Abstract
Background and Objectives
Pathogenic variants in PRRT2, encoding for the proline-rich transmembrane protein 2, were identified as the main cause of self-limiting sporadic and familial infantile epilepsy. Reported data on treatment response to antiseizure medications (ASMs) in defined monogenic epilepsies are limited. The aim of this study was to evaluate the treatment response of ASMs in children with monogenic PRRT2-associated infantile epilepsy.
Methods
A multicenter, retrospective, cross-sectional cohort study was conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology criteria. Inclusion criteria were occurrence of infantile seizures and genetic diagnosis of likely pathogenic/pathogenic PRRT2 variants.
Results
Treatment response data from 52 individuals with PRRT2-associated infantile epilepsy with a total of 79 treatments (defined as each use of an ASM in an individual) were analyzed. Ninety-six percent (50/52) of all individuals received ASMs. Levetiracetam (LEV), oxcarbazepine (OXC), valproate (VPA), and phenobarbital (PB) were most frequently administered. Sodium channel blockers were used in 22 individuals and resulted in seizure freedom in all but 1 child, who showed a reduction of more than 50% in seizure frequency. By contrast, treatment with LEV was associated with worsening of seizure activity in 2/25 (8%) treatments and no effect in 10/25 (40%) of treatments. LEV was rated significantly less effective also compared with VPA and PB. The retention rate for LEV was significantly lower compared with all aforementioned ASMs. No severe adverse events were reported, and no discontinuation of treatment was reported because of side effects.
Discussion
In conclusion, a favorable effect of most ASMs, especially sodium channel blockers such as carbamezepine and OXC, was observed, whereas the efficacy and the retention rate of LEV was lower in PRRT2-associated childhood epilepsy. Tolerability in these young children was good for all ASMs reported in the cohort.
Classification of Evidence
This study provides Class IV evidence that in individuals with PRRT2-associated infantile epilepsy, sodium channel blockers are associated with reduced seizure frequency but levetiracetam is not.
Pathogenic variants in PRRT2, encoding for the proline-rich transmembrane protein 2 (OMIM*614386), are identified as the leading genetic cause of self-limiting sporadic and familial infantile epilepsy (BFIS, OMIM#605751).1,2 PRRT2 has also been associated with paroxysmal kinesigenic dyskinesia (PKD, OMIM#128200)3 and the overlapping disorder of paroxysmal kinesigenic dyskinesia with infantile convulsions (PKD/IC, also known as ICCA [infantile convulsions and paroxysmal choreoathetosis], OMIM#602066).4 BFIS manifests with multiple seizure types in normally developing infants, beginning at a mean age of 6 months and usually remitting before the age of 2 years.5,6 Only limited data on treatment are available, and expert recommendations are derived from the allelic disorder PKD, where sodium channel blockers, e.g., carbamazepine (CBZ), have shown to be effective.7,8 To date, the International League Against Epilepsy (ILAE) recommendations for the management of benign infantile convulsions list carbamazepine, phenobarbital, and valproate as “possibly effective.”9-11 In a recent study on 20 individuals with BFIS, oxcarbazepine (OXC) was also considered to be effective.12 Levetiracetam is one of the most commonly used ASMs in this age group.9,13 In a review of 241 individuals with PRRT2- associated epilepsy by Ebrahimi-Fakhari et al.,5,14 the authors concluded that in most of the cases, a positive response to conventional anticonvulsive treatment was observed. No comprehensive comparative studies on the specific effects of antiseizure medications (ASM) on monogenic PRRT2-associated infantile epilepsy have been published.
PRRT2 is predominantly expressed in presynaptic membranes of excitatory neurons in the cerebral cortex, basal ganglia, cerebellum, and hippocampus.1,3,15,16 Within neuronal networks, loss of PRRT2 leads to an increase of spontaneous and evoked activity and increased excitability of neurons.17-20 PRRT2 interacts with several neuronal proteins, regulating Ca2+-mediated synchronous neurotransmitter release, directly affecting intrinsic neuronal excitability by negative modulation of Na+ channels, and modifying synaptic plasticity during brain development.21,22 The aim of this study was to evaluate treatment response of antiseizure medications in children with monogenic PRRT2-associated infantile epilepsy.
Methods
This retrospective, observational, cross-sectional analysis was conducted according to “Strengthening the Reporting of Observational Studies in Epidemiology” criteria.23 Data were collected within the German Network for Rare Neurologic Disorders in Childhood (“Erhebung Seltener Neurologischer Erkrankungen im Kindesalter”) and from collaborating geneticists in Denmark.24 The genetic diagnosis was established as part of the diagnostic workup. The cohort analyzed in this study overlaps with individuals from a study on the phenotypic spectrum of individuals with PRRT2-associated disease, where detailed clinical and sequence variation information is described.6 To analyze treatment effects in PRRT2-associated infantile epilepsy, we collected systematic data on epilepsy treatment in 52 cases (40 cases with previously reported clinical characteristics and 12 novel individuals). Only individuals with likely pathogenic or pathogenic variants according to the American College of Medical Genetics and Genomics criteria were included.25 Clinical data were collected using a web-based survey answered by the treating child neurologist. The survey included detailed questions on the type of treatment, dosage, response, and adverse events. Because of the retrospective, observational, cross-sectional design of the study, it did not influence the choice of treatment by pediatric neurologists. The medications administered represent a common practice for the treatment of childhood focal and generalized epilepsy. Levetiracetam is one of the most commonly used ASMs in this age group.9,13
Quantitative variables are described by sample size, mean or median, and SD. Statistical comparison between the mean values was performed with the Welch 2-sample t test. Frequencies of descriptive variables are illustrated with number, sample size, and percentages. Statistical comparison of frequencies was performed with the 2-tailed Fisher exact test. p < 0.05 was considered statistically significant. The p values resulting from the statistical analyses have a purely descriptive character. A treatment was defined as each single use of an ASM in an individual. The treatment effect was rated by the treating child neurologist based on medical notes and seizure diaries on a 5-step ordinal scale (worsening, no effect, less than 50% seizure reduction, more than 50% seizure reduction, and seizure freedom), comparing seizure frequencies before treatment and 3 months after start of the medication. If an ASM was exchanged within a shorter interval, seizure activity prior to the start of the following ASM was rated as treatment effect. In case of combination therapies, the treatment effect was attributed to the last added ASM. For statistical comparison, “effective” treatment was defined as >50% seizure reduction or seizure freedom vs “ineffective” treatment with <50% seizure reduction, no effect, or worsening of seizure frequency. To rule out confounding effects from age, the age at each start of ASMs was included in the analysis. Descriptive analysis was performed with IBM SPSS Statistics version 27.
Standard Protocol Approvals, Registrations, and Patient Consents
The study adheres to the principles set out in the Declaration of Helsinki. All individuals or guardians gave their informed consent for data acquisition, sharing, and genetic testing. The study was approved by the ethics committee of the University of Heidelberg (S-318/2018).
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
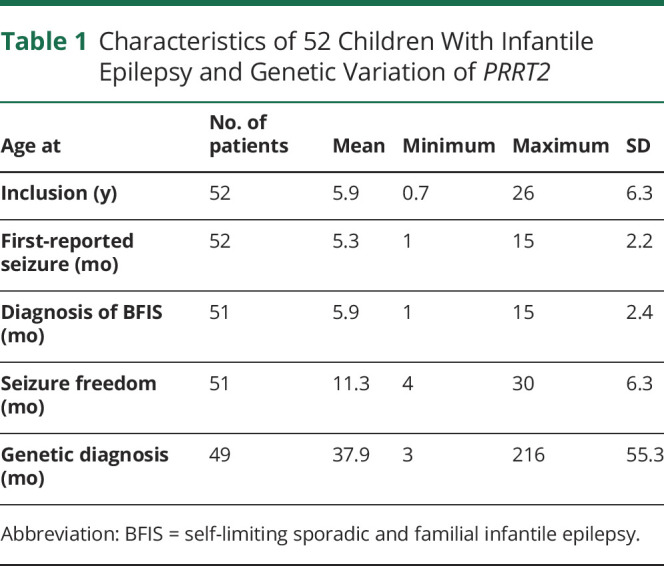
The cohort of individuals with PRRT2-associated infantile epilepsy analyzed in this study consisted of 52 cases. All individuals presented with an infantile seizure disorder in line with a clinical diagnosis of BFIS. Clinical characteristics from all included individuals are summarized in Table 1. Sixteen were male (30.8%) and 36 were female (69.2%) individuals. The mean age at inclusion into the study was 5.9 years (SD 6.3).
Table 1.
Characteristics of 52 Children With Infantile Epilepsy and Genetic Variation of PRRT2
Molecular Analysis
The median diagnostic delay from onset of infantile seizures to genetic diagnosis was 7 months (SD 75.6). Forty-nine individuals carried monoallelic variants, with the frameshift variant c.649dupC; p.Arg217Profs*8 most frequently identified. Three individuals presented with biallelic homozygous (1) or compound heterozygous (2) variants. 33/52 (63%) variants were of familial origin, 6/52 (12%) de novo, and in 13 cases, no data on inheritance were available. A summary of the spectrum of sequence variations is listed in eTable 1 (links.lww.com/NXG/A542).
Epilepsy
Most individuals developed clusters of seizures with a maximum seizure frequency of at least 2 seizures per day in 23/52 (44%) of cases and of more than 5 seizures per day in 8/52 (15%) of cases. The mean duration of active seizures was 5.7 months (SD 6.8). In single patients, seizures recurred later in life (2/52). Generalized-onset bilateral tonic-clonic or tonic seizures were reported in 31/52 (60%) individuals, focal motor and focal impaired awareness seizures in 11/52 (21%) of individuals, whereas 10/52 (19%) individuals displayed both focal and generalized seizures. One patient developed a convulsive status epilepticus at the age of 9 months.
Development
Prior to onset of seizures, all children developed normally. Despite the high seizure burden, cognitive and motor development was described as normal in 50/52 (96%) individuals during the course of disease. A mild delay of motor milestones and a mild language delay was described in single individuals. A girl with continuous spikes and waves during sleep developed a mild-moderate intellectual disability during childhood.
Treatment
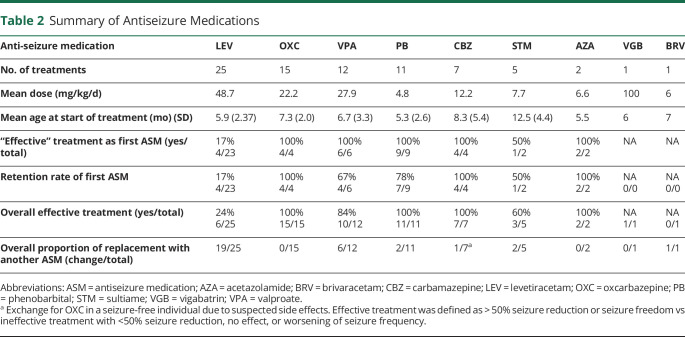
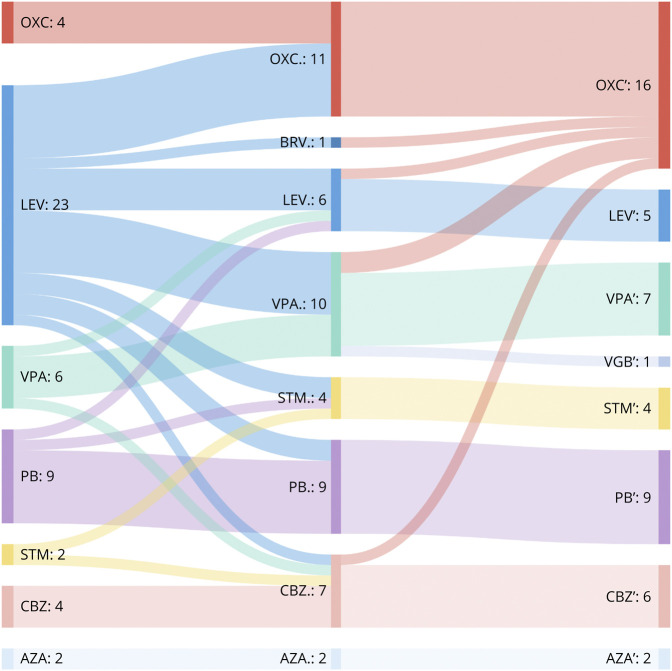
Ninety-six percent (50/52) of all individuals received ASMs. The use of additional rescue medications (diazepam and midazolam) was reported in 27/52 (52%) of all individuals during the course of the epilepsy. A total of 79 treatments were prescribed in the 52 children, and 9 different ASMs were used. Number of treatments, mean dosage, age at start of treatment, treatment effect of first ASM and overall treatment effects, and the proportion of changes to other ASM are summarized in Table 2. The mean delay to start of treatment was 1.1 months (SD 1.8). A total of 24/52 (46%) individuals received more than 1 ASM and 6/52 (12%) more than 2 in the course of the disease (Figure 1). By the time of study inclusion, 12/52 (23%) individuals still received ASM treatment (the median age of individuals with ongoing treatment: 27 months, SD 15.8). In 23/52 (44%) cases, the treatment was stopped, at a mean age of 26.4 (SD 15.1) months. In 17 cases, the information was missing.
Table 2.
Summary of Antiseizure Medications
Figure 1. Sankey Diagram of Treatment Changes From First-Line to Third-Line Therapy.
n = number of treated individuals. In cases with combination of ASM, the newly added ASM is shown in the diagram. AZA = acetazolamide; BRV = brivaracetam; CBZ = carbamazepine; LEV = levetiracetam; OXC = oxcarbazepine; PB = phenobarbital; STM = sultiame; VGB = vigabatrin; VPA = valproate.
Efficacy
Of all single treatments with ASMs, 55/79 (69%) were evaluated as “effective.” Of all cases, 25/52 (32%) were free of seizures within 1 month from the start of the first treatment. Compared with levetiracetam (LEV), the overall efficacy of treatment was rated higher for OXC (p < 0.0001), phenobarbital (PB) (p < 0.0001), CBZ (p = 0.0005), and valproate (VPA) (p = 0.0011) (Table 2, Figure 2, and eFigure 1, links.lww.com/NXG/A542). The isolated effect of the primarily used ASM also showed a clear difference between LEV and sodium channel blockers (OXC & CBZ, p < 0.0001), PB (p < 0.0001), and VPA (p = 0.0004). Sodium channel blockers were used in 22 individuals and resulted in seizure freedom in all but 1 child, who showed a reduction of more than 50% in seizure frequency. The use of LEV resulted in worsening of seizure activity in 2/25 (8%) individuals and no effect in 10/25 (40%) of treatments. To address a possible dose-dependent effect of LEV, we divided the LEV group into a low-dose subgroup and a high-dose subgroup based on the mean dose and compared the reported treatment effects: There was no difference between the 2 subgroups (p = 0.617). The mean age at the start of treatment did not differ significantly between LEV and OXC (6.08 months, SD 2.45 vs 7.4 months, SD 2.1, p = 0.19) (Figure 3). Since 2015, LEV was the most commonly used ASM in the cohort. It was started frequently as the first ASM (92%, 23/25), whereas OXC was started as the first ASM in 4/15 (27%) of treatments and was more often prescribed as the second or third choice (Figure 1).
Figure 2. Treatment Effect of Sodium Channel Blockers and Levetiracetam.
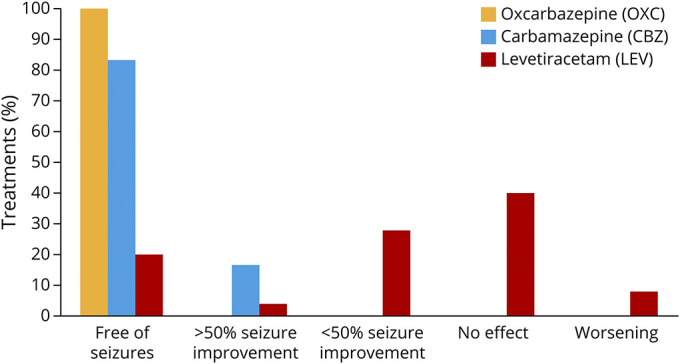
.
Figure 3. Boxplot, Displaying the Mean Age at Start of ASM.
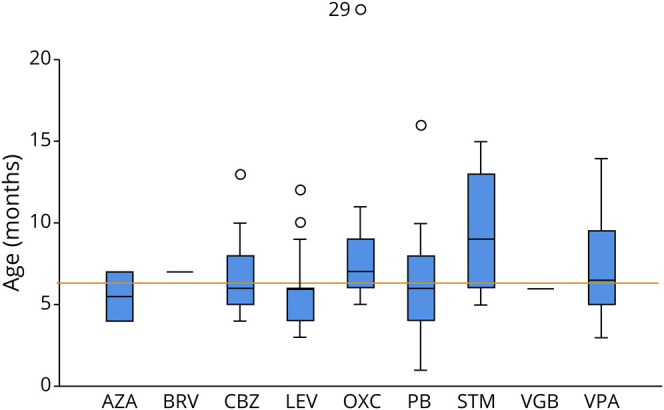
Orange line: mean age at start of ASM (6.4 months). ASM = antiseizure medication; AZA = acetazolamide; CBZ = carbamazepine; LEV = levetiracetam; OXC = oxcarbazepine; PB = phenobarbital; STM = sultiame; VGB = vigabatrin; VPA = valproate.
Drug Retention
Retention of the first ASM was high for sodium channel blockers (8/8, 100%) and PB (7/9, 78%). VPA was maintained in 4/6 initial treatments (67%). The retention rate for the initial treatment with LEV was 17% (4/19). Overall, retention to treatment with LEV was significantly different from that to OXC (p < 0.0001), PB (p = 0.0024), and CBZ (p = 0.0057).
Genetic confirmation of a variant in PRRT2 was reported to have affected further treatment decisions in 17/49 individuals (35%). Child neurologists reported that the genetic diagnosis justified less aggressive treatment approaches (5), led to a treatment with sodium channel blockers (6), early tapering of therapy (1), or no treatment at all (1). In 4 cases, no further specifications were given. Because the genetic diagnosis was performed after the onset of seizures, it did not affect the initial choice of medication.
The initial choice of treatment was also not influenced by concomitant movement disorders. Only 2 of 52 children had concomitant movement disorders in infancy. In all children, infantile seizures were the first symptom. One child developed dystonic episodes 1 month after the onset of seizures, although he was treated with sulthiame. Both the seizures and the dystonic movements were then controlled with oxcarbazepine.
Another girl showed benign myoclonus in infancy at 10 months of age, 6 months after the onset of seizures. Neither manifestation was controlled with levetiracetam, followed by valproate therapy. After identification of the pathogenic variant c.649dupC in PRRT2, treatment was successfully switched to oxcarbazepine.
Tolerability
No severe adverse events were reported, and no discontinuation of treatment was reported due to side effects. VPA was associated with more aggressive behavior in 1 individual and with increased tiredness in another. Sultiame was associated with restlessness and sleep disorder in 1 individual. CBZ was suspected to underly anemia and neutropenia in 1 patient, which was later diagnosed as autoimmune neutropenia. brivaracetam was associated with increased tiredness in 1 individual.
Classification of Evidence
Retrospective, noncontrolled, cross-sectional Study (Class of Evidence IV) to evaluate the treatment response to different ASMs in individuals with PRRT2-associated infantile seizures. Relevant outcomes were seizure frequency and retention to treatment.
Discussion
In this study, we analyzed the efficacy, retention, and tolerability of anti-seizure medications in a large multicenter cohort of 52 individuals with PRRT2-associated infantile seizures. Our study has several important findings: considering the retention rates and the evaluated efficacy of the treatments, not only sodium channel blockers (OXC and CBZ) in particular, but also PB and VPA, showed favorable effects in infants with PRRT2-associated epilepsy and had a good safety profile in this vulnerable age group. LEV, which is frequently used in neonatal and infantile epilepsies, was rated less effective for seizure control and had a lower drug retention rate.
In line with published data, age at onset, frequency of seizures, duration of seizure activity, semiology of seizures, and molecular distribution of variants in our cohort is representative for PRRT2-associated self-limiting sporadic and familial infantile epilepsy.5 One individual presented with status epilepticus, an exceptional feature in PRRT2-associated epilepsy at the age of 9 months,26 but the course of BFIS and treatment response did not deviate from the rest of the cohort.
Development before and in the course of the disease was unremarkable in almost all individuals (50/52), independent of the high seizure frequency over several months. Besides the positive developmental and epilepsy outcome, quality of life and psychosocial well-being of families with infants with seizures is generally reduced.27 Despite subsequent remission, seizure freedom through effective antiepileptic treatment is of great importance in this vulnerable group.
Ebrahimi et al.5 provided a comprehensive review on a historic cohort of 602 published individuals with PRRT2-associated BFIS and reported no superior ASMs. With long-term remission in virtually all children, the overall response to treatment reported for PRRT2-associated BFIS was excellent.5 It is important that the ASMs used for infantile seizures have changed recently. While before 2015, only 1 individual with LEV treatment was reported in all studies, LEV was now the most commonly used ASM in our cohort.
In this study, LEV, OXC, VPA, and PB were prescribed most frequently. Regarding the assessment of treatment effects, sodium channel blockers (OXC and CBZ) had excellent effects, and were rated as effective treatment in all respective children (22/22, 100%) with a high retention rate when used as first ASM. A specific effect on PRRT2-associated disease can be assumed because PRRT2 is a known negative modulator of sodium channels in excitatory neurons.21 Lack of PRRT2 leads to hyperactivity and increased expression of voltage-dependent Na+ channels, as well as increased Na+ current amplitude in homozygous PRRT2 knockout human and murine neurons, resulting in an overall enhancement of intrinsic excitability.21,22
Comparing the treatment effects of different ASMs, we found a comparable effectiveness for sodium channel blockers and PB, but a substantial difference with LEV, which has become one of the most commonly used ASMs in the first year of life.28 Treatment with LEV was frequently rated to be ineffective, and retention to treatment was low when used as the first ASM. Of interest, even worsening of seizure frequency and activation of multifocal epileptiform potentials in the electroencephalography (EEG) were reported in single cases. A change to OXC led to seizure freedom in all respective cases, an observation that has also been described in single familial cases and in a recent case series.12,29,30
The presynaptic vesicle protein 2A (SV2A) is the binding site for LEV.31 Similar to PRRT2, SV2A is involved in the presynaptic vesicle release machinery. To a lesser degree, LEV also binds to the calcium sensor synaptotagmin and calcium channels, leading to reduction of Ca2+-mediated glutamate and gamma-aminobutyric acid release in rapidly discharging neurons.32 Absence of PRRT2 results in opposite effects on excitatory and inhibitory synapses after short-term potentiation, with increased facilitation in excitatory transmission and increased depression in inhibitory transmission.17 LEV has been shown to cause a frequency-dependent reduction of both excitatory and inhibitory postsynaptic currents.32 The additional effect on inhibitory transmission might explain the lower effectiveness of LEV in this condition.
Considering the age-dependent course with possible initial deterioration in seizure frequency and spontaneous seizure remission over time, age at initiation and sequential use of individual ASM may confound our observations. To address this limitation, we compared the effect of the primarily used ASM and found a clear difference not only between LEV and sodium channel blockers (OXC and CBZ) but also to VPA and PB. This fact is also reflected in a lower retention rate to the initial therapy for LEV (Table 2). In addition, we compared the mean age at treatment start of each ASM, which was not significantly different for OXC, LEV, VPA, and PB (Figure 3). Still, because LEV was mostly prescribed as the first treatment, often followed by OXC (Figure 1), we cannot completely rule out a bias from this sequential use, also, because the genetic diagnosis of a PRRT2-associated epilepsy led to a change to sodium-channel blockers in single infants. Some of these potential biases in the evaluation of LEV can be excluded compared with PB: it was initiated very early in the course of epilepsy in most cases, it was the first ASM in almost all treatments, and there was no sequential use of LEV and PB. Still, a significant difference in perceived efficacy and retention between these 2 ASMs was found.
Ineffective ASMs were exchanged more quickly than 3 months after treatment initiation in most cases, which may have led to an underestimation of the full treatment effect. As effects of combinations of ASM were attributed to the treatment that was initiated last, effects from combinatorial and synergistic drugs may have escaped our analysis.
Given the young age at seizure onset and the unknown genetic etiology at the start of treatment, prospective controlled clinical trials are challenging, and the use of a placebo-control group seems unethical. Therefore, analysis of retrospective data is of great value. When assessing our findings, several limitations have to be considered, including the retrospective, unblinded design, the rating of efficacy by the caring pediatric neurologists with a risk of confirmation bias, and the lack of a control group. In conclusion, our data showed favorable effects of most ASM, especially sodium channel blockers, such as CBZ or OXC, but a low efficacy of LEV in PRRT2-associated monogentic infantile epilepsy (eFigure 1, links.lww.com/NXG/A542).
Acknowledgment
The authors thank the patients, families, and collaborators for their participation in our retrospective data collection.
Glossary
- ASM
antiseizure medication
- BFIS
self-limiting sporadic and familial infantile epilepsy
- CBZ
carbamazepine
- ICCA
infantile convulsions and paroxysmal choreoathetosis
- ILAE
International League Against Epilepsy
- OXC
oxcarbazepine
- PB
phenobarbital
- PKD
paroxysmal kinesigenic dyskinesia
- VPA
valproate
Appendix 1. Authors
Appendix 2. Coinvestigators
Footnotes
Class of Evidence: NPub.org/coe
Contributor Information
Jan H. Döring, Email: jan.doering@med.uni-heidelberg.de.
Afshin Saffari, Email: afshin.saffari@med.uni-heidelberg.de.
Thomas Bast, Email: tbast@diakonie-kork.de.
Knut Brockmann, Email: kbrock@med.uni-goettingen.de.
Laura Ehrhardt, Email: laura.ehrhardt@unimedizin-mainz.de.
Walid Fazeli, Email: walid.fazeli@ukbonn.de.
Wibke G. Janzarik, Email: wibke.janzarik@uniklinik-freiburg.de.
Annick Klabunde-Cherwon, Email: annick.klabunde-cherwon@med.uni-heidelberg.de.
Gerhard Kluger, Email: gkluger@schoen-klinik.de.
Hiltrud Muhle, Email: hiltrud.muhle@uksh.de.
Manuela Pendziwiat, Email: manuela.pendziwiat@uksh.de.
Rikke S. Møller, Email: rimo@filadelfia.dk.
Konrad Platzer, Email: konrad.platzer@medizin.uni-leipzig.de.
Joana Larupa Santos, Email: joana.santos@sund.ku.dk.
Julian Schröter, Email: julian.schroeter@med.uni-heidelberg.de.
Georg F. Hoffmann, Email: georg.hoffmann@med.uni-heidelberg.de.
Stefan Kölker, Email: stefan.koelker@med.uni-heidelberg.de.
Study Funding
Steffen Syrbe received funding from the Dietmar-Hopp-Stiftung (Grant 1DH1813319).
Disclosure
G. Kluger received consulting fee or honorarium from EISAI, GW Pharma and support for travel to meetings for the study or other purposes from Nutricia and Desitin. G.F. Hoffmann has received honoraria as a speaker from Takeda and consultancy honoraria from PTC Therapeutics International GT. All other authors declared no conflicts of interest that could be perceived to influence or that give the appearance of potentially influencing the submitted work. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NG.
References
- 1.Heron SE, Grinton BE, Kivity S, et al. . PRRT2 mutations cause benign familial infantile epilepsy and infantile convulsions with choreoathetosis syndrome. Am J Hum Genet. 2012;90(1):152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Symonds JD, Zuberi SM, Stewart K, et al. . Incidence and phenotypes of childhood-onset genetic epilepsies: a prospective population-based national cohort. Brain. 2019;142(8):2303-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen WJ, Lin Y, Xiong ZQ, et al. . Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat Genet. 2011;43(12):1252-1255. [DOI] [PubMed] [Google Scholar]
- 4.Lee HY, Huang Y, Bruneau N, et al. . Mutations in the gene PRRT2 cause paroxysmal kinesigenic dyskinesia with infantile convulsions. Cell Rep. 2012;1(1):2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebrahimi-Fakhari D, Saffari A, Westenberger A, Klein C. The evolving spectrum of PRRT2-associated paroxysmal diseases. Brain. 2015;138(pt 12):3476-3495. [DOI] [PubMed] [Google Scholar]
- 6.Doring JH, Saffari A, Bast T, et al. . The phenotypic spectrum of PRRT2-associated paroxysmal neurologic disorders in childhood. Biomedicines. 2020;8(11):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li HF, Chen WJ, Ni W, et al. . PRRT2 mutation correlated with phenotype of paroxysmal kinesigenic dyskinesia and drug response. Neurology. 2013;80(16):1534-1535. [DOI] [PubMed] [Google Scholar]
- 8.Huang XJ, Wang T, Wang JL, et al. . Paroxysmal kinesigenic dyskinesia: clinical and genetic analyses of 110 patients. Neurology. 2015;85(18):1546-1553. [DOI] [PubMed] [Google Scholar]
- 9.Wilmshurst JM, Gaillard WD, Vinayan KP, et al. . Summary of recommendations for the management of infantile seizures: task force report for the ILAE commission of pediatrics. Epilepsia. 2015;56(8):1185-1197. [DOI] [PubMed] [Google Scholar]
- 10.Espeche A. Benign infantile seizures: a prospective study. Epilepsy Res. 2010;89(1):96-103. [DOI] [PubMed] [Google Scholar]
- 11.Matsufuji H, Ichiyama T, Isumi H, Furukawa S. Low-dose carbamazepine therapy for benign infantile convulsions. Brain Dev. 2005;27(8):554-557. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Q, Hu Y, Liu Z, et al. . PRRT2 variants and effectiveness of various antiepileptic drugs in self-limited familial infantile epilepsy. Seizure. 2021;91:360-368. [DOI] [PubMed] [Google Scholar]
- 13.Wilmshurst JM, Burman R, Gaillard WD, Cross JH. Treatment of infants with epilepsy: common practices around the world. Epilepsia. 2015;56(7):1033-1046. [DOI] [PubMed] [Google Scholar]
- 14.Bureau M, Genton P, Delgado-Escueta AV, et al. . Epileptic Syndromes in Infancy, Childhood and Adolescence, 6th ed. John Libbey Eurotext; 2019. [Google Scholar]
- 15.Valente P, Castroflorio E, Rossi P, et al. . PRRT2 is a key component of the Ca(2+)-dependent neurotransmitter release machinery. Cell Rep. 2016;15(1):117-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagerberg L, Hallstrom BM, Oksvold P, et al. . Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13(2):397-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valente P, Romei A, Fadda M, et al. . Constitutive inactivation of the PRRT2 gene alters short-term synaptic plasticity and promotes network hyperexcitability in hippocampal neurons. Cereb Cortex. 2019;29(5):2010-2033. [DOI] [PubMed] [Google Scholar]
- 18.Ardiles AO, Grabrucker AM, Scholl FG, Rudenko G, Borsello T. Molecular and cellular mechanisms of synaptopathies. Neural Plast. 2017;2017:2643943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant SG. Synaptopathies: diseases of the synaptome. Curr Opin Neurobiol. 2012;22(3):522-529. [DOI] [PubMed] [Google Scholar]
- 20.Valtorta F, Benfenati F, Zara F, Meldolesi J. PRRT2: from paroxysmal disorders to regulation of synaptic function. Trends Neurosci. 2016;39(10):668-679. [DOI] [PubMed] [Google Scholar]
- 21.Fruscione F, Valente P, Sterlini B, et al. . PRRT2 controls neuronal excitability by negatively modulating Na+ channel 1.2/1.6 activity. Brain. 2018;141(4):1000-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binda F, Valente P, Marte A, Baldelli P, Benfenati F. Increased responsiveness at the cerebellar input stage in the PRRT2 knockout model of paroxysmal kinesigenic dyskinesia. Neurobiol Dis. 2021;152:105275. [DOI] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, et al. . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. [DOI] [PubMed] [Google Scholar]
- 24.Brockmann K. Erhebung Seltener Neurologischer Erkrankungen im Kindesalter. Neuropediatrics. 2014;45(S01):fp036. [Google Scholar]
- 25.Richards S, Aziz N, Bale S, et al. . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maini I, Iodice A, Spagnoli C, et al. . Expanding phenotype of PRRT2 gene mutations: a new case with epilepsy and benign myoclonus of early infancy. Eur J Paediatr Neurol. 2016;20(3):454-456. [DOI] [PubMed] [Google Scholar]
- 27.Ronen GM, Streiner DL, Rosenbaum P. Health-related quality of life in childhood epilepsy: moving beyond “seizure control with minimal adverse effects”. Health Qual Life Outcomes. 2003;1:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neininger MP, Ullmann M, Dahse AJ, et al. . Use of levetiracetam in neonates in clinical practice: a retrospective study at a German University Hospital. Neuropediatrics. 2015;46(5):329-334. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Q, Liu Z, Hu Y, et al. . Different experiences of two PRRT2-associated self-limited familial infantile epilepsy. Acta Neurol Belg. 2020;120(4):1025-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K, Zhao X, Du Y, He F, Peng G, Luo B. Phenotypic overlap among paroxysmal dyskinesia subtypes: lesson from a family with PRRT2 gene mutation. Brain Dev. 2013;35(7):664-666. [DOI] [PubMed] [Google Scholar]
- 31.Lynch BA, Lambeng N, Nocka K, et al. . The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101(26):9861-9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meehan AL, Yang X, Yuan LL, Rothman SM. Levetiracetam has an activity-dependent effect on inhibitory transmission. Epilepsia. 2012;53(3):469-476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.