Abstract
Purpose of Review
There is global increase in the incidence of mucormycosis. However, a sudden increase in the COVID-associated mucormycosis (CAM) was noted, particularly in India, during the second wave of the COVID-19 pandemic. The interplay of factors involved in the pathogenesis is complex. In this review, the influence of pre-existing disease, exaggerated risk factors, altered milieu due to COVID-19 itself and the consequences of its treatment on the host pathogen interactions leading to the disease and morphology of the fungus will be highlighted.
Recent Findings
Hyperglycemia, acidosis, available free iron, lowered host defenses, and the fungal virulence factors promote the growth of Mucorales. There is a high background prevalence of diabetes mellitus (DM) in India. Uncontrolled or undiagnosed DM, COVID-19 itself, and inappropriate administration of corticosteroids in high doses and for prolonged periods result in hyperglycemia. Diabetic ketoacidosis (DKA) and metabolic acidosis due to hypoxia or renal failure contribute to acidic pH and dissociate bound iron from serum proteins. The host defenses are lowered due to COVID-19-induced immune dysregulation, hyperglycemia itself, and administration of corticosteroids and immune suppressants for the treatment of COVID-19. The altered metabolic milieu in the local microenvironment of nose and paranasal sinuses (PNS) promotes specific interaction of glucose-regulated protein-78 (GRP-78) on host cells with spore coat protein homologue (CotH 3) on Mucorales resulting in rhino-orbito-cerebral mucormycosis (ROCM) as the predominant clinical form in CAM. The pathology is extensive soft tissue involvement with angioinvasion and perineural invasion. Melanized hyphae and sporangia were seen on histopathology, which is unique to CAM. While many factors favor the growth of Mucorales in CAM, hyperglycemia, hyperferritinemia, and administration of hyperbaric oxygen result in reactive oxygen species (ROS) and inadequate humidification results in dehydration. Melanization is possibly the adaptive and protective mechanism of Mucorales to escape the unfavorable conditions due to the treatment of COVID-19.
Summary
High background prevalence of DM, inappropriate administration of corticosteroids and immune dysregulation due to COVID-19 favor the growth of Mucorales in CAM. Melanization of Mucorales hyphae and sporangia on histopathology probably represent adaptive and protective mechanism due to the treatment with hyperbaric oxygen with inadequate humidification as well as the metabolic alterations.
Keywords: COVID-associated mucormycosis, Diabetes mellitus, Corticosteroids, Melanization of Mucorales hyphae, Sporangia on histopathology
Introduction
Mucormycosis was once considered a rare disease. However, the incidence is on the rise globally in the past few decades [1–10, 11••, 12•, 13]. The incidence of risk factors varies in different geographical regions contributing to the variation in the prevalence between developed and developing countries [8–10, 11••, 12•, 13]. In India, the prevalence is nearly 80 times higher and it is attributed to the high prevalence of diabetes mellitus (DM) [7, 11••, 12•, 13–15]. In the recent coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome virus 2 (SARS-CoV-2), during the second wave, there was a sudden increase in the number of cases of mucormycosis, particularly in India, which could not be explained by the high prevalence of DM alone [16–18, 19••, 20, 21•, 22, 23••, 24••]. The Government of India declared COVID-associated mucormycosis (CAM) an epidemic in many states and territories. In a multi-center study from India, the prevalence of CAM was reported to be 0.27% (range 0.05–0.57%) [19••].
The pathophysiology of COVID-19 and the associated secondary infections were described by many authors [19••, 25–30, 31•, 32]. Several publications described the epidemiology, risk factors, pathogenesis, and outcome of CAM [17, 18, 19••, 20, 21•, 23••, 24••, 33].
Uncontrolled DM, specifically ketoacidosis and administration of corticosteroids, were the key factors involved in the pathogenesis of CAM [19••, 24••]. Uncontrolled DM and newly diagnosed DM at the evaluation for CAM were reported in several series [19••, 24••, 34]. DKA and acute or chronic renal dysfunction were reported in 20% of patients with COVID-associated ROCM [35]. Corticosteroids were indicated for the treatment of hospitalized patients with COVID-19 pneumonia who required supplemental oxygen and higher levels of respiratory support [36]. Prolonged (> 3 weeks) high-dose use of corticosteroids was known to increase the risk to develop mucormycosis [37, 38]. Inappropriate and indiscriminate use of corticosteroids was reported in 63.3% of patients during the second wave of COVID-19 in India [19••, 34].
Comparison of studies on mucormycosis in pre-COVID times and CAM suggests that diabetic patients with COVID-19, receiving corticosteroids were at great risk of developing CAM [10, 11••, 19••, 24••, 33]. The outcome is poor with cerebral or pulmonary involvement and disseminated form of CAM [21•, 24••, 33]. Table 1 summarizes the demographic details, pre-existing disease, site of involvement, and outcome of pre-COVID-mucormycosis and CAM.
Table 1.
Demographic details, pre-existing disease, site of involvement, corticosteroid administration and outcome of pre-COVID- and COVID-associated mucormycosis
| Parameter | Pre-COVID mucormycosis | COVID-associated mucormycosis | |||
|---|---|---|---|---|---|
| Authors | Patel et al. 2020 [11••] n = 465 (India) |
Jeong et al. (2019) [10] n = 851 (31% Asia) |
Patel et al. (2021) [19••] n = 187 (India) |
Hoenigl et al. (2022) [24••] n = 80 (53% India) |
Watanabe et al. (2022) [33] n = 2312 (88.67% India) |
| Type of study | Multi-center study | Systematic review and meta-analysis | Multi-center study | Review of cases from 18 countries | Systematic review and meta-analysis |
| Study period | 1 January 2016–30 September 2017 | January 2000–January 2017 | 1 September 2020–1 December 2020 | 1 October 2019–12 April 2021 | Till 20 January 2022 |
| Age in years | 48 | 51 | 56.9 | 55 | 36–63 |
| Male gender (%) | 69.5 | 63 | 80.2 | 78 | 20–100 |
| Pre-existing disease (%) | |||||
| DM (DKA in % of DM patients) | 73.5 (14.6) | 40 (21; status unknown in 248/851) | 60.4 (8.6) | 83 (49: available in 55/66) | 82 (2.71) |
| Malignancy | 09 | 32 | 1.1 | 06 | 2.6 |
| Transplantation | 7.7 | 14 | 1.6 | - | - |
| Chronic kidney disease | 20 | - | 06 | 15 | |
| Trauma | 6.9 | 20 | 1.6 | - | - |
| Others | 0.6 | 03 | 2.7 | 19 | - |
| No predisposing disease | 11.8 | 18 | 32.6 (COVID-19 only) | 05 (COVID-19 only) | - |
| Site of involvement (%) | |||||
| ROM (ROCM) | 67.7 (32.7) | 34 (9) | 86.1 (27.33) | 74 (37) | 97 (25) |
| Pulmonary | 13.3 | 20 | 8.6 | 25 (includes 15% disseminated) | 2.7 |
| Cutaneous | 10.5 | 22 | 2.7 | - | < 1 |
| GIT | 2.6 | 8.5 | < 1 | ||
| Disseminated | 2.8 | 13 | 2.1 | - | |
| Others | 03 | 03 | 0.5 | ||
| Corticosteroid administration (%) | 3.7 | - | 78.7 | 79 | 77 |
| Mortality (%) | 52 | 46 | 44 | 49 | 29 |
Abbreviations: DM, diabetes mellitus; DKA, diabetic ketoacidosis; ROM, rhino-orbital mucormycosis; ROCM, rhino-orbito-cerebral mucormycosis; GIT, gastrointestinal tract
However, the interplay of factors involved in the pathogenesis and pathology of CAM are not completely understood. In this review, the influence of pre-existing disease, exaggerated risk factors, altered milieu due to COVID-19 itself, and the consequences of its treatment on the host pathogen interactions leading to the disease and morphology of the fungus will be highlighted.
Secondary Infections in COVID-19
COVID-19 causes immune dysregulation involving both innate and adaptive immunity and hence COVID-19 patients are more susceptible to develop secondary infections [19••, 27]. The rate of in-hospital secondary bacterial and fungal infection has been reported to be approximately 8% [25, 26, 28, 30, 32]. Severe COVID-19 disease, prolonged stay in intensive care unit (ICU), requirement of mechanical ventilation, treatment with broad-spectrum antibiotics, immunosuppressive agents along with pre-existing disease like DM, and de-compensated pulmonary function predispose to secondary infections by bacteria, viruses, and fungi [19••, 25–27, 39–41].
In a systematic review and meta-analysis of co-infections and super-infections with SARS-CoV-2, fungal infections were reported as co-infection in 4% and super-infection in 8% [31•]. Fungal infections were more likely to develop during the more advanced stages of COVID-19 infection [29]. CAM is the most common fungal infection reported from India, which is at variance to other countries where Aspergillus, Pneumocystis jiroveci, and Candida have been reported to be the major secondary fungal pathogens [23••, 27, 29, 42–46]. The other reported fungi causing disease in COVID-19 include Histoplasma spp., Cryptococcus spp., Fusarium spp., and Pneumocystis jiroveci [27, 29, 42–46].
Etiologic Agents
Mucormycosis is caused by fungi of the order Mucorales. The most common etiologic agents are Rhizopus spp., Mucor spp., and Lichtheimia spp., followed by Rhizomucor spp., Cunninghamella spp., Apophysomyces spp., and Saksenaea spp. [1, 9, 11••, 12•, 13, 47]. In CAM, Rhizopus spp. (Rhizopus arrhizus, Rhizomucor pusillus) were the most common agents isolated, followed by Apophysomyces variabilis, Lichtheimia corymbifera, and others [19••, 24••]. Rhizopus arrhizus was the predominant agent causing CAM in India [19••].
Pathogen Factors
Rhizopus spp. is aerobic, thermo-tolerant, fast-growing, saprotrophic, fungus, and has an optimal growth temperature of 39 °C under conditions of low pH and high glucose concentration [48]. The genome of Rhizopus spp. indicates an ancestral whole-genome duplication event, related to cell growth, signal transduction, and cell wall synthesis that facilitate its adaptation to different environmental conditions [49]. The virulence factors of Rhizopus spp. include the following [48, 50–55, 56••]:
The cell wall characters: The precise structure of the Mucorales cell wall for both the sporangiospores and hyphal form is not yet fully characterized, but the cell wall has been shown to consist of chitin/chitosan, β-1,3-glucans, mannan, mannose, extracellular polysaccharides (EPS), and other polysaccharides, e.g., mucoran and mucoric acid (hyphae) [54]. The cell wall is vital for integrity and viability and protects the fungus from harsh environment. The cell wall components are highly antigenic and elicit both humoral and cell-mediated immunity during infection and permits passage of nutrients [50, 52].
Growth conditions: Rhizopus spp. have optimal growth conditions at low pH and high glucose concentration, and are thermo-tolerant.
Nutrients: Rhizopus spp. elaborate enzymes required for iron acquisition by siderophores and iron permeases, and secrete proteases to digest extracellular matrix for invasion.
Host defenses: Rhizopus spp. cause infection when host defenses are lowered, particularly neutropenia, and evade host immune response, as Rhizopus spp. are able to survive within macrophages by the arrest of phagolysosome maturation.
Interaction with host: Rhizopus spp. interact with host epithelial cells by a specific recognition through GRP78 and Cot H proteins, which are exclusive to Mucorales, especially Rhizopus spp. and not found in Candida spp. and Aspergillus spp.
The pathophysiology of DM and the hyper-inflammatory state in COVID-19, along with the administration of corticosteroids offer favorable conditions for the successful replication of Rhizopus spp.
Risk Factors
The main risk factors for mucormycosis are the following [1, 6, 11••, 15, 57, 58•]:
-
i.
Neutropenia due to hematopoietic stem cell transplantation (HSCT), solid organ transplantation (SOT), immunosuppressive therapy (including corticosteroids) for malignancy, autoimmune diseases
-
ii.
Hyperglycemia due to uncontrolled/poorly controlled DM, administration of corticosteroids.
-
iii.
Acidosis due to DKA, chronic renal failure, decompensated pulmonary function leading to hypoxemia and metabolic acidosis
-
iv.
Disruption of mucosal/skin barrier due to trauma, burns, combat related injuries, natural disasters
-
v.
Iron overload due to deferoxamine therapy, multiple transfusions
-
vi.
Prematurity, malnutrition, dehydration
-
vii.
Antifungal therapy with voriconazole, posaconazole
-
viii.
Others
The most important risk factors in CAM were hyperglycemia due to uncontrolled DM, administration of corticosteroids for the treatment of COVID-19 and COVID-19 itself [19••, 24••, 58•].
In non-COVID mucormycosis, DM is the most common pre-existing disease in Asian and African countries, especially India and China, whereas neutropenia due to HSCT and SOT are the common factors in Europe, North & South America, Australia, and New Zealand [10, 13]. In addition to uncontrolled DM and DKA, chronic kidney disease and pulmonary tuberculosis were also reported to be frequent diseases in countries like India [12•]. Jeong et al. reported use of corticosteroid at the time of presentation in 33% (pre-COVID time), followed by neutropenia and trauma [10].
In a systematic review and meta-analysis, Watanabe et al. reported DM as the underlying disease in 82% and administration of corticosteroids in 77% patients of CAM [33]. In a multi-center epidemiologic study from India, Patel et al. reported DM in 60.4% and administration of corticosteroids in 78.7% patients with CAM [19••].
Route of Spread
The primary route of spread is by inhalation of the spores from the environment, which get deposited in the PNS. The other less common routes are by ingestion of contaminated food or inoculation of spores in skin or mucosa by trauma/injury or application of contaminated bandages/instruments. Although infection occurs usually in immunosuppressed hosts, cutaneous infections can occur in immunocompetent hosts [1, 9, 12•, 59]. In CAM, the predominant route of spread was inhalation and spread from PNS, most commonly resulting in rhino-orbital or rhino-orbito-cerebral mucormycosis (ROM or ROCM respectively) [19••, 24••].
Clinical Forms
The clinical forms described are (i) rhino-orbito-cerebral, (ii) pulmonary, (iii) cutaneous, (iv) gastrointestinal, (v) disseminated, and (vi) miscellaneous including isolated organ involvement such as isolated renal mucormycosis [1, 9, 12•, 59]. The clinical form depends on the risk factor and predisposing condition; ROCM is frequently observed in association with uncontrolled DM and DKA, whereas pulmonary involvement is often observed in patients having neutropenia, HSCT, SOT, and hematological malignancies, while gastrointestinal tract (GIT) gets involved more in malnourished individuals and cutaneous form following trauma [1, 10, 11••, 22]. The most common type of CAM was ROM or ROCM followed by pulmonary and others (cutaneous, renal, gastrointestinal, disseminated) [19••, 24••, 33].
Factors Favoring CAM
Hyperglycemia
Hyperglycemia in CAM may be due to uncontrolled or newly diagnosed DM, virus-induced damage to islets of pancreas or due to the administration of corticosteroids. SARS-CoV-2 can cause hyperglycemia by causing damage to pancreatic islet cells bearing acetyl cholinesterase 2 (ACE 2) receptors. The other mechanisms include insulin resistance due to cytokine storm and acute cortisol stress response [60–63]. Corticosteroids cause hyperglycemia by acting as a substrate for oxidative stress metabolism with lipolysis, proteolysis, and hepatic glucose production. They also increase insulin resistance in up to 60–80% of patients depending on the dose and type used [64].
Acidosis
Acidosis can occur due to the accumulation of ketone bodies, particularly, beta hydroxyl butyrate (BHB) in DKA. BHB-related acidosis exerts a direct effect on the virulence of Mucorales, favoring their growth but ketoacidosis does not predispose to aspergillosis [65]. Acute/chronic renal dysfunction and pre-existing destructive parenchymal lung disease can predispose to metabolic acidosis. In addition, SARS-CoV-2 also has renal tropism and direct infection can lead to acute kidney injury and renal failure [66]. In addition to direct infection, uncontrolled cytokine release, thrombosis, and ischemia, that occur in COVID-19, can also result in further kidney dysfunction, characterized by intra-renal inflammation, increased vascular permeability, and volume depletion [67]. Vitamin C, administered for treatment of COVID-19, if given in high doses and for prolonged periods, can also cause acidosis, especially in COVID-19 patients with compromised renal function [23••].
Availability of Free Iron
Hyperglycemia induces excessive glycosylation of ferritin and transferrin, leading to their decreased affinity for iron. The acidic pH impairs the ability of transferrin to chelate iron. Hence, iron, which is normally bound to serum proteins, gets dissociated in acidic pH leading to high serum concentrations of free iron [59]. Patients with renal failure undergoing deferoxamine chelation also have increased serum-free iron. The cytokine response, particularly secretion of interleukin-6 (IL-6), in COVID-19 stimulates ferritin and hepcidin synthesis and leads to sequestration of iron in macrophages. The resultant hyperferritinemia contributes to increased intracellular iron. Increased intracellular iron causes ROS, leading to tissue damage and release of free iron into circulation [68]. Vitamin C in excessive dose also increases intestinal absorption of iron [23••].
Hyperglycemia, acidosis, and availability of free iron increase the expression of GRP78 and its interaction with Cot H on the hyphae of Mucorales leading to growth and invasion of the fungus [65]. Moreover, Mucorales possess a ketone reductase enzyme and thus thrive in hyperglycemia and acidosis [69].
Immune Dysregulation
DM, along with COVID-19-induced systemic immune change and administration of corticosteroids and tocilizumab for the treatment of COVID-19, results in lowered host defenses and increases the risk for CAM [32, 70].
Hyperglycemia impairs innate immunity by the inhibition of neutrophil migration, chemotaxis, and phagocytosis. The diabetes-induced immune dysregulation may exacerbate the virus-activated hyper-inflammatory “cytokine storm,” which in turn leads to complications like adult respiratory distress syndrome (ARDS), shock, multiorgan failure, and death seen in severe COVID-19 [71].
COVID-19 causes immune dysregulation by involving both innate and adaptive immunity. It causes relative neutrophilia and lymphopenia, particularly involving CD4 + , CD8 + T cells, and natural killer cells [23••]. It causes overexpression of inflammatory cytokines, particularly secretion of IL-6 [72]. IL-6 promotes synthesis of ferritin and the hyperferritinemic state sustains a feed-forward loop of hyperinflammation and also modulates the lymphocyte response [73]. It interferes with T cell expansion, causes apoptosis of lymphocytes leading to T cell exhaustion. Lymphocytes are crucial to maintain immune homeostasis [74]. Severely infected individuals showed increased expression of activation and exhaustion markers of T cells. Hence, a prolonged period of immune dysregulation after SARS-CoV-2 infection persists and this may alter immune responsiveness to subsequent infections [75]. This also explains the occurrence of mucormycosis, in the post-COVID time period.
Platelets have antimicrobial and antifungal properties and suppress growth and dissemination of Mucorales. They inhibit germination of spores, produce cytokines/chemokines, promote phagocytosis, and activate B and T cells [76–78]. Thrombocytopenia in COVID-19 impairs the antifungal immune functions of platelets. In addition, the prothrombotic state associated with COVID-19 helps to propagate angio-invasive complications associated with CAM such as cavernous sinus thrombosis or stroke [79].
Corticosteroids impair immune mechanisms mainly through interaction with glucocorticoid receptors by causing functional impairment of neutrophils and macrophages by inhibiting chemotaxis, leukocyte migration, phagocytosis, and killing. They also impair phagolysosome fusion in macrophages. They downregulate the expression of proinflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-1β, IL-6, IL-8, and IL-12; interferon (IFN) α/β; and granulocyte–macrophage colony-stimulating factor (GM-CSF), chemokines, and the inflammatory enzymes (iNOS and COX2) secreted by macrophages [80–82, 83••]. Corticosteroids cause lymphopenia and T cell dysregulation [16]. They inhibit dendritic cell (DC) maturation, interfere with T cell signaling, thereby leading to immunosuppression [80–82, 83••].
Tocilizumab, an anti-IL-6 receptor monoclonal antibody, is used in COVID-19 patients with hypoxia and who are critically ill. It causes immune suppression and neutropenia and increases the risk of secondary infections [84].
Microenvironment of Nose/PNS
The portal of entry for both Mucorales and SARS-CoV-2 is through nasal cavity. Nasal epithelial cells were shown to express the highest levels of ACE 2 receptors and cellular serine protease TMPRS, which are the main entry receptors for SARS-CoV-2 following interaction with viral (S) pike protein [85]. Several variants of concern of SARS-CoV-2 (B.1.1.7 and B.6.177) were reported during the second wave globally and in India, which could use GRP78 as an alternate entry point for SARS-CoV-2 [86].
GRP78 is a heat shock protein present in the endoplasmic reticulum (ER) and helps in protein folding, maturation, and assembly [87–89]. Under stressful conditions, this is trans-located from ER to cell surface. Viral replication in the cell leads to the accumulation of excessively high number of unfolded viral structural proteins in ER leading to over expression of GRP78, at the cell surface [35]. GRP78 is also the receptor used by Mucorales, especially Rhizopus spp. for fungal invasion [65, 90]. Vascular endothelial injury and endothelitis caused by COVID-19-associated inflammation, also increases the susceptibility to mucormycosis [24••].
The intact mucosa of nose and PNS and the muco-ciliary action clear the inhaled spores of Mucorales and prevent their germination. Nasal epithelial damage can occur due to DM or chemotherapy. The other putative factors implicated in delay for muco-ciliary clearance include administration of broad-spectrum antibiotics and home remedies like repeated hot water steaming for COVID-19 (SARS-CoV-2). Nasal epithelial damage facilitates adherence of Mucorales spores to extracellular matrix proteins, laminin and collagen IV, and the spores begin to germinate [65, 90, 91].
Hyperglycemia, acidosis, and free iron alter the local microenvironment of nose and PNS and lead to overexpression of GRP78 on the nasal epithelial and endothelial cells as a response to stress. The hyphae of Mucorales specifically recognize the host receptor, GRP78 on endothelial cells, and this interaction is mediated by the fungal ligand CotH3 for endocytosis of the fungus [65, 90]. Angioinvasion by Mucorales causes endothelial injury and death leading to thrombosis, dissemination, and tissue necrosis. The toxic metabolites produced by Mucorales (e.g., mucoricin) have been shown to induce inflammation, vascular permeability, and tissue necrosis [92].
The overexpression of GRP78 occurs on nasal epithelial cells and not alveolar epithelial cells [56••, 93•, 94]. Cot H3 is exclusively present in all Mucorales but absent from other opportunistic fungi like Candida spp. and Aspergillus spp. [94, 95••]. Cot H3 protein is mainly expressed in the germination of R. arrhizus and shows a greater capacity to adhere and invade nasal epithelial cells. In contrast, Rhizopus spp. interact with alveolar epithelial cells by binding with integrin-β1 with CotH7 as the major ligand [93•]. GRP 78-mediated interaction is unique to Mucorales and not to other fungi like Aspergillus spp. and Candida spp. These factors possibly explain CAM during the second wave, frequency of infection with Mucorales over other fungi, and ROCM over other clinical forms and Rhizopus arrhizus as the most frequent etiological agent [96].
Other Putative Factors
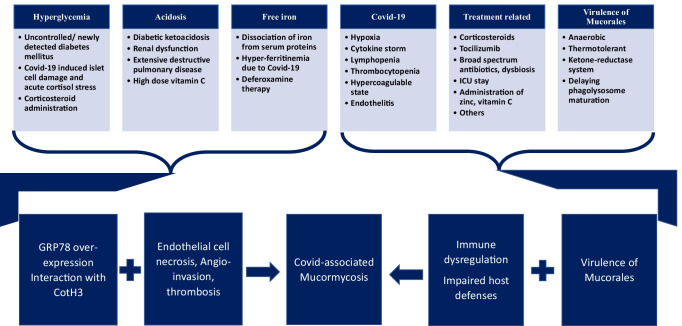
The environmental conditions like temperature, humidity, and spore content of Mucorales in the air were also implicated as possible contributing factors for CAM [83••, 97]. The contamination of masks and traditional medicine with hot water steaming possibly resulted in mucosal disruption and implantation of the spores. Due to the health crisis and inadequate health care facilities in resource-limited countries like India, unsupervised/indiscriminate use of steroids and antibiotics, administration of zinc and vitamin C in high doses for prolonged periods was done [23••, 83••]. These factors possibly contributed to CAM (Fig. 1).
Fig. 1.
Interplay of pathogenic factors in CAM
Pathology
Mucormycosis is an invasive disease and ROCM is the most common form. The surgical specimens in ROCM, usually include biopsy from sinus, resected specimens of maxillectomy, orbital exenteration, debrided soft tissues, or focal lesions in the brain (Fig. 2). In pulmonary mucormycosis, the samples are transbronchial/percutaneous biopsy, lobectomy, or pneumonectomy. In cutaneous form, it is biopsy or debridement specimens.
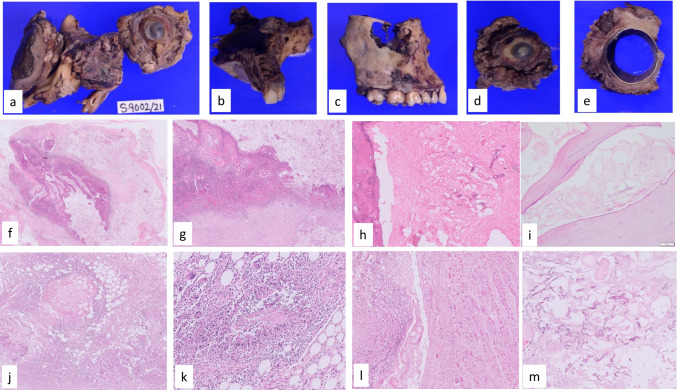
Fig. 2.
a The excised maxilla and the orbital exenteration specimen; b & c maxillectomy specimen showing black necrotic material filling the sinus and infarction of the bone; d & e the orbital structures show congested, unhealthy, brownish necrotic peri-orbital contents. External aspect shows brownish discolored necrotic tissue. On cut section of the specimen, the intra-ocular structures were uninvolved by infection; photomicrographs showing, f & g ulcerated and necrotic mucosa and acute inflammation; photomicrographs showing infarcted bony lamellae and invasion by numerous fungal hyphae: h maxilla; i mandible; j peri-orbital adipose tissue shows bland necrosis accompanied by numerous fungal hyphae; k both acute necrotizing inflammation and granulomatous response with multinucleate giant cells in soft tissue; l extensive myonecrosis of the extraocular muscles; m bland necrosis with minimal inflammation and numerous fungal hyphae in the soft tissues (H&E × 100)
The progression of disease is from nasal mucosa to PNS, orbital soft tissues, and to CNS [21•]. Pterygopalatine fossa is the largest reservoir for Mucorales hyphae [98]. Mucosal involvement is seen as edema and thickening. The sinus mucosa becomes ulcerated and necrotic. Both acute necrotizing inflammation and granulomatous response with multinucleate giant cells are seen in CAM (Fig. 2). Black, necrotic eschar on the palate indicates damage to the nasal turbinates, which is a significant clinical finding to suspect mucormycosis. Involvement of maxilla may occur as part of ROCM and the blackish necrotic material fills the sinus. The maxillary bone may show erosion, osteomyelitis, or infarction (Fig. 2). However, involvement of mandible with loosening and necrosis of teeth is uncommon but is reported in CAM [19••]. Osteomyelitis and infarction of the mandible either isolated or with concomitant involvement of maxilla in CAM were reported [99, 100].
The infection from the sinus erodes the bone and involves the orbital structures. Extensive soft tissue invasion and bone destruction occur in CAM. Orbital cellulitis, subperiosteal, and orbital abscess can occur. The fungus involves the blood vessels, nerves, and meninges. The peri-orbital adipose tissue shows either bland necrosis with numerous fungal hyphae or karyorrhexis and small neutrophilic infiltrates. The facial skin also shows ulceration and acute inflammatory infiltrate with numerous fungal hyphae. The extraocular muscles show myonecrosis with giant cell transformation (Fig. 2). Though there is extensive peri-orbital soft tissue involvement, intra-ocular involvement is rare [101].
The infection spreads posteriorly to involve sphenoid sinus and laterally to retro-orbital soft tissues and cavernous sinus, causing cranial nerve palsies [74]. The infection involves CNS through orbital apex or cribriform plate of the ethmoid bone [6]. In CAM, ROM was the most common form, whereas ROCM was reported in 21–37% cases [21•, 24••]. The spread also occurs through invasion of superior orbital fissure, ophthalmic vessels, cranial nerves, and carotid vessels [21•, 102, 103]. Cavernous sinus thrombosis is the most common route of CNS involvement in ROCM associated with COVID-19 [21•]. CNS parenchymal involvement is mainly in the diencephalic areas, sparing the infratemporal structures [104, 105].
Pulmonary involvement in CAM is as multiple large nodules with extensive cavitation and thick walls, necrotizing pneumonia, large consolidation, or pleural effusion [6, 24••].
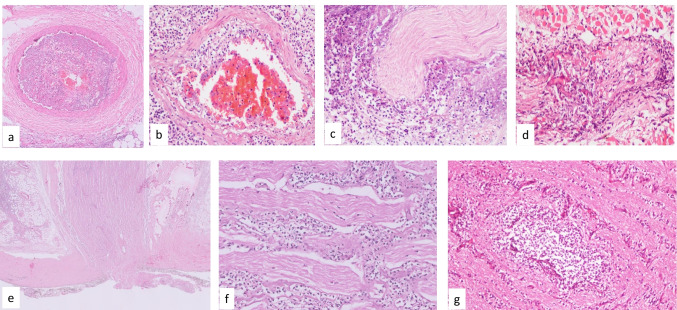
The hallmark of pathology is tissue necrosis and infarction due to angioinvasion [65]. The jugular veins and carotid artery may be involved. Thrombotic occlusion with narrowed lumen due to invasion by fungal hyphae and acute vasculitis with fungal hyphae within media and intima are seen in CAM. Perineural involvement is seen in disseminated infections along with angioinvasion [106–108]. Trigeminal and facial cranial nerve palsy can occur [104, 107]. Even though dura around the optic nerve is a tough barrier, the optic nerve can also be involved in CAM. Optic nerve is infiltrated by the fungal hyphae, accompanied by neutrophilic infiltrate. In fulminant cases of CAM, micro-abscesses have been noted within the optic nerve (Fig. 3).
Fig. 3.
Photomicrographs showing a thrombotic occlusion with narrowed lumen due to invasion by fungal hyphae; b acute vasculitis and fungal hyphae within media and intima; c & d perineural and intraneural invasion by fungus in the adjacent soft tissue; e involvement of the optic nerve by the fungal hyphae; f optic nerve with neutrophilic infiltrate; g micro-abscess in the optic nerve (H&E × 100)
CNS pathology is usually hemorrhagic infarct with cavitation involving the white matter or diencephalic nuclei or in the form of abscess involving frontal lobes [105]. The inflammatory response is neutrophilic in majority, with suppurating granuloma or minimal to absent inflammation in others [106] (Fig. 4). In the chronic invasive form, small micro-abscesses are seen surrounded by epitheloid cells, giant cells, eosinophils, and neutrophils, sometimes with Splendore-Hopple phenomenon [105]. Skull base osteomyelitis with epidural or subperiosteal abscess may be seen.
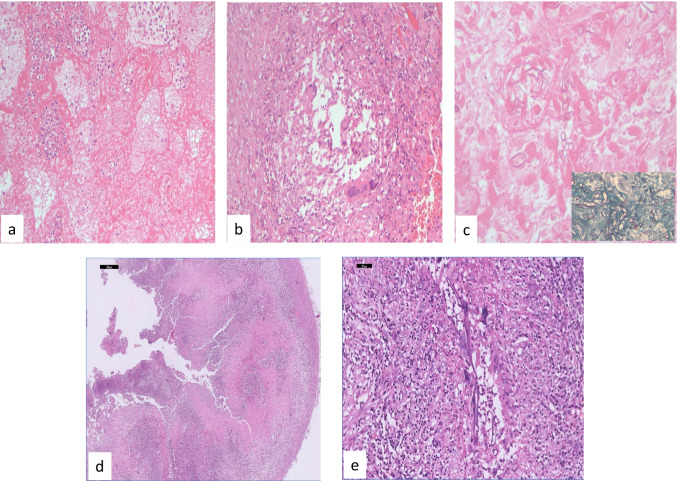
Fig. 4.
Photomicrographs from lobectomy specimen of lung showing a hemorrhagic infarct; H&E × 100; b granulomatous inflammation with fungal hyphae within multinucleated giant cells H&E × 100; c photomicrograph showing numerous hyphae in bland necrotic tissue H&Ex100; Inset showing broad aseptate and folded hyphae Gomori methenamine silver × 400; photomicrographs of brain abscess with neutrophilic infiltrate and broad aseptate hyphae of Mucorales: d H&E × 100; e H&E × 400
Pulmonary involvement is seen as hemorrhagic infarct, cavitary lesion, or abscess (Fig. 4).
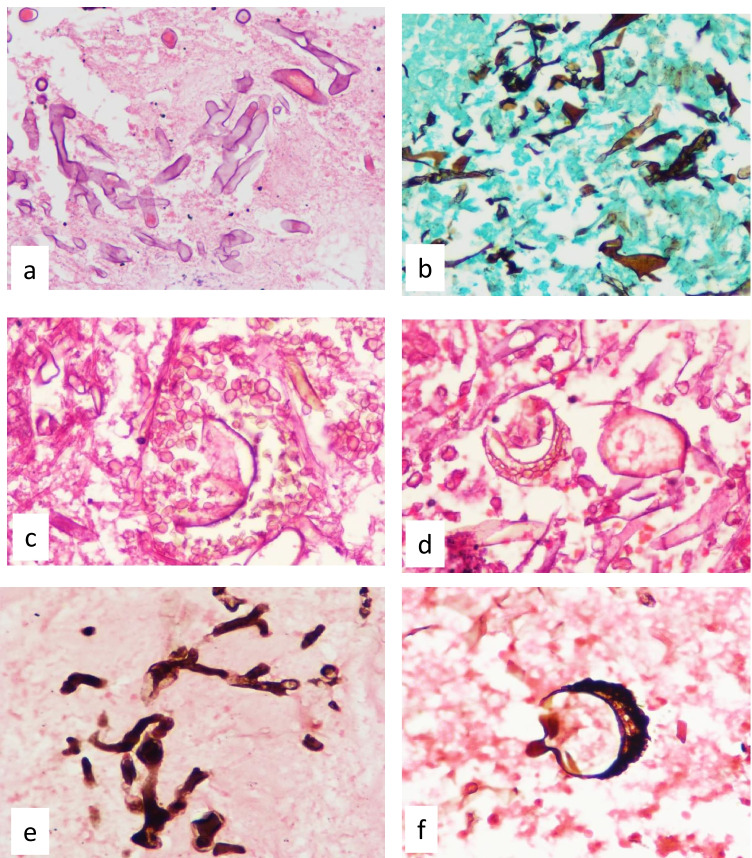
Histopathologic examination shows characteristic broad (3–25-µm diameter) thin-walled, hyaline aseptate/pauci-septate hyphae with irregular or right-angle branching. The hyphae are highlighted by Gomori methenamine stain (GMS) (Fig. 5) and periodic-acid Schiff stains (PAS) [109]. The pale eosinophilic hyphae are seen on hematoxylin and eosin (H&E) in the necrotic material or in the walls of the blood vessels or perineurium of the nerves. In CAM, pigmented/melanized hyphae and sporangia were seen in tissue sections. The melanized walls of fungus may be faintly seen on H&E stain, but they are better demonstrated and confirmed by Masson Fontana histochemical stain which gives black color to the fungus (Fig. 5).
Fig. 5.
Photomicrographs showing broad hyaline aseptate hyphae with irregular and right-angle branching, a H&E × 400; b Gomori methenamine silver × 400; c & d melanized hyphae and sporangia in tissue section (H&E × 400); e & f melanin pigment in the fungal cell walls and sporangia confirmed and highlighted on melanin stain (Masson Fontana × 400)
Sporangia are more commonly reported in colonizing Aspergillus spp. in lung cavities that have direct communication with environment and exposure to high oxygen tension [109]. Sporangia of Mucorales in tissues are extremely uncommon [110]. However, sporangia of Mucorales were seen in CAM, both in the sinus and lung tissues.
Possible Pathophysiology of Melanization of Mucorales Hyphae in CAM
Mucorales are classified as hyaline fungi, and this morphology helps in making the diagnosis as well as differentiate from other hyaline fungi like Aspergillus spp., Fusarium spp., or dematiaceous (pigmented) fungi in tissue sections stained with H&E [106, 109, 111]. However, melanin can appear under specific developmental phases (i.e., conidia) or can be induced in response to environmental queues or in vitro [112, 113]. Melanization represents a general adaptation mechanism to adverse conditions [114]. Melanization of hyaline fungi was rarely reported [115••, 116]. The possible factors in CAM that may induce melanization of hyphae are considered here.
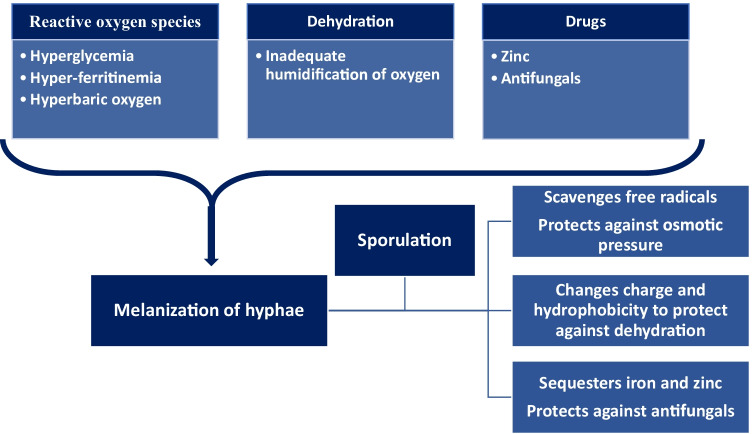
The factors like hyperglycemia, hypoxia, and acidosis promote the growth of Mucorales in the host. Hyperglycemia and hyperferritinemia, which are the key pathogenetic factors in CAM, produce ROS [117]. Hyperbaric oxygen therapy is a beneficial adjunct therapy for mucormycosis and is known to boost phagocytosis, alleviate acidosis, and improve the function of antifungals [118, 119]. The treatment of COVID-19 includes administration of oxygen under pressure to combat hypoxia. However, it causes oxidative stress and can induce ROS. Melanin, as a virulence factor, protects the fungus by scavenging the free radicals [120••, 121]. Melanin also protects against the high osmotic pressure. Inadequate humidification of oxygen was reported during the epidemic of CAM in India [30, 120••, 121]. Melanin exerts a protective effect to the resultant dehydration and dry microenvironment by altering the charge and hydrophobicity on the hyphae [122–125]. High doses of zinc were administered as part of treatment for COVID-19 [83••]. Administration of zinc in high doses and for prolonged periods enhances fungal virulence and promotes its growth [126]. Melanin also helps in the sequestration of iron and zinc, which are essential for the growth of fungus as well as to combat ROS [23••, 126]. Melanin also protects against antifungal drugs, which may be used in treatment of CAM [122–124]. Melanin as a virulence factor also helps the growth of the fungus by evading host immunity. It inhibits macrophage apoptosis and phagolysosome fusion, alters cytokine responses, and attenuates the host immune response [125]. The protective effects of melanin against the therapeutic measures in COVID-19, probably help in fungal development and conidiation [120••]. These factors possibly explain the melanization of the hyphae and sporangia in tissue sections in CAM (Fig. 6).
Fig. 6.
Pathophysiology of melanization of Mucorales hyphae
Conclusions
The pathogenesis of CAM is multi-factorial and complex. High prevalence of DM and undetected or newly detected DM during the pandemic was the most common pre-existing disease in CAM. Corticosteroid administration in high doses and prolonged periods is one of the known risk factors for mucormycosis and this was exaggerated by the indiscriminate use of steroids in this health crisis. COVID-19 itself can result in impaired immune system as well as aggravate hyperglycemia. The treatment of COVID-19 with corticosteroids and other immunomodulatory drugs contributes to immune dysfunction. Hyperglycemia and DKA make free available iron and, in the presence of lowered host defenses, promote specific interaction of host cells with Mucorales hyphae, resulting in CAM. The microenvironment of nose and PNS is affected by the antibiotics and metabolic alterations, contributing to CAM. ROCM was the most common clinical form of CAM with Rhizopus arrhizus as the most common etiologic agent. Extensive soft tissue involvement with angioinvasion and perineural invasion were seen in CAM. In addition, melanization of hyphae and sporangia were seen on histology in CAM, probably due to the virulence of Rhizopus spp. to protect it from ROS resulting from hyperglycemia, hyperferritinemia, hyperbaric oxygen, and the dehydration. CAM provided a unique opportunity to understand the pathogenesis of mucormycosis and virulence of Rhizopus spp.
Declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights
This article does not contain any studies with human and animal subjects performed by any of the authors.
Permissions
The figures and table are original and not published previously.
Footnotes
This article is part of the Topical Collection on Clinical Pathology
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bishan Radotra, Email: bishanradotra@gmail.com.
Sundaram Challa, Email: sundaramchalla@gmail.com.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 2.Chayakulkeeree M, Ghannoum MA, Perfect JR. Zygomycosis: the re-emerging fungal infection. Eur J Clin Microbiol Infect Dis. 2006;25(4):215–229. doi: 10.1007/s10096-006-0107-1. [DOI] [PubMed] [Google Scholar]
- 3.Lanternier F, Lortholary O. Zygomycosis and diabetes mellitus. Clin Microbiol Infect. 2009;15:21–25. doi: 10.1111/j.1469-0691.2009.02975.x. [DOI] [PubMed] [Google Scholar]
- 4.Roilides E, Zaoutis TE, Walsh TJ. Invasive zygomycosis in neonates and children. Clin Microbiol Infect. 2009;15:50–54. doi: 10.1111/j.1469-0691.2009.02981.x. [DOI] [PubMed] [Google Scholar]
- 5.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50(8):1091–100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 6.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl_1): S23–34. [DOI] [PubMed]
- 7.Chakrabarti A, Singh R. Mucormycosis in India: unique features. Mycoses. 2014;57:85–90. doi: 10.1111/myc.12243. [DOI] [PubMed] [Google Scholar]
- 8.Guinea J, Escribano P, Vena A, Muñoz P, Martínez-Jiménez MD, Padilla B, et al. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: Epidemiology and microbiological characterization of the isolates. PLoS One. 2017;12(6):e0179136. doi: 10.1371/journal.pone.0179136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SC, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–21. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DC, Chen SA. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25(1):26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 11.•• Patel A, Kaur H, Xess I, Michael JS, Savio J, Rudramurthy S, et al. A multicenter observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26:944.e9-944.e15. This article shows the status of mucormycosis in India in pre-COVID time and helps to compare and analyze the pathophysiology of CAM. [DOI] [PubMed]
- 12.• Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi (Basel). 2019;5(1):26. This article explains the increased incidence and prevalence of mucormycosis globally, mainly in DM patients. [DOI] [PMC free article] [PubMed]
- 13.Prakash H, Chakrabarti A. Epidemiology of mucormycosis in India. Microorganisms. 2021;9(3):523. doi: 10.3390/microorganisms9030523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chander J, Kaur M, Singla N, Punia RP, Singhal SK, Attri AK, et al. Mucormycosis: battle with the deadly enemy over a five-year period in India. J Fungi. 2018;4(2):46. doi: 10.3390/jof4020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skiada A, Pavleas I. Drogari-Apiranthitou M. J Fungi (Basel). 2020;6(265):10–3390. [DOI] [PMC free article] [PubMed]
- 16.Aranjani JM, Manuel A, Abdul Razack HI, Mathew ST. COVID-19–associated mucormycosis: evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PLoS Negl Trop Dis. 2021;15(11):e0009921. doi: 10.1371/journal.pntd.0009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi. 2021;7(4):298. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muthu V, Rudramurthy SM, Chakrabarti A, Agarwal R. Epidemiology and pathophysiology of COVID-19-associated mucormycosis: India versus the rest of the world. Mycopathologia. 2021;186(6):739–754. doi: 10.1007/s11046-021-00584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.•• Patel A, Agarwal R, Rudramurthy SM, Shevkani M, Xess I, Sharma R, et al. Multicenter epidemiologic study of coronavirus disease–associated mucormycosis, India. Emerg Infect Dis. 2021;27(9):2349. This article shows the scenario of CAM in India and highlights the pathophysiology. [DOI] [PMC free article] [PubMed]
- 20.Salmanton-García J, Sprute R, Stemler J, Bartoletti M, Dupont D, Valerio M, et al. COVID-19–associated pulmonary aspergillosis, March–August 2020. Emerg Infect Dis. 2021;27(4):1077. doi: 10.3201/eid2704.204895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.• Sen M, Honavar SG, Bansal R, Sengupta S, Rao R, Kim U, Sharma M, Sachdev M, Grover AK, Surve A, Budharapu A. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India-Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69(7):1670. A very large series of ROCM from India with detailed analysis of the ophthalmic manifestations of CAM. [DOI] [PMC free article] [PubMed]
- 22.Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15(4):102146. doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.•• Chakrabarti SS, Kaur U, Aggarwal SK, Kanakan A, Saini A, Agrawal BK, et al. The pathogenetic dilemma of post-COVID-19 mucormycosis in India. Aging Dis. 2022;13(1):24. Excellent paper that discusses the pathogenesis of CAM due to risk factors other than hyperglycemia and immune dysregulation. [DOI] [PMC free article] [PubMed]
- 24.•• Hoenigl M, Seidel D, Carvalho A, Rudramurthy SM, Arastehfar A, Gangneux JP, et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. 2022. This study of CAM from 18 countries shows the relative prevalence of risk factors in different countries and discusses the pathogenesis of CAM. [DOI] [PMC free article] [PubMed]
- 25.Hughes S, Troise O, Donaldson H, Mughal N, Moore LS. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(9):e10726. [DOI] [PMC free article] [PubMed]
- 27.Mohamed A, Rogers TR, Talento AF. COVID-19 associated invasive pulmonary aspergillosis: diagnostic and therapeutic challenges. Journal of fungi. 2020;6(3):115. doi: 10.3390/jof6030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rawson TM, Moore LS, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;185(4):599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garg D, Muthu V, Sehgal IS, Ramachandran R, Kaur H, Bhalla A, et al. Coronavirus disease (COVID-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186(2):289–298. doi: 10.1007/s11046-021-00528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.• Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS One. 2021;16(5):e0251170. A review on secondary infections in COVID-19. [DOI] [PMC free article] [PubMed]
- 32.Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135(5):442–447. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe A, So M, Mitaka H, Ishisaka Y, Takagi H, Inokuchi R, et al. Clinical features and mortality of COVID-19-associated mucormycosis: a systematic review and meta-analysis. Mycopathologia. 2022;187(2–3):271–289. doi: 10.1007/s11046-022-00627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arora U, Priyadarshi M, Katiyar V, Soneja M, Garg P, Gupta I, Bharadiya V, Berry P, Ghosh T, Patel L, Sarda R. Risk factors for coronavirus disease-associated mucormycosis. J Infect. 2022;84(3):383–390. doi: 10.1016/j.jinf.2021.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy YM, Yeduguri S, Parida S, Kamatham SN, Pidaparthi L, Jaiswal SK, Sadhvani B, Tourani V, Kumar S, Challa S, Murthy JM. Pathogenetic factors fanning the flames of COVID-19 to cause rhino-orbito-cerebral mucormycosis: An observational study. J Med Mycol. 2022;32(2):101252. doi: 10.1016/j.mycmed.2022.101252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Downloaded from https://www.covid19treatmentguidelines.nih.gov/ on 6/21/2022.
- 37.Kontoyiannis DP, Lewis RE. How I treat mucormycosis. Blood. 2011;118(5):1216–1224. doi: 10.1182/blood-2011-03-316430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moorthy A, Gaikwad R, Krishna S, Hegde R, Tripathi KK, Kale PG, Rao PS, Haldipur D, Bonanthaya K. SARS-CoV-2, uncontrolled diabetes and corticosteroids—an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg. 2021;20(3):418–425. doi: 10.1007/s12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selarka L, Sharma S, Saini D, Sharma S, Batra A, Waghmare VT, et al. Mucormycosis and COVID-19: an epidemic within a pandemic in India. Mycoses. 2021;64(10):1253–1260. doi: 10.1111/myc.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arastehfar A, Carvalho A, Nguyen MH, Hedayati MT, Netea MG, Perlin DS, et al. COVID-19-associated candidiasis (CAC): an underestimated complication in the absence of immunological predispositions? J Fungi. 2020;6(4):211. doi: 10.3390/jof6040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koehler P, Cornely OA, Böttiger BW, Dusse F, Eichenauer DA, Fuchs F, et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63(6):528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fekkar A, Lampros A, Mayaux J, Poignon C, Demeret S, Constantin JM, Marcelin AG, Monsel A, Luyt CE, Blaize M. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am J Respir Crit Care Med. 2021;203(3):307–317. doi: 10.1164/rccm.202009-3400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lahmer T, Kriescher S, Herner A, Rothe K, Spinner CD, Schneider J, et al. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: results from the prospective AspCOVID-19 study. PLoS One. 2021;16(3):e0238825. doi: 10.1371/journal.pone.0238825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roudbary M, Kumar S, Kumar A, Černáková L, Nikoomanesh F, Rodrigues CF. Overview on the prevalence of fungal infections, immune response, and microbiome role in COVID-19 patients. J Fungi. 2021;7(9):720. doi: 10.3390/jof7090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pamidimukkala U, Sudhaharan S, Kancharla A, Vemu L, Challa S, Karanam SD, Chavali P, Prakash H, Ghosh AK, Gupta S, Rudramurthy SM. Mucormycosis due to Apophysomyces species complex-25 years’ experience at a tertiary care hospital in southern India. Med Mycol. 2020;58(4):425–433. doi: 10.1093/mmy/myz081. [DOI] [PubMed] [Google Scholar]
- 48.Dolatabadi S, de Hoog GS, Meis JF, Walther G. Species boundaries and nomenclature of Rhizopus arrhizus (syn. R. oryzae). Mycoses. 2014;57:108–27. [DOI] [PubMed]
- 49.Ma LJ, Ibrahim AS, Skory C, Grabherr MG, Burger G, Butler M, et al. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 2009;5(7):e1000549. doi: 10.1371/journal.pgen.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arana DM, Prieto D, Román E, Nombela C, Alonso-Monge R, Pla J. The role of the cell wall in fungal pathogenesis. Microb Biotechnol. 2009;2(3):308–320. doi: 10.1111/j.1751-7915.2008.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghuman H, Voelz K. Innate and adaptive immunity to Mucorales. J Fungi (Basel) 2017;3(3):48. doi: 10.3390/jof3030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gow NAR, Latge JP, Munro CA. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr. 2017;5(3). 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PubMed]
- 53.Challa S. Mucormycosis: pathogenesis and pathology. Curr Fungal Infect Rep. 2019;13(1):11–20. doi: 10.1007/s12281-019-0337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanford FA, Voigt K. Iron assimilation during emerging infections caused by opportunistic fungi with emphasis on Mucorales and the development of antifungal resistance. Genes. 2020;11(11):1296. doi: 10.3390/genes11111296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soare AY, Watkins TN, Bruno VM. Understanding mucormycoses in the age of “omics”. Front Genet. 2020;11:699. doi: 10.3389/fgene.2020.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.•• Morales-Franco B, Nava-Villalba M, Medina-Guerrero EO, Sánchez-Nuño YA, Davila-Villa P, Anaya-Ambriz EJ, et al. Host-pathogen molecular factors contribute to the pathogenesis of Rhizopus spp. in diabetes mellitus. Curr Trop Med Rep. 2021;8(1):6–17. An excellent paper on the pathogenesis of mucormycosis. This article discusses the virulence of Rhizopus in DM. [DOI] [PMC free article] [PubMed]
- 57.Skiada A, Pagano LI, Groll A, Zimmerli S, Dupont B, Lagrou K, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17(12):1859–1867. doi: 10.1111/j.1469-0691.2010.03456.x. [DOI] [PubMed] [Google Scholar]
- 58.• Narayanan S, Chua JV, Baddley JW. Coronavirus disease 2019–associated mucormycosis: risk factors and mechanisms of disease. Clin Infect Dis. 2022;74(7):1279–83. This paper discusses the pathomechanisms in CAM. [DOI] [PMC free article] [PubMed]
- 59.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236–301. doi: 10.1128/CMR.13.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gianchandani R, Esfandiari NH, Ang L, Iyengar J, Knotts S, Choksi P, et al. Managing hyperglycemia in the COVID-19 inflammatory storm. Diabetes. 2020;69(10):2048–2053. doi: 10.2337/dbi20-0022. [DOI] [PubMed] [Google Scholar]
- 61.Wu L, Girgis CM, Cheung NW. COVID-19 and diabetes: insulin requirements parallel illness severity in critically unwell patients. Clin Endocrinol. 2020;93(4):390–393. doi: 10.1111/cen.14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kothandaraman N, Rengaraj A, Xue B, Yew WS, Velan SS, Karnani N, Leow MK. COVID-19 endocrinopathy with hindsight from SARS. Am J Physiol-Endocrinol Metab. 2021;320(1):E139–E150. doi: 10.1152/ajpendo.00480.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith SM, Boppana A, Traupman JA, Unson E, Maddock DA, Chao K, Dobesh DP, Brufsky A, Connor RI. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J Med Virol. 2021;93(1):409–415. doi: 10.1002/jmv.26227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamez-Pérez HE, Quintanilla-Flores DL, Rodríguez-Gutiérrez R, González-González JG, Tamez-Peña AL. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J Diabetes. 2015;6(8):1073. doi: 10.4239/wjd.v6.i8.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baldin C, Ibrahim AS. Molecular mechanisms of mucormycosis—the bitter and the sweet. PLoS Pathog. 2017;13(8):e1006408. doi: 10.1371/journal.ppat.1006408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020;16(6):308–310. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez-Rojas MA, Vega-Vega O, Bobadilla NA. Is the kidney a target of SARS-CoV-2? Am J Physiol-Renal Physiol. 2020;318(6):F1454–F1462. doi: 10.1152/ajprenal.00160.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tabassum T, Araf Y, Moin AT, Rahaman TI, Hosen MJ. COVID-19-associated-mucormycosis: possible role of free iron uptake and immunosuppression. Mol Biol Rep. 2022;49(1):747–754. doi: 10.1007/s11033-021-06862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bayram N, Ozsaygılı C, Sav H, Tekin Y, Gundogan M, Pangal E, Cicek A, Özcan İ. Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations. Jpn J Ophthalmol. 2021;65(4):515–525. doi: 10.1007/s10384-021-00845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhogireddy R, Krishnamurthy V, Pullaiah CP, Manohar S. Is Mucormycosis an inevitable complication of COVID-19 in India? Braz J Infect Dis. 2021;25(3):101597. doi: 10.1016/j.bjid.2021.101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodriguez-Morales AJ, Sah R, Millan-Oñate J, Gonzalez A, Montenegro-Idrogo JJ, Scherger S, et al. COVID-19 associated mucormycosis: the urgent need to reconsider the indiscriminate use of immunosuppressive drugs. Ther Adv Infect Dis. 2021;18:8. doi: 10.1177/20499361211027065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bohn MK, Hall A, Sepiashvili L, Jung B, Steele S, Adeli K. Pathophysiology of COVID-19: mechanisms underlying disease severity and progression. Physiology. 2020;35(5):288–301. doi: 10.1152/physiol.00019.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29(9):401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Azhar A, Khan WH, Khan PA, Al-hosaini K, Owais M, Ahmad A. Mucormycosis and COVID-19 pandemic: clinical and diagnostic approach. J Infect Public Health. 2022;15(4):466–79. [DOI] [PMC free article] [PubMed]
- 75.Files JK, Boppana S, Perez MD, Sarkar S, Lowman KE, Qin K, et al. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J Clin Investig. 2021;131(1):140491. [DOI] [PMC free article] [PubMed]
- 76.Perkhofer S, Kainzner B, Kehrel BE, Dierich MP, Nussbaumer W, Lass-Flörl C. Potential antifungal effects of human platelets against zygomycetes in vitro. J Infect Dis. 2009;200(7):1176–1179. doi: 10.1086/605607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jenne CN, Kubes P. Platelets in inflammation and infection. Platelets. 2015;26(4):286–292. doi: 10.3109/09537104.2015.1010441. [DOI] [PubMed] [Google Scholar]
- 78.Ghuman H, Shepherd-Roberts A, Watson S, Zuidscherwoude M, Watson SP, Voelz K. Mucor circinelloides induces platelet aggregation through integrin αIIbβ3 and FcγRIIA. Platelets. 2019;30(2):256–263. doi: 10.1080/09537104.2017.1420152. [DOI] [PubMed] [Google Scholar]
- 79.Portier I, Campbell RA. Phenotypic differences between polygenic and monogenic hypobetalipoproteinemia. Arterioscler Thromb Vasc Biol. 2021;41(1):70–78. doi: 10.1161/ATVBAHA.120.315491. [DOI] [PubMed] [Google Scholar]
- 80.Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362(9398):1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 81.Barshes NR, Goodpastor SE, Goss JA. Pharmacologic immunosuppression. Front Biosci -Landmark. 2004;9(1):411–420. doi: 10.2741/1249. [DOI] [PubMed] [Google Scholar]
- 82.Cain DW, Cidlowski JA. Immune regulation by corticosteroids. Nat Rev Immunol. 2017;17:233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.•• Kumar M, Sarma DK, Shubham S, Kumawat M, Verma V, Singh B, et al. Mucormycosis in COVID-19 pandemic: risk factors and linkages. Curr Res Microbial Sci. 2021;2:100057. Excellent paper that shows how different risk factors predispose to CAM. [DOI] [PMC free article] [PubMed]
- 84.Shovman O, Shoenfeld Y, Langevitz P. Tocilizumab-induced neutropenia in rheumatoid arthritis patients with previous history of neutropenia: case series and review of literature. Immunol Res. 2015;61(1):164–168. doi: 10.1007/s12026-014-8590-4. [DOI] [PubMed] [Google Scholar]
- 85.Gallo O, Locatello LG, Mazzoni A, Novelli L, Annunziato F. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol. 2021;14(2):305–316. doi: 10.1038/s41385-020-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaidyanathan G. Coronavirus variants are spreading in India—what scientists know so far. Nature. 2021;593(7859):321–322. doi: 10.1038/d41586-021-01274-7. [DOI] [PubMed] [Google Scholar]
- 87.Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal. 2009;11(9):2299–2306. doi: 10.1089/ars.2009.2568. [DOI] [PubMed] [Google Scholar]
- 88.Ibrahim IM, Abdelmalek DH, Elfiky AA. GRP78: A cell’s response to stress. Life Sci. 2019;226:156–163. doi: 10.1016/j.lfs.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elfiky AA, Baghdady AM, Ali SA, Ahmed MI. GRP78 targeting: hitting two birds with a stone. Life Sci. 2020;260:118317. doi: 10.1016/j.lfs.2020.118317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl_1):S16–22. [DOI] [PMC free article] [PubMed]
- 91.Bouchara JP, Oumeziane NA, Lissitzky JC, Larcher G, Tronchin G, Chabasse D. Attachment of spores of the human pathogenic fungus Rhizopus oryzae to extracellular matrix components. Eur J Cell Biol. 1996;70(1):76–83. [PubMed] [Google Scholar]
- 92.Soliman SS, Baldin C, Gu Y, Singh S, Gebremariam T, Swidergall M, Alqarihi A, Youssef EG, Alkhazraji S, Pikoulas A, Perske C. Mucoricin is a ricin-like toxin that is critical for the pathogenesis of mucormycosis. Nat Microbiol. 2021;6(3):313–326. doi: 10.1038/s41564-020-00837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.• Alqarihi A, Gebremariam T, Gu Y, Swidergall M, Alkhazraji S, Soliman SS, et al. GRP78 and integrins play different roles in host cell invasion during mucormycosis. MBio. 2020;11(3):e01087-e1120. This article discusses the interaction of GRP-78 on nasal epithelial cells via CotH3 on Rhizopus delemar to invade and damage the nasal epithelial cells. [DOI] [PMC free article] [PubMed]
- 94.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.•• Jose A, Singh S, Roychoudhury A, et al. Current understanding in the pathophysiology of SARS-CoV-2-associated Rhino-orbito-cerebral mucormycosis: a comprehensive review. J Maxillofac Oral Surg. 2021;20:373–80. 10.1007/s12663-021-01604-2. An excellent paper that focuses on the pathogenesis of rhino-orbito-cerebral mucormycosis. It highlights the role of ferritin, endothelitis and GRP-78. [DOI] [PMC free article] [PubMed]
- 96.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia–a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prakash H, Singh S, Rudramurthy SM, Singh P, Mehta N, Shaw D, Ghosh AK. An aero mycological analysis of mucormycetes in indoor and outdoor environments of northern India. Med Mycol. 2020;58(1):118–123. doi: 10.1093/mmy/myz031. [DOI] [PubMed] [Google Scholar]
- 98.Hosseini SM, Borghei P. Rhinocerebral mucormycosis: pathways of spread. Eur Arch Otorhinolaryngol Head Neck. 2005;262(11):932–938. doi: 10.1007/s00405-005-0919-0. [DOI] [PubMed] [Google Scholar]
- 99.Valvi B, Shah K, Pandilwar P, Parmar S, Shaikh W. Post COVID 19-mucormycosis and osteomyelitis of the mandible-a rare case report. J Oral Med. 2022;4:230–234. [Google Scholar]
- 100.Ambereen A, Rahman SA, Rehman S, Zaidi K, Arif SH. Mandibular mucormycosis following SARS-CoV-2 infection–a case report and review of literature. Clin Infect Pract. 2021;12:100099. doi: 10.1016/j.clinpr.2021.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nayak S, Das T, Parameswarappa D, Sharma S, Jakati S, Jalali S, Narayanan R, Basu S, Tyagi M, Dave VP, Pappuru RR. Sight-threatening intraocular infection in patients with COVID-19 in India. Indian J Ophthalmol. 2021;69(12):3664. doi: 10.4103/ijo.IJO_1474_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Talmi YP, Goldschmied-Reouven A, Bakon M, Barshack I, Wolf M, Horowitz Z, Berkowicz M, Keller N, Kronenberg J. Rhino-orbital and rhino-orbito-cerebral mucormycosis. Otolaryngol-Head Neck Surg. 2002;127(1):22–31. doi: 10.1067/mhn.2002.126587. [DOI] [PubMed] [Google Scholar]
- 103.Prabhu RM, Patel R. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect. 2004;10:31–47. doi: 10.1111/j.1470-9465.2004.00843.x. [DOI] [PubMed] [Google Scholar]
- 104.Kaushik KS, Ananthasivan, Acharya UV. Rawat S, Patil UD, Shankar B, et al. Spectrum of intracranial complications of rhino-orbito-cerebral mucormycosis — resurgence in the era of COVID-19 pandemic: a pictorial essay. Emerg Radiol 2021;28:1097–1106. [DOI] [PMC free article] [PubMed]
- 105.Shankar SK, Mahadevan A, Sundaram C, Sarkar C, Chacko G, Lanjewar DN, Santosh V, Yasha TC, Radhakrishnan VV. Pathobiology of fungal infections of the central nervous system with special reference to the Indian scenario. Neurol India. 2007;55(3):198–215. doi: 10.4103/0028-3886.35680. [DOI] [PubMed] [Google Scholar]
- 106.Cornely O, Arikan-Akdagli SE, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano LI, Skiada A, Akova M, Arendrup MC. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014;20:5–26. doi: 10.1111/1469-0691.12371. [DOI] [PubMed] [Google Scholar]
- 107.Frater JL, Hall GS, Procop GW. Histologic features of zygomycosis: emphasis on perineural invasion and fungal morphology. Arch Pathol Lab Med. 2001;125(3):375–378. doi: 10.5858/2001-125-0375-HFOZ. [DOI] [PubMed] [Google Scholar]
- 108.Sravani T, Uppin SG, Uppin MS, Sundaram C. Rhinocerebral mucormycosis: pathology revisited with emphasis on perineural spread. Neurol India. 2014;62(4):383–386. doi: 10.4103/0028-3886.141252. [DOI] [PubMed] [Google Scholar]
- 109.Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247–280. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chandler FW, Watts JC. Pathologic diagnosis of fungal infections ASCP Press. Chicago: IL; 1987. Pathologic diagnosis of fungal infections; p. 303. [Google Scholar]
- 111.Chavez JA, Brat DJ, Hunter SB, Velazquez Vega J, Guarner J. Practical diagnostic approach to the presence of hyphae in neuropathology specimens with three illustrative cases. Am J Clin Pathol. 2018;149(2):98–104. doi: 10.1093/ajcp/aqx144. [DOI] [PubMed] [Google Scholar]
- 112.Bell AA, Wheeler MH. Biosynthesis and functions of fungal melanins. Annu Rev Phytopathol. 1986;24(1):411–451. doi: 10.1146/annurev.py.24.090186.002211. [DOI] [Google Scholar]
- 113.Sephton-Clark PCS, Muñoz JF, Ballou ER, Cuomo CA, Voelz K. 2018. Pathways of pathogenicity: transcriptional stages of germination in the fatal fungal pathogen Rhizopus delemar. mSphere 3:e00403–18. 10.1128/mSphere.00403-18 [DOI] [PMC free article] [PubMed]
- 114.Roulin A. Melanin-based colour polymorphism responding to climate change. Glob Change Biol. 2014;20(11):3344–3350. doi: 10.1111/gcb.12594. [DOI] [PubMed] [Google Scholar]
- 115.•• Chongkae S, Nosanchuk JD, Pruksaphon K, Laliam A, Pornsuwan S, Youngchim S. Production of melanin pigments in saprophytic fungi in vitro and during infection. J Basic Microbiol. 2019;59(11):1092–104. Excellent paper that discusses the production of melanin pigment. [DOI] [PubMed]
- 116.Oddó D, Cisternas D, Méndez GP. Pseudodematiaceous fungi in rhinosinusal biopsies: report of 2 cases with light and electron microscopy analysis. Clin Pathol. 2019;12:2632010X19874766. [DOI] [PMC free article] [PubMed]
- 117.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.John BV, Chamilos G, Kontoyiannis DP. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin Microbiol Infect. 2005;11(7):515–517. doi: 10.1111/j.1469-0691.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 119.Kontoyiannis DP, Lewis RE. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect Dis Clin. 2006;20(3):581–607. doi: 10.1016/j.idc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 120.•• Cordero RJ, Casadevall A. Functions of fungal melanin beyond virulence. Fungal Biol Rev. 2017;31(2):99–112. This paper gives a beautiful and detailed analysis on the role of melanin in fungal biology. [DOI] [PMC free article] [PubMed]
- 121.Lin L, Xu J. Fungal pigments and their roles associated with human health. J Fungi. 2020;6(4):280. doi: 10.3390/jof6040280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gómez BL, Nosanchuk JD. Melanin and fungi. Curr Opin Infect Dis. 2003;16(2):91–96. doi: 10.1097/00001432-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 123.Nosanchuk JD, Casadevall A. The contribution of melanin to microbial pathogenesis. Cell Microbiol. 2003;5(4):203–223. doi: 10.1046/j.1462-5814.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- 124.Nosanchuk JD, Casadevall A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob Agents Chemother. 2006;50(11):3519–3528. doi: 10.1128/AAC.00545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Andrianaki AM, Kyrmizi I, Thanopoulou K, Baldin C, Drakos E, Soliman SS, Shetty AC, McCracken C, Akoumianaki T, Stylianou K, Ioannou P. Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species. Nat Commun. 2018;9(1):3333. doi: 10.1038/s41467-018-05820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Staats CC, Kmetzsch L, Schrank A, Vainstein MH. Fungal zinc metabolism and its connections to virulence. Front Cell Infect Microbiol. 2013;3:65. doi: 10.3389/fcimb.2013.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]