was treated with intravenous high-dose methylprednisolone (1 g/day) with physiotherapy for 5 days, followed by daily oral prednisolone for 1 month. Follow-up MRI revealed that LN size had almost normalized (Fig. 2). In addition, consecutive follow-up electrophysiological tests showed nearly normal CMAP and SNAP 1.5 months after symptom initiation (Fig. 1and Supplementary Table 1, only online). The patient’s wrist drop recovered completely.
DISCUSSION
LAP is a normal immunological reaction in the body, indicating that an effective and active immune response to foreign antigens is in progress. Vaccination-associated LAP (VA-LAP) has often been considered a successful immune response.4 Most adverse effects are self-limiting minor symptoms, such as localized swelling, redness, or tenderness at the injection site.5 Previously, VA-LAP on the same side of vaccination has been reported for other vaccinations.6,7 However, little is known about the neurological complications caused by VA-LAP, particularly in COVID-19 vaccination. Meanwhile, research has shown that COVID-19 vaccination can induce stronger immunological responses in immunocompetent young patients than in older adult patients:8 Müller, et al.9 reported lower frequencies of neutralizing antibodies in an older adults after Pfizer–BioNTech COVID-19 vaccination.
Currently, mRNA COVID-19 vaccines include the Pfizer–BioNTech and Moderna vaccines. These vaccines are both composed of mRNA, which delivers genetic information that encodes the SARS-CoV-2 spike (S) glycoprotein, and lipid nanoparticles, which safely transport the mRNA into the cells of the human body. They amplify the immune responses by producing S proteins using mRNA genetic information and induce rapid and robust immune reactions in the body. For these reasons, LN reactions occur quickly after inoculation, and the duration of LAP remains longer than that of previous VA-LAP clinically.10
In our patient, wrist drop occurred within half a day after the second dose. Albeit direct nerve compression was not demonstrated on MRI, axillary ultrasonography can reveal compression of the radial nerve by enlarged LN. All clinical evaluations excluded differential diagnoses of brachial plexopathy, idiopathic neuralgic amyotrophy, and vasculitic peripheral neuropathy. In addition, the clinical findings and course were different from those etiologies. In the healthy young male patient, multiple LAP following an explosive immune reaction after the second vaccination may be presumed to have induced acute nerve injury in the compressed radial nerve. Meanwhile, in most COVID-19 cases, VA-LAP has been reported to spontaneously resolve after 4–12 weeks of inoculation, according to sex, age, and immune status.7 In the present patient, wrist drop fully recovered as the reactive LAP resolved in approximately 6 weeks. However, if compressive neuropathy is prolonged, severe axonal injury can occur, leading to irreversible nerve damage.11 Therefore, we believe that the administration of urgent systemic steroids was probably the principal treatment that decreased the LAP size in the initial period.
In conclusion, this is the first report of ipsilateral radial neuropathy related to VA-LAP. Even in extremely rare cases, compressive neuropathy associated with reactive LAP may occur after COVID-19 vaccination. Although the disease progression was self-limiting, we emphasize that careful surveillance and increased awareness are required for its early diagnosis and accumulation of evidence of causality with vaccination in the field of neurology.
ACKNOWLEDGEMENTS
This work was supported by Research Resettlement Fund for the new faculty of Konyang University Hospital.
The authors sincerely thank Dr. Sang Young Oh in Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, for providing radiological advice on this report.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Seon-Min Lee and Sang-Jun Na.
- Data curation: Jun Yeong Hong and Si-Yeon Kim.
- Formal analysis: Si-Yeon Kim.
- Funding acquisition: Seon-Min Lee.
- Investigation: Jun Yeong Hon.
- Methodology: Seon-Min Lee.
- Project administration: Sang-Jun Na.
- Resources: Jun Yeong Hong and Si-Yeon Kim.
- Software: Jun Yeong Hong and Si-Yeon Kim.
- Supervision: Sang-Jun Na.
- Validation: Seon-Min Lee and Sang-Jun Na.
- Visualization: Si-Yeon Kim.
- Writing—original draft: Seon-Min Lee.
- Writing—review & editing: Seon-Min Lee and Sang-Jun Na.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Serial Nerve Conduction Studies of the Patient
Electromyography Summary in the Left Upper Limb (on the 17th Day from 2nd Vaccination)
References
- 1.Ganga K, Solyar AY, Ganga R. Massive cervical lymphadenopathy post-COVID-19 vaccination. Ear Nose Throat J. 2021 Oct 02; doi: 10.1177/01455613211048984. [Epub]. Available at: [DOI] [PubMed] [Google Scholar]
- 2.Mortazavi S. COVID-19 vaccination–associated axillary adenopathy: imaging findings and follow-up recommendations in 23 women. AJR Am J Roentgenol. 2021;217:857–858. doi: 10.2214/AJR.21.25651. [DOI] [PubMed] [Google Scholar]
- 3.Waheed W, Carey ME, Tandan SR, Tandan R. Post COVID-19 vaccine small fiber neuropathy. Muscle Nerve. 2021;64:E1–E2. doi: 10.1002/mus.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewer KD, DeBay DR, Dude I, Davis C, Lake K, Parsons C, et al. Using lymph node swelling as a potential biomarker for successful vaccination. Oncotarget. 2016;7:35655–35669. doi: 10.18632/oncotarget.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadali RAK, Janagama R, Peruru S, Gajula V, Madathala RR, Chennaiahgari N, et al. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: a randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol. 2021;93:4420–4429. doi: 10.1002/jmv.26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagen C, Nowack M, Messerli M, Saro F, Mangold F, Bode PK. Fine needle aspiration in COVID-19 vaccine-associated lymphadenopathy. Swiss Med Wkly. 2021;151:w20557. doi: 10.4414/smw.2021.20557. [DOI] [PubMed] [Google Scholar]
- 7.Keshavarz P, Yazdanpanah F, Rafiee F, Mizandari M. Lymphadenopathy following COVID-19 vaccination: imaging findings review. Acad Radiol. 2021;28:1058–1071. doi: 10.1016/j.acra.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner JS, O’Halloran JA, Kalaidina E, Kim W, Schmitz AJ, Zhou JQ, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73:2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettini E, Locci M. SARS-CoV-2 mRNA vaccines: immunological mechanism and beyond. Vaccines (Basel) 2021;9:147. doi: 10.3390/vaccines9020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thatte MR, Mansukhani KA. Compressive neuropathy in the upper limb. Indian J Plast Surg. 2011;44:283–297. doi: 10.4103/0970-0358.85350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serial Nerve Conduction Studies of the Patient
Electromyography Summary in the Left Upper Limb (on the 17th Day from 2nd Vaccination)