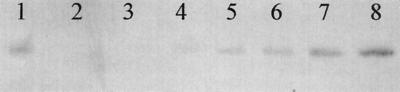Abstract
Cytochrome c′ from Rhodobacter capsulatus has been shown to confer resistance to nitric oxide (NO). In this study, we demonstrated that the amount of cytochrome c′ synthesized for buffering of NO is insufficient to account for the resistance to NO but that the cytochrome-dependent resistance mechanism involves the catalytic breakdown of NO, under aerobic and anaerobic conditions. Even under aerobic conditions, the NO removal is independent of molecular oxygen, suggesting cytochrome c′ is a NO reductase. Indeed, we have measured the product of NO breakdown to be nitrous oxide (N2O), thus showing that cytochrome c′ is behaving as a NO reductase. The increased resistance to NO conferred by cytochrome c′ is distinct from the NO reductase pathway that is involved in denitrification. Cytochrome c′ is not required for denitrification, but it has a role in the removal of externally supplied NO. Cytochrome c′ synthesis occurs aerobically and anaerobically but is partly repressed under denitrifying growth conditions when other NO removal systems are operative. The inhibition of respiratory oxidase activity of R. capsulatus by NO suggests that one role for cytochrome c′ is to maintain oxidase activity when both NO and O2 are present.
Cytochrome c′ is a periplasmic heme-containing protein found in a metabolically diverse set of proteobacteria. The cytochrome has been identified biochemically in photosynthetic organisms of the Rhodospirillaceae and Chromatiaceae families, denitrifiers, sulfur oxidizers, and methylotrophs. Additionally, analyses of partial and completed genome sequences have revealed that a number of pathogenes, including Neisseria meningitidis, Bordatella pertussis, and Pseudomonas aeruginosa have genes encoding cytochrome c′ (4).
The heme iron in cytochrome c′ has been shown to bind preferentially to small uncharged ligands such as nitric oxide (NO) and carbon monoxide (CO) (10). In keeping with the observed ligand binding properties of the isolated cytochrome, cytochrome c′ also binds to NO in intact cells (17, 18). The expression of the cytochrome has been shown to confer an increased resistance to NO in intact cells of Rhodobacter capsulatus (4).
In this paper we address the question of the mechanism by which cytochrome c′ is capable of increasing the resistance of bacteria to the potentially toxic free-radical gas NO. Firstly, the cytochrome may reversibly bind NO in the intact cell, lowering the internal concentration of the gas and hence relieving its toxic effects on metabolic processes such as respiration. Support for this putative mechanism comes from the observation that cytochrome c′ is capable of binding and then releasing NO gas at physiologically relevant concentrations (12). This mechanism has also been suggested to be employed by a hexaheme protein in the gram-positive bacterium Bacillus halodenitrificans (5). Secondly, the cytochrome may enzymatically remove NO from cells by binding and converting the molecule to a nontoxic or less-toxic product(s). Precedent for such a mechanism is the enzymatic turnover of NO by hemoglobins. The function of the hemoglobin from the nematode Ascaris lumbricoides has been proposed to be the reaction of NO with molecular oxygen to produce nitrate (11). Bacterial flavohemoglobins have been shown to catalyze the oxygen-dependent conversion of NO to nitrate (6) and the anoxic reduction of NO to nitrous oxide (N2O) (7).
MATERIALS AND METHODS
Growth of bacteria.
R. capsulatus PAS100 (a wild-type strain) and MC111 (an isogenic strain mutated in the gene cycP encoding cytochrome c′ [4]) were grown photosynthetically under anaerobic conditions in RCV medium (15) supplemented with 25 mM malate at 30°C. R. capsulatus BK5DNIT, a denitrifying strain (13), and R. capsulatus MC206, an isogenic denitrifying strain deficient in cycP (described below), were grown under anaerobic denitrifying conditions in RCV medium supplemented with 10 mM butyrate as a carbon and energy source, with nitrate or nitrite included as a terminal electron acceptor (13). R. capsulatus strains were grown aerobically in 20 ml of RCV medium-malate in 250-ml conical flasks shaken at 200 rpm at 30°C. Growth rate experiments were carried out by taking samples from cultures at measured time intervals, and the rates of growth of the cultures were calculated from readings of the optical density at 600 nm measured in a Jenway 6105 UV-visible light spectrophotometer.
Measurements of NO and oxygen.
Suspensions of intact bacterial cells were maintained in a 50-ml water-jacketed vessel at 30°C. At the base of this chamber was a Clark-type electrode (Rank Brothers, Bottisham, United Kingdom) for measurement of the [O2]. The top of the chamber was stoppered with a rubber bung. Inserted into the bung was an isoNO electrode (World Precision Instruments) for measurement of the [NO]. The bung contained two other ports, one for sparging samples with N2 (for maintenance of complete anaerobiosis) and one for injection of samples into the reaction mixture via Hamilton syringe. Changes in the [NO] and the [O2] over time were recorded using a Lloyd Graphic, 1002 two-channel chart recorder.
Saturated solutions of NO were generated by sparging 5 ml of distilled water in a 10-ml bijou container fitted with a rubber septum, firstly with N2 and then with NO gas (Aldrich). NO was supplied to some experiments via sodium nitroprusside (SNP).
Measurements of NO.
Noninvasive, continuous measurements of the changes in the N2O concentration in a liquid assay system were made using membrane-inlet mass spectrometry essentially as described previously (8, 9). The probe for the mass spectrometry was inserted into a magnetically stirred reaction vessel with a working volume of 5 ml. The reaction vessel also contained an isoNO electrode probe to allow simultaneous measurements of NO to be made.
Purification of cytochrome c′ and preparation and use of antibodies.
Cytochrome c′ was purified from periplasmic extracts of R. capsulatus using three chromatographic steps. Periplasmic extract from 10 liters of R. capsulatus was loaded onto a 1.7- by 15-cm DEAE-Sepharose CL6B column (Pharmacia) equilibrated with 100 mM Tris-HCl (pH 8), and the cytochrome c′ was eluted with a gradient of NaCl. The partially purified cytochrome was concentrated into a volume of 5 ml and purified further on a 1.7- by 100-cm Sephacryl S100 column (Pharmacia) equilibrated with 100 mM Tris-HCl (pH 8). Fractions containing cytochrome c′ were dialyzed against 10 mM sodium phosphate (pH 7) and loaded onto a 1- by 10-cm Macro-Prep hydroxyapatite column (Bio-Rad) equilibrated with the same buffer. This column was developed with a gradient from 10 to 100 mM sodium phosphate (pH 7), and the peak cytochrome-containing fractions were pure as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Purified cytochrome c′ was used to raise antibodies in a rabbit. The presence of cytochrome c′ in R. capsulatus grown under different conditions and an estimate of the concentration of cytochrome c′ in intact cells of R. capsulatus was determined by Western blotting. Protein extracts (purified cytochrome c′ and/or extracts of intact cells) were separated by SDS-PAGE and blotted onto polyvinylidene difluoride (PVDF) membranes. Anti-cytochrome c′ antibody was used as the primary antibody, and anti-rabbit immunoglobulin G conjugated to alkaline phosphatase (Sigma) used as the secondary antibody. Blots were developed with nitroblue tetrazolium and 5-bromo-4-chloro3-indolyl phosphate (Sigma). Cytochrome c′ was quantified by comparisons of band intensities. The concentration of the purified cytochrome c′ was estimated from UV-visible light absorption spectra using extinction coefficients calculated previously (16).
Insertional mutagenesis of cycP from R. capsulatus BK5DNIT.
To make an insertional mutation in cycP, both uptake of a plasmid bearing a disrupted copy of cycP and homologous recombination with the chromosomal copy of cycP are required. Wild-type R. capsulatus BK5DNIT possesses a restriction-modification system which makes rates of plasmid uptake very low. Therefore, in order to get sufficiently high rates of plasmid uptake and recombination to generate a cycP mutant it was necessary to select for a restriction-deficient strain of R. capsulatus BK5DNIT. Transconjugative matings of R. capsulatus BK5DNIT with Escherichia coli S17-1 containing broad-host-range plasmid pRK415 yielded tetracycline-resistant R. capsulatus (i.e., a strain carrying pRK415) at a frequency of 1 in 1010. A tetracycline-resistant R. capsulatus colony was picked, and the plasmid was cured by replating and selecting for tetracycline-sensitive colonies. The cured strain was found to receive plasmid DNA via conjugation at a high frequency and was used for construction of a cycP-negative strain. The inactivation of cycP was accomplished with the use of plasmid construct pCP301 and methods described previously (4) to obtain a kanamycin-resistant strain, named R. capsulatus MC206, that was unable to make cytochrome c′.
RESULTS AND DISCUSSION
Rapid NO removal by bacterial cells containing cytochrome c′.
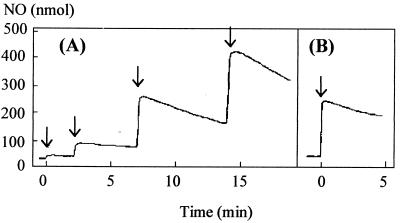
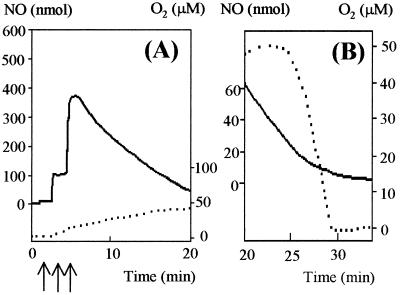
Suspensions of R. capsulatus PAS100 (wild type) and MC111 (cytochrome c′-deficient strain) were maintained at 30°C in a water-jacketed vessel. These suspensions were allowed to become anaerobic via the innate respiratory activity of the bacteria, and anaerobiosis was maintained by sparging the headspace with a stream of oxygen-free nitrogen gas. Aliquots of a saturated solution of NO were added to the suspension via a Hamilton syringe, which fitted into a port in the stoppered vessel. The NO concentration was monitored with an electrode. Figure 1 shows results typical of such experiments. R. capsulatus PAS100 was capable of rapidly consuming two 300-nmol aliquots of NO added to the cell suspension (Fig. 1A). The rate of removal was faster than the response time of the electrode, such that the initial accumulation of the NO was not observed. Subsequent additions of NO led to an accumulation of NO and then a slow rate of removal. In contrast, when an aliquot of NO was added to a suspension of R. capsulatus that lacks cytochrome c′ (Fig. 1B), the NO removal rate was slow and measurable, as had been observed after the third aliquot of NO was added to the wild-type strain.
FIG. 1.
Metabolism of NO after addition of NO to suspensions of R. capsulatus. Fifty-milliliter suspensions of RCV growth medium-malate containing 0.5 mg of R. capsulatus PAS100 (A) or MC111 (B) per ml were allowed to become anaerobic by their innate respiratory activity and were kept anaerobic by sparging with nitrogen gas, and then aliquots of NO were added (300-nmol aliquots added, as marked by arrows). The accumulation of NO was measured using the apparatus described in Materials and Methods.
Clearly the possession of cytochrome c′ enables the bacteria to remove NO either by sequestering the molecule so that it is not visible to the NO electrode or by catalytically breaking down the molecule. The cytochrome c′-dependent removal of NO appears to have a limited capacity, as indicated by the exhaustion of rapid NO removal after the repeated addition of aliquots of NO to the suspension of wild-type R. capsulatus.
Cytochrome c′ content of intact cells.
In order to determine whether sequestration of NO by cytochrome c′ could account for the apparent rapid removal of NO by suspensions of R. capsulatus, the cytochrome c′ content of R. capsulatus used in a typical NO removal experiment was estimated by Western blotting. Samples of purified cytochrome c′ and a known amount of R. capsulatus cell extract were subjected to SDS-PAGE, blotted onto PVDF membranes, and probed with antibodies to R. capsulatus cytochrome c′ (Fig. 2). From the blot shown in Fig. 2, it is possible to estimate the cytochrome c′ content of intact cells to be 0.3 ± 0.15 μmol/g (dry weight) (5 to 10% of total periplasmic protein). The data in Fig. 1A indicate that 25 mg of wild-type R. capsulatus is capable of cytochrome c′-dependent removal of around 0.5 μmol of NO, i.e., 20 μmol NO/g (dry weight). Each molecule of cytochrome c′ is therefore capable of removing at least 60 molecules of NO, demonstrating that sequestration of NO by cytochrome c′ cannot account for the NO removal observed. Therefore, the logical conclusion is that c′ removes NO via some enzymatic mechanism.
FIG. 2.
Cytochrome c′ content of R. capsulatus. Suspensions of sonicated anaerobically grown R. capsulatus and samples of purified cytochrome c′ were subjected to SDS-PAGE and Western blotting with antibodies to cytochrome c′. Lane 1, 2 μg of a cell extract of R. capsulatus; lanes 2 to 8, 0.075, 0.15, 0.225, 0.3, 0.75, and 1.5 pmol, respectively, of purified cytochrome c′.
Effect of oxygen concentration on cytochrome c′-dependent NO removal.
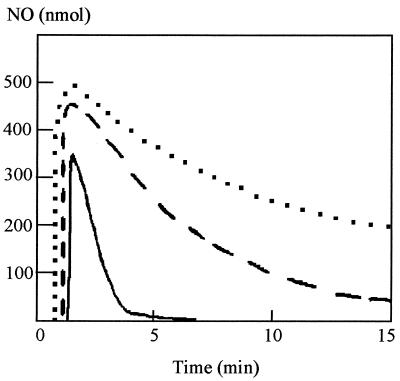
In the initial experimental setup described above, R. capsulatus suspensions were kept anaerobic. We therefore decided to investigate whether oxygen affected the cytochrome c′-dependent NO removal. To maintain aerobiosis in cell suspensions, the stopper that was normally fitted over the chamber was removed and the stirrer speed was increased to give an [O2] of 150 μM. Aliquots of NO were added, and the rate of removal was monitored (Fig. 3). In the presence of oxygen there was removal of NO even in sterile growth media and in suspensions of R. capsulatus MC111 due to the reaction of the molecule with O2. However, it was evident that NO was more rapidly removed from suspensions of PAS100 than from suspensions of MC111, i.e., cytochrome c′ aided NO removal under aerobic conditions.
FIG. 3.
NO removal under aerobic conditions. Twenty-milliliter suspensions of RCV growth medium-malate containing 0.1 mg of R. capsulatus PAS100 per ml (solid line), 0.1 mg of R. capsulatus MC111 per ml (broken line), or no cells (dotted line) were maintained under aerobic conditions (150 μM O2). Aliquots (500 nmol) of NO were added to each of the suspensions, and the accumulation and subsequent disappearance of NO was monitored using the apparatus described earlier.
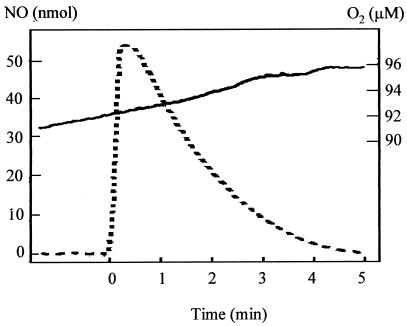
To determine whether aerobic cytochrome c′-dependent NO removal uses molecular oxygen as a substrate, we measured NO and O2 simultaneously. To simplify interpretation of data from this experiment, we wanted to remove any interfering effects due to changes in oxygen respiration rate and therefore included 50 μM KCN in the cell suspension in order to inhibit the respiratory oxidases. Cyanide inhibited oxygen respiration by >95% but had no effect on the capacity of cytochrome c′ to remove NO (in keeping with the observation that cyanide is, at best, a weak ligand to the cytochrome). During NO removal via cytochrome c′ there was no observed drop in oxygen concentration (Fig. 4), indicating that cytochrome c′ does not function as an oxygenase. Therefore, the mechanism of NO removal is distinct from that via the flavohemoglobin Hmp, which acts as an NO oxygenase under aerobic conditions (6) and has only a very low rate of NO reduction to N2O under anaerobic conditions (7).
FIG. 4.
Cytochrome c′-dependent NO removal is independent of molecular oxygen. A 20-ml suspension of 0.2 mg of R. capsulatus PAS100 per ml was kept aerobic in the presence of 50 μM KCN. The removal of NO (60 nmol added at time zero) was monitored (dotted line). During this period there was no drop in oxygen concentration (solid line), indicating that oxygen is not used up by the cytochrome c′-dependent NO removal reaction.
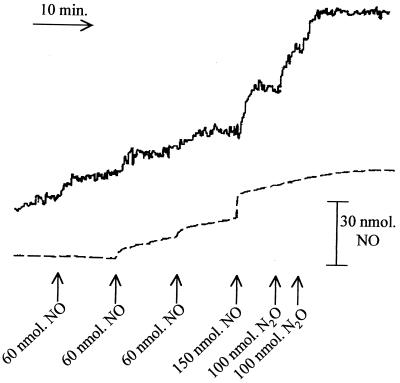
Product of NO removal by cytochrome c′ is N2O.
Having determined that cytochrome c′ was capable of catalytic removal of NO in an oxygen-independent fashion, trying to determine the product of NO breakdown was of considerable interest. The removal of NO and the production of N2O were measured simultaneously in a 5-ml reaction vessel containing a suspension of R. capsulatus PAS100 (Fig. 5). Upon addition of aliquots of 60 nmol of NO to the cell suspension, there was an immediate increase in the level of N2O as measured by mass spectrometry, while NO was removed so rapidly via cytochrome c′ that only a minor part of the NO added was ever seen to accumulate. Subsequent additions of 150 nmol of NO elicited a more marked increase in the NO detected by the electrode, accompanied by greater production of N2O. Aliquots of N2O alone did not influence the output of the NO electrode but were readily detectable by mass spectrometry. From this observation we can conclude that cytochrome c′ converts NO to N2O and is, therefore, a NO reductase. The limited capacity of the reaction (since cells expressing cytochrome c′ only remove a certain amount of NO) indicates that cytochrome c′ has a specific pool of reductant, the supply of which is limited. The nature of this reductant is unclear, but it is noteworthy that we have not been able to reconstitute a cytochrome c′-dependent NO reductase activity with the purified protein in vitro.
FIG. 5.
N2O is the product of NO disappearance. A 5-ml suspension of 1 mg of R. capsulatus PAS100 per ml was stirred anaerobically in a 5-ml reaction vessel containing probes for N2O (solid line) measurement (mass spectrometer measuring m/z = 44) and NO (dashed line) measurement (via isoNO electrode). NO removal was so rapid that it was not observed after the first addition of NO, and subsequently only small accumulations of NO were observed. However, each addition of NO gives rise to the production of N2O concomitant with the rapid cytochrome c′-dependent removal of NO.
Effect of NO and cytochrome c′ on oxygen metabolism.
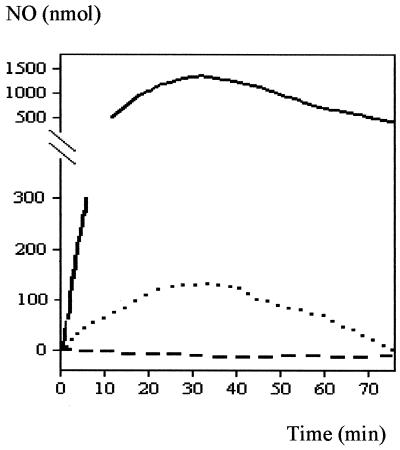
When suspensions of R. capsulatus were established in the electrode reaction chamber and stoppered but not sparged with N2, oxygen accumulated in response to the presence of NO (Fig. 6A). The stopper that was fitted to the top of the reaction chamber contains ports through which oxygen from the air can gain access to the chamber. Once NO begins to accumulate, the respiratory oxidases are inhibited and hence O2 is able to accumulate.
FIG. 6.
Oxygen metabolism in the presence of NO. A 50-ml suspension of RCV growth medium-malate containing 0.5 mg of R. capsulatus PAS100 per ml was allowed to become anaerobic by its own respiratory activity, in the absence of sparging with nitrogen gas. NO (solid line) and O2 (dotted line) were monitored simultaneously with electrodes. NO additions (300-nmol aliquots) are marked with arrows. After enough NO had been added so that NO had accumulated, the oxygen also began to accumulate (panel A and continuing into panel B). Panel B shows that once the [NO] had fallen below approximately 25 nmol in the 50-ml cell suspension (i.e., 0.5 μM NO), oxygen was consumed by the cell suspension.
NO and O2 concentrations were monitored simultaneously during the slow removal of NO that occurs in both wild-type and cytochrome c′ mutant strains of R. capsulatus as shown in Fig. 1 (presumably a consequence of low-level NO reductase activity [2]) in order to determine the concentration of NO which inhibited oxidase activity. Figure 6B shows that the oxygen concentration decreased as a consequence of oxygen respiration once the [NO] dropped below ∼0.5 μM. This concentration of NO is similar to the concentration of NO that inhibits mitochondrial oxidase activity (3). Furthermore, in E. coli cells lacking the NO-protective flavohemoglobin, Hmp, the half-maximal inhibition of oxygen respiration occurs at around 0.8 μM NO (14).
R. capsulatus is a versatile microorganism, capable of growth under anaerobic and aerobic conditions. Existence on the anaerobic-aerobic interface may depend upon the ability to utilize O2 in the presence of NO, which is most stable under anaerobic conditions. The capacity of cytochrome c′ to prevent accumulation of NO and hence allow oxygen respiration to occur may therefore be central to its physiological and ecological function.
Impact of constant supply of NO on cytochrome c′-dependent NO removal.
In previous experiments addition of NO to cultures was achieved using saturated NO solutions, i.e., the addition of a bolus of NO. To see the effect of a constant supply of NO, SNP was used in the experiment. The accumulation of NO released from SNP was monitored using an isoNO electrode. Figure 7 shows that R. capsulatus which cannot synthesize cytochrome c′ is unable to prevent the accumulation of NO, unlike a wild-type strain of R. capsulatus in which no accumulated NO was observed. It is notable that NO did not accumulate to as great an extent in a suspension of the mutant strain of R. capsulatus as it did in growth medium supplemented with SNP, indicating that mechanisms other than cytochrome c′ contribute to control of NO toxicity.
FIG. 7.
Effect of SNP on NO accumulation. NO accumulation was measured as described in Materials and Methods after the addition of 25 mM SNP to 20-ml suspensions of RCV growth medium-malate containing 1 mg of R. capsulatus PAS100 per ml (broken line), 1 mg of R. capsulatus MC111 per ml (dotted line), or no cells (solid line).
Cytochrome c′ is not involved in denitrification.
As cytochrome c′ was demonstrated to have an effect on the removal of a constant supply of NO (see above) the importance of cytochrome c′ in denitrification was examined. Growth experiments were carried out with denitrifying cultures of R. capsulatus strains BK5DNIT and the cytochrome c′ mutant MC206, as described in Materials and Methods. It was found that there was no significant difference in growth rates between these strains under denitrifying conditions with either nitrate or nitrite as the electron acceptor (data not shown). Cytochrome c′ is therefore not involved in denitrification despite NO being produced by this process. This suggests there is an alternative pathway to remove this NO from denitrifying cultures, most likely NO reductase.
The observation that cytochrome c′ is not required for denitrification was clarified when Western blots of cell extracts from BK5DNIT strains grown under anaerobic, aerobic, and denitrifying conditions were carried out. As shown in Fig. 8, the amount of cytochrome c′ in the sample grown under denitrifying conditions was considerably lower than that for samples grown under the other conditions. It is also worth noting that cytochrome c′ was present at slightly lower amounts in the sample grown aerobically than it was in the anaerobic sample. This is an observation which is in partial agreement with a previous report, which suggested that expression of cytochrome c′ in the closely related organism Rhodobacter sphaeroides is repressed 10-fold in the presence of oxygen (1).
FIG. 8.
Expression of cytochrome c′ under different growth conditions. Suspensions of R. capsulatus BK5DNIT were grown under different conditions, and the cell extract was subjected to SDS-PAGE and Western blotting with antibodies to cytochrome c′. Each lane contains 0.5 μg of total protein. Lane 1, extract from an aerobically grown culture; lane 2, extract from an anaerobically grown culture; lane 3, extract from a denitrifying culture.
Concluding remarks.
The capacity of cytochrome c′ to remove NO is such that it cannot be explained by simple sequestration of the free radical, i.e., the cytochrome acts as an enzyme. The catalytic removal of NO by cytochrome c′ occurs both aerobically and anaerobically. Cytochrome c′ acts independently of molecular oxygen and synthesizes N2O as a product, demonstrating that it is a NO reductase (albeit with limited capacity for NO reduction in the intact cell). This is not the only mechanism for NO toxicity control that occurs in R. capsulatus and it is noteworthy that under denitrifying conditions cytochrome c′ is not required to control the accumulation of NO generated by the cell itself. Rather, cytochrome c′ is a constitutively produced protein (slightly repressed in the presence of oxygen) whose capacity to reduce the toxic effects of NO is associated with externally generated NO.
ACKNOWLEDGMENTS
This work was supported by Biotechnology and Biological Sciences Research Council (BBSRC) grant P08290, awarded to J.W.B.M. and R.K.P.
ADDENDUM IN PROOF
Since submission of this paper, the structure of the NO-bound form of cytochrome c′ from Alcaligenes xylosoxidans has been published (D. M. Lawson, C. E. M. Stevenson, C. R. Andrew, and R. R. Eady, EMBO J. 19:5661–5671, 2000). The structure reveals two possible NO binding positions on the heme, with clear mechanistic implications for the reduction of NO to N2O by cytochrome c′.
REFERENCES
- 1.Bartsch R G, Ambler R P, Meyer T E, Cusanovich M A. Effect of aerobic growth-conditions on the soluble cytochrome content of the purple phototrophic bacterium Rhodobacter sphaeroides: induction of cytochrome c554. Arch Biochem Biophys. 1989;271:433–440. doi: 10.1016/0003-9861(89)90293-2. [DOI] [PubMed] [Google Scholar]
- 2.Bell L C, Richardson D J, Ferguson S J. Identification of nitric oxide reductase activity in Rhodobacter capsulatus: the electron transport pathway can either use or bypass both cytochrome c2 and the cytochrome bc1 complex. J Gen Microbiol. 1992;138:437–443. doi: 10.1099/00221287-138-3-437. [DOI] [PubMed] [Google Scholar]
- 3.Brown G C, Cooper C E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 4.Cross R, Aish J, Paston S J, Poole R K, Moir J W B. Cytochrome c′ from Rhodobacter capsulatus confers increased resistance to nitric oxide. J Bacteriol. 2000;182:1442–1447. doi: 10.1128/jb.182.5.1442-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denariaz G, Ketchum P A, Payne W J, Liu M-Y, LeGall J, Moura I, Moura J J. An unusual hemoprotein capable of reversible binding of nitric oxide from the gram-positive Bacillus halodenitrificans. Arch Microbiol. 1994;162:316–322. [Google Scholar]
- 6.Gardner P R, Gardner A M, Martin L A, Salzman A L. Nitric oxide dioxygenase: an enzymic function for flavohemoglobin. Proc Natl Acad Sci USA. 1998;95:10378–10383. doi: 10.1073/pnas.95.18.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S O, Orii Y, Lloyd D, Hughes M N, Poole R K. Anoxic function of the Escherichia coli flavohaemoglobin (Hmp): reversible binding of nitric oxide and reduction to nitrous oxide. FEBS Lett. 1999;445:389–394. doi: 10.1016/s0014-5793(99)00157-x. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd D, Scott R I. Mass spectrometric monitoring of dissolved gases. In: Poole R K, Dow C S, editors. Microbial gas metabolism. London, United Kingdom: Academic Press; 1985. pp. 239–262. [Google Scholar]
- 9.Lloyd D, Thomas K, Price D, O'Neil B, Oliver K, Williams T N. A membrane-inlet mass spectrometer miniprobe for the direct simultaneous measurement of multiple gas species with spatial resolution of 1 mm. Microbiol Methods. 1996;25:145–151. [Google Scholar]
- 10.Meyer T E, Kamen M D. New perspectives on c-type cytochromes, Adv. Prot Chem. 1982;35:105–212. doi: 10.1016/s0065-3233(08)60469-6. [DOI] [PubMed] [Google Scholar]
- 11.Minning D M, Gow A J, Bonaventura J, Braun R, Dewhirst M, Goldberg D E, Stamler J S. Ascaris haemoglobin is a nitric oxide-activated ‘deoxygenase.’. Nature. 1999;401:497–502. doi: 10.1038/46822. [DOI] [PubMed] [Google Scholar]
- 12.Moir J W B. Cytochrome c′ from Paracoccus denitrificans: spectroscopic studies consistent with a role for the protein in nitric oxide metabolism. Biochim Biophys Acta. 1999;1430:65–72. doi: 10.1016/s0167-4838(98)00276-3. [DOI] [PubMed] [Google Scholar]
- 13.Richardson D J, Bell L C, Moir J W B, Ferguson S J. A denitrifying strain of Rhodobacter capsulatus. FEMS Microbiol Lett. 1994;120:323–328. [Google Scholar]
- 14.Stevanin T M, Ioannidis N, Mills C E, Kim S O, Hughes M N, Poole R K. Flavohemoglobin Hmp affords inducible protection for Escherichia coli respiration, catalysed by cytochromes bo or bd, from nitric oxide. J Biol Chem. 2000;275:35868–35875. doi: 10.1074/jbc.M002471200. [DOI] [PubMed] [Google Scholar]
- 15.Weaver P F, Wall J D, Gest H. Characterisation of Rhodopseudomonas capsulata. Arch Microbiol. 1975;105:207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura T, Suzuki S, Iwasaki H, Takakuwa S. Spectral properties of cytochrome c′ from Rhodopseudomonas capsulata B100 and its CO complex. Biochim Biophys Acta. 1987;144:224–231. doi: 10.1016/s0006-291x(87)80499-0. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura T, Iwasaki H, Shidara S, Suzuki S, Nakahara A, Matsubara T. Nitric oxide complex of cytochrome c′ in cells of denitrifying bacteria. J Biochem. 1988;103:1016–1019. doi: 10.1093/oxfordjournals.jbchem.a122372. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura T, Shidara S, Ozaki T, Kamada H. 5-Coordinated nitrosylhemoprotein in the whole cells of denitrifying bacterium, Achromobacter xylosoxidans NCIB 11015. Arch Microbiol. 1993;160:498–500. [Google Scholar]