Abstract
The timing of first period of slow wave sleep (SWS) is often used as a proxy for determining if and when Disorders of Arousal (DOA) such as sleepwalking are likely to occur or did occur in the past. In criminal cases employing a “sleepwalking defense” the prosecution may argue that nocturnal violence or sexually aggressive behavior occurred too early in the sleep period to be associated with SWS. Expert witness opinion on the expected latency to SWS (LSWS) has varied from minutes after sleep onset to ≥60 min. A search of PubMed was conducted for LSWS and for any reports of DOAs occurring from stage N2. A total of 21 studies reported LSWS in normal controls, clinically diagnosed sleepwalkers, in otherwise normal sleepers following different types of sleep deprivation and due to the effects of alcohol. Five studies reported episodes of DOA from N2 sleep. The shortest mean LSWS of 6.4 min was found with a combination of total sleep deprivation and alcohol. In a group of normal research subjects, a LSWS mean of 10.7 min was noted. LSWS in DOA patients occurred as early as a mean of 12.4 min. Two sleep studies performed on Kenneth Parks, acquitted of the murder of his mother-in-law by a sleepwalking defense, reported LSWSs of 9.7 and 10 min. Sleep deprivation but not alcohol was found to decrease LSWS significantly. Expert opinions on LSWS should be based on scientific peer reviewed publications documenting empirical sleep evidence and can be much shorter than is generally reported.
Keywords: Slow wave sleep, N3, Disorder of arousal, Sleepwalking defense, Latency to slow wave sleep, Kenneth parks, Sleep deprivation, Alcohol, Forensic evaluation, Sleepwalking violence, Sexual behavior in sleep
Abbreviations: DOA, Disorder of Arousal; NREM, Non-Rapid Eye Movement Sleep; SWS, Slow Wave Sleep; LSWS, Latency to Slow Wave Sleep
Highlights
-
•
Detailed review of methods and problems of determining the onset of Slow Wave Sleep.
-
•
Reviews the application of latency to slow wave sleep in criminal cases invoking a sleepwalking defense.
-
•
Reviews the influence of sleep deprivation and alcohol on slow wave sleep and sleepwalking.
1. Introduction
Disorders of Arousal (DOA) are a subset of NREM parasomnias that includes sleepwalking, confusional arousal and sleep terrors [1]. DOAs are most often reported to be associated with the presence of delta wave EEG that characterizes slow wave sleep (SWS) also known as N3 sleep [1,2]. DOA behaviors may manifest in a variety of ways including violence and sexual behavior [3,4]. However, when violent behavior results in the injury of a bedpartner or others, or sexually aggressive behavior involves a non-consenting individual, criminal charges may result [5]. Under these circumstances criminal defendants may invoke a “sleepwalking defense”. The basis of this defense is that during sleepwalking episodes cognition is impaired or absent, and there is an also an absence of conscious awareness and intention. Executive functions such as planning are absent due to deactivation of the prefrontal cortex (PFC) [6,7]. Depending on the jurisdiction, this defense has the advantage – if accepted by the jury – of avoiding a criminal conviction and jail.
Forensic sleep evaluations differ in many ways from clinical evaluations for DOAs [5]. As a first step, a forensic evaluation for or against a sleepwalking defense often involves evaluating whether the charged episode of alleged sleep related complex behavior -violence, sexual assault, etc.- occurred at a time when SWS was likely to have been present [5]. In forensic settings, complex behaviors that are estimated to have occurred earlier than SWS is expected to occur are not thought to be consistent with a DOA, undermining a defense of sleepwalking violence or sexual behavior in sleep.
SWS is based on the presence of delta frequency EEG. The scientific assumption is that the underlying brain state represented by delta EEG activity measured at the scalp is associated with an increased propensity and susceptibility for sleepwalking in an individual who has a genetic predisposition. It is not known how many delta waves or what delta wave amplitude or duration are most consistent with the onset of sleepwalking. However, the presence of SWS and the occurrence of DOAs are often perceived as so strongly linked that the presence of one suggests the strong probability of the second. Although the occurrence of a DOA cannot be predicted, the timing of SWS after sleep onset is often estimated to fall at a certain point in the first or second sleep cycles. If DOAs occur primarily during SWS, this would provide a general means of estimating when a DOA is most likely to occur.
In many criminal cases using a sleepwalking defense, the timeline of sleep onset to SWS is often a critical element. This was emphasized in the recent homicide trial of State of Florida v Randy A Herman -see documentary Dead Asleep [8]) where this element was described by a juror as the most important piece of prosecution evidence.
As part of the prosecution's theory of the crime, they argued that there was insufficient time for SWS to have occurred to allow for a sleepwalking episode to be triggered, suggesting the violent behavior could not have been part of a sleepwalking episode. However, the expert witnesses disagreed on how soon after sleep onset SWS could occur. The defense expert stated SWS could occur minutes after sleep onset while the prosecution expert stated that 60–120 min, or more was required, but that LSWS of 30 min had been reported. Both experts discussed how much time was required for the onset of SWS as if it were identical to the time required for an episode of DOA to occur.
Review articles or book chapters on DOAs also provide wide estimations of latency to SWS; 60–120 min after sleep onset [5], first third of night [9] or limited to 1–3 h after sleep onset [10]. These appear to be estimations or generalizations often without a basis in or with reference to peer reviewed empirical sleep science. Additionally, these estimates appear to be associated with sleep cycles found in otherwise young normal sleepers with regular sleep/wake schedules in the absence of alcohol, drugs, sleep deprivation, sleep fragmentation or any other process that might advance or delay the occurrence of SWS.
The current analysis of sleep is based on the application of rules mandated by the AASM (American Academy of Sleep Medicine) Manual for The Scoring of Sleep and Associated Events required for use by all accredited sleep disorders centers in the United States [11]. The “scoring” of sleep stages or wakefulness is determined by rules for each consecutive 30 s “epoch” of EEG data as wake, one of three stages of non-REM (NREM) sleep – N1, N2, N3 - or REM sleep [2,12]. However, the measurement and report of latency to SWS or N3 is not required or defined by The AASM manual. Following the pattern of other latency definitions, it is the time from sleep onset defined as the first epoch of sleep, typically light stage N1, to the first epoch scored as N3. The current use of SWS is identical to the AASM defined N3.
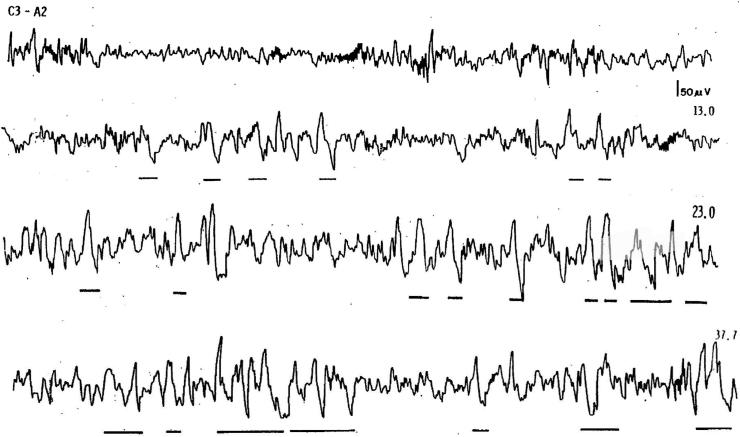
As seen in Fig. 1, current rules for SWS require the scorer to identify all delta EEG waves that are 75 uv. in amplitude peak to peak with a frequency of 0.5–2.0 Hz in each 30 s epoch.
Fig. 1.
Four 30 s epochs originally published in the Rechtshaffen and Kales manual demonstrating the change from N2 sleep to N3sleep based on % of delta EEG waves [11]. Underlining indicates delta waves that met 75 uv. amplitude and 0.2–2.0 frequency. The top tracing appears in Figure 14 The other tracings appear in Fig. 2. The first 2 tracings have less than 20% delta EEG and are thus scored as Stage 2. The second 2 tracings have more than 20% delta EEG and are thus scored as stage 3.
The duration in seconds of all delta waves meeting this definition is then totaled up. Any 30 s epoch with ≥20% or more delta wave EEG activity is scored as N3 [13]. 20% of a 30 s epoch is 6 s. Once the threshold of ≥6 s is met, all other EEG data is discarded for that epoch and that entire epoch is scored as N3. Thus, epochs scored as N3 may contain 6 s or 16 s or 30 s of delta EEG activity, but a value of 30 s will be included in the sleep stage summary for that epoch.
Expert witnesses in Florida v Herman apparently did not rely on published empirically derived sleep data using standard visual scoring techniques for LSWS and they were not pressed to provide a reviewable basis for their definitions or identify published articles. Nevertheless, citing peer reviewed empirical data would appear to be a first step in placing the forensic evaluation of a sleepwalking defendant on valid and reliable scientific grounds. In an attempt to fill in this gap in forensic sleep science this study first locates published empirical sleep studies that report LSWS and attempts to revise the general statements made in the absence of empirical sleep data. Further, methodological problems with N3 assessment are reviewed and suggestion make for their revision.
2. Methods
An initial search of PubMed was conducted for the search terms delta sleep latency, latency to slow wave sleep, deep sleep and N3. This did not result in a return of any results. This was followed by a detailed online search of the PubMed medical database for all empirical studies of Disorders of Arousal, NREM parasomnias, alcohol and sleep, and partial and total sleep deprivation, sleep restriction, extended wakefulness in which full polysomnographic studies were conducted and reported. The abstracts of these studies were reviewed and the full published reports of 45 studies were retrieved. Tables of sleep stages results were reviewed, and 26 studies were found to include latency to slow wave sleep or latency to delta sleep. All but 2 reported the latency from sleep onset to the first epoch of N3. The remaining 2 were older studies who reported latency to stage 4 sleep. One study used N2 as the onset for sleep.
A second review was conducted among all retrieved articles for Disorders of Arousal to determine if they reported how many episodes of DOA occurred from SWS and N2 sleep. 5 studies reported the number of DOA related arousals that occurred from N2 sleep.
3. Results
A total of 26 studies were reviewed and subdivided in categories of DOA from N2, Latency to SWS in patients with DOA, effects of sleep deprivation on latency to SWS in normal patients and effects of alcohol on latency to SWS. A single publication reported the effects of partial and total sleep deprivation combined with alcohol on latency to DOA.
3.1. DOA from N2
Five articles reporting DOA episodes occurring from N2 sleep were identified. Two of the 5 also measured the effects of 25 h and 38 h of total sleep deprivation prior to recovery sleep. The percentage of total episodes of DOA occurring from N2 ranged from 9 to 25% of all episodes. Total sleep deprivation did not result in a statistically significant increase in N2 DOA episodes (see Table 1).
Table 1.
DOA episodes from stage N2.
| Table 1: DOA Episodes from Stage N2 | Baseline | Recovery after sleep deprivation | |
|---|---|---|---|
| Joncas et al. [14] | Effects of 38 h of total sleep deprivation on 10 clinically diagnosed sleepwalkers and 10 matched normal controls. Sleep studies performed at baseline and on recovery. | % N2 baseline = 18.8% | % N2 recovery = 19.6% |
| Kavey [15] | 10 adult sleepwalkers underwent 1–3 diagnostic sleep studies | 4 of 41 episodes = 9% | |
| Guilleminault et al. [16] | Retrospective review of 38 adult sleepwalkers who underwent diagnostic sleep testing | 7 of 37episodes or 18.9% | |
| Zucconi [17] | 21 adult sleepwalkers | 22 episodes or 14% | |
| Zadra et al. [18] | 30 adult sleepwalkers were studied baseline and after 25 h of total sleep deprivation | Entire Group: Baseline - 5 of 24 episodes 21% Subgroup with Periodic Leg Movements in Sleep and mild sleep-disordered breathing Baseline – 2 of 8 25% |
Recovery – 8 of 69 episodes 12% Subgroup with Periodic Leg Movements in Sleep and mild sleep-disordered breathing Recovery − 4 of 23 = 17% |
Prior wakefulness/Total sleep deprivation/Partial sleep deprivation or sleep restriction – As noted in Table 2 nine empirical sleep studies involving some form of sleep deprivation and in which latency to SWS was reported in healthy subjects. These included extended periods of prior wakefulness, total sleep deprivation, partial sleep deprivation, sleep fragmentation and sleep restriction over several nights. The lowest mean Latency to SWS on baseline nights was 14.8 ±7 min. Both partial and total sleep deprivation were found to result in statistically significant decreases in LSWS minutes. The shortest mean LSWS was noted to be 12.4 ± 1.8 min (p < 0.01) after 24 h of wakefulness. After partial sleep deprivation limited to 2 h of sleep per night LSWS was reduced to 13.1± 1.9 min.
Table 2.
Effects of sleep deprivation/sleep restriction/sleep fragmentation/first night effect on latency to SWS.
| Baseline | Sleep Deprivation and/or Recovery Sleep | ||
|---|---|---|---|
| Gillberg and Akerstedt [19] | 8 healthy male subjects mean age of 33.6 Subjects slept on 4 occasions initially for 8 h then 4 h, then 2 h and then 0 h. At 11 a.m. the next day they were permitted to sleep until the awakened spontaneously. |
8 h. TST = 32.2 ± 6.9 | 4 h. TST = 27.1 ± 4.6 min 2 h. TST = 13.1 ± 1.9 min 11 a.m. recovery sleep after 0 h. TST = 13.1 ± 1.9 min p < 0.01 |
| Benoit et al. 1983 [20] | 33 healthy subjects aged 19–26 yrs short sleepers, after 36 h of sleep deprivation and after 12 h of wakefulness. Also, broken down into habitual short, regular and long sleepers. Based on S4 latency without standard amplitude rule. | Baseline 18 ±7.7 min Habitual Sleep Short = 18.4 ± 15.6 Regular = 14.8 ± 7 Long = 28.8 ± 21.6 |
36 h TSD 13.7 ± 6.8 12 h waking = 15.7 ±10.7 |
| Borbely et al. 1981 [21] | 8 healthy subjected with mean age of 24.4 yrs. 2 baselines night followed by 24 h of TSD, followed by 2 recovery nights. | B1 = 31.5 ± 3.7 B2 = 31.1 ± 3.7 |
Recover after 24 h. TSD R1 = 12.4 ± 1.8 P < 0.01 R2 = 26.6 ± 3.0 |
| Issa and Sullivan 1986 [22] | 12 patients with a dx of moderate to severe obstructive sleep apnea treated with CPAP Diagnostic PSG vs. CPAP treatment | DPSG 80 ± 17.4 | First CPAP Night = 3-±14.2 Third CPAP Night ±45 ± 14.2 |
| Ratnavadivel et al. [23] | 171 patients with OSA, 14 with CSA, and 68 non-OSA patients. OSA patients took significantly longer to achieve slow wave and REM sleep (p < 0.001) than non-OSA patients. | Controls 25.6 ± 2.6 min, p = 0.005) | OSA = 39.3 ± 2.6 P < 0.005 |
| Webb and Agnew 1974 [24] | 15 male subjects were studied for 4 baseline nights and then 1x week for 60 days while restricting total sleep time to 5.5 h | Mean latency to S4 for 3 baseline nights = 38 min | Latency to S4 reduced to 22 min. P < 0.01 Overall reduction in S4 latency over 8 weeks of study was 26% |
| Akerstedt et al. 2008 [25] | 9 healthy males aged 21–38 years underwent 1 baseline night followed by 5 night of partial sleep deprivation of 5 h. TST followed by 4 recovery nights. | Mean latency to S3 Baseline = 36 min∗∗ |
Mean latency to S3 Partial Sleep Deprivation P1 = 20 min P < 0.05 P2 = 19 min P3 = 18 min P4 = 17 min P5 = 19 min Recovery R1 = 19 min R2 = 25 min R3 = 26 min R7 = 25 min |
| Ferrara et al. 2002 [26] | 10 male subjects mean age of 23 years. Baseline, 2 nights selective suppression of SWS by acoustic stimuli and then a recovery night. SWS suppression was effective with only 4.3 min remaining. | Baseline S3 Lat. = 39 ± 6.9 | Recovery S3 = 28(3.7) P < 0.02 |
| Carskadon & Dement [27] 1985 | 10 older subjects mean age 69.3 years. Baseline night followed by 38 h of total sleep deprivation followed by 2 recovery nights | Baseline = 20 ± 7 | Recovery 1 = 12 ± 8 p < 0.05 Recovery 2 = 34 ± 3 |
The reverse pattern was present in patients with severe sleep apnea, later effectively treated with Continuous Positive Airway Pressure (CPAP). The latency to SWS was delayed to a mean of 80 min by apnea related sleep fragmentation during baseline and significantly reduced with administration of CPAP. Sleep deprivation that reduces or eliminates SWS may result in SWS rebound once sleep is permitted to recover. This study suggests that sleep fragmentation may delay the onset SWS by disrupting the normal progression of sleep stages. CPAP eliminates this disruption allowing for SWS rebound and shortening of SWS latency.
Alcohol- See Table 3: Administration of alcohol to normal sleepers resulted in a small but statistically significant change in latency to SWS in 1 of 8 studies [28]. Data from Rundell et al was not included as N2 was used as marker for sleep onset. Arnedt et al. is also the only published study in which alcohol resulted in a statistically significant increase in total SWS as a % of total sleep time [29]. Further, a significant reduction LSWS and increase in %SWS was found only in female subjects. Even in the 3 of 4 studies in which alcohol is associated with a statistically significant increase in SWS% only in the first ½ of the sleep period, latency to SWS is not reduced significantly suggesting that alcohol did not increase SWS, but rather resulted in change in the timing of SWS periods so that more of the usual share of total SWS occurred earlier in the sleep period.
Table 3.
Effect of alcohol on latency to SWS.
| Citation | Alcohol | Baseline or Placebo | |
|---|---|---|---|
| Van Reen et al. 2006 [29] | 7 women aged 23.5 yrs. | Alcohol = 11.3 min | Placebo = 14.3 min NS |
| Chan et al. 2013 [30] | 24 female health subjects mean aged 19.1 years. Pre sleep ETOH with target of 0.01% BAC and placebo | Alcohol = 11.3 ± 2.09 | Placebo = 11.4 ± 1.07 NS |
| Stone 1980 [31] | 6 healthy male volunteers aged 20–31 years. Baseline vs. 3 alcohol doses | Alcohol 0.16 = 16.8 min 0.32 = 13.4 0.64 = 11.8 |
Placebo 16.6 min NS |
| Rundell [32] | 10 subjects baseline and ETOH. With sleep latency at N2. to N3 BAC 50–90% | 15.4 min | 23.7 min |
| MacLean [33] | 10 men aged 23.6 years baseline and 4 levels of BAC | Alcohol by Grams per Kg of Weight 0.25 = 31.3 ± 17.5 0.50 = 20.4 ± 15.2 0.75 = 25.8 ± 22.1 1.00 = 22.8 ± 13.7 all NS |
Baseline = 32.5 min |
| Rouhani et al. 1989 [34] | 14 healthy volunteers PSG during 90-min afternoon naps. Baseline vs. 0.25 g 95% ETOH/kg body weight | ETOH 39.97 ± 21.96 NS | Baseline 25.69 ± 10.37 |
| Williams and MacLean [10] | 11 women mean age 19.5 years. Baseline and 2 levels of BAC | BAC 0.50 = 10.6 ± 2.4 0.75 = 9.2 ± 3.0 NS |
Baseline = 10.6 ± 2. |
| Arnedt et al. 2011 [28] | 93 healthy subjects mean age 24.4 years. Placebo then alcohol with BRAC of 0.11 g% | Alcohol Men = 15.2 ± 11.0 Women = 14.7 ± 10.9 p < 0.01 |
Placebo Men = 23.15 ± 15.2 Women = 17.0±9.2 |
|
Alcohol Plus Sleep Deprivation | |||
| Lobo et al. 1997 [35] Alcohol and sleep deprivation |
Sleep deprivation plus alcohol. 12 healthy mail subjects mean age of 27.3 years. Baseline followed by sleep deprivation then placebo or ethanol, Recovery. Randomized, cross over. Partial SD focused on REM sleep. | Latency to SWS Baseline = 18.6 ± 14.4 Sleep deprivation – Partial Sleep deprivation11.6 ± 4.8 Recovery 13.3 ± 18 Ethanol 12.9 ± 4.0 Recover23.4 ± 61.1 all NS |
Latency to SWS Baseline = 15.0 ± 7.3 Sleep deprivation – Total 9.7 ± 7.3 p < 0.05 Recovery 18.5 ± 8.1 Ethanol 6.7 ± 3.4 P < 0.01 Recover 18.6 = 6.9 |
Alcohol with Sleep Deprivation: Lobo et al. 1997 was the only study that combined alcohol with partial and total sleep deprivation. Total sleep deprivation plus alcohol resulted in a latency to SWS of 6.7 ± 3.4 p < 0.01, the shortest latency of all studies reviewed.
3.2. Disorders of Arousal
See Table 4. The latency to SWS was reported in 3 studies of DOA. A fourth publication reported detailed results of sleep studies performed as part of the forensic evaluation of Kenneth Parks, accused and later acquitted of the murder of his mother-in-law while in a sleepwalking state [36]. The mean latency to SWS in the empirical studies ranged from 17.9 to 28 min. Latencies for Mr. Parks from 2 sleep studies conducted as part of his pre-trial evaluation were 9.7 and 10 min. These studies were conducted in the presence of benzodiazepine medication often prescribed as a treatment for DOA and known to reduce SWS duration and delta wave amplitude.
Table 4.
Latency to SWS in DOA.
| Citation | Baseline | Normal Controls | |
|---|---|---|---|
| Barros et al. 202 [37] | 52 patients with Dx of DOA and 52 Age and sex matched normal controls. 1 DPSG | DOA = 17.9 ± 14.9 min | Control 25.7 ± 32.5 NS |
| Perrault et al. 2014 [38] | 12 clinically diagnosed sleepwalkers mean age of 27.4 years. One DSPG. | 28.6 ± 35.7 min | |
| Broughton et al. 1994 [36] | 2 diagnostic studies and 1 follow up for Kenneth Parks as part of his sleepwalking defense for the murder of his mother-in law | 22 January 1988–10 min∗ 23 January 1988–9.7 min∗ 25 July 1989–24 min∗∗ |
4. Discussion
A review of published data on the latency to slow wave sleep based on visual sleep scoring methods finds latencies shorter than was suggested by the prosecution in Florida v. Herman and longer than suggested by the defense expert. The latencies are also much shorter than is often estimated in court cases and academic sleep review articles with a focus on Disorders of Arousal. Mean LSWSs of 10.6 ±2.4, 11.4 ± 07 and 16.6 min have been reported for baseline or placebo-based sleep studies [10,30,39]. Even shorter LSWSs were reported in sleep studies performed as part of a successful sleepwalking defense in the R v Parks, 1985 murder case [36]. Two diagnostic sleep studies were performed with latencies to SWS of 10.0 and 9.7 min. As he was being treated with a benzodiazepine medication on both nights that is well documented to decrease SWS amplitude as well as SWS % it seems reasonable to suggest his LSWS might be even shorter without medication [40].
The effects of different types of sleep deprivation were especially effective in reducing LSWS. This is consistent with reports that as prior wakefulness increases % of SWS also increases [21,24,[41], [42], [43], [44], [45], [46]]. The latency to SWS following 24 h of sleep deprivation was reported as short as a mean of 12.4±1.8 min.
The effects of alcohol alone were quite limited although a combination of total sleep deprivation and alcohol resulted in a mean LSWS latency of 6.7 ± 3.4 min, the shortest LSWS found in any of the studies reported here [35]. Only 1 of 5 studies of alcohol and sleep reporting LSWS found a statistically significant decrease in LSWS latency [28]. Alcohol has been reported in the past to act as a trigger for DOAs but a statistically significant increase in the % of SWS as function of total sleep time has been reported in only 1 of 20 published studies [47]. Three of 4 other studies reported a statistically significant increase in SWS only during variously defined periods in the first ½ of the sleep period even when SWS was not increased as a % of total sleep time [47]. Thus, the overwhelming number of sleep and alcohol studies did not find that alcohol increased SWS as % of TST or decreased SWS latency, severely undermining the theory that alcohol triggers DOA by increasing SWS. Unfortunately, the overwhelming majority of reviews of the effects of alcohol on sleep have failed to report negative findings regarding increased SWS. In clinically diagnosed sleepwalkers studied in the sleep laboratory studies mean latency to SWS was as low as 17.9 ± 14.9 min. These studies did not provide the range of latency values acquired. However, the high standard deviations reported suggest some individual subject latencies were shorter and longer.
The published sleep data also reported in 5 empirical studies that 9–25% of episodes of sleepwalking or confusional arousals noted to occurred in stage N2. Thus, N3 was not required for the occurrence of a DOA. However, these studies do not specify if the episodes of DOA during N2 occurred in association with the 5 s of delta EEG permitted by AASM rules or whether the N2 occurred prior to the first period of N3 that would also shorten the time from sleep onset to the time when sleepwalking episodes become possible. In any event, these data indicate DOAs may occur in the absence of AASM defined N3 or SWS.
In forensic settings the use of general statements by the prosecution that complex violent or sexual aggressive behavior attributed to the defendant occurred too early in the sleep period to be consistent with the presence of SWS should be carefully examined. Statements that DOAs cannot occur until 60 min or longer after sleep onset or any other specific time period cannot be supported. Published peer reviewed empirical sleep laboratory data should be relied upon. General statements about DOA and SWS should always state that episodes have been reported to occur from N2 as well as N3 sleep.
An initial problem in cases other than Florida v Herman in determining latency to SWS is in first determining the starting point or timing of sleep onset. Although this is easily done in the sleep laboratory, it may not be possible to determine exactly when consciousness was lost especially when the episode in question may have occurred months or even years in the past. It is essentially an anecdotal report without benefit of polysomnography or CCTV in the defendant's home setting or other circumstances that are considerably different from the published studies of latency to SWS. The defendant's estimation is typically based on the approximate time he/she entered their bed if known or the last memory before losing consciousness if a time can be recalled. Sometimes defendant and victims will estimate time by how much of a movie or TV show they were watching they can remember. The victim can contribute by reporting that the defendant was asleep at a certain time, based on changes in breathing or the presence of snoring and sometimes other family members snoring, but not the exact moment. Often there is a range of possible times of sleep onset, greatly affecting when slow wave sleep onset could have occurred [48].
Estimating the latency to N3 sleep by AASM rules is at best a general and inexact method for determining if the neurophysiological substrate for sleepwalking correlated disorders might have been present at the time a complex sleep related behavior was reported to have occurred. However, it may overestimate the latency, a problem that can be serious in forensic cases. LSWS is measured to the first epoch scored as N3, not to the first delta wave or waves noted irregardless of amplitude and frequency requirements. There is no empirical data linking the onset of DOAs to the first 6 s of delta EEG wave activity or to delta waves of a particular amplitude.
The R&K manual and the current AASM manual do not provide an explanation for the 20% of epoch rule or the 75 uv. amplitude rule. In the absence of an empirical based rationale for these cut-offs or for any other cut-off, no reason for a change could be agreed upon. However, it is noted that these rules have been applied in thousands of published sleep related publications and for purposes of continuity maintaining the R&K cut-offs rules was thought to be of value [12,13]. A more recent publication has also determined that the method for scoring N3 is unreliable and differs significantly between interlaboratory sleep labs scoring the same sleep studies [48].
“Manual scoring of non-rapid eye movement sleep stages is highly unreliable among highly trained, experienced technologists.”
The current AASM scoring rules with regard to SWS do not allow for deviation. However, the predecessor of this manual – the Rechtshaffen and Kales Manual – allowed for some variance under other conditions. The R&K committee acknowledged that “alternative measures of slow wave activity might have a usefulness and empirical significance not enjoyed by the measure chosen” and that “this should not deter investigators from using measures of slow wave activity other than the one suggested here.” (Page 8).
LSWS is generally not computed or reported for clinical diagnostic purposes. The quantity of SWS also has limited diagnostic significance especially in that there are no generally accepted norms for %SWS. In the absence of normative data, the determination of abnormality is not possible. Nevertheless, based on general knowledge, reductions in SWS as a % of total sleep time may be a result of sleep fragmentation by sleep disorders such as sleep apnea and periodic leg movements. SWS is also reduced in patients with DOAs a result of frequent arousals. However, this appears to be a normal feature of DOA sleep of unknown origin [49]. Medications, especially CNS depressants or sedatives such as benzodiazepines -are known to reduce delta EEG amplitudes and % of SWS [40,50]. Delta amplitude is also reported to decrease with age. An early study reported the LSWS on the first night in the sleep laboratory was also delayed [51]. In a clinical evaluations, higher than expected %SWS most often takes the form of rebound sleep following the end of sleep deprivation or sleep fragmentation due to effective treatment of sleep disorders such as sleep apnea. The change in %SWS may suggest to the sleep clinician that the sleep study was preceded by sleep deprivation or indicate the presence of medication. However, this type of finding is not in and of itself diagnostic and cannot be used to rule -out or confirm a diagnosis.
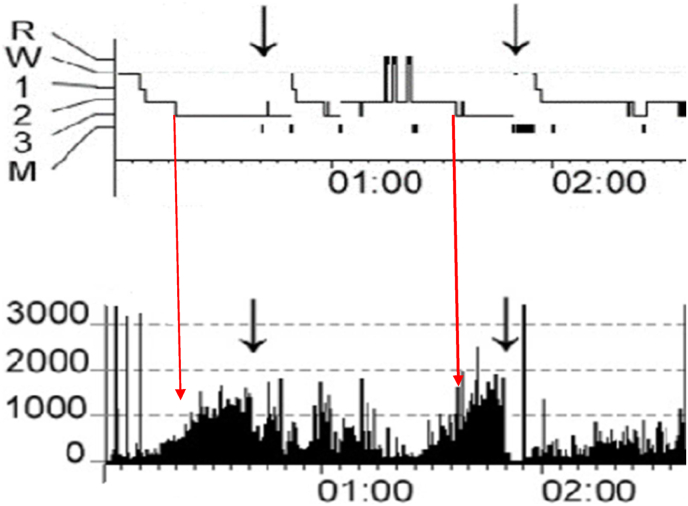
SWS defined by the presence of delta wave of amplitudes less than 75 uv. would occur earlier than the LSWS based on an epoch of currently defined N3. Both N2 and delta EEG may occur before the minimum requirements for N3 are met especially if the 75 uv amplitude rule is applied as required. This may indicate a change is needed for the definition of LSWS in forensic cases. Delta EEG activity measured via spectral analysis occurs earlier than the LSWS as determined by visual analysis (see Fig. 2). However, there is no specific level of Slow Wave Activity (SWA) or power in microvolts squared for the frequency band of 0.5–4.0 Hz analyzed that has been shown to be associated DOAs or are predictive of DOAs. However, initial increases in SWA have been noted to start within minutes of sleep onset although episodes of DOA occur later as the intensity increases. However, this level varies widely in the reported data [52].
Fig. 2.
Standard visual scoring of sleep stages compared with slow wave activity (SWA) acquired at the same time for the same subject – in power in microvolts squared in the 0.5–4.0 hz frequency. Histograms are combined and modified from Fig. 1, Fig. 2, for subject S6 pages 533-4 in Janusko et al. [51].
Black arrows indicate timing of an episode of sleepwalking or confusional arousal. Red arrows indicated the start of N3 in the upper tracing and its relationship to SWA at the same time. SWA occurs prior to the onset of N3.
Subject 6 is a clinically diagnosed with sleepwalking or confusional arousal with a mean age of 31.2 + 2.2 years. The subject was free of sleep disorders, kept a regular sleep/wake schedule, no psychotropic drug. Large arrows indicate SWA level at the time N3 is first scored.
Further, in forensic settings it cannot be assumed that N3 latency will be identical to that reported for normal controls or in untreated sleepwalkers who maintain a regular sleep/wake schedule with total sleep time in the normal range. The effects of the sleep laboratory, quantity of total sleep time for several days before the sleep study and withdrawal from alcohol and drugs all need to be taken into account. The circumstances of the charged episode cannot be duplicated in the sleep laboratory. Thus, the use of the LSWS in otherwise normal sleepers to individuals who are sleep deprived, living on disordered sleep/wake schedule, etc. may not be a fair comparison.
5. Conclusion
It is clear that the visual rules for scoring N3 were not devised, intended or are compatible with forensic applications such as in the Herman case. Nevertheless, even under current rules, published sleep laboratory studies have demonstrated mean LSWSs that are considerably shorter than 60–120 min and indeed may be l0 minutes or less even for subjects who are not sleep deprived. DOAs have been reported to occur during sleep stage N2 in the absence of delta EEG or SWS. Thus, SWS may not always be required for the occurrence of DOAs. Several different types of sleep deprivation have been shown to reduce LSWS while alcohol has only a limited effect.
The 75 uv amplitude requirement for determination of individual delta waves was formulated without considering how it might affect LSWS or forensic applications. The use of polysomnography for forensic use was not even considered in 1968 when R&K was first published. Indeed, there is no empirical evidence that a lower delta wave amplitude would not be just as effective as a priming factor for DOAs.
A review of the available empirical data and methodology suggests SWS as scored by R&K or current AASM rules may occur earlier than is often indicated and that the current AASM rules for determination of SWS may not reflect underlying delta wave EEG activity that could be related to DOA susceptibility or propensity. Reliance on sleep data from young normal controls is unlikely to be a proper point of reference in forensic cases. Thus, the latency to slow wave sleep cannot and should not be considered as the proverbial “smoking gun” type of evidence in criminal cases.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
References
- 1.Academy_of_Sleep_Medicine A. 2014. International classificatin of sleep disorders version 3: diagnostic and coding manual. [Google Scholar]
- 2.Berry R.B.Q.S., Abreu A.R., Bibbs M.L., DelRosso L., Harding S.M., Mao M., Plante D.T., Pressman M.R., Troester M.M. 6 ed. American Academy of Sleep Medicine.; Darien, IL: 2020. Vaughn the AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 3.Pressman M.R. Disorders of arousal from sleep and violent behavior: the role of physical contact and proximity. Sleep. 2007;30:1039–1047. doi: 10.1093/sleep/30.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schenck C.H., Arnulf I., Mahowald M.W. Sleep and sex: what can go wrong? A review of the literature on sleep related disorders and abnormal sexual behaviors and experiences. Sleep. 2007;30:683–702. doi: 10.1093/sleep/30.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pressman Mark., Ph.D. American Psychological Association; Washington, D.c.: 2018. Sleepwalking, Criminal behavior and reliable scientific evidence: a guide for experts. [Google Scholar]
- 6.Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 8.Borgman S. Dead asleep. Hulu2022.
- 9.Avidan A.Y. In: Principles and practice of sleep medicie. 6 ed. Krieger M., Roth, Thomas, Dement William C., editors. Elsevier; Philadelphia, PA: 2017. Non-rapid eye movement parasomnias: clinical spectrum, diagnostic features, and management; pp. 981–999. [Google Scholar]
- 10.Williams D.L., MacLean A.W., Cairns J. Dose-response effects of ethanol on the sleep of young women. J Stud Alcohol. 1983;44:515–523. doi: 10.15288/jsa.1983.44.515. [DOI] [PubMed] [Google Scholar]
- 11.Medicine AaoS . 2020. AASM facility standards of accreditation 2020 standard F-5 PSG scoring. [Google Scholar]
- 12.Rechtschaffen A KA. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Brain Information Service/Brain Research Institute. Los Angeles, CA1968.
- 13.Silber M.H., Ancoli-Israel S., Bonnet M.H., Chokroverty S., Grigg-Damberger M.M., Hirshkowitz M., et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3:121–131. [PubMed] [Google Scholar]
- 14.Joncas S., Zadra A., Paquet J., Montplaisir J. The value of sleep deprivation as a diagnostic tool in adult sleepwalkers. Neurology. 2002;58:936–940. doi: 10.1212/wnl.58.6.936. [DOI] [PubMed] [Google Scholar]
- 15.Kavey N.B., Whyte J., Resor S.R., Jr., Gidro-Frank S. Somnambulism in adults. Neurology. 1990;40:749–752. doi: 10.1212/wnl.40.5.749. [DOI] [PubMed] [Google Scholar]
- 16.Guilleminault C., Moscovitch A., Leger D. Forensic sleep medicine: nocturnal wandering and violence. Sleep. 1995;18:740–748. doi: 10.1093/sleep/18.9.740. [DOI] [PubMed] [Google Scholar]
- 17.Zucconi M., Oldani A., Ferini-Strambi L., Smirne S. Arousal fluctuations in non-rapid eye movement parasomnias: the role of cyclic alternating pattern as a measure of sleep instability. J Clin Neurophysiol. 1995;12:147–154. [PubMed] [Google Scholar]
- 18.Zadra A., Pilon M., Montplaisir J. Polysomnographic diagnosis of sleepwalking: effects of sleep deprivation. Ann Neurol. 2008;63:513–519. doi: 10.1002/ana.21339. [DOI] [PubMed] [Google Scholar]
- 19.Gillberg M., Akerstedt T. The dynamics of the first sleep cycle. Sleep. 1991;14:147–154. [PubMed] [Google Scholar]
- 20.Benoit O., Foret J., Bouard G. The time course of slow wave sleep and REM sleep in habitual long and short sleepers: effect of prior wakefulness. Hum Neurobiol. 1983;2:91–96. [PubMed] [Google Scholar]
- 21.Borbely A.A., Baumann F., Brandeis D., Strauch I., Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–495. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 22.Issa F.G., Sullivan C.E. The immediate effects of nasal continuous positive airway pressure treatment on sleep pattern in patients with obstructive sleep apnea syndrome. Electroencephalogr Clin Neurophysiol. 1986;63:10–17. doi: 10.1016/0013-4694(86)90056-8. [DOI] [PubMed] [Google Scholar]
- 23.Ratnavadivel R., Chau N., Stadler D., Yeo A., McEvoy R.D., Catcheside P.G. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009;5:519–524. [PMC free article] [PubMed] [Google Scholar]
- 24.Webb W.B., Agnew H.W., Jr. The effects of a chronic limitation of sleep length. Psychophysiology. 1974;11:265–274. doi: 10.1111/j.1469-8986.1974.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 25.Akerstedt T., Kecklund G., Ingre M., Lekander M., Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep. 2009;32:217–222. doi: 10.1093/sleep/32.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrara M., De Gennaro L., Curcio G., Cristiani R., Corvasce C., Bertini M. Regional differences of the human sleep electroencephalogram in response to selective slow-wave sleep deprivation. Cerebr Cortex. 2002;12:737–748. doi: 10.1093/cercor/12.7.737. [DOI] [PubMed] [Google Scholar]
- 27.Carskadon M.A., Dement W.C. Sleep loss in elderly volunteers. Sleep. 1985;8:207–221. doi: 10.1093/sleep/8.3.207. [DOI] [PubMed] [Google Scholar]
- 28.Arnedt J.T., Rohsenow D.J., Almeida A.B., Hunt S.K., Gokhale M., Gottlieb D.J., et al. Sleep following alcohol intoxication in healthy, young adults: effects of sex and family history of alcoholism. Alcohol Clin Exp Res. 2011;35:870–878. doi: 10.1111/j.1530-0277.2010.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Reen E., Jenni O.G., Carskadon M.A. Effects of alcohol on sleep and sleep Electroencephalogram in healthy young women. Alcohol Clin Exp Res. 2006;30:974–981. doi: 10.1111/j.1530-0277.2006.00111.x. [DOI] [PubMed] [Google Scholar]
- 30.Chan J.K., Trinder J., Andrewes H.E., Colrain I.M., Nicholas C.L. The acute effects of alcohol on sleep architecture in late adolescence. Alcohol Clin Exp Res. 2013;37:1720–1728. doi: 10.1111/acer.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meilman P.W., Stone J.E., Gaylor M.S., Turco J.H. Alcohol consumption by college undergraduates: current use and 10-year trends. J Stud Alcohol. 1990;51:389–395. doi: 10.15288/jsa.1990.51.389. [DOI] [PubMed] [Google Scholar]
- 32.Rundell O.H., Lester B.K., Griffiths W.J., Williams H.L. Alcohol and sleep in young adults. Psychopharmacologia. 1972;26:201–218. doi: 10.1007/BF00422697. [DOI] [PubMed] [Google Scholar]
- 33.MacLean A.W., Cairns J. Dose-response effects of ethanol on the sleep of young men. J Stud Alcohol. 1982;43:434–444. doi: 10.15288/jsa.1982.43.434. [DOI] [PubMed] [Google Scholar]
- 34.Rouhani S., Tran G., Leplaideur F., Durlach J., Poenaru S. EEG effects of a single low dose of ethanol on afternoon sleep in the nonalcohol-dependent adult. Alcohol. 1989;6:87–90. doi: 10.1016/0741-8329(89)90078-5. [DOI] [PubMed] [Google Scholar]
- 35.Lobo L.L., Tufik S. Effects of alcohol on sleep parameters of sleep-deprived healthy volunteers. Sleep. 1997;20:52–59. doi: 10.1093/sleep/20.1.52. [DOI] [PubMed] [Google Scholar]
- 36.Broughton R., Billings R., Cartwright R., Doucette D., Edmeads J., Edwardh M., et al. Homicidal somnambulism: a case report. Sleep. 1994;17:253–264. [PubMed] [Google Scholar]
- 37.Barros A., Uguccioni G., Salkin-Goux V., Leu-Semenescu S., Dodet P., Arnulf I. Simple behavioral criteria for the diagnosis of disorders of arousal. J Clin Sleep Med. 2020;16:121–128. doi: 10.5664/jcsm.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perrault R., Carrier J., Desautels A., Montplaisir J., Zadra A. Electroencephalographic slow waves prior to sleepwalking episodes. Sleep Med. 2014;15:1468–1472. doi: 10.1016/j.sleep.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 39.Stone B.M. Sleep and low doses of alcohol. Electroencephalogr Clin Neurophysiol. 1980;48:706–709. doi: 10.1016/0013-4694(80)90427-7. [DOI] [PubMed] [Google Scholar]
- 40.Schenck C.H., Mahowald M.W. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333–337. doi: 10.1016/S0002-9343(97)89493-4. [DOI] [PubMed] [Google Scholar]
- 41.Brunner D.P., Dijk D.J., Borbely A.A. Repeated partial sleep deprivation progressively changes in EEG during sleep and wakefulness. Sleep. 1993;16:100–113. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- 42.Tilley A.J., Wilkinson R.T. The effects of a restricted sleep regime on the composition of sleep and on performance. Psychophysiology. 1984;21:406–412. doi: 10.1111/j.1469-8986.1984.tb00217.x. [DOI] [PubMed] [Google Scholar]
- 43.Feinberg I., Baker T., Leder R., March J.D. Response of delta (0-3 Hz) EEG and eye movement density to a night with 100 minutes of sleep. Sleep. 1988;11:473–487. [PubMed] [Google Scholar]
- 44.Moses J.M., Johnson L.C., Naitoh P., Lubin A. Sleep stage deprivation and total sleep loss: effects on sleep behavior. Psychophysiology. 1975;12:141–146. doi: 10.1111/j.1469-8986.1975.tb01264.x. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds C.F., 3rd, Kupfer D.J., Hoch C.C., Stack J.A., Houck P.R., Berman S.R. Sleep deprivation in healthy elderly men and women: effects on mood and on sleep during recovery. Sleep. 1986;9:492–501. doi: 10.1093/sleep/9.4.492. [DOI] [PubMed] [Google Scholar]
- 46.Feinberg I., Floyd T.C., March J.D. Effects of sleep loss on delta (0.3-3 Hz) EEG and eye movement density: new observations and hypotheses. Electroencephalogr Clin Neurophysiol. 1987;67:217–221. doi: 10.1016/0013-4694(87)90019-8. [DOI] [PubMed] [Google Scholar]
- 47.Pressman M.R., Grunstein R.R., Mahowald M.W., Schenck C.H., Montplaisir J.Y., Bornemann M.C., et al. Alcohol and sleep review: flawed design, methods, and statistics cannot support conclusions. Alcohol Clin Exp Res. 2015;39:941–943. doi: 10.1111/acer.12712. [DOI] [PubMed] [Google Scholar]
- 48.Younes M., Kuna S.T., Pack A.I., Walsh J.K., Kushida C.A., Staley B., et al. Reliability of the American Academy of sleep medicine rules for assessing sleep depth in clinical practice. J Clin Sleep Med. 2018;14:205–213. doi: 10.5664/jcsm.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaudreau H., Joncas S., Zadra A., Montplaisir J. Dynamics of slow-wave activity during the NREM sleep of sleepwalkers and control subjects. Sleep. 2000;23:1–6. [PubMed] [Google Scholar]
- 50.Fisher C., Kahn E., Edwards A., Davis D.M. A psychophysiological study of nightmares and night terrors. The suppression of stage 4 night terrors with diazepam. Arch Gen Psychiatr. 1973;28:252–259. doi: 10.1001/archpsyc.1973.01750320082013. [DOI] [PubMed] [Google Scholar]
- 51.Agnew H.W., Jr., Webb W.B., Williams R.L. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 52.Januszko P., Niemcewicz S., Gajda T., Wolynczyk-Gmaj D., Piotrowska A.J., Gmaj B., et al. Sleepwalking episodes are preceded by arousal-related activation in the cingulate motor area: EEG current density imaging. Clin Neurophysiol. 2016;127:530–536. doi: 10.1016/j.clinph.2015.01.014. [DOI] [PubMed] [Google Scholar]