Abstract
This was a cross-sectional community-based serological survey of polio antibodies assessing the immunogenicity of inactivated poliovirus vaccine (IPV) focusing on poliovirus serotype 2. IPV was administered to 5-month-old children. Type 2 antibody seroprevalence when measured 1 month after IPV administration was >95%. One IPV dose successfully closed the immunity gap.
Keywords: inactivated poliovirus vaccine, polio eradication, poliomyelitis, routine immunization, Vietnam
This cross-sectional community-based serological survey of polio antibodies demonstrates that one IPV dose successfully closes the type 2 immunity gap from the global switch from tOPV to bOPV. Type 2 antibody seroprevalence one month after IPV administration was >95%.
Afghanistan and Pakistan are the only 2 remaining endemic countries for wild poliovirus (WPV); 5 paralytic WPV cases have been reported from these 2 countries in 2021; however, in the same period, 774 paralytic cases of polio were caused by vaccine-derived poliovirus [1].
The Sabin poliovirus strains in the live oral poliovirus vaccine (OPV) may, in rare circumstances, genetically revert into a form causing paralytic disease. This circulating vaccine-derived poliovirus (cVDPV) can cause outbreaks that are clinically indistinguishable from outbreaks caused by WPV [2]. The “Polio Eradication & Endgame Strategic Plan 2013-2018” laid out the framework for interruption of WPV transmission and the phased withdrawal of OPV in order to eliminate the risk of VDPV [3]. In April 2016, there was a globally synchronized switch from trivalent OPV (tOPV) to bivalent OPV (bOPV). Since that date, no live poliovirus type 2 (PV2) vaccine has been used in routine immunization (RI) programs anywhere in the world [4].
In parallel with OPV withdrawal, the Strategic Advisory Group of Experts (SAGE) on Immunization recommended the universal introduction of at least 1 dose of inactivated poliovirus vaccine (IPV) to provide base immunity for PV2 and to mitigate the risks caused by cVDPV2 [5, 6]. This recommendation has now been modified and includes 2 IPV doses [7]. Almost 2 years after the tOPV to bOPV switch, a large number of countries have not been able to introduce IPV because of IPV supply constraints leading to country-wide IPV stock-outs [8, 9]. Vietnam was unable to introduce IPV until October 2018 leaving more than 2 birth cohorts of children unprotected from PV2. A study from 2018 in Vietnam showed a rapid decline of PV2 neutralizing antibodies in these cohorts of children [10]. As IPV became available, 1 dose of IPV was introduced in the Vietnamese RI schedule at 5 months of age, following a schedule of 3 doses of bOPV administered at 2, 3, and 4 months of age.
The objective of this study was to demonstrate the impact of IPV introduction on PV2 immunity. As the secondary objective, we compared the achieved seroprevalence through regular RI activities versus through vaccination in the controlled setting of this study. In addition, we report seroprevalence of antibodies against poliovirus types 1 and 3. The data were collected in the second half of 2020.
METHODS
This study was a cross-sectional community-based serological survey of polio antibodies in the Nga Sơn district of the Thanh Hoa province in Vietnam. The Thanh Hoa province is located ~160 km from Hanoi with a population of ~3.4 million in 27 districts.
The primary objective of the study was to quantify the level of serological protection (seroprevalence) against PV2 in children who received 1 IPV dose either as part of the study or as part of the RI schedule. The secondary objective was to quantify the seroprevalence against PV1 and PV3 in children who received 1 IPV dose and 3 bOPV doses.
Children in 2 age groups were enrolled: 5-11 months (group 1)—these children had not yet received IPV—and children aged 12-15 months (group 2), who had received IPV dose as part of RI approximately 7 months before being enrolled in this study—the receipt of IPV was documented by vaccination card and verified through the National Immunization Information System.
A sample size was calculated to estimate seroprevalence assuming 60% seroprevalence after 1 dose of IPV at 5 months. This was calculated at the 95% confidence level with 10% precision using assumed seroprevalence levels. The resulting sample size was 93, which was inflated to 120 to account for possible challenges with blood draws, withdrawals, and sample management.
Parents/guardians of eligible children were approached by community health workers in 10 selected health centers and provided informed consent for enrollment. In group 1, one IPV dose was administered as part of the study, and 2 mL of peripheral blood was drawn in 2 instances: at enrollment (at the same time of IPV administration) and 30 days later. In group 2, blood was only drawn once at enrollment, no vaccine was administered, and no follow-up visit was organized.
Sera were analyzed at the Centers for Disease Control and Prevention (CDC) in Atlanta for the presence of neutralizing antibodies against all 3 polio serotypes using standard micro-neutralization assays [11]. Antibody titers were reported on a log2 scale. Seropositivity was defined as reciprocal titers of poliovirus neutralizing antibodies ≥1:8 (≥3 on log2 scale). The proportion of seropositive children and median antibody titers was calculated for each group. Highest reported antibody titer was 1:1448 (10.5 on log2 scale). In group 1, seroconversion for each serotype was defined as a change from seronegative at enrollment to seropositive post-IPV administration among children who were seronegative at enrollment. The seroprevalence and seroconversion, expressed as percentages, were presented along with Clopper-Pearson adjusted 95% confidence intervals (CIs) for proportions. The comparison of seroprevalence was performed using Fisher’s exact test. The median titers across the groups were compared using the Wilcoxon rank-sum test. P-value < .05 was considered as statistically significant. STATA 17.0 was used for the statistical analysis of data.
RESULTS
A total of 128 children between the age of 5 and 11 months (group 1) and 130 children between the age of 12 and15 months (group 2) were enrolled. The analyzable number of children was 119/128 (93.0%) in group 1 and 130/130 (100%) in group 2; the sera from the remaining children were insufficient to complete the analysis or the children were lost to follow-up between the first and second study visits in group 1. The baseline study characteristics are presented in Table 1. All children had a vaccination card available with a date of IPV vaccine administration. The median age at IPV administration in group 1 was 6 months (95% CI, 6-7 months) compared with 5 months (95% CI, 5-6 months) in group 2 (P = .043).
Table 1.
Distribution of Baseline Characteristics in the 2 Groups
| Group 1 N = 128 (5-11 mo) |
Group 2 N = 130 (12-15 mo) |
P-value | |||
|---|---|---|---|---|---|
| n/N | % or IQR | n/N | % or IQR | ||
| Female child | 65/128 | 50.8 | 71/130 | 54.6 | .541 |
| Median age at enrollment (months, IQR) | 6 | 5-7 | 13 | 12-14 | <.001 |
| Child received 3 doses of pentavalent vaccine | 84/128 | 65.6 | 130/130 | 100.0 | <.001 |
| Child did not receive any pentavalent vaccine | 1/128 | 0.8 | 0/130 | 0 | .307 |
| Child received IPV | 0/128 | 0 | 130/130 | 100.0 | <.001 |
| Age at IPV administration (median months, 95% CI) |
6 (6-7) | 5 (5-6) | .043 | ||
| Child received 3 doses of bOPV | 80/128 | 62.5 | 129/130 | 99.2 | <.001 |
| Child did not receive any bOPV | 1/128 | 0.8 | 0/130 | 0 | .307 |
All children had a vaccination card available with a date of IPV vaccine administration. Abbreviations: IQR, interquartile range; IPV, inactivated poliovirus vaccine; bOPV, bivalent oral poliovirus vaccine.
Bold indicates statistical significance at P < 0.05.
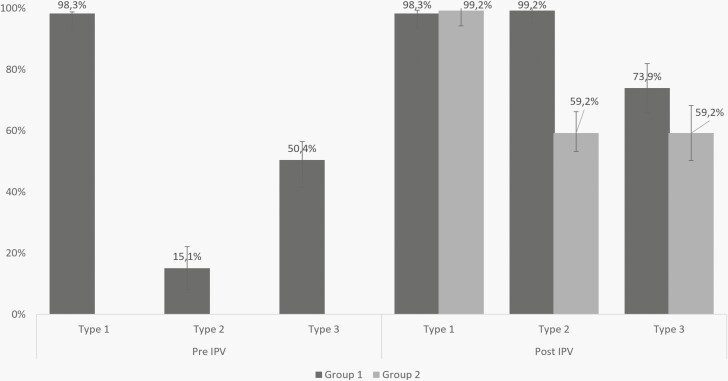
Seroprevalence of PV2 antibodies in group 1 was 18 out of 119 (15.1%) before IPV (corresponding with the expected prevalence of maternal antibodies at 6 months of age) and 118/119 (99.2%) one month after verified administration of 1 IPV dose. In group 2, the type 2 seroprevalence was 77 out of 130 (59.2%) measured approximately 7 months after the IPV dose was administered as part of RI (Figure 1).
Figure 1.
Seroprevalences of types 1, 2, and 3 by age groups (group 1: 5-11 months; group 2: 12-15 months). Samples were collected 1 month after IPV administration in group 1, and 7-10 months after IPV administration in group 2. IPV, inactivated poliovirus vaccine.
Seroprevalence of type 1 polio antibodies was 117/119 (98.3%) before and after IPV administration in group 1 and 129/130 (99.2%) in group 2. Type 3 seroprevalence was 60/119 (50.4%) before IPV; and 88/119 (73.9%) after IPV in group 1; and 77/130 (59.2%) in group 2 (Figure 1).
We assessed seroconversion in group 1 for all 3 serotypes. For serotype 2, it was 99% (100/101, 95% CI, 94.6-100); while for type 1 and type 3 it was 50% (1/2; 95% CI, 1.2-98.7) and 47.5% (28/59; 95% CI, 35.3-60.0), respectively.
Median type 2 antibody titers were significantly higher in group 1 compared with group 2 (6.50 vs 3.17; P < 0.001). Median antibody titers and reverse cumulative distribution curves for PV2 antibodies can be found in the Supplementary Material. In bivariate analysis, we did not find any risk factors for type 2 seronegativity.
DISCUSSION
Our study demonstrated that 1 dose of IPV administered at 5 months of age or later efficiently closed the immunity gap for poliovirus type 2. We were surprised at the finding that type 2 seroprevalence and antibody titers were significantly different when measured approximately 7 months after IPV compared with measurement 1 month after IPV administration (59.2% vs 99.2%, respectively), although this difference is in 2 separate cohorts of children. We were unable to distinguish whether this difference was due to waning of humoral immunity during the 7-month period between IPV administration and blood draw in group 2, or due to children in group 1 having received IPV on average 1 month later in life than in group 2, or because the history of IPV administration in RI is not certain—vaccination records may not be precise. Previous studies in Pakistan and elsewhere described antibody waning after IPV but not at such scale nor speed [10, 12, 13]. IPV improper storage or low batch efficacy must also be considered as potential causes of this difference, albeit quite unlikely in the case of Vietnam where the immunization program is well supervised.
Recently, SAGE recommended that 2 doses of IPV are included in all RI schedules worldwide to provide better immunogenicity [14]; however, our findings suggest that 1 IPV dose administered at a later age (≥5 months) is sufficiently immunogenic.
A limitation of the study was the acquisition of the vaccination history record of the children. The history was provided by the parents/guardian through immunization cards (100% children with card) and verified against the Vietnamese information system. It is possible that the vaccination cards were not a correct reflection of vaccination history. The fact that children in group 1 were sampled 1 month after IPV and children in group 2 were sampled approximately 7-10 months after IPV administration introduced bias due to possible waning of antibodies and comparison of possibly disparate groups of children.
Our study provides evidence regarding the importance of IPV in closing the type 2 immunity gap. Furthermore, the study findings underline the imperative to catch up cohorts of Vietnamese children that had been missed by IPV because of stock-outs and provided evidence that 1 dose of IPV, when administered at 5 months of age or later, is sufficient to provide close to 100% serological protection in case of VDPV2 emergence or importation.
Supplementary Material
Notes
Acknowledgments. We thank the staff of the Vietnamese Ministry of Health, enterovirus laboratories of the National Institute of Hygiene and Epidemiology, Pasteur Institute in Ho Chi Minh City, provincial and district health institutes, and the staff of the Division of Viral Diseases at the Centers for Disease Control and Prevention in Atlanta, namely Kathryn Jones, Sharla McDonald, Deborah Moore, Ashley Smith, and Yiting Zhang, for timely analysis of the sera. We acknowledge the staff of the health facilities in the selected districts for their extraordinary efforts to complete enrollments and study procedures within a tight timeline. We would also like to thank the Western Pacific Region Office and the Vietnam Country Office of the World Health Organization and their staff for their support and technical assistance, namely Tigran Avagyan, Nihal Singh, Makiko Iijima, and others. Most of all, we wish to acknowledge the children and their parents for agreeing to participate in the study.
Financial support . This work was supported by the World Health Organization through an IPPC Rotary Grant.
Potential conflicts of interest . All authors—no conflict of interest declared. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the contributing agencies. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Dang Thi Thanh Huyen, Expanded Program on Immunizations Department, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
Dang Duc Anh, Expanded Program on Immunizations Department, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
Nguyen Thanh Trung, Expanded Program on Immunizations Department, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
Duong Thi Hong, Expanded Program on Immunizations Department, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
Tran Trung Thanh, Expanded Program on Immunizations Department, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam.
Luong Ngoc Truong, Thanh Hoa Provincial CDC, Thanh Hoa, Vietnam.
Visalakshi Jeyaseelan, Polio Eradication Department, World Health Organization, Geneva, Switzerland.
Rocio Lopez Cavestany, Polio Eradication Department, World Health Organization, Geneva, Switzerland.
William S Hendley, CNA, Contracting Agency to the Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Bernardo A Mainou, Division of Viral Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Ondrej Mach, Polio Eradication Department, World Health Organization, Geneva, Switzerland.
References
- 1. WHO, GPEI. Global Wild Poliovirus 2016-2021 GPEI2021 [updated November 30, 2021]. https://polioeradication.org/wp-content/uploads/2021/12/weekly-polio-analyses-WPV-20211130.pdf. Accessed May 10, 2022.
- 2. GPEI. Fact sheet: vaccine-derived poliovirus. In: World Health Organization, ed. 2019. https://polioeradication.org/wp-content/uploads/2018/07/GPEI-cVDPV-Fact-Sheet-20191115.pdf. Accessed May 10, 2022.
- 3. GPEI. Polio Eradication & Endgame Strategic Plan 2013-2018. In: World Health Organization, ed. WHO Press, Geneva, Switzerland; 2013:51–72. https://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf. Accessed May 10, 2022. [Google Scholar]
- 4. GPEI. Polio Eradication & Endgame Strategic Plan 2019-2023. In: World Health Organization, ed. World Health Organization on behalf of the Global Polio Eradication Initiative,Switzerland; 2019: 28–33. https://polioeradication.org/wp-content/uploads/2019/06/english-polio-endgame-strategy.pdf. Accessed May 10, 2022. [Google Scholar]
- 5. GPEI. Planning for IPV introduction FAQs. In: World Health Organization, editor. 2014. https://www.paho.org/hq/dmdocuments/2014/IPV-IntroductionFAQ-e.pdf. Accessed May 10, 2022.
- 6. Strategic Advisory Group of Experts on Immunization. Meeting of the Strategic Advisory Group of Experts on immunization, November 2013 – conclusions and recommendations. Wkly Epidemiol Rec 2014; 89:1–20. [PubMed] [Google Scholar]
- 7. WHO. 18th Meeting of the SAGE Polio Working Group. 2019. Note for record. Available at https://polioeradication.org/wp-content/uploads/2020/11/1-18th-Meeting-SAGE-WG-on-Polio-Note-for-Record.pdf. Accessed May 10, 2022. [Google Scholar]
- 8. SAGE discussion and statement in relation with the IPV supply situation. In: World Health Organization, editor. 2016. https://cdn.who.int/media/docs/default-source/immunization/sage/2017/sage-meeting-of-april-2017/background-docs/session-report-from-ivb-director/3.-sage-tracking-record-of-recommendations-and-action-points-pdf-548kb.pdf?sfvrsn=9299b971_3. Accessed May 10, 2022.
- 9. Sutter RW, Cochi SL.. Inactivated poliovirus vaccine supply shortage: is there light at the end of the tunnel? J Infect Dis 2019; 220:1545–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huyen DTT, Mach O, Trung NT, et al. Rapid disappearance of poliovirus type 2 (PV2) immunity in young children following withdrawal of oral PV2-containing vaccine in Vietnam. J Infect Dis 2019; 220:386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weldon WC, Oberste MS, Pallansch MA.. Standardized methods for detection of poliovirus antibodies. Methods Mol Biol 2016; 1387:145–76. [DOI] [PubMed] [Google Scholar]
- 12. Grassly NC, Jafari H, Bahl S, et al. Waning intestinal immunity after vaccination with oral poliovirus vaccines in India. J Infect Dis 2021; 205:1554–61. [DOI] [PubMed] [Google Scholar]
- 13. Bandyopadhyay AS, Gast C, Rivera L, et al. Safety and immunogenicity of inactivated poliovirus vaccine schedules for the post-eradication era: a randomised open-label, multicentre, phase 3, non-inferiority trial. Lancet Infect Dis 2021; 21:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. WHO, UNICEF. Considerations for the introduction of a second dose of Inactivated polio vaccine (IPV2) in routine immunization programmes from 2021. 2020. https://www.paho.org/sites/default/files/faqs_ipv2_eng_10_june_2020.pdf. Accessed May 10, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.