Abstract
Pure trigeminal motor neuropathy (PTMN) is characterized by trigeminal motor weakness without signs of trigeminal sensory dysfunction or involvement of other cranial nerves. We describe a rare case of an 83-year-old man with weakness and atrophy of the right masticatory muscle without any sensory disturbance. Brain computed tomography and magnetic resonance imaging revealed atrophy and fatty infiltration of the right masticatory muscle. Electromyography revealed abnormal spontaneous activity, chronic neurogenic motor unit potentials, and reduced interference patterns in the right temporalis and the masseter muscles. The patient was diagnosed with PTMN based on the clinical symptoms and examinations. Our case presents a rare clinical manifestation with unclear etiology.
Keywords: Unilateral masticatory muscle atrophy, Trigeminal nerve, Pure trigeminal motor neuropathy, Malocclusion, Magnetic resonance imaging
Introduction
The trigeminal nerve is the largest of the 12 cranial nerves and has both sensory and motor components [1]. When trigeminal motor paralysis is not accompanied by trigeminal sensory or signs of other cranial nerve involvement, it is termed pure trigeminal motor neuropathy (PTMN). Few patients reported with PTMN [1,2]. PTMN is a rare medical condition, and the underlying mechanism remains unclear. PTMN is usually diagnosed based on radiological examinations as well as specific clinical features [2,3]. Potential etiological factors for PTMN are viral infection, tumor, trauma, stroke, and other unknown causes [2,[4], [5], [6], [7], [8], [9], [10]. Herein, we describe a rare case of unilateral PTMN accompanied by widespread atrophy of the masticatory muscles.
Case report
An 83-year-old man presented with a 1-year history of malocclusion. The patient had no history of head trauma, craniofacial injury, or surgery. The patient showed facial asymmetry, atrophy, and weakness of the right masticatory muscle caused by trigeminal motor nerve involvement (Fig. 1). Manifestations of trigeminal sensory nerve abnormalities, such as loss of sensation in the ophthalmic, maxillary, and mandibular divisions of the trigeminal nerve, were absent. The findings and functions of other cranial nerves were normal. No evidence of additional motor nerve involvement or polyneuropathy was detected. Muscle strength, deep tendon reflexes, and sensory functions of limb muscles were normal. The patient was only affected by the motor component of the right mandibular division of the trigeminal nerve.
Fig. 1.
Extraoral photograph of the patient. Right lateral view (A), frontal view (B), and left lateral view (C) showing asymmetry.
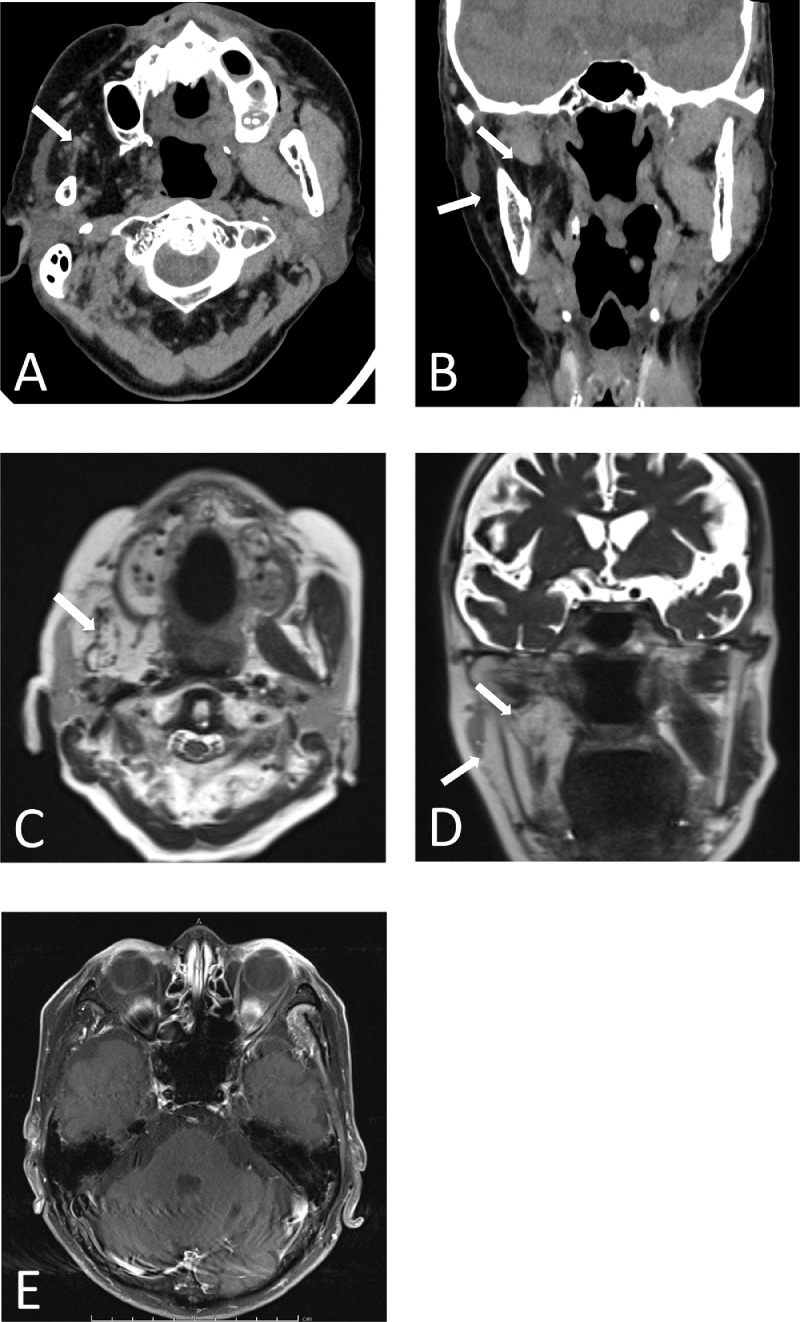
All hematological and biochemical tests were normal. The autoantibody profile was negative for antinuclear antibody, anti-proteinase 3 neutrophil cytoplasmic antibody, myeloperoxidase neutrophil cytoplasmic antibody, anti-Sjögren's syndrome-related antigen A and B antibodies, and angiotensin-converting enzyme. Brain computed tomography (CT) and magnetic resonance imaging (MRI) revealed atrophy and fatty infiltration of the masticatory muscles, including the right masseter, medial pterygoid, and lateral pterygoid muscles (Figs. 2A-D). Gadolinium-enhanced T1-weighted MRI did not reveal any abnormal mass lesions involving the trigeminal nerve or mandibular division (Fig. 2E). Electromyography (EMG) revealed abnormal spontaneous activity, chronic neurogenic motor unit potentials, and reduced interference patterns in the right temporalis and the masseter muscles. On the other hand, no abnormalities were observed on the right biceps brachii and the first dorsal interosseous muscle, as well as the left temporalis and the masseter muscles (Table 1). There was no abnormality in the pons or the course of the trigeminal nerve along the prepontine cistern.
Fig. 2.
Brain computed tomography (CT) and magnetic resonance imaging (MRI). Axial (A) and coronal (B), T2 FSE axial (C) and coronal (D), and gadolinium-enhanced T1 (E). Brain CT and MRI revealed atrophy and fatty infiltration of the masticatory muscles, including the right masseter and medial and lateral pterygoid muscles (A-D, arrows). Gadolinium-enhanced T1-weighted MRI did not reveal any abnormal mass lesions involving the trigeminal nerve.
Table 1.
Electromyographic findings.
| Spontaneous |
MUAP |
Recruitment pattern | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Muscle | Nerve | IA | Fib | PSW | Fasc | Amp | Dur | Polyphasia | |
| R. Temporalis | Mandibular | None | 1+ | None | None | High 2+ | Long 2+ | 1+ | Reduced |
| R. Masseter | Mandibular | None | 1+ | None | None | High 2+ | Long 2+ | 1+ | Reduced |
| R. Biceps | Musculocutaneous | None | None | None | None | Normal | Normal | Normal | Normal |
| R. FDI | Ulnar | None | None | None | None | Normal | Normal | Normal | Normal |
| L. Temporalis | Mandibular | None | None | None | None | Normal | Normal | Normal | Normal |
| L. Masseter | Mandibular | None | None | None | None | Normal | Normal | Normal | Normal |
R, right; L, left; IA, insertion activity; Fib, fibrillation potentials; PSW, positive sharp waves; Fasc, fasciculation potentials; MUAP, motor unit action potential; Amp, amplitude; Dur, duration; FDI, first dorsal interosseous.
The patient was diagnosed with PTMN based on the symptoms, examinations, and exclusion of other diseases. He was treated with a stabilization splint, following which, a favorable prognosis was achieved.
Discussion
PTMN is characterized by mandibular branch motor weakness without any signs of trigeminal sensory or other cranial nerve involvement [2,3]. The trigeminal nerve has its nucleus in the pontine tegmentum and divides into 3 major branches: the ophthalmic, maxillary, and mandibular nerves [1]. The mandibular branch contains both sensory and motor fibers and supplies the masticatory muscles, including the masseter, temporalis, medial pterygoid muscle, and lateral pterygoid muscle [1]. A lesion anywhere along its course from the pons to distal peripheral nerve-innervating muscles can produce symptoms and signs of trigeminal sensory and motor involvement [1,9]. Our patient showed weakness of the right masticatory muscle caused by the trigeminal motor nerve and no signs of trigeminal sensory nerve abnormality. Only the motor component of the mandibular branch was believed to be affected.
Peripheral motor neurons structurally differ from sensory neurons. Trigeminal neuropathy commonly presents as a sensory disturbance. The main neurological finding is hypesthesia in the territory of one or more trigeminal nerve divisions [1]. In contrast, PTMN causes paralysis of the motor branch of the trigeminal nerve without sensory nerve disturbances [2,3]. Synaptobrevin is a membrane protein in synaptic vesicles that plays a key role in exocytosis [11]. Synaptobrevin I is predominant in motor neurons, whereas Synaptobrevin II is present in sensory neurons [11]. The difference in the specificity of this membrane protein in motor and sensory neurons may account for the susceptibility of motor neurons to viral infections [2,4]. In patients with PTMN, as seen in our case, mandibular muscle atrophy is caused by anterograde degeneration of the efferent fibers of the affected trigeminal motor neurons.
The EMG showed neurogenic changes consisting of fibrillation potentials (FP) and large motor unit action potentials with reduced recruitment patterns. The 7-10 days after nerve damage, abnormal spontaneous activity, such as FP, is detectable [12]. In neurologic disease, abnormal motor unit action potentials with high amplitude and long duration is detected after 6 months of pathological changes [13]. Our patient presented with a 1-year history of malocclusion. In this case, the EMG findings are not inconsistent with the clinical findings that the patient has a chronic course of the disease.
The etiology of PTMN, which is a rather rare neuropathy, is poorly understood. Viral infection was reported the one of the etiological factors for PTMN [2,[4], [5], [6], [7], [8], [9], [10]. It has been suggested that viral infections and post-viral autoimmune processes may focally affect the motor branch of the trigeminal nerve [2,4]. In our patient, clinical symptoms and examinations showed no signs of infection and no mass lesion affecting the trigeminal nerve. Hence the cause of PTMN remains obscure. However, we speculated that the autoimmune response to a past viral infection without the patient's notice might have been involved.
Diagnostic criteria and therapeutic protocols for PTMN have not yet been established. PMTN is usually diagnosed based on specific clinical features such as wasting and weakness of the masticatory muscles, without any sensory facial disturbances [9]. Paresis and atrophy of the masticatory muscles are confirmed by CT, MRI of the head, and electromyography [9]. We evaluated the patient using clinical, biochemical, radiologic, and electrophysiological examinations to diagnose PTMN. There is currently no effective treatment for PTMN [3,8]. A previous report suggested that physiotherapy and stabilization splints were effective for the patient [14]. Our patient was treated with a stabilization splint and a favorable prognosis was achieved. Additional studies are required to develop diagnostic criteria and therapeutic protocols for PTMN. Thus, our case presents a rare clinical manifestation with unclear etiology. It can guide the design of standard diagnostic and therapeutic protocols, which will improve treatment and patient management.
Author contributions
KK and TM contributed to the study's conception and design. KK, TM, YN, and NH collected the clinical data. KK and TM wrote the manuscript. KS supervised this case. All the authors have read and approved the final version of the manuscript.
Patient consent
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Acknowledgments
The authors are grateful to Miki Zaizen and Nobuhiro Ueda (Department of Oral and Maxillofacial Surgery, Nara Medical University) for oral and dental examinations. The authors also thank Tadaaki Kirita (Department of Oral and Maxillofacial Surgery, Nara Medical University) for his help and supervision.
Footnotes
Competing Interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Funding: This work received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Smith JH, Cutrer FM. Numbness matters: a clinical review of trigeminal neuropathy. Cephalalgia. 2011;31:1131–1144. doi: 10.1177/0333102411411203. [DOI] [PubMed] [Google Scholar]
- 2.Chia LG. Pure trigeminal motor neuropathy. Br Med J (Clin Res Ed) 1988;296:609–610. doi: 10.1136/bmj.296.6622.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei Y, Yang M. Surgical therapy for pure trigeminal motor neuropathy accompanied by limited mouth opening: a retrospective study. Dentistry. 2015;5(10) doi: 10.4172/2161-1122.1000337. [DOI] [Google Scholar]
- 4.Kang YK, Lee EH, Hwang M. Pure trigeminal motor neuropathy: a case report. Arch Phys Med Rehabil. 2000;81:995–998. doi: 10.1053/apmr.2000.6273. [DOI] [PubMed] [Google Scholar]
- 5.Park KS, Chung JM, Jeon BS, Park SH, Lee KW. Unilateral trigeminal mandibular motor neuropathy caused by tumor in the foramen ovale. J Clin Neurol. 2006;2:194–197. doi: 10.3988/jcn.2006.2.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko KF, Chan KL. A case of isolated pure trigeminal motor neuropathy. Clin Neurol Neurosurg. 1995;97:199–200. doi: 10.1016/0303-8467(95)00027-h. [DOI] [PubMed] [Google Scholar]
- 7.Wilson MH, Hodgson EJ, Felstead AM. Focal atrophy of the masticatory muscles caused by pure trigeminal motor neuropathy: case report. Br J Oral Maxillofac Surg. 2016;54:e13–e14. doi: 10.1016/j.bjoms.2015.08.265. [DOI] [PubMed] [Google Scholar]
- 8.Nagure P, Urunkar B, Nikam V, Tiwale S, Pawal S. Unilateral trigeminal motor nerve neuropathy. Radiol Case Rep. 2022;17:3111–3114. doi: 10.1016/j.radcr.2022.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiba M, Echigo S. Unilateral atrophy of the masticatory muscles and mandibular ramus due to pure trigeminal motor neuropathy: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:e30–e34. doi: 10.1016/j.oooo.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Tsugawa J, Ouma S, Fukae J, Tsuboi Y, Maki Y, Hokezu Y. Pure trigeminal motor neuropathy with an antecedent infection: a case report. Rinsho Shinkeigaku. 2014;54:515–517. doi: 10.5692/clinicalneurol.54.515. [DOI] [PubMed] [Google Scholar]
- 11.Li JY, Edelmann L, Jahn R, Dahlstrom A. Axonal transport and distribution of synaptobrevin I and II in the rat peripheral nervous system. J Neurosci. 1996;16:137–147. doi: 10.1523/JNEUROSCI.16-01-00137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mills KR. The basics of electromyography. J Neurol Neurosurg Psychiatry. 2005;76(suppl 2):ii32–ii35. doi: 10.1136/jnnp.2005.069211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daube JR, Rubin DI. Needle electromyography. Muscle Nerve. 2009;39:244–270. doi: 10.1002/mus.21180. [DOI] [PubMed] [Google Scholar]
- 14.Elsayed N, Shimo T, Tashiro M, Nakayama E, Nagayasu H. Disuse atrophy of masticatory muscles after intracranial trigeminal schwannoma resection: a case report and review of literature. Int J Surg Case Rep. 2020;75:23–28. doi: 10.1016/j.ijscr.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]