Abstract
This study aimed to investigate the effects of time access to post-hatch feeding on the growth performance, hormone secretion, intestinal morphology, and intestinal microbiota structure of broilers. A total of 900 broilers were randomly allocated to 3 treatment groups, with 6 replicates of 50 broilers each. The 3 treatments were: immediate feeding (Group 2 h), delayed access to feed for 24 h (Group 24 h), and delayed access to feed for 48 h (Group 48 h). The experiment lasted for 50 d. Results revealed that Group 2 h had a higher average daily gain (ADG) and average daily feed intake (ADFI) as well as a lower feed-to-gain ratio (F/G) than Group 48 h during the starter period (P < 0.05). Compared with Group 48 h, broilers in Group 2 h exhibited significantly elevated villus height (VH) and villus height to crypt depth ratio (VH: CD) in the duodenum, increased Occludin, and Claudin-1 mRNA expression in the jejunum but decreased crypt depth (CD) in the duodenum at 50 d (P < 0.05). Meanwhile, broilers in Groups 2 h and 24 h had increased glycogen (Gn) and protein (Pro) levels in breast muscle and TG levels in the liver, as well as a higher concentration of serum T3, T4, and IGF-1 compared with Group 48 h at 21 d (P < 0.05). Besides, intestinal microbiota consisted primarily of Firmicutes, Bacteroidetes, and Proteobacteria at the phylum level at 21 d and 50 d; at the genus level, broilers in Group 2 h displayed significantly reduced abundance of Escherichia at 21 d and Bacteroides at 50 d compared with Group 48 h (P < 0.05). Collectively, these findings signal that early post-hatch feeding measures, especially at 21 d, improve hormone secretion, intestinal morphology, and the growth performance of broilers by enhancing intestinal health and modulating the intestinal microbiota.
Key words: early post-hatch feeding, growth performance, metabolism, intestinal microbiota, broilers
INTRODUCTION
Contemporary broilers are chiefly subject to rapid growth and a high feed conversion ratio, resulting in a shortened feeding cycle and fast listing (Dixon, 2020; Güz et al., 2021). Accordingly, the nutritional and health status of broilers in the starter periods is increasingly instrumental to their health throughout the life cycle (Ravindran and Abdollahi, 2021). The period from late chick embryonic development to the first few days following hatching is a critical period for the development of the gastrointestinal tract and immune system in poultry (Wang et al., 2020a). Noy and Sklan (2001) established that chicks are more dependent on absorbable nutrients to cope with a turbulent external environment, while the utilization efficiency of three major nutrients (carbohydrates, protein, and lipid) is continuously improved. In contrast, Van der Wagt et al. (2020) reported that nutrient source gradually shifts from the internal yolk itself to exogenous feed, and this adjustment indirectly promotes the development of the digestive system in chicks. Moreover, the later exogenous feeding is initiated, the lower the absorption efficiency of the yolk by newborn chicks, and the poorer the growth and development during the yolk absorption period, ultimately influencing the growth homeostasis of the organism (Proszkowiec-Weglarz et al., 2019; Wang et al., 2020b).
After chicks hatch out of the incubation machine, there is an interval in the hatching window during which chicks are deprived of feeds and water for up to 24 h (Liu et al., 2020). Besides, owing to farm transportation and other factors, chicken feed deprivation time can attain 48 h or even 72 h (Boyner et al., 2021). Some previous studies have corroborated that long-term feed deprivation after hatching can reduce the weight of chick organs (Lamot et al., 2014), delay the development of the gastrointestinal tract (Liu et al., 2020), damage intestinal health and immune system development (Panda et al., 2015), and lower the survival rate of chicks (Wijnen et al., 2021). Besides, Proszkowiec-Weglarz et al. (2020) observed that delayed access to feed post-hatch may impact the structure and function of mucus and epithelial cells with tight junction, thereby indirectly affecting gut barrier function and overall health of the small intestine while reducing the absorption and utilization of carbohydrate energy molecules in the intestinal tract of broilers. However, feed early acquisition can effectively stimulate the absorption of residual yolk in chicks, especially the digestion and utilization of hydrophilic compounds such as glucose and protein (Wang et al., 2020b), and then ameliorate gastrointestinal development (Reicher et al., 2020), which is conducive to the growth and development of chicks and the maintenance of homeostasis (Jha et al., 2019, Lingens et al., 2021).
Nevertheless, it remains to be determined whether the early nutritional consequence of newborn chicks persists throughout the growth cycle of broilers (1–50 d) and the mechanism by which it affects intestinal health and intestinal microbiota structure. Therefore, the primary objective of the present work was to investigate the effects of early post-hatch feeding on the growth performance, hormone secretion, intestinal morphology, and intestinal microbiota composition of broilers.
MATERIALS AND METHODS
The present study followed Chinese guidelines for animal welfare and was approved by the Animal Care and Welfare Committee and the Scientific Ethical Committee of the Zhejiang University (No. ZJU2013105002, Hangzhou, China).
Experimental Design and Bird Husbandry
Hatching eggs were collected from breeders of 31-wk-old Lingnan Yellow at a local hatchery (Qunda Breeder Company, Jiaxing, China). A total of 900 chicks with similar body weight (43 g) hatched within 2 h were collected and randomly allocated to three groups with 6 replicates of 50 each after weighing. After placement at the farm (Xingjian Culture-Farm, Jiaxing, China), Group 2 h was immediately fed ad-libitum. Chicks in Groups 24 h and 48 h had delayed access to feed for 24 and 48 h, respectively. All chicks were reared on the floor with 5-cm deep wood shavings and were provided with water and food ad libitum after placement. The whole experiment lasted for 50 d with a light schedule of 23 h light and 1 h darkness. The temperature of the chicken house was set to 35°C on d 1, then decreased by 0.5°C each day, to a final temperature of 21 to 24°C with a humidity of 60 to 65%. The composition and nutrient concentration of the basal diet are listed in Table 1, which were formulated to meet the nutritional requirements recommended by the National Research Council (NRC, 1994). All chicks in each replicate were weighed individually after a 12 h feed deprivation at 21 d and 50d of age, and the consumption of feed by the chicks and body weight were recorded on the replicate basis to calculate average daily feed intake (ADFI), average daily gain (ADG), and feed to gain ratio (F/G) during the starter (1–21 d), grower (22–50 d), and overall (1–50 d) periods.
Table 1.
Composition and nutrient levels of the basal diets for broilers.
| Items | 1 to 21 d | 22 to 50 d |
|---|---|---|
| Ingredients (%) | ||
| Corn | 59.20 | 65.50 |
| Soybean meal | 29.00 | 23.00 |
| CGM | 6.00 | 6.00 |
| Soybean oil | 1.50 | 1.50 |
| NaCl | 0.30 | 0.30 |
| CaHPO4 | 1.70 | 1.50 |
| Limestone | 1.30 | 1.20 |
| Premix1 | 1.00 | 1.00 |
| Total | 100.0 | 100.0 |
| Nutrient levels2 (%) | ||
| ME (MJ/kg) | 12.35 | 12.68 |
| CP | 20.92 | 18.95 |
| Ca | 0.98 | 0.88 |
| TP | 0.65 | 0.60 |
| Lys | 1.10 | 0.95 |
| Met | 0.50 | 0.40 |
| Met+Cys | 0.85 | 0.72 |
Abbreviations: CGM, corn gluten meal; CP, crude protein; ME, metabolizable energy; TP, total phosphorus.
Supplied per kilogram of diet: vitamin A, 9,600 IU; vitamin D3, 2,700 IU; vitamin E, 36 mg; vitamin K3, 3.0 mg; vitamin B1, 3.0 mg; vitamin B2, 10.5 mg; vitamin B6, 4.2 mg; vitamin B12, 0.03 mg; folic acid, 1.5 mg; nicotinamide, 60 mg; D-calcium pantothenate, 18 mg; biotin, 0.225 mg; choline chloride, 1,000 mg; Fe, 80 mg; Cu, 8.0 mg; Mn, 80 mg; Zn, 60 mg; I, 0.35 mg; Se, 0.15 mg.
ME is calculated value, and other nutrient levels are the measured values.
Sample Collection
At 22 d and 51 d, four birds of moderate weight were randomly selected from each replicate (24 birds from each group) and weighted and slaughtered for sampling, respectively. Blood samples were collected from the underwing vein. After standing at 37°C for 2 h, the serum was separated by centrifugation to 4,000 × and stored in a refrigerator at −80°C for further analysis. The broilers were euthanized by cervical dislocation and immediately necropsied. Then, the birds were slaughtered to acquire the liver (part of the left liver lobe) and the left breast muscle samples, which were swiftly collected into self-sealing bags with corresponding labels and frozen at −80°C for subsequent analysis. Meanwhile, about 2 cm segment of the middle region of the duodenum was collected, flushed gently with ice-cold PBS (pH 7.4) to remove the intestinal contents, and immediately fixed in a 4% paraformaldehyde solution for histological measurement. Besides, approximately 2 cm segment of the mid-jejunum was fixed with 2.5% glutaraldehyde for microvillus analysis under a transmission electron microscope. Lastly, the residual jejunum was stored at −80°C for the gene expression analysis of tight junction proteins.
Measurements of Hormone and Primary Metabolite
The levels of triiodothyronine (T3), tetraiodothyronine (T4), and insulin-like growth factor 1 (IGF-1) in serum were quantified using an enzyme-linked immunosorbent (ELISA) assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's protocols. Besides, the concentrations of protein (Pro) and triglyceride (TG) in the liver, as well as glycogen (Gn) and protein (Pro) in the muscle were determined by a normal kit (Nanjing Jiancheng Bioengineering Institute).
Intestinal Morphological Analyses
After fixation in 4% paraformaldehyde for 24 h, the duodenal segments were dehydrated and embedded in paraffin blocks under standard procedures. The intestines were cut into 5-µm thick sections and then stained with hematoxylin-eosin. Photographs of the stained sections were taken through a Nikon microscope (Nikon Corp., Tokyo, Japan). The measurements of villus height (VH) and crypt depth (CD) were conducted with the Image-Pro software (MediaCybernetics, Rockville, MD) and the former was measured from the top of the villus to the junction of the villus crypt (Guo et al., 2020). The jejunum samples for transmission electron microscope (TEM) observation were processed according to the previously reported protocols (Mo et al., 2019). Samples were observed using a Hitachi Model H-7650 TEM (Hitachi, Tokyo, Japan).
Quantitative Reverse-Transcription PCR Analysis
Jejunum samples were determined for the mRNA expression of Occludin and Claudin-1. Total RNA was extracted from the jejunum samples using Trizol reagent kit (TaKaRa Biotechnology, Beijing, China) according to the manufacturer's instructions. RNA was inversely converted to cDNA using the TaKaRa PrimeScript RT Reagent Kit (TaKaRa Biotechnology). quantitative reverse-transcription PCR (qRT-PCR) was carried out using TB Green Premix Ex Taq TM (TaKaRa Biotechnology) in CFX96 Real-Time PCR Detection System (Bio-Rad., CA) with specific bird primers (Table 2). The results were normalized to the housekeeping 18S ribosomal RNA gene using the 2–ΔΔCt method (Livak and Schmittgen., 2001).
Table 2.
Primers used for the genes.
| Genes | Forward | Reverse |
|---|---|---|
| 18S ribosomal RNA | 5’-ATTCCGATAACGAACGAGACT-3’ | 5’-GGACATCTAAGGGCATCACA-3’ |
| Occludin | 5’-TCATCGCCTCCATCGTCTAC-3’ | 5’-TCTTACTGCGCGTCTTCTGG-3’ |
| Claudin-1 | 5’-TGGAGGATGACCAGGTGAAGA-3’ | 5’-CGAGCCACTCTGTTGCCATA-3’ |
16S rDNA Sequencing and Gut Microbiota Analysis
Total DNA of the gut microbiota was extracted from the cecum chyme samples, and 16S rDNA was amplified. Sequencing was conducted on an Illumina Miseq platform (LC-Bio Technology Co., Ltd, Hangzhou, China) based on the version of QIIME 1. Amplicon data were denoised using Vsearchv (2.3.4), and clustered to present a 97% sequence identity. All the results were based on the OTUs. OTUs abundance was normalized by the SILVA classifier. Alpha diversity and Beta diversity of all the samples were calculated by QIIME1. Sequence alignment was performed by Blast, and OTUs sequences were annotated in the SILVA database. The diagrams were illustrated by the R package (v3.5.2).
Statistical Analysis
Data from the present study were analyzed by one-way ANOVA using SPSS statistical software (version 20.0 for Windows; SPSS Inc., Chicago, IL). Differences among treatments were examined using Tukey's multiple range tests, and a probability of P < 0.05 was considered to be significant. Results were presented as standard deviation (SD). Figures were made by GraphPad Prism 8.00 software (GraphPad Software, San Diego, CA).
RESULTS
Growth Performance
Compared with Group 48 h, the ADFI and ADG of broilers were significantly increased in Group 2 during the starter period (P < 0.05; Table 3). Moreover, Group 2 h and Group 24 h had lower F/G than Group 48 h during the starter period (P < 0.05). However, there were no differences in ADG, ADFI, and F/G among three groups during the grower period and the overall periods, but broilers fed early had higher BW, ADG, and ADFI (P > 0.05).
Table 3.
Effects of initial feeding time on growth performance and feed utilization in broilers1.
| Items | Treatments2 |
P-value | ||
|---|---|---|---|---|
| Group h | Group 24 h | Group 48 h | ||
| Starter (1 to 21 d) | ||||
| 1d BW(g) | 42.97 ± 0.45 | 43.03 ± 0.45 | 42.89 ± 0.71 | 0.936 |
| 21d BW(g) | 474.1 ± 27.53a | 455.0 ± 16.05ab | 421.1 ± 20.77b | 0.011 |
| ADG (g/d) | 20.53 ± 1.29a | 19.62 ± 0.75ab | 18.02 ± 1.00b | 0.011 |
| ADFI (g/d) | 28.45 ± 1.86a | 27.00 ± 0.73ab | 25.76 ± 1.22b | 0.031 |
| F/G | 1.386 ± 0.01b | 1.377 ± 0.03b | 1.430 ± 0.02a | 0.008 |
| Grower (22 to 50 d) | ||||
| 50d BW(g) | 1744 ± 118.72 | 1723 ± 70.54 | 1674 ± 122.38 | 0.519 |
| ADG (g/d) | 44.19 ± 2.99 | 43.74 ± 2.18 | 42.84 ± 3.53 | 0.729 |
| ADFI (g/d) | 111.6 ± 4.22 | 109.3 ± 109.25 | 106.7 ± 7.51 | 0.321 |
| F/G | 2.530 ± 0.09 | 2.498 ± 0.06 | 2.495 ± 0.04 | 0.615 |
| Overall (1 to 50 d) | ||||
| ADG (g/d) | 34.02 ± 2.37 | 33.61 ± 33.61 | 32.61 ± 2.45 | 0.516 |
| ADFI (g/d) | 74.33 ± 3.94 | 72.90 ± 2.68 | 70.97 ± 4.69 | 0.345 |
| F/G | 2.188 ± 0.05 | 2.170 ± 0.02 | 2.178 ± 0.03 | 0.694 |
Means with different superscript differ significantly (P < 0.05) within a same row.
Each group n = 6.
2 h group (immediately feeding), 24 h (delayed access to feed for 24 h), 48 h (delayed access to feed for 48 h).
Primary Metabolites and Serum Hormones
Compared with Group 48 h, significantly increased levels of Gn and Pro were observed in breast muscle as well as TG levels in liver in Groups 2 h and 24 h at 21 d (P < 0.05; Figures 1A–C). Meanwhile, Group 2 h and Group 24 h had higher concentrations of serum T3, T4, and IGF-1 than Group 48 h (P < 0.05; Figures 1E–G). Nevertheless, there were no statistically significant differences in Gn and Pro contents of muscle, hepatic Pro and TG levels, as well as serum T3 and T4 concentrations between the groups (P > 0.05) at 50 d.
Figure 1.
Effects of initial feeding time on primary metabolite contents and hormone levels. (A) Contents of glycogen in breast muscle; (B) contents of protein in breast muscle; (C) contents of triglyceride in liver; (D) Contents of protein in liver; (E) concentration of T3 in serum; (F) concentration of T4 in serum; (G) concentration of IGF-1 in serum. The different letters represent significant differences among each group (P < 0.05). Mean values with their SD (n = 6). a, b, c Mean values within a row with unlike letters are significantly different (P < 0.05). Abbreviations: Gn, glycogen; Group 2 h, immediately feeding; Group 24 h, delayed access to feed for 24 h; Group 48 h, delayed access to feed for 48 h; IGF-1, insulin-like growth factor 1; Pro, protein; TG, triglyceride; T3, triiodothyronine; T4, thyroxine.
Small Intestinal Histomorphology
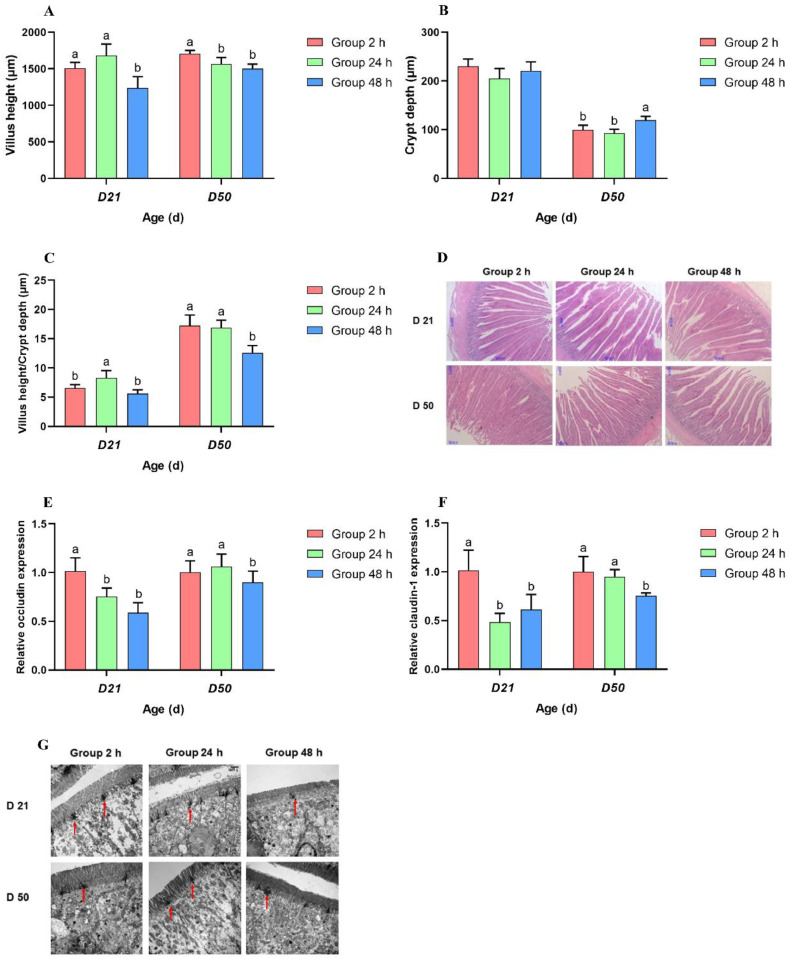
Compared with the Group 48 h, lower levels of CD but increased VH:CD values were found in the duodenum of broilers in Group 2 h and Group 24 h at 50 d (P < 0.05; Figures 2B–D). Meanwhile, Group 2 h had higher VH values in the duodenum of broilers than Group 24 h and Group 48 h at 50 d (P < 0.05); Figures 2A and D). Besides, there were no differences in duodenal CD and VH:CD values at 21 d (P > 0.05; Figures 2B–D)).
Figure 2.
Effects of initial feeding time on intestinal health. (A) Villus height in duodenum; (B) crypt depth in duodenum; (C) villus height to crypt depth ratio in the duodenum; (D) light microscopy of the cross-sections of the duodenum (40 ×); (E) occludin mRNA abundance in jejunum; (F) claudin-1 mRNA abundance in jejunum; (G) transmission electron-photomicrograph of tight junction in the jejunum (12,000 ×), red arrow means tight junction and adhesion belt. Mean values with their SD (n = 6). a, b Mean values within a row with unlike letters are significantly different (P < 0.05). Abbreviations: Group 2 h, immediately feeding; Group 24 h, delayed access to feed for 24 h; and Group 48 h, delayed access to feed for 48 h.
Jejunum Tight Junction Protein Expression
Compared with Group 48 h and Group 24 h, the mRNA levels of Occludin and Claudin-1 in the jejunal epithelium were significantly increased in Group 2 h at 21 d. Meanwhile, Group 48 h showed lower mRNA levels of Occludin and Claudin-1 in the jejunal epithelium in comparison with Groups 2 h and 24 h at 50 d (P > 0.05; Figures 2E and F). Moreover, ultrastructural analysis of the jejunum specimens displayed that the microvilli on the cell surface were well-arranged, and the marginal zone of the tight junction was clear and complete in Group 2 h at 21 d. In the corresponding period, the microvilli on the cell surface of Group 24 h and 48 h were shorter in length and looser in arrangement relative to Group 2h. However, broilers with delayed access to feed for 24 h or 48 h showed a normal arrangement of microvilli compared with that in immediate feeding at 50 d (Figure 2G).
16S rDNA Sequencing of Caecal Microbiota
On the one hand, the α-diversity index was used to evaluate the richness and species diversity of gut microbiota. As illustrated in Figure 3A, the Chao1 index and observed species index richness of the Group 48 h were significantly lower than those of Group 2 h at 21 d (P < 0.05). On the other hand, β-diversity is a metric for comparing the similarity of bacterial composition between groups, as measured by the distance between them. Figure 3B depicts that Group 48 h had a different microbiota structure compared with Group 2 h at 21 d (P < 0.01). The intestinal microbiota predominantly comprised Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria (>95%) at the phylum level at 21 d, while the dominant populations (relative abundances >5%) in the intestinal microbiota of the chickens were Lachnospiraceae, Ruminococcaceae, Alistipes, Faecaligranulum, and Subbdoligranulum at the genus level at 21d. Besides, compared with Group 48h, significantly decreased the abundance of Proteobacteria at the phylum level and Escherichia at the genus level were observed in Group 2 h at 2 1d (P < 0.05; Figures 4C and D).
Figure 3.
Effects of initial feeding time on the composition of the gut microbiota at 21d. (A) α-diversity of the Observed species index(a) and Chao1 index(b); (B) β-diversity assessed by PCoA analysis; (C) column diagram of the microbial composition at the phylum level(a), and the relative abundances of Proteobacteria(b); and (D) column diagram of the microbial composition at the genus level (a), and the relative abundances of Escherichia(b). Each plot represents one sample (n = 10). The different letters represent significant differences among each group (P < 0.05). Abbreviations: 2 h, immediately feeding; 24 h, delayed access to feed for 24 h; and 48 h, delayed access to feed for 48 h.
Figure 4.
Effects of initial feeding time on the composition of the gut microbiota at 50d. (A) α-diversity of the observed species index(a) and Chao1 index(b); (B) β-diversity assessed by PCoA analysis; (C) column diagram of the microbial composition at the phylum level(a), and the relative abundances of Actinobacteria (b); and (D) column diagram of the microbial composition at the genus level(a), and the relative abundances of Bacteroides (b). Each plot represents one sample (n = 10). The different letters represent significant differences among each group (P < 0.05). Abbreviation: 2 h, immediately feeding; 24 h, delayed access to feed for 24 h; and 48 h, delayed access to feed for 48 h.
Figure 4A delineates that the Chao1 and Observed species index richness of Group 48 h were significantly lower than those of the Group 2 h and Group 24 h at 50 d (P < 0.01). The results exposed that the gut microbiota structure was significantly altered in Group 48 h at 50 d (P < 0.01), whereas that in Group 2 h and Group 24 h were similar, as shown in Figure 4B. Figure 4(C) depicts the bacterial composition at the phylum level; the gut microbiota at 50 d was mainly composed of Firmicutes and Bacteroidetes. Interestingly, the relative abundance of Actinobacteria at the phylum level (P < 0.05) was significantly decreased in Group 2 h. At the genus level, the dominant populations (relative abundances >5%) in the intestinal microbiota of the chickens were essentially composed of Lachnospiraceae, Ruminococcaceae, Alistipes, and Acetivibrio (Figure 4D). Besides, the relative abundance of Bacteroides was significantly decreased in Group 2 h (P < 0.05). These findings imply that early feeding could increase microbial community diversity and optimize the structure of the microbial flora.
DISCUSSION
With advances of breeding genetics and the shortening of the feeding period, the nutritional status of broilers following shell release is particularly significant for the overall growth performance (Zuidhof et al., 2014). Jha et al. (2019) reported that early nutrition is a nutritional management approach used to protect the developing embryo or the newborn chick from acquiring the required nutrients before the digestive system matures. Several studies have established that early post-hatch feeding treatment can increase broiler body weight and feed intake at 7 d (Lamot et al., 2014), whereas early fasting for 24 h is detrimental to the start phase weight gain (Zulkifli et al., 2016). Additionally, Willemsen et al. (2010) also revealed that chick growth performance was reduced by early fasting, which is not conducive to chest muscle production and can result in death in extremely circumstances. Herein, our results determined that early feeding significantly increased ADG and ADFI and decreased F/G during the starter period. The growth performance of Group 2 h was superior to that of Group 24 h, which is consistent with the above findings. However, there were no significant differences in the ADG and FCR of the three treatments during the overall period; nonetheless, the growth performance of Group 2 h was still better than Group 48 h, indicating that compensatory growth occurred in the fasting group but did not return to that of the early post-hatch feeding group. Similarly, Zubair and Leeson (1996) uncovered that broilers may receive insufficient compensation gain from feed management. The earlier the chicken fasted, the less resistant it was, and the longer the adjustment time required to correct for development.
Earlier studies have shown that early post-hatch feeding promotes the absorption of leftover egg yolk in chicks, as well as the absorption and utilization of hydrophilic substances such as Gn and Pro, thereby stimulating early gastrointestinal development and broiler performance (Bigot et al., 2003, Noy and Uni, 2010), whereas delayed nutrition lowers glycogen levels in the breast muscle (Kornasio et al., 2011) and liver of chicks (Wang et al., 2014). Besides, Knowles et al. (1995) showed that plasma glucose and total plasma protein levels were reduced in birds deprived of food or water. Early post-hatch feeding significantly boosted the levels of Gn and Pro in breast muscle as well as TG levels in the liver at 21 d, which is in line with previous studies. T3 is distinguished by high activity in vivo at a low concentration, while thyroxine deiodinases (DIOs) can catalyze the conversion of T4 to T3, regulate body metabolism, stimulate protein synthesis and transport, facilitate the metabolism of other nutrients and induce animal growth and development (Lin et al., 2014; Mojadadi et al., 2021). In this study, early nutrition raised the serum T3 and T4 concentrations during the starter stage, which concurs with the findings of Lu et al. (2007), who discovered a positive correlation between chick BW and T3 and T4 levels. Meanwhile, IGF-1 can mediate the growth-promoting impact of GH (Vélez and Unniappan, 2021), and given that the liver is the most critical site for IGF-1 synthesis, it is a key target tissue for GH ( Ohlsson et al., 2009). According to relevant research, IGF-1 promotes skeletal muscle growth by increasing the rate of protein synthesis, and its concentration is usually positively related to broiler body weight (Boschiero et al., 2013; Wen et al., 2014). Moreover, Kita et al. (2002) also identified that feed fasting lowered plasma IGF-1 concentrations in chick. In our experiment, early post-hatch feeding improved serum IGF-1 levels at 21 d, supporting other studies that demonstrated that early feeding encouraged broiler growth and development in the early growth stage. Notably, IGF-1 levels in Group 24 h were significantly higher than in the other 2 groups at 50 d, which was possibly attributable to compensatory growth in the 24 h open feeding group.
The morphology of intestine has become a typical biomarker of intestinal development (Lilburn and Loeffler, 2015; Lv et al., 2022). Following chick hatching, the intestine length, weight, and digestive enzyme activity rapidly increase, whereas fasting can impair the growth and development of the intestine and hence limit food absorption (Yegani and Korver, 2008; Jha and Berrocoso, 2015; Liu et al., 2020). However, early nourishment can accelerate yolk absorption, stimulate intestinal health and maintain healthy growth (Henderson et al., 2008). Previous research uncovered that the villus surface area of the duodenum and VH of the duodenum and jejunum of broilers declined after fasting(Gonzales et al., 2003; Potturi et al., 2005; Mahmoud and Edens, 2012). In comparison, our research demonstrated that early post-hatch feeding significantly enhanced the VH/CD of broilers over the course of the study, suggesting that early feeding can stimulate and maintain intestine growth, digestion, and absorption.
Gastrointestinal epithelial cells are the main barrier between the body and the external environment, and can prevent hazardous substances such as bacteria and endotoxins from entering the bloodstream and participating in bodily circulation through the gut mucosa (Ornelas et al., 2022). Meanwhile, the impenetrable connections between adjacent intestinal epithelial cells that make it impossible for massive molecules to pass through are known as tight junctions (Van Itallie and Anderson, 2006). However, studies on the effects of early post-hatch fasting on intestinal barrier function are limited. Horn et al. (2014, 2017) discovered that water and feed restriction for 24 h, or water deprivation for 24 h lowered the mRNA expression of the tightly related genes Occludin, Claudin-1, and ZO-1 in weaned pigs. Additionally, Gilani et al. (2017) noted that early fasting substantially increased intestinal permeability in broilers after 21 d. In our experiment, early post-hatch feeding enhanced the mRNA expression level of Occludin and Claudin-1 of jejunum epithelial tight junction genes, which is analogous to previous works. Furthermore, Reicher et al. (2020) shared that intestinal morphology ultrastructure is a powerful part to evaluate intestinal health of broilers, microvilli clumps, and crypt structure anomalies of unhealthy animals. The results of our research also showed that the jejunal microvilli in the early feeding group were well-arranged, and the marginal zone of the tight junction was clear and complete in Group 2 h (2 h>24 h>48 h). While the microvilli on the surface of jejunum epithelial cells in each group displayed a neat arrangement at 50d, with complete close-connection and increased density, only the microvilli length was shorter in Group 48h. The results revealed that early post-hatch feeding extensively enhanced the morphology of jejunal villi in the early stage, and the compensatory growth adaptation to the external environment appeared broiler suited to in the later period, which was consistent with the growth performance.
Broiler intestinal microbiota colonization occurred chiefly in the early stages after hatching, with host and environmental factors impacting the makeup and activity of future microbiota (Kers et al., 2018). Moreover, fluctuations in gut microbiota diversity, composition, and general community structure of chickens are caused by differences in feedstuffs, digestion of substances, and eating or feeding behaviors ( Tan et al., 2019; Berrocoso et al., 2017, Yadav and Jha, 2019). Furthermore, a relatively stable and sophisticated gut microbiota evolved throughout time (Kogut, 2022). In our study, the intestinal microflora richness was higher in Group 2 h compared with Group 48 h, and the community structure composition exhibited significant variations. Additionally, after environmental adaptation, the bacterial community structure of Group 24 h shifted from between Group 2 h and Group 48 h to near to Group 2 h. These findings suggest that various feeding times influence the variety, composition, and overall community structure of the chicken intestinal microbiota. Additionally, the early post-hatch feeding group provides a species-rich microecosystem to withstand external stressors and maintain the healthy development of the intestinal tract (Clarke et al., 2014, Valdes et al., 2018 ).
Similar to previous studies, the top three most abundant bacteria at the phylum level were Firmicutes, Bacteroidetes, and Proteobacteria ( Yadav et al., 2021). In addition, our research found that early post-hatch feeding lowered the relative abundance of Proteobacteria and Escherichia in the starter stage. The former is a gram-negative bacterium that includes numerous significant pathogens such as Salmonella, Vibrio, and Helicobacter, as well as several Cyanobacteria species, which can create a variety of neurotoxins that cause sickness (Codd et al., 2005). Dai et al. (2018) pointed out that a lower percentage of Proteobacteria phylum indicates a healthy intestinal environment and probably contributes to the higher performance observed in poultry. The Escherichia genus belongs to the Proteobacteria family and is composed of Escherichia albertii, E. fergusonii, 5 cryptic Escherichia clades, and E. coli sensu stricto (Beghain et al., 2018). More importantly, Mellata (2013) revealed that most Escherichia members have detrimental effects on maintaining the health of organisms. Dai et al. (2018) reported that microbial species richness and diversity in caeca were considerably reduced in co-infected birds, whereas the abundance of the Escherichia, Helicobacter, and Bacteroides groups was relatively higher. Moreover, Escherichia coli in the cecal lumen penetrated deeper layers in infected birds, which eventually led to death (Abdelhamid et al., 2020). In brief, early post-hatch feeding inhibited the growth of harmful bacteria and improved organic intestinal development throughout the early phases of development in the starter phase. In line with earlier studies, adult poultry intestinal microbiota is dominated by Bacteroidetes and Firmicutes, whereas Actinobacteria and Proteobacteria are present only in minor proportions (Zhai et al., 2020 2021). Conversely, feed deprivation for 48 h significantly raised the relative abundance of Actinobacteria and Bacteroides at 50 d in our research. Barka et al. (2015) reported that Actinobacteria are Gram-positive bacteria that constitute one of the largest bacterial phyla, and they are ubiquitously distributed globally. The abundant genes for the metabolism of xenobiotics, terpenoids, and polyketides in the chicken gut are relevant to Actinobacteria, which decompose organic matter and create diverse natural medicines, enzymes, and potentially vast secondary metabolites (Ventura et al., 2007). Besides, researchers have discovered that a range of Bacteroidetes members play a favorable role in broiler digestion by improving nutrient digestion and absorption, and that the quantity of Bacteroidetes is positively correlated with broiler growth (Li et al., 2016; Huang et al., 2021; Wang et al., 2021). In short, the high abundance of Actinobacteria and Bacteroides in Group 48 h at 50 d was related to compensatory expansion, and this intricate mechanism warrants further exploration.
In conclusion, early feeding after hatching can regulate hormone secretion and material metabolism and promote the digestion and absorption of feed-in broilers by optimizing the structure of intestinal microflora and improving intestinal development, thereby enhancing the growth performance of broilers. Notably, age-related compensatory growth occurred in the feed deprivation groups to allow the chickens to adapt to their environment.
ACKNOWLEDGMENTS
This study was supported by Collaborative Extension Plan of Major Agricultural Technologies in Zhejiang Province (project: 2021XTTGXM04) and China Agriculture Research System of MOF and MARA (project: CARS-41, Beijing, China).
Disclosures
No conflict of interest exits in the submission of this manuscript, and the manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
REFERENCES
- Abdelhamid M.K., Quijada N.M., Dzieciol M., Hatfaludi T., Bilic I., Selberherr E., Liebhart D., Hess C., Hess M., Paudel S. Co-infection of chicken layers with histomonas meleagridis and avian pathogenic escherichia coli is associated with dysbiosis, cecal colonization and translocation of the bacteria from the gut lumen. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.586437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barka E.A., Vatsa P., Sanchez L., Gaveau-Vaillant N., Jacquard C., Meier-Kolthoff J.P., Klenk H.P., Clément C., Ouhdouch Y., van Wezel G.P. Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 2015;80:1–43. doi: 10.1128/MMBR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghain J., Bridier-Nahmias A., Nagard H.Le, Denamur E., Clermont O. ClermonTyping: an easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microb. Genom. 2018;4 doi: 10.1099/mgen.0.000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocoso J.D., Kida R., Singh A.K., Kim Y.S., Jha R. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken. Poult. Sci. 2017;96:1575–1580. doi: 10.3382/ps/pew430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigot K., Mignon-Grasteau S., Picard M., Tesseraud S. Effects of delayed feed intake on body, intestine, and muscle development in neonate broilers. Poult. Sci. 2003;82:781–788. doi: 10.1093/ps/82.5.781. [DOI] [PubMed] [Google Scholar]
- Boschiero C., Jorge E.C., Ninov K., Nones K., do Rosário M.F., Coutinho L.L., Ledur M.C., Burt D.W., Moura A.S. Association of IGF1 and KDM5A polymorphisms with performance, fatness and carcass traits in chickens. J. Appl. Genet. 2013;54:103–112. doi: 10.1007/s13353-012-0129-6. [DOI] [PubMed] [Google Scholar]
- Boyner M., Ivarsson E., Franko M.A., Rezaei M., Wall H. Effect of hatching time on time to first feed intake, organ development, enzymatic activity and growth in broiler chicks hatched on-farm. Animal. 2021;15 doi: 10.1016/j.animal.2020.100083. [DOI] [PubMed] [Google Scholar]
- Clarke S.F., Murphy E.F., O’Sullivan O., Lucey A.J., Humphreys M., Hogan A., Hayes P., O’Reilly M., Jeffery I.B., Wood-Martin R., Kerins D.M., Quigley E., Ross R.P., O’Toole P.W., Molloy M.G., Falvey E., Shanahan F., Cotter P.D. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- Codd G.A., Morrison L.F., Metcalf J.S. Cyanobacterial toxins: risk management for health protection. Toxicol. Appl. Pharmacol. 2005;203:264–272. doi: 10.1016/j.taap.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Dai P., Yan Z., Ma S., Yang Y., Wang Q., Hou C., Wu Y., Liu Y., Diao Q. The herbicide glyphosate negatively affects midgut bacterial communities and survival of honey bee during larvae reared in vitro. J. Agric. Food Chem. 2018;66:7786–7793. doi: 10.1021/acs.jafc.8b02212. [DOI] [PubMed] [Google Scholar]
- Dixon L.M. Slow and steady wins the race: The behaviour and welfare of commercial faster growing broiler breeds compared to a commercial slower growing breed. PLoS One. 2020;15 doi: 10.1371/journal.pone.0231006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani S., Howarth G.S., Kitessa S.M., Tran C.D., Forder R.E.A., Hughes R.J. New biomarkers for increased intestinal permeability induced by dextran sodium sulphate and fasting in chickens. Anim. Physiol. Anim. Nutr. (Berl). 2017;101:e237–e245. doi: 10.1111/jpn.12596. [DOI] [PubMed] [Google Scholar]
- Gonzales E., Kondo N., Saldanha E.S., Loddy M.M., Careghi C., Decuypere E. Performance and physiological parameters of broiler chickens subjected to fasting on the neonatal period. Poult. Sci. 2003;82:1250–1256. doi: 10.1093/ps/82.8.1250. [DOI] [PubMed] [Google Scholar]
- Guo M., Li M., Zhang C., Zhang X., Wu Y. Dietary administration of the bacillus subtilis enhances immune responses and disease resistance in chickens. Front. Microbiol. 2020;11:1768. doi: 10.3389/fmicb.2020.01768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güz B.C., de Jong I.C., Da Silva C.S., Veldkamp F., Kemp B., Molenaar R., van den Brand H. Effects of pen enrichment on leg health of fast and slower-growing broiler chickens. PLoS One. 2021;16 doi: 10.1371/journal.pone.0254462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S.N., Vicente J.L., Pixley C.M., Hargis B.M., Tellez G. Effect of an early nutritional supplement on broiler performance. Int. J. Poult. Sci. 2008;7:211–214. [Google Scholar]
- Horn N., Miller G., Ajuwon K.M., Adeola O. Ability of garlic-derived diallyl disulfide and diallyl trisulfide supplemented by oral gavage to mitigate effects of an acute postweaning feed and water deprivation event in nursery pigs. J. Anim. Sci. 2017;95:3579–3590. doi: 10.2527/jas.2017.1545. [DOI] [PubMed] [Google Scholar]
- Horn N., Ruch F., Miller G., Ajuwon K.M., Adeola O. Impact of acute water and feed deprivation events on growth performance, intestinal characteristics, and serum stress markers in weaned pigs. J. Anim. Sci. 2014;92:4407–4416. doi: 10.2527/jas.2014-7673. [DOI] [PubMed] [Google Scholar]
- Huang Y., Lv H., Song Y., Sun C., Zhang Z., Chen S. Community composition of cecal microbiota in commercial yellow broilers with high and low feed efficiencies. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Berrocoso J.D. Review: dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal. 2015;9:1441–1452. doi: 10.1017/S1751731115000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Singh A.K., Yadav S., Berrocoso J.F.D. Early Nutrition Programming (in ovo and Post-hatch Feeding) as a Strategy to Modulate Gut Health of Poultry [e-pub ahead of print] Front Vet. Sci. 2019;6 doi: 10.3389/fvets.2019.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kers J.G, Velkers F.C., Fischer E.A.J., Hermes G.D.A., Stegeman J.A., Smidt H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018;9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita K., Nagao K., Taneda N., Inagaki Y., Hirano K., Shibata T., Yaman M.A., Conlon M.A., Okumura J. Insulin-like growth factor binding protein-2 gene expression can be regulated by diet manipulation in several tissues of young chickens. J. Nutr. 2002;132:145–151. doi: 10.1093/jn/132.2.145. [DOI] [PubMed] [Google Scholar]
- Knowles T.G., Warriss P.D., Brown S.N., Edwards J.E., Mitchell M.A. Response of broilers to deprivation of food and water for 24 hours. Br. Vet. J. 1995;151:197–202. doi: 10.1016/s0007-1935(95)80011-5. [DOI] [PubMed] [Google Scholar]
- Kogut M.H. Role of diet-microbiota interactions in precision nutrition of the chicken: facts, gaps, and new concepts [e-pub ahead of print] Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornasio R., Halevy O., Kedar O., Uni Z. Effect of in ovo feeding and its interaction with timing of first feed on glycogen reserves, muscle growth, and body weight. Poult. Sci. 2011;90:1467–1477. doi: 10.3382/ps.2010-01080. [DOI] [PubMed] [Google Scholar]
- Lamot D.M., van de Linde I.B., Molenaar R., van der Pol C.W., Wijtten P.J., Kemp B., van den Brand H. Effects of moment of hatch and feed access on chicken development. Poult. Sci. 2014;93:2604–2614. doi: 10.3382/ps.2014-04123. [DOI] [PubMed] [Google Scholar]
- Li Y., Xu Q., Huang Z., Lv L., Liu X., Yin C., Yan H., Yuan J. Effect of bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 2016;120:195–204. doi: 10.1111/jam.12972. [DOI] [PubMed] [Google Scholar]
- Lilburn M.S., Loeffler S. Early intestinal growth and development in poultry. Poult. Sci. 2015;94:1569–1576. doi: 10.3382/ps/pev104. [DOI] [PubMed] [Google Scholar]
- Lin S.L., Wang C.W., Tan S.R., Liang Y., Yao H.D., Zhang Z.W., Xu S.W. Selenium deficiency inhibits the conversion of thyroidal thyroxine (T4) to Triiodothyronine (T3) in chicken thyroids. Biol. Trace Elem. Res. 2014;161:263–271. doi: 10.1007/s12011-014-0083-8. [DOI] [PubMed] [Google Scholar]
- Lingens J.B., Abd El-Wahab A., Ahmed M.F.E., Schubert D.C., Sürie C., Visscher C. Effects of early nutrition of hatched chicks on welfare and growth performance: a pilot study. Animals (Basel) 2021;11:2888. doi: 10.3390/ani11102888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Jia M., Wong E.A. Delayed access to feed affects broiler small intestinal morphology and goblet cell ontogeny. Poult. Sci. 2020;99:5275–5285. doi: 10.1016/j.psj.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu J.W., McMurtry J.P., Coon C.N. Developmental changes of plasma insulin, glucagon, insulin-like growth factors, thyroid hormones, and glucose concentrations in chick embryos and hatched chicks. Poult. Sci. 2007;86:673–683. doi: 10.1093/ps/86.4.673. [DOI] [PubMed] [Google Scholar]
- Lv J., Guo L., Chen B., Hao K., Ma H., Liu Y., Min Y. Effects of different probiotic fermented feeds on production performance and intestinal health of laying hens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud K.Z., Edens F.W. Breeder age affects small intestine development of broiler chicks with immediate or delayed access to feed. Br. Poult. Sci. 2012;53:32–41. doi: 10.1080/00071668.2011.652596. [DOI] [PubMed] [Google Scholar]
- Mellata M. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013;10:916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Q., Fu A., Deng L., Zhao M., Li Y., Zhang H., Feng F. High-dose glycerol monolaurate up-regulated beneficial indigenous microbiota without inducing metabolic dysfunction and systemic inflammation: new insights into its antimicrobial potential. Nutrients. 2019;11:1981. doi: 10.3390/nu11091981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojadadi A., Au A., Salah W., Witting P., Ahmad G. Role for selenium in metabolic homeostasis and human reproduction. Nutrients. 2021;13:3256. doi: 10.3390/nu13093256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 9th ed. National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Noy Y., Sklan D. Yolk and exogenous feed utilization in the posthatch chick. Poult. Sci. 2001;80:1490–1495. doi: 10.1093/ps/80.10.1490. [DOI] [PubMed] [Google Scholar]
- Noy Y., Uni Z. Early nutritional strategies. Worlds Poult. Sci. J. 2010;66:639–646. [Google Scholar]
- Ohlsson C., Mohan S., Sjögre K., Tivesten A., Isgaard J., Isaksson O., Jansson J.O., Svensson J. The role of liver-derived insulin-like growth factor-I. Endocr. Rev. 2009;30:494–535. doi: 10.1210/er.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelas A., Dowdell A.S., Lee J.S., Colgan S.P. Microbial metabolite regulation of epithelial cell-cell interactions and barrier function. Cells. 2022;11:944. doi: 10.3390/cells11060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda A.K., Bhanja S.K., Shyam Sunder G. Early post hatch nutrition on immune system development and function in broiler chickens. Worlds Poult. Sci. J. 2015;71:285–296. [Google Scholar]
- Potturi P.V., Patterson J.A., Applegate T.J. Effects of delayed placement on intestinal characteristics in turkey poults. Poult. Sci. 2005;84:816–824. doi: 10.1093/ps/84.5.816. [DOI] [PubMed] [Google Scholar]
- Proszkowiec-Weglarz M., Schreier L.L., Miska K.B., Angel R., Kahl S., Russell B. Effect of early neonatal development and delayed feeding post-hatch on jejunal and ileal calcium and phosphorus transporter genes expression in broiler chickens. Poult. Sci. 2019;98:1861–1871. doi: 10.3382/ps/pey546. [DOI] [PubMed] [Google Scholar]
- Proszkowiec-Weglarz M., Schreier L.L., Kahl S., Miska K.B., Russell B., Elsasser T.H. Effect of delayed feeding post-hatch on expression of tight junction- and gut barrier-related genes in the small intestine of broiler chickens during neonatal development. Poult. Sci. 2020;99:4714–4729. doi: 10.1016/j.psj.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran V., Abdollahi M.R. Nutrition and digestive physiology of the broiler chick: state of the art and outlook. Animals (Basel) 2021;11:2795. doi: 10.3390/ani11102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reicher N., Melkman-Zehavi T., Dayan J., Uni Z. It’s all about timing: early feeding promotes intestinal maturation by shifting the ratios of specialized epithelial cells in chicks. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.596457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z., Luo L., Wang X., Wen Q., Zhou L., Wu K. Characterization of the cecal microbiome composition of Wenchang chickens before and after fattening. PLoS One. 2019;14 doi: 10.1371/journal.pone.0225692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wagt I., de Jong I.C., Mitchell M.A., Molenaar R., van den Brand H. A review on yolk sac utilization in poultry. Poult. Sci. 2020;99:2162–2175. doi: 10.1016/j.psj.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes A.M., Walter J., Segal E., Spector T.D. Role of the gut microbiota in nutrition and health. BMJ. 2018;361 doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie C.M., Anderson J.M. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- Vélez E.J., Unniappan S. A comparative update on the neuroendocrine regulation of growth hormone in vertebrates. Front Endocrinol (Lausanne) 2021;11 doi: 10.3389/fendo.2020.614981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura M., Canchaya C., Tauch A., Chandra G., Fitzgerald G.F., Chater K.F., van Sinderen D. Genomics of Actinobacteria: tracing the evolutionary history of an ancient phylura. Microbiol. Mol. Biol. R. 2007;71:495–548. doi: 10.1128/MMBR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Xiao Y., Yu L., Tian F., Zhao J., Zhang H., Chen W., Zhai Q. Protective effects of different Bacteroides vulgatus strains against lipopolysaccharide-induced acute intestinal injury, and their underlying functional genes. J. Adv. Res. 2021;36:27–37. doi: 10.1016/j.jare.2021.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.S., Wang D.C., Li K.X., Xia L., Wang Y.Y., Jiang L., Heng C.N., Guo X.Y., Liu W., Zhan X.A. Effects of first feed administration on small intestinal development and plasma hormones in broiler chicks. Animals (Basel) 2020;10:1568. doi: 10.3390/ani10091568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.S., Hu H.J., Xu Y.B., Wang D.C., Jiang L., Li K.X., Wang Y.Y., Zhan X.A. Effects of posthatch feed deprivation on residual yolk absorption, macronutrients synthesis, and organ development in broiler chicks. Poult. Sci. 2020;99:5587–5597. doi: 10.1016/j.psj.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li Y., Willems E., Willemsen H., Franssens L., Koppenol A., Guo X., Tona K., Decuypere E., Buyse J., Everaert N. Spread of hatch and delayed feed access affect post hatch performance of female broiler chicks up to day 5. Animal. 2014;8:610–617. doi: 10.1017/S175173111400007X. [DOI] [PubMed] [Google Scholar]
- Wen C., Wu P., Chen Y., Wang T., Zhou Y. Methionine improves the performance and breast muscle growth of broilers with lower hatching weight by altering the expression of genes associated with the insulin-like growth factor-I signalling pathway. Br. J. Nutr. 2014;111:201–206. doi: 10.1017/S0007114513002419. [DOI] [PubMed] [Google Scholar]
- Wijnen H.J., van der Pol C.W., van Roovert-Reijrink I.A.M., De Smet J., Lammers A., Kemp B., van den Brand H., Molenaar R. Low incubation temperature during late incubation and early feeding affect broiler resilience to necrotic enteritis in later life. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.784869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen H., Debonne M., Swennen Q., Everaert N., Careghi C., Han H., Bruggeman V., Tona J.K., Decuypere E. Delay in feed access and spread of hatch: importance of early nutrition. Worlds Poult. Sci. J. 2010;66:177–188. [Google Scholar]
- Yadav S., Caliboso K.D., Nanquil J.E., Zhang J., Kae H., Neupane K., Mishra B., Jha R. Cecal microbiome profile of Hawaiian feral chickens and pasture-raised broiler (commercial) chickens determined using 16S rRNA amplicon sequencing. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegani M., Korver D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008;87:2052–2063. doi: 10.3382/ps.2008-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S., Zhu Y., Feng P., Li M., Wang W., Yang L., Yang Y. Ochratoxin A: its impact on poultry gut health and microbiota, an overview. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S.S., Ruan D., Zhu Y.W., Li M.C., Ye H., Wang W.C., Yang L. Protective effect of curcumin on ochratoxin A-induced liver oxidative injury in duck is mediated by modulating lipid metabolism and the intestinal microbiota. Poult. Sci. 2020;99:1124–1134. doi: 10.1016/j.psj.2019.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair A., Leeson S. Compensatory growth in the broiler chicken: a review. Worlds Poult. Sci. J. 1996;52:189–201. [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulkifli I., Shakeri M., Soleimani A.F. Dietary supplementation of L-glutamine and L-glutamate in broiler chicks subjected to delayed placement. Poult. Sci. 2016;95:2757–2763. doi: 10.3382/ps/pew267. [DOI] [PubMed] [Google Scholar]