Abstract
Most consumers are aware that climate change is a growing problem and admit that action is needed. However, research shows that consumers’ behavior often does not conform to their value and orientations. This value-behavior gap is due to contextual factors such as price, product design, and social norms as well as individual factors such as personal and hedonic values, environmental beliefs, and the workload capacity an individual can handle. Because of this conflict of interest, consumers have a hard time identifying the true drivers of their behavior, as they are either unaware of or unwilling to acknowledge the processes at play. Therefore, consumer neuroscience methods might provide a valuable tool to uncover the implicit measurements of pro-environmental behavior (PEB). Several studies have already defined neurophysiological differences between green and non-green individuals; however, a behavior change intervention must be developed to motivate PEB among consumers. Motivating behavior with reward or punishment will most likely get users engaged in climate change action via brain structures related to the reward system, such as the amygdala, nucleus accumbens, and (pre)frontal cortex, where the reward information and subsequent affective responses are encoded. The intensity of the reward experience can be increased when the consumer is consciously considering the action to achieve it. This makes goal-directed behavior the potential aim of behavior change interventions. This article provides an extensive review of the neuroscientific evidence for consumer attitude, behavior, and decision-making processes in the light of sustainability incentives for behavior change interventions. Based on this review, we aim to unite the current theories and provide future research directions to exploit the power of affective conditioning and neuroscience methods for promoting PEB engagement.
Keywords: pro-environmental behavior (PEB), consumer neuroscience, intention-behavior gap, conditioning, cognitive dissonance, neuromarketing, decision-making, sustainability
Introduction
The behavior of people on a daily basis has an effect on their own health and well-being, but also on the health and well-being of other individuals, groups, and on society at large (Fishbein and Ajzen, 2011). There is a growing awareness that human behavior can both cause and alleviate social problems in a variety of domains such as health, safety, and the environment (Fishbein and Ajzen, 2011). When considering the effects of human behavior on the environment, the Intergovernmental Panel on Climate Change has made this clear: “It is unequivocal that human influence has warmed the atmosphere, ocean, and land. Widespread and rapid changes in the atmosphere, ocean cryosphere, and biosphere have occurred” (SPM, p. 5). Pollution from fossil-based plastic waste may take up to 1,000 years to decompose completely (Sumrin et al., 2021), and thereby waste related to packaging has a devastating effect on the quality of air, soil, and water, which accelerates climate change (Boz et al., 2020; Phelan et al., 2021). Food consumption of humans contributes to deforestation and up to 30% of greenhouse gas emissions (Theurl et al., 2020). Hence one of the solutions to reduce the negative impact of food consumption on the environment is stimulating consumers to purchase more environmentally friendly products (Ischen et al., 2022), for example, products with sustainable packaging that have a lower environmental impact (Granato et al., 2021).
Consumers have become increasingly aware of their environmental impact, but excessive consumption patterns still contribute to current ecological challenges (Stolz et al., 2013). Although consumers value sustainable products (Rokka and Uusitalo, 2008), they do not always purchase them (Jerzyk, 2016). The discrepancy between what people say and what they do is labeled as the attitude-behavior gap (Kennedy et al., 2009), value-action gap (Van der Linden and Weber, 2021), or intention-behavior gap (Hassan et al., 2016). It is essential to differentiate between these definitions, as they indicate a discrepancy in different levels of behavior. Behavior (change) typically is initiated in four steps: when the user becomes aware of the issue (i.e., has knowledge), (s)he forms an attitude or value about the issue and then starts contemplating about performing an action, which is also called intention forming (Michaelsen and Esch, 2021). This intention may indicate the readiness for the execution of the actual behavior or the “subjective probability” that the user would find it relevant to perform the behavior (Fishbein and Ajzen, 2011). Thus, knowledge of the issue can exist without an attitude change and attitude can exist without an intention to act on it.
The discrepancy between a person’s attitude or intention and their behavior can give rise to “cognitive dissonance” (Szmigin et al., 2009), which refers to the situation where cognition and behavior contradict each other, triggering a discomforting psychological tension (Festinger, 1957). Following this cognitive dissonance, people try to rationalize their behavior or cognition. For example, eating meat is dissonant with liking animals and in order to reduce the discomfort of this discrepancy, the meat-eater might: (1) dichotomize: “It seems wrong that people in some cultures eat dogs and cats”, (2) deny: “Meat is processed so that animal pain and discomfort is minimized and avoided”, (3) dissociate: “I do not like to think about where the meat I eat comes from”, or (4) justify: “We need the protein we can only get in meat for healthy development” (Rothgerber, 2013). This explains why intentions cannot be evaluated in retrospect once the behavior is completed, however, measurements of cognitive dissonance (if exist) could function as a signal as to whether the subject experienced the action to be in line with their intentions (Harmon-Jones et al., 2015; Zangemeister et al., 2019).
The choice to buy a sustainable product often confronts consumers with a difficult decision. Similar to the meat consumption example, an imbalance exists in fashion shopping where consumers may want to act pro-environmentally but are unwilling to sacrifice fashionability (Newman et al., 2014), branding of the product (Cairns et al., 2022), or pay a higher price (Li and Kallas, 2021). Typically, cognitive dissonance emerges when consumers have to compromise between biospheric, altruistic, egoistic, and hedonic values, and specific climate actions have contradicting implications for each of these values (Bouman et al., 2021). Thus, individuals might not consistently engage in climate action because acting on their biospheric values can threaten other relevant values (Steg, 2016; Bouman et al., 2021), thereby contributing to the value-behavior gap. In this way, even when an individual prioritizes biospheric values over egoistic values, for example by wanting to travel by train, it might be that the investment costs are too high (the travel time is doubled when comparing it to taking a plane), which consequently prevent them from adopting such practices. The cognitive dissonance tension experienced in this situation is regulated by the individual’s environmental self-concept (Cairns et al., 2022).
In order to understand and consequently close the value-behavior gap, it is important to first elucidate how consumers develop and use strategies for decision-making. Decision-making is thought to occur mainly under implicit and automatic processes, with only a minority of actions performed by the reflective system (Chaiken, 1980; Petty and Cacioppo, 1986; Kahneman, 2003). Although there is still controversy around this dual-process model (Evans, 2009; Keren and Schul, 2009; Foxall, 2016; Melnikoff and Bargh, 2018; Grayot, 2020), researchers agree that choices cannot be deduced from the rationality of choices alone; the diversity of emotional (Brosch, 2021; Schneider et al., 2021), social (Cialdini and Jacobson, 2021), attentional (Luo and Zhao, 2021), motivational (Bayes and Druckman, 2021), habitual (Verplanken and Whitmarsh, 2021), behavioral (Thøgersen, 2021), and neural (Sawe and Chawla, 2021) factors that contribute to the decision-making and actions of the consumers (Van der Linden and Weber, 2021) prevent them from being able to explicitly identify and state how they make decisions. This is also evidenced by previous research in the area of health and food consumption indicating that day-to-day eating behavior is often shaped by implicit emotions and automatic motives rather than explicit willpower (Sheeran et al., 2013; Michaelsen and Esch, 2021).
For this reason, self-report measures are only valid to the extent that people are willing or able to provide accurate reports (Cacioppo et al., 2018). The difference between attitudes, intentions, and behavior might be hard for consumers to conceptualize; when they indicate a positive attitude towards zero waste packaging, this does not imply that they are planning to reduce their waste. Additionally, self-reports are prone to biases that play part in explicit measurements, especially when concerning a topic vulnerable to social desirability such as engagement in environmental behavior (Kaiser et al., 1999), although the effects of social desirability strongly vary between cases (Vesely and Klöckner, 2020). This does not happen consciously; biases and assumptions come into play when people are reflecting on their cognitive processes (Nisbett and Wilson, 1977). For example, consumers might be hesitant to address their concerns about the pricing of a green product out loud and therefore this is not reflected in survey outcomes (Vezich et al., 2017). Another limiting resource might be the amount of working memory, which is shown to impact the extent to which consumers engage in pro-environmental behavior (PEB; Langenbach et al., 2020). This underlines the fact that decision-making under conflicting circumstances might be better investigated using implicit measures.
To this end, neuroscience tools can provide an additional implicit measurement when verbalized attitudes and intentions are not consistent with the performed behavior. Behavioral interventions have been focused on the final outcome and therefore do not contribute to the understanding of the mechanisms and factors that underlie the formation of behavior (Van Dessel et al., 2022). In order to explore the (implicit) steps before an action takes place, neuroscientific tools could provide an additional asset (Leeuwis et al., 2022). Consumer neuroscience aims to gain insights into consumers’ motivations, preferences, and decision processes through neural and behavioral measures (Javor et al., 2013). Neuroscience tools deliver less biased data for choices/actions that are performed automatically (Ariely and Berns, 2010; Vezich et al., 2017), and hence can provide an insightful measure for the processes and drivers underlying PEB (Van Geffen et al., 2016; Goucher-Lambert et al., 2017; Sawe and Chawla, 2021; Wang and van den Berg, 2021). For instance, by measuring cortical activation during the resting state (Baumgartner et al., 2019) or during the viewing of green products (Lee et al., 2014) or climate change images (Van Geffen et al., 2016), it has been shown that individuals with pro-environmental beliefs display differentiated neural patterns as compared to their peers. While neuroscience methods entail limitations in terms of prediction of affect and attitudes (for example, the reverse inference fallacy; Poldrack, 2006), the lack of unified theories and definitions and the underpowered sample sizes many studies suffer from (Alvino, 2019), they still show promise for preference prediction (Hakim and Levy, 2019), which allows us to identify neural markers of the decision-making processes.
Literature is already picking up neuroscience for environmental research as is shown by the increasing number of publications and several recent reviews (Pagan et al., 2020; Sawe and Chawla, 2021; Wang and van den Berg, 2021). For instance, Pagan et al. (2020) suggest that researchers should investigate the drivers and barriers to the adoption of neuromarketing in sustainability studies; Sawe and Chawla (2021) focus primarily on neuroeconomics and the neuroscience of affect to gather insights for environmental policymakers who need to characterize and anticipate public’s responses to sustainable decisions; Wang and van den Berg (2021) focus on the neuroscience of self vs. others and suggest to investigate this predominantly using event-related potentials; and White et al. (2019) propose five psychological routes (i.e., social influence, habit formation, the individual self, feelings and cognition, and tangibility) for encouraging sustainable consumer behavior change.
While all the above studies conclude that more research needs to be done, a unified framework in which the adoption of neuroscience tools in environmental research can be operationalized remains missing. This article distinguishes itself from previous studies by integrating neuroscientific theories of decision-making, cognitive dissonance, and behavior change interventions into one comprehensive framework. We aim to provide a scoping review of the application of neuroscience tools in environmental research. Using this literature, we lay out open research questions and future research directions that could deepen our understanding of consumer attitude-behavior relationship in ecological settings and eventually lead to the development of successful interventions that promote more sustainable decision-making among consumers.
Background
Studies looking into the neuroscience of sustainability are scarce and isolated. The current landscape of the literature is fragmented, which creates the need for a comprehensive framework that guides future research. In the following sections, the current state of the literature will be elaborated by providing a model of behavior change and investigating the neuroscience of decision-making, cognitive dissonance, and learning.
Behavior change and motivation
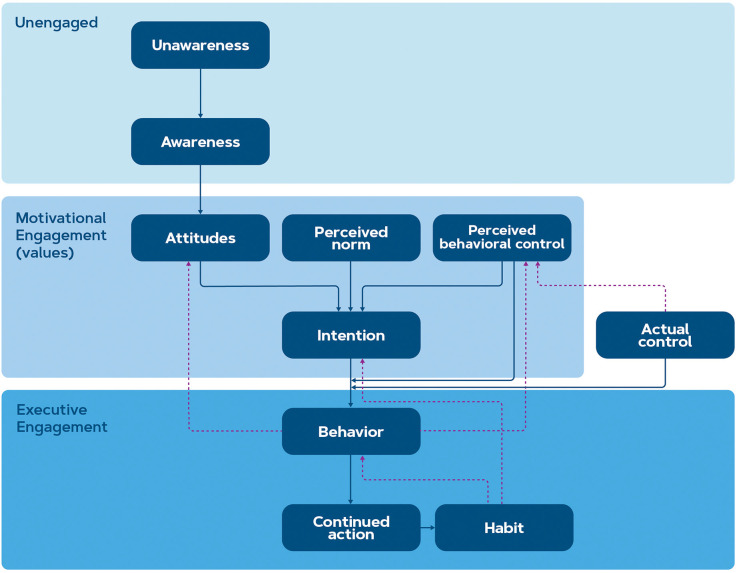
To reduce the impact of human factors on the climate crisis, behavior change from consumers is essential. Researchers in the climate and sustainability research domain can borrow ideas from the health domain where intentions to stay healthy are ubiquitous but lifestyle change is not easily achieved. Just like environmental intentions suffer from individual and contextual influences, behavior change for a healthy lifestyle is also submissive to social, biological, psychological, cognitive, and contextual factors (Van Cappellen et al., 2018). In a recent study, Michaelsen and Esch (2021) proposed a three-stage model of health behavior change that might, therefore, be similarly applicable to pro-environmental behavior change interventions. Their model is comprised of past models of behavior change such as the Transtheoretical Model (Prochaska, 2008), the Rubicon Model of Action Phases (Gollwitzer, 1990), Precaution Adoption Process Model (Weinstein et al., 2020), Four Phases of the Behavior Change Process (Rothman et al., 2011), and Health Action Process Approach (Schwarzer and Luszczynska, 2008). For our proposed framework, we adopted the model of Michaelsen and Esch (2021) and combined it with the Reasoned Action Approach by Fishbein and Ajzen (2011), to sufficiently reflect the steps between intention and behavior. This framework is depicted in Figure 1.
Figure 1.
The proposed framework for pro-environmental behavior change intervention is based on the models of Michaelsen and Esch (2021) and Fishbein and Ajzen (2011). The stages within motivational engagement are sometimes also referred to as values or beliefs.
This framework defines three stages of behavior change, namely: (1) non-engagement, where the consumer is either unaware or aware of the benefits of behavior change but does not intend to take action, (2) motivational engagement, where the consumer contemplates or plans the action and (3) executive engagement where the action is initiated, continued and ideally maintained until it becomes a habit (Michaelsen and Esch, 2021). Within these stages, several sub-stages take part: for instance, in the stage of non-engagement, the consumer first becomes aware of the issue (gains knowledge) and then forms an attitude. In the stage of motivational engagement, other factors than attitude such as the perception of norm and behavioral control (according to the Reasoned Action Approach by Fishbein and Ajzen, 2011) may play a role before the consumer forms an intention to act.
Moreover, this framework considers behavioral control as a factor that contributes to the transition from intention to behavior. Perceived behavioral control refers to the belief a person has regarding his/her control over the action (i.e., perception of environmental factors that may facilitate or impede the action such as financial or physical capacity) whereas actual control refers to the person having sufficient skills, resources, or abilities to actually carry out the action. Explicitly stated intentions predict only about 30% of subsequent behavior (Sheeran, 2002). This percentage can be partially explained by the difference between perceived behavioral control and actual control, which ultimately inhibits the consumer from behaving according to their intentions (Fishbein and Ajzen, 2011). The (perceived) control incorporates socioeconomic status, expected cognitive effort, lack of expertise, lack of availability or lack of trust (Sheeran, 2002; Fishbein and Ajzen, 2011; Gleim et al., 2013), which thereby coincides with economic choice models (McFadden, 1986; Padoa-Schioppa, 2011; Yousuf et al., 2019). Green consumption intentions are positively impacted by positive attitudes towards sustainability and environmental concerns while the intentions and actions are negatively impacted by price sensitivity (Yue et al., 2020). This might indicate that interventions could only apply to consumers with a certain level of economic stability. These and other internal and external factors are discussed in the list of open questions in the next chapter.
The transition from non-engagement to motivational engagement can occur either by conscious involvement or without being explicitly aware of it (Michaelsen and Esch, 2021). From there, three types of motivational states seem to provide the driver for taking action: approach, avoidance, and assertion motivation (Michaelsen and Esch, 2021). The first type, approach motivation, is directed towards stimuli or goals that are associated with positive affect, joy, and reward (expectation). These positive emotions are experienced through psychological and neurobiological processes that occur with anticipation and as a reaction to the reward (Berridge and Kringelbach, 2008; Schultz, 2015). Approach motivation may unconsciously lead to executive engagement due to appetitive stimuli that increase the desire to obtain the reward (Berridge, 2018). The second category, avoidance motivation, is related to the avoidance of threat or punishment either by fight, flight, or freeze responses (Seymour et al., 2007). The punishment is associated with aversive and negative emotions such as anxiety, fear, and disgust (Elliot et al., 2013). Finally, the third motivational state, assertion motivation, is often not distinguished from approach motivation, while the affect is related to not-wanting instead of wanting (McCall and Singer, 2012) and the behavior is characterized by the absence of action because it happens mainly internally by consenting towards the new state (e.g., by inaction or acceptance).
All the above-mentioned motivations can arise either from internal or external stimuli, where the difference in actions is characterized as goal- or stimulus-driven behavior, respectively. The trigger might either be observed consciously or be unnoticed and the resulting action might be the same, only the intensity of the reward would be stronger when the action was cognitively pursued, especially in the phase of continued action (Carver and White, 1994; Van Cappellen et al., 2018; Michaelsen and Esch, 2021). Therefore, in creating interventions for behavior change, attention should be paid to emotion-driven motivation that transitions the user from intention to goal-directed action and ultimately to habit. Hereby, we assume that most consumers have knowledge about the climate change issue (Arshad et al., 2021; Calculli et al., 2021). Moreover, focus on approach motivation is recommended since increasing the appeal is easier than decreasing appeal (Marteau, 2017). The distinction of these motivations is measured regularly using implicit methods of consumer neuroscience. The exact neural parameters will be discussed in the following section.
When motivations are strong enough, the behavior takes place, and the consumer (temporarily) transitions to the stage of executive engagement. Carrying out this behavior could possibly raise two different responses; a positive emotion, which subsequently encourages the consumer to continue the action in anticipation of positive affect (Brosch, 2021), or a negative emotion or psychological tension in the form of cognitive dissonance that reduces attitudes towards the behavior (Brosch, 2021). Moreover, the experience of performing the behavior can impact the perceived behavioral control when it was easier or more complicated than expected to carry out. When the action is regularly and frequently performed, a habit emerges (Verplanken and Orbell, 2022). Habits are automatic responses from memory that led to behavior in the past. They may derive from cue-response associations in memory that were learned through repeated coupling (Verplanken, 2018; Verplanken and Orbell, 2022).
The discussion of habits requires a distinction between habit and goal-directed behavior, which is still an ongoing debate in literature. Goal-directed behavior is defined as an action that is consciously performed in order to reach the outcome, while habits are automatic stimulus-response reactions where the outcome of the action is not considered (Kruglanski and Szumowska, 2020). But recently, it has been argued that habits can be goal-directed too; for instance, when a more attractive reward appears, habits might be abandoned in order to strive for a new goal. Habitual change is essential in order to maintain sustainable decision-making in combatting the climate crisis (Verplanken and Whitmarsh, 2021), but in real life, it may not be possible to draw a strict distinction between habit and goal-directed behavior because attitude or motivation-based behavior is needed to form new habits (Verplanken and Orbell, 2022). Therefore, in this context, we reason from the viewpoint that goal-directed behavior is sufficient to form a sustainable or break an unsustainable habit. Automatically caried out habits can also override consciously set intentions: only in the absence of strong habits, intentions are predictive of actual behavior (Ji and Wood, 2007; Smith, 2021), which means habits also act on behavior directly. Thus, the behavioral control that impacts the transition from intention to behavior in the Reasoned Action Approach might also be interpreted as (un)conscious habits playing a part.
To summarize, motivations are important drivers of consumer’s behavior, but it is difficult to measure motivations and consumer’s intentions subjectively. Therefore, neuroscience tools can be used for a more objective evaluation of consumer’s motivations and decision-making processes.
The neuroscience of consumer decision-making
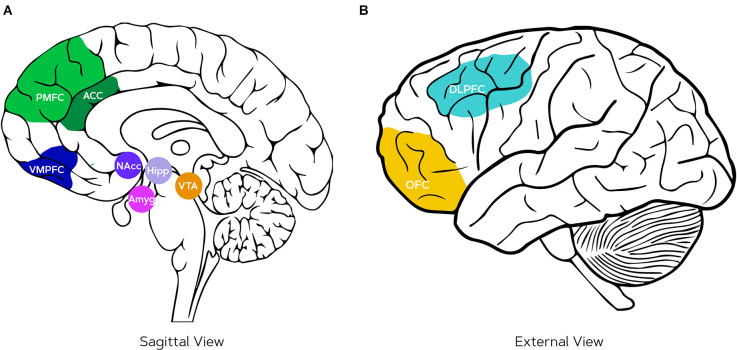
The neuroscience of decision-making is often based on the perspective that reward and loss are the drivers of human decision-making (Javor et al., 2013), which is comparable to the discussion of approach, avoidance, and assertion motivation that was proposed by Michaelsen and Esch (2021). Within this domain, several measurements have been proposed. Measurements from brain areas related to reward systems such as the ventral striatum were found to relate most to population-wide shopping behavior (Venkatraman et al., 2015). The neural pathway of reward learning (approach motivation) includes the ventral tegmental area (VTA), the amygdala, and the hippocampus (see Figure 2); VTA projects dopamine to the nucleus accumbens (NAcc; part of the ventral striatum; Nestler, 2001) where the reward is anticipated (Berridge and Kringelbach, 2008), the amygdala processes the intensity of the reward and generates a link between the stimulus and reward by projecting to the hippocampus, and the hippocampus remembers the experience and strengthens the reward anticipation for the next time the action is performed (Esch and Stefano, 2004). The connection between the ventral striatum and hippocampus is suggested to facilitate the consolidation of location-reward associations information (Lansink et al., 2009).
Figure 2.
Neural pathways underlying reward and punishment processing, decision-making, and cognitive dissonance: (A) the anterior cingulate cortex (ACC) which is part of the posterior medial frontal cortex (PMFC) that (among other functions) detects the psychological tension during cognitive dissonance and thus serves for conflict monitoring, ventromedial prefrontal cortex (VMPFC) related to reward-based decision-making, nucleus accumbens (NAcc) where the reward is anticipated, amygdala (Amyg) that processes the intensity of the rewards, hippocampus (Hipp) that stores a representation of the behavior and subsequent reward, ventral tegmental area (VTA) that projects dopamine to the Nacc, (B) orbitofrontal cortex (OFC) for reward expectation, and dorsolateral prefrontal cortex (DLPFC) for integration of goals and is, therefore, also active during the rationalization phase of cognitive dissonance.
The neural pathway of punishment (or withdrawal motivation) works either via the active fight or flight stress response involving the brain stem and cortisol or (nor)adrenaline, whereby the relief of response that follows from the action triggers the amygdala to couple the emotional relief to the performed action and store it in the hippocampus (Esch and Stefano, 2004; Schultz, 2015). In this way, anticipation of emotions following actions plays a role in decision-making both in approach and withdrawal contexts (Nestler, 2001; Michaelsen and Esch, 2021).
When the stimuli are cognitively processed, the orbitofrontal (OFC) and ventromedial prefrontal cortex (VMPFC) are involved (Michaelsen and Esch, 2021). The OFC is associated to reward expectations (O’Doherty et al., 2001), whereas the role of the VMPFC is in the representation of reward-based decision-making and also the generation of negative emotions and aspects of social cognition (Hiser and Koenigs, 2018; Lieberman et al., 2019). Additionally, integration of goals is observed in the dorsolateral prefrontal cortex (DLPFC; Miller and Cohen, 2001). A recent comparison using transcranial direct current stimulation (tDCS) suggested that control and regulation of valence of emotional experiences (either positive or negative, evidence is unclear) are mediated by the DLPFC and that the extinction of arousal in response to emotional stimuli might be related to the VMPFC (Nejati et al., 2021). Several experiments where consumers watch anti-smoking campaigns found that VMPFC activity was predictive of individual quitting (Falk et al., 2011) and group-level quitting intentions (Falk et al., 2016). Emotional responses in the amygdala correlated with individual quitting intentions as well as population-wide interest but this relation was mediated by the activation of the VMPFC on both individual and group levels (Falk et al., 2016; Doré et al., 2019). Studies where people were persuaded to use sun-screen, also consistently showed that the VMPFC is related to message-consistent behavior change even when controlling for prior behavior and self-reported intentions (Falk et al., 2010; Burns et al., 2018). Moreover, activity in the right DLPFC was found in consumers that were counterarguing persuasive messages and this correlated negatively to behavior change (Burns et al., 2018). The frontal brain regions discussed serve in many more cognitive processes and might, therefore, not be completely diagnostic (Poldrack, 2011) but are often engaged in decision-making by integrating goals and regulation of emotional responses.
While the previous regions mainly derive from fMRI experiments, another metric that is essential to discuss within the context of approach-avoidance is the EEG measurement of frontal asymmetry. This is the relative difference between activity in the left compared to the right hemisphere in the frontal electrodes (Coan and Allen, 2003). The investigation of cerebral asymmetry proposed by Davidson (1984) is considered to reflect the valence of emotions (Gable and Poole, 2012). Positive emotions often coexist with approach-related motivation and more left frontal brain activity, whereas negative emotions often correlate with withdrawal-related motivation and more right frontal brain activity (Harmon-Jones and Sigelman, 2001; Rohlfs and Ramírez, 2006; Liu et al., 2018). For example, using electroencephalogram (EEG), Liu et al. (2018) found that frontal alpha asymmetry (FAA) in electrode positions F3-F4 distinguished between positive and negative emotions (induced by movie clips) while AF3-AF4 asymmetry differentiated positive emotions from neutral ones. Positive affect can induce increased approach motivation in pursuit of a goal and will be decreased when the pursued goal is achieved (Knutson and Greer, 2008).
Several studies have investigated frontal asymmetry as a measurement of decision-making, which strongly responds to affective stimuli—especially when they are engaging (Sabu et al., 2022). This metric is also correlated to activity in the amygdala (Zotev et al., 2016), which is related to evaluating the intensity of a reward (Michaelsen and Esch, 2021). Moreover, individuals with a higher baseline asymmetry in favor of the left prefrontal alpha activity were more sensitive to rewards than individuals with a lower FAA at the baseline (Pizzagalli et al., 2005; De Pascalis et al., 2018). Similarly, FAA is an important measurement in consumer neuroscience; studies have shown that FAA dynamics when subjects watch images of products are related to sales (Baldo et al., 2015), willingness to buy (Golnar-Nik et al., 2019), and individual preference (Touchette and Lee, 2017; Di Gruttola et al., 2021). FAA measured during sub-sections of commercials was also related to individual preference (Ohme et al., 2010; Vecchiato et al., 2014), investment decision-making (Di Gruttola et al., 2021), and consumers’ product choice (Golnar-Nik et al., 2019). Moreover, the reverse effect has also been verified in neurofeedback studies, where users learned to upregulate their frontal alpha asymmetry, which reduced anxiety (Mennella et al., 2017) or increased ratings of neutral and positive films as more positive (Allen et al., 2001).
This effect may not be limited to the alpha frequency band as frontal asymmetry in the gamma band has also been used to predict willingness to pay for a product (Ramsøy et al., 2018) and tourist destination preference (Ramsøy et al., 2019). Another metric that is gaining attention in neuroeconomics both in fMRI and EEG experiments is neural similarity within and between subjects, which is predictive of product preference not only for a certain individual but also on a population-level (out of sample; Genevsky et al., 2017; Chan et al., 2019; Leeuwis et al., 2021). However, since these are employed on a group level, they are beyond the scope of this discussion. These (and other) metrics provide value for neuromarketing applications where marketers improve their communication based on these brain responses.
In the environmental literature, there is a growing consensus that neuroscientific measurements provide an objective and promising tool to understand the neural mechanisms underlying sustainable behavior (Van Geffen et al., 2016; Goucher-Lambert et al., 2017; Sawe and Chawla, 2021; Wang and van den Berg, 2021). For instance, Vezich et al. (2017) conducted an fMRI study where subjects watched advertisements for sustainable and regular products and indicated their product preference on a survey. They found more positive ratings for sustainable products compared to regular ones; however, data from brain activity showed the opposite patterns: activations in the ventromedial prefrontal cortex and ventral striatum, which are associated with reward and personal value, were higher for regular products compared to green ones. This confirms that subjective reports might not reflect true personal value but rather a socially acceptable answer.
Moreover, in an EEG study, Lee et al. (2014) found increased theta activations in the frontal electrodes when comparing green to non-green consumers while they were processing an advertising message for a sustainable product, but this brain activity did not reflect the difference between the consumer groups during the processing of price information. The increase in frontal theta activity is related to attention (Aftanas and Golocheikine, 2001) and working memory (Bastiaansen and Hagoort, 2003), whereby these results might indicate that when consumers read an advertisement that matches their environmental goals, they are faced with greater demand for working memory resources as they have to activate their personal values and reward system (Lee et al., 2014).
The discussion provided in this section mainly focused on the neuroscientific findings that explain how values and motivations could lead to decision-making, however, they do not necessarily reflect the intention to act. Some studies suggest that neural indicators of intention can be located, for example in the form of the readiness potential which is related to the motor actions required for the action (Schmidt et al., 2016). We argue that behavioral intentions might also be inferred in hindsight by looking at neural indicators of cognitive dissonance as will be discussed in the following section.
Neural indicators of cognitive dissonance
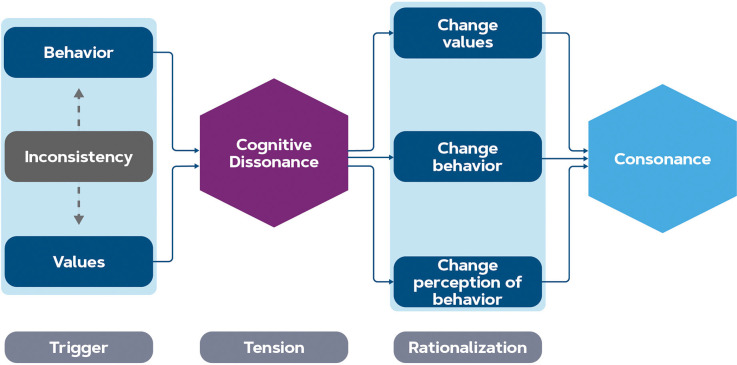
As the study of Lee et al. (2014) shows, there is a discrepancy in the brain patterns associated with green products when consumers process product advertisements in the absence of pricing information. When values are contradicting each other, this creates a trade-off, and subjects may experience cognitive dissonance. This means that cognitive dissonance typically happens when transitioning between values and behavior in the model discussed in Figure 1. Factors that impact this experience might be both individual and contextual. The theory of cognitive dissonance distinguishes three stages: a trigger (the inconsistency of contradicting values and behavior), psychological tension which is the state of cognitive dissonance, and the rationalization to relieve that tension (Vaidis and Bran, 2019), see Figure 3. The tension can be relieved in three ways; by changing values such that they line up with the behavior, changing the (future) behavior such that it lines up with the values, or changing a cognitive element that changes the perception of the behavior (Festinger, 1957). In our discussion, the term cognitive dissonance will reflect this state of psychological tension, and the trigger and rationalization will be discussed separately.
Figure 3.
Stages of cognitive dissonance relief. The inconsistency between behavior and values (motivational engagement stage) triggers a psychological tension called cognitive dissonance. Rationalization is a way of relieving this tension: changing values, changing the behavior, or changing the perception of the behavior help to reduce the dissonance.
From a neuroscience standpoint, detection of cognitive dissonance between behavior and values is mostly reported from the posterior medial frontal cortex (PMFC; Izuma and Murayama, 2019). In an fMRI study, it was found that the subject group in which cognitive dissonance was induced, changed their attitudes towards the task as compared to the group where the task was consonant with their values and the PMFC was activated when this justification happened (van Veen et al., 2009). More specifically, this activation was seen in the dorsal anterior cingulate cortex (ACC), which is the region of the ACC neighboring the PMFC (see Figure 2). The activations of the PMFC have been reported in fMRI studies with other tasks as well, e.g., during the free choice task (Izuma et al., 2010; Kitayama et al., 2013) and in Stroop tasks (Botvinick et al., 2001) where cognitive control in the presence of conflict is examined (in a Stroop task, subjects watch a list of color names that are printed in a color incongruent to the meaning of the word and must name the color and not the printed word). The reverse has also been shown: using TMS on the PMFC, Izuma et al. (2015) showed that down-regulation of the PMFC reduced the choice rationalization, while the control group still changed attitudes after a cognitive dissonance inducing task. Similar results have been found in the anterior insula (Izuma and Murayama, 2019), which is interpreted as the representation of negative emotion (Jarcho et al., 2011; Qin et al., 2011; Kitayama et al., 2013). Thus, the PMFC is associated with conflict monitoring and the negative outcome of it (the tension), making its function to act as a detector of cognitive dissonance in the brain (Izuma, 2013; Izuma and Murayama, 2019). The PMFC is also associated with reward prediction error and thereby might guide behavior in the future by updating predictions of behavior outcomes (Holroyd et al., 2003; Niv, 2009). However, these results must be interpreted with caution as the PMFC and anterior insula can be active during various cognitive functions and thereby their activity alone is not indicative of cognitive dissonance (Poldrack, 2011; Izuma and Murayama, 2019).
The brain functions of dorsal ACC and PMFC are explained by two different theories. The cognitive control theory of the dorsal ACC posits that its activity reflects conflict monitoring processes, e.g., when participants make responses that are dissonant with their intentions (emerging from their attitudes), it is sent to the DLPFC to adjust the level of cognitive control accordingly (rationalization; Harmon-Jones et al., 2008; Izuma and Murayama, 2019). Other theories propose reinforcement learning as an explanation: the dorsal ACC is activated by cognitive dissonance because it is processed as a negative outcome, thus the following attitude change that is aimed to prevent this negative outcome can be interpreted as reinforcement learning (Izuma, 2013).
Other neural correlates of cognitive dissonance that overlap with the decision-making regions include the DLPFC, posterior cingulate cortex, ventral striatum, and the hippocampus (Jarcho et al., 2011; Izuma and Murayama, 2019), see Figure 2 for their locations. Although the DLPFC is involved in multiple brain processes and, therefore, is not exclusively a predictor of cognitive dissonance (Poldrack, 2011; Izuma and Murayama, 2019), its relation with cognitive dissonance has been previously evidenced by neurofeedback training where two groups of subjects learned to either increase or decrease their relative left DPLFC activity (Harmon-Jones et al., 2008). After the training, subjects who trained to increase their left DLPFC changed their values, whereas the other group did not show any sign of rationalization of their choices compared to measurements before the training (Harmon-Jones et al., 2008). While such outcomes from neurofeedback training may not directly prove a causal relation (Kvamme et al., 2022), the effects were replicated also with direct brain stimulation using tDCS. This showed that stimulating the DLPFC increased cognitive control and subsequently the rationalization of responses by adjusting behavior or values (Mengarelli et al., 2015). The posterior cingulate cortex is also correlated to preference change after cognitive dissonance situations (Jarcho et al., 2011; Kitayama et al., 2013; Izuma and Murayama, 2019), although this is not replicated in all studies (Qin et al., 2011). The ventral striatum (and especially the nucleus accumbens) tracks changes in preferences by means of reward anticipation (Jarcho et al., 2011; Izuma and Murayama, 2019). While there is no evidence for the hippocampus being involved, it is unlikely that cognitive dissonance can arise without a memory of past behaviors (Izuma and Murayama, 2019).
Results regarding reward prediction error were also found with EEG experiments: stronger cognitive dissonance evoked a larger event-related potential in the frontocentral areas, similar to error-related negativity (ERN) when subjects selected products in a choice task (Colosio et al., 2017). This also happens when subjects want to present themselves favorably in the experiment (social desirability): individuals who had strong personal motivation to respond without prejudice but accidentally made responses that suggested they are racists showed stronger ERN and ACC activation (Amodio et al., 2004, 2008). ERN is generated in the ACC (Dehaene et al., 1994) and has been associated with errors in reward prediction (Holroyd et al., 2003), monitoring of action outcomes (Luu et al., 2004), and behavioral adjustments (Gehring et al., 2012). The activity observed in the (pre)frontal regions during the processing of cognitive dissonance shows that conscious conflict monitoring and reward prediction are responsible for the rationalization leading to attitude change, even when the subject is not conscious of the attitude change that has taken place (Mengarelli et al., 2015). Thus, in order to close the gap between intention and behavior, the cognitive control mechanisms in these regions might be targeted by an intervention.
Cognitive dissonance might provide a promising approach for behavior change: either the behavior is changed, and an attitude change follows; or the other way around where an attitude change evokes altered behavior (De Vos and Singleton, 2020) because once an individual commits to a given action, any information inconsistent with that commitment is likely to arouse dissonance and prevent the action from occurring (Harmon-Jones and Mills, 2019). Previous studies have already shown examples of attitude change through the experience of (un)pleasant actions (De Vos and Singleton, 2020). In this way, (neural) measurements of increased cognitive dissonance could help indicate if the consumer’s attitude has an impact on their behavior (or the other way around; Harmon-Jones et al., 2015), for example after an intervention has taken place. Especially when reported intentions cannot predict behavior (Sheeran, 2002), neural indicators of psychological tension might help interpret or even predict the outcome of the behavior change interventions. Since such a measure does not yet exist, future research investigating the neural markers of cognitive dissonance is encouraged (Izuma and Murayama, 2019).
Interventions for behavior change
In order for the behavior change to happen, it is essential that the reward and punishment effects are stored in the memory. Long-term memory consists of two types of learning: declarative (explicit) and non-declarative (implicit). Explicit memories can be intentionally and consciously recalled, and they encompass the memory of life events (episodic) and facts (semantic; Purves et al., 2013). On the other hand, implicit memories involve perceptional and emotional episodes that are unconsciously recalled and expressed through performance. Such implicit processes are theorized to govern behavior without awareness and are seen as important as explicit processes in self-regulation and behavior change (Nigg, 2017). The terms memory and learning are closely related and sometimes used interchangeably: memory is the series of processes whereby the nervous system acquires, retains, and uses new information to eventually guide behavior (Purves et al., 2013). Learning is used to describe memory encoding: it entails experiences that alter the nervous system, either spontaneously or via training (Purves et al., 2013). Because implicit memories may exist without the awareness of the person, this memory is expressed through changed behavior rather than self-reported measurements. The distinctions between the two terminologies are usually made from the neuroscientific perspective of memory and learning (Squire and Dede, 2015). Learning in the case of behavior change can aim at learning a new attitude or behavior, but can also serve to disrupt an existing habit by discontinuing the behavior (Verplanken and Orbell, 2022), which can be effectively triggered with interventions (Papies, 2017) as a facilitator of control over the existing behavior (Figure 1).
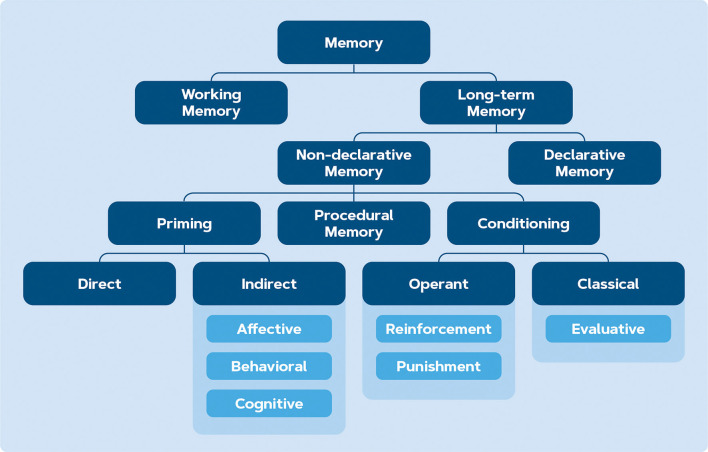
Implicit or non-declarative memory encompasses three types (see Figure 4): conditioning, skill learning, and priming (Purves et al., 2013). While skill learning might not be relevant within the context of behavior change and decision-making, the distinction between priming and conditioning needs to be elaborated upon. In conditioning, a neutral stimulus is paired with an appetitive/aversive stimulus over multiple trials in order to elicit a response (Hollands et al., 2011). With priming, prior exposure to a stimulus affects a subject’s reaction to a subsequent stimulus unconsciously (Weingarten et al., 2016). This effect is short-lived and happens unconsciously, while conditioning involves multiple trials and therefore its effects tend to last longer and on a more voluntary basis (Purves et al., 2013). Besides conditioning and priming, another commonly observed method in applied behavioral science is nudging: a technique in which, rather than changing the incentive, an addition or modification of the environment is made and then how this alteration influences the observer behavior is measured (Sunstein, 2021). Theoretically, nudging falls under the branch of conditioning because it steers behavior from a reinforcement perspective (Simon and Tagliabue, 2018). However, because it involves a single exposure, nudging will be discussed separately in this section. All three types of learning will be elaborated in the following sections.
Figure 4.
Branches of memory according to Purves et al. (2013).
Priming
Priming refers to an unconscious process where exposure to a stimulus influences the reaction to a subsequent stimulus (Weingarten et al., 2016). Three techniques of priming exist; cognitive, affective, and behavioral priming (Minton et al., 2017). The classic design of priming paradigms falls under the umbrella of cognitive priming and aimed at semantic or lexical associations: i.e., showing the word nurse primed the identification of doctor as a word (Fazio, 2001). Affective priming entails a reaction time procedure; when the processing of an emotional target (e.g., the word love) happens faster and more accurate when it is preceded by a consistent prime (e.g., sunshine) compared to an inconsistent word (e.g., death; Klauer and Musch, 2003). Behavioral or social priming is aimed at participation in the primed behavior (Minton et al., 2017); it investigates how the presentation attributes can impact behavior. For example, consumers primed with low-quality brand names were more likely to shop for low-value products (Laran et al., 2011), although the reliability of these results has been put into question (Sherman and Rivers, 2021).
Various brain patterns have been observed in priming studies. For example, the neural response is distinguished between (in)congruent responses: affectively incongruent trials had larger and more negative N200 activation and a later and more negative N400 than those in neutral trials (Zhang et al., 2006). Other affective priming studies using neuroscience have shown that brain activity was impacted after emotional primes: the aforementioned frontal asymmetry index during the informational part of the commercial was positively impacted after adding an emotional scene to the commercial, and on top of that, this increased the product preference of the subjects (Ohme et al., 2010). Subliminally (i.e., presenting a stimulus for such a short time that the subject cannot consciously perceive the stimulus) adding smileys in a promo video could alter theta and beta waves and influence the explicit preference rating of hotels (Hsu and Chen, 2020). More specifically, adding a happy smiley to promo videos improved the rankings for the corresponding hotels, and viewers of these videos showed increases in theta activity and decreases in beta power.
In environmental research, several studies have aimed at priming consumers towards more sustainable products. In one fMRI study, Lee et al. (2020) showed that priming the subject with a “green” label on fashion products (as opposed to climate change information in general) improved subjects’ preference for sustainable products and increased brain activations in regions that reflect relational reasoning when the subject was subsequently presented with green-labeled fashion products. This relational reasoning is reflected in the brain activity in the lingual gyrus and superior parietal lobule and evidences that consumers can be primed towards the ecologically friendly label (Lee et al., 2020). For instance, affective priming with a positive or negative environmental awareness cue impacted hotel booking intentions, moderated by environmental beliefs (Kim et al., 2021).
In another study, Bimonte et al. (2019) showed either a video where a smartphone was used in an urban environment and ended in the trash or a video where the smartphone was used in a natural environment and then recycled. They concluded that affective priming of the natural scene could increase Willingness to Pay (WTP) for recycled smartphones. Moreover, affective priming on social media using positively-valenced images and sustainability-framed advertisements increased hotel ratings and booking intentions, which were moderated by the environmental beliefs of the individual (Tanford et al., 2020). Priming could also be implemented to remind green consumers about their motivations and goals, both consciously and unconsciously (Custers and Aarts, 2005; Papies, 2017); when primed with a pro-environmental message, people tend to select the pro-environmental choice of unpackaged products compared to plastic-wrapped products (Tate et al., 2014) or products with a lower carbon footprint (Panzone et al., 2021).
Conditioning
Conditioning is the pairing of a neutral stimulus with an appetitive/aversive stimulus over multiple trials in order to elicit a response (Purves et al., 2013). The investigation of conditioning typically consists of two lines of experiments; Pavlovian conditioning (also known as classical conditioning) and operant conditioning (see Figure 4). Pavlov (1927) worked on Pavlovian conditioning, which is aimed at modifying the behavior by pairing two unrelated stimuli, one of which is likely to evoke the behavior. Thorndike (1898) and Skinner (1938) worked on a paradigm called operant or instrumental conditioning where the probability of a behavioral response is altered by associating the behavior with a reward or punishment (Purves et al., 2013). Typically, conditioning is applied for behavior change, but emotional applications have also emerged in the form of evaluative conditioning (also called affective or emotional conditioning), which aims to change the liking of the conditioned stimulus (Hofmann et al., 2010). For example, a neutral face paired with an attractive face makes the neutral face more positively valenced (Baeyens et al., 1992).
Evaluative conditioning is a form of classic conditioning and can be used for changing preferences by creating a relationship between actions and emotional responses (Eder et al., 2019; De Houwer and Hughes, 2020). In the health domain, evaluative conditioning has been successfully implemented in several studies for behavior change although the results have not been fully exclusive (Houben et al., 2010; Hollands et al., 2011; Hollands and Marteau, 2016; Papies, 2017). For example, when healthy food was paired with affective images (Halbeisen and Walther, 2021) or unhealthy food was paired with aversive images (Hollands et al., 2011), the preferences for products were changed, and subjects were more likely to pick a piece of fruit instead of the snack they would have chosen before the conditioning. Evaluative conditioning has also been used in promoting pro-vaccination attitudes: aversive cues (e.g., images showing sickness or death) in ads promoting flu vaccine products could enhance attitudes towards a co-occurring vaccine brand, but only when people were under a low attentional load (Fan et al., 2021). Another effect was found for drinking: after evaluative conditioning training, participants showed more negative attitudes toward beer, experienced less craving, and consumed less both in the lab during the taste test and outside the lab during the week following the manipulation (Houben et al., 2010). In the context of environmental research, images showing the environmental impact of products in virtual reality (VR) supermarket could influence the self-reported buying behavior toward more pro-environmental choices up to two weeks after the intervention (Meijers et al., 2021).
The neuroscience of operant conditioning is shown to rely on the hippocampus (Yin and Knowlton, 2006; Purves et al., 2013) as well as differential activity in the OFC and VMPFC, which are related to the perceived value of events (Valentin et al., 2007; Huang et al., 2020), see Figure 2. The fact that the OFC is essential for inferring the value of expected outcomes in action-outcome behavior is also shown by Howard et al. (2020), where TMS was used to attenuate activity in this region, and as a result, individuals exhibited more habitual behavior than the control condition. Applied studies investigating the neural reactions to evaluative conditioning include Bosshard et al. (2019), who found that when (dis)liked brand names were paired with (un)pleasant sounds explicit ratings of brand preference remained the same however EEG frontal asymmetry was impacted; it increased when disliked brands were coupled to pleasant sounds and similarly FAA was attenuated when preferred brands were coupled to unpleasant sounds. This indicates that during evaluative conditioning neural and behavioral responses are susceptible to change even though such a difference is not reflected in subjective measures.
Nudging
Nudging is aimed to increase the attractiveness of the behavior while freedom of choice still exists. Nudges might be used as reinforcement strategies by priming the goals/intentions of an individual (Michaelsen and Esch, 2021). They may heighten appetitive or aversive salience and hence lead to more motivation (either to approach or to avoid). In the area of healthy eating, the most effective nudges were behaviorally oriented, such as presenting the healthy option on a bigger plate or making it more convenient to select or consume the healthy option (Cadario and Chandon, 2020). Within the affect-oriented nudges, hedonic enhancements were most effective: a vivid description or attractive display of healthy food choices drove the selection of healthy options (Ensaff et al., 2015; Turnwald et al., 2017; Cadario and Chandon, 2020). Studies using VR showed that increasing the salience of the healthy options led to more healthy choices (Blom et al., 2021), also when the prices of healthy options increased (Hoenink et al., 2020).
Within environmental research, a typical nudge would aim to make the desired choice easier, e.g., a utility company provides energy from green sources unless otherwise requested, or the size and colors of waste bins are changed to make recycling more appealing (Grilli and Curtis, 2021). Nudging has been shown effective in 75% of the 85 case studies reviewed by Grilli and Curtis (2021), although most of these studies were aimed at energy conservation and waste management and only five studies aimed at changing people’s behaviors or general lifestyles. Other studies presented greener options as default, which also nudges toward more sustainable choices (Vetter and Kutzner, 2016; Ghesla et al., 2019; Sunstein, 2021). For reduction of energy consumption, nudging interventions such as providing feedback, default setting, or communication of norms have shown to be moderately effective (Composto and Weber, 2022). Particularly, feedback nudges seem to be quite effective in the short-term, but their effect fades over time (Ma et al., 2018). Byerly et al. (2018) provided an overview of nudges that have been investigated for several modalities of climate change actions such as meat consumption, transportation choices, and water use and showed that commitment nudges might be promising for behavior change in these areas: asking people to make a commitment will increase the likelihood of the behavior (Loy et al., 2016). However, there was no consensus with respect to nudging techniques and the related climate change action, which means that research must further elaborate the effects of nudging in underexposed climate actions such as lifestyle changes (Byerly et al., 2018).
While nudging may be a good solution for direct impact, such as selecting an organic product during online grocery shopping, there is no spillover to situations where the nudge is absent, such as other products in the same online store (Kuhn et al., 2021). This implicates that nudging techniques can be limited by temporary effects, although the frequent repetition of nudged behavior may lead to a new habit that is eventually independent of the nudge (Verplanken and Aarts, 1999; Lieberoth et al., 2018; Michaelsen and Esch, 2022).
Nudging experiments vary in terms of the experimental environment with studies conducted in real life (Ensaff et al., 2015; Elshiewy and Boztug, 2018), lab environments (Ghesla et al., 2019), online environments (Kuhn et al., 2021), and immersive VR (Hoenink et al., 2020; Blom et al., 2021; Meijers et al., 2021). The modality of nudging stimuli may impact the behavioral outcomes as learning effects could be mediated by the induced arousal or ecological validity of images, videos (Höffler and Leutner, 2007), or virtual reality (Alimardani et al., 2016; Liang et al., 2016; Coogan and He, 2018). Especially within PEB research, the difference between abstract and concrete visualizations is essential (Paswan et al., 2017; Duan et al., 2019; White et al., 2019), and therefore, it is important not only to consider the intervention paradigm but also the media type and environment through which the intervention is implemented.
What intervention is the most effective for longer-term behavior change?
In the previous sections, we discussed three intervention types for behavior change (i.e., priming, conditioning, and nudging, Figure 4) which target implicit processes underlying behavior change. Conditioning, nudging, and priming are types of non-declarative learning where the response is evoked by another stimulus. With conditioning, this pairing of stimulus and response often happens consciously because it involves multiple trials, while the response to a priming or nudging stimulus might happen unconsciously (Purves et al., 2013). Theoretically, this is why conditioning effects tend to last longer. However, there is scarce longitudinal evidence to compare the longer-term effects of each intervention type. For example in a 3-week priming experiment, the increase in green online shopping decisions was marginally observable in the first week but already absent in the second week of the priming intervention (Panzone et al., 2021). Conditioning in the virtual supermarket led to shopping decision changes until 2 weeks after the experiment (Meijers et al., 2021). Nudging showed some short-term longitudinal effect but this effect also faded over time (Ma et al., 2018). Since most studies only evaluate the direct effects of their employed intervention (e.g., Hollands et al., 2011; Tate et al., 2014; Bimonte et al., 2019), the longer-term effects cannot be directly compared.
The brain structures and neural patterns associated with each intervention type (as discussed in “Interventions for behavior change” Section) serve as examples that brain activity could change after PEB interventions. Particularly the reward system plays an important role in memory formation for behavior change (Michaelsen and Esch, 2021). As followed from Michaelsen and Esch (2021), the experience of a reward is most intense when the behavior to acquire it was cognitively processed. This could be the case for conditioning as well as priming and nudging: while priming and nudging are stimulus-directed and happen mostly unconsciously, the behavior might still be cognitively executed, for example after goal priming. Conditioning is a coupling between action and outcome that may be learned implicitly, whereafter the behavior is executed consciously. The brain structure sensitive to the distinction between goal-directed and stimulus-directed rewards is the ventromedial prefrontal cortex (VMPFC). Typical reinforcement learning happens through the amygdala that projects reward information to the hippocampus (Esch and Stefano, 2004), however, the conditioning effect is moderated by the cognitive integration of reward signals that happens in the VMPFC (Falk et al., 2010; Burns et al., 2018; Doré et al., 2019; Lieberman et al., 2019). The VMPFC relates to the personal valuation of both primary (e.g., food) and secondary (e.g., money) rewards (Bartra et al., 2013) and thereby provides a reward prediction that strengthens reinforcement learning (Schultz, 2006; Falk and Scholz, 2018). This means that the prediction error between the expected reward and the actual reward impacts future behavior: the execution of climate change actions might trigger positive emotions afterward and thereby activate a positive feedback loop where the behavior is maintained in anticipation of positive affect (Brosch, 2021). The activation in VMPFC is able to predict both individual and population-wide behavior (Falk et al., 2016) and thus provides a possible measure for intervention success. Additionally, frontal alpha asymmetry, which is an EEG measure of the approach motivation experienced by the individual, might infer attitude change with evaluative conditioning (De Pascalis et al., 2018; Harmon-Jones and Harmon-Jones, 2019).
To sum up, we demonstrated that implicit memory interventions have been shown effective in several areas (Hollands and Marteau, 2016; Meijers et al., 2021) and can further be strengthened by neuroscientific tools that enable monitoring of implicit processes before actual behavior change takes place. However, changing behavior via attitudes may have a limited impact on behavior change if they fail to translate the new behavior into long-term habits, or if they try to change existing habits that are insensitive to attitude (Verplanken and Orbell, 2022). Therefore, advancing knowledge on the relations between attitudes, intentions, behavior, and habits requires more research into individual factors of habits, the cues that are needed to trigger or disrupt the habit and the effectiveness of interventions (Gardner et al., 2021). Our review of different behavioral interventions indicated that there is a limited understanding of the longitudinal effects of each intervention type. In order to sufficiently compare the effectiveness of these intervention types, future research is encouraged. In the following section, we will elaborate on research directions that present open questions to further advance this field of research.
Research Gap and Future Directions
In the previous section, we reviewed studies that have shown the power of neuroscience and behavioral science in environmental research by either identifying how brain patterns vary between green and non-green consumers or how (implicit) interventions can alter the perception, attitude, and ultimately behavior of the consumers toward more sustainable choices. However, as illustrated in Section “Background”, the current literature is scattered over a multitude of measurements, intervention techniques, and outcome effects that make it difficult to draw firm conclusions. Therefore, in the light of the growing body of studies on this topic, it is important to develop a unified framework for the integration of neuroscience tools in PEB research in order to objectively examine the factors that play a role in consumer decision-making and hence effectively combat the human contribution to climate crisis.
Subsequently, an important undertaking for future research is the design and evaluation of an intervention that can effectively promote engagement in PEB among consumers. Interventions for more pro-environmental behavior have been analyzed by Grilli and Curtis (2021), who concluded that most studies were aimed at energy conservation and waste, whereas there were only five studies aimed at the change of lifestyle. From our literature review, we conclude that in order to close the gap between attitude and behavior and to motivate consumers to make the transition from intention to action, effective behavior change interventions must be designed and their long-term impacts must be investigated. Furthermore, neural markers might serve as implicit indicators of attitude change or cognitive dissonance related to the rationalization of subjects’ behavior, and therefore, future research is required to examine the empirical validity of these markers. By measuring brain responses during the interventions, we gather more insights into the neural processes that drive behavior change as well as the subconscious emotional variations that govern a consumer’s decision-making. To generalize the outcomes to the public-level finding, neuroforecasting could be an interesting tool for assessing the effectiveness of pro-environmental interventions (Knutson and Genevsky, 2018). In the following paragraphs, we summarize the open questions in this domain and based on them lay out research directions for future studies.
Open questions
Our review of the existing literature on environmental neuroscience indicated several open questions that remain unanswered. The most relevant research questions are presented in Table 1. They serve to support the outlined research directions and provide insight for future research in this field. Roughly, the open questions are divided into two domains: individual factors and contextual factors. The domain of individual factors focuses on questions about the human and neural mechanisms that drive PEB and thus could regulate the effectiveness of behavioral interventions. Within this domain, the relevant questions investigate how internal factors such as motivation, attitudes, and associations are related to brain activity and behavior of the consumer and whether these are subjective to interventions that aim to promote sustainable behavior. The domain of contextual factors focuses on the impact of external contributors that shape consumer’s decision-making and consumption behavior. In this domain, relevant questions include the effect of price, packaging, social norms, and stimuli for communication and conditioning.
Table 1.
Open questions in environmental neuroscience.
| Factor | Research questions |
|---|---|
| Individual factors |
|
| Contextual factors |
|
With the framework presented in Figure 1, behavior change interventions should be explored and compared in order to provide an overview of the outcome effects and their interaction with the indicated individual and contextual factors. Consumer neuroscience measurements could guide hypotheses in this field and evaluate the effectiveness of the intervention on attitude or behavior change and provide insight into the neural dynamics between green attitudes and behavior. The equilibrium of cognitive dissonance where attitude change might evoke behavior change or the other way around, is an interesting venue for future researchers. These questions aim to establish the potential of a joint model between behavioral interventions and consumer neuroscience in exploring pro-environmental decision-making.
Research directions
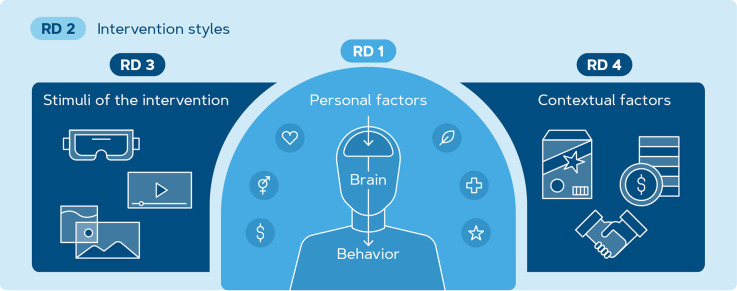
Following the literature review and the subsequent open questions, we outline four research directions we deem necessary to advance the integration of neuroscience tools in the investigation of PEB in consumers (Figure 5). They include: (1) identifying individual factors that affect emotional, behavioral, and neural responses to climate change communication, (2) studying the effectiveness of behavior change interventions on PEB engagement using neural and behavioral measures, (3) finding the most compelling and ecologically valid stimulus environment for communication of climate change messages to enhance the effectiveness of sustainable behavior change interventions, and (4) investigating the intensity and robustness of intervention effects when subjects are exposed to contextual factors.
Figure 5.
Schematic overview of factors that play a role in sustainable decision-making. Four research directions are outlined and elaborated based on identified factors. RD 1 focuses on individual factors contributing to PEB such as socio-economic status, demographics, attitudes, and beliefs of an individual. RD 2 aims to investigate the possibilities of an intervention for behavior change. In RD 3, the effect of learning stimuli and corresponding media type such as VR, video, or images is explored. RD 4 focuses on contextual factors within the learning paradigm for example packaging design, pricing, and social norms.
The first research direction (RD 1) is aimed at investigating the variability among consumers’ individual factors and how that interacts with emotional, behavioral, and neural responses when they are exposed to visualizations of climate change impact or a sustainable decision. These account for the degree of knowledge, attitude, normative compliance, and (perceived) control a user possesses to migrate from intention to behavior. Individual factors related to the sustainability of the consumer include but are not limited to: age, which is related to climate change engagement (Geiger et al., 2021); positionality in society, such as gender, socioeconomic status (Tichenor et al., 1970; Sligo and Jameson, 2000), and other social variables (Wolf and Moser, 2011); pro-environmental beliefs (Van Geffen et al., 2016) or the fact that the individual is already engaged in pro-environmental behavior (Brosch, 2021); climate change risk perceptions and climate anxiety (Clayton, 2020; Verplanken et al., 2020); knowledge of the climate crisis and products’ environmental impact (White et al., 2019); health consciousness (Koenig-Lewis et al., 2022); implementation intentions (Fennis et al., 2011); conceptions related to the self, e.g., self-concept, self-consistence, self-interest, and self-efficacy (White et al., 2019), self-relevance (Falk and Scholz, 2018), and self-control (Zhang and Zhang, 2022); positive or negative emotions that the individual experiences towards climate change or climate change actions (White et al., 2019; Brosch, 2021; Shiota et al., 2021); coping strategies (Taddicken and Wolff, 2020) and many more. The neural differentiations that have been found in individuals with higher/lower environmental beliefs, include the gamma (Van Geffen et al., 2016) and theta band (Lee et al., 2014) and areas in the DLPFC (Baumgartner et al., 2019). Further research is needed to establish the nature of these individual differences. For example, predictive modeling of the individual neural responses can contribute to pinpointing the neural markers of pro-environmental behavior “in the wild” and may help in predicting the success of the intervention on a population scale (Genevsky et al., 2017). If successful, these metrics can serve as reliable indicators of neural and behavior change for generalization of the proposed interventions to larger populations.
In RD 2, we propose to investigate interventions as a tool to change the consumer’s perception of the products and ultimately promote pro-environmental decision-making. This research direction could include investigation of the various interventions that were previously discussed: priming, conditioning, and nudging could be compared to each other as well as the modality of the intervention (i.e., affective, behavioral or cognitive). In studies investigating the cognitive dissonance that arises when animal-lovers eat meat, Rothgerber and Rosenfeld (2021) argue that tailoring interventions according to different populations might provide the most effective solution, as providing information on health or environment, lifestyle counseling, and daily text messaging have shown little to no effects. This tailoring to individual factors also provides an open question within this research direction where paradigms are designed and compared based on their emotional, cognitive, and behavioral impact both in the short and longer-term. For instance, visualizations of climate change impact have been shown to induce emotional response (Lehman et al., 2019; O’Neill and Nicholson-Cole, 2009) and engagement (O’Neill, 2020) in viewers, and therefore could be used as stimuli for conditioning or priming of consumers towards more positive emotions and reward associations towards green products. Prior environmental studies suggest that subjects with a higher environmental belief show differentiating activations in the gamma band as compared to the group with lower environmental concerns when they are exposed to images related to climate change (Van Geffen et al., 2016). However, the interaction between brain responses during exposure to such images and the behavior change that follows has sparsely been explored in sustainability research. Thus, it is important for future research to investigate the neural and behavioral responses before and after the intervention in the lab and real environments. In doing so, future researchers should employ data science techniques that can generalize beyond the sample (Genevsky et al., 2017) and predict the intervention outcome based on individual and contextual factors.
When investigating the different intervention types, the question would arise as to which stimulus type is most effective in eliciting emotional, neural, and behavioral responses. For example, stronger learning effects have been found for video compared to static images (Höffler and Leutner, 2007) or when learners are exposed to realistic settings in virtual reality (Alimardani et al., 2016; Liang et al., 2016; Coogan and He, 2018). The vividness of the proposed scenery is important in persuading consumers to intend behavior change (Fennis et al., 2011). Also, abstract or concrete visualizations have different impacts on the urge an individual feels to engage in climate change action (Paswan et al., 2017; White et al., 2019). Duan et al. (2019) found that concrete images of climate change such as floods or fires help people perceive the problem closer whereas those who viewed abstract images such as graphs and comics were more likely to perceive climate change as a spatially and temporally distant issue. The recent study by Meijers et al. (2021) examined the potential of VR technology by creating an immersive supermarket where subjects saw images of the corresponding climate change impact when they grabbed (un)sustainable products from the shelf. They found that green consumer choices increased up to 2 weeks after the intervention. Moreover, one can think about studies using apps and mobile notifications, where feedback nudges have shown to be able to provide a reminder for the behavior (Composto and Weber, 2022). This suggests that learning via immersive environments and new media design could be an effective tool and hence an interesting exploration avenue for future research as indicated by RD 3.
Finally, RD 4 targets the impact of several external factors that are not always accounted for in lab experiments. In real-life, various contextual factors compete for consumer’s attention during the decision-making in the supermarket (Newman et al., 2014; Boz et al., 2020; Wandosell et al., 2021), which might lead to the user having to compromise between their values (Bouman et al., 2021) and display a discrepancy between their attitudes and their behavior (Kennedy et al., 2009). These contextual factors include but are not limited to: the pricing of the products (Martinho et al., 2015; Li and Kallas, 2021); the persuasive design of the packaging (Steenis et al., 2017; Ischen et al., 2022); labels and communications on the packaging (Verplanken and Holland, 2002; Jin et al., 2018; White et al., 2019); social influences from others (van Riper et al., 2019; Bouman et al., 2021); visibility of the behavior (Brick et al., 2017); distractions due to choice overload in the supermarket (Grandi and Cardinali, 2020), social norms (Miller and Prentice, 2016; Steg, 2016; Farrow et al., 2017; Cialdini and Jacobson, 2021), and many more (Goucher-Lambert et al., 2017).
While the contribution of each individual or contextual factor to sustainable consumer behavior may be investigated individually, many of these factors can serve as a moderator of each other: gender, geographical region, sustainable attributes, and food categories influence the premium willingness of consumers to pay for a sustainable product (Li and Kallas, 2021). On the other hand, the habituation of behavior might limit the impact of these internal and external factors: while price increases are generally known to affect buying behavior (WHO,. 2016), the effect of raised prices is attenuated when consumers are habituated to buying a certain product or are heavy consumers, as shown for example on sugar-sweetened beverages (Cabrera Escobar et al., 2013) and alcohol (Chaloupka et al., 2002). Cognitive dissonance can be an important factor in mediating between these internal and external factors and therefore, we propose to investigate its neural indicators and their relation with PEB interventions using both comparative and predictive analyses. Neuroscience can provide a powerful tool in assessing the effect of individual and contextual factors to corroborate the impact of the proposed PEB interventions when they interplay in real-world decision-making, although future research is needed to uncover how we truly might exploit this tool.
Conclusion
In order to turn the tide of climate change crisis, consumer behavior change is essential. While consumers are increasingly aware of the need for sustainable action, this is not always executed. The gap between consumers’ intentions and actions gives rise to a state of cognitive dissonance, which makes it hard for individuals to rationalize their behavior or authentically reflect on it. This calls for the application of implicit measurements in environmental research to examine climate change attitudes and behavior in an objective manner. Neuroscience studies have shown to be able to predict behavior above and beyond self-reports by finding that (pre)frontal regions modulate the emotional responses to reward and punishment. We conclude that the cognitive dissonance that arises from the intention-behavior gap could be attenuated by evoking goal-directed behavior change. In this article, we reviewed conditioning, priming, and nudging as possible interventions for PEB and following consumer neuroscience literature we discussed their pros and cons for longer-term behavior change. Additionally, we identified open questions in the field and proposed four research directions in order to further investigate the role of neuroscience in consumer decision-making and PEB interventions. These include the individual and contextual factors that impact PEB as well as the type of learning and the stimulus types it uses. Combining the literature from environmental, behavioral, and neuroscience, we pave the path for future researchers to collaborate on designing an effective intervention to promote pro-environmental behavior among consumers.
Author Contributions
All authors contributed on the conception of the ideas and theories presented in this manuscript. NL wrote the draft of the manuscript under supervision of MA and TB. All authors contributed to the article and approved the submitted version.
Conflict of Interest
TB and NL are employed by the company Unravel Research. Authors’ employment does not depend on the outcomes or publication of this study nor does it compromise the scientific integrity of the study in any way. Authors adhere to the code of conduct and research integrity guidelines of Tilburg University. The study was reviewed by the ethics board of the department of Tilburg School of Humanities and Digital Sciences. The authors declare no financial or competing interest, that is, the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aftanas L. I., Golocheikine S. A. (2001). Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neurosci. Lett. 310, 57–60. 10.1016/s0304-3940(01)02094-8 [DOI] [PubMed] [Google Scholar]
- Alimardani M., Nishio S., Ishiguro H. (2016). The importance of visual feedback design in BCIs; from embodiment to motor imagery learning. PLoS One 11:e0161945. 10.1371/journal.pone.0161945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. J., Harmon-Jones E., Cavender J. H. (2001). Manipulation of frontal EEG asymmetry through biofeedback alters self-reported emotional responses and facial EMG. Psychophysiology 38, 685–693. 10.1111/1469-8986.3840685 [DOI] [PubMed] [Google Scholar]
- Alvino L. (2019). “How can we improve consumer behavior research? A critical literature review on the contributions and the limitations of consumer neuroscience,” in Proceedings of the 33rd International Business Information Management Association Conference, IBIMA 2019: Education Excellence and Innovation Management Through Vision 2020 (Granada, Spain: ). [Google Scholar]
- Amodio D. M., Devine P. G., Harmon-Jones E. (2008). Individual differences in the regulation of intergroup bias: the role of conflict monitoring and neural signals for control. J. Pers. Soc. Psychol. 94:60. 10.1037/0022-3514.94.1.60 [DOI] [PubMed] [Google Scholar]
- Amodio D. M., Harmon-Jones E., Devine P. G., Curtin J. J., Hartley S. L., Covert A. E., et al. (2004). Neural signals for the detection of unintentional race bias. Psychol. Sci. 15, 88–93. 10.1111/j.0963-7214.2004.01502003.x [DOI] [PubMed] [Google Scholar]
- Ariely D., Berns G. S. (2010). Neuromarketing: the hope and hype of neuroimaging in Business. Nat. Rev. Neurosci. 11:284. 10.1038/nrn2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad H. M., Saleem K., Shafi S., Ahmad T., Kanwal S. (2021). Environmental awareness, concern, attitude and behavior of university students: a comparison across academic disciplines. Pol. J. Environ. Stud. 30, 561–570. 10.15244/pjoes/122617 [DOI] [Google Scholar]
- Baeyens F., Eelen P., Van den Bergh O., Crombez G. (1992). The content of learning in human evaluative conditioning: acquired valence is sensitive to US-revaluation. Learn. Motiv. 23, 200–224. 10.1016/0023-9690(92)90018-H [DOI] [Google Scholar]
- Baldo D., Parikh H., Piu Y., Müller K. M. (2015). Brain waves predict success of new fashion products: a practical application for the footwear retailing industry: a practical application for the footwear retailing industry. J. Creating Value 1, 61–71. 10.1177/2394964315569625 [DOI] [Google Scholar]
- Bartra O., McGuire J. T., Kable J. W. (2013). The valuation system: a coordinate-based meta-analysis of bold fMRI experiments examining neural correlates of subjective value. Neuroimage 76, 412–427. 10.1016/j.neuroimage.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen M., Hagoort P. (2003). Event-induced theta responses as a window on the dynamics of memory. Cortex 39, 967–992. 10.1016/s0010-9452(08)70873-6 [DOI] [PubMed] [Google Scholar]
- Baumgartner T., Langenbach B. P., Gianotti L. R. R., Müri R. M., Knoch D. (2019). Frequency of everyday pro-environmental behavior is explained by baseline activation in lateral prefrontal cortex. Sci. Rep. 9, 1–7. 10.1038/s41598-018-36956-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayes R., Druckman J. N. (2021). Motivated reasoning and climate change. Curr. Opin. Behav. Sci. 42, 27–35. 10.1016/j.cobeha.2021.02.009 [DOI] [Google Scholar]
- Berridge K. C. (2018). Evolving concepts of emotion and motivation. Front. Psychol. 1647:1647. 10.3389/fpsyg.2018.01647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K. C., Kringelbach M. L. (2008). Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology 199, 457–480. 10.1007/s00213-008-1099-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte S., Bosco L., Stabile A. (2019). Nudging pro-environmental behavior: evidence from a web experiment on priming and WTP. J. Environ. Plann. Manage. 63, 651–668. 10.1080/09640568.2019.1603364 [DOI] [Google Scholar]
- Blom S. S., Gillebaart M., De Boer F., van der Laan N., De Ridder D. T. (2021). Under pressure: nudging increases healthy food choice in a virtual reality supermarket, irrespective of system 1 reasoning. Appetite 160:105116. 10.1016/j.appet.2021.105116 [DOI] [PubMed] [Google Scholar]
- Bosshard S., Koller M., Walla P. (2019). Can evaluative conditioning change well-established attitudes towards popular brands? Your brain says yes even though your mouth says no. Brain Sci. 9:106. 10.3390/brainsci9050106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M. M., Braver T. S., Barch D. M., Carter C. S., Cohen J. D. (2001). Conflict monitoring and cognitive control. Psychol. Rev. 108:624. 10.1037/0033-295x.108.3.624 [DOI] [PubMed] [Google Scholar]
- Bouman T., Steg L., Perlaviciute G. (2021). From values to climate action. Curr. Opin. Psychol. 42, 102–107. 10.1016/j.copsyc.2021.04.010 [DOI] [PubMed] [Google Scholar]
- Boz Z., Korhonen V., Sand C. K. (2020). Consumer considerations for the implementation of sustainable packaging: a review. Sustainability 12:2192. 10.3390/su12062192 [DOI] [Google Scholar]
- Brick C., Sherman D. K., Kim H. S. (2017). “Green to be seen” and “brown to keep down”: visibility moderates the effect of identity on pro-environmental behavior. J. Environ. Psychol. 51, 226–238. 10.1016/j.jenvp.2017.04.004 [DOI] [Google Scholar]
- Brosch T. (2021). Affect and emotions as drivers of climate change perception and action: a review. Curr. Opin. Behav. Sci. 42, 15–21. 10.1016/j.cobeha.2021.02.001 [DOI] [Google Scholar]
- Burns S. M., Barnes L. N., Katzman P. L., Ames D. L., Falk E. B., Lieberman M. D., et al. (2018). A functional near infrared spectroscopy (fNIRS) replication of the sunscreen persuasion paradigm. Soc. Cogn. Affect. Neurosci. 13, 628–636. 10.1093/scan/nsy030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly H., Balmford A., Ferraro P. J., Wagner C. H., Palchak E., Polasky S. (2018). Nudging pro-environmental behavior: evidence and opportunities. Front. Ecol. Environ. 16, 159–168. 10.1093/scan/nsy030 [DOI] [Google Scholar]
- Cabrera Escobar M. A., Veerman J. L., Tollman S. M., Bertram M. Y., Hofman K. J. (2013). Evidence that a tax on sugar sweetened beverages reduces the obesity rate: a meta-analysis. BMC Public Health 13, 1–10. 10.1186/1471-2458-13-1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo J. T., Cacioppo S., Petty R. E. (2018). The neuroscience of persuasion: a review with an emphasis on issues and opportunities. Soc. Neurosci. 13, 129–172. 10.1080/17470919.2016.1273851 [DOI] [PubMed] [Google Scholar]
- Cadario R., Chandon P. (2020). Which healthy eating nudges work best? A meta-analysis of field experiments. Marketing Sci. 39, 465–486. 10.1287/mksc.2018.1128 [DOI] [Google Scholar]
- Cairns H. M., Ritch E. L., Bereziat C. (2022). Think eco, be eco? The tension between attitudes and behaviors of millennial fashion consumers. Int. J. Conscious Stud. 46, 1262–1277. 10.1111/ijcs.12756 [DOI] [Google Scholar]
- Calculli C., D’Uggento A. M., Labarile A., Ribecco N. (2021). Evaluating people’s awareness about climate changes and environmental issues: a case study. J. Cleaner Prod. 324:129244. 10.1016/j.jclepro.2021.129244 [DOI] [Google Scholar]
- Carver C. S., White T. L. (1994). Behavioral inhibition, behavioral activation and affective responses to impending reward and punishment: the BIS/BAS scales. J. Pers. Soc. Psychol. 67:319. [Google Scholar]
- Chaiken S. (1980). Heuristic versus systematic information processing and the use of source versus message cues in persuasion. J. Pers. Soc. Psychol. 39:752. [Google Scholar]
- Chaloupka F. J., Grossman M., Saffer H. (2002). The effects of price on alcohol consumption and alcohol-related problems. Alcohol Res. Health 26:22. [PMC free article] [PubMed] [Google Scholar]
- Chan H.-Y., Smidts A., Schoots V. C., Dietvorst R. C., Boksem M. A. (2019). Neural similarity at temporal lobe and cerebellum predicts out-of-sample preference and recall for video stimuli. Neuroimage 197, 391–401. 10.1016/j.neuroimage.2019.04.076 [DOI] [PubMed] [Google Scholar]
- Cialdini R. B., Jacobson R. P. (2021). Influences of social norms on climate change-related behaviors. Curr. Opin. Behav. Sci. 42, 1–8. 10.1016/j.cobeha.2021.01.005 [DOI] [Google Scholar]
- Clayton S. (2020). Climate anxiety: psychological responses to climate change. J. Anxiety Disord. 74:102263. 10.1016/j.janxdis.2020.102263 [DOI] [PubMed] [Google Scholar]
- Coan J. A., Allen J. J. B. (2003). Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology 40, 106–114. 10.1111/1469-8986.00011 [DOI] [PubMed] [Google Scholar]
- Colosio M., Shestakova A., Nikulin V. V., Blagovechtchenski E., Klucharev X. (2017). Neural mechanisms of cognitive dissonance (revised): an EEG study. J. Neurosci. 37, 5074–5083. 10.1523/JNEUROSCI.3209-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Composto J. W., Weber E. U. (2022). Effectiveness of behavioral interventions to reduce household energy demand: a scoping review. Environ. Res. Lett. 17:063005. 10.1088/1748-9326/ac71b8 [DOI] [Google Scholar]
- Coogan C. G., He B. (2018). Brain-computer interface control in a virtual reality environment and applications for the internet of things. IEEE Access 6, 10840–10849. 10.1109/ACCESS.2018.2809453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custers R., Aarts H. (2005). Positive affect as implicit motivator: on the nonconscious operation of behavioral goals. J. Pers. Soc. Psychol. 89:129. 10.1037/0022-3514.89.2.129 [DOI] [PubMed] [Google Scholar]
- Davidson R. J. (1984). “Affect, cognition and hemispheric specialization,” in Emotions, Cognition and Behavior, eds InIzard C. E., Kagan J., Zajonc R. (New York: Cambridge University Press; ), 320–365. [Google Scholar]
- De Houwer J., Hughes S. (2020). Learning to like or dislike: revealing similarities and differences between evaluative learning effects. Curr. Dir. Psychol. Sci. 29, 487–491. 10.1177/0963721420924752 [DOI] [Google Scholar]
- De Pascalis V., Sommer K., Scacchia P. (2018). Resting frontal asymmetry and reward sensitivity theory motivational traits. Sci. Rep. 8, 1–9. 10.1038/s41598-018-31404-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos J., Singleton P. A. (2020). Travel and cognitive dissonance. Trans. Res. A Policy Prac. 138, 525–536. 10.1016/j.tra.2020.06.014 [DOI] [Google Scholar]
- Dehaene S., Posner M. I., Tucker D. M. (1994). Localization of a neural system for error detection and compensation. Psychol. Sci. 5, 303–305. [Google Scholar]
- Di Gruttola F., Malizia A. P., D’Arcangelo S., Lattanzi N., Ricciardi E., Orfei M. D. (2021). The relation between consumers’ frontal alpha asymmetry, attitude and investment decision. Front. Neurosci. 14:1414. 10.3389/fnins.2020.577978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré B. P., Tompson S. H., O’Donnell M. B., An L. C., Strecher V., Falk E. B. (2019). Neural mechanisms of emotion regulation moderate the predictive value of affective and value-related brain responses to persuasive messages. J. Neurosci. 39, 1293–1300. 10.1523/JNEUROSCI.1651-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan R., Takahashi B., Zwickle A. (2019). Abstract or concrete? The effect of climate change images on people’s estimation of egocentric psychological distance. Public Underst. Sci. 28, 828–844. 10.1177/0963662519865982 [DOI] [PubMed] [Google Scholar]
- Eder A. B., Krishna A., Van Dessel P. (2019). Operant evaluative conditioning. J. Exp. Psychol. Anim. Learn. Cogn. 45, 102–110. 10.1037/xan0000189 [DOI] [PubMed] [Google Scholar]
- Elliot A. J., Eder A. B., Harmon-Jones E. (2013). Approach-avoidance motivation and emotion: convergence and divergence. Emot. Rev. 5, 308–311. 10.1177/1754073913477517 [DOI] [Google Scholar]
- Elshiewy O., Boztug Y. (2018). When back of pack meets front of pack: how salient and simplified nutrition labels affect food sales in supermarkets. J. Public Policy Market. 37, 55–67. 10.1509/jppm.16.100 [DOI] [Google Scholar]
- Ensaff H., Homer M., Sahota P., Braybrook D., Coan S., McLeod H. (2015). Food choice architecture: an intervention in a secondary school and its impact on students’ plant-based food choices. Nutrients 7, 4426–4437. 10.3390/nu7064426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch T., Stefano G. B. (2004). The neurobiology of pleasure, reward processes, addiction and their health implications. Neuro Endocrinol. Lett. 25, 235–251. [PubMed] [Google Scholar]
- Evans J. S. B. (2009). “How many dual-process theories do we need? One, two, or many?,” in Two Minds: Dual Processes and Beyond, eds Evans J. S. B. T., Frankish K. (Oxford: Oxford University Press; ), 33–54. [Google Scholar]
- Falk E. B., Berkman E. T., Mann T., Harrison B., Lieberman M. D. (2010). Predicting persuasion-induced behavior change from the brain. J. Neurosci. 30, 8421–8424. 10.1523/JNEUROSCI.0063-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E. B., Berkman E. T., Whalen D., Lieberman M. D. (2011). Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychol. 30:177. 10.1037/a0022259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E. B., O’Donnell M. B., Tompson S., Gonzalez R., Dal Cin S., Strecher V., et al. (2016). Functional brain imaging predicts public health campaign success. Soc. Cogn. Affect. Neurosci. 11, 204–214. 10.1093/scan/nsv108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E., Scholz C. (2018). Persuasion, influence and value: perspectives from communication and social neuroscience. Annu. Rev. Psychol. 69, 329–356. 10.1146/annurev-psych-122216-011821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Bodenhausen G. V., Lee A. Y. (2021). Acquiring favorable attitudes based on aversive affective cues: examining the spontaneity and efficiency of propositional evaluative conditioning. J. Exp. Soc. Psychol. 95:104139. 10.1016/j.jesp.2021.104139 [DOI] [Google Scholar]
- Farrow K., Grolleau G., Ibanez L. (2017). Social norms and pro-environmental behavior: a review of the evidence. Ecol. Econ. 140, 1–13. 10.1016/j.ecolecon.2017.04.017 [DOI] [Google Scholar]
- Fazio R. H. (2001). On the automatic activation of associated evaluations: an overview. Cogn. Emot. 15, 115–141. 10.1080/02699930125908 [DOI] [Google Scholar]
- Fennis B. M., Adriaanse M. A., Stroebe W., Pol B. (2011). Bridging the intention-behavior gap: inducing implementation intentions through persuasive appeals. J. Consumer Psychol. 21, 302–311. 10.1016/j.jcps.2010.12.003 [DOI] [Google Scholar]
- Festinger L. (1957). A Theory of Cognitive Dissonance. Redwood City, CA: Stanford University Press. [Google Scholar]
- Fishbein M., Ajzen I. (2011). Predicting and Changing Behavior: The Reasoned Action Approach. New York: Psychology press. [Google Scholar]
- Foxall G. R. (2016). Metacognitive control of categorial neurobehavioral decision systems. Front. Psychol. 7:170. 10.3389/fpsyg.2016.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable P. A., Poole B. D. (2012). Time flies when you’re having approach-motivated fun: effects of motivational intensity on time perception. Psychol. Sci. 23, 879–886. 10.1177/0956797611435817 [DOI] [PubMed] [Google Scholar]
- Gardner B., Arden M. A., Brown D., Eves F. F., Green J., Hamilton K., et al. (2021). Developing habit-based health behavior change interventions: twenty-one questions to guide future research. Psychol. Health 14, 1–23. 10.1080/08870446.2021.2003362 [DOI] [PubMed] [Google Scholar]
- Gehring W. J., Liu Y., Orr J. M., Carp J. (2012). The error-related negativity (ERN/Ne),” in Oxford Handbook of Event-Related Potential Components, eds Luck S. J., Kappenman E. S. (New York, NY: Oxford University Press; ), 231–291. [Google Scholar]
- Geiger N., McLaughlin B., Velez J. (2021). Not all boomers: temporal orientation explains inter-and intra-cultural variability in the link between age and climate engagement. Climatic Change 166, 1–20. 10.1007/s10584-021-03116-x [DOI] [Google Scholar]
- Genevsky A., Yoon C., Knutson B. (2017). When brain beats behavior: neuroforecasting crowdfunding outcomes. J. Neurosci. 37, 8625–8634. 10.1523/JNEUROSCI.1633-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghesla C., Grieder M., Schmitz J. (2019). Nudge for good? choice defaults and spillover effects. Front. Psychol. 10:178. 10.3389/fpsyg.2019.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleim M. R., Smith J. S., Andrews D., Cronin Jr J. J. (2013). Against the green: a multi-method examination of the barriers to green consumption. J. Retailng 89, 44–61. 10.1016/j.jretai.2012.10.001 [DOI] [Google Scholar]
- Gollwitzer P. M. (1990). Action phases and mind-sets. Handb. Motiv. Cogn. Found. Soc. Behav. 2, 53–92. [Google Scholar]
- Golnar-Nik P., Farashi S., Safari M.-S. (2019). The application of EEG power for the prediction and interpretation of consumer decision-making: a neuromarketing study. Physiol. Behav. 207, 90–98. 10.1016/j.physbeh.2019.04.025 [DOI] [PubMed] [Google Scholar]
- Goucher-Lambert K., Moss J., Cagan J. (2017). Inside the mind: using neuroimaging to understand moral product preference judgments involving sustainability. J. Mech. Des. 139:41103. 10.1115/1.4035859 [DOI] [Google Scholar]
- Granato G., Fischer A. R. H., van Trijp H. C. M. (2021). A meaningful reminder on sustainability: when explicit and implicit packaging cues meet. J. Environ. Psychol. 79:101724. 10.1016/j.jenvp.2021.101724 [DOI] [Google Scholar]
- Grandi B., Cardinali M. G. (2020). Choice overload in the grocery setting: results from a laboratory experiment. Int. Bus. Res. 14:94. 10.5539/ibr.v14n1p94 [DOI] [Google Scholar]
- Grayot J. D. (2020). Dual process theories in behavioral economics and neuroeconomics: a critical review. Rev. Phil. Psychol. 11, 105–136. 10.1007/s13164-019-00446-9 [DOI] [Google Scholar]
- Grilli G., Curtis J. (2021). Encouraging pro-environmental behaviors: a review of methods and approaches. Renewable Sustainable Energy 135:110039. 10.1016/j.rser.2020.110039 [DOI] [Google Scholar]
- Hakim A., Levy D. J. (2019). A gateway to consumers’ minds: achievements, caveats and prospects of electroencephalography-based prediction in neuromarketing. Wiley Interdiscip. Rev. Cogn. Sci. 10:e1485. 10.1002/wcs.1485 [DOI] [PubMed] [Google Scholar]
- Halbeisen G., Walther E. (2021). How to promote healthy eating in preschool children: evidence from an associative conditioning procedure with non-food stimuli. Appetite 166:105472. 10.1016/j.appet.2021.105472 [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Harmon-Jones C., Fearn M., Sigelman J. D., Johnson P. (2008). Left frontal cortical activation and spreading of alternatives: tests of the action-based model of dissonance. J. Pers. Soc. Psychol. 94:101. 10.1037/0022-3514.94.1.1 [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Harmon-Jones C., Levy N. (2015). An action-based model of cognitive-dissonance processes. Curr. Direct. Psychol. Sci. 24, 184–189. 10.1177/0963721414566449 [DOI] [Google Scholar]
- Harmon-Jones C., Harmon-Jones E. (2019). A broad consideration of motivation, with a focus on approach motivation. Psychol. Inquiry 30, 132–135. 10.1080/1047840X.2019.1646043 [DOI] [Google Scholar]
- Harmon-Jones E., Mills J. (2019). “An introduction to cognitive dissonance theory and an overview of current perspectives on the theory,” in Cognitive Dissonance: Reexamining a Pivotal Theory in Psychology. ed E. Harmon-Jones (Washington, DC: American Psychology Association; ), 3–24. 10.1037/0000135-001 [DOI] [Google Scholar]
- Harmon-Jones E., Sigelman J. (2001). State anger and prefrontal brain activity: evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. J. Pers. Soc. Psychol. 80, 797–803. 10.1037//0022-3514.80.5.797 [DOI] [PubMed] [Google Scholar]
- Hassan L. M., Shiu E., Shaw D. (2016). Who says there is an intention-behavior gap? Anssessing the empirical evidence of an intention-behavior gap in ethical consumption. J. Bus. Ethics 136, 219–236. 10.1007/s10551-014-2440-0 [DOI] [Google Scholar]
- Hiser J., Koenigs M. (2018). The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition and psychopathology. Biol. Psychiatry 83, 638–647. 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenink J. C., Mackenbach J. D., Waterlander W., Lakerveld J., van der Laan N., Beulens J. W. J. (2020). The effects of nudging and pricing on healthy food purchasing behavior in a virtual supermarket setting: a randomized experiment. Int. J. Behav. Nutr. Phys. Activ. 17:98. 10.1186/s12966-020-01005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höffler T. N., Leutner D. (2007). Instructional animation versus static pictures: a meta-analysis. Learn. Instruct. 17, 722–738. 10.1016/j.learninstruc.2007.09.013 [DOI] [Google Scholar]
- Hofmann W., De Houwer J., Perugini M., Baeyens F., Crombez G. (2010). Evaluative conditioning in humans: a meta-analysis. Psychol. Bull. 136, 390–421. 10.1037/a0018916 [DOI] [PubMed] [Google Scholar]
- Hollands G. J., Marteau T. M. (2016). Pairing images of unhealthy and healthy foods with images of negative and positive health consequences: impact on attitudes and food choice. Health Psychol. 35:847. 10.1037/hea0000293 [DOI] [PubMed] [Google Scholar]
- Hollands G. J., Prestwich A., Marteau T. M. (2011). Using aversive images to enhance healthy food choices and implicit attitudes: an experimental test of evaluative conditioning. Health Psychol. 30:195. 10.1037/a0022261 [DOI] [PubMed] [Google Scholar]
- Holroyd C. B., Nieuwenhuis S., Yeung N., Cohen J. D. (2003). Errors in reward prediction are reflected in the event-related brain potential. Neuroreport 14, 2481–2484. 10.1097/00001756-200312190-00037 [DOI] [PubMed] [Google Scholar]
- Houben K., Schoenmakers T. M., Wiers R. W. (2010). I didn’t feel like drinking but I don’t know why: the effects of evaluative conditioning on alcohol-related attitudes, craving and behavior. Addict. Behav. 35, 1161–1163. 10.1016/j.addbeh.2010.08.012 [DOI] [PubMed] [Google Scholar]
- Howard J. D., Reynolds R., Smith D. E., Voss J. L., Schoenbaum G., Kahnt T. (2020). Targeted stimulation of human orbitofrontal networks disrupts outcome-guided behavior. Curr. Biol. 30, 490–498. e494. 10.1016/j.cub.2019.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L., Chen Y. J. (2020). Neuromarketing, subliminal advertising and hotel selection: an EEG study. Aus. Market. J. 28, 200–208. 10.1016/j.ausmj.2020.04.009 [DOI] [Google Scholar]
- Huang Y., Yaple Z. A., Yu R. (2020). Goal-oriented and habitual decisions: neural signatures of model-based and model-free learning. Neuroimage 215:116834. 10.1016/j.neuroimage.2020.116834 [DOI] [PubMed] [Google Scholar]
- Ischen C., Meijers M. H., Vandeberg L., Smit E. G. (2022). Seen as green? Assessing the salience and greenness of environmentally friendly packaging cues. J. Food Prod. Market. 10, 1–18. 10.1080/10454446.2022.2038757 [DOI] [Google Scholar]
- Izuma K. (2013). The neural basis of social influence and attitude change. Curr. Opin. Neurobiol. 23, 456–462. 10.1016/j.conb.2013.03.009 [DOI] [PubMed] [Google Scholar]
- Izuma K., Akula S., Murayama K., Wu D.-A., Iacoboni M., Adolphs R. (2015). A causal role for posterior medial frontal cortex in choice-induced preference change. J. Neurosci. 35, 3598–3606. 10.1523/JNEUROSCI.4591-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K., Murayama K. (2019). “Neural basis of cognitive dissonance,” in Cognitive Dissonance: Reexamining a Pivotal Theory in Psychology, eds Harmon-Jones E. (Washington, DC: American Psychology Association; ), 227–245. [Google Scholar]
- Izuma K., Matsumoto M., Murayama K., Samejima K., Sadato N., Matsumoto K. (2010). Neural correlates of cognitive dissonance and choice-induced preference change. Proc. Natl. Acad. Sci. U S A 107, 22014–22019. 10.1073/pnas.1011879108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho J. M., Berkman E. T., Lieberman M. D. (2011). The neural basis of rationalization: cognitive dissonance reduction during decision-making. Soc. Cogn. Affect. Neurosci. 6, 460–467. 10.1093/scan/nsq054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javor A., Koller M., Lee N., Chamberlain L., Ransmayr G. (2013). Neuromarketing and consumer neuroscience: contributions to neurology. BMC Neurol. 13, 1–12. 10.1186/1471-2377-13-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerzyk E. (2016). Design and communication of ecological content on sustainable packaging in young consumers’ opinions. J. Food Prod. Market. 22, 707–716. 10.1080/10454446.2015.1121435 [DOI] [Google Scholar]
- Ji M. F., Wood W. (2007). Purchase and consumption habits: not necessarily what you intend. J. Consumer Psychol. 17, 261–276. 10.1016/S1057-7408(07)70037-2 [DOI] [Google Scholar]
- Jin J., Dou X., Meng L., Yu H. (2018). Environmental-friendly eco-labeling matters: evidences from an ERPs study. Front. Hum. Neurosci. 12:417. 10.3389/fnhum.2018.00417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. (2003). A perspective on judgment and choice: mapping bounded rationality. Am. Psychol. 58:697. 10.1037/0003-066X.58.9.697 [DOI] [PubMed] [Google Scholar]
- Kaiser F. G., Ranney M., Hartig T., Bowler P. A. (1999). Ecological behavior, environmental attitude and feelings of responsibility for the environment. Eur. Psychol. 4:59. 10.1027//1016-9040.4.2.59 [DOI] [Google Scholar]
- Kennedy E. H., Beckley T. M., McFarlane B. L., Nadeau S. (2009). Why we don’t “walk the talk”: understanding the environmental values/behavior gap in Canada. Hum. Ecol. Rev. 1, 151–160. [Google Scholar]
- Keren G., Schul Y. (2009). Two is not always better than one: a critical evaluation of two-system theories. Perspect. Psychol. Sci. 4, 533–550. 10.1111/j.1745-6924.2009.01164.x [DOI] [PubMed] [Google Scholar]
- Kim E. J., Tanford S., Book L. A. (2021). The effect of priming and customer reviews on sustainable travel behaviors. J. Travel Res. 60, 86–101. 10.1177/0047287519894069 [DOI] [Google Scholar]
- Kitayama S., Chua H. F., Tompson S., Han S. (2013). Neural mechanisms of dissonance: an fMRI investigation of choice justification. Neuroimage 69, 206–212. 10.1016/j.neuroimage.2012.11.034 [DOI] [PubMed] [Google Scholar]
- Klauer K. C., Musch J. (2003). “Affective priming: Findings and theories,” in The Psychology of Evaluation: Affective Processes in Cognition and Emotion, eds Musch J., Klauer K. C. (New York: Lawrence Erlbaum Associates Publishers; ), 7–49. [Google Scholar]
- Knutson B., Genevsky A. (2018). Neuroforecasting aggregate choice. Curr. Dir. Psychol. Sci. 27, 110–115. 10.1177/0963721417737877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Greer S. M. (2008). Anticipatory affect: neural correlates and consequences for choice. Philos. Trans. R Soc. Lond. B Biol. Sci. 363, 3771–3786. 10.1098/rstb.2008.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig-Lewis N., Grazzini L., Palmer A. (2022). Cakes in plastic: a study of implicit associations of compostable bio-based versus plastic food packaging. Resources Conserv. Recycling 178:105977. 10.1016/j.resconrec.2021.105977 [DOI] [Google Scholar]
- Kruglanski A. W., Szumowska E. (2020). Habitual behavior is goal-driven. Perspect. Psychol. Sci. 15, 1256–1271. 10.1177/1745691620917676 [DOI] [PubMed] [Google Scholar]
- Kuhn S., Ihmels M., Kutzner F. (2021). Organic defaults in online-shopping: immediate effects but no spillover to similar choices. J. Consumer Behav. 20, 271–287. 10.1002/cb.1850 [DOI] [Google Scholar]
- Kvamme T. L., Ros T., Overgaard M. (2022). Can neurofeedback provide evidence of direct brain-behavior causality? Neuroimage 258:119400. 10.1016/j.neuroimage.2022.119400 [DOI] [PubMed] [Google Scholar]
- Langenbach B. P., Berger S., Baumgartner T., Knoch D. (2020). Cognitive resources moderate the relationship between pro-environmental attitudes and green behavior. Environ. Behav. 22, 979–995. 10.1177/0013916519843127 [DOI] [Google Scholar]
- Lansink C. S., Goltstein P. M., Lankelma J. V., McNaughton B. L., Pennartz C. M. (2009). Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 7:e1000173. 10.1371/journal.pbio.1000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laran J., Dalton A. N., Andrade E. B. (2011). The curious case of behavioral backlash: why brands produce priming effects and slogans produce reverse priming effects. J. Consumer Res. 37, 999–1014. 10.1086/656577 [DOI] [Google Scholar]
- Lee E. J., Choi H., Han J., Kim D. H., Ko E., Kim K. H. (2020). How to “Nudge” your consumers toward sustainable fashion consumption: an fMRI investigation. J. Bus. Res. 117, 642–651. 10.1016/j.jbusres.2019.09.050 [DOI] [Google Scholar]
- Lee E. J., Kwon G., Shin H. J., Yang S., Lee S., Suh M. (2014). The spell of green: can frontal EEG activations identify green consumers? J. Bus. Ethics 122, 511–521. 10.1007/s10551-013-1775-2 [DOI] [Google Scholar]
- Leeuwis N., Alimardani M., Bommel T. (2022). “Neuromarketing as a tool for environmental conditioning and sustainable consumption,” in Neuroergonomics and Cognitive Engineering. AHFE (2022) International Conference, Vol. 42, ed H. Ayaz (USA: AHFE International). 10.54941/ahfe100182331993656 [DOI] [Google Scholar]
- Leeuwis N., Pistone D., Flick N., van Bommel T. (2021). A sound prediction: EEG-based neural synchrony predicts online music streams. Front. Psychol. 12:672980. 10.3389/fpsyg.2021.672980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman B., Thompson J., Davis S., Carlson J. M. (2019). Affective images of climate change. Front. Psychol. 10:960. 10.3389/fpsyg.2019.00960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Kallas Z. (2021). Meta-analysis of consumers’ willingness to pay for sustainable food products. Appetite 163:105239. 10.1016/j.appet.2021.105239 [DOI] [PubMed] [Google Scholar]
- Liang S., Choi K. S., Q26n J., Pang W. M., Wang Q., Heng P. A. (2016). Improving the discrimination of hand motor imagery via virtual reality based visual guidance. Comput. Methods Programs Biomed. 132, 63–74. 10.1016/j.cmpb.2016.04.023 [DOI] [PubMed] [Google Scholar]
- Lieberman M. D., Straccia M. A., Meyer M. L., Du M., Tan K. M. (2019). Social, self, (situational) and affective processes in medial prefrontal cortex (MPFC): causal, multivariate and reverse inference evidence. Neurosci. Biobehav. Rev. 99, 311–328. 10.1016/j.neubiorev.2018.12.021 [DOI] [PubMed] [Google Scholar]
- Lieberoth A., Jensen N. H., Bredahl T. (2018). Selective psychological effects of nudging, gamification and rational information in converting commuters from cars to buses: a controlled field experiment. Transport. Res. F Traffic Psychol. Behav. 55, 246–261. 10.1016/j.trf.2018.02.016 [DOI] [Google Scholar]
- Liu Y. J., Yu M., Zhao G., Song J., Ge Y., Shi Y. (2018). Real-time movie-induced discrete emotion recognition from EEG signals. IEEE Transact. Affect. Comput. 9, 550–562. 10.1109/TAFFC.2017.2660485 [DOI] [Google Scholar]
- Loy L. S., Wieber F., Gollwitzer P. M., Oettingen G. (2016). Supporting sustainable food consumption: mental contrasting with implementation intentions (MCII) aligns intentions and behavior. Front. Psychol. 7:607. 10.3389/fpsyg.2016.00607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Zhao J. (2021). Attentional and perceptual biases of climate change. Curr. Opin. Behav. Sci. 42, 22–26. 10.1016/j.cobeha.2021.02.010 [DOI] [Google Scholar]
- Luu P., Tucker D. M., Makeig S. (2004). Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin. Neurophysiol. 115, 1821–1835. 10.1016/j.clinph.2004.03.031 [DOI] [PubMed] [Google Scholar]
- Ma G., Lin J., Li N. (2018). Longitudinal assessment of the behavior-changing effect of app-based eco-feedback in residential buildings. Energy Buildings 159, 486–494. 10.1016/j.enbuild.2017.11.019 [DOI] [Google Scholar]
- Marteau T. M. (2017). Towards environmentally sustainable human behavior: targeting non-conscious and conscious processes for effective and acceptable policies. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 375:20160371. 10.1098/rsta.2016.0371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinho G., Pires A., Portela G., Fonseca M. (2015). Factors affecting consumers’ choices concerning sustainable packaging during product purchase and recycling. Resources Conserv. Recycling 103, 58–68. 10.1016/j.resconrec.2015.07.012 [DOI] [Google Scholar]
- McCall C., Singer T. (2012). The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat. Neurosci. 15, 681–688. 10.1038/nn.3084 [DOI] [PubMed] [Google Scholar]
- McFadden D. (1986). The choice theory approach to market research. Market. Sci. 5, 275–297. [Google Scholar]
- Meijers M. H. C., Smit E. S., de Wildt K., Karvonen S. G., van der Plas D., van der Laan L. N., et al. (2021). Stimulating sustainable food choices using virtual reality: taking an environmental vs. health communication perspective on enhancing response efficacy beliefs. Environ. Commun. 1, 1–22. 10.1080/17524032.2021.1943700 [DOI] [Google Scholar]
- Melnikoff D. E., Bargh J. A. (2018). The mythical number two. Trends Cogn. Sci. 22, 280–293. 10.1016/j.tics.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Mengarelli F., Spoglianti S., Avenanti A., Di Pellegrino G. (2015). Cathodal tDCS over the left prefrontal cortex diminishes choice-induced preference change. Cereb. Cortex 25, 1219–1227. 10.1093/cercor/bht314 [DOI] [PubMed] [Google Scholar]
- Mennella R., Patron E., Palomba D. (2017). Frontal alpha asymmetry neurofeedback for the reduction of negative affect and anxiety. Behav. Res. Ther. 92, 32–40. 10.1016/j.brat.2017.02.002 [DOI] [PubMed] [Google Scholar]
- Michaelsen M. M., Esch T. (2021). Motivation and reward mechanisms in health behavior change processes. Brain Res. 1757:147309. 10.1016/j.brainres.2021.147309 [DOI] [PubMed] [Google Scholar]
- Michaelsen M. M., Esch T. (2022). Functional mechanisms of health behavior change techniques: a conceptual review. Front. Psychol. 13:725644. 10.3389/fpsyg.2022.725644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. K., Cohen J. D. (2001). An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202. 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Miller D. T., Prentice D. A. (2016). Changing norms to change behavior. Annu. Rev. Psychol. 67, 339–361. 10.1146/annurev-psych-010814-015013 [DOI] [PubMed] [Google Scholar]
- Minton E. A., Cornwell T. B., Kahle L. R. (2017). A theoretical review of consumer priming: prospective theory, retrospective theory and the affective-behavioral-cognitive model. J. Consumer Behav. 16, 309–321. 10.1002/cb.1624 [DOI] [Google Scholar]
- Nejati V., Majdi R., Salehinejad M. A., Nitsche M. A. (2021). The role of dorsolateral and ventromedial prefrontal cortex in the processing of emotional dimensions. Sci. Rep. 11:1971. 10.1038/s41598-021-81454-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J. (2001). Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2, 119–128. 10.1038/35053570 [DOI] [PubMed] [Google Scholar]
- Newman G. E., Gorlin M., Dhar R. (2014). When going green backfires: how firm intentions shape the evaluation of socially beneficial product enhancements. J. Consumer Res. 41, 823–839. 10.1086/677841 [DOI] [Google Scholar]
- Nigg J. T. (2017). Annual research review: on the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking and inhibition for developmental psychopathology. J. Child Psychol. Psychiatry 58, 361–383. 10.1111/jcpp.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbett R. E., Wilson T. D. (1977). Telling more than we can know: verbal reports on mental processes. Psychol. Rev. 84:231. [Google Scholar]
- Niv Y. (2009). Reinforcement learning in the brain. J. Math. Psychol. 53, 139–154. 10.1016/j.jmp.2008.12.005 [DOI] [Google Scholar]
- O’Doherty J., Kringelbach M. L., Rolls E. T., Hornak J., Andrews C. (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 4, 95–102. 10.1038/82959 [DOI] [PubMed] [Google Scholar]
- Ohme R., Reykowska D., Wiener D., Choromanska A. (2010). Application of frontal EEG asymmetry to advertising research. J. Econ. Psychol. 31, 785–793. 10.1016/j.joep.2010.03.008 [DOI] [Google Scholar]
- O’Neill S. (2020). More than meets the eye: a longitudinal analysis of climate change imagery in the print media. Climate Change 163, 9–26. 10.1183/16000617.0087-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill S., Nicholson-Cole S. (2009). “Fear won’t do it”: promoting positive engagement with climate change through visual and iconic representations. Sci. Commun. 30, 355–379. 10.1177/1075547008329201 [DOI] [Google Scholar]
- Padoa-Schioppa C. (2011). Neurobiology of economic choice: a good-based model. Annu. Rev. Neurosci. 34:333. 10.1146/annurev-neuro-061010-113648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan N. M., Pagan K. M., Teixeira A. A., de Moura Engracia Giraldi J., Stefanelli N. O., de Oliveira J. H. C. (2020). Application of neuroscience in the area of sustainability: mapping the territory. Global J. Flexible Syst. Manage. 21, 61–77. 10.1007/s40171-020-00243-9 [DOI] [Google Scholar]
- Panzone L. A., Ulph A., Hilton D., Gortemaker I., Tajudeen I. A. (2021). Sustainable by design: choice architecture and the carbon footprint of grocery shopping. J. Public Policy Market. 40, 463–486. 10.1177/07439156211008898 [DOI] [Google Scholar]
- Papies E. K. (2017). Situating interventions to bridge the intention-behavior gap: a framework for recruiting nonconscious processes for behavior change. Soc. Pers. Psychol. Compass 11:e12323. 10.1111/spc3.12323 [DOI] [Google Scholar]
- Paswan A., Guzmán F., Lewin J. (2017). Attitudinal determinants of environmentally sustainable behavior. J. Consumer Market. 10.1108/JCM-02-2016-1706 [DOI] [Google Scholar]
- Pavlov P. I. (1927). Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex, ed. Anrep G. V.. London: Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty R. E., Cacioppo J. T. (1986). The elaboration likelihood model of persuasion. Adv. Exp. Soc. Psychol. 19, 123–205. 10.1016/S0065-2601(08)60214-2 [DOI] [Google Scholar]
- Phelan A. A., Meissner K., Humphrey J., Ross H. (2021). Plastic pollution and packaging: corporate commitments and actions from the food and beverage sector. J. Cleaner Prod. 133:129827. 10.1016/j.jclepro.2021.129827 [DOI] [Google Scholar]
- Pizzagalli D. A., Sherwood R. J., Henriques J. B., Davidson R. J. (2005). Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychol. Sci. 16, 805–813. 10.1111/j.1467-9280.2005.01618.x [DOI] [PubMed] [Google Scholar]
- Poldrack R. A. (2006). Can cognitive processes be inferred from neuroimaging data. Trends Cognit. Sci. 10, 59–63. 10.1016/j.tics.2005.12.004 [DOI] [PubMed] [Google Scholar]
- Poldrack R. A. (2011). Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron 72, 692–697. 10.1016/j.neuron.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska J. O. (2008). Decision making in the transtheoretical model of behavior change. Med. Decis. Making 28, 845–849. 10.1177/0272989X08327068 [DOI] [PubMed] [Google Scholar]
- Purves D., Cabeza R., Huettel S. A., LaBar K. S., Platt M. L., Woldorff M. G., et al. (2013). Principles of Cognitive Neuroscience. 2nd Edn. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Qin J., Kimel S., Kitayama S., Wang X., Yang X., Han S. (2011). How choice modifies preference: neural correlates of choice justification. Neuroimage 55, 240–246. 10.1016/j.neuroimage.2010.11.076 [DOI] [PubMed] [Google Scholar]
- Ramsøy T. Z., Michael N., Michael I. (2019). A consumer neuroscience study of conscious and subconscious destination preference. Sci. Rep. 9, 1–8. 10.1093/tas/txac099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsøy T. Z., Skov M., Christensen M. K., Stahlhut C. (2018). Frontal brain asymmetry and willingness to pay. Front. Neurosci. 12:138. 10.3389/fnins.2018.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfs P., Ramírez J. M. (2006). Aggression and brain asymmetries: a theoretical review. Aggress. Viol. Behav. 11, 283–297. 10.1016/j.avb.2005.09.001 [DOI] [Google Scholar]
- Rokka J., Uusitalo L. (2008). Preference for green packaging in consumer product choices-do consumers care. Int. J. Consumer Stud. 32, 516–525. 10.1111/j.1470-6431.2008.00710.x [DOI] [Google Scholar]
- Rothgerber H. (2013). Real men don’t eat (vegetable) quiche: masculinity and the justification of meat consumption. Psychol. Men Masculinities 14, 363–375. 10.1037/a0030379 [DOI] [Google Scholar]
- Rothgerber H., Rosenfeld D. L. (2021). Meat-related cognitive dissonance: the social psychology of eating animals. Soc. Pers. Psychol. Compass 15:e12592. 10.1111/spc3.12592 [DOI] [Google Scholar]
- Rothman A. J., Baldwin A. S., Hertel A. W., Fuglestad P. T. (2011). “Self-regulation and behavior change: disentangling behavioral initiation and behavioral maintenance,” in Handbook of Self-Regulation: Research, Theory, and Applications, eds Vohs K. D., Baumeister R. F. (New York: Guilford Press), 106–122. [Google Scholar]
- Sabu P., Stuldreher I., Kaneko D., Brouwer A.-M. (2022). A review on the role of affective stimuli in event-related frontal alpha asymmetry. Front. Comput. Sci. 4:869123. 10.3389/fcomp.2022.8691 [DOI] [Google Scholar]
- Sawe N., Chawla K. (2021). Environmental neuroeconomics: how neuroscience can inform our understanding of human responses to climate change. Curr. Opin. Behav. Sci. 42, 147–154. 10.1016/j.cobeha.2021.08.002 [DOI] [Google Scholar]
- Schmidt S., Jo H.-G., Wittmann M., Hinterberger T. (2016). “Catching the waves” – slow cortical potentials as moderator of voluntary action. Neurosci. Biobehav. Rev. 68, 639–650. 10.1016/j.neubiorev.2016.06.023 [DOI] [PubMed] [Google Scholar]
- Schneider C. R., Zaval L., Markowitz E. M. (2021). Positive emotions and climate change. Curr. Opin. Behav. Sci. 42, 114–120. 10.1016/j.cobeha.2021.04.009 [DOI] [Google Scholar]
- Schultz W. (2006). Behavioral theories and the neurophysiology of reward. Annu. Rev. Psychol. 57, 87–115. 10.1146/annurev.psych.56.091103.070229 [DOI] [PubMed] [Google Scholar]
- Schultz W. (2015). Neuronal reward and decision signals: from theories to data. Physiol. Rev. 95, 853–951. 10.1152/physrev.00023.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer R., Luszczynska A. (2008). How to overcome health-compromising behaviors: the health action process approach. Eur. Psychol. 13:141. 10.1027/1016-9040.13.2.141 [DOI] [Google Scholar]
- Seymour B., Singer T., Dolan R. (2007). The neurobiology of punishment. Nat. Rev. Neurosci. 8, 300–311. 10.1038/nrn2119 [DOI] [PubMed] [Google Scholar]
- Sheeran P. (2002). Intention—behavior relations: a conceptual and empirical review. Eur. Rev. Soc. Psychol. 12, 1–36. 10.1080/14792772143000003 [DOI] [Google Scholar]
- Sheeran P., Gollwitzer P. M., Bargh J. A. (2013). Nonconscious processes and health. Health Psychol. 32:460. 10.1037/a0029203 [DOI] [PubMed] [Google Scholar]
- Sherman J. W., Rivers A. M. (2021). There’s nothing social about social priming: derailing the “train wreck”. Psychol. Inquiry 32, 1–11. 10.1080/1047840X.2021.1889312 [DOI] [Google Scholar]
- Shiota M. N., Papies E. K., Preston S. D., Sauter D. A. (2021). Positive affect and behavior change. Curr. Opin. Behav. Sci. 39, 222–228. 10.1016/j.cobeha.2021.04.022 [DOI] [Google Scholar]
- Simon C., Tagliabue M. (2018). Feeding the behavioral revolution: contributions of behavior analysis to nudging and vice versa. J. Behav. Econom. Policy 2, 91–97. [Google Scholar]
- Skinner B. F. (1938). The Behavior of Organisms: An Experimental Analysis, Cambridge: BF Skinner Foundation. [Google Scholar]
- Sligo F. X., Jameson A. M. (2000). The knowledge—behavior gap in use of health information. J. Am. Soc. Info. Sci. 51, 858–869. [DOI] [Google Scholar]
- Smith M. E. (2021). Inspiring Green Consumer choices: Leverage Neuroscience to Reshape Marketplace Behavior. New York, NY: Kogan Page Publishers. [Google Scholar]
- Squire L. R., Dede A. J. (2015). Conscious and unconscious memory systems. Cold Spr. Harb. Perspect. Biol. 7:a021667. 10.1101/cshperspect.a021667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenis N. D., van Herpen E., van der Lans I. A., Ligthart T. N., van Trijp H. C. (2017). Consumer response to packaging design: the role of packaging materials and graphics in sustainability perceptions and product evaluations. J. Cleaner Prod. 162, 286–298. 10.1016/j.jclepro.2017.06.036 [DOI] [Google Scholar]
- Steg L. (2016). Values, norms and intrinsic motivation to act proenvironmentally. Annu. Rev. Environ. Res. 41, 277–292. 10.1146/annurev-environ-110615-085947 [DOI] [Google Scholar]
- Stolz J., Molina H., Ramírez J., Mohr N. (2013). Consumers’ perception of the environmental performance in retail stores: an analysis of the G erman and the S panish consumer. Int. J. Consumer Stud. 37, 394–399. 10.1111/ijcs.12028 [DOI] [Google Scholar]
- Sumrin S., Gupta S., Asaad Y., Wang Y., Bhattacharya S., Foroudi P. (2021). Eco-innovation for environment and waste prevention. J. Bus. Res. 122, 627–639. 10.1016/j.jbusres.2020.08.001 [DOI] [Google Scholar]
- Sunstein C. R. (2021). Green defaults can combat climate change. Nat. Hum. Behav. 5, 548–549. 10.1038/s41562-021-01071-2 [DOI] [PubMed] [Google Scholar]
- Szmigin I., Carrigan M., McEachern M. G. (2009). The conscious consumer: taking a flexible approach to ethical behavior. Int. J. Consumer Stud. 33, 224–231. 10.1111/j.1470-6431.2009.00750.x [DOI] [Google Scholar]
- Taddicken M., Wolff L. (2020). ‘Fake News’ in science communication: emotions and strategies of coping with dissonance online. Media Commun. 8, 206–217. 10.17645/mac.v8i1.2495 [DOI] [Google Scholar]
- Tanford S., Kim M., Kim E. J. (2020). Priming social media and framing cause-related marketing to promote sustainable hotel choice. J. Sustainable Tourism 28, 1762–1781. 10.1080/09669582.2020.1760287 [DOI] [Google Scholar]
- Tate K., Stewart A. J., Daly M. (2014). Influencing green behavior through environmental goal priming: the mediating role of automatic evaluation. J. Environ. Psychol. 38, 225–232. 10.1016/j.jenvp.2014.02.004 [DOI] [Google Scholar]
- Theurl M. C., Lauk C., Kalt G., Mayer A., Kaltenegger K., Morais T. G., et al. (2020). Food systems in a zero-deforestation world: dietary change is more important than intensification for climate targets in 2050. Sci. Total Environ. 735:139353. 10.1016/j.scitotenv.2020.139353 [DOI] [PubMed] [Google Scholar]
- Thøgersen J. (2021). Consumer behavior and climate change: consumers need considerable assistance. Curr. Opin. Behav. Sci. 42, 9–14. 10.1016/j.cobeha.2021.02.008 [DOI] [Google Scholar]
- Thorndike E. L. (1898). Animal intelligence: an experimental study of the associative processes in animals. Psychol. Monogr. 24:8. 10.1037/10780-000 [DOI] [Google Scholar]
- Tichenor P. J., Donohue G. A., Olien C. N. (1970). Mass media flow and differential growth in knowledge. Public Opin. Q. 34, 159–170. 10.1086/267786 [DOI] [Google Scholar]
- Touchette B., Lee S.-E. (2017). Measuring neural responses to apparel product attractiveness: an application of frontal asymmetry theory. Clothing Textiles Res. J. 35, 3–15. 10.1177/0887302X16673157 [DOI] [Google Scholar]
- Turnwald B. P., Boles D. Z., Crum A. J. (2017). Association between indulgent descriptions and vegetable consumption: twisted carrots and dynamite beets. JAMA Intern. Med. 177, 1216–1218. 10.1001/jamainternmed.2017.1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidis D. C., Bran A. (2019). Respectable challenges to respectable theory: cognitive dissonance theory requires conceptualization clarification and operational tools. Front. Psychol. 10:1189. 10.3389/fpsyg.2019.01189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin V. V., Dickinson A., O’Doherty J. P. (2007). Determining the neural substrates of goal-directed learning in the human brain. J. Neurosci. 27, 4019–4026. 10.1523/JNEUROSCI.0564-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cappellen P., Rice E. L., Catalino L. I., Fredrickson B. L. (2018). Positive affective processes underlie positive health behavior change. Psychol. Health 33, 77–97. 10.1080/08870446.2017.1320798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linden S., Weber E. U. (2021). Editorial overview: can behavioral science solve the climate crisis? Curr. Opin. Behav. Sci. 42, iii–viii. 10.1016/j.cobeha.2021.09.001 [DOI] [Google Scholar]
- Van Dessel P., Boddez Y., Hughes S. (2022). Nudging societally relevant behavior by promoting cognitive inferences. Sci. Rep. 12:9201. 10.1038/s41598-022-12964-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Geffen L. E. J., Hollup S. A., Klöckner C. A. (2016). How do people with weak and strong pro-environmental worldviews process visual climate change information? An EEG study. Psyecology 7, 262–281. 10.1080/21711976.2016.1242229 [DOI] [Google Scholar]
- van Riper C., Winkler-Schor S., Foelske L., Keller R., Braito M., Raymond C., et al. (2019). Integrating multi-level values and pro-environmental behavior in a US protected area. Sustainability Sci. 14, 1395–1408. 10.1007/s11625-019-00677-w [DOI] [Google Scholar]
- van Veen V., Krug M. K., Schooler J. W., Carter C. S. (2009). Neural activity predicts attitude change in cognitive dissonance. Nat. Neurosci. 12, 1469–1474. 10.1038/nn.2413 [DOI] [PubMed] [Google Scholar]
- Vecchiato G., Maglione A. G., Cherubino P., Wasikowska B., Wawrzyniak A., Latuszynska A., et al. (2014). Neurophysiological tools to investigate consumer’s gender differences during the observation of TV commercials. Comput. Math. Methods Med. 2014:912981. 10.1155/2014/912981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V., Dimoka A., Pavlou P. A., Vo K., Hampton W., Bollinger B., et al. (2015). Predicting advertising success beyond traditional measures: new insights from neurophysiological methods and market response modeling. J. Market. Res. 52, 436–452. 10.1509/jmr.13.0593 [DOI] [Google Scholar]
- Verplanken B., Aarts H. (1999). Habit, attitude and planned behavior: is habit an empty construct or an interesting case of goal-directed automaticity? Eur. Rev. Soc. Psychol. 10, 101–134. 10.1080/14792779943000035 [DOI] [Google Scholar]
- Verplanken B., Holland R. W. (2002). Motivated decision making: effects of activation and self-centrality of values on choices and behavior. J. Pers. Soc. Psychol. 82, 434–447. 10.1037//0022-3514.82.3.434 [DOI] [PubMed] [Google Scholar]
- Verplanken B., Orbell S. (2022). Attitudes, habits and behavior change. Ann. Rev. Psychol. 73, 327–352. 10.1146/annurev-psych-020821-011744 [DOI] [PubMed] [Google Scholar]
- Verplanken B., Whitmarsh L. (2021). Habit and climate change. Curr. Opin. Behav. Sci. 42, 42–46. 10.1016/j.cobeha.2021.02.020 [DOI] [Google Scholar]
- Verplanken B., Marks E., Dobromir A. I. (2020). On the nature of eco-anxiety: how constructive or unconstructive is habitual worry about global warming? J. Environ. Psychol. 72:101528. 10.1016/j.jenvp.2020.101528 [DOI] [Google Scholar]
- Verplanken B. Ed. (2018). The Psychology of Habit. Theory, Mechanisms, Change, and Contexts. Cham: Springer. 10.1007/978-3-319-97529-0 [DOI] [Google Scholar]
- Vesely S., Klöckner C. A. (2020). Social desirability in environmental psychology research: three meta-analyses. Front. Psychol. 11:1395. 10.3389/fpsyg.2020.01395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter M., Kutzner F. (2016). Nudge me if you can-how defaults and attitude strength interact to change behavior. Compr. Results Soc. Psychol. 1, 8–34. 10.1080/23743603.2016.1139390 [DOI] [Google Scholar]
- Vezich I. S., Gunter B. C., Lieberman M. D. (2017). The mere green effect: an fMRI study of pro-environmental advertisements. Soc. Neurosci. 12, 400–408. 10.1080/17470919.2016.1182587 [DOI] [PubMed] [Google Scholar]
- Wandosell G., Parra-Meroño M. C., Alcayde A., Baños R. (2021). Green packaging from consumer and business perspectives. Sustainability 13:1356. 10.3390/su13031356 [DOI] [Google Scholar]
- Wang S., van den Berg B. (2021). Neuroscience and climate change: how brain recordings can help us understand human responses to climate change. Curr. Opin. Psychol. 42, 126–132. 10.1016/j.copsyc.2021.06.023 [DOI] [PubMed] [Google Scholar]
- Weingarten E., Chen Q., McAdams M., Yi J., Hepler J., Albarracín D. (2016). From primed concepts to action: a meta-analysis of the behavioral effects of incidentally presented words. Psychol. Bull. 142, 472–497. 10.1037/bul0000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein N. D., Sandman P. M., Blalock S. J. (2020). The precaution adoption process model. Wiley Encyclopedia Health Psychol. 495–506. 10.1002/9781119057840.ch100 [DOI] [Google Scholar]
- White K., Habib R., Hardisty D. J. (2019). How to SHIFT consumer behaviors to be more sustainable: a literature review and guiding framework. J. Market. 83, 22–49. 10.1177/0022242919825649 [DOI] [Google Scholar]
- WHO (2016). Fiscal Policies For Diet And Prevention Of Noncommunicable Diseases. Geneva, Switzerland: WHO Technical Meeting Report. [Google Scholar]
- Wolf J., Moser S. C. (2011). Individual understandings, perceptions and engagement with climate change: insights from in-depth studies across the world. Wiley Interdiscip. Rev. 2, 547–569. 10.1002/wcc.120 [DOI] [Google Scholar]
- Yin H. H., Knowlton B. J. (2006). The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 7, 464–476. 10.1038/nrn1919 [DOI] [PubMed] [Google Scholar]
- Yousuf J. B., Bose S., Kotagama H., Boughanmi H. (2019). Preferences and intentions of seafood consumers in Oman: an empirical analysis. J. Int. Food Agribusiness Market. 31, 175–203. 10.1080/08974438.2018.1497565 [DOI] [Google Scholar]
- Yue B., Sheng G., She S., Xu J. (2020). Impact of consumer environmental responsibility on green consumption behavior in China: the role of environmental concern and price sensitivity. Sustainability 12:2074. 10.3390/su12052074 [DOI] [Google Scholar]
- Zangemeister L., Grabenhorst F., Schultz W. (2019). Neural activity in human ventromedial prefrontal cortex reflecting the intention to save reward. Soc. Cogn. Affect. Neurosci. 14, 1255–1261. 10.1093/scan/nsaa013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Lawson A., Guo C., Jiang Y. (2006). Electrophysiological correlates of visual affective priming. Brain Res. Bull. 71, 316–323. 10.1016/j.brainresbull.2006.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhang H. (2022). High construal level enhances perceived self-control capacity in intrinsic but not extrinsic goals. Pers. Individual Diff. 197:111761. 10.1016/j.paid.2022.111761 [DOI] [Google Scholar]
- Zotev V., Yuan H., Misaki M., Phillips R., Young K. D., Feldner M. T., et al. (2016). Correlation between amygdala BOLD activity and frontal EEG asymmetry during real-time fMRI neurofeedback training in patients with depression. Neuroimage Clin. 11, 224–238. 10.1016/j.nicl.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]