Abstract
Pentavalent rotavirus vaccine has been associated with a small increase in intussusception, but pre- and post-introduction data are lacking in many low-resource settings. Using chart review and prospective surveillance data, intussusception incidence was estimated in Bamako, Mali. The mean annual intussusception incidence post-introduction was not significantly different from that of pre-introduction.
Keywords: immunization, intussusceptions, Mali, post-marketing surveillance, RotaTeq
The association between rotavirus vaccines and intussusception has raised concern about the risk-benefit ratio of these vaccines in low-resource settings. We describe rates of intussusception among infants in Bamako, Mali, before and after the introduction of pentavalent rotavirus vaccine.
The introduction of rotavirus vaccine has reduced hospitalization and death from rotavirus-specific as well as all cause diarrhea globally. In Mali, where rotavirus has been shown to be an important cause of morbidity and mortality [1, 2], RotaTeq (RV5), Merck, West Point, PA, USA, was introduced into the infant immunization schedule in 2014, with doses scheduled for administration at 6, 10, and 14 weeks of age, and high levels of vaccine coverage were rapidly attained [3]. Although the benefits of rotavirus vaccination are expected to far outweigh any vaccine-associated risks, RV5 was associated with a small increased risk of intussusception during the 7 days following receipt of the first dose of either rotavirus vaccine [4–7]. This association has been found primarily in high-resource settings, while data on the risk in low-income countries are sparse.
This study aims to estimate the incidence of intussusception among infants residing in Bamako, Mali, before and after RV5 introduction, to describe trends in intussusception incidence, and to characterize the care-seeking behavior, management, and outcomes of infants with intussusception.
METHODS
Post-Vaccine Introduction Assessment
Between August 1, 2015, and September 30, 2017, we prospectively identified infants less than 12 months of age with possible intussusception who resided in Bamako, Mali. Because of the severe symptoms associated with intussusception, it was assumed that most intussusception cases would present to 1 of the 9 hospitals or referral centers in Bamako for acutely ill children. Study staff visited these sites daily to review the admission, consultation, and surgical procedure logs for infants who might be eligible for the study. Infants diagnosed with possible abdominal obstruction, bowel resection, or suspected intussusception were eligible. Medical records of enrolled children were abstracted, and parents were interviewed to obtain a detailed history of the child’s illness. An adjudication committee (AC) composed of 3 physicians with expertise in pediatrics, intussusception, and surgery reviewed these documents to reach a consensus. An independent radiologist interpreted all imaging studies. Confirmed intussusceptions fulfilled Brighton Collaboration level 1 criteria [8].
Pre-Vaccine Introduction Assessment
To estimate the incidence rate of intussusception prior to vaccine introduction, we retrospectively identified children hospitalized with intussusception between January 2099 and December 2014 by reviewing admission and discharge logs, case history records, ultrasonography, radiology logs, and surgery reports from lʹHôpital Gabriel Touré (HGT), the primary pediatric referral hospital in Mali and one of the sites included in the prospective study. Bamako residents <12 months of age who met Brighton Collaboration level 1 criteria were included. A retrospective review of records from the other centers participating in the prospective study was not performed because HGT was the sole pediatric surgery service managing cases of intussusception before RV5 introduction.
The annual incidence of intussusception was estimated using the number of confirmed cases as the numerator, while the denominator was derived from the enumerated infants residing in Bamako during the Mali 2009 national census. Annual subsequent estimates of the infant birth cohort were adjusted using 5.4% as the annual growth rate, provided by the Mali National Institute of Statistics.
For all incidence estimates, sensitivity analyses were performed to estimate the impact of estimated birth cohort growth, possible enrollment refusal, and the potential omission of other surveillance sites from record review. All statistical analyses were conducted using R version 3.2.2 [9].
These studies were approved by the Institutional Review Board of the University of Maryland, Baltimore, and by the Ethics Committee of the Faculté de Médecine, de Pharmacie et dʹOdonto-Stomatologie, University of Mali. A parent or guardian gave consent for the participation of each child in the prospective study.
RESULTS
Demographic and Clinical Features of Active Surveillance Cohort (Post-RV5 Vaccine Introduction)
Between August 1, 2015, and September 30, 2017, when active surveillance for intussusception was taking place, 365 records were identified at surveillance centers. In total, 246 records did not meet age or residency eligibility requirements, and 9 represented the same child recognized at a second surveillance center. Thirteen eligible children could not be located to obtain consent for enrollment, and the parents of 5 eligible children declined to participate. All 83 suspected intussusceptions enrolled were reviewed by the AC, and 59 were confirmed as intussusceptions. Nearly all (55/59) children with intussusception were ultimately referred to HGT.
Demographics and clinical management of confirmed intussusception cases are presented in Table 1. Of the 59 confirmed cases, nearly all cases were treated surgically, the median age was 6 months, and nearly two-thirds were male. In total, 11 children (18.6%) died from complications of intussusception; these children did not present significantly later for care. The median age of RV5 was 1.5 months (dose 1), 2.8 months (dose 2), and 3.8 months (dose 3). All but one of the infants with intussusception had received at least one dose of RV5; 81% (48/59) had received the complete 3-dose series.
Table 1.
Demographics, Clinical Characteristics, and Healthcare-Seeking Behavior of Infants With Intussusception Identified by Active Surveillance Performed Following Rotavirus Vaccine Introduction
| All (N = 59) |
Outcome | |||
|---|---|---|---|---|
| Survived (N = 48) |
Died (N = 11) |
P-value | ||
| Median age in months (range) | 6 (1-10) | 6 (1-10) | 6 (5-9) | .64 |
| N (%) Female sex | 21 (35.6) | 17 (35.4) | 4 (36.4) | 1 |
| Rotavirus vaccination | ||||
| No doses | 1 (1.7) | 1 (2.1) | 0 (0) | .47 |
| 1 dose | 2 (3.4) | 1 (2.1) | 1 (9.1) | |
| 2 doses | 8 (13.6) | 6 (12.5) | 2 (18.2) | |
| 3 doses | 48 (81.4) | 40 (83.3) | 8 (72.7) | |
| Mean number of days between last dose and intussusception (sd) | 90.9 (58.7) | 86.7 (60.7) | 107.2 (49.3) | .24 |
| Treatment | ||||
| Infant died before surgery | 1 (1.7) | 0 (0) | 1 (9.1) | .19 |
| Spontaneous reduction before surgery | 1 (1.7) | 1 (2.1) | 0 (0) | 1 |
| Spontaneous reduction noted during surgery | 3 (5.1) | 3 (6.2) | 0 (0) | 1 |
| Surgical reduction | 40 (67.8) | 35 (72.9) | 5 (45.5) | .15 |
| Surgical resection | 14 (23.7) | 9 (18.8) | 5 (45.5) | .11 |
| Median number of times care sought prior to admission (range) | 2 (0-6) | 2 (0-6) | 3 (0-4) | .35 |
| Median number of days between symptom onset and hospital admission (range) | 3 (0-21) | 3 (0-21) | 4 (1-10) | .15 |
Demographic and Clinical Features of Retrospective Surveillance Cohort (Pre-RV5 Vaccine Introduction)
A total of 219 cases met Brighton Collaboration level 1 criteria. Age did not differ significantly between the pre-introduction (6.4 months) and post-introduction periods (6.3 months) (P = .75); neither did mortality (11.0% vs 16.9%, P = .07). No child in the active surveillance cohort experienced an intussusception within 21 days of the administration of the first dose of RV5. Four intussusceptions occurred during the 21 days after the second dose of RV5, and 5 intussusceptions occurred during the 21 days after the third dose of RV5, but visual inspection of the data did not suggest any clustering of cases after a specific dose.
Incidence of Intussusception Before and After Vaccine Introduction
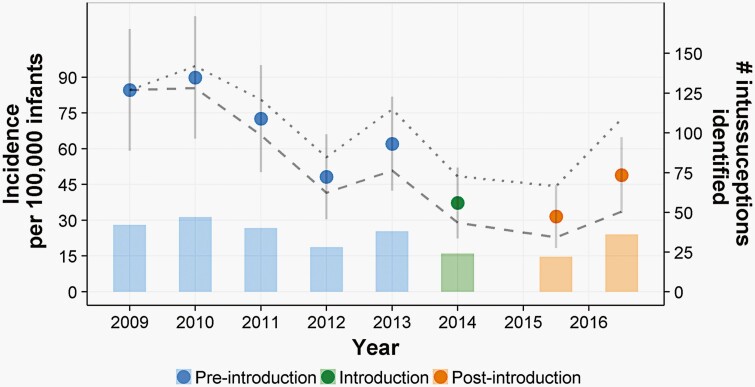
The overall incidence of intussusception over the period of active surveillance was estimated to be 40.4 (95% confidence interval, 25.7-55.1) per 100 000 live births, with an average of 29 cases per year. In the retrospective record review, an average of 36.5 cases of intussusception per year were identified, with cases falling year over year through 2014, when RV5 was introduced in Mali (Figure 1). Treating 2014 as a washout year, the incidence rate ratio (IRR) for the post-RV5 years relative to the pre-RV5 years was 0.57 (95% CI, 0.49-1.27; P = .33). The observed IRR suggested that there was no statistically significant difference in the incidence rates. This inference was not altered by adjustments to estimates of the size of the underlying infant population, adjustments for non-enrollment during the active surveillance portion of the study, or adjustments for cases not presenting to HGT during the record review time period.
Figure 1.
Incidence and counts of intussusception cases among Bamako infants, before and after RV5 introduction. Lines estimate incidence under alternative population growth models. The lower dashed line estimates incidence under the assumption that the population would grow twice as quickly as estimated, and the upper dotted line represents a population that has remained static since 2009. Bars estimate the number of intussusception cases identified annually.
DISCUSSION
We provide here the first description of intussusception incidence among infants residing in Bamako, Mali, a country with high infant mortality that implemented routine RV5 vaccination. We did not find evidence for an increased risk of intussusception following RV5 introduction. The observed incidences of intussusception before and after the programmatic introduction of RV5 were not significantly different, nor was there a trend showing a post-introduction increase. Moreover, the post-introduction rates were consistent with pre-rotavirus vaccine introduction incidence estimates from other countries [10], and the peak age of episodes post-vaccine introduction (6 months) was comparable to the background rates in our study and in other African countries [11].
Post-licensure safety surveillance is a useful tool for detecting rare adverse events from vaccination. World Health Organization and other regulatory bodies have recommended post-licensure studies to evaluate the risk of intussusception to assure that the long-term benefit and safety of rotavirus vaccines are adequately assessed. However, there are numerous challenges that impact data collection in high-mortality countries in sub-Saharan Africa, including limited access to health care where cases can be detected, low awareness of intussusception among healthcare workers, and the unavailability of noninvasive radiographic tools for diagnosis and treatment. It is reassuring that a safety signal was not detected among recipients of RV1 in a large multi-center self-controlled case series conducted post-licensure in 7 countries in sub-Saharan Africa [12]. Although underpowered to detect very small changes in intussusception risk, the absence of a safety signal in our study is reassuring.
This study has several limitations. Retrospective cases could have been undercounted because we conducted the retrospective chart review only at HGT, whereas the prospective surveillance took place at all major district, regional, and national health facilities in Bamako. However, the prospective surveillance demonstrated that 93% of all cases of intussusception were ultimately referred to HGT, suggesting that the retrospective review identified most cases. In addition, under-reporting may have occurred in both retrospective and prospective surveillance if children died from intussusception without seeking medical care. We believe that this would be rare given the severity of symptoms prior to death. Finally, even if intussusception cases were underestimated during pre-vaccination retrospective surveillance, the observation that the incidence of intussusception did not increase after the introduction of RV5 would not have been impacted.
In conclusion, our findings show that in Mali, a high-mortality country in sub-Saharan Africa, RV5 introduction is associated with no apparent increased risk of intussusception among vaccinated infants.
Notes
Acknowledgments . The authors thank the families of the infants who participated in this study, Dr Mamadou Sylla, and the efforts of the adjudication committee, Drs. Gregory Bates, Julie Bines, Hope Glover-Addy, Tatiana Keita, Sebastian King, and Margaret Rennels.
Author contributions . A. R. drafted the manuscript; S. O. S. and A. M. K. collected the data; M. D. T., T. C. M., and K. L. K. designed the study and reviewed the manuscript. All authors had full access to the data and approved the final manuscript.
Financial support. This work was supported by Merck & Co, Inc. The funding source contributed to the design of the trial and reviewed and approved the final manuscript.
Potential conflicts of interest . All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Anna Roose, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Adama Mamby Keita, Centre for Vaccine Development, Bamako, Mali.
Milagritos D Tapia, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA; Department of Pediatrics and Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Samba O Sow, Centre for Vaccine Development, Bamako, Mali.
T Christopher Mast, Merck & Co., Kenilworth, New Jersey, USA.
Karen L Kotloff, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA; Department of Pediatrics and Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland, USA.
References
- 1. Kotloff KL, Nataro JP, Blackwelder WC, et al. . Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 2. Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD.. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis 2017; 215:1666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roose A, Onwuchekwa U, Tapia M, et al. . Assessing vaccine coverage and timeliness in Bamako, Mali after the introduction of rotavirus vaccine: a modified immunization cluster survey. Am J Trop Med Hyg 2021; 105:1594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rotavirus Vaccines. WHO position paper – January 2013. Releve Epidemiol Hebd 2013; 88:49–64. [PubMed] [Google Scholar]
- 5. Tate JE, Steele AD, Bines JE, Zuber PLF, Parashar UD.. Research priorities regarding rotavirus vaccine and intussusception: a meeting summary. Vaccine 2012; 30:179–84. [DOI] [PubMed] [Google Scholar]
- 6. Buttery JP, Danchin MH, Lee KJ, et al. . Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine 2011; 29:3061–6. [DOI] [PubMed] [Google Scholar]
- 7. Patel MM, López-collada VR, Mattos M, et al. . Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med 2011; 228:3–92. [DOI] [PubMed] [Google Scholar]
- 8. Bines JE, Ivanoff B, Justice F, Mulholland K.. Clinical case definition for the diagnosis of acute intussusception. J Pediatr Gastroenterol Nutr 2004; 39:511–8. [DOI] [PubMed] [Google Scholar]
- 9. R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Core Team; 2016. [Google Scholar]
- 10. Clark AD, Hasso-Agopsowicz M, Kraus MW, et al. . Update on the global epidemiology of intussusception: a systematic review of incidence rates, age distributions and case-fatality ratios among children aged <5 years, before the introduction of rotavirus vaccination. Int J Epidemiol 2019; 48:1316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mpabalwani EM, Mwenda JM, Tate JE, Parashar UD.. Review of naturally occurring intussusception in young children in the WHO African region prior to the era of rotavirus vaccine utilization in the expanded programme of immunization. J Trop Pediatr 2017; 63:221–8. [DOI] [PubMed] [Google Scholar]
- 12. Tate JE, Mwenda JM, Armah G, et al. . Evaluation of intussusception after monovalent rotavirus vaccination in Africa. N Engl J Med 2018; 378:1521–8. [DOI] [PubMed] [Google Scholar]