Summary
Background
Although air pollution has been frequently linked to a range of cardiometabolic diseases, its association with the onset, progression, and prognosis of cardiometabolic multimorbidity (CMM) has never been studied.
Methods
We conducted this prospective analysis based on the UK Biobank cohort. CMM was defined as the coexistence of at least two cardiometabolic diseases, including type 2 diabetes, ischemic heart disease and stroke. Multi-state model was used to analyze the association between air pollution and the trajectory of CMM.
Findings
410,494 middle- and old-age participants were included. During a median follow-up of 12.0 years, 56,877 participants developed first cardiometabolic disease (FCMD), 8616 developed CMM, and 22,423 died. The risks of transitions from baseline to FCMD, from FCMD to CMM, and transitions from baseline and FCMD to all-cause mortality increased by 3% (2%, 5%), 3% (1%, 6%), 5% (2%, 7%) and 2% (−1%, 6%), respectively, per interquartile range increase of fine particulate matter. The corresponding increases were 3% (2%, 5%), 6% (3%, 9%), 4% (2%, 7%) and 6% (2%, 10%), respectively, for nitrogen dioxide. Older participants, males, and individuals with excessive alcohol drinking and lower economic levels were more likely to experience these risks.
Interpretation
Air pollution exposures could play important roles in almost all transition phases of CMM development. Our results highlight clean air as an upstream approach to mitigate both initiation and progression of CMM, especially in vulnerable populations.
Funding
Shanghai Municipal Science and Technology Commission (21TQ015); The National Natural Science Foundation of China (92143301 and 92043301).
Keywords: Air pollution, Fine particulate matter, Nitrogen dioxide, Cardiometabolic multimorbidity, Cardiovascular diseases, Diabetes
Research in context.
Evidence before this study
Cardiometabolic multimorbidity (CMM), which refers to the co-presence of at least two cardiometabolic diseases (CMDs) typically including diabetes, ischemic heart disease, and stroke, has become a rising public health challenge in an era of aging. Air pollution has been frequently linked to the incidence or mortality for a range of single CMDs. We searched PubMed and Google Scholar for studies on the association between air pollution and CMM, published up to August 31, 2022, using the terms “air pollution”, “fine particulate matter”, “fine particles”, “PM2.5”, “nitrogen dioxide”, “oxynitride” and “NO2 in combination with “cardiometabolic multimorbidity”, “multimorbidity”, “comorbidity”, “cardiometabolic disease”, “cardiovascular diseases”, “diabetes” and “stroke”. We found that most previous studies have focused on single CMDs when investigating the adverse effects of air pollution. Only one study in China has assessed the association between PM2.5 and CMM, reporting an increased risk of CMM associated with PM2.5 exposure (HR, 95% CI: 1.03, 1.03–1.04). The relationship between air pollution and CMM was largely unknown. Moreover, no studies have evaluated the role of air pollution in the onset, progression and prognosis of CMM.
Added value of this study
The present study was based on 410,494 middle- and old-age participants from the UK Biobank, a large, prospective cohort. We explore the impacts of air pollution on the trajectory of CMM. We found that air pollution exposure increased the risk of almost all phases of CMM progression, including developing first cardiometabolic disease (FCMD), transition from FCMD to CMM, and death from baseline and FCMD. The effects of air pollutants on disease-specific transitions differed by sub-types of FCMD (diabetes, ischemic heart disease, and stroke). In addition, older participants, males, and individuals with excessive alcohol drinking and lower economic levels were more likely to experience these risks.
Implications of all the available evidence
Our study demonstrates the role of long-term exposure to air pollution in almost all transition phases of CMM progression. Our findings suggested that clean air might be helpful for the primary and secondary prevention of CMM and for reducing the societal burden of aging. Taking CMM into consideration when assessing air pollution-related disease burden and developing health protection strategies is highly proposed in the future. The chronic disease state may be monitored more frequently for participants living in polluted areas.
Alt-text: Unlabelled box
Introduction
Cardiometabolic multimorbidity (CMM) refers to the co-presence of at least two cardiometabolic diseases (CMDs), typically including type 2 diabetes (T2D), ischemic heart disease (IHD), and stroke.1, 2, 3 Compared to single CMDs occurring on their own, combination of multiple CMDs has been found to be associated with multiplicative increase in mortality risk and a substantial reduction in life expectancy.3 What makes this issue even worse is the aging of population. In 2019, there were 703 million persons older than 65 years over the world and by 2050, the number of elders is projected to double to 1.5 billion.4 CMM is an issue of great public concern in an era of aging. It was reported that the prevalence of CMM was several-fold higher among population aged 60 years and older than population aged 40 years and older.5 Thus, the identification of potential risk factors of CMM is of great importance to alleviate the health burden and promote healthy aging. Several studies suggested that obesity and lack of physical activity were important risk factors of developing CMM.1,6 However, very few studies have considered the impacts of environmental exposures such as ambient air pollution on the development of CMM.
Ambient air pollution has been recognized as the fourth leading risk factor and the largest environmental risk factor of all-cause mortality globally.7 As two of the most important air pollutants, particulate matter with an aerodynamic diameter ≤ 2.5 µm (PM2.5) and nitrogen dioxide (NO2), have been linked to increased morbidity and mortality of single CMDs in numerous epidemiological studies.8,9 However, their potential effects on the development of CMM are largely unknown. Furthermore, air pollution may theoretically play roles in all key stages of CMM including transitions from a disease-free state to single CMD, subsequently to CMM, and finally to death. Nevertheless, previous studies only focused on the adverse effects of air pollution on one of these transitions (mainly from health to single CMD), which would underestimate the disease burden attributable to air pollution. To the best of our knowledge, no previous studies have evaluated and compared the effects of air pollutants on the incidence, progress, and prognosis of CMM simultaneously, which would have significant implications in the evidence-based prevention and intervention. In addition, premature death from other causes than CMDs may mask the risk of CMD and CMM,10 resulting in a competing risk from death when evaluating the association between air pollution and CMM.11 However, no prior studies had taken competing risk into consideration, which may lead to overestimation of the estimates.12,13
In this study, we sought to evaluate the impacts of PM2.5 and NO2 on the trajectory of CMM, including the transitions from free of CMD to first cardiometabolic diseases (FCMD), then to CMM and further to mortality in the UK Biobank, a large, prospective cohort.14 We further compared the associations across transition paths by different FCMD. We also examined modification effects of sociodemographic characteristics to identify potential vulnerable populations.
Methods
Study participants and outcome identification
During 2006–2010, the UK Biobank cohort recruited over 500,000 middle- to old- age participants in 22 assessment centers across the UK (17 in England, 2 in Scotland and 3 in Wales), covering populations with different genetic backgrounds, socio-economics and lifestyles. Information on demographics, lifestyle and socioeconomic status was collected by questionnaires at baseline. Physical measurements were performed as well to obtain anthropometric data. This study was in accordance with Declaration of Helsinki. All participants in the UK Biobank provided informed consent. The UK Biobank was approved by the North West Multi-Centre Research Ethics Committee (Ref: 11/NW/0382). This work was performed under the UK Biobank application numbers “66251” and “80741”.
In the current study, we defined CMM as copresence of at least two of three CMDs (i.e., T2D, IHD and stroke) in line with many previous studies.1, 2, 3 Incidence of these events was obtained from hospital inpatient visits and coded according to the International Classification of Diseases, 10th Revision (ICD 10th): T2D (E11), IHD (I20–I25) and stroke (I60–I69). Diabetes coded as E14 (unspecified diabetes) was also assigned as T2D because only middle- and old-age participants were recruited in the UK Biobank cohort, and unspecified diabetes was primarily T2D.1,15 Incident cases of all-cause death were identified through linking to national death registries. Details of UK Biobank have been described previously.14
Environmental data
Long-term exposures to PM2.5 and NO2 were measured using a land use regression model developed for the European Study of Cohorts for Air Pollution Effects (ESCAPE).16,17 This model was developed using the ESCAPE monitoring data from January 2010 to January 2011 and covered 36 study areas in Europe, including the UK (Manchester and London). Model validation results showed that this model can explain a large fraction of spatial variability of air pollutants (median cross-validation R2 = 0.77 and 0.87 for PM2.5 and NO2, respectively). Given that this model only used monitoring data collected in 2010, we mapped the model-predicted annual averages of PM2.5 and NO2 for 2010 to the participant's geocoded residential address at baseline to represent long-term exposures to PM2.5 and NO2.
Covariates
We considered age, sex, race, body mass index (BMI), education, socioeconomic status, alcohol drinking, smoking, physical activity, diet at baseline and recruitment centers as candidate covariates, in accordance to priori knowledges.18,19 A directed acyclic graph (DAG) was then generated using DAGitty's online tool (www.dagitty.net) to determine whether a candidate covariate should be adjusted in the models. Specifically, race was classified into White, Asian or Asian British, Black or Black British, Mixed and others. BMI was calculated by dividing weight (kg) by height (m) squared. Education was dichotomized as college degree or above, and high school or below. Socioeconomic status was measured using the Townsend Deprivation Index (TDI), a composite score based on unemployment, overcrowded household, non-car ownership, and non-home ownership. A lower TDI value indicates a higher socioeconomic level.20 Frequency of alcohol drinking was categorized as never, at special occasions only, one to three times a month, once or twice a week, three or four times a week, and daily or almost daily. Self-reported smoking status was divided into never smoking and current/ever smoking. Being physically active was determined using the 2017 UK Physical Activity Guidelines as having 150 minutes of moderate activity or 75 min of vigorous activity per week. A cumulative dietary risk factors score was created using the same method as reported in a previous study from UK Biobank.21 Briefly, a total of 9 food items were used to create the diet score, including processed meat, red meat, total fish, milk, spread type, cereal intake, salt added to food, water, and fruits and vegetables. Each food item was dichotomized as meeting or not meeting recommendations as suggested by the UK and European dietary guidelines. Participants were given 1 point for each unhealthy category. Finally, a diet score ranging from 0 (healthiest) to 9 (least healthy) was derived by summing the points for each participant. A minimally sufficient adjustment set including age, sex, race, education, TDI and recruitment center was finally identified based on DAG (Figure S1).
Statistical analysis
We excluded participants with prevalent diabetes (n=10,063), stroke (n=3878) or IHD (n=20,438) at baseline. We also excluded individuals with cancer (ICD 10 code: C00–C97, n=23,967) at baseline as did in many cohort studies investigating the health effects of environmental risk factors on CMD.1,22 Additionally, participants with missing data on exposures and important covariables, including PM2.5 (n=37,217), NO2 (n=6648), race (n=2279) and TDI (n=482) were also excluded. We included a missing category for education and physical activity, respectively, as there were a large proportion of missing data on them. Finally, 410,494 participants were included in the primary analysis (Figure S2).
Participants were followed from enrollment until death, loss to follow-up, or May 31, 2021, whichever came first. In main analyses, PM2.5 and NO2 were introduced into models as continuous variables. We also introduced air pollutants into models as quartiles and tested the trend by assigning the quartile number as a continuous variable.23,24 All models were adjusted for age, sex, race, education, TDI and recruitment centers.
In the initial analyses, we used traditional Cox proportional hazards models to estimate the associations of air pollution with FCMD, CMM and all-cause mortality. The proportional hazards assumption was checked using Schoenfeld residual plots and no violations were detected. Thereafter, in main analyses, we further decomposed these associations and explored the roles of air pollutants in each transitional phase of CMM progression and prognosis, i.e., from baseline free of all the three CMDs to FCMD, CMM and then to death by performing multi-state models. Multi-state model is an extension of traditional Cox proportional hazards model that can be considered the simplest multi-state model with only two states (i.e., from baseline to event). By including multiple subsequent or competing events as states of transitions, multi-state models offer a unique advantage in investigating the influence of risk factors on different stages of disease progression simultaneously, with the consideration of competing risks.11,25 In line with previous studies,1,2 five transition stages (transition pattern A, Figure 1) were constructed as 1) baseline to FCMD, 2) FCMD to CMM, 3) baseline to death, 4) FCMD to death, 5) and CMM to death. The entering date of CMM was defined as the date when the second CMD was diagnosed. For participants entering different stages on the same date, we calculated the entering date of theoretically prior state as the date of the latter state minus 0.5 day based on previous study.1 For example, for patients with first diagnosis as CMM, the entering date of FCMD equaled the date of CMM minus 0.5 day.
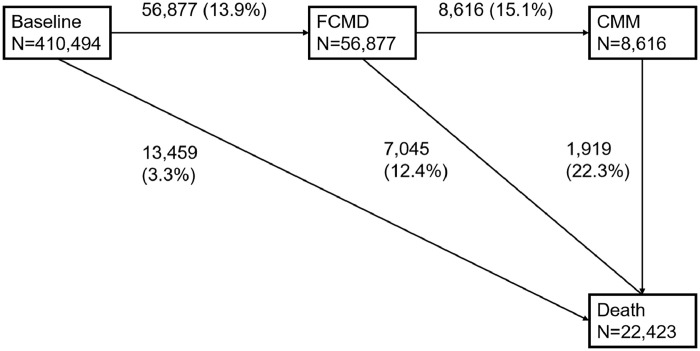
Figure 1.
Numbers (percentages) of participants in five transition stages of transition pattern A*.
Abbreviation: FCMD, first cardiometabolic disease; CMM, cardiometabolic multimorbidity;
Cardiometabolic diseases included type 2 diabetes, ischemic heart disease and stroke. CMM was defined as the occurrence of at least two of the above-mentioned diseases;
*, transition pattern A was defined as transition from baseline to FCMD, then to CMM, and subsequently to death.
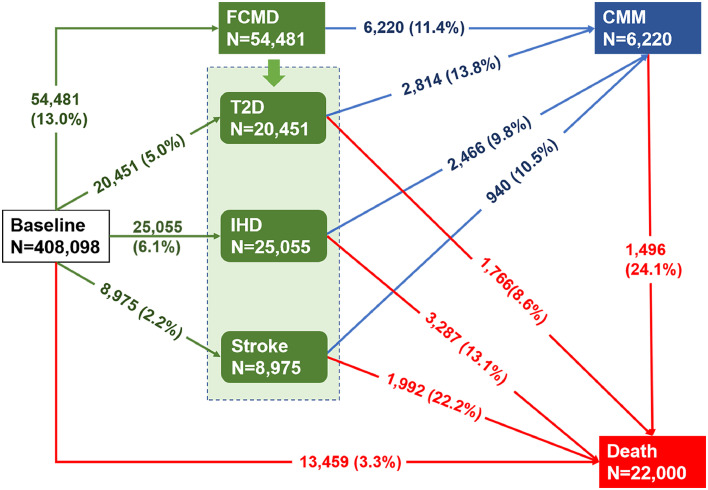
To further examine possible differential associations of PM2.5 and NO2 with the progression by individual first CMDs, we further split the multi-stage paths by subtypes of FCMD, and constructed 11 transitions (transition pattern B, Figure 2). Participants who received more than one new diagnosis of CMD on the same date after enrollment (n=2396) were excluded for this disease-specific analysis because we could not ascertain the temporal sequence of CMDs.
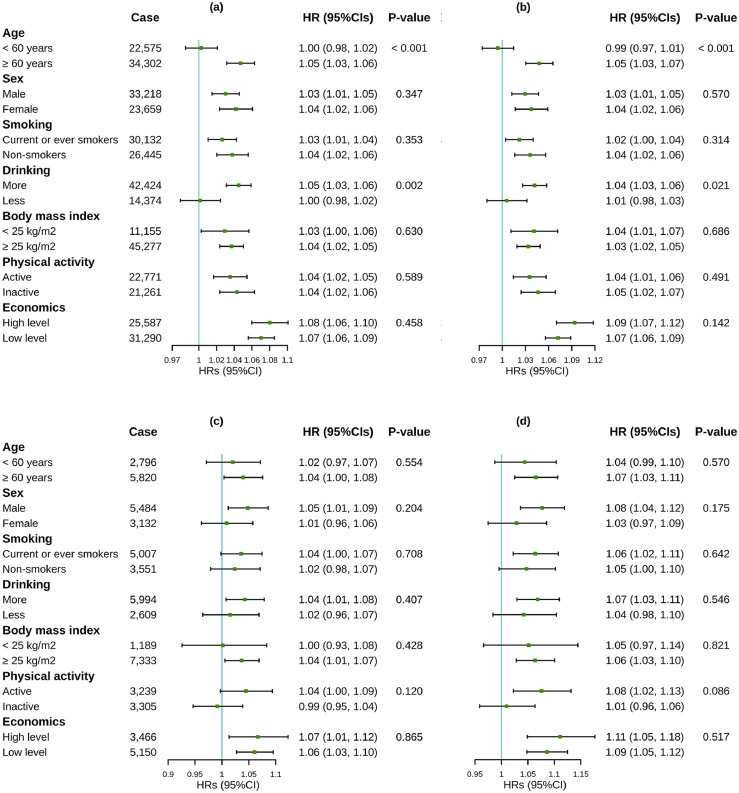
Figure 3.
Associations of air pollutants with morbidity transitions among 410,494 participants, stratified by potential modifiers.
(a) Associations of PM2.5 with transition from baseline to FCMD;
(b) Associations of NO2 with transition from baseline to FCMD;
(c) Associations of PM2.5 with transition from FCMD to CMM;
(d) Associations of NO2 with transition from FCMD to CMM.
Abbreviation: HR, hazard ratios; CI, confidence interval; PM2.5, particulate matter with an aerodynamic diameter ≤ 2.5 µm; NO2: nitrogen dioxide; FCMD, first cardiometabolic disease; CMM, cardiometabolic multimorbidity.
Associations were expressed as HR (95% CI) per interquartile range increase in PM2.5 (1.3 μg/m3) and NO2 (9.9 μg/m3).
P-value < 0.05 (Z-test) indicated significant modifications.
Cardiometabolic diseases included type 2 diabetes, ischemic heart disease and stroke. CMM was defined as the occurrence of at least two of the above-mentioned diseases.
Models were adjusted for age, sex, race, education, Townsend Deprivation Index and recruitment centers.
Figure 2.
Numbers (percentages) of participants in eleven transition stages of transition pattern B*.
Abbreviation: T2D, type 2 diabetes; IHD, ischemic heart disease; CMM, cardiometabolic multimorbidity;
Cardiometabolic diseases included type 2 diabetes, ischemic heart disease and stroke. CMM was defined as the occurrence of at least two of the above-mentioned diseases;
*, transition pattern B was defined as baseline to one of specific cardiometabolic diseases, then to CMM, and subsequently to death.
To identify subgroups susceptible to air pollution, we conducted stratified analyses by age (< 60 vs ≥ 60 years), sex, BMI (< 25 vs ≥ 25 kg/m2), physical activity (active vs inactive), smoking status (current/ever smoking vs never smoking), alcohol drinking (one to three times a month vs never or special occasions only) and economic level (high: −6.3∼−2.2 vs low: −2.2∼10.6). Effect modification by these factors was tested using the Z-statistic from the formula proposed by Altman et al.26 as:
Where Coef2 and Coef1 were coefficients, i.e., the log-hazard per unit increment in air pollutants, of two subgroups; SE1 and SE2 were standard errors for Coef1 and Coef2. P value was obtained by looking up the Z score on a standard normal distribution (N (0,1)). |Z score| > 1.96 would be considered significant (i.e., P < 0.05). We also evaluated interactions by incorporating multiplicative interaction terms of air pollution and some demographic characteristics into models.
We considered several sensitivity analyses to evaluate the robustness of the results for transition pattern A. (1) To further examine the influence of patients who reached multiple disease states on the same day, we tried several different analytical strategies, including 1) calculating the entering date of the prior state using additional four different time intervals instead of 0.5 day, i.e., 0.5-year, 1 year, 3 years and 5 years; 2) excluding participants who entered different states on the same date; and 3) adding a transition from baseline directly to CMM.1 (2) To exclude possible influence of delayed diagnosis of an existing cardiometabolic condition at baseline, we repeated the analysis after excluding participants with FCMD diagnosed within two years since enrollment. (3) We also excluded participants who relocated during follow-up, identified by comparing the residential addresses provided at enrollment and during the follow-up. (4) To test the influence of unspecific diabetes on the results, we redefined T2D as E11 instead of E11 and E14. (5) We also explored a broader definition of CMDs by including more cardiac (ICD 10: I00–I99) and metabolic (diabetes: E10–E14; obesity: E66; dyslipidemia: E78) outcomes. (6) In addition, we further adjusted for BMI, alcohol drinking frequency, smoking status, physical activity, and diet in models to be in keeping with priori knowledges and many previous studies.18,19 (7) We also additionally included participants with cancer at baseline to test the robustness of the results.
All analyses were conducted in R (version 3.6.3). The multi-state models were constructed using the “mstate” package. The hazard ratios (HRs) were estimated per interquartile range (IQR, µg/m3) increase in PM2.5 and NO2 in all analyses. All statistical tests were two-sided. P-values < 0.05 were considered statistically significant in all analyses.
Role of the funding source
The funders of this study had no role in the study design, in the collection, analysis, or interpretation of the data, or in drafting the manuscript.
Results
Descriptive analysis
The mean age of the included participants was 56.1 years (standard deviation: 8.1 years) at enrollment. Approximately 55.4 % of them were females. The median concentrations of PM2.5 and NO2 at residential address were 9.9 (IQR: 9.3–10.6) µg/m3 and 26.1 (IQR: 21.3–31.2) µg/m3, respectively. During a median follow–up of 12.0 years (IQR: 11.2–12.8 years; total person-years (PYs) 4,626,805), a total of 56,877 (13.9%) participants developed at least one CMD (122.9/10,000 PYs). Among those with at least one CMD, 8,616 (15.1%) further developed CMM (153.2/10,000 PYs). A total of 22,423 deaths were identified during follow-up. Among them, 7045 (31.4%) died with experiencing FCMD, and 1919 (8.6%) died after CMM (Figure 1). When further dividing FCMD into specific CMDs, 20,451 (37.5%) participants had T2D, 25,005 (45.9%) had IHD, 8975 (16.5%) had stroke, and 2814 (13.8%), 2466 (9.8%), and 940 (10.5%) of them developed CMM afterwards, respectively (Figure 2). Compared with survivors free of CMDs during follow-up, those who experienced one or more CMDs were older and had higher BMI, lower economic level, lower education level, and higher smoking rate (Table S1). Compared to the overall cohort, participants who received more than one new diagnosis of CMD on the same date were more likely to be older, males, smokers, non-Caucasians, obese, and with lower economic levels (Table S2).
Multi-state analysis
Results from traditional Cox proportional hazards models showed significantly positive associations of air pollution with FCMD, CMM and all-cause mortality (Table S3). By using multi-state models, we further observed different roles of air pollution in each transition stage of the CMM trajectories (Table 1). Both PM2.5 and NO2 could increase the risk of transition from baseline to FCMD, as well as the risk of transition to CMM. The risk estimates per IQR increase in air pollutant concentrations for transition from FCMD to CMM [HR (95% CI): 1.03 (1.01, 1.06) for PM2.5; 1.06 (1.03, 1.09) for NO2] were similar to estimates for transition from baseline to FCMD [1.03 (1.02, 1.05) for PM2.5; 1.03 (1.02, 1.05) for NO2]. For transition to death, PM2.5 was associated with mortality from baseline [HR (95% CI): 1.05 (1.02, 1.07)], but not from FCMD [1.02 (0.99, 1.06)] or CMM [1.00 (0.95, 1.07)]. NO2 was significantly associated with death from baseline and FCMD but not death from CMM. The corresponding HRs (95% CIs) associated with each IQR increase in NO2 were 1.04 (1.02, 1.07), 1.06 (1.02, 1.10) and 1.04 (0.97, 1.11), respectively.
Table 1.
Associations between air pollutants and transitions from baseline to FCMD, CMM, and then death.
| Case | HR (95% CI) | P-value | |
|---|---|---|---|
| PM2.5 | |||
| Baseline → FCMD | 56,877 | 1.03 (1.02, 1.05) | < 0.001 |
| FCMD → CMM | 8616 | 1.03 (1.01, 1.06) | 0.020 |
| Baseline → Death | 13,459 | 1.05 (1.02, 1.07) | < 0.001 |
| FCMD → Death | 7045 | 1.02 (0.99, 1.06) | 0.139 |
| CMM → Death | 1919 | 1.00 (0.95, 1.07) | 0.897 |
| NO2 | |||
| Baseline → FCMD | 56,877 | 1.03 (1.02, 1.05) | < 0.001 |
| FCMD → CMM | 8616 | 1.06 (1.03, 1.09) | < 0.001 |
| Baseline → Death | 13,459 | 1.04 (1.02, 1.07) | 0.001 |
| FCMD → Death | 7045 | 1.06 (1.02, 1.10) | 0.001 |
| CMM → Death | 1919 | 1.04 (0.97, 1.11) | 0.230 |
Abbreviation: HR, hazard ratio; CI, confidence interval; FCMD, first cardiometabolic disease; CMM, cardiometabolic multimorbidity; PM2.5, particulate matter with an aerodynamic diameter ≤ 2.5 µm; NO2: nitrogen dioxide.
Cardiometabolic diseases included type 2 diabetes, ischemic heart disease and stroke. CMM was defined as the occurrence of at least two of the above-mentioned diseases.
Associations were presented as HR (95 CI%) per interquartile range increases in concentrations of PM2.5 (1.3 μg/m3) and NO2 (9.9 μg/m3) for the transitions among 410,494 participants.
Models were adjusted for age, sex, race, education, Townsend Deprivation Index and recruitment center.
Exposure to PM2.5 and NO2 showed differential associations with disease transition by specific FCMDs (i.e., T2D, IHD, and stroke). Specifically, for transition from baseline to FCMDs, PM2.5 and NO2 had the strongest association with stroke [HR (95% CI): 1.05 (1.02, 1.08) for PM2.5; 1.08 (1.04, 1.11) for NO2], followed by T2D [1.04 (1.02, 1.06) for PM2.5; 1.04 (1.02, 1.06) for NO2] and IHD [1.02 (1.01, 1.04) for PM2.5; 1.01 (0.99, 1.03) for NO2]. For transition from FCMD to CMM, participants who were first diagnosed with T2D were more likely to develop CMM induced by higher PM2.5 and NO2 exposure, although the 95% CI for PM2.5 included the null [HR (95% CI): 1.05 (1.00, 1.10) for PM2.5; 1.07 (1.02, 1.13) for NO2]. No significant associations were found between PM2.5 and NO2 and transitions from IHD or stroke to CMM. For transitions from FCMD to death, both PM2.5 and NO2 were associated with transition to death from IHD [HR (95% CI): 1.05 (1.01, 1.10) for PM2.5; 1.09 (1.04, 1.15) for NO2] but not from T2D and stroke (Table 2).
Table 2.
Associations between air pollutants and transitions from baseline to single CMD, CMM, and then death.
| Case | PM2.5 |
NO2 |
|||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Baseline → FCMD | |||||
| Baseline → T2D | 20,451 | 1.04 (1.02, 1.06) | < 0.001 | 1.04 (1.02, 1.06) | < 0.001 |
| Baseline → IHD | 25,055 | 1.02 (1.01, 1.04) | 0.008 | 1.01 (0.99, 1.03) | 0.216 |
| Baseline → stroke | 8975 | 1.05 (1.02, 1.08) | < 0.001 | 1.08 (1.04, 1.11) | < 0.001 |
| FCMD → CMM | |||||
| T2D → CMM | 2814 | 1.05 (1.00, 1.10) | 0.075 | 1.07 (1.02, 1.13) | 0.009 |
| IHD → CMM | 2466 | 1.03 (0.98, 1.08) | 0.290 | 1.06 (1.00, 1.12) | 0.054 |
| Stroke → CMM | 940 | 1.01 (0.93, 1.10) | 0.780 | 0.99 (0.90, 1.09) | 0.882 |
| Baseline → Death | 13,459 | 1.05 (1.02, 1.07) | < 0.001 | 1.05 (1.02, 1.07) | < 0.001 |
| FCMD → Death | |||||
| T2D → Death | 1766 | 0.98 (0.92, 1.05) | 0.574 | 1.02 (0.95, 1.09) | 0.676 |
| IHD → Death | 3287 | 1.05 (1.01, 1.10) | 0.021 | 1.09 (1.04, 1.15) | 0.001 |
| Stroke → Death | 1992 | 1.00 (0.94, 1.06) | 0.973 | 1.03 (0.96, 1.10) | 0.404 |
| CMM → Death | 1496 | 0.98 (0.92, 1.05) | 0.627 | 1.01 (0.94, 1.09) | 0.786 |
Abbreviation: HR, hazard ratios; CI, confidence interval; FCMD, first cardiometabolic disease; CMM, cardiometabolic multimorbidity; PM2.5, particulate matter with an aerodynamic diameter ≤ 2.5 µm; NO2: nitrogen dioxide; CMD, cardiometabolic disease; T2D, type 2 diabetes; IHD, ischemic heart disease.
Cardiometabolic diseases included T2D, IHD and stroke. CMM was defined as the occurrence of at least two of the above-mentioned diseases.
Associations were presented as HR (95 CI%) per interquartile range increases in concentrations of PM2.5 (1.3 μg/m3) and NO2 (9.9 μg/m3) for the transitions among 408,098 participants.
Models were adjusted for age, sex, race, education, Townsend Deprivation Index and recruitment center.
The above associations remained in models using quartiles of exposures (Table S4). Compared to those in the lowest quartiles of exposures, participants in the highest quartiles had increased risk of transitions from baseline to FCMD, from FCMD to CMM, and transitions from baseline and FCMD to all-cause mortality. The corresponding HRs (95% CIs) were 1.09 (1.06, 1.12), 1.09 (1.02, 1.17), 1.08 (1.03, 1.14) and 1.06 (0.98, 1.14), respectively for PM2.5, and were 1.07 (1.04, 1.10), 1.12 (1.04, 1.20), 1.08 (1.02, 1.14) and 1.12 (1.04, 1.21), respectively for NO2.
Effect modification and interaction
We observed significant effect modification of PM2.5 and NO2 by age, alcohol drinking, economic levels and sex on one or more transitions (Figure 3; Figure S3). The older groups had higher risk of developing FCMD in association with exposure to PM2.5 and NO2. Excessive alcohol drinking amplified the impacts of air pollutants on the transitions from baseline to FCMD, and from FCMD to death. Individuals with lower economic levels had a higher risk of death from baseline associated with exposure to PM2.5 and NO2. Males were more vulnerable to death from FCMD associated with PM2.5 compared to females. The conclusions from multiplicative interaction models were generally consistent with those from stratified analyses (Table S5). Specifically, alcohol drinking, smoking and male have synergistic effects, while BMI, physical activity and economic levels have antagonistic effects with PM2.5 and NO2 for at least one transition from baseline to FCMD, then to CMM, and finally to mortality.
Sensitivity analyses
Results from sensitivity analyses remained relatively robust by considering the influence of participants who were diagnosed with multiple CMDs on the same day, excluding participants with CMD events occurred within the first two years of follow-up, excluding participants who were relocated during the follow-up, redefining T2D by excluding unspecific diabetes, additionally including participants with cancer at baseline, and extending the set of covariate adjustment. When a broader definition of CMDs was applied, we obtained robust associations of PM2.5 and NO2 with all transitions except for the transition from baseline to death, which turned insignificant (Table S6).
Discussion
In this large-scale, prospective UK Biobank cohort, we examined the impact of air pollution on the whole course of CMM, from onset, progression, to prognosis. We found that PM2.5 and NO2 played roles in multiple transition stages, including from baseline to FCMD, FCMD to CMM and baseline to death. Exposure to NO2 additionally increased the risk of transition from FCMD to death. When disease-specific transitions were considered, the impacts of air pollution within certain transition stages varied depending on disease types. Specifically, the strongest effects of air pollution were observed on stroke for the transition from baseline to FCMD, on T2D for the transition from FCMD to CMM, while on IHD for the transition from FCMD to death. In addition, we identified several subgroups susceptible to one or more CMM transitions.
The adverse effects of air pollution on some transitions of CMM observed in the current study were generally consistent with prior studies which reported the associations between air pollution and single disease stage of CMM. For example, numerous epidemiologic studies have linked air pollution to increased risks of morbidity of single CMDs in general population, which were consistent with the increased risk for the transition from baseline to FCMD found in the current study.8,9 Moreover, a number of studies also reported an increased risk of PM2.5-related CVD in people with diabetes,27,28 indicating the role of PM2.5 in the transition from FCMD to CMM. However, these studies merely focused on single disease stage, and failed to evaluate the effects of air pollution on different transition stages of the whole course of CMM, i.e., from CMD-free to FCMD, then to CMM and further to death. In addition, these studies did not consider the competing risk of from death. As air pollution is a well-known risk factor of mortality from CMDs and other causes,29,30 simply regarding participants who died from baseline or FCMD during follow-up as censored might result in a deviation of the morbidity risk related to air pollution. To address these issues, we applied the multi-state model, a model considering both competing risk and the transitions of various disease stages. To our knowledge, the multi-state model has only been used to explore the role of several risk factors in the progression of CMM, including lifestyle, clinical and behavioral factors,1,2 but not environmental factors. More studies with multi-state models in investigating the chronic health effects of air pollution are warranted to validate our findings.
We found that the associations between air pollution and the incidence of FCMD and later the transition to CMM differed by specific types of diseases. Our data suggested the effects of both PM2.5 and NO2 on the transitions from health to FCMD were strongest for stroke, followed by T2D and IHD. Several pooled analyses also suggested smaller estimates for the associations of PM2.5 with IHD morbidity compared to stroke.19,31 For example, Alexeeff et al. reported an increased risk of 8% (−1% to 18%) for incident myocardial infarction and 13% (11% to 15%) for incident stroke per 10 µg/m3 increase of PM2.5 in a meta-analysis.31 In terms of transitions from specific CMDs to CMM, we found that the risks of air pollution were only significant for participants who were first diagnosed with T2D but not for those who were first diagnosed with IHD and stroke. This might be explained by that IHD and stroke are more likely to cause disability,32,33 resulting in reduced outdoor activities, which may lead to potential misclassification of exposure and underestimation of effects.34 The smaller sample size may also limit the power to detect the associations, if any, between air pollution and transitions from IHD or stroke to CMM.
We found that both PM2.5 and NO2 were significantly associated with risk of transition to death from IHD, but not from stroke and T2D. The lack of associations between air pollution and death from stroke and T2D seems to be not consistent with some previous studies that indicated significant, and even stronger associations of air pollution with mortality among population with pre-existing conditions than general population.35,36 The inconsistency may be explained by the insufficient statistical power resulting from the much smaller sample size of cases with pre-existing conditions in the present study than in previous studies.35,36 Additionally, the findings obtained from previous studies cannot be compared directly to our estimates due to the distinct analytic strategies and statistical models. Traditional Cox regression models in prior studies evaluated effects of air pollution on rough transition from pre-existing conditions to mortality. Nevertheless, the multi-state model used in our study decomposed the rough transition into several continuous and mutually exclusive phases, and had the advantage of assessing the independent effects on each transition phase. Besides, the potential interaction between medications and air pollution may also account for the null associations. Previously, several epidemiologic and experimental studies have reported that medications commonly prescribed to patients with T2D and stroke may mitigate the detrimental effects of air pollution, such as metformin and aspirin.37,38
We identified potential vulnerable sub-populations to the impacts of air pollution on CMM transitions, which is of importance to develop evidence-based plans for CMM prevention and intervention. We observed increased susceptibility to air pollutants among the older population and persons with excessive alcohol drinking, which may result from unbalanced immune system, disturbed metabolism and worse health condition of them.2,39,40 A lower economic level was found to amplify the mortality risk in relation to air pollution in our study, possibly due to poorer health care. The pronounced impacts of PM2.5 among males were also observed in many environmental epidemiological studies,41,42 which may be explained by the fact that males are more likely to have unhealthy lifestyles such as alcohol drinking in the UK Biobank cohort (Table S7).
Our findings had significant public health implications. First, we identified significant associations between air pollution and CMDs. Although the HRs associated with air pollution are smaller than some conventional risk factors of CMDs, the disease burden attributable to air pollution is very high because of ubiquitous exposures to air pollution in the world.7 Second, by using multi-state model, we observed that air pollution had non-negligible impacts on the transition from CMD-free to FCMD that had been well characterized in previous researches,8,9 as well as on the transition further to CMM that had not been considered previously. In view of the excess risk of morbidity and mortality of CMM related to air pollution, we proposed to take CMM into consideration when assessing air pollution-related disease burden and developing health protection strategies. Third, as a global public health challenge, population aging is a global phenomenon, which is always accompanied by an increased burden of chronic diseases especially CMDs43 and decreased disability-adjusted life-years. Our results suggested the important role of clean air in prolonging healthy life span of the elderly and reducing the societal burden of aging. Lastly, we found stronger impacts of air pollution on CMM progression among participants with excessive alcohol drinking. Presumably, reduced alcohol drinking is potentially helpful in mitigating the risk of CMM in association with exposure to air pollution.
Our study presents several strengths. The major strength is the use of multi-state models rather than conventional Cox proportional hazards models, which enables us to explore the impacts of air pollution on different stages of the whole course of CMM and rule out the competing risk from death. Furthermore, the large sample size of the UK Biobank provided substantial power and allowed to further investigate all transitions of specific CMDs. Additionally, the prospective nature of the analysis has a clear temporal order between air pollution exposure and CMM incidence, thus potential reverse confounding can be reduced. Finally, the wide range of individual-level information on lifestyle and sociodemographic characteristics collected in the UK Biobank makes it possible to investigate the potential modifiers.
Our study also has some limitations. First, as PM2.5 data were only publicly available in 2010 in the UK Biobank, we used annual average concentrations of air pollutants in 2010 as a proxy for long-term exposure in line with most air pollution studies in the UK Biobank cohort.44,45 Although we may reasonably assume that the spatial contrasts in air pollution concentrations did not change substantially during recruitment (2006–2010) and follow-up in UK, the exposure measurement misclassifications could not be fully excluded. Second, the changes in exposure levels due to residential relocation were not captured, but their impacts were very small according to our sensitivity analysis. Third, we didn't perform two-pollutant analyses due to the very high correlation between PM2.5 and NO2 (correlation coefficient: 0.85) and thus the possible independent effects need to be clarified in further investigations. Finally, UK Biobank mainly includes Caucasians from developed countries with relatively low air pollution exposures and high levels of access to healthcare, limiting the generalizability of the study findings to population with other genetic backgrounds, high exposure levels and relatively low socioeconomic levels.
In conclusion, using data from a large, prospective cohort, we found that long-term exposures to PM2.5 and NO2 were associated with elevated risks of transitions from a disease-free state to single CMD, CMM, and death, suggesting the importance of reducing air pollution exposures in the primary and secondary prevention of CMM. Older participants, males, and individuals with excessive alcohol drinking and lower economic levels were more susceptible to air pollution-related CMM progression, further highlighting the significance of clean air action in vulnerable sub-populations.
Contributors
H.L, Data verification, Formal analysis, Writing - Original Draft & Review & Editing. Q.Z, Data verification, Formal analysis, Writing - Original Draft & Review & Editing. K.Y, Writing - Review & Editing. X.M, Writing - Review & Editing. H.K, Data verification, Funding acquisition. R.C, Conceptualization, Data verification, Funding acquisition, Supervision, Writing - Review & Editing. All authors have read and approved the final version of the manuscript. All authors have final responsibility for the decision to submit for publication.
Data sharing statement
The data that support the findings of this study are available from the UK Biobank but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the UK Biobank (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access).
Declaration of interests
We declare no competing interests.
Acknowledgments
We thank the Shanghai Municipal Science and Technology Commission (21TQ015) and the National Natural Science Foundation of China (92143301 and 92043301). This research has been conducted using the UK Biobank Resource under Application Numbers 66251 and 80741. The chief acknowledgment is to the team members and participants of UK Biobank.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104282.
Appendix. Supplementary materials
References
- 1.Han Y., Hu Y., Yu C., et al. Lifestyle, cardiometabolic disease, and multimorbidity in a prospective Chinese study. Eur Heart J. 2021;42(34):3374–3384. doi: 10.1093/eurheartj/ehab413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh-Manoux A., Fayosse A., Sabia S., et al. Clinical, socioeconomic, and behavioural factors at age 50 years and risk of cardiometabolic multimorbidity and mortality: a cohort study. PLoS Med. 2018;15(5) doi: 10.1371/journal.pmed.1002571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Angelantonio E., Kaptoge S., Wormser D., et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314(1):52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World population ageing 2019: Highlights. United Nations, Department of Economic and Social Affairs; 2019. Report No.: ST/ESA/SER.A/430.
- 5.Zhang D., Tang X., Shen P., et al. Multimorbidity of cardiometabolic diseases: prevalence and risk for mortality from one million Chinese adults in a longitudinal cohort study. BMJ Open. 2019;9(3) doi: 10.1136/bmjopen-2018-024476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freisling H., Viallon V., Lennon H., et al. Lifestyle factors and risk of multimorbidity of cancer and cardiometabolic diseases: a multinational cohort study. BMC Med. 2020;18(1):5. doi: 10.1186/s12916-019-1474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang F., Liu F., Huang K., et al. Long-term exposure to fine particulate matter and cardiovascular disease in China. J Am Coll Cardiol. 2020;75(7):707–717. doi: 10.1016/j.jacc.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q., Liu C., Wang Y., et al. Associations of long-term exposure to ambient nitrogen dioxide with indicators of diabetes and dyslipidemia in China: a nationwide analysis. Chemosphere. 2021;269 doi: 10.1016/j.chemosphere.2020.128724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin H.H., Maquiling A., Thomson E.M., Park I.W., Stieb D.M., Dehghani P. Sex-difference in air pollution-related acute circulatory and respiratory mortality and hospitalization. Sci Total Environ. 2022;806(Pt 3) doi: 10.1016/j.scitotenv.2021.150515. [DOI] [PubMed] [Google Scholar]
- 11.Putter H., Fiocco M., Geskus R.B. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 12.Ethier J.L., Anderson G.M., Austin P.C., et al. Influence of the competing risk of death on estimates of disease recurrence in trials of adjuvant endocrine therapy for early-stage breast cancer: a secondary analysis of ma.27, ma.17 and ma.17r. Eur J Cancer. 2021;149:117–127. doi: 10.1016/j.ejca.2021.02.034. [DOI] [PubMed] [Google Scholar]
- 13.Lacny S., Wilson T., Clement F., et al. Kaplan-Meier survival analysis overestimates the risk of revision arthroplasty: a meta-analysis. Clin Orthop Relat Res. 2015;473(11):3431–3442. doi: 10.1007/s11999-015-4235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudlow C., Gallacher J., Allen N., et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin K.H., Glintborg D., Nybo M., Abrahamsen B., Andersen M. Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(10):3848–3857. doi: 10.1210/jc.2017-01354. [DOI] [PubMed] [Google Scholar]
- 16.Beelen R., Hoek G., Vienneau D., et al. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe-the ESCAPE project. Atmos Environ. 2013;72:10–23. [Google Scholar]
- 17.Eeftens M., Beelen R., de Hoogh K., et al. Development of land use regression models for PM(2.5), PM(2.5) absorbance, PM(10) and PM(coarse) in 20 European study areas; results of the ESCAPE project. Environ Sci Technol. 2012;46(20):11195–11205. doi: 10.1021/es301948k. [DOI] [PubMed] [Google Scholar]
- 18.Liu L., Zhang Y., Yang Z., Luo S., Zhang Y. Long-term exposure to fine particulate constituents and cardiovascular diseases in Chinese adults. J Hazard Mater. 2021;416 doi: 10.1016/j.jhazmat.2021.126051. [DOI] [PubMed] [Google Scholar]
- 19.Wolf K., Hoffmann B., Andersen Z.J., et al. Long-term exposure to low-level ambient air pollution and incidence of stroke and coronary heart disease: a pooled analysis of six European cohorts within the ELAPSE project. Lancet Planet Health. 2021;5(9):e620–e632. doi: 10.1016/S2542-5196(21)00195-9. [DOI] [PubMed] [Google Scholar]
- 20.Blane D., Townsend P., Phillimore P., Beattie A. Health and deprivation: inequality and the North. Br J Sociol. 1987;40:344. [Google Scholar]
- 21.Petermann-Rocha F., Ho F.K., Foster H., et al. Nonlinear associations between cumulative dietary risk factors and cardiovascular diseases, cancer, and all-cause mortality: a prospective cohort study from UK Biobank. Mayo Clin Proc. 2021;96(9):2418–2431. doi: 10.1016/j.mayocp.2021.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Odegaard A.O., Koh W.P., Gross M.D., Yuan J.M., Pereira M.A. Combined lifestyle factors and cardiovascular disease mortality in Chinese men and women: the Singapore Chinese health study. Circulation. 2011;124(25):2847–2854. doi: 10.1161/CIRCULATIONAHA.111.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosnijeh F.S., Lan Q., Rothman N., et al. Mitochondrial DNA copy number and future risk of B-cell lymphoma in a nested case-control study in the prospective EPIC cohort. Blood. 2014;124(4):530–535. doi: 10.1182/blood-2013-10-532085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan X., Peters B.A., Jacobs E.J., et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome. 2018;6(1):59. doi: 10.1186/s40168-018-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meira-Machado L., de Uña-Alvarez J., Cadarso-Suárez C., Andersen P.K. Multi-state models for the analysis of time-to-event data. Stat Methods Med Res. 2009;18(2):195–222. doi: 10.1177/0962280208092301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altman D.G., Bland J.M. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su P.F., Sie F.C., Yang C.T., Mau Y.L., Kuo S., Ou H.T. Association of ambient air pollution with cardiovascular disease risks in people with type 2 diabetes: a Bayesian spatial survival analysis. Environ Health. 2020;19(1):110. doi: 10.1186/s12940-020-00664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinault L., Brauer M., Crouse D.L., et al. Diabetes status and susceptibility to the effects of PM2.5 exposure on cardiovascular mortality in a national Canadian cohort. Epidemiology. 2018;29(6):784–794. doi: 10.1097/EDE.0000000000000908. [DOI] [PubMed] [Google Scholar]
- 29.Strak M., Weinmayr G., Rodopoulou S., et al. Long-term exposure to low-level air pollution and mortality in eight European cohorts within the ELAPSE project: pooled analysis. BMJ. 2021;374:n1904. doi: 10.1136/bmj.n1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J., Hoek G. Long-term exposure to PM and all-cause and cause-specific mortality: a systematic review and meta-analysis. Environ Int. 2020;143 doi: 10.1016/j.envint.2020.105974. [DOI] [PubMed] [Google Scholar]
- 31.Alexeeff S.E., Liao N.S., Liu X., Van Den Eeden S.K., Sidney S. Long-term PM2.5 exposure and risks of ischemic heart disease and stroke events: review and meta-analysis. J Am Heart Assoc. 2021;10(1) doi: 10.1161/JAHA.120.016890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cucchiara B., George D.K., Kasner S.E., et al. Disability after minor stroke and TIA: a secondary analysis of the SOCRATES trial. Neurology. 2019;93(7):e708–e716. doi: 10.1212/WNL.0000000000007936. [DOI] [PubMed] [Google Scholar]
- 33.Institute of Medicine . National Academies Press (US); Washington (DC): 2010. Cardiovascular Disability: Updating the Social Security Listings; p. 304 p. [PubMed] [Google Scholar]
- 34.Grandjean P., Budtz-Jørgensen E., Keiding N., Weihe P. Underestimation of risk due to exposure misclassification. Int J Occup Med Environ Health. 2004;17(1):131–136. [PubMed] [Google Scholar]
- 35.Paul L.A., Burnett R.T., Kwong J.C., et al. The impact of air pollution on the incidence of diabetes and survival among prevalent diabetes cases. Environ Int. 2020;134 doi: 10.1016/j.envint.2019.105333. [DOI] [PubMed] [Google Scholar]
- 36.Chen G., Wang A., Li S., et al. Long-term exposure to air pollution and survival after ischemic stroke. Stroke. 2019;50(3):563–570. doi: 10.1161/STROKEAHA.118.023264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao J., Yuan J., Wang Q., et al. Metformin protects against PM2.5-induced lung injury and cardiac dysfunction independent of AMP-activated protein kinase α2. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao X., Coull B., Lin X., Vokonas P., Schwartz J., Baccarelli A.A. Nonsteroidal anti-inflammatory drugs modify the effect of short-term air pollution on lung function. Am J Respir Crit Care Med. 2020;201(3):374–378. doi: 10.1164/rccm.201905-1003LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu M., Xu C.-X., Zhu H.-H., et al. Associations of cigarette smoking and alcohol consumption with metabolic syndrome in a male Chinese population: a cross-sectional study. J Epidemiol. 2014;24(5):361–369. doi: 10.2188/jea.JE20130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salminen A. Activation of immunosuppressive network in the aging process. Ageing Res Rev. 2020;57 doi: 10.1016/j.arr.2019.100998. [DOI] [PubMed] [Google Scholar]
- 41.Wang M., Hou Z.H., Xu H., et al. Association of estimated long-term exposure to air pollution and traffic proximity with a marker for coronary atherosclerosis in a nationwide study in China. JAMA Netw Open. 2019;2(6) doi: 10.1001/jamanetworkopen.2019.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li N., Chen G., Liu F., et al. Associations between long-term exposure to air pollution and blood pressure and effect modifications by behavioral factors. Environ Res. 2020;182 doi: 10.1016/j.envres.2019.109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng X.J., Hu G.Q. Progress in research of burden of disease attributed to population ageing. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(11):1915–1920. doi: 10.3760/cma.j.cn112338-20191220-00905. [DOI] [PubMed] [Google Scholar]
- 44.Cai Y., Zijlema W.L., Doiron D., et al. Ambient air pollution, traffic noise and adult asthma prevalence: a BioSHaRE approach. Eur Respir J. 2017;49(1) doi: 10.1183/13993003.02127-2015. [DOI] [PubMed] [Google Scholar]
- 45.Doiron D., de Hoogh K., Probst-Hensch N., et al. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J. 2019;54(1) doi: 10.1183/13993003.02140-2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.