This cohort study examines data from a pharmacoepidemiologic database of US medical claims for patients who filled a folic acid prescription to investigate an association with risk of suicide attempt.
Key Points
Question
Is folic acid associated with decreased suicide attempts and intentional self-harm?
Findings
In this cohort study, a within-person pharmacoepidemiologic study that included 866 586 adults, folic acid treatment was associated with a significantly reduced rate of suicidal events. This large-scale observational study confirmed results of an earlier signal-generation study.
Meaning
Folic acid may be an inexpensive and widely available suicide prevention tool; a large-scale randomized clinical trial is warranted.
Abstract
Importance
Suicide is a leading cause of death in the United States, having increased more than 30% from 2000 to 2018. An inexpensive, safe, widely available treatment for preventing suicidal behavior could reverse this trend.
Objective
To confirm a previous signal for decreased risk of suicide attempt following prescription fills for folic acid in a national pharmacoepidemiologic study of patients treated with folic acid.
Design, Setting, and Participants
A within-person exposure-only cohort design was used to study the dynamic association between folic acid (vitamin B9) prescription fills over a 24-month period and suicide attempts and intentional self-harm. Data were collected from a pharmacoepidemiologic database of US medical claims (MarketScan) for patients with private health insurance who filled a folic acid prescription between 2012 and 2017. The same analysis was repeated with a control supplement (cyanocobalamin, vitamin B12). Data were analyzed from August 2021 to June 2022.
Exposure
Folic acid prescription fills.
Main Outcome and Measure
Suicide attempt or intentional self-harm resulting in an outpatient visit or inpatient admission as identified by codes from the International Statistical Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification.
Results
Data on 866 586 patients were collected; 704 514 (81.30%) were female, and 90 296 (10.42%) were 60 years and older. Overall, there were 261 suicidal events during months covered by a folic acid prescription (5 521 597 person-months) for a rate of 4.73 per 100 000 person-months, compared with 895 suicidal events during months without folic acid (8 432 340) for a rate of 10.61 per 100 000 person-months. Adjusting for age and sex, diagnoses related to suicidal behavior, diagnoses related to folic acid deficiency, folate-reducing medications, history of folate-reducing medications, and history of suicidal events, the hazard ratio (HR) for folic acid for suicide events was 0.56 (95% CI, 0.48-0.65), with similar results for the modal dosage of 1 mg of folic acid per day (HR, 0.57; 95% CI, 0.48-0.69) and women of childbearing age (HR, 0.60; 95% CI, 0.50-0.73). A duration-response analysis (1-mg dosage) revealed a 5% decrease in suicidal events per month of additional treatment (HR, 0.95; 95% CI, 0.93-0.97). The same analysis for the negative control, cyanocobalamin, found no association with suicide attempt (HR, 1.01; 95% CI, 0.80-1.27).
Conclusions and Relevance
This large-scale pharmacoepidemiologic study of folic acid found a beneficial association in terms of lower rates of suicide attempts. The results warrant the conduct of a randomized clinical trial with suicidal ideation and behavior as outcomes of interest. If confirmed, folic acid may be a safe, inexpensive, and widely available treatment for suicidal ideation and behavior.
Introduction
We developed a novel statistical drug safety signal-generation algorithm known as iDEAS (High Dimensional Empirical Bayes Screening) and illustrated its use by examining the association of suicide attempt with all 922 drugs on the market that had 3000 or more prescriptions in 2014.1 We found 10 drugs associated with greater suicide attempt risk and 44 drugs associated with decreased suicide attempt risk. The strongest associations with increased risk were for alprazolam, butalbital, hydrocodone, and the combination codeine/promethazine. The strongest associations with decreased risk were for folic acid, mirtazapine, hydroxyzine, disulfiram, and naltrexone.
The decreased risk of suicidal events in patients taking folic acid was not predicted.1 Although many people who take folic acid purchase it over the counter (OTC), the population receiving it via prescription may be different. In the original article,1 we found that 52% of patients receiving prescriptions for folic acid had a diagnosis of pain, 16% had a mood disorder diagnosis, 31% filled a prescription for methotrexate, and approximately 60% received anti-inflammatories or analgesics. Only 8% received antidepressants. We noted that methotrexate is commonly prescribed for rheumatoid arthritis pain, and methotrexate depletes folate,2 so folic acid is often prescribed to prevent folic acid deficiency. We hypothesized that low folate levels produced by methotrexate may increase suicide risk, which is then decreased after folic acid supplementation. In addition, prednisone (21%) and hydrocodone (20%) were also commonly prescribed in the year before a folic acid prescription is filled. Both drugs were associated with higher risk of suicide attempts, and we hypothesized that folic acid may reverse this increased risk.
In terms of mechanism, folate deficiency predicts poorer clinical response to selective serotonin reuptake inhibitors,3 and folate may enhance effects of antidepressants acting via monoamine neurotransmitter systems by its involvement in methylation pathways in the 1-carbon cycle.4 Folate levels are reportedly low in blood and red cells in future suicide decedents5 but not in cerebrospinal fluid.6 Improvement with folinic acid treatment in treatment-resistant depression was associated with cerebrospinal fluid evidence of brain folate deficiency.7 More broadly, folate is a member of the vitamin B family and prevents neural tube and heart defects in the fetus during pregnancy8 and may prevent strokes9 and reduce age-related hearing loss in adults.10 It is essential for neurogenesis, nucleotide synthesis, and methylation of homocysteine.
We previously proposed that signals related to individual drugs identified by iDEAS should be tested using more rigorous pharmacoepidemiologic studies and, if confirmed, further studied in randomized clinical trials (RCTs). Given the possibility of suicide prevention properties of folic acid, and its potential as a new, safe, and inexpensive preventive, we further explored this association in a well-controlled large-scale pharmacoepidemiologic study using cyanocobalamin (vitamin B12) as a negative control supplement. A meta-analysis of 35 prospective studies including more than 14 000 adults did not find an association between vitamin B12 and cognitive impairment or dementia,11 and our iDEAS study did not detect an association with suicidal events.1
Methods
Patients
This study was reviewed and deemed exempt from review by the University of Chicago institutional review board. Data were obtained from the MarketScan Commercial Claims and Encounters databases12 distributed by IBM Watson and included inpatient, outpatient, and prescription claims from more than 100 insurers in the United States (164 million unique enrollee observations between 2005 and 2017). Codes from the International Statistical Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9-CM and ICD-10-CM), were used to identify suicide attempt and intentional self-harm (including deaths by suicide after a medical claim), as well as other diagnoses relevant to suicide risk or folate deficiency, from service claims (eTable in the Supplement). Diagnoses relevant to suicide risk included depression, anxiety, attention-deficit/hyperactivity disorder, bipolar disorder, schizophrenia, sleep disorders, and pain.13,14
The data were extracted for the period of 2010 to 2018. The cohort was restricted to patients filling a folic acid prescription in 2012 to 2017, and the first folic acid prescription was considered the index date. The 2018 data were not used in defining the cohort so that all patients would have at least 1 year of follow-up. The sample was restricted to adults 18 years and older. The baseline period data (2 years before the index date) were used to identify folate-reducing medications (Table 115,16,17) and identify previous users of folic acid, which were used to define an incident-user cohort. Patients with private health insurance who filled a folic acid prescription between 2012 and 2017 were followed up until disenrollment (including death) or suicide attempt or intentional self-harm. The entire process (including identification of the index prescription) was repeated for the negative control, cyanocobalamin. Data were analyzed from August 2021 to June 2022.
Table 1. Indications for Folate Prescribing and Folate Drug Interactions.
| Medication group | Generic name | Folic acid indication |
|---|---|---|
| Antimetabolite | Methotrexate | Rheumatoid arthritis,15,16 psoriasis. |
| Proton pump inhibitor | Dexlansoprazole, esomeprazole, lansoprazole, lansoprazole/naproxen, omeprazole, omeprazole/sodium bicarbonate, pantoprazole, rabeprazole | |
| Antihistamine and antacid | Cimetidine, cimetidine/dextrose, cimetidine/sodium chloride, famotidine, nizatidine, ranitidine, ranitidine/sodium chloride | |
| Antidiabetes | Metformin | |
| Antirheumatic | Sulfasalazine | |
| Antimicrobial | Sulfamethizole/trimethoprim, sulfamethoxazole/trimethoprim | |
| Antibiotic | Trimethoprim | |
| Antidepressant | Citalopram, escitalopram, fluoxetine, olanzapine, fluvoxamine, paroxetine, sertraline, desvenlafaxine, venlafaxine, levomilnacipran, duloxetine, doxepin, trazodone, isocarboxazid, phenelzine, tranylcypromine, amitriptyline, bupropion, fluoxetine, mirtazapine, nefazodone, vilazodone, vortioxetine, amitriptyline, amoxapine, clomipramine, desipramine, imipramine, maprotiline, nortriptyline, protriptyline, trimipramine | |
| Antiepileptic | Acetazolamide, carbamazepine, divalproex, eslicarbazepine acetate, ethanol/phenobarbital, ethosuximide, ethotoin, felbamate, fosphenytoin, lacosamide, levetiracetam, methsuximide, oxcarbazepine, perampanel, phenobarbital, phenytoin, primidone, rufinamide, tiagabine, topiramate, valproate sodium, valproic acid, vigabatrin, zonisamide | Any use. May reduce folate, but folate may also impede mechanism of action.a |
Effects are not well understood. The prescriber may use discretion in supplementation with folic acid when prescribing antiepileptic drugs.17
Folic Acid Exposure
Analyses were restricted to folic acid products, including multivitamins, prescribed by a health care professional. As previously noted, the majority of patients prescribed folic acid had a pain disorder.1 In this study, 48.0% of folic acid prescriptions were single agent at a dosage of 1 mg/d. Other single-agent daily dosages ranging from 0.4 mg to 5 mg accounted for 0.11% of all prescriptions, and the remainder were multivitamins. To have a negative control, we selected the supplement cyanocobalamin (vitamin B12). Cyanocobalamin is a man-made form of vitamin B12 that is essential for metabolism, blood cell synthesis, and the nervous system. It is also available both OTC and by prescription. It does not contain folic acid and is commonly used to treat anemia.
Outcomes
Our primary end point was suicide attempt or intentional self-harm resulting in an outpatient visit or inpatient admission as identified by the ICD-9-CM and ICD-10-CM codes listed in the eTable in the Supplement. We refer to these as suicidal events in the rest of the article.
Statistical Analysis
The primary analysis used a discrete-time survival model,18 based on a logistic regression with complementary log-log link function, so that the exponential of the estimated regression coefficient is a hazard ratio (HR). Patients were followed up for 24 months after the index folic acid prescription month, which was designated as month zero and not used in the analysis. We selected a 24-month follow-up period because patients change insurance carriers or discontinue service on average every 2 years. Month was the unit of analysis and treated as a categorical variable. Folic acid is a time-varying treatment variable, so we compared suicidal events during months with and without folic acid prescription coverage within individuals.
This is a proportional hazards model in that the effect of folic acid is assumed to be constant over time. We tested this assumption by adding folic acid × month interactions to the model, which allows the effect of folic acid on the hazard function to take on month-specific values. In our view, the target trial19 is a sequential randomized trial with rerandomization to treatment and control on a monthly basis. In this connection, the non–proportional hazard model can be used to assess period and carryover effects, in which the folic acid × month interaction allows the effect of treatment to vary over time.
Models were adjusted for age, sex, baseline use of folate-reducing drugs (eg, methotrexate), prior suicidal events, diagnoses related to suicide attempt (depression, anxiety, attention-deficit/hyperactivity disorder, bipolar disorder, schizophrenia, sleep disorders, pain) and diagnoses related to intestinal folate absorption (Crohn disease and celiac disease) or both (substance use disorder), and any other use of folate-reducing drugs (antimetabolites, proton pump inhibitors, antihistamines and antacids, antidiabetics, antirheumatics, antimicrobials, antibiotics, antiepileptics, and antidepressants) (Table 1). Diagnostic covariates were assessed at baseline, but pharmacologic covariates were treated as time-varying covariates. As such, covariation between folic acid prescription and antidepressant and antimanic (antiepileptic) drug prescriptions were adjusted for in the analysis.
To determine if the association between folic acid and suicidal events was moderated by folate-reducing drugs, a separate model with interactions between folic acid and folate-reducing drugs was also fit to these data. A model with interactions between folic acid and sex and age was used to determine if the effects were different in women and/or elderly individuals. Sex and age have large effects on suicide and suicide attempt rates, and women frequently take folic acid during pregnancy. These 2 variables were specifically examined in our original article1 and were included here based on that prespecification and to determine whether any identified association was restricted to pregnant women. Age was dichotomized at younger than 60 years.
As sensitivity analyses, we first repeated the main analysis in patients taking the modal 1-mg dosage and second in women of childbearing age. Third, a duration-response analysis was conducted in patients taking the modal 1-mg dosage by treating the cumulative number of months on treatment as a time-varying covariate. Fourth, we restricted the analysis to enrollment from 2015 to 2017 so that there was no overlap with the original signal-generation study. Fifth, we conducted an incident-user analysis by eliminating data for patients with previous use (past 2 years) of folic acid. Sixth, we eliminated data for patients with a history of suicide attempt and reanalyzed both using the primary analysis and the incident-user cohort.
In terms of multiplicity, we designate the primary analysis as the only hypothesis test of the association between folic acid and suicide attempts, and all of the other analyses as secondary/sensitivity analyses to test the robustness and generalizability of our conclusions. Furthermore, to have a negative control, we performed comparable analyses in patients prescribed cyanocobalamin (vitamin B12), excluding those also taking folic acid.
Results
Data on 866 586 patients were collected; 704 514 (81.30%) were female, and 90 296 (10.42%) were 60 years and older. Table 2 provides characteristics of the sample in terms of diagnoses, concomitant medications, and demographic data. Most folic acid doses were for the upper tolerable limit for adults (including in pregnancy and lactation) of 1 mg/d.
Table 2. Baseline Characteristics of Study Cohort (N = 866 586).
| Variable | No. (%) |
|---|---|
| Age, y | |
| 18-29 | 223 294 (25.77) |
| 30-39 | 236 551 (27.30) |
| 40-49 | 144 852 (16.72) |
| 50-59 | 171 593 (19.80) |
| ≥60 | 90 296 (10.42) |
| Female | 704 514 (81.30) |
| Male | 162 072 (18.70) |
| Prior suicide attempt and intentional self-harm | 1283 (0.15) |
| Medication | |
| Folic acid | 125 870 (14.52) |
| Antimetabolite (methotrexate) | 161 789 (18.67) |
| Proton pump inhibitor | 151 295 (17.46) |
| Antihistamine and antacid | 33 046 (3.81) |
| Antidiabetic | 60 913 (7.03) |
| Antirheumatic | 15 992 (1.85) |
| Antimicrobial | 76 298 (8.80) |
| Antibiotic | 5565 (0.64) |
| Antidepressant | 208 804 (24.10) |
| Antiepileptic | 47 016 (5.43) |
| Any of above drugs excluding folic acid | 464 541 (53.61) |
| Comorbidity or other diagnosis | |
| Substance use disorder | 41 019 (4.73) |
| Depression | 103 954 (12.00) |
| Anxiety | 126 425 (14.59) |
| Attention-deficit/hyperactivity disorder | 17 680 (2.04) |
| Bipolar disorder | 21 234 (2.45) |
| Schizophrenia | 7301 (0.84) |
| Sleep disorder | 82 004 (9.46) |
| Crohn disease | 10 369 (1.20) |
| Celiac disease | 3177 (0.37) |
| Pain | 399 019 (46.04) |
Overall, there were 261 suicidal events during months covered by a folic acid prescription (5 521 597 person-months) for a rate of 4.73 per 100 000 person-months, and 895 suicidal events during months without folic acid (8 432 340) for a rate of 10.61 per 100 000 person-months. The observed relative risk is 0.45, which is comparable with the previously reported iDEAS odds ratio (OR) of 0.40 (95% CI, 0.28-0.59), and within its 95% CI.1 The total number of patients in this data set is 866 586 for an overall suicidal event rate of 133 per 100 000 population. This rate is lower than the reported national rate of 600 per 100 000.20
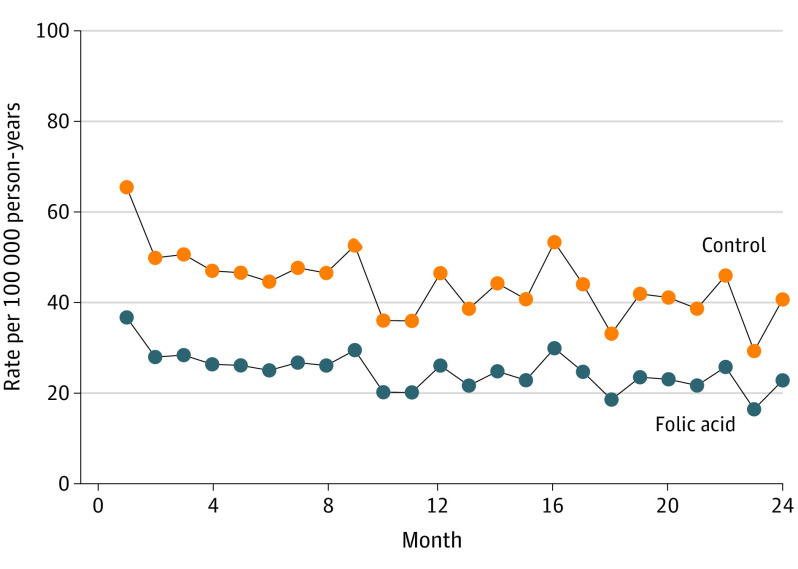
The adjusted estimated HR for folic acid for suicide events was 0.56 (95% CI, 0.48-0.65) (Figure). The unadjusted estimated HR for folic acid for suicide events was 0.39 (95% CI, 0.34-0.45).
Figure. Hazard Function for Suicide Attempts for Folic Acid vs Control: Proportional Hazards Model.
Tests of the moderating effects of folate-reducing drugs were all nonsignificant. Two- and 3-way interactions between age, sex, and folic acid were also nonsignificant. Including folic acid × month interactions in the model to test for nonproportional hazards did not significantly improve the fit of the model to the data (χ223 = 28.21, P = .21), which also suggests that there were no period or crossover effects.
A duration-response analysis restricted to patients taking the 1-mg/d dosage revealed a steady 5% decrease in suicidal event rates for each additional month of treatment (HR, 0.95; 95% CI, 0.93-0.97). The median (IQR) number of months of treatment was 6 months (2-13).
A total of 259 600 individuals took cyanocobalamin, the negative control, during the study period. After excluding those who also took folic acid, 236 610 individuals remained, with 1 460 534 person-months of cyanocobalamin use (128 suicide attempts) and 2 440 834 months of no use (206 suicide attempts), for suicide event rates of 8.76 and 8.44 per 100 000 person-months, respectively. No association was found between cyanocobalamin and suicidal events in the adjusted analysis (HR, 1.01; 95% CI, 0.80-1.27) or unadjusted analysis (HR, 1.02; 95% CI, 0.80-1.28).
Discussion
This large-scale pharmacoepidemiologic study showed an association with the signal originally detected using the iDEAS methodology.1 The unadjusted HR of 0.39 was comparable with the iDEAS OR of 0.40. These estimates are not unbiased, and the adjusted estimated HR, 0.56, was associated with a 44% reduction in suicidal events. Although both studies used MarketScan claims data, eliminating any overlap in years yielded virtually identical results (HR, 0.55; 95% CI, 0.45-0.66). Our hypothesis that folic acid decreases suicidal event risk by increasing folate levels in people taking folate-reducing drugs was not confirmed in this study. Age and sex did not moderate the association, and a similar association was found in women of childbearing age. No association was found for our negative control supplement, cyanocobalamin.
Adding to the validity of our findings was the demonstration of a significant inverse duration-response association (1-mg dosage subcohort). Every additional month of treatment was associated with a 5% reduction in the suicidal event rate. The validity is also supported by lack of an association between our negative control supplement, cyanocobalamin, and suicidal events. The HR of 1.01 found in our analysis of cyanocobalamin is also similar to that found in our previous study, iDEAS, in an all-drug screening analysis (HR, 0.98; 95% CI, 0.53-1.81), which was based on 301 188 individuals.1
Several studies have found associations between folate levels and folic acid supplementation and depression and suicidality. In a case-control study involving 110 patients with depression and 220 matched controls, a healthy dietary pattern was associated with a 25% reduced risk of depression, and the effect was mediated by folate levels.21 In a meta-analysis of 43 studies including 8519 individuals with depression and 27 282 individuals without depression, individuals with depression had lower folate levels than those without depression (effect size = 0.24 SD units).22 In an RCT of folic acid augmentation therapy for depression in patients treated with lithium for 1 year,23 patients who achieved folate levels of 13 ng/mL or above at the end point had a 40% greater reduction in Affective Morbidity Index scores relative to placebo augmentation. A recent study in South Korea24 found an association between serum folate levels and fatal and nonfatal suicide attempts during follow-up (area under the curve, 0.77). Using a cutpoint of 6 ng/mL, an adjusted OR of 2.69 (95% CI, 1.27-5.69) was found for dichotomized serum folate levels and OR 2.84 (95% CI, 1.19-6.77) for folate deficiency defined as less than 3 ng/mL. A case-control study7 found cerebral folate deficiency in 36% of patients with refractory depression. All individuals with cerebral folate deficiency and depression were treated with folinic acid (leucovorin calcium) for at least 6 weeks, and 83.3% showed improvement at follow-up (including reduction in suicidal ideation). However, an RCT that randomized 475 patients to receive either 5 mg of folic acid daily (223 patients) or placebo (217 patients) as an adjunct to antidepressant treatment for 12 weeks found no significant difference between groups on suicide ratings.25
The role of folate in depression and cognition has been recognized for more than a decade, leading to recommendations for folate augmentation in patients with low or normal levels at the start of any depression treatment.4 Polymorphisms have been hypothesized to account for individual differences in response to antidepressants, including treatment-resistant depression; however, in 1 study, no association between MTHFR polymorphisms and fluoxetine treatment response were found.26
We believe that these results justify advocating for an RCT to study the effect of folic acid on suicidality. That study could be conducted in a high-risk population, which would maximize the number of suicidal events, and could also use longitudinal assessments of suicidal events using previously validated adaptive tests for suicidality.27 The Computerized Adaptive Test Suicide Scale (CAT-SS)27 has recently been demonstrated to be feasible in a group-level intervention (n = 1485) in the US Air Force Wingman Connect Study28 and validated in a population of US veterans.29 In the Veterans Affairs study, the baseline suicide attempt rate over the past 3 months was 6%. To detect a 50% reduction in suicide attempt rate with power of 0.8 (6% vs 4%) would require 4000 participants. Sample size requirements for the CAT-SS would be in the hundreds for a moderate effect size.
Limitations
There are several limitations of this study. First, it is an observational study, and selection effects may be present. However, because all members of the cohort filled a folic acid prescription in the month before the start of the study period, if confounding does exist, it is dynamic and related to the progression of a disease and increased use of folic acid and suicidal events. Second, claims data (ICD-9-CM and ICD-10-CM) likely underrepresent the number of suicidal events because of incomplete reporting. Indeed, our rate of suicidal events is one-fourth of the national rate reported by the National Institutes of Health. Third, the association between folic acid and suicidal events may be explained by healthy user bias. While this is likely for OTC folic acid use, it is less true for filled prescriptions, where more than half of these prescriptions were associated with pain disorders. The within-person nature of our design and analysis further insulates the treatment effect from this bias. Fourth, in addition to OTC, folate may be provided as leucovorin calcium (folinic acid) as in Pan et al7 or as 5-MTHF (levomefolate calcium). However, this would lead us to underestimate the association because some of our nonuse periods might actually be use periods. Fifth, while we conducted a sensitivity analysis in women of childbearing age, we did not have data on women actively planning for pregnancy. Nevertheless, we found the same association in men and women and no evidence of a sex × age × folic acid interaction, making confounding unlikely.
Conclusions
This large-scale well-controlled pharmacoepidemiologic study of folic acid found a beneficial association in terms of lowering rates of suicide attempts. These results warrant the conduct of an RCT with suicidal ideation and behavior as outcomes of interest. If confirmed, folic acid may be a safe, inexpensive, and widely available treatment for suicidal ideation and behavior.
eTable. ICD-9-CM and ICD-10-CM diagnostic codes for identifying suicide attempt, intentional self-harm, and other diagnoses included in the statistical models
References
- 1.Gibbons RD, Hur K, Lavigne J, Wang J, Mann JJ. Medications and suicide: high dimensional empirical Bayes screening (iDEAS). Harvard Data Science Review. Published November 1, 2019. 10.1162/99608f92.6fdaa9de [DOI]
- 2.Selhub J, Seyoum E, Pomfret EA, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon liver folate content and distribution. Cancer Res. 1991;51(1):16-21. [PubMed] [Google Scholar]
- 3.Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. 2008;13(3):216-226. [PubMed] [Google Scholar]
- 4.Fava M, Mischoulon D. Folate in depression: efficacy, safety, differences in formulations, and clinical issues. J Clin Psychiatry. 2009;70(suppl 5):12-17. doi: 10.4088/JCP.8157su1c.03 [DOI] [PubMed] [Google Scholar]
- 5.Wolfersdorf M, Keller F, Maier V, Fröscher W, Kaschka WP. Red-cell and serum folate levels in depressed inpatients who commit violent suicide: a comparison with control groups. Pharmacopsychiatry. 1995;28(3):77-79. doi: 10.1055/s-2007-979594 [DOI] [PubMed] [Google Scholar]
- 6.Engström G, Träskman-Bendz L. Blood folate, vitamin B12, and their relationships with cerebrospinal fluid monoamine metabolites, depression, and personality in suicide attempters. Nord J Psychiatry. 1999;53:131-137. doi: 10.1080/080394899426837 [DOI] [Google Scholar]
- 7.Pan LA, Martin P, Zimmer T, et al. Neurometabolic disorders: potentially treatable abnormalities in patients with treatment-refractory depression and suicidal behavior. Am J Psychiatry. 2017;174(1):42-50. doi: 10.1176/appi.ajp.2016.15111500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czeizel AE, Dudás I, Vereczkey A, Bánhidy F. Folate deficiency and folic acid supplementation: the prevention of neural-tube defects and congenital heart defects. Nutrients. 2013;5(11):4760-4775. doi: 10.3390/nu5114760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Qin X, Demirtas H, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369(9576):1876-1882. doi: 10.1016/S0140-6736(07)60854-X [DOI] [PubMed] [Google Scholar]
- 10.Durga J, Verhoef P, Anteunis LJ, Schouten E, Kok FJ. Effects of folic acid supplementation on hearing in older adults: a randomized, controlled trial. Ann Intern Med. 2007;146(1):1-9. doi: 10.7326/0003-4819-146-1-200701020-00003 [DOI] [PubMed] [Google Scholar]
- 11.O’Leary F, Allman-Farinelli M, Samman S. Vitamin B12 status, cognitive decline and dementia: a systematic review of prospective cohort studies. Br J Nutr. 2012;108(11):1948-1961. doi: 10.1017/S0007114512004175 [DOI] [PubMed] [Google Scholar]
- 12.Hansen L. The MarketScan databases for life sciences researchers [MarketScan white paper]. Truven Health Analytics. Published 2016.
- 13.Nock MK, Hwang I, Sampson NA, Kessler RC. Mental disorders, comorbidity and suicidal behavior: results from the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(8):868-876. doi: 10.1038/mp.2009.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldessarini RJ, Tondo L. Suicidal risks in 12 DSM-5 psychiatric disorders. J Affect Disord. 2020;271:66-73. doi: 10.1016/j.jad.2020.03.083 [DOI] [PubMed] [Google Scholar]
- 15.Duhra P. Treatment of gastrointestinal symptoms associated with methotrexate therapy for psoriasis. J Am Acad Dermatol. 1993;28(3):466-469. doi: 10.1016/0190-9622(93)70069-6 [DOI] [PubMed] [Google Scholar]
- 16.Ortiz Z, Shea B, Suarez Almazor M, Moher D, Wells G, Tugwell P. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2000;(2):CD000951. [DOI] [PubMed] [Google Scholar]
- 17.Linnebank M, Moskau S, Semmler A, et al. Antiepileptic drugs interact with folate and vitamin B12 serum levels. Ann Neurol. 2011;69(2):352-359. doi: 10.1002/ana.22229 [DOI] [PubMed] [Google Scholar]
- 18.Efron B. Logistic regression, survival analysis, and the Kaplan-Meier curve. J Am Stat Assoc. 1988;83(402):414-425. doi: 10.1080/01621459.1988.10478612 [DOI] [Google Scholar]
- 19.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758-764. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute of Mental Health . Suicide. Retrieved December 6, 2021. https://www.nimh.nih.gov/health/statistics/suicide
- 21.Khosravi M, Sotoudeh G, Amini M, Raisi F, Mansoori A, Hosseinzadeh M. The relationship between dietary patterns and depression mediated by serum levels of folate and vitamin B12. BMC Psychiatry. 2020;20(1):63. doi: 10.1186/s12888-020-2455-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bender A, Hagan KE, Kingston N. The association of folate and depression: a meta-analysis. J Psychiatr Res. 2017;95:9-18. doi: 10.1016/j.jpsychires.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 23.Coppen A, Chaudhry S, Swade C. Folic acid enhances lithium prophylaxis. J Affect Disord. 1986;10(1):9-13. doi: 10.1016/0165-0327(86)90043-1 [DOI] [PubMed] [Google Scholar]
- 24.Kim JM, Kim HY, Lee HJ, et al. Prediction of suicidality according to serum folate levels in depressive patients receiving stepwise pharmacotherapy. Front Psychiatry. 2021;12:747228. doi: 10.3389/fpsyt.2021.747228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bedson E, Bell D, Carr D, et al. Folate Augmentation of Treatment–Evaluation for Depression (FolATED): randomised trial and economic evaluation. Health Technol Assess. 2014;18(48):vii-viii,1-159. doi: 10.3310/hta18480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mischoulon D, Lamon-Fava S, Selhub J, et al. Prevalence of MTHFR C677T and MS A2756G polymorphisms in major depressive disorder, and their impact on response to fluoxetine treatment. CNS Spectr. 2012;17(2):76-86. doi: 10.1017/S1092852912000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbons RD, Kupfer D, Frank E, Moore T, Beiser DG, Boudreaux ED. Development of a computerized adaptive test suicide scale: the CAT-SS. J Clin Psychiatry. 2017;78(9):1376-1382. doi: 10.4088/JCP.16m10922 [DOI] [PubMed] [Google Scholar]
- 28.Wyman PA, Pisani AR, Brown CH, et al. Effect of the Wingman-Connect upstream suicide prevention program for Air Force personnel in training: a cluster randomized clinical trial. JAMA Netw Open. 2020;3(10):e2022532. doi: 10.1001/jamanetworkopen.2020.22532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner LA, Betthauser LM, Penzenik M, Bahraini N, Gibbons RD. Validation of a computerized adaptive test suicide scale (CAT-SS) among United States military veterans. PLoS One. 2022;17(1):e0261920. doi: 10.1371/journal.pone.0261920 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. ICD-9-CM and ICD-10-CM diagnostic codes for identifying suicide attempt, intentional self-harm, and other diagnoses included in the statistical models