This cohort study investigated the association of early (<24 hours) surgical decompression with neurologic and functional outcomes in patients with central cord syndrome.
Key Points
Question
What is the association between early (<24 hours) surgical decompression and neurologic and functional outcomes for patients with central cord syndrome (CCS)?
Findings
This was a secondary analysis of 3 independent prospective multicenter data sets. In this propensity score–matched analysis of 186 patients with CCS, early surgical decompression was associated with significantly improved recovery in upper limb (mean difference [MD] 2.3), but not lower limb (MD, 1.1), motor function, as compared with late (≥24 hours) surgery.
Meaning
Treatment paradigms for patients with severe CCS should be redefined to encompass early surgical decompression as a neuroprotective therapy.
Abstract
Importance
The optimal clinical management of central cord syndrome (CCS) remains unclear; yet this is becoming an increasingly relevant public health problem in the face of an aging population.
Objective
To provide a head-to-head comparison of the neurologic and functional outcomes of early (<24 hours) vs late (≥24 hours) surgical decompression for CCS.
Design, Setting, and Participants
Patients who underwent surgery for CCS (lower extremity motor score [LEMS] − upper extremity motor score [UEMS] ≥ 5) were included in this propensity score–matched cohort study. Data were collected from December 1991 to March 2017, and the analysis was performed from March 2020 to January 2021. This study identified patients with CCS from 3 international multicenter studies with data on the timing of surgical decompression in spinal cord injury. Participants were included if they had a documented baseline neurologic examination performed within 14 days of injury. Participants were eligible if they underwent surgical decompression for CCS.
Exposures
Early surgery was compared with late surgery.
Main Outcomes and Measures
Propensity scores were calculated as the probability of undergoing early compared with late surgery using the logit method and adjusting for relevant confounders. Propensity score matching was performed in a 1:1 ratio by an optimal-matching technique. The primary end point was motor recovery (UEMS, LEMS, American Spinal Injury Association [ASIA] motor score [AMS]) at 1 year. Secondary end points were Functional Independence Measure (FIM) motor score and complete independence in each FIM motor domain at 1 year.
Results
The final study cohort consisted of 186 patients with CCS. The early-surgery group included 93 patients (mean [SD] age, 47.8 [16.8] years; 66 male [71.0%]), and the late-surgery group included 93 patients (mean [SD] age, 48.0 [15.5] years; 75 male [80.6%]). Early surgical decompression resulted in significantly improved recovery in upper limb (mean difference [MD], 2.3; 95% CI, 0-4.5; P = .047), but not lower limb (MD, 1.1; 95% CI, −0.8 to 3.0; P = .30), motor function. In an a priori–planned subgroup analysis, outcomes were comparable with early or late decompressive surgery in patients with ASIA Impairment Scale (AIS) grade D injury. However, in patients with AIS grade C injury, early surgery resulted in significantly greater recovery in overall motor score (MD, 9.5; 95% CI, 0.5-18.4; P = .04), owing to gains in both upper and lower limb motor function.
Conclusions and Relevance
This cohort study found early surgical decompression to be associated with improved recovery in upper limb motor function at 1 year in patients with CCS. Treatment paradigms for CCS should be redefined to encompass early surgical decompression as a neuroprotective therapy.
Introduction
The epidemiology of acute spinal cord injury (SCI) is changing.1,2 With the aging population, the incidence of central cord syndrome (CCS) is increasing, and it is anticipated this will become the most common subcategory of acute traumatic SCI.3,4 The hallmark feature of CCS is disproportionate weakness of the upper compared with the lower limbs.5 This most classically occurs after a hyperextension injury, such as a fall forward, in an older patient with cervical spondylosis.6,7 The identification of treatment strategies that mitigate disability and improve functional status in this vulnerable population is a key public health priority.8
The role of early surgery for CCS remains controversial, with the current evidence comprising underpowered studies.9,10,11,12,13,14 Surgical intervention in the setting of CCS has traditionally been discouraged, owing to fear of causing further iatrogenic injury to the spinal cord and historical reports of an otherwise favorable natural history.15 Nonetheless, many of the original fundamental concepts underpinning CCS have recently been challenged, and there is consequently a strong impetus to revisit treatment paradigms for this condition.16 To this end, using a large prospective multicenter data set and after adjusting for relevant confounders with propensity score matching, we evaluated the efficacy of early surgical decompression, within 24 hours of injury, in the context of CCS. We hypothesized that early surgery would result in improved long-term neurologic and functional recovery compared with late surgery.
Methods
Data Source
This cohort study was a secondary analysis of data derived from a merger of 3 independent large prospective multicenter data sets of patients with SCIs: (1) the North American Clinical Trials Network (NACTN) SCI Registry17; (2) the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS)18; and (3) the National Acute Spinal Cord Injury Study (NASCIS) III19 (eTable 1 and eAppendix in the Supplement). This study underwent institutional review at the University Health Network in Toronto and did not require ethics approval or informed consent from patients as this was a secondary analysis of previously collected data sets. Each of the patients in these data sets were unique, and there were no duplications. This study was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Patient Population
All patients must have had a documented baseline American Spinal Injury Association (ASIA)/International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)20 neurologic examination performed within 14 days of injury. Eligible patients were those who underwent surgical decompression for CCS, defined as a motor incomplete SCI (ASIA Impairment Scale [AIS] grade C or D), with a cervical neurologic level of injury, and a 5 or more point difference between baseline ASIA lower extremity motor score (LEMS) and upper extremity motor score (UEMS) in favor of the lower limbs ([LEMS − UEMS] ≥5). As per ASIA standards, a motor incomplete SCI (AIS grade C or D) is one with (1) preserved motor function in the most caudal sacral segments (ie, voluntary anal contraction) or (2) sensory preservation in the most caudal sacral segments (ie, light touch or pin prick at S4-5 or deep anal pressure) plus preserved motor function more than 3 levels below the motor level on either side of the body. If less than half of the key muscles below the neurologic level of injury have a grade of 3 or more, the injury is graded as an AIS C; if half or more of the key muscles below the neurologic level of injury have a grade of 3 or more, the injury is graded as an AIS D. Notably, varying definitions of CCS have been described in the literature, ranging from any difference between LEMS and UEMS (LEMS > UEMS) to a 10 or more point difference ([LEMS − UEMS] ≥10); we adopted an intermediate definition, which the authors agreed was the most fair and reasonable.5,21 The definition of CCS for this study was made a priori.
Baseline Characteristics
Data pertaining to baseline patient demographics (eg, age, sex), injury characteristics (eg, mechanism of injury, AIS grade, neurologic level, ASIA motor score [AMS] and sensory [light touch and pinprick] scores), and treatment (eg, administration of steroids, time to surgery) were obtained. Data on race and ethnicity were variably collected in the included data sets and hence could not be merged for analysis. Mechanism of injury was categorized as fall, motor vehicle accident, sports accident, or other. Time to surgery was dichotomized as early (<24 hours) or late (≥24 hours) based on the number of hours elapsed from injury to surgical decompression procedure.18
Outcomes
Neurologic recovery was assessed in accordance with ASIA/ISNCSCI standards.20 Functional outcomes were scored by the Functional Independence Measure (FIM).22 The primary end point was motor recovery, as assessed by UEMS, LEMS, and AMS, at 1 year. Secondary end points were FIM motor score and complete independence at 1 year. The latter was assessed individually for each of the FIM motor domains (eating; grooming; bathing; dressing of the upper body; dressing of the lower body; toileting; bladder management; bowel management; bed, chair, and wheelchair transfers; toilet transfers; tub and shower transfers; walking/wheelchair; and stairs) and defined by a score of 7 of 7. Data relating to UEMS, LEMS, AMS, and FIM were collected prospectively as part of the 3 data sets.
Statistical Analysis
All statistical analyses were performed using Stata, version 15 (Stata Corp) and R, version 3.6.0 (R Foundation for Statistical Computing) with an a priori specified significance level of P = .05 (2-tailed). Descriptive statistics were by mean and SD or median and IQR for continuous variables and count and percentage for categorical variables. Assumptions of normal distribution were tested by visual inspection of histograms. The analysis was performed from March 2020 to January 2021.
Propensity Score Matching
Propensity scores were calculated as the probability of undergoing early (<24 hours) compared with late (≥24 hours) surgery using the logit method with data source (categorical), age (continuous), mechanism of injury (categorical), and baseline AMS (continuous), AIS grade (categorical), and neurologic level (continuous) as covariates. These covariates were selected based on hypothesized influence on choice of treatment (ie, early vs late surgical decompression) and also potential impact on neurologic recovery. Age at and measures of severity of injury (ie, AMS, AIS, neurologic level) factor into clinical decision-making in patients with acute SCI, and it is also recognized that both factors have an important effect on neurologic recovery, particularly in the setting of CCS.12,23,24,25,26,27 Similarly, the phenotype of patients with a low- vs high-energy trauma and resultant CCS is different, and the treatment considerations are varied.6,15 Further, we hypothesized that there may be differences between data sources with regard to propensity for early surgical decompression, especially given these data sets spanned multiple decades, and treatment paradigms for acute SCI have evolved over time.8 Propensity score matching was performed in a 1:1 ratio using the optimal-matching technique to minimize the average absolute distance across all matched pairs. This resulted in 2 treatment groups (early surgery vs late surgery) adjusted for the baseline variables specified previously. Baseline characteristics were compared between treatment groups by t test for continuous variables and χ2 test for categorical variables.
Analysis of Outcomes
Missing 1-year outcome scores were first imputed by a last observation carried forward approach, a practice that has been used and validated in prior studies of acute SCI.28,29,30 Thereafter, a multiple imputation procedure with 10 iterations was performed using Markov chain Monte Carlo methods to account for remaining missing outcome data. Such imputation is recommended and has been found to be less susceptible to bias than omitting cases with incomplete data.31,32 Outcomes were compared between treatment groups by linear (for UEMS, LEMS, AMS, FIM motor score) or logistic (for complete independence) regression. Regression models for UEMS, LEMS, and AMS were adjusted for baseline score as a covariate. Effect sizes for each outcome measure were summarized by mean difference (MD) or odds ratio (OR) and associated 95% CIs. Multicollinearity was assessed by variance inflation factor, with a threshold set at 10. The assumption of linearity and outliers were evaluated by visual inspection of a scatterplot of the dependent variable vs the covariate. A preplanned subgroup analysis stratified by AIS grade was performed, owing to our secondary hypothesis that the apparent efficacy of early surgery may be dependent on the severity of injury, with a ceiling effect encountered in patients with AIS grade D injury.
Sample Size/Power Calculation
The reported minimum clinically important difference (MCID) has been estimated to be 5 points for AMS and 2 points for UEMS.33 Using an SD for AMS change of 10.9 points, a sample size of 152 patients (76 in each group) would have 80% power to detect a 5-point difference at a 2-sided α level of .05.
Results
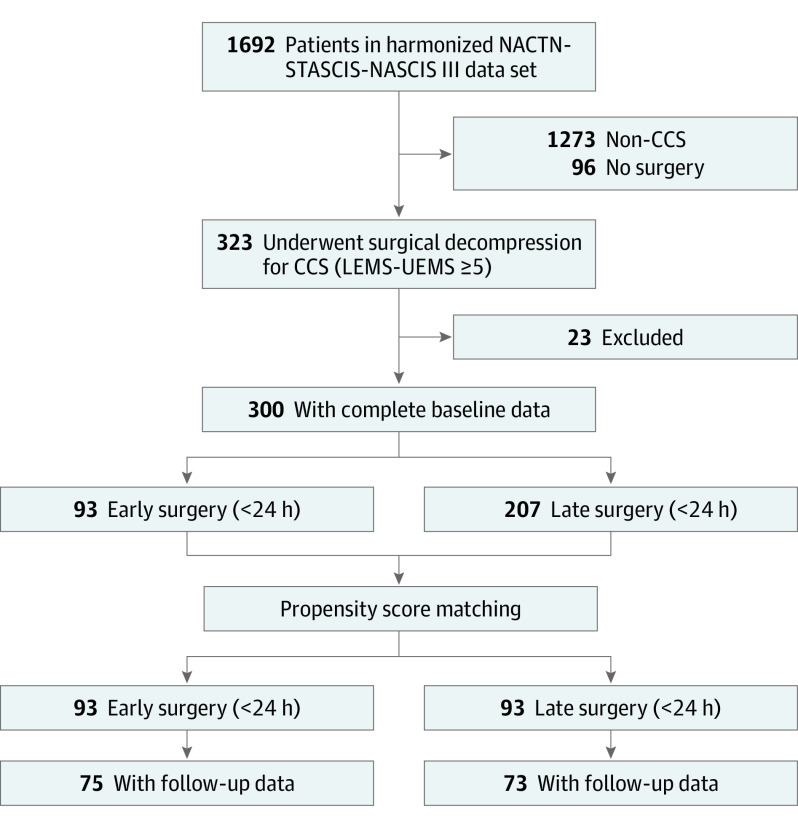
Prospective data from the NACTN, STASCIS, and NASCIS III SCI registries were combined to produce a single harmonized data set of 1692 patients who underwent surgical decompression for SCI. Of these, 300 patients fulfilled eligibility criteria and had complete baseline data available for analysis (eTable 2 in the Supplement). Propensity score matching produced a final study cohort of 186 patients. The early-surgery group included 93 patients (mean [SD] age, 47.8 [16.8] years; 66 male [71.0%]; 27 female [29.0%]), and the late-surgery group included 93 patients (mean [SD] age, 48.0 [15.5] years; 75 male [80.6%]; 18 female [19.4%]). A flowchart of study eligibility appears in Figure 1. Figure 2 presents the distribution of propensity scores before and after matching. Patient demographic, injury, and treatment characteristics were balanced between matched early- and late-surgery groups (Table 1).
Figure 1. Flowchart of Patient Eligibility and Enrollment.
CCS indicates central cord syndrome; LEMS, lower extremity motor score; NACTN, North American Clinical Trials Network; NASCIS, National Acute Spinal Cord Injury Study; STASCIS, Surgical Timing in Acute Spinal Cord Injury Study; UEMS, upper extremity motor score.
Figure 2. Distribution of Propensity Scores .
Distribution of propensity scores among raw-treated (A), match-treated (B), raw-control (C), and match-control (D) study groups with 1:1 optimal matching.
Table 1. Baseline Characteristics in Propensity Score–Matched Cohort (N = 186).
| Variable | No. (%) | P value | |
|---|---|---|---|
| Early surgery (n = 93) | Late surgery (n = 93) | ||
| Age, mean (SD), y | 47.8 (16.8) | 48.0 (15.5) | .94 |
| Sex | |||
| Female | 27 (29.0) | 18 (19.4) | .12 |
| Male | 66 (71.0) | 75 (80.6) | |
| Mechanism of injury | .76 | ||
| Fall | 37 (39.8) | 40 (43.0) | |
| Motor vehicle injury | 41 (44.1) | 43 (46.2) | |
| Sports injury | 6 (6.5) | 4 (4.3) | |
| Other | 9 (9.7) | 6 (6.5) | |
| AIS grade C | 20 (21.5) | 24 (25.8) | .50 |
| Neurologic level, median (IQR) | C5 (C4-C5) | C5 (C4-C5) | .86 |
| UEMS, mean (SD) | 25.2 (12.5) | 25.0 (14.0) | .90 |
| LEMS, mean (SD) | 39.5 (11.2) | 38.1 (12.2) | .40 |
| AMS, mean (SD) | 64.8 (22.2) | 63.0 (25.1) | .62 |
| Light touch score, mean (SD) | 91.9 (24.3) | 90.2 (27.1) | .67 |
| Pin prick score, mean (SD) | 86.1 (28.9) | 86.2 (28.9) | <.99 |
| Administration of steroids | 58 (62.4) | 53 (57.0) | .46 |
Abbreviations: AIS, American Spinal Injury Association Impairment Scale; AMS, American Spinal Injury Association motor score; FIM, functional independence measure; LEMS, lower extremity motor score; UEMS, upper extremity motor score.
Follow-up data were available for 148 patients (80%). Motor outcomes at 1 year by treatment group are presented in Table 2. Early surgery was significantly associated with improved recovery in upper limb (MD, 2.3; 95% CI, 0-4.5; P = .047), but not lower limb (MD, 1.1; 95% CI, −0.8 to 3.0; P = .30), motor function compared with late surgery. However, early surgery was not associated with improved overall motor score (MD, 3.2; 95% CI, −0.2 to 6.6; P = .07).
Table 2. Motor and Functional Recovery at 1 Year.
| Variable | Mean (SE) score | MD (95% CI)a | P value | |
|---|---|---|---|---|
| Early surgery (n = 93) | Late surgery (n = 93) | |||
| Overall cohort | ||||
| UEMS | 43.6 (0.8) | 41.2 (0.9) | 2.3 (0.03 to 4.5) | .047b |
| LEMS | 47.6 (0.7) | 46.2 (0.8) | 1.1 (−0.8 to 3.0) | .26 |
| AMS | 91.2 (1.2) | 87.4 (1.3) | 3.2 (−0.2 to 6.6) | .07 |
| FIM motor score | 82.7 (2.5) | 78.2 (2.3) | 4.4 (−2.1 to 11.0) | .18 |
| AIS grade C | ||||
| UEMS | 38.0 (2.2) | 32.4 (2.1) | 4.8 (−1.3 to 11.0) | .12 |
| LEMS | 45.6 (1.6) | 41.3 (1.5) | 4.2 (0.1 to 8.4) | .046b |
| AMS | 83.5 (3.2) | 73.7 (3.0) | 9.5 (0.5 to 18.4) | .04b |
| FIM motor score | 70.8 (5.5) | 59.6 (5.1) | 11.1 (−4.1 to 26.3) | .15 |
| AIS grade D | ||||
| UEMS | 45.1 (0.9) | 44.3 (1.0) | 1.3 (−1.1 to 3.8) | .28 |
| LEMS | 48.2 (0.8) | 47.9 (0.8) | 0.1 (−2.1 to 2.3) | .91 |
| AMS | 93.3 (1.3) | 92.2 (1.4) | 1.4 (−2.4 to 5.1) | .47 |
| FIM motor score | 85.9 (2.3) | 84.7 (2.4) | 1.2 (−5.4 to 7.9) | .71 |
Abbreviations: AIS, American Spinal Injury Association Impairment Scale; AMS, American Spinal Injury Association motor score; FIM, functional independence measure; LEMS, lower extremity motor score; MD, mean difference; UEMS, upper extremity motor score.
Comparing early surgery with late surgery.
Statistically significant difference.
On subgroup analysis (Table 2), in patients with AIS grade D injury (142 [76.3%]), there was no difference in motor or functional recovery with early vs late surgery. However, in patients with AIS grade C injury (44 [23.7%]), early surgery was associated with significantly greater recovery in overall motor score (MD, 9.5; 95% CI, 0.5-18.4; P = .04), owing to gains in both upper (MD, 4.8; 95% CI, −1.3 to 11.0; P = .10) and lower (MD, 4.2; 95% CI, 0.1-8.4; P = .046) limb motor function. No significant difference was observed in FIM motor score at 1 year (MD, 11.1; 95% CI, −4.1 to 26.3; P = .10).
A higher proportion of patients in the early-surgery group appeared to achieve complete independence in various functional activities, particularly those involving upper limb function; however, none of these associations reached statistical significance, and there was no difference in 1-year FIM motor score (MD, 4.4; 95% CI, −2.1 to 11.0; P = .20) (Tables 2 and 3).
Table 3. Complete Independence in Functional Activities at 1 Year.
| FIM motor domain | Complete independence, No. (%) | OR (95% CI)a | P value | |
|---|---|---|---|---|
| Early surgery (n = 93) | Late surgery (n = 93) | |||
| Eating | 74 (79.4) | 63 (67.5) | 1.9 (0.9-3.9) | .10 |
| Grooming | 70 (75.3) | 61 (65.4) | 1.6 (0.8-3.3) | .19 |
| Bathing | 65 (70.0) | 56 (59.7) | 1.6 (0.8-3.1) | .19 |
| Dressing, upper body | 66 (71.1) | 57 (61.6) | 1.5 (0.8-3.0) | .21 |
| Dressing, lower body | 61 (66.1) | 53 (57.5) | 1.4 (0.8-2.8) | .27 |
| Toileting | 67 (72.3) | 62 (66.5) | 1.3 (0.6-2.7) | .46 |
| Bladder management | 73 (78.4) | 67 (71.7) | 1.4 (0.6-3.2) | .37 |
| Bowel management | 71 (76.8) | 69 (73.9) | 1.2 (0.5-2.8) | .72 |
| Bed, chair, wheelchair transfers | 72 (77.6) | 65 (70.0) | 1.5 (0.7-3.1) | .28 |
| Toilet transfers | 68 (73.2) | 62 (67.0) | 1.4 (0.6-2.9) | .44 |
| Tub, shower transfers | 65 (70.3) | 60 (64.2) | 1.3 (0.7-2.7) | .43 |
| Walking, wheelchair | 63 (67.4) | 61 (65.2) | 1.1 (0.5-2.3) | .78 |
| Stairs | 49 (52.5) | 42 (45.2) | 1.3 (0.7-2.7) | .41 |
Abbreviations: FIM, functional independence measure; OR, odds ratio.
Comparing early surgery with late surgery.
Discussion
The biological rationale for early surgical decompression after acute SCI comes from the mitigation of secondary injury.34,35,36,37 In fact, there is robust basic science data to indicate that surgical decompression of the spinal cord after SCI may attenuate secondary injury and improve neurologic outcomes and that this effect is inversely related to time (ie, duration of compression).38,39,40,41,42 This concept has also borne out in clinical studies, although the quality of the evidence is variable.8,18,43 Weighing the available evidence, the recent AO Spine clinical practice guidelines for acute SCI offer a weak recommendation: “We suggest that early surgery be offered as an option for adult acute SCI patients regardless of level.”44(p196S)
Focusing on CCS in particular, the systematic review informing the AO Spine guidelines identified 1 study meeting eligibility criteria that examined this specific subset of patients.43 Lenehan et al10 reported superior improvements in AMS and FIM at 1 year in 17 patients with CCS who underwent early surgery (<24 hours) compared with 56 patients who received late surgery (≥24 hours). Other studies that have indirectly examined this question have failed to demonstrate a beneficial effect of early surgical decompression.11,12,13,45 The AO Spine guidelines hence provide a weak recommendation: “We suggest that early surgery be considered as a treatment option in adult patients with traumatic central cord syndrome.”44(p196S) Notably, a more recent small exploratory randomized trial comparing early with delayed surgery in 70 patients with motor incomplete cervical SCI with canal stenosis noted similar motor recovery at 1 year after injury but accelerated recovery within the first 6 months in the early surgery group.12 The current study complements and extends these results. Our results suggest a benefit of early surgical intervention for patients with AIS grade C CCS. In contrast, the benefit of surgical intervention within 24 hours for patients with less severe (AIS D) CCS is less clear. The latter finding may reflect the insensitivity and a ceiling effect of the ASIA scoring system.
Although prior reports chronicled a favorable natural history, it is increasingly recognized that many patients with CCS have significant residual impairment, particularly in hand function.12 The fact that early surgery was associated with superior recovery in upper limb motor scores in the overall cohort of patients included in this study is therefore a highly relevant finding. In parallel with this, we observed trends toward better functional independence in activities involving upper limb use (eg, eating, grooming, bathing, dressing) with early surgery, although this was not statistically significant. The lack of statistical significance here may reflect a power issue, or alternatively, with the magnitude of the effect size for recovery in UEMS being relatively small (2.3 points), it is possible that the additional motor recovery provided by early surgery was not large enough to produce a corresponding significant change in functional abilities. However, at the same time, patients with tetraplegia rate recovery in upper limb motor function as their top priority,46,47 and even small sensorimotor gains here may translate into improved function and quality of life.47,48 In fact, the reported MCID for UEMS in patients with cervical AIS C or D injury is 2 points.33 A challenge is that current outcome measures for acute SCI demonstrate a strong ceiling effect in patients with AIS grade D injury, which comprised the majority of the current cohort.49 Accordingly, when patients were stratified by AIS grade, we observed only a marginal, nonsignificant association (1.4 points for AMS; 1.2 points for FIM motor score) for early surgery for all outcomes examined for AIS D injuries. By contrast, effect sizes were relatively sizeable in patients with AIS grade C injury (9.5 points for AMS; 11.1 points for FIM motor score). However, because of the small sample size of patients with AIS grade C injury (n = 44), power was limited, and the CIs around these treatment effects were wide; the difference in FIM motor subscore was hence not statistically significant, despite the large magnitude. We observed an analogous outcome with regard to recovery in lower limb motor scores. That is, in the overall cohort, early surgery did not have a significant association with recovery in LEMS, likely because most patients had relatively preserved lower limb strength at baseline by virtue of experiencing CCS. When this ceiling effect was removed, in patients with AIS grade C injury, who did have significant lower limb motor deficits, we observed a significant association with early surgery. What we can reasonably infer from, then, is that early surgery was associated with an improvement in upper limb motor recovery; however, the magnitude of this association may be small, and there may not be a corresponding change in functional capacity. However, if we consider patients with AIS grade C injury specifically, early surgery may have an association with overall motor recovery.
Of note, early surgical decompression in patients with CCS may not always be feasible. In general, this is an older patient population often with medical comorbidities, and hence, the acute medical condition of the patient may preclude early surgery or necessitate some degree of medical optimization. In addition, there may be delays in recognition and diagnosis of CCS, which are often attributable to lower energy mechanisms that do not cause vertebral fractures that may be found by computed tomography (CT) scan and radiography. Further, there is literature to suggest that older age in patients with CCS may be associated with poorer neurologic and functional outcomes.26,50,51,52,53 Recently, there has been increasing interest in the effect of frailty, conceptualized as a measure of physiological reserve; increasing frailty has been linked with greater morbidity and mortality in patients with acute SCI.54,55 Our results taken in the context of the literature suggest that early surgical decompression may be associated with improved neurologic recovery in the setting of CCS, all things being equal. However, the practitioner must exercise sound clinical judgment and tailor the decision-making surrounding surgical intervention and its timing to the individual patient and clinical scenario, accounting for the important potential effects of age and frailty.
This study used a threshold of 24 hours to define early surgery after SCI because this is the most consistent and best-studied time window used in the literature.56 However, the concept of time is spine, or earlier is better, is increasingly recognized.8,56,57 Biologically, one would not expect a binary effect of time on recovery based on a 24-hour cutoff, but rather a continuous one. Indeed, recent literature has validated this concept, with a large-scale pooled analysis demonstrating progressively improved neurologic recovery with progressively shorter time to decompression after acute SCI.58 Other smaller studies, similarly, have shown superior recovery using shorter time thresholds, such as 8 or 12 hours after injury.59,60,61,62 Hence, although statistical power limitations precluded a more granular examination of the continuous effect of time in this and other studies examining CCS specifically, considering the literature relating to acute SCI more broadly, it should be recognized that any potential benefit to early surgical decompression may be greater with time thresholds even shorter than 24 hours.
Limitations
This study had notable limitations. First, despite the relatively large overall sample size, this study was underpowered in subgroup analyses. Indeed, sample size constraints have been the greatest barrier to the close study of CCS. Second, data for the current study were derived from 3 different sources. Although common data elements were harmonized and analytic methods uniformly applied, some of the variability within our cohort could be attributable to between-study factors. Further, there is the potential for within-institution and within-clinician correlations in outcomes that could not be accounted for in this study design. One must also account for the uncertainty introduced in outcome assessment methods and the statistical modeling process in propensity score matching and imputation of missing data. Third, an objective and agreed upon definition of CCS is lacking, and diagnosis of this clinical syndrome has traditionally relied on physician impression. One proposed criterion has been a 10 or more point difference between upper and lower extremity motor scores,5 although many authors continue to consider a difference of any magnitude to be diagnostic of CCS.10,12,21,53 Here, we used a 5 or more point difference between UEMS and LEMS as a diagnostic criterion, which represents a compromise between the 2 previously mentioned conflicting definitions. It was the opinion of the authors that this definition may be most clinically relevant. Nonetheless, it should be recognized that the findings of the present study may therefore be generalizable only to patients meeting this criterion. Finally, although we did examine neurologic and functional recovery using validated measures, we could not examine hand dexterity or pain outcomes, which are important considerations in this population of patients.12
Conclusions
In this cohort study of patients with CCS, results suggest that early surgical decompression, within 24 hours of injury, was associated with improved recovery in upper limb motor function at 1 year. In patients with AIS grade C injury, early surgery was associated with a parallel improvement in lower extremity motor function, resulting in superior overall motor score. Improvements in functional abilities in the latter group did not reach statistical significance. Treatment paradigms and clinical care pathways for CCS may need to be redefined to encompass early surgical decompression as a neuroprotective therapy.
eTable 1. Summary of Data sets
eAppendix. Description of Each Data set
eTable 2. Baseline, Treatment, and Follow-up Variables Included in Combined Data set
eReferences
References
- 1.Badhiwala JH, Wilson JR, Fehlings MG. Global burden of traumatic brain and spinal cord injury. Lancet Neurol. 2019;18(1):24-25. doi: 10.1016/S1474-4422(18)30444-7 [DOI] [PubMed] [Google Scholar]
- 2.Jain NB, Ayers GD, Peterson EN, et al. Traumatic spinal cord injury in the US, 1993-2012. JAMA. 2015;313(22):2236-2243. doi: 10.1001/jama.2015.6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson C, Mutch J, Parent S, Mac-Thiong JM. The changing demographics of traumatic spinal cord injury: an 11-year study of 831 patients. J Spinal Cord Med. 2015;38(2):214-223. doi: 10.1179/2045772314Y.0000000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehlings MG, Tetreault L, Nater A, et al. The aging of the global population: the changing epidemiology of disease and spinal disorders. Neurosurgery. 2015;77(suppl 4):S1-S5. doi: 10.1227/NEU.0000000000000953 [DOI] [PubMed] [Google Scholar]
- 5.Pouw MH, van Middendorp JJ, van Kampen A, et al. ; EM-SCI study group . Diagnostic criteria of traumatic central cord syndrome. part 1: a systematic review of clinical descriptors and scores. Spinal Cord. 2010;48(9):652-656. doi: 10.1038/sc.2009.155 [DOI] [PubMed] [Google Scholar]
- 6.Harrop JS, Sharan A, Ratliff J. Central cord injury: pathophysiology, management, and outcomes. Spine J. 2006;6(6)(suppl):198S-206S. doi: 10.1016/j.spinee.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 7.Schneider RC, Crosby EC, Russo RH, Gosch HH. Chapter 32: traumatic spinal cord syndromes and their management. Clin Neurosurg. 1973;20:424-492. doi: 10.1093/neurosurgery/20.CN_suppl_1.424 [DOI] [PubMed] [Google Scholar]
- 8.Badhiwala JH, Ahuja CS, Fehlings MG. Time is spine: a review of translational advances in spinal cord injury. J Neurosurg Spine. 2018;30(1):1-18. doi: 10.3171/2018.9.SPINE18682 [DOI] [PubMed] [Google Scholar]
- 9.Guest J, Eleraky MA, Apostolides PJ, Dickman CA, Sonntag VK. Traumatic central cord syndrome: results of surgical management. J Neurosurg. 2002;97(1)(suppl):25-32. doi: 10.3171/spi.2002.97.1.0025 [DOI] [PubMed] [Google Scholar]
- 10.Lenehan B, Fisher CG, Vaccaro A, Fehlings M, Aarabi B, Dvorak MF. The urgency of surgical decompression in acute central cord injuries with spondylosis and without instability. Spine (Phila Pa 1976). 2010;35(21)(suppl):S180-S186. doi: 10.1097/BRS.0b013e3181f32a44 [DOI] [PubMed] [Google Scholar]
- 11.Stevens EA, Marsh R, Wilson JA, Sweasey TA, Branch CL Jr, Powers AK. A review of surgical intervention in the setting of traumatic central cord syndrome. Spine J. 2010;10(10):874-880. doi: 10.1016/j.spinee.2010.07.388 [DOI] [PubMed] [Google Scholar]
- 12.Aarabi B, Alexander M, Mirvis SE, et al. Predictors of outcome in acute traumatic central cord syndrome due to spinal stenosis. J Neurosurg Spine. 2011;14(1):122-130. doi: 10.3171/2010.9.SPINE09922 [DOI] [PubMed] [Google Scholar]
- 13.Anderson DG, Sayadipour A, Limthongkul W, Martin ND, Vaccaro A, Harrop JS. Traumatic central cord syndrome: neurologic recovery after surgical management. Am J Orthop (Belle Mead NJ). 2012;41(8):E104-E108. [PubMed] [Google Scholar]
- 14.Chikuda H, Koyama Y, Matsubayashi Y, et al. ; OSCIS investigators . Effect of early vs delayed surgical treatment on motor recovery in incomplete cervical spinal cord injury with preexisting cervical stenosis: a randomized clinical trial. JAMA Netw Open. 2021;4(11):e2133604. doi: 10.1001/jamanetworkopen.2021.33604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider RC, Cherry G, Pantek H. The syndrome of acute central cervical spinal cord injury; with special reference to the mechanisms involved in hyperextension injuries of cervical spine. J Neurosurg. 1954;11(6):546-577. doi: 10.3171/jns.1954.11.6.0546 [DOI] [PubMed] [Google Scholar]
- 16.Badhiwala JH, Wilson JR, Fehlings MG. The case for revisiting central cord syndrome. Spinal Cord. 2020;58(1):125-127. doi: 10.1038/s41393-019-0354-5 [DOI] [PubMed] [Google Scholar]
- 17.Grossman RG, Toups EG, Frankowski RF, Burau KD, Howley S. North American Clinical Trials Network for the treatment of spinal cord injury: goals and progress. J Neurosurg Spine. 2012;17(1)(suppl):6-10. doi: 10.3171/2012.4.AOSPINE1294 [DOI] [PubMed] [Google Scholar]
- 18.Fehlings MG, Vaccaro A, Wilson JR, et al. Early vs delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One. 2012;7(2):e32037. doi: 10.1371/journal.pone.0032037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury: results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277(20):1597-1604. doi: 10.1001/jama.1997.03540440031029 [DOI] [PubMed] [Google Scholar]
- 20.Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34(6):535-546. doi: 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Middendorp JJ, Pouw MH, Hayes KC, et al. ; EM-SCI Study Group Collaborators . Diagnostic criteria of traumatic central cord syndrome. part 2: a questionnaire survey among spine specialists. Spinal Cord. 2010;48(9):657-663. doi: 10.1038/sc.2010.72 [DOI] [PubMed] [Google Scholar]
- 22.Kidd D, Stewart G, Baldry J, et al. The Functional Independence Measure: a comparative validity and reliability study. Disabil Rehabil. 1995;17(1):10-14. doi: 10.3109/09638289509166622 [DOI] [PubMed] [Google Scholar]
- 23.Dahdaleh NS, Lawton CD, El Ahmadieh TY, et al. Evidence-based management of central cord syndrome. Neurosurg Focus. 2013;35(1):E6. doi: 10.3171/2013.3.FOCUS13101 [DOI] [PubMed] [Google Scholar]
- 24.Penrod LE, Hegde SK, Ditunno JF Jr. Age effect on prognosis for functional recovery in acute, traumatic central cord syndrome. Arch Phys Med Rehabil. 1990;71(12):963-968. [PubMed] [Google Scholar]
- 25.Roth EJ, Lawler MH, Yarkony GM. Traumatic central cord syndrome: clinical features and functional outcomes. Arch Phys Med Rehabil. 1990;71(1):18-23. [PubMed] [Google Scholar]
- 26.Dvorak MF, Fisher CG, Hoekema J, et al. Factors predicting motor recovery and functional outcome after traumatic central cord syndrome: a long-term follow-up. Spine (Phila Pa 1976). 2005;30(20):2303-2311. doi: 10.1097/01.brs.0000182304.35949.11 [DOI] [PubMed] [Google Scholar]
- 27.Pouw MH, van Middendorp JJ, van Kampen A, Curt A, van de Meent H, Hosman AJ. Diagnostic criteria of traumatic central cord syndrome. part 3: descriptive analyses of neurological and functional outcomes in a prospective cohort of traumatic motor incomplete tetraplegics. Spinal Cord. 2011;49(5):614-622. doi: 10.1038/sc.2010.171 [DOI] [PubMed] [Google Scholar]
- 28.van Middendorp JJ, Hosman AJ, Donders AR, et al. ; EM-SCI Study Group . A clinical prediction rule for ambulation outcomes after traumatic spinal cord injury: a longitudinal cohort study. Lancet. 2011;377(9770):1004-1010. doi: 10.1016/S0140-6736(10)62276-3 [DOI] [PubMed] [Google Scholar]
- 29.van Middendorp JJ, Hosman AJ, Pouw MH, Van de Meent H; EM-SCI Study Group . Is determination between complete and incomplete traumatic spinal cord injury clinically relevant? validation of the ASIA sacral sparing criteria in a prospective cohort of 432 patients. Spinal Cord. 2009;47(11):809-816. doi: 10.1038/sc.2009.44 [DOI] [PubMed] [Google Scholar]
- 30.Wilson JR, Grossman RG, Frankowski RF, et al. A clinical prediction model for long-term functional outcome after traumatic spinal cord injury based on acute clinical and imaging factors. J Neurotrauma. 2012;29(13):2263-2271. doi: 10.1089/neu.2012.2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrell FE. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression and Survival Analysis. Springer-Verlag; 2001. [Google Scholar]
- 32.Little RJA. Regression with missing X’s: a review. J Am Stat Assoc. 1992;87(420):1227-1237. doi: 10.1080/01621459.1992.10476282 [DOI] [Google Scholar]
- 33.Scivoletto G, Tamburella F, Laurenza L, Molinari M. Distribution-based estimates of clinically significant changes in the International Standards for Neurological Classification of Spinal Cord Injury motor and sensory scores. Eur J Phys Rehabil Med. 2013;49(3):373-384. [PubMed] [Google Scholar]
- 34.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75(1):15-26. doi: 10.3171/jns.1991.75.1.0015 [DOI] [PubMed] [Google Scholar]
- 35.Tator CH. Review of experimental spinal cord injury with emphasis on the local and systemic circulatory effects. Neurochirurgie. 1991;37(5):291-302. [PubMed] [Google Scholar]
- 36.Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995;5(4):407-413. doi: 10.1111/j.1750-3639.1995.tb00619.x [DOI] [PubMed] [Google Scholar]
- 37.Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. doi: 10.1038/nrdp.2017.18 [DOI] [PubMed] [Google Scholar]
- 38.Dimar JR II, Glassman SD, Raque GH, Zhang YP, Shields CB. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine (Phila Pa 1976). 1999;24(16):1623-1633. doi: 10.1097/00007632-199908150-00002 [DOI] [PubMed] [Google Scholar]
- 39.Carlson GD, Gorden CD, Oliff HS, Pillai JJ, LaManna JC. Sustained spinal cord compression: part I: time-dependent effect on long-term pathophysiology. J Bone Joint Surg Am. 2003;85(1):86-94. doi: 10.2106/00004623-200301000-00014 [DOI] [PubMed] [Google Scholar]
- 40.Furlan JC, Noonan V, Cadotte DW, Fehlings MG. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of preclinical and clinical studies. J Neurotrauma. 2011;28(8):1371-1399. doi: 10.1089/neu.2009.1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guha A, Tator CH, Endrenyi L, Piper I. Decompression of the spinal cord improves recovery after acute experimental spinal cord compression injury. Paraplegia. 1987;25(4):324-339. [DOI] [PubMed] [Google Scholar]
- 42.Batchelor PE, Wills TE, Skeers P, et al. Meta-analysis of preclinical studies of early decompression in acute spinal cord injury: a battle of time and pressure. PLoS One. 2013;8(8):e72659. doi: 10.1371/journal.pone.0072659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson JR, Tetreault LA, Kwon BK, et al. Timing of decompression in patients with acute spinal cord injury: a systematic review. Global Spine J. 2017;7(3)(suppl):95S-115S. doi: 10.1177/2192568217701716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fehlings MG, Tetreault LA, Wilson JR, et al. A clinical practice guideline for the management of patients with acute spinal cord injury and central cord syndrome: recommendations on the timing (≤24 hours vs >24 hours) of decompressive surgery. Global Spine J. 2017;7(3)(suppl):195S-202S. doi: 10.1177/2192568217706367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aarabi B, Akhtar-Danesh N, Simard JM, et al. Efficacy of early (≤ 24 hours), late (25-72 hours), and delayed (>72 hours) surgery with magnetic resonance imaging–confirmed decompression in American Spinal Injury Association Impairment Scale grades C and D acute traumatic central cord syndrome caused by spinal stenosis. J Neurotrauma. 2021;38(15):2073-2083. doi: 10.1089/neu.2021.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371-1383. doi: 10.1089/neu.2004.21.1371 [DOI] [PubMed] [Google Scholar]
- 47.Jones LAT, Bryden A, Wheeler TL, et al. Considerations and recommendations for selection and utilization of upper extremity clinical outcome assessments in human spinal cord injury trials. Spinal Cord. 2018;56(5):414-425. doi: 10.1038/s41393-017-0015-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Consortium for Spinal Cord Medicine . Outcomes following traumatic spinal cord injury: clinical practice guidelines for health care professionals. J Spinal Cord Med. 2000;23(4):289-316. doi: 10.1080/10790268.2000.11753539 [DOI] [PubMed] [Google Scholar]
- 49.Marino RJ, Ditunno JF Jr, Donovan WH, Maynard F Jr. Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch Phys Med Rehabil. 1999;80(11):1391-1396. doi: 10.1016/S0003-9993(99)90249-6 [DOI] [PubMed] [Google Scholar]
- 50.Aito S, D’Andrea M, Werhagen L, et al. Neurological and functional outcome in traumatic central cord syndrome. Spinal Cord. 2007;45(4):292-297. doi: 10.1038/sj.sc.3101944 [DOI] [PubMed] [Google Scholar]
- 51.Furusawa K, Tokuhiro A, Ikeda A, et al. Effect of age on bowel management in traumatic central cord syndrome. Spinal Cord. 2012;50(1):51-56. doi: 10.1038/sc.2011.90 [DOI] [PubMed] [Google Scholar]
- 52.Newey ML, Sen PK, Fraser RD. The long-term outcome after central cord syndrome: a study of the natural history. J Bone Joint Surg Br. 2000;82(6):851-855. doi: 10.1302/0301-620X.82B6.0820851 [DOI] [PubMed] [Google Scholar]
- 53.Lenehan B, Street J, O’Toole P, Siddiqui A, Poynton A. Central cord syndrome in Ireland: the effect of age on clinical outcome. Eur Spine J. 2009;18(10):1458-1463. doi: 10.1007/s00586-009-1107-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banaszek D, Inglis T, Marion TE, et al. Effect of frailty on outcome after traumatic spinal cord injury. J Neurotrauma. 2020;37(6):839-845. doi: 10.1089/neu.2019.6581 [DOI] [PubMed] [Google Scholar]
- 55.Elsamadicy AA, Sandhu MRS, Freedman IG, et al. Impact of frailty on morbidity and mortality in adult patients presenting with an acute traumatic cervical spinal cord injury. World Neurosurg. 2021;153:e408-e418. doi: 10.1016/j.wneu.2021.06.130 [DOI] [PubMed] [Google Scholar]
- 56.Wilson JR, Witiw CD, Badhiwala J, Kwon BK, Fehlings MG, Harrop JS. Early surgery for traumatic spinal cord injury: where are we now? Global Spine J. 2020;10(1)(suppl):84S-91S. doi: 10.1177/2192568219877860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahuja CS, Badhiwala JH, Fehlings MG. “Time is spine”: the importance of early intervention for traumatic spinal cord injury. Spinal Cord. 2020;58(9):1037-1039. doi: 10.1038/s41393-020-0477-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Badhiwala JH, Wilson JR, Witiw CD, et al. The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol. 2021;20(2):117-126. doi: 10.1016/S1474-4422(20)30406-3 [DOI] [PubMed] [Google Scholar]
- 59.Jug M, Kejžar N, Vesel M, et al. Neurological recovery after traumatic cervical spinal cord injury is superior if surgical decompression and instrumented fusion are performed within 8 hours vs 8 to 24 hours after injury: a single center experience. J Neurotrauma. 2015;32(18):1385-1392. doi: 10.1089/neu.2014.3767 [DOI] [PubMed] [Google Scholar]
- 60.Grassner L, Wutte C, Klein B, et al. Early decompression (< 8 h) after traumatic cervical spinal cord injury improves functional outcome as assessed by spinal cord independence measure after 1 year. J Neurotrauma. 2016;33(18):1658-1666. doi: 10.1089/neu.2015.4325 [DOI] [PubMed] [Google Scholar]
- 61.Burke JF, Yue JK, Ngwenya LB, et al. Ultra-early (<12 hours) surgery correlates with higher rate of American Spinal Injury Association Impairment Scale conversion after cervical spinal cord injury. Neurosurgery. 2019;85(2):199-203. doi: 10.1093/neuros/nyy537 [DOI] [PubMed] [Google Scholar]
- 62.Jug M, Kejžar N, Cimerman M, Bajrović FF. Window of opportunity for surgical decompression in patients with acute traumatic cervical spinal cord injury. J Neurosurg Spine. Published online December 27, 2019. doi: 10.3171/2019.10.SPINE19888 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Summary of Data sets
eAppendix. Description of Each Data set
eTable 2. Baseline, Treatment, and Follow-up Variables Included in Combined Data set
eReferences