This randomized clinical trial investigates the efficacy and safety of dimethyl fumarate vs interferon β-1a for treatment of pediatric-onset multiple sclerosis.
Key Points
Question
What are the efficacy and safety of dimethyl fumarate (DMF) vs interferon β-1a (IFNβ-1a) in patients with pediatric-onset multiple sclerosis (POMS)?
Findings
In this randomized clinical trial of 150 patients aged 10 to 17 years, the proportion of patients with no new or newly enlarging T2 lesions among trial completers was 16.1% for DMF vs 4.9% for IFNβ-1a at 96 weeks relative to baseline. Treatment-emergent adverse events (TEAEs), serious TEAEs, and trial drug discontinuation due to TEAEs were similar in DMF and IFNβ-1a groups.
Meaning
This study found that DMF was more efficacious than IFNβ-1a in patients with POMS and the drugs had similar safety profiles.
Abstract
Importance
With few approved multiple sclerosis therapies in the pediatric population, there is a need for further approved treatment options. Limited data exist for dimethyl fumarate (DMF) treatment in pediatric-onset multiple sclerosis (POMS).
Objective
To compare the efficacy, safety, and tolerability of DMF vs intramuscular interferon β-1a (IFNβ-1a) in POMS.
Design, Setting, and Participants
The CONNECT study was an active-controlled, open-label, rater-blinded 96-week randomized clinical trial in patients with POMS aged 10 to less than 18 years treated between August 2014 and November 2020. Data were analyzed from January through October 2021.
Interventions
Patients were randomized to DMF or IFNβ-1a.
Main Outcomes and Measures
The primary end point was the proportion of patients free of new or newly enlarging (N or NE) T2 hyperintense lesions at week 96 among trial completers. Secondary end points included number of N or NE T2 lesions, proportion of patients free of relapse, annualized relapse rate (ARR), and safety. The estimated proportion of participants who were relapse free up to week 96 was calculated based on the Kaplan-Meier method. Adjusted ARR was obtained from a negative binomial regression adjusted for baseline relapse rate, baseline Expanded Disability Status Scale (EDSS) score, and age group.
Results
Among 150 patients with POMS in the intention-to-treat (ITT) population (median [range] age, 15 [10-17] years; 101 [67.3%] female patients), 78 individuals received DMF and 72 individuals received IFNβ-1a. At week 96, the proportion of patients with no N or NE T2 hyperintense lesions among 103 trial completers was 16.1% (95% CI, 8.0%-27.7%) for DMF vs 4.9% (95% CI, 0.6%-16.5%) for IFNβ-1a, and in a sensitivity analysis among the ITT population, the proportions were 10 patients receiving DMF (12.8%) vs 2 patients receiving IFNβ-1a (2.8%). The estimated proportion of patients who remained relapse free at week 96 was 66.2% for DMF vs 52.3% for IFNβ-1a. Adjusted ARR (95% CI) at week 96 was 0.24 (95% CI, 0.15-0.39) for DMF vs 0.53 (95% CI, 0.33-0.84) for IFNβ-1a; the rate ratio for DMF vs IFNβ-1a was 0.46 (95% CI, 0.26-0.80; P = .006). The number of treatment-emergent adverse events (TEAEs; 74 patients [94.9%] vs 69 patients [95.8%]), serious TEAEs (18 patients [23.1%] vs 21 patients [29.2%]), and treatment discontinuations due to TEAEs (5 patients [6.4%] vs 8 patients [11.1%]) was similar for DMF vs IFNβ-1a.
Conclusions and Relevance
This study found that more pediatric patients with POMS treated with DMF were free of new or newly enlarging T2 lesions and that the adjusted ARR was lower among these patients compared with those treated with interferon β-1a. DMF was well tolerated.
Trial Registration
ClinicalTrials.gov Identifier: NCT02283853
Introduction
Pediatric-onset multiple sclerosis (POMS) is rare, with incidence ranging from 0.05 to 2.85 and prevalence from 0.69 to 26.92 per 100 000 pediatric patients.1 Children and adolescents with POMS have an active inflammatory disease course with a higher relapse rate than adults.2,3,4 Hospitalization rates were higher among pediatric patients with POMS than matched pediatric patients in control groups,5 and POMS was associated with cognitive impairment.6 This backdrop highlights the importance of timely intervention with disease-modifying therapy (DMT).7,8
Because of low disease prevalence and difficulties in patient recruitment into randomized clinical trials, it is challenging to obtain long-term safety and efficacy data for regulatory approval of DMTs.9 As a result, there is a shortage of approved treatment options for POMS, and therapies licensed for use in adults, including interferon β, dimethyl fumarate (DMF), fingolimod, glatiramer acetate, natalizumab, and teriflunomide, have been used off label. However, licensed therapies for pediatric patients are now emerging, with fingolimod approved in 2018 by the US Food and Drug Administration and the European Medicines Agency for patients with multiple sclerosis (MS) aged 10 to 17 years,10 while teriflunomide was approved by the European Medicines Agency in 2021 for use in patients with relapsing-remitting MS (RRMS) aged 10 to 17 years.9,11
DMF is an oral DMT approved for adults with relapsing forms of MS. It has demonstrated clinically meaningful and sustained efficacy with a favorable benefit-risk profile in the pivotal phase 3 studies. Determination of the Efficacy and Safety of Oral Fumarate in Relapsing-Remitting MS (DEFINE)12 and Comparator and an Oral Fumarate in Relapsing-Remitting Multiple Sclerosis (CONFIRM),13 and in studies in clinical practice of patients with relapsing forms of MS.14,15As of June 30, 2021, more than 537 000 patients have been treated with DMF, representing more than 1 100 000 patient-years of exposure. Single-arm studies in small numbers of patients with POMS found that DMF was well tolerated and had similar efficacy and safety to that observed in adults.16,17,18 In addition, studies in clinical practice of POMS have shown better disease activity control with newer DMTs (including fingolimod, DMF, teriflunomide, natalizumab, rituximab, and ocrelizumab) than with injectables (ie, interferon and glatiramer).19,20 CONNECT (ClinicalTrials.gov NCT02283853) is a 96-week randomized clinical trial comparing the efficacy, safety, and tolerability of DMF vs intramuscular IFNβ-1a in pediatric patients with MS.
Methods
This randomized clinical trial was conducted in accordance with relevant European Union federal regulations, the Declaration of Helsinki, and the International Conference on Harmonisation Guideline for Good Clinical Practice. Approvals were granted by relevant institutional ethics committees for trial protocol and amendments. The trial protocol and statistical analysis plan can be found in Supplement 1. Written assent and consent forms were obtained from each patient and the patient’s parent or legal guardian. The trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Trial Design and Participants
CONNECT is an open-label, rater-blinded, 96-week (2-year), multicenter, active-controlled, parallel group randomized clinical trial designed to compare the safety and efficacy of DMF vs IFNβ-1a, the active comparator, in patients with POMS (eFigure 1 in Supplement 2; clinical trial protocol in Supplement 1). The trial was initiated on August 28, 2014, and completed on November 12, 2020. When the trial began, there were no approved DMTs for patients with POMS; however, interferons were commonly used in clinical practice.21,22,23,24,25 In this trial, eligible patients were randomized to DMF or IFNβ-1a in a 1:1 ratio at baseline (day 1). Patients were stratified by age (10 to <13 years, 13 to <15 years, or 15 to <18 years). Race and ethnicity were self-reported on the electronic case report form in Canada, Israel, Serbia, and Turkey at the screening visit; this information was not collected in other countries in the trial due to European Union and European Economic Area data privacy regulations. Race and ethnicity options were American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or other Pacific-Islander, White, and other. Only Asian and White groups were reported because these were the only race and ethnicity groups with patients; other and unknown or missing were also reported. Race and ethnicity were collected to provide an understanding of the distribution of study participants and if it was representative of the general population.
At 62 study sites in Europe, North America, and the Middle East, patients in the DMF group received oral DMF 120 mg twice daily for the first 7 days and 240 mg twice daily thereafter. In the active-control group, patients self-administered (or administered via proxy) IFNβ-1a 7.5 μg once by intramuscular injection on day 1, with subsequent doses increased by 7.5 μg each week for 3 weeks until the recommended dose of 30 μg once weekly was achieved.
Eligible patients were aged 10 to 17 years, with a body weight of 30 kg or more and a diagnosis of RRMS according to the consensus definition for pediatric RRMS,26 which was later amended to the 2013 International Paediatric Multiple Sclerosis Study Group criteria for pediatric MS.27 Patients were ambulatory, with an Expanded Disability Status Scale (EDSS) score between 0 and 5.5. Eligible patients had experienced 1 or more relapse within the past 12 months or 2 or more relapses within the past 24 months, with a prior brain magnetic resonance imaging (MRI) scan demonstrating lesions consistent with MS or evidence of gadolinium-enhancing (Gd+) lesions of the brain on an MRI in the past 6 weeks. Additionally, patients could not have relapsed in the past 50 days. Key exclusion criteria included history of premalignant or malignant disease and alanine aminotransferase (ALT), aspartate aminotransferase (AST), or γ-glutamyl transferase levels increased by 2-fold or more vs the upper limit of the reference range.
Trial Assessments and End Points
Efficacy outcomes of MS lesions on MRI brain scans, MS relapse, and EDSS scores were assessed by raters who were blinded. MRI scans were read at local sites and forwarded to an independent, blinded, central MRI center for assessment. An independent neurologist, blinded to treatment, confirmed MS relapse and performed EDSS assessment.
The primary end point was the proportion of patients free of new or newly enlarging (N or NE) T2 hyperintense lesions on MRI scans at week 96 relative to baseline among trial completers (ie, patients who completed week 96 of the trial and had MRI data at week 96). Secondary MRI outcome end points included the number of N or NE T2 hyperintense lesions from MRI scans at week 96 and the proportion of patients free of new MRI activity (defined as free of Gd+ lesions and N or NE T2 MRI lesions). Exploratory MRI outcomes included the proportion of patients free of Gd+ lesions at week 96 and the number of new T1 hypointense lesions at week 96.
Clinical secondary end points were time to first relapse, proportion of patients who remained relapse free up to week 96, and annualized relapse rate (ARR) at week 96. Relapse was defined as new or recurrent neurologic symptoms not associated with fever or infection, lasting 24 hours or more, accompanied by new objective neurologic findings by the treating neurologist, and separated from the onset of other confirmed relapses by 30 or more days.12,13
Change in EDSS score from baseline to week 96 was a secondary end point, and an exploratory outcome was EDSS progression (defined as ≥1.0-point increase on EDSS from a baseline EDSS score ≥1.0 that was sustained for 12 weeks or a ≥1.5-point increase on EDSS from a baseline EDSS score of 0 that was sustained for 12 weeks). Patient-reported outcomes (eAppendix in Supplement 2) of fatigue were measured by Pediatric Quality of Life Inventory (PedsQL) Multidimensional Fatigue Scale scores (eTable 1 in Supplement 2) and quality of life by PedsQL Quality of Life Scale scores (eTable 2 in Supplement 2). Safety outcomes included evaluation of treatment-emergent adverse events (TEAEs), serious TEAEs, TEAEs leading to treatment discontinuation, and TEAEs of special interest.
Analysis Populations
The intention-to-treat (ITT) population included all patients who were assigned to treatment and received 1 or more doses of DMF or IFNβ-1a. The completers population was defined as patients from the ITT population who completed week 96 of the trial and who had MRI data for week 96. Patients in the ITT population who were not included in the completers population were trial dropouts. Among patients in the IFNβ-1a group who dropped out, 1 patient had an MRI measurement that fell into the week 96 MRI analysis window; this patient was included in selected MRI analyses. The completers population for the primary end point analysis included patients from the ITT population who completed week 96 of the trial and had MRI data for week 96. Secondary efficacy analyses were performed on the ITT population, except for analyses for secondary MRI end points, which were performed among patients with MRIs at the time of interest. Patients who received 1 or more doses of DMF 240 mg BID or IFNβ-1a were included in the safety analysis population.
Statistical Analysis
Sample size was based on feasibility, with the goal of having 50 evaluable patients at 96-weeks for each treatment group. Analysis of the primary end point included summary statistics and exact CIs using the Clopper-Pearson method for the proportion of patients who were free of N or NE T2 hyperintense lesions at week 96 for each treatment group in the completer population. Using the same methods, a sensitivity analysis for the primary end point included the ITT population (150 patients) at week 96. In addition to completer and ITT analyses, an analysis of the ITT population with multiple imputation was performed. For each visit, a multiple imputation model was estimated from all observed data on N or NE T2 hyperintense lesions at week 96 relative to baseline using a negative binomial regression adjusted for treatment group, age group, and baseline number of T2 lesions. Proportions were calculated as means of 30 estimates. Each estimate was based on the analysis of 1 imputed data set. Statistical inferences considered within-imputation and between-imputation variance. CIs were calculated using t distribution. Imputation was done for patients in the ITT population who did not have week 96 MRI measurement (30 patents in the IFNβ-1a group and 16 patients in the DMF group).
Summary statistics for the number of N or NE T2 hyperintense lesions were presented by treatment group. A negative binomial regression model was used to analyze the number of N or NE T2 hyperintense lesions, with adjustments for age group and baseline number of T2 lesions.
Proportions of patients who remained relapse free were estimated using the Kaplan-Meier method; time to first relapse was analyzed using the Cox proportional hazards model, adjusted for baseline relapse rate, baseline EDSS score, and age group. The estimated proportion of participants who were relapse free up to week 96 was calculated based on the Kaplan-Meier method. Adjusted ARR was obtained from a negative binomial regression adjusted for baseline relapse rate, baseline EDSS score, and age group. Nominal P values are presented and are not corrected for multiplicity.
Statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute). Statistical tests were 2-sided at the 0.05 level of significance. Data were analyzed from January through October 2021.
Results
Patients
Of 156 patients randomized, 150 patients with POMS (ITT population; median [range] age, 15.0 [10-17] years; 101 [67.3%] female patients) received treatment with DMF (78 patients) or IFNβ-1a (72 patients) (Table 1 and Figure 1). There were 2 Asian patients (1.3%), 25 White patients (16.7%), and 53 patients with race data unreported due to confidentiality regulations (35.3%). Overall, the mean (SD) time since first MS symptoms was 1.6 (0.8) years. The mean (SD) number of relapses in the past 12 months was 1.6 (1.0) relapses for DMF and 1.5 (0.7) relapses for IFNβ-1a. Demographic and baseline disease characteristics were similar by treatment group.
Table 1. Baseline and Demographic Patient Characteristicsa.
| Characteristic | Patients, No. (%) (N = 150) | ||
|---|---|---|---|
| IFNβ-1a (n = 72) | DMF (n = 78) | Total (N = 150) | |
| Age category, y | |||
| 10-12 | 8 (11.1) | 7 (9.0) | 15 (10.0) |
| 13-14 | 14 (19.4) | 18 (23.1) | 32 (21.3) |
| 15-17 | 50 (69.4) | 53 (67.9) | 103 (68.7) |
| Age, y | |||
| Mean (SD) [range] | 15.0 (1.6) [10-17] | 14.9 (1.6) [10-17] | 14.9 (1.6) [10-17] |
| Median (IQR) | 15.0 (14.0-16.0) | 15.0 (14.0-16.0) | 15.0 (14.0-16.0) |
| Sex | |||
| Male | 23 (31.9) | 26 (33.3) | 49 (32.7) |
| Female | 49 (68.1) | 52 (66.7) | 101 (67.3) |
| Raceb | |||
| Asian | 1 (1.4) | 1 (1.3) | 2 (1.3) |
| White | 14 (19.4) | 11 (14.1) | 25 (16.7) |
| Not reported due to confidentiality regulations | 26 (36.1) | 27 (34.6) | 53 (35.3) |
| Otherc | 0 | 3 (3.8) | 3 (2.0) |
| Unknown or missing | 31 (43.1) | 36 (46.2) | 67 (44.7) |
| Weight, kg | |||
| Mean (SD) [range] | 63.5 (14.0) [35.5-101.2] | 64.2 (15.2) [36.6-112.0] | 63.9 (14.6) [35.5-112.0] |
| Median (IQR) | 62.5 (53.0-71.0) | 63.0 (53.0-72.6) | 63.0 (53.0-71.3) |
| Height, cm | |||
| Mean (SD) [range] | 165 (9.2) [143-185] | 164 (8.2) [143-180] | 164.5 (8.7) [143-185] |
| Median (IQR) | 164.2 (160.0-171.5) | 163.0 (159.2- 168.5) | 164.0 (159.5-170.0) |
| Time since first MS symptoms, y | |||
| Mean (SD) [range] | 1.2 (1.3) [0-6] | 1.7 (1.9) [0-10] | 1.5 (1.6) [0-10] |
| Median (IQR) | 1 (0-2) | 1 (1-2) | 1 (1-2) |
| Time since diagnosis of MS, y | |||
| Mean (SD) [range] | 0.5 (0.7) [0-4] | 0.9 (1.6) [0-7] | 0.8 (1.2) [0-7] |
| Median (IQR) | 0 (0-1) | 1 (0-1) | 0 (0-1) |
| Relapses within past 12 mo, No. | |||
| Mean (SD) [range] | 1.5 (0.7) [0-3] | 1.6 (1.0) [0-5] | 1.6 (0.8) [0-5] |
| Median (IQR) | 1 (1-2) | 1 (1-2) | 1 (1-2) |
| Baseline EDSS score | |||
| Mean (SD) [range] | 1.1 (1.0) [0-4] | 1.2 (1.1) [0-5] | 1.1 (1.0) [0-5] |
| Median (IQR) | 1.0 (0-1.5) | 1.0 (0-2) | 1.0 (0-2) |
| Prior MS therapy | 23 (31.9) | 23 (29.5) | 46 (30.7) |
| IFNβ-1a | 4 (5.6) | 10 (12.8) | 14 (9.3) |
| Glatiramer acetate | 3 (4.2) | 3 (3.8) | 6 (4.0) |
| IFNβ-1b | 2 (2.8) | 3 (3.8) | 5 (3.3) |
| Natalizumab | 0 | 3 (3.8) | 3 (2.0) |
| Otherd | 16 (22.2) | 11 (14.1) | 27 (18.0) |
| Exposure to trial treatment, wk | |||
| Mean (SD) [range] | 72 (32) [0-98] | 84 (26) [1-109] | 79 (30) [0-109] |
| Median (IQR) | 95 (47-95) | 96 (95-96) | 95 (70-96) |
Abbreviations: DMF, dimethyl fumarate; EDSS, Expanded Disability Status Scale; IFNβ-1a, interferon β-1a; IFNβ-1b, interferon β-1ba; MS, multiple sclerosis.
Among intent-to-treat population.
Only Asian and White categories included because patients did not report any other categories.
Other race included Middle Eastern (2 individuals) and Iraqi (1 individual). Patients reported other, and data came from the electronic case report form.
Other MS medications included methylprednisolone sodium succinate, corticosteroid, prednisone, methylprednisolone, dexamethasone, plasmapheresis, prednisolone, and steroids.
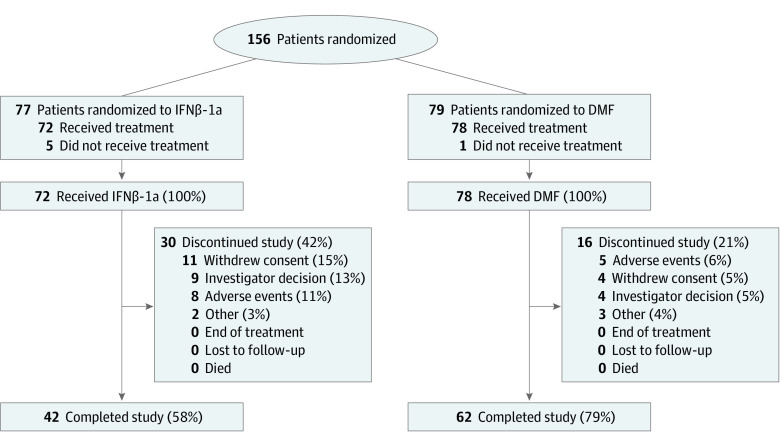
Figure 1. Patient Disposition.
The number of individuals screened and excluded prior to randomization is not available.
In total, 103 patients (68.6%) in the ITT population completed 96 weeks of treatment and were included in the primary analysis (ie, the completers population); of 78 patients who received DMF, 62 patients completed the 96-week study (79.5%), and of 72 patients who received IFNβ-1a, 41 patients completed the 96-week study (56.9%) and were included in the primary analysis. Baseline characteristics were similar between groups of completers (eTable 3 in Supplement 2). In the ITT population, the most common reasons for trial treatment discontinuation were consent withdrawal (15 patients [10.0%]), TEAEs (14 patients [9.3%]), and investigator decision (13 patients [8.7%]).
Efficacy
The proportion of patients, with 95% Clopper-Pearson exact CI, in the completers population with no N or NE T2 hyperintense lesions relative to baseline at week 96 was 16.1% (95% CI, 8.0%-27.7%) for DMF vs 4.9% (95% CI, 0.6%-16.5%) for IFNβ-1a (Figure 2A). In a sensitivity analysis of the ITT population, 10 patients treated with DMF (12.8%) and 2 patients treated with IFNβ-1a (2.8%) had no N or NE T2 hyperintense lesions at week 96. Additional sensitivity analysis on the ITT population, with missing T2 lesion data at week 96 imputed by multiple imputation, yielded consistent results: 16.1% (95% CI, 6.9%-25.3%) of patients treated with DMF and 6.7% (95% CI, 0%-25.3%) of patients treated with IFNβ-1a had no N or NE T2 hyperintense lesions at week 96.
Figure 2. New or Newly Enlarging (N or NE) T2 Lesion and Adjusted Annualized Relapse Rate (ARR) Outcomes.
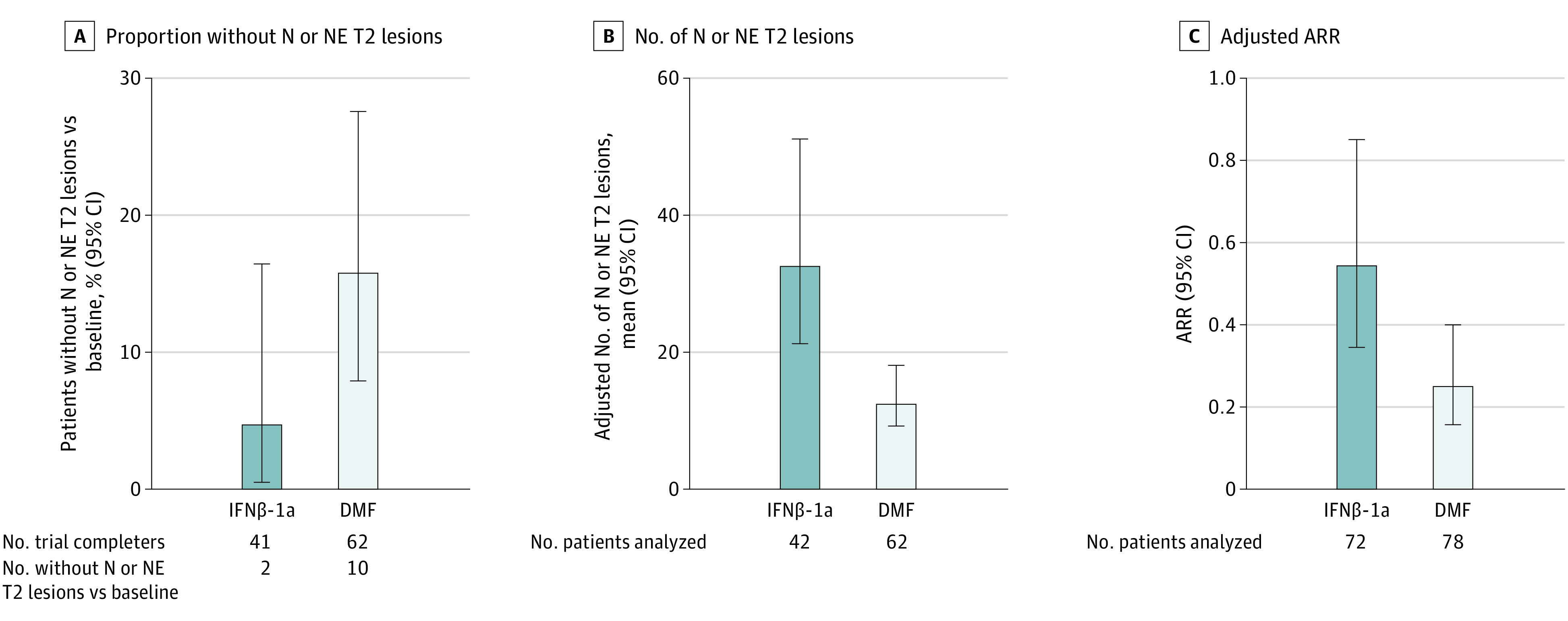
A, The proportions of patients from the completers population with no N or NE hyperintense T2 lesions at week 96 relative to baseline, with Clopper-Pearson exact 95% CIs, are shown. B, The adjusted mean number of N or NE T2 hyperintense lesions at week 96 relative to baseline and percent reduction of T2 hyperintense lesions were estimated from a negative binomial regression model, adjusted for age group and baseline number of T2 hyperintense lesions, in the intention-to-treat population with week 96 magnetic resonance imaging measurements. C, Adjusted ARR and 95% CI were estimated from a negative binomial regression model adjusted for the baseline relapse rate, baseline Expanded Disability Status Scale score, and age group in the intention-to treat population. DMF indicates dimethyl fumarate; IFNβ-1a, interferon β-1a.
More N or NE T2 hyperintense lesions at week 96 relative to baseline were observed among patients treated with IFNβ-1a than among those treated with DMF, with a significant relative reduction of 62.0% (95% CI, 37.9%-76.7%; P < .001) in the number of N or NE T2 hyperintense lesions with DMF vs IFNβ-1a (Table 2). The proportion of patients free of new MRI activity at week 96 was greater among patients treated with DMF than among those treated with IFNβ-1a, although the difference between treatment groups was not significant for the proportion of patients free of Gd+ lesions alone (Table 2). There were fewer new T1 hypointense lesions at week 96 among patients treated with DMF than among those treated with IFNβ-1a (Table 2).
Table 2. Secondary and Exploratory Outcomes at Week 96a.
| Outcome | IFNβ-1a (n = 72) | DMF (n = 78) | Total (N = 150) |
|---|---|---|---|
| Relapses, No. (%) | |||
| 0 | 42 (58.3) | 53 (67.9) | 95 (63.3) |
| 1 | 17 (23.6) | 16 (20.5) | 33 (22.0) |
| 2 | 8 (11.1) | 6 (7.7) | 14 (9.3) |
| 3 | 3 (4.2) | 2 (2.6) | 5 (3.3) |
| ≥4 | 2 (2.8) | 1 (1.3) | 3 (2.0) |
| Total relapses, No. | 53 | 38 | 91 |
| Total patient-years followed, No. | 101.9 | 129.0 | 230.8 |
| Adjusted ARR (95% CI)b | 0.53 (0.33-0.84) | 0.24 (0.15-0.39) | NA |
| Patient relapse ratec | |||
| Mean (SD) | 0.697 (1.269) | 0.407 (1.005) | 0.546 (1.145) |
| Median (IQR) | 0 (0-1.088) | 0 (0-0.543) | 0 (0-0.546) |
| Estimated proportion relapse free at week 96 (95% CI)d | 0.523 | 0.662 | NA |
| HR (95% CI) | NA | NA | 0.57 (0.33-1.00) |
| P value | NA | NA | .05 |
| N or NE T2 hyperintense lesions at week 96, mean (95% CI)e | 32.7 (21.0-50.9) | 12.4 (8.8-17.5) | NA |
| % Reduction (95% CI) | NA | NA | 62.0 (37.9-76.7) |
| P value | NA | NA | <.001 |
| Proportion free of new MRI activity, No./No. (%)f | 2/42 (4.8) | 9/62 (14.5) | NA |
| OR (95% CI) | NA | NA | 8.8 (1.5-81.4) |
| P value | NA | NA | .01 |
| Proportion free of new Gd+ lesions, No./No. (%) | 25/42 (59.5) | 47/62 (75.8) | NA |
| Odds ratio (95% CI) | NA | NA | 0.43 (0.18-1.03) |
| P value | NA | NA | .06 |
| New T1 hypointense lesions, mean (95% CI)g | 18.8 (11.2-31.8) | 7.8 (5.3-11.6) | NA |
| % Reduction (95% CI) | NA | NA | 58.4 (25.1-76.9) |
| P value | NA | NA | .004 |
| Change in EDSS score (week 0 to week 96) | |||
| No. | 44 | 60 | 104 |
| Mean (SD) | 0.13 (0.75) | −0.03 (1.05) | 0.03 (0.93) |
| Median (IQR) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
Abbreviations: ARR, annualized relapse rate; DMF, dimethyl fumarate; EDSS, Expanded Disability Status Scale; HR, hazard ratio; Gd+, gadolinium enhancing; IFNβ-1a, interferon β-1a; MRI, magnetic resonance imaging; N or NE, new or newly enlarging; NA, not applicable; OR, odds ratio.
Among the intent-to-treat population.
Estimated from a negative binomial regression model adjusted for the baseline relapse rate, baseline EDSS score, and age group. Baseline relapse rate was calculated as the total number of relapses in the 3 years prior to trial entry divided by 3.
Number of relapses for each patient divided by the number of years followed up in the trial for that patient.
Based on Kaplan-Meier product limit method, with HR based on a Cox proportional hazards model adjusted for baseline relapse rate (the number of relapses in the 3 years prior to the trial divided by 3), age group, and baseline EDSS score. In the DMF group, 1 patient was excluded due to missing data on number of relapses prior to the trial.
Estimated from a negative binomial regression model, adjusted for age group and baseline number of T2 hyperintense lesions.
Free of new magnetic resonance imaging activity was defined as free of Gd+ lesions at all postbaseline visits up to and including the specified visit and free of N or NE T2 lesions relative to baseline.
Estimated from a negative binomial regression model, adjusted for age group and baseline number of T1 lesions.
In the ITT population, higher proportions of patients treated with IFNβ-1a (30 patients [41.7%]) than DMF (25 patients [32.1%]) relapsed over the trial period. The hazard ratio for risk of relapse for DMF vs IFNβ-1a was 0.574 (95% CI, 0.329-1.001; P = .05), which was not statistically significant (Table 2), indicating a 42.6% decreased risk of relapse for DMF vs IFNβ-1a. By week 96, the estimated percentage of patients who were relapse free by Kaplan-Meier was 66.2% for DMF vs 52.3% for IFNβ-1a. Adjusted ARR at 96 weeks was 0.24 (95% CI, 0.15-0.39) with DMF vs 0.53 (95% CI, 0.33-0.84) with IFNβ-1a; the rate ratio was 0.46 (95% CI, 0.26-0.80; P = .006) (Figure 2C). At week 96 in the ITT population, the mean (SD) change from baseline EDSS score was −0.03 (1.05) in the DMF group and 0.13 (0.75) in the IFNβ-1a group. Few patients in either group (DMF: 7 patients [9.0%]; IFNβ-1a: 4 patients [5.6%]) experienced disability progression as measured by EDSS score at week 96.
Safety
In the CONNECT ITT population, 74 patients in the DMF-treated group (94.9%) and 69 patients in the IFNβ-1a–treated group (95.8%) experienced TEAEs (eTable 4 in Supplement 2). Most TEAEs were mild (29 patients [37.1%] receiving DMF and 27 patients [37.5%] receiving IFNβ-1a) or moderate (42 patients [53.8%] receiving DMF and 32 patients [44.4%] receiving IFNβ-1a) in severity. No deaths were reported. The most commonly reported AEs are shown in Table 3. There were 18 patients (23.1%) receiving DMF and 21 patients (29.2%) receiving IFNβ-1a who experienced serious TEAEs (eTable 4 in Supplement 2). MS relapse was the only serious TESAE reported by more than 1% of patients in either treatment group (13 patients receiving DMF [16.7%] and 18 patients receiving IFNβ-1a [25.0%]). Of TEAEs leading to discontinuation of trial treatment (5 patients [6.4%] in the DMF group and 8 patients [11.1%] in the IFNβ-1a group) (eTable 4 in Supplement 2), MS relapse was reported by a similar percentage in the treatment groups (2 patients receiving DMF [2.6%] and 3 patients receiving IFNβ-1a [4.2%]; flushing was reported only in the DMF group (2 patients).
Table 3. Most Common TEAEs Occurring in ≥20% of Patients and TEAEs of Special Interest.
| Eventa | Patients, No. (%) | ||
|---|---|---|---|
| IFNβ-1a (n = 72) | DMF (n = 78) | Total (N = 150) | |
| TEAEb | |||
| MS relapse | 33 (45.8) | 27 (34.6) | 60 (40.0) |
| Headache | 26 (36.1) | 22 (28.2) | 48 (32.0) |
| Influenza-like illness | 37 (51.4) | 2 (2.6) | 39 (26.0) |
| Abdominal pain | 5 (6.9) | 32 (41.0) | 37 (24.7) |
| Flushing | 1 (1.4) | 30 (38.5) | 31 (20.7) |
| TEAE of special interestb | |||
| Flushing or other related symptom | 1 (1.4) | 30 (38.5) | 31 (20.7) |
| GI tolerabilityc | 22 (30.6) | 58 (74.4) | 80 (53.3) |
| Hepatic disorder | 1 (1.4) | 1 (1.3) | 2 (1.3) |
| Infection or infestation | 30 (41.7) | 41 (52.6) | 71 (47.3) |
| Vascular disorder | 5 (6.9) | 36 (46.2) | 41 (27.3) |
| Leukopenia | 0 | 0 | 0 |
| Lymphopenia | 0 | 2 (2.6) | 2 (1.3) |
| Malignant neoplasm | 0 | 0 | 0 |
| Kidney disorder | 7 (9.7) | 4 (5.1) | 11 (7.3) |
Abbreviations: DMF, dimethyl fumarate; GI, gastrointestinal; IFNβ-1a, interferon β-1a; MS, multiple sclerosis; TEAE, treatment-emergent adverse event.
There were no deaths during the trial.
Patients can appear in more than 1 category.
GI tolerability included nausea, abdominal pain, diarrhea, and other conditions.
Overall, 126 patients in the ITT population (73 patients receiving DMF [93.5%] and 53 patients receiving IFNβ-1a [73.6%]) experienced 1 or more TEAEs of special interest (Table 3). Most TEAEs of special interest that were related to GI events resolved by the end of the trial. Among patients treated with DMF, 2 patients (2.7%) experienced a TEAE of lymphopenia, 1 of which was a severe serious TEAE considered related to treatment by the investigator, which led to drug interruption. The event occurred at the same time as a serious TEAE of tonsillitis; lymphocyte levels were in the reference range 2 weeks before (1470/μL) and 2 weeks after (2000/μL) the hospital visit and were 400/μL on the date of hospital admission (to convert to ×109/L, multiply by 0.001).
In the DMF group, mean (SD) lymphocyte values decreased from baseline (1970/μL [500/μL]) to nadir (1560/μL [680/μL]) at week 60, for a mean (SD) percent change of −18.5% (31.9%), then stabilized at 1640/μL (700/μL) by week 96 (eFigure 2 in Supplement 2). Lymphocyte counts of less than 500/μL were experienced by 4 patients during the trial: 1 patient in the IFNβ-1a group and 3 patients in the DMF group. There were no patients with prolonged, severe lymphopenia (absolute lymphocyte count < 500/μL for ≥6 months).
In each treatment group, 5 patients experienced ALT or AST levels outside the reference range during the trial; 3 patients treated with IFNβ-1a- and 2 patients treated with DMF had AST or ALT levels of less than 3 × the upper limit of normal (ULN), and 1 additional patient treated with DMF had total bilirubin levels of less than 2 × ULN. No patients had both ALT or AST values of more 3 × ULN and bilirubin levels of more than 2 × ULN. Details on patient-reported outcomes can be found in Supplement 2.
Discussion
In this randomized clinical trial of children and adolescents with POMS, DMF had beneficial effects on radiological outcomes, as indicated by a higher proportion of patients treated with DMF being free of N or NE T2 hyperintense lesions at week 96 among trial completers compared with those receiving intramuscular IFNβ-1a. This was also true across other radiological and clinical outcomes in trial completers and the ITT population.
It is recognized that recruiting patients with POMS into randomized clinical trials is challenging.9 In view of this, the sample size in CONNECT was based on feasibility and was not powered to detect statistical differences between treatments on the primary end point. Although statistical inference cannot be drawn, the proportion of patients who were free from N or NE T2 hyperintense lesions at 96 weeks was higher among 68 patients who completed DMF treatment than 42 patients who completed IFNβ-1a treatment. Furthermore, despite an imbalance in the number of patients in the treatment groups for the completer analysis, the higher proportion of patients who were free from N or NE T2 hyperintense lesions with DMF vs IFNβ-1a was confirmed by the sensitivity analysis in the ITT population (10 of 78 patients receiving DMF [12.8%] vs 2 of 72 patients receiving IFNβ-1a [2.8%]). A statistically significant decrease was found in the mean number of N or NE T2 hyperintense lesions among patients treated with DMF vs IFNβ-1a, which suggests a beneficial effect of DMF vs IFNβ-1a on radiographical outcomes in POMS, as do data for other secondary MRI end points.
Clinical outcomes regarding risk of relapse, proportion of patients who were relapse free at week 96, and AAR were also more favorable with DMF than IFNβ-1a. MRI and clinical end points for DMF in our trial were consistent with the phase 2, 24-week FOCUS study16 and its 96-week extension study, CONNECTED,18 in patients with POMS. Our data were also consistent with data from an integrated post hoc analysis in young adults aged 18 to 25 years from 2 pivotal phase 3 DMF studies, DEFINE and CONFIRM.28 The ARR of 0.20 (95% CI, 0.12-0.34) in those studies was similar to our trial’s ARR of 0.24 (95% CI, 0.15-0.39) even though young adults in DEFINE and CONFIRM treated with DMF had a longer mean time after MS diagnosis (2.1 vs 0.9 years) and higher baseline EDSS (2.1 vs 1.2) compared with patients treated with DMF in CONNECT.
Safety profiles of IFNβ-1a and DMF in this trial were consistent with the established safety profile in adults.12,13,15,16,18,23,24,29 Safety findings among pediatric patients treated with DMF in our trial were also consistent with those reported for patients with POMS treated with DMF in the FOCUS16 and CONNECTED18 studies. Decreases in lymphocytes noted in our trial were of a similar magnitude to those observed in previous pediatric studies of DMF,16,18 as well as studies in adults.12,13
Outside of efficacy and safety data, the observation that a higher proportion of patients in the DMF group (79.5%) vs the IFNβ-1a group (56.9%) completed the 96-week trial period is worthy of discussion. In terms of baseline disease characteristics, the population who completed the trial was similar to the randomized population and therefore may be representative of the overall randomized population. The imbalance in patients completing the trial may be partially explained by 2 factors. First, although disease activity was similar at baseline by treatment group, the higher rates of discontinuation were likely due to lower efficacy for IFN, as suggested by higher relapse rates. In addition, the open-label nature of the trial and the development of an alternative therapy that was licensed and became available while the trial was ongoing may have driven a higher discontinuation rate in the interferon group.
Given the highly active and inflammatory disease course of POMS, with frequent relapses in the first years and different MRI lesion patterns,8,30,31 there is a need for treatments that are effective at reducing inflammatory disease activity early in the disease course. Early treatment is important to delay the eventual accrual of disability and prevent diminished cognition.32,33 While new treatments for POMS are becoming available, there is still a need for new effective and well-tolerated DMTs for this patient population.
Limitations
This CONNECT trial has several limitations. As discussed previously, the primary end point was not powered to detect a difference between treatment groups, and no adjustments were made for multiplicity. Therefore, it is important that the directional concordance of results favoring DMF align across a multitude of end points (reducing the potential that the effect seen was by chance and statistically biased) and be reflected by patient experience (differences in discontinuations and completer numbers). Additionally, CONNECT was designed as an open-label trial to reduce patient burden and increase compliance given that a double dummy study may have high patient burden and dropout rates; thus, the open-label design could be considered a limitation. However, this may have been mitigated by MRI data, relapse confirmation, and EDSS rater–blinding analysis, which minimized potential bias arising from knowledge of treatment assignment for efficacy. However, the limitation could not be mitigated for safety.
Conclusions
The CONNECT randomized clinical trial found that DMF led to meaningful improvements in radiological and clinical outcomes in patients with POMS, with a positive benefit-risk balance. The CONNECT trial is ongoing, and part 2 will describe long-term outcomes of DMF in patients with POMS who completed the 96-week part of the trial.
Trial Protocol and Statistical Analysis Plan
eAppendix. Patient-Reported Outcomes
eTable 1. Pediatric Quality of Life Inventory Multidimensional Fatigue Scale Scores Comparisons With Participant Self-report and Parent Proxy Report
eTable 2. Pediatric Quality of Life Inventory Quality of Life Scale Score Comparisons With Participant Self-report and Parent Proxy-Report
eTable 3. Baseline and Demographic Patient Characteristics in Completers Population
eTable 4. Summary of Treatment-Emergent Adverse Events in Intention-to-Treat Population
eFigure 1. CONNECT Study Design
eFigure 2. Mean Lymphocyte Count Over 96-Week Treatment Period
Data Sharing Statement
References
- 1.Jeong A, Oleske DM, Holman J. Epidemiology of pediatric-onset multiple sclerosis: a systematic review of the literature. J Child Neurol. 2019;34(12):705-712. doi: 10.1177/0883073819845827 [DOI] [PubMed] [Google Scholar]
- 2.Alroughani R, Boyko A. Pediatric multiple sclerosis: a review. BMC Neurol. 2018;18(1):27. doi: 10.1186/s12883-018-1026-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson LA, Healy BC, Gorman MP, et al. Elevated relapse rates in pediatric compared to adult MS persist for at least 6 years. Mult Scler Relat Disord. 2014;3(2):186-193. doi: 10.1016/j.msard.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 4.Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol. 2009;66(1):54-59. doi: 10.1001/archneurol.2008.505 [DOI] [PubMed] [Google Scholar]
- 5.Marrie RA, O’Mahony J, Maxwell C, et al. ; Canadian Pediatric Demyelinating Disease Network . Factors associated with health care utilization in pediatric multiple sclerosis. Mult Scler Relat Disord. 2020;38:101511. doi: 10.1016/j.msard.2019.101511 [DOI] [PubMed] [Google Scholar]
- 6.Amato MP, Goretti B, Ghezzi A, et al. ; Multiple Sclerosis Study Group of the Italian Neurological Society . Cognitive and psychosocial features of childhood and juvenile MS. Neurology. 2008;70(20):1891-1897. doi: 10.1212/01.wnl.0000312276.23177.fa [DOI] [PubMed] [Google Scholar]
- 7.Pohl D, Waubant E, Banwell B, et al. ; International Pediatric MS Study Group . Treatment of pediatric multiple sclerosis and variants. Neurology. 2007;68(16)(suppl 2):S54-S65. doi: 10.1212/01.wnl.0000259407.40023.ab [DOI] [PubMed] [Google Scholar]
- 8.Ghezzi A, Banwell B, Boyko A, et al. The management of multiple sclerosis in children: a European view. Mult Scler. 2010;16(10):1258-1267. doi: 10.1177/1352458510375568 [DOI] [PubMed] [Google Scholar]
- 9.Ghezzi A, Amato MP, Edan G, et al. The introduction of new medications in pediatric multiple sclerosis: open issues and challenges. Mult Scler. 2021;27(3):479-482. doi: 10.1177/1352458520930620 [DOI] [PubMed] [Google Scholar]
- 10.Feng J, Rensel M. Review of the safety, efficacy and tolerability of fingolimod in the treatment of pediatric patients with relapsing-remitting forms of multiple sclerosis (RRMS). Pediatric Health Med Ther. 2019;10:141-146. doi: 10.2147/PHMT.S220817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paik J. Teriflunomide: pediatric first approval. Paediatr Drugs. 2021;23(6):609-613. doi: 10.1007/s40272-021-00471-1 [DOI] [PubMed] [Google Scholar]
- 12.Gold R, Kappos L, Arnold DL, et al. ; DEFINE Study Investigators . Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098-1107. doi: 10.1056/NEJMoa1114287 [DOI] [PubMed] [Google Scholar]
- 13.Fox RJ, Miller DH, Phillips JT, et al. ; CONFIRM Study Investigators . Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087-1097. doi: 10.1056/NEJMoa1206328 [DOI] [PubMed] [Google Scholar]
- 14.Cohan SL, Moses H, Calkwood J, et al. Clinical outcomes in patients with relapsing-remitting multiple sclerosis who switch from natalizumab to delayed-release dimethyl fumarate: a multicenter retrospective observational study (STRATEGY). Mult Scler Relat Disord. 2018;22:27-34. doi: 10.1016/j.msard.2018.02.028 [DOI] [PubMed] [Google Scholar]
- 15.Gold R, Arnold DL, Bar-Or A, et al. Long-term safety and efficacy of dimethyl fumarate for up to 13 years in patients with relapsing-remitting multiple sclerosis: final ENDORSE study results. Mult Scler. 2022;28(5):801-816. doi: 10.1177/13524585211037909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alroughani R, Das R, Penner N, Pultz J, Taylor C, Eraly S. Safety and efficacy of delayed-release dimethyl fumarate in pediatric patients with relapsing multiple sclerosis (FOCUS). Pediatr Neurol. 2018;83:19-24. doi: 10.1016/j.pediatrneurol.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 17.Makhani N, Schreiner T. Oral dimethyl fumarate in children with multiple sclerosis: a dual-center study. Pediatr Neurol. 2016;57:101-104. doi: 10.1016/j.pediatrneurol.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 18.Alroughani R, Huppke P, Mazurkiewicz-Beldzinska M, et al. Delayed-release dimethyl fumarate safety and efficacy in pediatric patients with relapsing-remitting multiple sclerosis. Front Neurol. 2021;11:606418. doi: 10.3389/fneur.2020.606418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krysko KM, Graves JS, Rensel M, et al. ; US Network of Pediatric MS Centers . Real-world effectiveness of initial disease-modifying therapies in pediatric multiple sclerosis. Ann Neurol. 2020;88(1):42-55. doi: 10.1002/ana.25737 [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Mannan OA, Manchoon C, Rossor T, et al. ; UK-Childhood Inflammatory Disease Network . Use of disease-modifying therapies in pediatric relapsing-remitting multiple sclerosis in the United Kingdom. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1008. doi: 10.1212/NXI.0000000000001008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghezzi A. Childhood-juvenile multiple sclerosis: clinical characteristics and treatment. Expert Rev Neurother. 2005;5(3):403-411. doi: 10.1586/14737175.5.3.403 [DOI] [PubMed] [Google Scholar]
- 22.Adams AB, Tyor WR, Holden KR. Interferon beta-1b and childhood multiple sclerosis. Pediatr Neurol. 1999;21(1):481-483. doi: 10.1016/S0887-8994(99)00007-7 [DOI] [PubMed] [Google Scholar]
- 23.Mikaeloff Y, Moreau T, Debouverie M, et al. Interferon-beta treatment in patients with childhood-onset multiple sclerosis. J Pediatr. 2001;139(3):443-446. doi: 10.1067/mpd.2001.117004 [DOI] [PubMed] [Google Scholar]
- 24.Pohl D, Rostasy K, Gärtner J, Hanefeld F. Treatment of early onset multiple sclerosis with subcutaneous interferon beta-1a. Neurology. 2005;64(5):888-890. doi: 10.1212/01.WNL.0000153570.33845.6A [DOI] [PubMed] [Google Scholar]
- 25.Waubant E, Chabas D. Pediatric multiple sclerosis. Curr Treat Options Neurol. 2009;11(3):203-210. doi: 10.1007/s11940-009-0024-6 [DOI] [PubMed] [Google Scholar]
- 26.Krupp LB, Banwell B, Tenembaum S; International Pediatric MS Study Group . Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(16)(suppl 2):S7-S12. doi: 10.1212/01.wnl.0000259422.44235.a8 [DOI] [PubMed] [Google Scholar]
- 27.Krupp LB, Tardieu M, Amato MP, et al. ; International Pediatric Multiple Sclerosis Study Group . International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261-1267. doi: 10.1177/1352458513484547 [DOI] [PubMed] [Google Scholar]
- 28.Soman T, Castrillo-Viguera C, Zhang A, Potts J, Marantz J. Efficacy of delayed-release dimethyl fumarate in young adults with RRMS: an integrated analysis of DEFINE and CONFIRM. Paper presented at: Congress of the European Committee for Treatment and Research in Multiple Sclerosis; October 7-10, 2015; Barcelona, Spain. [Google Scholar]
- 29.Ghezzi A, Deplano V, Faroni J, et al. Multiple sclerosis in childhood: clinical features of 149 cases. Mult Scler. 1997;3(1):43-46. doi: 10.1177/135245859700300105 [DOI] [PubMed] [Google Scholar]
- 30.Chitnis T, Tenembaum S, Banwell B, et al. ; International Pediatric Multiple Sclerosis Study Group . Consensus statement: evaluation of new and existing therapeutics for pediatric multiple sclerosis. Mult Scler. 2012;18(1):116-127. doi: 10.1177/1352458511430704 [DOI] [PubMed] [Google Scholar]
- 31.Waubant E, Chabas D, Okuda DT, et al. Difference in disease burden and activity in pediatric patients on brain magnetic resonance imaging at time of multiple sclerosis onset vs adults. Arch Neurol. 2009;66(8):967-971. doi: 10.1001/archneurol.2009.135 [DOI] [PubMed] [Google Scholar]
- 32.Johnen A, Elpers C, Riepl E, et al. Early effective treatment may protect from cognitive decline in paediatric multiple sclerosis. Eur J Paediatr Neurol. 2019;23(6):783-791. doi: 10.1016/j.ejpn.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 33.Deiva K, Huppke P, Banwell B, et al. Consistent control of disease activity with fingolimod versus IFN β-1a in paediatric-onset multiple sclerosis: further insights from PARADIGMS. J Neurol Neurosurg Psychiatry. 2020;91(1):58-66. doi: 10.1136/jnnp-2019-321124 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix. Patient-Reported Outcomes
eTable 1. Pediatric Quality of Life Inventory Multidimensional Fatigue Scale Scores Comparisons With Participant Self-report and Parent Proxy Report
eTable 2. Pediatric Quality of Life Inventory Quality of Life Scale Score Comparisons With Participant Self-report and Parent Proxy-Report
eTable 3. Baseline and Demographic Patient Characteristics in Completers Population
eTable 4. Summary of Treatment-Emergent Adverse Events in Intention-to-Treat Population
eFigure 1. CONNECT Study Design
eFigure 2. Mean Lymphocyte Count Over 96-Week Treatment Period
Data Sharing Statement