Version Changes
Revised. Amendments from Version 1
This new version of the paper includes the FTIR spectra of the bush tea leaf extract (Figure 6) as well as an addition to the UV-Visible data, the bandgap energy of the ZnO nanoparticles.
Abstract
Background: Nanoparticles are globally synthesized for their antimicrobial, anti-inflammatory, wound healing, catalytic, magnetic, optical, and electronic properties that have put them at the forefront of a wide variety of studies. Among them, zinc oxide (ZnO) has received much consideration due to its technological and medicinal applications. In this study, we report on the synthesis process of ZnO nanoparticles using Athrixia phylicoides DC natural extract as a reducing agent.
Methods: Liquid chromatography–mass spectrometry (LC-MS) was used to identify the compounds responsible for the synthesis of ZnO nanoparticles. Structural, morphological and optical properties of the synthesized nanoparticles have been characterized through X-ray diffraction (XRD), Ultraviolet-visible spectroscopy (UV-Vis), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS).
Results: LC-MS results showed that different flavonoids and polyphenols, as well as Coumarin, an aromatic compound, reacted with the precursor to form ZnO nanoparticles. XRD and UV-Vis analysis confirmed the synthesis of ZnO nanoparticles, with a spherical shape showed in SEM images. The quasi-spherical ZnO crystals had an average crystallite size of 24 nm. EDS and FTIR analysis confirmed that the powders were pure with no other phase or impurity.
Conclusions: This study successfully demonstrated that the natural plant extract of A. phylicoides DC. can be used in the bio-reduction of zinc nitrate hexahydrate to prepare pure ZnO nanoparticles, thus, extending the use of this plant to an industrial level.
Keywords: ZnO nanoparticles, green synthesis, bush tea, reducing agent, natural extract.
Introduction
Materials with a diameter of less than 100 nm are classified as nanoparticles. These particles have a reduced size associated with their high surface/volume ratio that increases as their size decreases ( Ohno et al., 2010). They are considered as the borderline between single molecules and bulk materials and present more properties compared to their bulk counterpart ( Mansoori and Soelaiman, 2005). Nanoparticles are globally synthesized for their various properties, such as antimicrobial, anti-inflammatory, wound healing, catalytic, magnetic, optical, and electronic properties, that have put them at the forefront of a wide variety of studies ( Gunalan et al., 2012; Jamdagni et al., 2018).
Nanoparticles have been incorporated into numerous consumer industries such as industrial, health, food, space, chemical, and cosmetics, necessitating a green and environmentally responsible strategy for their production ( Rao and Gautam, 2016). Among all nanoparticles, metal oxides and dioxides such as zinc oxide, silver, gold and titanium dioxide have received copious consideration because of their multiple properties and applications ( Dobrucka and Długaszewska, 2016). Numerous physicochemical methods of the synthesis of nanoparticles such as laser ablation, microwave irradiation and vapour deposition have been reported to date ( Satyanarayana and Reddy, 2018). The physical and chemical methods involve forces of condensation, dispersion, or fragmentation of bulk particles into nanoparticles ( Dhandapani et al., 2014; Krupa and Vimala, 2016). Hence, chemical methods often require toxic chemicals that are harmful to the environment due to the difficulty of removing them from the nanoparticles after synthesis, thus a new, safe and cost-effective method is needed ( Dhandapani et al., 2014).
Synthesis of nanomaterials through biological systems assisted by some biotechnological tools is an emerging field of nanotechnology called green nanotechnology ( Shinwari and Maaza, 2017). Plants, diatoms, fungi, yeast, algae, bacteria, and human cells have been used to reduce metal ions into nanoparticles. Their proteins and other metabolites have been well reported to have a reductive capacity that can transform metal ions into metal nanoparticles ( Dobrucka and Długaszewska, 2016; Parveen et al., 2016). The biological synthesis of nanoparticles provides more advantages than chemical and physical ones ( Kharissova et al., 2013). Numerous metal oxide nanoparticles, such as TiO 2, CuO, and ZnO have been produced by total green chemistry. Among them ZnO, an n-type semiconductor, has gained interest owing to its easy production, cost-effectiveness, and safety of synthesis and usage ( Agarwal et al., 2017). Several studies have successfully been led to synthesize ZnO nanoparticles using different organisms such as bacteria, fungi, algae, and plants ( Agarwal et al., 2017).
Among all biological systems, plant phytosynthesis of nanoparticles using plants has shown great potential. Plant-mediated nanoparticle synthesis is simple, eco-friendly, and provides antibacterial assets ( Gunalan et al., 2012; Iravani et al., 2015; Thema et al., 2015). A variety of metabolites such as terpenoids, polyphenols, sugars, alkaloids, phenolic acids and proteins have been reported to have metal ion reduction assets ( Parveen et al., 2016). Several studies dedicated to the green synthesis of ZnO nanoparticles using plant extracts as capping or reducing agents have shown the use of different plant aerial parts, such as leaves and fruits of different species such as Aloe vera, Hibiscus sabdariffa, Allium sativum, Allium cepa, Petroselinum crispum, Moringa oleifera and Camellia sinensis, for the synthesis of nanoparticles ( Mahendiran et al., 2017; Matinise et al., 2017; Senthilkumar and Sivakumar, 2014; Stan et al., 2015). Bush tea, mostly known as a medicinal tea plant in southern Africa where it originates has high concentrations of phenolic compounds such as tannins and flavonoids ( Lerotholi et al., 2017). However, data explaining the synthesis processes of nanoparticles using this plant are lacking. Hence, the objective of this study was to contribute to the explanation of the compounds induced in the synthesis process of ZnO nanoparticles using Athrixia phylicoides leaf extract.
Methods
Material
Leaves of bush tea ( A. phylicoides DC) were used to reduce zinc nitrate hexahydrate. Analytical grade Zn (NO 3) 2.6H 2O of 99% purity was purchased from Sigma-Aldrich, South Africa. Bush tea leaves were harvested from the wild in Thohoyandou (22.8785°S; 30.4818°E) in the Limpopo province, South Africa. Following the harvest, the leaves were washed with deionized water and freeze-dried for 72 hours at -50°C at a pressure of 0.32 Kpa, hereafter they were ground and kept for further usage.
Material preparation
Extract preparation
Ten grams of ground bush tea leaves were weighed and mixed with 300 ml of deionized water. The mixture was heated at 60°C for 30 minutes until the water changed to a dark green colour. After centrifugation using a Hermle Labortecnik GmbH Z 216-M benchmark centrifuge at 4000 rpm for 10 minutes, the mixture was filtered twice using Whatman filter paper number 1, and the extract was kept in an airtight container in a fridge at ≈4°C for analysis and ZnO nanoparticles synthesis.
Synthesis of ZnO nanoparticles
In this study, zinc nitrate hexahydrate [Zn (NO 3) 2.6H 2O] was used as the precursor. One gram of the precursor was mixed with 25 ml of A. phylicoides extract. The mixture was kept on a magnetic stirrer at 300 rpm at 60°C for 30 minutes then left to cool down at room temperature for 12 hours, a precipitate was observed. The mixture was centrifuged for 15 minutes at 4000 rpm. The supernatant was collected and transferred to LC-MS vials for analysis and the precipitate was dried at 60°C for one hour then annealed at 800°C for two hours. The obtained powder was then kept for characterization.
Bush tea compounds profiling and identification
The determination and profiling of different compounds present in the extract before the synthesis as well as the supernatant after synthesis were performed using Liquid chromatography quadrupole time-of-flight mass spectrometry (LC-Q-TOF-MS) using a Bruker impact II (Germany). After peak integration and Pareto scaling, the liquid chromatography–mass spectrometry (LC-MS) data were transformed into buckets using the Bruker Compass data analysis programme version 4.3.110 ( https://www.bruker.com/en/). Peaks were determined using real mass, MS/MS, and retention time (RT). The accuracy of the mass and MS/MS spectral data was compared to the Kyoto standard Encyclopaedia of Genes and Genomes (KEGG) and ChemSpider databases using the MetFrag 2.2 online software ( Tete et al., 2020). Principal component analysis (PCA) and T-tests were performed using MetaboAnalyst 4.0.
ZnO nanoparticle characterization
The characterization of the obtained ZnO nanopowders was done using X-ray diffraction (XRD), Ultraviolet-visible spectroscopy (UV-Vis), Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS). The crystallite size of ZnO nanoparticles was estimated using the modified Scherrer equation:
where is the X-ray wavelength, β the peak width at half maximum weight, K = 0.9, the Scherrer constant ( Monshi et al., 2012).
Results
Assessment of the synthesis process
Evaluation of extract composition relative to the synthesis of ZnO nanoparticles using LC-Q-TOF-MS
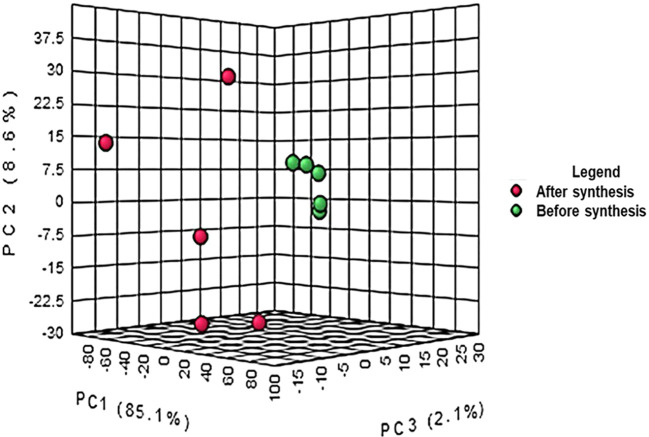
The crude extract from bush tea leaves and the supernatant after the synthesis of ZnO nanoparticles were investigated. The differences between the composition of the crude extract and the supernatant after synthesis is represented in Figure 1. The results from the PCA co-variance of data show that two distinct groups were observed from the three principal components with 85.1%, 8.6% and 2.1% respectively for principal components 1, 2 and 3. The compounds (represented in red) resulting from the supernatant after synthesis of ZnO nanoparticles clustered together following the Y-axis of the PCA while the compounds of crude extract were on the Z-axis. The differences observed are due to the reaction between the plant extract and the precursor to form ZnO nanoparticles. The synthesis of ZnO nanoparticles involves a reaction between the plant extract and the precursor resulting in the reduction of Zn +2 ions into ZnO nanoparticles ( Hussain et al., 2019).
Figure 1. Principal component analysis of liquid chromatography quadrupole time-of-flight mass spectrometry (LC-Q-TOF-MS) peak intensities of 10 bush tea ( Athrixia phylicoides DC.) leaf extracts before and after ZnO nanoparticle synthesis.
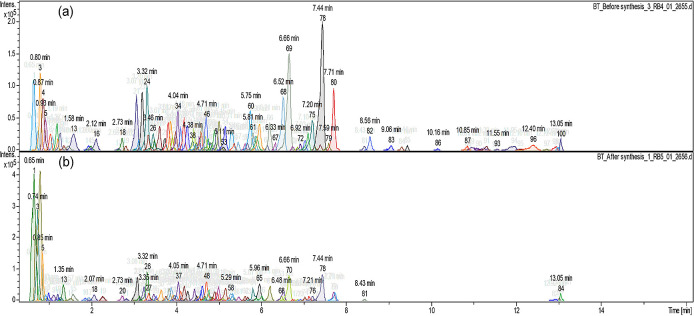
Figure 2 shows the different compound peaks observed using LC-Q-TOF-MS analysis. The dissection of observed spectra into compounds produced 100 different peaks for the crude extract ( Figure 2a) and 84 peaks for the supernatant after synthesis ( Figure 2b). The reduction in the number of compounds confirms that the synthesis took place and secondary metabolites from A. phylicoides DC. extract has reacted with the precursor reducing Zn 2+ ions into ZnO nanoparticles.
Figure 2. Compound dissection (a) before the synthesis process (100 peaks) (b) after the synthesis process (84 peaks).
Bush tea extract compounds identification before and after synthesis
Different peaks identified, after chromatogram dissection ( Figure 2), from LC-Q-TOF-MS revealed the presence of several compounds in both the crude extract and the supernatant after ZnO nanoparticle synthesis, with a reduction of the compound’s amount in the supernatant collected after synthesis. Thus, revealing the presence of an interaction between the precursor and the extract mainly by the oxidation, reduction or degradation of the phytochemical compounds that occur during nanoparticle formation ( Jeevanandam et al., 2016). Table 1 presents the secondary metabolites investigated for both the crude bush tea extract and the supernatant solution after synthesis, respectively.
Table 1. Liquid chromatography quadrupole time-of-flight mass spectrometry (LC-Q-TOF-MS) bush tea extract compounds identified before ZnO nanoparticles synthesis using MetFrag software (KEGG and ChemSpider databases, 50 ppm).
| Compound name | Formula | RT [sec] | |
|---|---|---|---|
| 1 | (+)-7-Isojasmonic acid | C 12H 18O 3 | 294 |
| 2 | (6Z,9Z,12Z)-Octadecatrienoic acid | C 18H 30O 2 | 543.6 |
| 3 | 10-Oxo-11,15-phytodienoic acid | C 18H 28O 3 | 357.6 |
| 4 | 13-hydroxy-9Z,11E-octadecadienoic acid | C 18H 32O 3 | 652.2 |
| 5 | 17-Hydroxylinolenic acid | C 18H 30O 3 | 340.8 |
| 6 | 1-O,6-O-Digalloyl-beta-D-glucose (tannin) | C 20H 20O 14 | 351 |
| 7 | 3,6-Anhydroglucose | C 6H 10O 5 | 228.6 |
| 8 | 3-hydroperoxy-4-phenyl-pentan-1-ol/Loliolide | C 11H 16O 3 | 309 |
| 9 | 3-tert-Butyl-5-methylcatechol | C 11H 16O 2 | 426 |
| 10 | 4-Heptyloxyphenol | C 13H 20O 2 | 282.6 |
| 11 | 4”-Hydroxyacetophenone | C 8H 8O 2 | 71.4 |
| 12 | 4-Hydroxyestradiol-17beta | C 18H 24O 3 | 443.4 |
| 13 | 5,7,3'-Trimethoxy-6,4',5'-trimethoxyisoflavone | C 18H 16O 8 | 690 |
| 14 | 7-Hydroxy-2”,4”,5”-trimethoxyisoflavone | C 18H 16O 6 | 726 |
| 15 | Naringenin 7-O-beta-D-glucoside | C 21H 22O 10 | 435.6 |
| 16 | 17-Hydroxylinolenic acid | C 18H 30O 3 | 372.6 |
| 17 | Adenine | C 5H 5N 5 | 783 |
| 18 | alpha-Curcumene | C 15H 22 | 609.6 |
| 19 | 5S-Hydroperoxy-18R-HEPE | C 20H 30O 5 | 274.8 |
| 20 | Atropaldehyde | C 9H 8O | 345 |
| 21 | Scullcapflavone II | C 19H 18O 8 | 462.6 |
| 22 | Cinnamaldehyde | C 9H 8O | 354 |
| 23 | Cisapride | C 29H 27N 3O 3 | 115.8 |
| 24 | Coumaric acid/Caffeic Aldehyde | C 9H 8O 3 | 285 |
| 25 | Coumarin | C 9H 6O 2 | 76.8 |
| 26 | D-Norvaline | C 5H 11NO 2 | 48 |
| 27 | Homovanillate/Dihydrocaffeic acid | C 9H 10O 4 | 288.6 |
| 28 | Lancerin | C 19H 18O 10 | 348.6 |
| 29 | Lophophorine/Stovaine | C 13H 17NO 3 | 55.8 |
| 30 | Mallotophenone | C 21H 24O 8 | 432 |
| 31 | Malonyldaidzin | C 24H 22O 12 | 207.6 |
| 32 | Melampodin A | C 21H 24O 9 | 399.6 |
| 33 | Montanol | C 21H 36O 4 | 513.6 |
| 34 | Myrcene/(E)-beta-Ocimene | C 10H 16 | 321.6 |
| 35 | Nafenopin glucuronide | C 26H 30O 9 | 291 |
| 36 | Neocnidilide/4-Hexyloxyphenol | C 12H 18O 2 | 421.8 |
| 37 | Pentalen-13-ol/Nonylphenol | C 15H 24O | 411 |
| 38 | Petasin/Cafestol | C 20H 28O 3 | 558.6 |
| 39 | Pinosylvin | C 14H 12O 2 | 276.6 |
| 40 | Quinestrol | C 25H 32O 2 | 232.2 |
| 41 | Traumatic acid | C 12H 20O 4 | 403.8 |
| 42 | Tricin | C 17H 14O 7 | 379.8 |
| 43 | Umbelliferone/4-Hydroxycoumarin | C 9H 6O 3 | 209.4 |
| 44 | 4”-Hydroxyacetophenone | C 8H 8O 2 | 1.19 |
Table 2 present the compounds identified from the supernatant after synthesis of ZnO nanoparticles. The secondary metabolites investigated present a reduced number compared to the ones from the crude extract, thus revealing that a reaction has taken place between bush tea natural extract metabolites and the precursor resulting in the formation of ZnO nanoparticles.
Table 2. Liquid chromatography quadrupole time-of-flight mass spectrometry (LC-Q-TOF-MS) bush tea extract compounds identified after ZnO nanoparticles synthesis using MetFrag software (KEGG and ChemSpider, 50 ppm).
| Compound name | Formula | RT [sec] | |
|---|---|---|---|
| 1 | Indanone | C 9H 8O | 348.6 |
| 2 | Mallotophenone | C 21H 24O 8 | 432.6 |
| 3 | Melampodin A | C 21H 24O 9 | 399.6 |
| 4 | Sterigmatocystin | C 18H 12O 6 | 268.8 |
| 5 | Umbelliferone | C 9H 6O 3 | 211.8 |
| 6 | Salicylate | C 7H 6O 3 | 182.4 |
| 7 | Resolvin E2 | C 20H 30O 4 | 265.8 |
| 8 | Scullcapflavone II | C 19H 18O 8 | 463.8 |
| 9 | Myrtenol | C 10H 16O | 306 |
| 10 | 3-tert-Butyl-5-methylcatechol | C 11H 16O 2 | 427.8 |
| 11 | (+)-7-Isojasmonic acid | C 12H 18O 3 | 404.4 |
| 12 | Traumatic acid | C 12H 20O 4 | 91.2 |
| 13 | 4-Heptyloxyphenol | C 13H 20O 2 | 282.6 |
| 14 | 4,4”-Dihydroxystilbene | C 14H 12O 2 | 276.6 |
| 15 | 1,3-Diphenylpropane | C 15H 16 | 309.6 |
| 16 | Geranyl hydroquinone | C 16H 22O 2 | 781.2 |
| 17 | Syringin | C 17H 24O 9 | 232.8 |
| 18 | 3-Hydroxybenzaldehyde | C 7H 6O 2 | 280.2 |
| 19 | 6-Hydroxyluteolin 7-glucoside | C 21H 20O 12 | 256.2 |
| 20 | 6-Methoxyaromadendrin 3-O-acetate | C 18H 16O 8 | 388.8 |
| 21 | Adenine | C 5H 5N 5 | 72.6 |
| 22 | 9S-hydroxy-10E,12Z,15Z-octadecatrienoic acid | C 18H3 0O 3 | 372.6 |
| 23 | 9E-Heptadecenoic acid | C 17H 32O 2 | 337.2 |
| 24 | Carboxymethyloxysuccinate | C 6H 8O 7 | 81 |
| 25 | Coumarin | C 9H 6O 2 | 219 |
| 26 | Pent-7alpha-Hydroxykaur-16-en-19-oic acid | C 20H 30O 3 | 319.8 |
| 27 | Etherolenic acid | C 18H 28O 3 | 357.6 |
| 28 | Icariin | C 33H 40O 15 | 240 |
Assessment of the implication of bush tea compounds in ZnO nanoparticles synthesis
In this study, compound identification was carried out using Bruker data analysis and data profiling tools. The KEGG and ChemSpider databases were consulted to find the name and the chemical formula of each identified compound. The different compounds with mass to ratio (m/z) values as well as their retention time (in seconds) were shown with a variable importance in progression (VIP) score plot ( Figure 3). The concentration of eight compounds were found to be high in the crude extract compared to the supernatant after synthesis of ZnO nanoparticles where their concentrations were low.
Figure 3. Variable importance in progression (VP) score plot of different compounds found in the bush tea crude extract before synthesis and the supernatant after synthesis of ZnO nanoparticles.
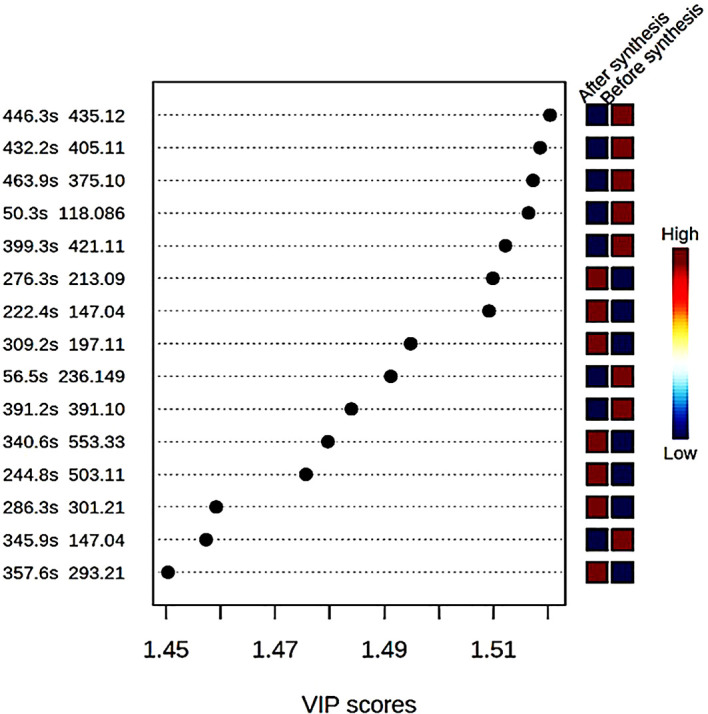
Table 3 present the various compounds that were involved in the synthesis process of ZnO nanoparticles including five flavonoids and two polyphenol compounds, as well as one aromatic compound, which highly reacted with the precursor to form ZnO nanoparticles. Studies have shown that the synthesis of nanoparticles using plant extracts involves terpenoids, flavonoids, alkaloids and phenolic acid, which act as reducing, capping, and stabilizing agents ( Kuppusamy et al., 2016).
Table 3. Identified compounds reported having mostly interacted with the precursor to form ZnO nanoparticles.
| Compound name | Formula | Type |
|---|---|---|
| Naringenin 7-O-beta-D-glucoside | C 21H 22O 10 | Flavonoid |
| Scullcapflavone II | C 19H 18O 8 | Flavonoid |
| Mallotophenone | C 21H 24O 8 | Polyphenol |
| 6-Methoxyaromadendrin 3-O-acetate | C 18H 16O 8 | Flavonoid |
| 2-Phenylacetamide | C 8H 9NO | Polyphenol group |
| 7-Hydroxy-2”,4”,5”-trimethoxyisoflavone | C 18H 16O 6 | Flavonoid |
| Coumarin | C 9H 6O 2 | Aromatic |
| Malonyldaidzin | C 24H 22O 12 | Flavonoid |
ZnO nanoparticles characterization
XRD analysis
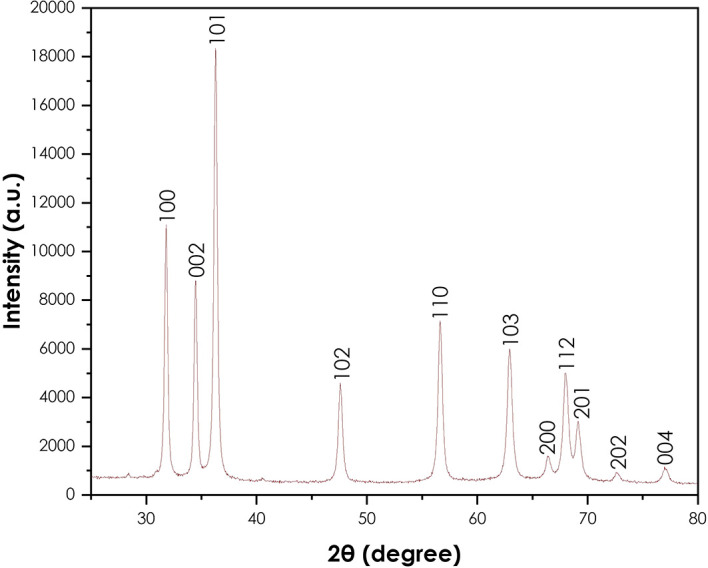
The XRD analysis was done to confirm the crystallinity of the synthesized ZnO nanoparticles using a Bruker AXS (Germany) D8 advance X-ray diffractometer. Figure 4 presents the XRD pattern of the ZnO nanoparticles. The crystallinity of the powder resulting from the synthesis using A. phylicoides DC extract. The peaks (100), (002), (101), (102), (110), (103), (200), (112), (201), (004) and (202) are lattice planes. The diffraction peaks reveal that the synthesized ZnO nanoparticles are essentially crystalline, in accord with the ICDD #897102 in the wurtzite structure ( Noman et al., 2020). The same results have been observed by the green synthesis of ZnO nanoparticles using Ocimum basilicum ( Salam et al., 2014) and Agathosma betulina ( Thema et al., 2015). The average crystallite size of obtained ZnO nanoparticles calculated using the modified Scherrer equation was approximately 24.53 nm.
Figure 4. X-ray diffraction pattern of ZnO nanoparticles.
Fourier-transform infrared spectroscopy
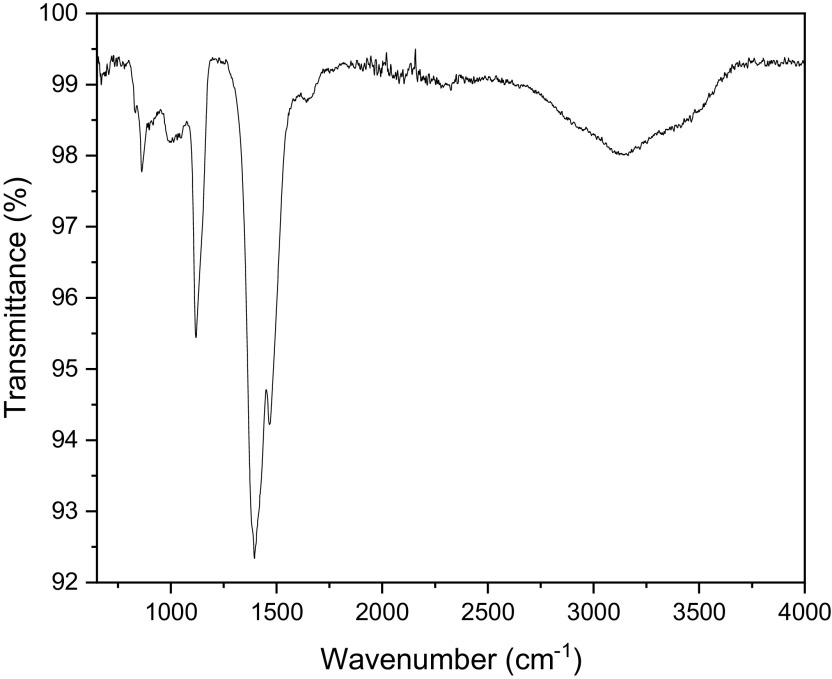
The PerkinElmer Frontier FTIR spectrometer was used to perform FTIR analyses using Potassium bromide (KBr) (Potassium bromide) optics. The presence of ZnO nanoparticles was confirmed by the peak at 479 cm −1 as shown in Figure 5. The other observed peaks are attributed to the phytochemical components present in the extract solution. The peak at 1113 cm −1 is attributed to the C-O stretching of primary alcohols. The peak at 1427 cm −1 corresponds to the O-H bending of the carboxylic acid. The peak observed at 2351 cm −1 is attributed to the O=C=O stretching of carbon dioxide. The FTIR spectra of bush tea extract, presented in Figure 6, show the presence of carboxylic acid bonding, primary alcohol stretching as well as the intramolecular hydrogen bond.
Figure 5. Fourier-transform infrared spectra of ZnO powder annealed at 600°C.
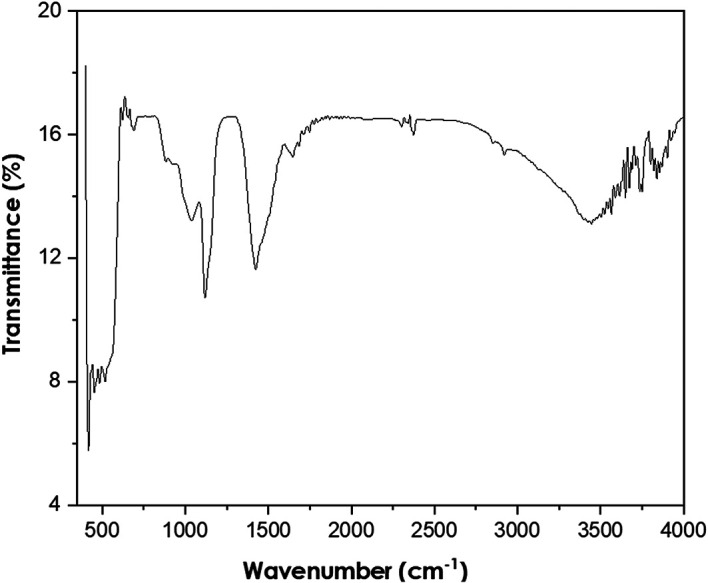
Figure 6. Fourier-transform infrared spectra of Bush tea leaf extract.
UV-Vis analysis
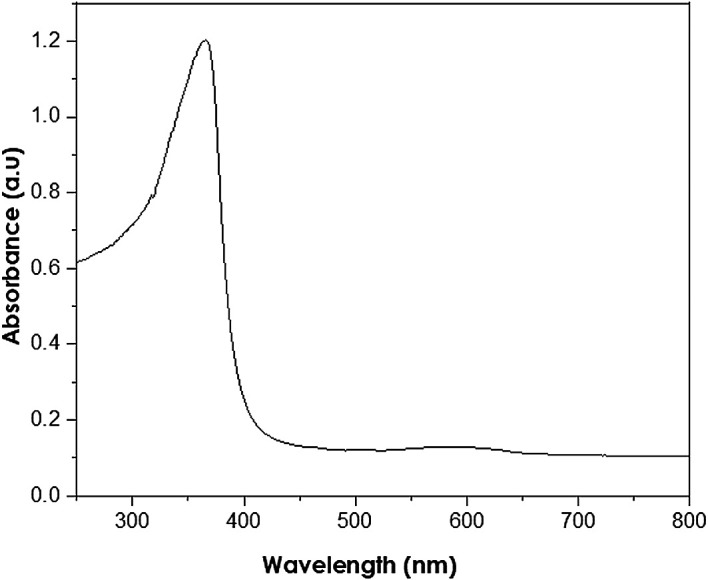
UV-Vis analyses were performed at a resolution of 1 nm at 250–800 nm wavelength range using a PerkinElmer Lambda 650S UV-Vis spectrometer. The absorption of ZnO nanoparticles is observed in the wavelength range of 250–400 nm ( Kolekar et al., 2011). The measured peak at 380 nm (as shown in Figure 7) reveals the presence of ZnO nanoparticles with a band gap energy of 3.11 eV, smaller than the bulk ZnO of 3.37 eV. Thus, the presence of hexagonal wurtzite structures in the analysed samples is indicated, in accordance with the XRD results.
Figure 7. Ultraviolet-visible spectra of as-synthesized ZnO nanoparticles.
SEM and EDS analyses
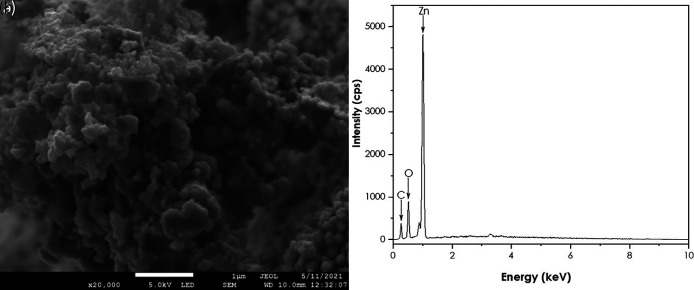
A JEOL JSM-7500F field-emission scanning electron microscope (FE-SEM) coupled with a JXA-8230/SXEDS/EDS/WDS energy-dispersive X-ray spectrometer (EDS) was used to get the morphology and the purity of the ZnO nanoparticles. SEM results are represented in Figure 8. The image shows quasi-spherical shaped ZnO nanoparticles agglomerated together. The EDS confirmed the presence of Zn and O. These findings are supported by Nethavhanani (2017) using natural extracts of Aspalathus linearis as a reducing agent ( Nethavhanani, 2017).
Figure 8. (a) Scanning electron microscopy image and (b) Energy-dispersive X-ray spectra of ZnO nanoparticles.
Discussion
Understanding the process of nanoparticles synthesis using the green route is key to the efficiency of the process and the outcome. Following the lack of data on chemical interactions of plant extracts with different metals to form nanoparticles, this study aimed to investigate the interaction of compounds with zinc nitrate to form ZnO nanoparticles. The identification of plant metabolites was performed using LC-MS tools by means of different databases such as KEGG, ChemSpider or Metfrag ( Cecilia et al., 2020). Henceforth, the differences in the extracts resulting from the synthesis of ZnO nanoparticles were shown by means of PCA and the VIP score plot. Bush tea leaves contain a high percentage of flavonoids and tannins, apart from non-structural carbohydrates, proteins, fatty acids, and minerals, such as calcium, magnesium, phosphorus, potassium, sodium, iron, manganese, zinc, copper, aluminium, sulphur and fluoride ( Lerotholi et al., 2017). Hence, the synthesis process resulted in the complete use of some metabolites as shown in Figure 2. The supernatant recorded low quantities of 8-C-Glucosylnaringenin/Naringenin 7-O-beta-D-glucoside, Scullcapflavone II, Mallotophenone, 6-Methoxyaromadendrin 3-O-acetate, 2-Phenylacetamide, 7-Hydroxy-2″,4″,5″-trimethoxyisoflavone, Coumarin, Malonyldaidzin ( Figure 3). A variety of metabolites, such as terpenoids, polyphenols, sugars, alkaloids, phenolic acids, and proteins can reduce metal ions into nanoparticles ( Marslin et al., 2018). Flavonoids, polyphenols as well as an aromatic compound interacted most with the precursor to form ZnO nanoparticles ( Table 2). UV-Vis is a wonderful tool for the examination of the size and the shape of nanoparticles ( Raut Rajesh et al., 2009). The analysed samples show the presence of a wurtzite structure at 380 nm. These findings are supported by ( Krupa and Vimala, 2016) who reported the synthesis of ZnO nanoparticles absorbing light at 368 nm. The wavelength of 380 nm corresponds to the bulk band-edge of 3.2 eV for ZnO ( Kolekar et al., 2011).
Conclusion
In this study, bush tea metabolites were screened to understand their interaction with metal ions to form nanoparticles. The LC-MMS peaks in both the crude extract before ZnO nanoparticles synthesis and the supernatant after synthesis revealed a significant difference, shown by the PCAs. Different flavonoids, polyphenols and an aromatic compound were found to react with zinc nitrate to form zinc nanoparticles. The FTIR as well as the XRD and UV-Vis analyses confirmed the formation of ZnO nanoparticles with a hexagonal wurtzite structure.
Data availability
All data underlying the results are available as part of the article and no additional source data are required.
Funding Statement
This research was generously supported by the University of South Africa, iThemba LABS, the UNESCO-UNISA Africa Chair in Nanosciences and Nanotechnology, to whom we are all grateful.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 1 approved with reservations
References
- Agarwal H, Kumar SV, Rajeshkumar S: A review on green synthesis of zinc oxide nanoparticles–An eco-friendly approach. Resour. Technol. 2017;3:406–413. 10.1016/j.reffit.2017.03.002 [DOI] [Google Scholar]
- Cecilia MK, Abrha AH, Hlengilizwe N, et al. : Untargeted profiling of field cultivated bush tea (Athrixia phylicoides DC.) based on metabolite analysis. Cell. Mol. Biol. 2020;66:104–109. 10.14715/cmb/2020.66.4.14 [DOI] [PubMed] [Google Scholar]
- Dhandapani P, Siddarth AS, Kamalasekaran S, et al. : Bio-approach: ureolytic bacteria mediated synthesis of ZnO nanocrystals on cotton fabric and evaluation of their antibacterial properties. Carbohydr. Polym. 2014;103:448–455. 10.1016/j.carbpol.2013.12.074 [DOI] [PubMed] [Google Scholar]
- Dobrucka R, Długaszewska J: Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J. Biol. Sci. 2016;23:517–523.2016. 10.1016/j.sjbs.2015.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunalan S, Sivaraj R, Rajendran V: Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int. 2012;22:693–700. 10.1016/j.pnsc.2012.11.015 [DOI] [Google Scholar]
- Hussain A, Oves M, Alajmi MF, et al. : Biogenesis of ZnO nanoparticles using Pandanus odorifer leaf extract: anticancer and antimicrobial activities. RSC Adv. 2019;9:15357–15369. 10.1039/C9RA01659G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani S, Korbekandi H, Zolfaghari B: Phytosynthesis of nanoparticles. Nanotechnol. Plant Sci. 2015;203–258. 10.1007/978-3-319-14502-0_11 [DOI] [Google Scholar]
- Jamdagni P, Khatri P, Rana JS: Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ. 2018;30:168–175. 10.1016/j.jksus.2016.10.002 [DOI] [Google Scholar]
- Jeevanandam J, Chan YS, Danquah MK: Biosynthesis of metal and metal oxide nanoparticles. ChemBioEng Rev. 2016;3:55–67. 10.1002/cben.201500018 [DOI] [Google Scholar]
- Kharissova OV, Dias HVR, Kharisov BI, et al. : The greener synthesis of nanoparticles. Trends Biotechnol. 2013;31:240–248. 10.1016/j.tibtech.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Kolekar TV, Yadav HM, Bandgar SS, et al. : Synthesis by sol–gel method and characterization of ZnO nanoparticles. Indian Streams Res. J. 2011;1:1–4. [Google Scholar]
- Krupa AND, Vimala R: Evaluation of tetraethoxysilane (TEOS) sol–gel coatings, modified with green synthesized zinc oxide nanoparticles for combating microfouling. Mater. Sci. Eng. C. 2016;61:728–735. 10.1016/j.msec.2016.01.013 [DOI] [PubMed] [Google Scholar]
- Krupa AND, Vimala R: Evaluation of tetraethoxysilane (TEOS) sol–gel coatings, modified with green synthesized zinc oxide nanoparticles for combating microfouling. Mater. Sci. Eng. C. 2016;61:728–735. 10.1016/j.msec.2016.01.013 [DOI] [PubMed] [Google Scholar]
- Kuppusamy P, Yusoff MM, Maniam GP, et al. : Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications–An updated report. Saudi Pharm. J. 2016;24:473–484. 10.1016/j.jsps.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerotholi L, Chaudhary SK, Combrinck S, et al. : Bush tea (Athrixia phylicoides): A review of the traditional uses, bioactivity and phytochemistry. South African J. Bot. 2017;110:4–17. 10.1016/j.sajb.2016.06.005 [DOI] [Google Scholar]
- Mahendiran D, Subash G, Selvan DA, et al. : Biosynthesis of zinc oxide nanoparticles using plant extracts of Aloe vera and Hibiscus sabdariffa: Phytochemical, antibacterial, antioxidant and anti-proliferative studies. Bionanoscience. 2017;7:530–545. 10.1007/s12668-017-0418-y [DOI] [Google Scholar]
- Mansoori GA, Soelaiman TAF: Nanotechnology—an introduction for the standards community. J. ASTM Int. 2005;2:13110–13122. 10.1520/JAI13110 [DOI] [Google Scholar]
- Marslin G, Siram K, Maqbool Q, et al. : Secondary metabolites in the green synthesis of metallic nanoparticles. Materials (Basel). 2018;11:940. 10.3390/ma11060940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matinise N, Fuku XG, Kaviyarasu K, et al. : ZnO nanoparticles via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Appl. Surf. Sci. 2017;406:339–347. 10.1016/j.apsusc.2017.01.219 [DOI] [Google Scholar]
- Monshi A, Foroughi MR, Monshi MR: Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012;02:154–160. 10.4236/wjnse.2012.23020 [DOI] [Google Scholar]
- Nethavhanani T: Synthesis of zinc oxide nanoparticles by a green process and the investigation of their physical properties. 2017.
- Noman MT, Petru M, Amor N, et al. : Thermophysiological comfort of zinc oxide nanoparticles coated woven fabrics. Sci. Rep. 2020;10:1–12. 10.1038/s41598-020-78305-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Akashi T, Huang Y, et al. : Surface-initiated living radical polymerization from narrowly size-distributed silica nanoparticles of diameters less than 100 nm. Macromolecules. 2010;43:8805–8812. 10.1021/ma1018389 [DOI] [Google Scholar]
- Parveen K, Banse V, Ledwani L: Green synthesis of nanoparticles: their advantages and disadvantages. AIP Conference Proceedings. AIP Publishing LLC;2016. p.20048. [Google Scholar]
- Rao MD, Gautam P: Synthesis and characterization of ZnO nanoflowers using C hlamydomonas reinhardtii: A green approach. Environ. Prog. Sustain. Energy. 2016;35:1020–1026. 10.1002/ep.12315 [DOI] [Google Scholar]
- Raut Rajesh W, Lakkakula Jaya R, Kolekar Niranjan S, et al. : Phytosynthesis of silver nanoparticle using Gliricidia sepium (Jacq.). Curr. Nanosci. 2009;5:117–122. [Google Scholar]
- Salam HA, Sivaraj R, Venckatesh R: Green synthesis and characterization of zinc oxide nanoparticles from Ocimum basilicum L. var. purpurascens Benth.-Lamiaceae leaf extract. Mater. Lett. 2014;131:16–18. 10.1016/j.matlet.2014.05.033 [DOI] [Google Scholar]
- Satyanarayana T, Reddy SS: A review on chemical and physical synthesis methods of nanomaterials. Int. J. Res. Appl. Sci. Eng. Technol. 2018;6:2885–2889. 10.22214/ijraset.2018.1396 [DOI] [Google Scholar]
- Senthilkumar SR, Sivakumar T: Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int J Pharm Pharm Sci. 2014;6:461–465. [Google Scholar]
- Shinwari ZK, Maaza M: The study of structural, physical and electrochemical activity of Zno nanoparticles synthesized by green natural extracts of sageretia thea. Arch. Med. 2017;3:9. [Google Scholar]
- Stan M, Popa A, Toloman D, et al. : Enhanced photocatalytic degradation properties of zinc oxide nanoparticles synthesized by using plant extracts. Mater. Sci. Semicond. Process. 2015;39:23–29. [Google Scholar]
- Tete VS, Nyoni H, Mamba BB, et al. : Occurrence and spatial distribution of statins, fibrates and their metabolites in aquatic environments. Arab. J. Chem. 2020;13:4358–4373. 10.1016/j.arabjc.2019.08.003 [DOI] [Google Scholar]
- Thema FT, Manikandan E, Dhlamini MS, et al. : Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater. Lett. 2015;161:124–127. 10.1016/j.matlet.2015.08.052 [DOI] [Google Scholar]