Key Points
Question
Is interprofessional discharge planning associated with safely reducing length of stay among medical inpatients?
Findings
In this nonrandomized controlled trial including 493 486 hospitalizations at 7 intervention hospitals and 75 control hospitals, the implementation of an electronic interprofessional-led discharge planning tool was associated with a monthly reduction in length of stay by 0.9 hours, resulting in a cumulative benefit of 10.5 hours after the 1-year intervention phase. The intervention did not increase the risk of hospital readmission, in-hospital mortality, or facility discharge.
Meaning
These findings support the broader implementation of discharge planning programs to reduce length of stay.
This nonrandomized controlled trial evaluates the outcomes and safety associated with an electronic interprofessional-led discharge planning tool designed to reduce length of stay among medical inpatients with multimorbidity.
Abstract
Importance
Whether interprofessional collaboration is effective and safe in decreasing hospital length of stay remains controversial.
Objective
To evaluate the outcomes and safety associated with an electronic interprofessional-led discharge planning tool vs standard discharge planning to safely reduce length of stay among medical inpatients with multimorbidity.
Design, Setting, and Participants
This multicenter prospective nonrandomized controlled trial used interrupted time series analysis to examine medical acute hospitalizations at 82 hospitals in Switzerland. It was conducted from February 2017 through January 2019. Data analysis was conducted from March 2021 to July 2022.
Intervention
After a 12-month preintervention phase (February 2017 through January 2018), an electronic interprofessional-led discharge planning tool was implemented in February 2018 in 7 intervention hospitals in addition to standard discharge planning.
Main Outcomes and Measures
Mixed-effects segmented regression analyses were used to compare monthly changes in trends of length of stay, hospital readmission, in-hospital mortality, and facility discharge after the implementation of the tool with changes in trends among control hospitals.
Results
There were 54 695 hospitalizations at intervention hospitals, with 27 219 in the preintervention period (median [IQR] age, 72 [59-82] years; 14 400 [52.9%] men) and 27 476 in the intervention phase (median [IQR] age, 72 [59-82] years; 14 448 [52.6%] men) and 438 791 at control hospitals, with 216 261 in the preintervention period (median [IQR] age, 74 [60-83] years; 109 770 [50.8%] men) and 222 530 in the intervention phase (median [IQR] age, 74 [60-83] years; 113 053 [50.8%] men). The mean (SD) length of stay in the preintervention phase was 7.6 (7.1) days for intervention hospitals and 7.5 (7.4) days for control hospitals. During the preintervention phase, population-averaged length of stay decreased by −0.344 hr/mo (95% CI, −0.599 to −0.090 hr/mo) in control hospitals; however, no change in trend was observed among intervention hospitals (−0.034 hr/mo; 95% CI, −0.646 to 0.714 hr/mo; difference in slopes, P = .09). Over the intervention phase (February 2018 through January 2019), length of stay remained unchanged in control hospitals (slope, −0.011 hr/mo; 95% CI, −0.281 to 0.260 hr/mo; change in slope, P = .03), but decreased steadily among intervention hospitals by −0.879 hr/mo (95% CI, −1.607 to −0.150 hr/mo; change in slope, P = .04, difference in slopes, P = .03). Safety analyses showed no change in trends of hospital readmission, in-hospital mortality, or facility discharge over the whole study time.
Conclusions and Relevance
In this nonrandomized controlled trial, the implementation of an electronic interprofessional-led discharge planning tool was associated with a decline in length of stay without an increase in hospital readmission, in-hospital mortality, or facility discharge.
Trial Registration
isrctn.org Identifier: ISRCTN83274049
Introduction
Hospital length of stay (LOS) is a quality and reimbursement measure for efficient hospital management used by many health care systems. Worldwide, hospitals have aimed at reducing LOS to better match demand with capacity for new admissions and to improve turnaround of patients.1 Furthermore, prolonged LOS might be associated with increased inpatient complications and negative patient and staff experience.2 To overcome these problems, hospitals have specifically targeted patients, mostly those with multimorbidity, who tend to have longer LOS by reducing the number of long-stay beds, shifting care to the outpatient setting, and implementing policies such as discharge planning.3 In 2012, following a global trend, Switzerland classified patient hospital stays into diagnosis-related groups (SwissDRGs) with fixed reimbursements that further incentivized hospitals to reduce LOS to maintain operating margins. As in other countries, this raised safety concerns.4
Several approaches have been developed to optimize LOS, some focusing on aspects of patient management, such as clinical care,5,6 and others targeting staffing models7,8 and logistics of care coordination (eg, discharge planning and application of interprofessional care).3,9,10,11 Nevertheless, hospital discharge planning procedures have not been widely standardized, carrying the risk of poor communication, delayed assessments for postacute care demands, medication errors, or unprepared patient discharges.12,13,14
Health policy makers have called for the use of interprofessional collaboration as an essential element in improving safety of patient discharge and optimizing LOS,15,16 involving different health and social care professionals. Working together aims to overcome the fragmented, siloed, and burdensome provision of care that patients with medical complexity typically face.17,18 However, in Switzerland, interprofessional rounds are not necessarily standard, and evidence is still limited, especially regarding the effectiveness of interventions in older and multimorbid populations at risk for prolonged LOS and poor outcomes.19
To address this need, we developed a bundled hospital discharge planning tool that was embedded in the electronic medical records (EMRs) for use by the existing interprofessional ward teams. We hypothesized that this multifaceted intervention to shorten waiting times during hospital stay through an improved interprofessional collaboration would be associated with decreased LOS without increased hospital readmission and other adverse patient outcomes among medical inpatients with multimorbidity.
Methods
Overview
This nonrandomized controlled trial was part of the National Research Program Smarter Health Care, launched by the Swiss National Science Foundation to promote innovative health services research and to tackle practical challenges of caring for patients with multimorbidity in Switzerland.20 As a quality improvement study, the institutional review board of Northwestern Switzerland approved the study and waived the need for individual informed consent by formulating a declaration of no objection. Eligible patients were informed by clinical staff and an information flyer about their study participation within 24 hours after hospital admission. This study follows the Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) reporting guideline.21
Study Design and Participants
The Integrative Hospital Treatment in Older Patients to Benchmark and Improve Outcome and Length of Stay (In-HospiTOOL) study was an investigator-initiated, multicenter quality improvement study. The trial protocol appears in Supplement 1. It investigated whether the implementation of an electronic interprofessional-led discharge planning tool (In-HospiTOOL) would be associated with decreased LOS compared with standard discharge planning without negatively affecting relevant safety outcomes. Using a controlled interrupted time series design, this study compared outcomes among patients in hospitals that implemented In-HospiTOOL (intervention group) vs hospitals without such an additional intervention (control group). The overall 24-month study period was divided into a 12-month preintervention phase, from February 2017 to January 2018, and a 12-month intervention phase, from February 2018 to January 2019. From August 2017 to January 2019, patients were followed up for 30 days after hospital admission by structured telephone interviews to assess patient-reported outcomes, such as activities of daily living and satisfaction with care, among others not discussed in this article (eFigure 1 in Supplement 2). The rationale for this study, design details, and eligibility features have been previously published.22
Of 10 invited hospitals, 7 agreed to participate in the study. Declining hospitals were not asked to provide reasons for not participating but were not different in terms of patient characteristics and setting of care when compared with participating hospitals. Invitations were extended given prior scientific collaboration and the availability of the same EMR to facilitate technical implementation of the tool. Although not using the same EMR, participation of the University Hospital Basel was included because it would increase the generalizability of the findings. Control hospitals followed their individual standard discharge planning strategies.
The 7 intervention hospitals were secondary and tertiary care hospitals and included the University Clinic in Aarau, the University Hospital in Basel, the cantonal hospitals in Baden and Muensterlingen, and the hospitals in Interlaken, Muri, and Zofingen. The remaining 75 secondary and tertiary care hospitals in Switzerland with comparable characteristics were included in the control group.
Consecutively admitted medical patients aged 18 years or older with at least 2 diagnoses in need of acute treatment were prospectively included in the study. This requirement was chosen to better address the level of multimorbidity in the target study population. Eligible patients were admitted via the emergency department and were required to be hospitalized for at least 24 hours. Patients treated in an intensive care unit (ICU) only were excluded, given that the intervention was not applied to ICU patients. Patients with outlier hospitalization stays (ie, >100 days) were excluded from the analysis. The same eligibility criteria were retrospectively applied to patients among control hospitals.
The In-HospiTOOL
The In-HospiTOOL is an EMR-based application that standardizes interprofessional communication and was designed for team-based rounding at patient’s bedside. The tool was developed based on a sounding board consensus involving national stakeholders from the health care, information technology, and governmental sectors. The tool encompassed a bundle of interventions performed by physicians, nurses, and social workers that took place in the emergency department (step 1), at the medical ward (step 2), and before hospital discharge (step 3). In step 1, the main factors for improving discharge planning were an early estimation of the potential discharge date and the identification of potential delaying factors in diagnostics or treatment. The information from the emergency department was immediately applicable to all involved professions (eg, at the ward) on an EMR application. In step 2, physicians reevaluated the patient’s potential discharge date daily based on clinical condition, diagnostics, and therapeutics; nurses assessed any postacute care needs using the postacute care discharge (PACD) score23 during the first day on medical ward; and, if needed, social workers were required to contact postacute care facilities early to reduce transfer waiting times. In step 3, physicians and nurses instructed patients about the most important findings during the hospitalization, pending in-hospital diagnostics, changes to their medication plan, strategies in case of clinical worsening at home, and next clinical appointments following elements of the BOOST (Better Outcomes by Optimizing Safe Transitions) checklist.13 Details of the tool are illustrated and described in Figure 1.
Figure 1. Activities of the Integrative Hospital Treatment in Older Patients to Benchmark and Improve Outcome and Length of Stay Study Along the Patient Transition Pathway.
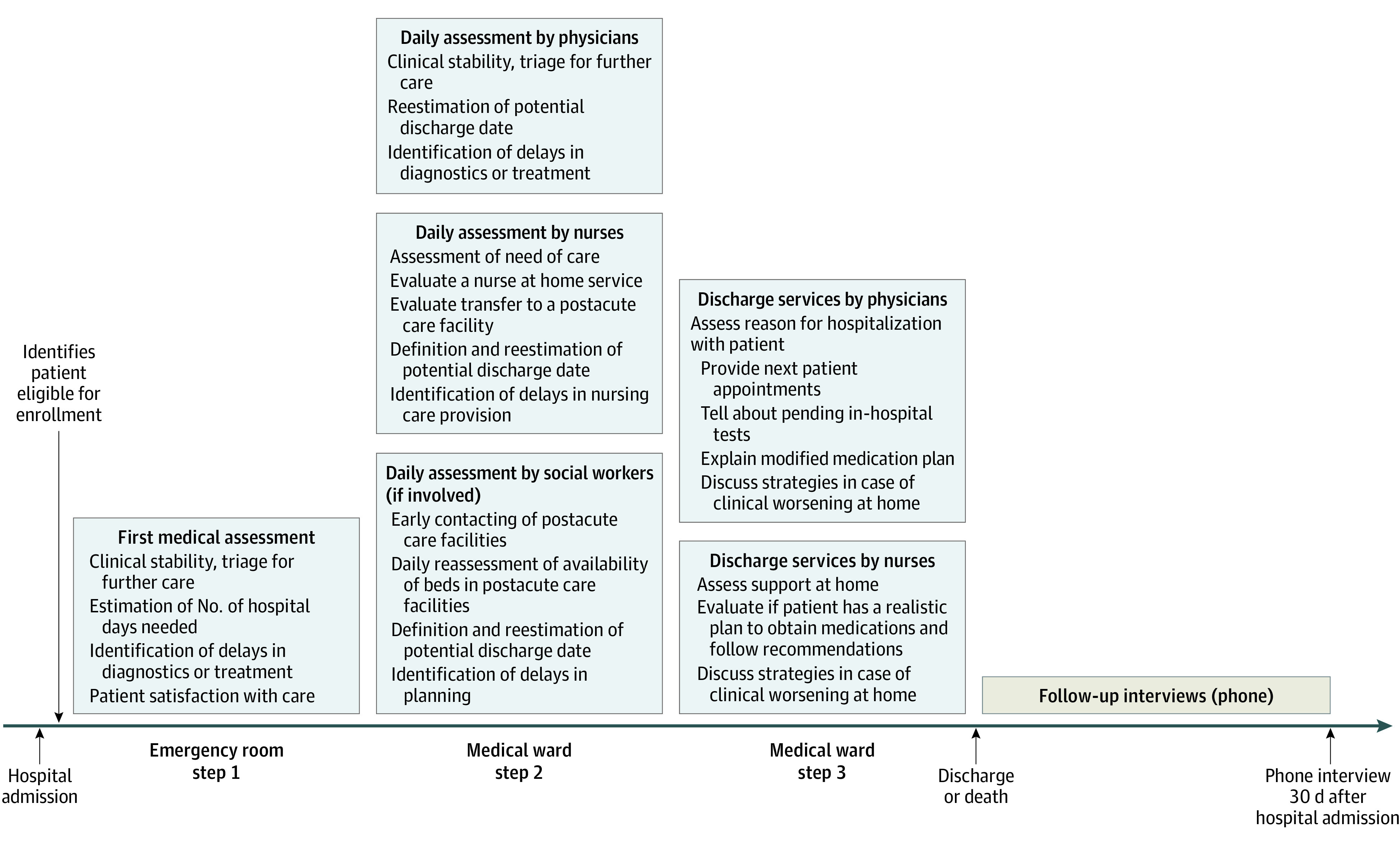
Step 1 included an early discharge date estimation done by the emergency care team; step 2 involved a systematic assessment of delaying factors (diagnostics and therapy) during hospital stay, a modification of the potential discharge date, and an early assessment and contacting of postacute care facilities; step 3, physicians and nurses performed a timely discussion of pending in-hospital tests, out-of-hospital appointments, changes in medication plan, and strategies in case of clinical worsening at home with patients and relatives. Patient comprehension was assessed using the teach-back methodology. Information was recorded by either physicians, nurses, or social workers and was immediately available to all involved professionals using the EMR.
Interventions
We evaluated the implementation process of the intervention using the Reach, Effectiveness, Adoption, Implementation, Maintenance (RE-AIM) framework. Table 1 provides domain definitions.24,25 This process evaluation allows insight into how study interventions were implemented and might be optimized to aid future dissemination.
Table 1. Definition of RE-AIM Domains Along the Study Intervention.
| RE-AIM domain | Definition in In-HospiTOOL implementation evaluation |
|---|---|
| Reach |
|
| Effectiveness |
|
| Adoption |
|
| Implementation |
|
| Maintenance |
|
Abbreviations: In-HospiTOOL, Integrative Hospital Treatment in Older Patients to Benchmark and Improve Outcome and Length of Stay; EMR, electronic medical records; RE-AIM, Reach, Effectiveness, Adoption, Implementation, Maintenance.
Although not monitored by the study team, control hospitals followed their own discharge planning procedures, usually (but not mandatorily) including an interdisciplinary assessment of patient’s need for follow-up care after leaving the hospital and arrangements for that care.
Data Collection
To provide regular feedback regarding frequency of tool use, all intervention hospitals implemented a structured data export that was sent monthly to the involved hospital staff. These data were sourced from the hospital-specific EMR. To assess patient covariates and outcomes of interest, for both intervention and control hospitals, anonymous inpatient claims data were obtained from the Federal Statistical Office in Neuchâtel, Switzerland. Among patients who were transferred between acute care hospitals, hospital stays were combined into a single episode of care, and the patient outcome was attributed to the first hospital.
Measures and Outcomes
As a measure of adherence, the frequency of tool use was defined as the proportion of patients for whom the tool was used in the numerator and eligible patients in the denominator. Tool use was defined as filling at least 1 element of the tool; however, step 2 was mandatory because it included the core elements of the intervention with a daily reassessment of discharge-relevant aspects by all involved professionals, as described previously.
The primary outcome was LOS, defined as days spent in the hospital during the index hospitalization. The index hospitalization included all readmissions into the index hospital within 18 days after discharge from the index hospitalization if the main reason for rehospitalization was related to the main diagnosis from the index hospitalization. According to the SwissDRG definition, every readmission into the same hospital after 18 days from discharge or any readmission into another hospital was defined as a new index hospitalization.26 Thus, a single patient may have more than 1 index admission during the study period. Hospitalizations that crossed from 1 study phase to another or that went past the study end date were attributed to the study phase applicable during hospital admission.
Secondary outcomes included any all-cause hospital readmission within 30 days after discharge, all-cause in-hospital mortality, and facility discharge (ie, long-term care facility, convalescent home, or rehabilitation clinic). Regarding hospital readmission, both planned and emergency readmissions were included in the analysis. Out-of-hospital mortality data were not available in the claims data set and could not be linked to the national death registry. We did not evaluate the relative outcomes associated with the separate elements of this bundled intervention. Blinding of patients, staff, and study personnel was not feasible due to the hospital ward-based nature of the intervention.
Statistical Analysis
We descriptively examined patient and hospital characteristics of index hospitalizations for intervention and control hospitals for the preintervention phase and the season-matched intervention phase. The index hospitalization was the unit of analysis, and the statistical analysis was on an intention-to-treat basis.
To analyze population-averaged trends in LOS, 30-day hospital readmission, in-hospital mortality, and facility discharge, we performed segmented regression analyses of interrupted time-series data from February 2017 through January 2019. To model trends in LOS, we used a multivariable mixed-effects linear regression, including study time, modeled continuously as a linear spline with 1 knot location chosen at the start of the intervention phase (February 2018). In addition, we added a parameter to assess a change in level in LOS at the start of the intervention phase. The model included random effects for hospital and patient-specific intercept and slope to allow for individual trends for each hospital and patient, which improved models’ fit over random intercept only models, based on log likelihood ratio tests, with statistical significance set at P < .05. At the patient-level, the model was adjusted for age, sex, housing conditions, level of health care insurance coverage, Elixhauser comorbidity index, and the Hospital Frailty Risk Score.27 Time coefficients can be interpreted as monthly mean change in hours of hospitalization.
To explore whether the intervention was associated with patient safety, we assessed the previously mentioned secondary outcomes using multivariable mixed-effects logistic regression analyses following the same specification as described for the linear model. Resulting time coefficients can be interpreted as monthly mean change in percentage. We censored patients who died during hospitalization when assessing hospital readmission and facility discharge. There were no missing data for patient characteristics and study outcomes. Statistical significance was based on 95% CIs, and all P values are two-sided. All statistical analyses were performed using Stata version 17.0 (StataCorp).
Results
Index Hospital Admissions
Among 54 695 hospitalizations in 7 intervention hospitals, 27 219 (49.8%) were included during the preintervention phase and 27 476 (50.2%) during the intervention phase. Among the 75 nonintervention hospitals, 216 261 (49.3%) and 222 530 (50.7%) hospitalizations, respectively, served as controls (eFigure 2 in Supplement 2).
Baseline characteristics were generally similar between intervention and control hospitals (Table 2). In the 7 intervention hospitals, the median (IQR) age was 72 [59-82] years, and 14 400 patients (52.9%) were men during the preintervention phase; the median (IQR) age was 72 (59-82) years and 14 448 patients (52.6%) were men in the intervention phase. In the control hospitals, the median (IQR) age was 74 (60-83) years, and 109 770 patients (50.8%) were men in the preintervention phase; the median (IQR) age was 74 (60-83) years, and 113 053 patients (50.8%) were men in intervention phase. Of note, the proportion of patients treated in a university hospital was larger among intervention hospitals. Patients hospitalized in intervention hospitals were more likely to be male and being admitted from a nursing home. They were also hospitalized more frequently for a cardiovascular main diagnosis and had a slightly higher burden of comorbidities and frailty than patients in control hospitals. Between the preintervention and intervention phase, there were only minimal differences in baseline characteristics for both study groups.
Table 2. Baseline Characteristics of Patients.
| Characteristic | Patients, No. (%) | |||
|---|---|---|---|---|
| Intervention hospitals | Control hospitals | |||
| Preintervention phase, Feb 2017 to Jan 2018 (n = 27 219) | Intervention phase, Feb 2018 to Jan 2019 (n = 27 476) | Preintervention phase, Feb 2017 to Jan 2018 (n = 216 261) | Intervention phase, Feb 2018 to Jan 2019 (n = 222 530) | |
| Sociodemographic | ||||
| Age, median (IQR), y | 72 (59-82) | 72 (59-82) | 74 (60-83) | 74 (60-83) |
| Gender | ||||
| Male | 14 400 (52.9) | 14 448 (52.6) | 109 770 (50.8) | 113 053 (50.8) |
| Female | 12 819 (47.1) | 13 028 (47.4) | 106 491 (49.2) | 109 477 (49.2) |
| Swiss residents | 22 113 (81.2) | 22 237 (80.9) | 178 391 (82.5) | 182 729 (82.1) |
| Private health insurance | 5669 (20.8) | 5678 (20.7) | 48 625 (22.5) | 48 177 (21.6) |
| Living at home before admission | 22 467 (82.5) | 22 632 (82.4) | 192 116 (88.8) | 197 881 (88.9) |
| Nursing home residents | 1867 (6.9) | 1905 (6.9) | 9212 (4.3) | 10159 (4.6) |
| Hospital | ||||
| Quarter of hospital admission | ||||
| Jan-Mar | 7425 (27.3) | 7333 (26.7) | 56 915 (26.3) | 59 450 (26.7) |
| Apr-Jun | 6526 (24.0) | 6711 (24.4) | 51 398 (23.8) | 53 256 (23.9) |
| Jul-Sep | 6767 (24.9) | 6702 (24.4) | 52 613 (24.3) | 53 784 (24.2) |
| Oct-Dec | 6501 (23.9) | 6730 (24.5) | 55 335 (25.6) | 56 040 (25.2) |
| Hospital teaching level | ||||
| University hospitals | 6679 (24.5) | 6220 (22.6) | 31 153 (14.4) | 32 800 (14.7) |
| Nonuniversity tertiary care hospitals | 17 510 (64.3) | 17 940 (65.3) | 142 752 (66.0) | 146 513 (65.8) |
| Secondary care hospitals | 3030 (11.1) | 3316 (12.1) | 42 356 (19.6) | 43 217 (19.4) |
| Level of morbidity | ||||
| Comorbidities | ||||
| Hypertension | 14 613 (53.7) | 14 704 (53.5) | 110 018 (50.9) | 115 012 (51.7) |
| Diabetes | 5519 (20.3) | 5692 (20.7) | 42 330 (19.6) | 44 072 (19.8) |
| Congestive heart failure | 4063 (14.9) | 4245 (15.4) | 32 441 (15.0) | 34 380 (15.4) |
| Coronary artery disease | 6482 (23.8) | 6554 (23.9) | 47 393 (21.9) | 49 576 (22.3) |
| Cerebrovascular disease | 3368 (12.4) | 3486 (12.7) | 18 276 (8.5) | 19 404 (8.7) |
| Chronic kidney disease | 6314 (23.2) | 6416 (23.4) | 47 325 (21.9) | 50 883 (22.9) |
| Liver disease | 1161 (4.3) | 1229 (4.5) | 9319 (4.3) | 9864 (4.4) |
| COPD | 2661 (9.8) | 2737 (10.0) | 20 732 (9.6) | 22 100 (9.9) |
| Cancer | 3775 (13.9) | 3739 (13.6) | 28 434 (13.1) | 29 142 (13.1) |
| Dementia | 1634 (6.0) | 1667 (6.1) | 12 790 (5.9) | 13 178 (5.9) |
| Groups of main diagnoses during hospital stay | ||||
| Cardiovascular | 7445 (27.4) | 7331 (26.7) | 53 891 (24.9) | 55 033 (24.7) |
| Respiratory | 3610 (13.3) | 3766 (13.7) | 30 197 (14.0) | 32 097 (14.4) |
| Oncology | 2098 (7.7) | 2155 (7.8) | 15 718 (7.3) | 15 858 (7.1) |
| Gastroenterology | 2351 (8.6) | 2324 (8.5) | 17 275 (8.0) | 17 732 (8.0) |
| Infectious disease | 2018 (7.4) | 2090 (7.6) | 16 491 (7.6) | 17 252 (7.7) |
| Elixhauser Comorbidity Index, mean (SD)a | 3.0 (2.1) | 3.1 (2.1) | 2.8 (2.0) | 2.9 (2.0) |
| Hospital Frailty Risk Scoreb | ||||
| <5, Low risk | 17 698 (65.0) | 17 636 (64.2) | 143 041 (66.1) | 145 014 (65.2) |
| 5-15, Intermediate risk | 8433 (31.0) | 8729 (31.8) | 66 582 (30.8) | 70 072 (31.5) |
| >15, High risk | 1088 (4.0) | 1111 (4.0) | 6638 (3.1) | 7444 (3.3) |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Scores range from −7 to 12, with higher scores indicating greater comorbidity.
Scores range from 0 to 99, with higher scores indicating greater frailty.
Frequency of Tool Use
During the intervention phase, the tool was used by either physicians, nurses, or both for mean (SD) 81.9% (38.5) of patients. The mean (SD) frequency of tool use among physicians was 77.6% (41.7), and among nurses, it was 75.5% (43.0%) (eFigure 3 in Supplement 2).
Hospital Length of Stay
The mean (SD) LOS during the preintervention phase was 7.5 (7.4) days for control hospitals and 7.6 (7.1) days for intervention hospitals. It was 7.4 (7.2) days and 7.3 (7.0) days, respectively, during the intervention phase. The eTable in Supplement 2 shows crude estimates per study month.
Over the year before implementing the tool, in control hospitals, LOS declined 0.344 hr/mo (95% CI, −0.599 to −0.090 hr/mo; P = .01), while there was no change in LOS among intervention hospitals (0.034 hr/mo; 95% CI, −0.646 to 0.714 hr/mo; P = .92) during the same time (Table 3 and Figure 2A). The difference in slopes between control and intervention hospitals was not statistically significant (P = .09). During the intervention phase, the decline ceased among control hospitals (−0.011 hr/mo; 95% CI, −0.281 to 0.260 hr/mo; P = .94; change from prior slope, P = .03), but it was more pronounced in intervention hospitals with a monthly reduction in LOS by 0.879 hours (95% CI, −1.607 to −0.150 hr/mo; change from prior slope, P = .04; difference in intervention phase slopes, P = .03). There was no evidence for a sudden change in level at the start of the intervention phase.
Table 3. Multivariable Random Slope Mixed-Effects Model of Changes in Hospital Length of Stay, 30-day Hospital Readmission, In-Hospital Mortality, and Facility Discharge for Intervention and Control Hospitals Between February 2017 and January 2019.
| Outcome and study phasea | Coefficient (95% CI)b | Slope (95% CI)c | P value | ||
|---|---|---|---|---|---|
| Slope differs from zero | Change in slope from prior slope | Difference in slopes (control vs intervention hospitals) | |||
| Hospital length of stayd | |||||
| Control hospitals, reference group | |||||
| Preintervention phase, Feb 2017 to Jan 2018 | −0.344 (−0.599 to −0.090) | −0.344 (−0.599 to −0.090) | .01 | .03 | NA |
| Intervention phase, Feb 2018 to Jan 2019 | 0.334 (0.026 to 0.642) | −0.011 (−0.281 to 0.260) | .94 | NA | |
| Intervention hospitals | |||||
| Change in level in Feb 2017 | 1.086 (−26.399 to 28.571) | NA | NA | NA | NA |
| Intervention hospital by time interaction during preintervention phase, Feb 2017 to Jan 2018 | 0.378 (−0.332 to 1.089) | 0.034 (−0.646 to 0.714) | .92 | .04 | .09 |
| Intervention hospital by time interaction during intervention phase, Feb 2018 to Jan 2019 | −1.247 (−2.160 to −0.334) | −0.879 (−1.607 to −0.150) | .02 | .03 | |
| 30-d hospital readmission | |||||
| Control hospitals, reference group | |||||
| Preintervention phase, Feb 2017 to Jan 2018 | 0.042 (0.011 to 0.074) | 0.042 (0.011 to 0.074) | .01 | .88 | NA |
| Intervention phase, Feb 2018 to Jan 2019 | −0.005 (−0.063 to 0.054) | 0.038 (−0.023 to 0.099) | .22 | NA | |
| Intervention hospitals | |||||
| Change in level in Feb 2017 | −0.277 (−2.264 to 1.710) | NA | NA | NA | NA |
| Intervention hospital by time interaction during pre-intervention phase, Feb 2017 to Jan 2018 | −0.041 (−0.141 to 0.058) | 0.001 (−0.094 to 0.096) | .99 | .54 | .15 |
| Intervention hospital by time interaction during intervention phase, Feb 2018 to Jan 2019 | 0.061 (−0.129 to 0.251) | 0.058 (−0.136 to 0.251) | .56 | .85 | |
| In-hospital mortality | |||||
| Control hospitals, reference group | |||||
| Preintervention phase, Feb 2017 to Jan 2018 | 0.002 (−0.018 to 0.021) | 0.002 (−0.018 to 0.021) | .88 | .67 | NA |
| Intervention phase, Feb 2018 to Jan 2019 | 0.007 (−0.025 to 0.039) | 0.008 (−0.023 to 0.040) | .60 | NA | |
| Intervention hospitals | |||||
| Change in level in Feb 2017 | −0.340 (−2.121 to 1.442) | NA | NA | NA | NA |
| Intervention hospital by time interaction during pre-intervention phase, Feb 2017 to Jan 2018 | 0.001 (−0.055 to 0.058) | 0.003 (−0.051 to 0.057) | .92 | .39 | >.99 |
| Intervention hospital by time interaction during intervention phase, Feb 2018 to Jan 2019 | 0.036 (−0.066 to 0.137) | 0.045 (−0.052 to 0.143) | .36 | .48 | |
| Facility discharge | |||||
| Control hospitals, reference group | |||||
| Preintervention phase, Feb 2017 to Jan 2018 | 0.024 (−0.023 to 0.072) | 0.024 (−0.023 to 0.072) | .32 | .24 | NA |
| Intervention phase, Feb 2018 to Jan 2019 | −0.041 (−0.110 to 0.028) | −0.017 (−0.088 to 0.055) | .65 | NA | |
| Intervention hospitals | |||||
| Change in level in February 2017 | 3.410 (−1.184 to 8.004) | NA | NA | NA | NA |
| Intervention hospital by time interaction during pre-intervention phase, Feb 2017 to Jan 2018 | −0.001 (−0.148 to 0.146) | 0.023 (−0.117 to 0.163) | .74 | .58 | .77 |
| Intervention hospital by time interaction during intervention phase, Feb 2018 to Jan 2019 | −0.017 (−0.233 to 0.200) | −0.034 (−0.257 to 0.188) | .76 | .88 | |
Abbreviation: NA, not applicable.
Time is treated continuously; coefficients and slopes are reported in monthly estimates (eg, change per month).
Model includes patient’s age, sex, housing condition, level of health care insurance coverage, Elixhauser comorbidity index, and Hospital Frailty Risk Score.
Slope during intervention phase can be derived by summing coefficient from preintervention phase and intervention phase.
Regression coefficients and slopes for hospital length of stay are provided in hours.
Figure 2. Trends in Hospital Length of Stay, 30-day Hospital Readmission, In-Hospital Mortality, and Facility Discharge for Intervention and Control Hospitals Before and After the Tool Implementation.
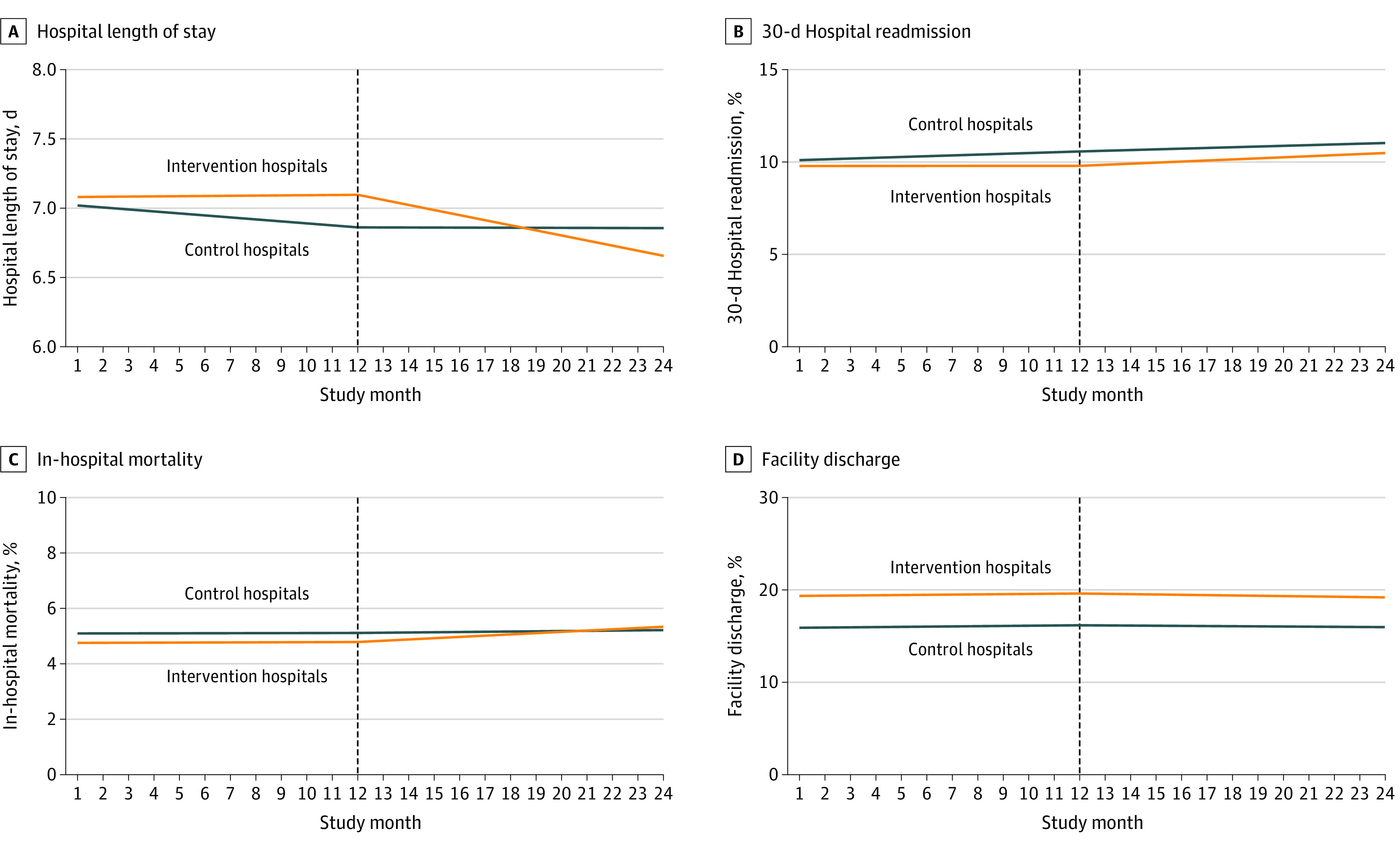
Graphs show population-averaged means for outcomes 1 year before and after the implementation of the intervention. The tool implementation in February 2018 is indicated with dashed vertical lines.
Secondary Safety Outcomes
During the preintervention phase, the mean (SD) 30-day hospital readmission rate was 10.3% (12.5) among both control and intervention hospitals, and it was 10.4% (12.5) and 10.1% (12.3), respectively, during the intervention phase (eTable in Supplement 2). Mean in-hospital mortality was 5.2% (10.3) and 4.7% (10.4), respectively, during the preintervention phase, and 5.1% (10.1) and 4.9% (10.3), respectively, during the intervention phase. Discharge to a postacute care facility occurred in a mean (SD) 18.3% (23.1) and 20.3% (25.6), respectively, of hospitalizations during the preintervention phase and was comparable with the intervention phase (18.7% [23.2] and 20.3% [25.7], respectively). Segmented regression analyses revealed no differences in trends for all secondary outcomes of interest, except for a small increase in hospital readmission rate among control hospitals during the preintervention phase (slope, 0.042% per month; 95% CI, 0.011%-0.074%; P = .01) (Table 3 and Figure 2B-D).
Discussion
In this multicenter study involving 493 486 hospitalizations, the implementation of an electronic interprofessional-led discharge planning tool embedded in the EMRs of 7 hospitals was associated with a decline in LOS among patients with multimorbidity compared with a control group of hospitals receiving standard discharge planning. There were no increases in hospital readmission, in-hospital mortality, or postacute facility discharge.
This study found that a prospective multicenter implementation of an electronic interprofessional-led discharge planning tool in a challenging and siloed environment was feasible. This is important, as many previous improvement studies were limited by complex interventions or cumbersome implementations, and it has been suggested that team-based care approaches would have limited success.19,28,29,30 Given the clinical setting with a heterogeneous patient population, this nationwide large-scale study, showing high tool use and systematic assessment of outcomes with no missing data, may fill an important gap in the current evidence about the effectiveness of interprofessional-led discharge planning to reduce LOS.
Second, given the fact that intention-to-treat analyses arguably better evaluate the effectiveness of interventions in clinical pragmatic studies, assessment of intervention fidelity is critical.31 Although we could not systematically assess whether the intervention was implemented as intended, the frequency of tool use in this study was approximately 80%, which is higher compared with other pragmatic studies.32 Nonetheless, a more comprehensive comparison with previous studies is largely limited, as many studies did not assess the adherence to intervention use and, thus, had an increased risk of rejecting potentially effective interventions that failed to work because they were poorly implemented or of accepting ineffective interventions that achieved desired effects caused by factors other than the intervention.33
Third, this bundled intervention was associated with a reduction in LOS of approximately 10.5 hours over 12 months, which was not seen in the control group. This is relevant not only for patients and clinicians but also for hospital authorities, as even a small decrease in LOS could unlock capacity for subsequent admissions in a hospital with a shortage of acute care beds. This is notable because previous interventions were not consistently associated with a decrease in LOS for patients with medical complexity.19 While our findings validate results from a previous meta-analysis3 showing that LOS was reduced for medical patients who were allocated to a discharge planning program, other investigations found no or even an increasing effect on LOS among unselected medical patients.6,34 Interventions in smaller and more selected patient populations, such as in those with heart failure, were more likely to be successful in reducing LOS.35 To increase reach, however, this study included a broad and heterogeneous population of medically complex patients with multiple acute and chronic morbidities. Fourth, the study intervention was not associated with safety concerns. This was also true for 30-day hospital readmissions, an outcome that was negatively affected by the SwissDRG implementation, the so far largest strategy in Switzerland to improve hospital efficiency.36 Previous hospital-based discharge planning interventions showed mixed results among medical patients. One systematic review34 found improved readmission rates while LOS increased among the intervention groups. Another systematic review35 found no difference in readmission, without, however, assessing LOS. In-hospital mortality and facility discharge, 2 balancing outcomes of this study, did not change in the intervention hospitals, supporting the safety of our intervention, and did not explain the reduction in LOS.
The large multicenter design, the heterogeneity in medical patients with multimorbidity, and the adaption of the intervention to local settings are all characteristics of a pragmatic clinical intervention, revealing the robust external validity of this study. We provided a standardized description of all interventions that can be adapted and transferred to different health care settings. The intervention’s adaptability was proven by this study, as local settings varied among the 7 intervention hospitals, even though 6 had the same EMR and some already had experience in research collaboration. We found that the implementation of In-HospiTOOL was associated with a significant reduction in LOS without an increase in hospital readmission and other balancing safety outcomes. Thus, these findings provide evidence of the potential effectiveness of interventions to safely reduce LOS and will also support hospital authorities and health care decision-makers in implementing interprofessional-led discharge planning interventions tailored to their local setting and patient spectrum.
Areas of Further Research
Further research into the effectiveness of our intervention would be beneficial. To better evaluate the main factors associated with more efficient discharge planning, active comparisons may help to identify single elements of this bundled intervention that are useful to organizations. These could include experimental and nonexperimental comparative studies and look at the specific influence of potentially important moderators (eg, training methods, intensity of social worker rounding to detect postacute care demands early, monitoring strategies to evaluate intervention’s fidelity in terms of plausibility). Moreover, given the fact that there was still a progressive decline in LOS at the end of study period, the potential benefit of the tool might be underestimated. Therefore, potential sustainability of the intervention should be evaluated after the study intervention was stopped in most centers.
Limitations
Our study has several limitations. First, although our findings are consistent with the implementation of the discharge planning tool, the nonrandomized design limits the ability to draw a causal link. However, it is unlikely that the changes in trend among the intervention group were solely caused by secular trends in the presence of a comparison group without such changes. Second, while the average LOS at baseline in this study was much longer than seen in many other countries’ hospitals, generalizability could be compromised. However, even in a setting of shorter LOS, the use of a structured interprofessional collaboration as an essential element in preserving safety of patient discharge seems justified. In addition, LOS might be overestimated in Switzerland by counting certain readmissions within 18 days of discharge as a single hospitalization; however, any resulting bias would affect the intervention and control groups similarly. Third, while the analyses were adjusted for several important measured confounders, unmeasured confounding, such as differences in socioeconomic status or practice variations between wards and hospitals, is likely and may have influenced outcomes. Fourth, the selection of intervention hospitals based on prior scientific collaboration may have introduced selection bias and could have reduced generalizability. Fifth, involved health care professionals, hospital authorities, and research assistants could not be blinded, which may have introduced reporting and participation bias favoring the intervention. Sixth, although the frequency of tool use was systematically assessed, we were not able to evaluate whether the tool was used in a plausible and intended way. Seventh, we did not perform a cost-effectiveness analysis nor an exploration on extra workload that both could be barriers for a broader tool use. Similarly, caregivers were not systematically interviewed to assess whether they perceived a change in their work satisfaction during the study. Furthermore, a certain risk of misclassification cannot be excluded given that administrative data were used; however, we do not expect that it influenced the outcomes, as both study groups would have been similarly affected.
Conclusions
This nonrandomized controlled trial found that the implementation of an electronic interprofessional-led discharge planning tool was associated with a reduction in LOS among medical patients with multimorbidity without increasing the risk of hospital readmission, in-hospital mortality, or facility discharge. The findings of this study strongly support a broader implementation of embedded discharge planning programs to safely reduce LOS in unselected hospitalized patients with multiple medical conditions as routinely treated.
Trial Protocol
eTable. Crude Estimates of Length of Stay, 30-Day Hospital Readmission, In-Hospital Mortality, and Facility Discharge Among Intervention and Control Hospitals
eFigure 1. Timeline and Study Design
eFigure 2. Study Flow Diagram
eFigure 3. Frequency of Tool Use Among Physicians and Nurses
Data Sharing Statement
References
- 1.Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303(21):2141-2147. doi: 10.1001/jama.2010.748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rojas-García A, Turner S, Pizzo E, Hudson E, Thomas J, Raine R. Impact and experiences of delayed discharge: a mixed-studies systematic review. Health Expect. 2018;21(1):41-56. doi: 10.1111/hex.12619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonçalves-Bradley DC, Lannin NA, Clemson LM, Cameron ID, Shepperd S. Discharge planning from hospital. Cochrane Database Syst Rev. 2016;(1):CD000313. doi: 10.1002/14651858.CD000313.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fässler M, Wild V, Clarinval C, Tschopp A, Faehnrich JA, Biller-Andorno N. Impact of the DRG-based reimbursement system on patient care and professional practise: perspectives of Swiss hospital physicians. Swiss Med Wkly. 2015;145:w14080. doi: 10.4414/smw.2015.14080 [DOI] [PubMed] [Google Scholar]
- 5.Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: a quality-improvement project. J Hosp Med. 2016;11(5):341-347. doi: 10.1002/jhm.2546 [DOI] [PubMed] [Google Scholar]
- 6.Mudge AM, McRae P, Banks M, et al. Effect of a ward-based program on hospital-associated complications and length of stay for older inpatients: the cluster randomized CHERISH trial. JAMA Intern Med. 2022;182(3):274-282. doi: 10.1001/jamainternmed.2021.7556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercedes A, Fairman P, Hogan L, Thomas R, Slyer JT. Effectiveness of structured multidisciplinary rounding in acute care units on length of stay and satisfaction of patients and staff: a quantitative systematic review. JBI Database System Rev Implement Rep. 2016;14(7):131-168. doi: 10.11124/JBISRIR-2016-003014 [DOI] [PubMed] [Google Scholar]
- 8.Butler M, Schultz TJ, Halligan P, et al. Hospital nurse-staffing models and patient- and staff-related outcomes. Cochrane Database Syst Rev. 2019;4:CD007019. doi: 10.1002/14651858.CD007019.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 2017;19(11):1427-1443. doi: 10.1002/ejhf.765 [DOI] [PubMed] [Google Scholar]
- 10.Zhu QM, Liu J, Hu HY, Wang S. Effectiveness of nurse-led early discharge planning programmes for hospital inpatients with chronic disease or rehabilitation needs: a systematic review and meta-analysis. J Clin Nurs. 2015;24(19-20):2993-3005. doi: 10.1111/jocn.12895 [DOI] [PubMed] [Google Scholar]
- 11.Pannick S, Davis R, Ashrafian H, et al. Effects of interdisciplinary team care interventions on general medical wards: a systematic review. JAMA Intern Med. 2015;175(8):1288-1298. doi: 10.1001/jamainternmed.2015.2421 [DOI] [PubMed] [Google Scholar]
- 12.Poulos CJ, Magee C, Bashford G, Eagar K. Determining level of care appropriateness in the patient journey from acute care to rehabilitation. BMC Health Serv Res. 2011;11:291. doi: 10.1186/1472-6963-11-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178-187. doi: 10.7326/0003-4819-150-3-200902030-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345-349. [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . Framework for action on interprofessional education and collaborative practice. Accessed August 24, 2022. https://apps.who.int/iris/handle/10665/70185
- 16.NHS England . Improvement guidance for writing a criteria-led discharge policy. September 2021. Accessed August 30, 2022. https://www.england.nhs.uk/wp-content/uploads/2021/10/B0928-criteria-led-discharge-guidance-v2.pdf
- 17.Fuji KT, Abbott AA, Norris JF. Exploring care transitions from patient, caregiver, and health-care provider perspectives. Clin Nurs Res. 2013;22(3):258-274. doi: 10.1177/1054773812465084 [DOI] [PubMed] [Google Scholar]
- 18.Blaum CS, Rosen J, Naik AD, et al. Feasibility of implementing patient priorities care for older adults with multiple chronic conditions. J Am Geriatr Soc. 2018;66(10):2009-2016. doi: 10.1111/jgs.15465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddique SM, Tipton K, Leas B, et al. Interventions to reduce hospital length of stay in high-risk populations: a systematic review. JAMA Netw Open. 2021;4(9):e2125846. doi: 10.1001/jamanetworkopen.2021.25846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smarter Health Care. National Research Programme. Accessed March 18, 2021. http://www.nfp74.ch/en/Pages/Home.aspx
- 21.Des Jarlais DC, Lyles C, Crepaz N; TREND Group . Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94(3):361-366. doi: 10.2105/AJPH.94.3.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kutz A, Koch D, Conca A, et al. ; Clinical Trial Protocol Personal Narrative . Integrative hospital treatment in older patients to benchmark and improve outcome and length of stay—the In-HospiTOOL study. BMC Health Serv Res. 2019;19(1):237. doi: 10.1186/s12913-019-4045-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis Simonet M, Kossovsky MP, Chopard P, Sigaud P, Perneger TV, Gaspoz JM. A predictive score to identify hospitalized patients’ risk of discharge to a post-acute care facility. BMC Health Serv Res. 2008;8:154. doi: 10.1186/1472-6963-8-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.BZP. In HospiTOOL Vs3. January 18, 2018. Accessed August 29, 2022. https://www.youtube.com/watch?v=bNyRPucs-FQ [Google Scholar]
- 25.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322-1327. doi: 10.2105/AJPH.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DRG . Regeln und Definitionen zur Fallabrechnung unter SwissDRG. Accessed October 24, 2019. https://www.swissdrg.org/application/files/4714/8111/3146/160620_SwissDRG_Falldefinitionen_v5.pdf
- 27.Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz JI, Gonzalez-Colaso R, Gan G, et al. Structured interdisciplinary bedside rounds improve interprofessional communication and workplace efficiency among residents and nurses on an inpatient internal medicine unit. J Interprof Care. Published online January 21, 2021. doi: 10.1080/13561820.2020.1863932 [DOI] [PubMed] [Google Scholar]
- 29.Gilman SC, Chokshi DA, Bowen JL, Rugen KW, Cox M. Connecting the dots: interprofessional health education and delivery system redesign at the Veterans Health Administration. Acad Med. 2014;89(8):1113-1116. doi: 10.1097/ACM.0000000000000312 [DOI] [PubMed] [Google Scholar]
- 30.Moore JE, Mascarenhas A, Marquez C, et al. ; MOVE ON Team . Mapping barriers and intervention activities to behaviour change theory for Mobilization of Vulnerable Elders in Ontario (MOVE ON), a multi-site implementation intervention in acute care hospitals. Implement Sci. 2014;9:160. doi: 10.1186/s13012-014-0160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernán MA, Robins JM. Per-protocol analyses of pragmatic trials. N Engl J Med. 2017;377(14):1391-1398. doi: 10.1056/NEJMsm1605385 [DOI] [PubMed] [Google Scholar]
- 32.Smith J, Green J, Siddiqi N, et al. Investigation of ward fidelity to a multicomponent delirium prevention intervention during a multicentre, pragmatic, cluster randomised, controlled feasibility trial. Age Ageing. 2020;49(4):648-655. doi: 10.1093/ageing/afaa042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ginsburg LR, Hoben M, Easterbrook A, Anderson RA, Estabrooks CA, Norton PG. Fidelity is not easy! challenges and guidelines for assessing fidelity in complex interventions. Trials. 2021;22(1):372. doi: 10.1186/s13063-021-05322-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mabire C, Dwyer A, Garnier A, Pellet J. Meta-analysis of the effectiveness of nursing discharge planning interventions for older inpatients discharged home. J Adv Nurs. 2018;74(4):788-799. doi: 10.1111/jan.13475 [DOI] [PubMed] [Google Scholar]
- 35.Bryant-Lukosius D, Carter N, Reid K, et al. The clinical effectiveness and cost-effectiveness of clinical nurse specialist-led hospital to home transitional care: a systematic review. J Eval Clin Pract. 2015;21(5):763-781. doi: 10.1111/jep.12401 [DOI] [PubMed] [Google Scholar]
- 36.Kutz A, Gut L, Ebrahimi F, Wagner U, Schuetz P, Mueller B. Association of the Swiss diagnosis-related group reimbursement system with length of stay, mortality, and readmission rates in hospitalized adult patients. JAMA Netw Open. 2019;2(2):e188332. doi: 10.1001/jamanetworkopen.2018.8332 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Crude Estimates of Length of Stay, 30-Day Hospital Readmission, In-Hospital Mortality, and Facility Discharge Among Intervention and Control Hospitals
eFigure 1. Timeline and Study Design
eFigure 2. Study Flow Diagram
eFigure 3. Frequency of Tool Use Among Physicians and Nurses
Data Sharing Statement


