Abstract
Low-risk prostate cancer has traditionally seen a preference towards avoiding treatment-related harms with active surveillance (AS) and multimodal monitoring protocols utilized to assess for disease progression. Large trials have shown variations in mortality and cancer survival benefit between AS and radical treatment, which has prompted further trials into the management of low-risk disease. Nonradical treatments for men on AS have been an emerging field and yet to enter mainstream guidelines or practice. These include pharmacological treatments, focal therapy, nutraceuticals, immunotherapy, and exercise. We present a review of all current major randomized clinical trials for nonradical treatment of men on AS and summarize their findings.
Keywords: Active surveillance, low risk, nonradical treatment, progression, prostate cancer, survival
1. Introduction
The management of low-risk prostate cancer (PCa) has seen several updates recently. Active surveillance (AS) has been the standard of care in managing cT1a-c to T2a disease for decades. While the overarching theme of current guidelines is to avoid over treatment of low-risk disease, there is interest from several groups trying to demonstrate the role nonradical treatments such as antiandrogen monotherapy, focal therapy, and alternative therapies. AS protocols possess regional differences influenced by resource availability and local guidelines for patients suitable for AS. The systematic review by Willemse et al made recommendations in addition to the current European Association of Urology (EAU) guidelines towards the pathology that should be considered for AS and the protocol for surveillance.1 The review of 375 included articles (N = 264,852 patients) found that AS should be recommended for men as per Table 1.
Table 1.
Summary of recommendations from the Willemse group review on patient suitability for AS
| Domain | Current EAU 2020 guidelines | Willemse et al 2022 Recommendations | Strength of evidence |
|---|---|---|---|
| Inclusion criteria | mpMRI prior to biopsy | Low volume International Society of Urological Pathology (ISUP) Grade 2 disease with 2-3 positive cores and <50% cancer involvement per core (Favorable) | Weak |
| ISUP 1 disease only | ISUP 2 with >3 cores positive should be excluded | Weak | |
| Surveillance | PSA every 6 months | Confirmatory biopsy within the first 2 years | Weak |
| DRE every 6 months | Surveillance biopsy at least every 3 years for 10 years | Weak | |
| No need for confirmatory biopsy if mpMRI done prior to diagnostic biopsy | If mpMRI is not available repeat biopsy | Weak | |
| mpMRI prior to all surveillance biopsy | Any increase in core involvement at repeat biopsy should be reclassified | Weak |
mpMRI, multiparametric magnetic resonance imaging; DRE, digital rectal examination.
Both the EAU and Willemse group recommendation feature transperineal prostate biopsy (TPBx) prominently as part of the AS protocol. Previous recommendations of either transrectal or transperineal approach to surveillance biopsy but there has been recent evidence to suggest that the transrectal approach is more likely to miss clinically significant PCa in the anterior prostate.2 AS protocols rely on accurate staging and diagnosis of low-risk PCa to minimize risk of progression, and TPBx remains the standard of care within the Asia-Pacific region to provide this reassurance to the patient. Prior to the Willemse recommendations, a review of PCa prediction models using multiparametric magnetic resonance imaging (mpMRI) and TPBx was conducted to compare the performance of existing risk calculators for men on AS. This review found existing calculators (European Randomized Study of Screening for Prostate Cancer risk calculator ¾ (ERSPC-RC3/4), Prostate Biopsy Collaborative Group risk calculator (PBCG-RC)) were comparable to the Leeuwen model that combines key clinical features with mpMRI to determine the need for TPBx.3 The value of these models is as a decision-making tool for urologists and can provide reassurance for men progressing to an AS pathway. Previous large studies have successfully randomized men to AS, surgery, or radiation (ProtecT).4 ProtecT was able to demonstrate that men on AS were more likely to suffer disease-related events including reduced overall cancer survival and progression of disease when compared to radical treatment arms. The reduced life expectancy of men on AS was a surprising development in the management of low-risk disease. The basis for AS in localized PCa was established well before ProtecT with the PIVOT trial 2012 demonstrating no overall survival benefit to patients who received radical prostatectomy over monitoring of PSA.5 Just prior to ProtecT, there was further strong evidence for AS as demonstrated by Hamdy et al.6 who found no overall reduction in 10-year PCa-specific survival for AS versus radical treatment of low-risk disease. Since this analysis of 10-year outcomes in 2016 AS has formed the basis for the management of low-risk disease around the world. The drawback to this large study being the retrospective nature of the study design and the inclusion of men with locally advanced disease. Practice still differs regionally depending on local guidelines. Within Australia and New Zealand alone, there is a large variation in the management of low-risk disease across jurisdictions. For example a recent analysis found patient receiving care in metropolitan private settings were more likely to undergo active treatment than conservative management (AS or watchful waiting(WW)) (OR 0.71, 95% CI 0.58–0.87; P = 0.001).7 Differences in practice could be explained by variations in patient demographic and comorbid states who pursue private healthcare over public management of disease. The variations in practice vary again when compared to overseas cohorts with the US-based Surveillance Epidemiology and End Results study showing only 42% of men with low-risk PCa receiving AS or WW compared to nearly 70% in Australia.8 International differences in low-risk disease management are multifactorial and government policy and funding of surveillance protocols play a large role. Australian government funding of all mpMRI prior to prostate biopsy has reduced the number of prostate biopsies performed and improved patient adherence to AS protocol with reassuring radiological findings.9 Since ProtecT in 2020, there have been several attempts to explore the role of nonradical treatments in true low-risk disease. Broadly, they can be categorized into pharmacological agents, focal therapy, nutraceuticals, immunotherapy, and lifestyle interventions.
2. Comparing AS to pharmacological agents
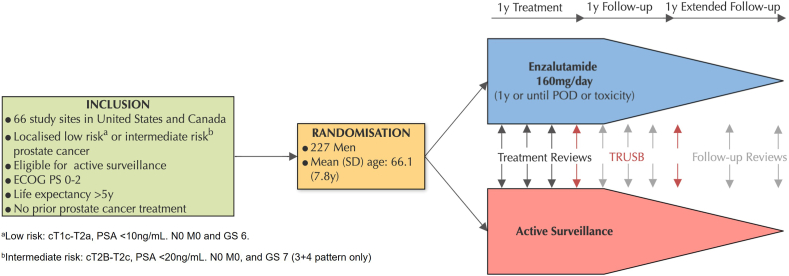
The ENACT randomized control trial allocated men to AS and antiandrogen monotherapy (Enzalutamide) traditionally reserved for treatment of advanced and metastatic disease (Fig. 1). ENACT was able to demonstrate a 46% reduction in cancer progression for the treatment arm compared to the AS group. This trial employed a nonradical treatment for low-risk PCa that has established high rates of drug toxicity. While the study found most men tolerated the monotherapy well, it established a precedent for further trials of antiandrogen agents in the management of low-risk disease. AS is an option selected by men to avoid treatment-related harms, and this trial 88.4% of men suffered adverse effects directly related to enzalutamide with 7.1% discontinuing the drug.10
Fig. 1.
Summary of enzalutamide versus AS (ENACT study) protocol.
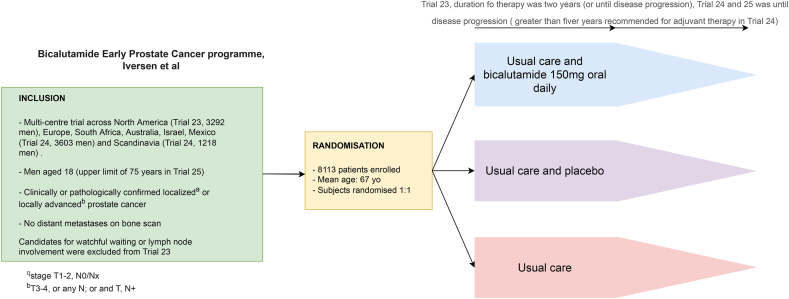
Other antiandrogen monotherapy trials for the treatment of low-risk disease have included bicalutamide. McLeod et al.11 conducted the largest randomized control trial with this drug on a mixed population of men with both localized and locally advanced PCa. This population, while not truly a population suitable for AS, had mixed response to bicalutamide in the trial (Fig. 2). Localized disease that was defined as T1-2Nx/0 had no improved progression free survival (PFS) while patients with locally advanced disease (T3-4) did derive a benefit to PFS on bicalutamide. The study, including the outcomes analysis at 9.7-year follow-up, should not be considered to reflect on a true AS cohort given the mixed pathology included in the trial and the use of radiotherapy in one of the treatment arms. Therefore, bicalutamide has not been shown to benefit patients who would be suitable for AS.
Fig. 2.
Summary of bicalutamide versus AS (Early prostate cancer programme trial) protocol.
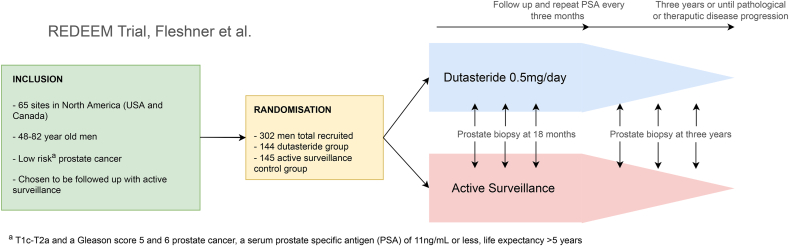
REDEEM is a double blinded randomized placebo-control trial analyzing the effect of dutasteride in the management of localized PCa.12 5α reductase inhibitors block the conversion of testosterone to dihydrotestosterone that can lead to a reduction prostate volume and Prostate specific antigen (PSA) levels. REDEEM builds on the previous results of REDUCE which demonstrated the effect of dutasteride in reducing PCa detection on biopsy.13 Over 3 years the investigators randomized men with low-risk disease (low volume Gleason 5-6 PCa) to dutasteride and a placebo drug (Fig. 3). REDEEM found a 10% reduction in PCa progression for the dutasteride group with a hazard ration of 0.62 95% CI 0·43–0·89: log-rank p = 0·009. As a secondary outcome man in the dutasteride group also showed less cancer on final biopsy at 3 years compared to the placebo group.
Fig. 3.
Summary of dutasteride versus AS (REDEEM trial) protocol.
3. Comparing AS to focal therapy
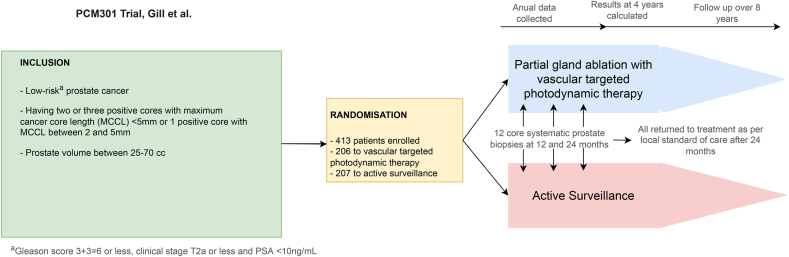
Focal therapy is an emerging treatment modality for low-risk PCa with low volume or single focus intraprostatic disease (Fig. 4). The prospective PCM301 randomized trial highlighted that the partial ablation of low-risk PCa photodynamic therapy significantly reduced the subsequent finding of higher-grade cancer on biopsy.14 Consequently, fewer cases were converted to radical therapy, a clinically meaningful benefit that lowered treatment-related morbidity.
Fig. 4.
Summary of focal therapy (PCM301 trial) protocol.
4. Nutraceuticals
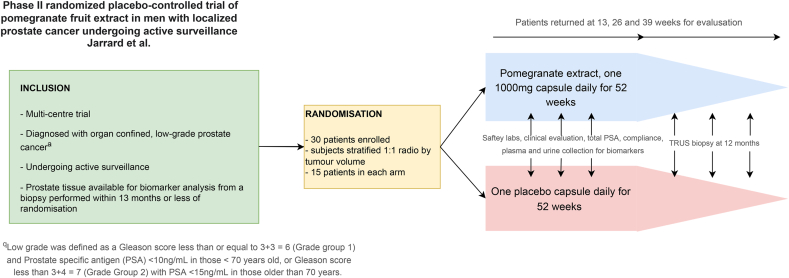
Nutraceuticals have long been utilized in the setting of low-risk PCa.15 Prominent regimes have included green tea, Vitamin D, pomegranate and selenium. Many of these studies are retrospective and there is a paucity of randomized clinical trials in the area. The underlying theme to nutraceutical treatment resides in the reduction of circulating androgens. Pomegranate fruit extract (PFE) is an experimental treatment in the nutraceutical sphere for localized PCa (Fig. 5). Phase II randomized control trials have shown PFE does have an antiproliferative and proapoptotic effect in men on AS with a significant reduction in DNA damage markers and androgen receptor expression over 12 months.16 Further Phase III trials are required to assess the effect of PFE on reducing cancer progression. Other nutraceuticals that have seen some traction in the literature include Vitamin D. Vitamin D supplementation is thought to reduce circulating androgens including testosterone and dihydrotestosterone, reduce circulating PSA and inhibit cell growth of hormone sensitive PCa cell lines. However systematic review and meta-analysis of clinical trials in this area suggest that high dose vitamin D supplementation does not benefit patients with low-risk disease and there is no significant benefit to PFS, overall cancer-specific survival or PSA reduction compared to placebo groups. As such Vitamin D should not be recommended as an alternative treatment to AS.17
Fig. 5.
Summary of pomegranate fruit extract versus AS (PFE trial) protocol.
5. Immunotherapy
Other novel therapies still in development are immunotherapy regimes for PCa. PROSTVAC, a viral vector–based immunotherapy trial, has been proposed as a novel treatment for T1-2 PCa. The trial will assess the expression of CD4+ and CD8+ tumour markers as a surrogate for PCa progression ion a true AS population.18 The trial results are still pending and have not been finalized despite over 3 years of analysis since the published completion date.
6. Exercise
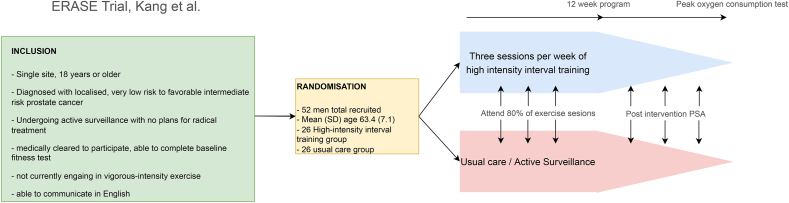
There are several studies looking at the effect of exercise on PCa progression in an AS cohort. The largest of these is the ERASE trial which looks at the isolated effects of exercise on patients undergoing AS (Fig. 6). The contention of the trial is to reduce disease progression via a combination of three mechanisms.
-
1.
A biological pathway where tumour suppression occurs through modulation of systemic biomarkers including natural killer cell function, metabolic markers such as insulin and insulin growth factor and inflammatory cytokines
-
2.
Psychological pathway; reduction in fear of cancer progression to remain on AS
-
3.
Functional pathway; pre-habilitation with improvements in physical conditioning. Previous studies have looked the effect of exercise in conjunction with other nonradical and radical treatments but the ERASE trial when finalized will deliver insights into compartmentalized effects of exercise on low-risk disease progression.19 ERASE employs a model of high-intensity interval training (HIIT) as part of its training regime (Fig. 6). Previously HIIT as well as resistance training have been compared to AS in promotion of circulating tumour suppression markers (serum insulin-like binding protein 3 (IGFBP -3) and interferon-γ). A 3-arm feasibility randomized control trial by the Papadopolous group 2021 found both HIIT and resistance training improved serum tumour suppression markers and could have a role to play in low-risk disease.20 Developing on this work the Brassetti 2021 group conducted a prospective trial using the Physical Activity Scale for the Elderly (PASE) to assess the effect of exercise on PSA doubling time and reclassification of disease. They found that the higher the PASE score the lower the risk of re-classification of PCa for men on AS.21 Large prospective and feasibility trials provide the impetus for randomized control trials such as ERASE to proceed to fruition. Aside from the potential benefit in preventing PCa progression exercise has overall morbidity benefits and reduction in all-cause mortality independent of cancer risk and should form an important part of any patient counselling as part of a healthy lifestyle.
Fig. 6.
Summary of exercise versus AS (ERASE trial) protocol.
7. Conclusion and future directions
Non radical treatments for low-risk PCa encompass a spectrum of options ranging from well-established and documented to emerging and experimental (Table 2). This is a dynamic and developing field of treatment with many trials still requiring validation and phase III components to demonstrate mortality and morbidity benefit in low-risk PCa. Overall, the main player in this field are pharmacological agents with the highest level of evidence. Although many of these agents have some benefit on reducing cancer-specific progression, they also subject a population of men to adverse effects directly related to their treatment. Future studies in nonradical treatments for low-risk PCa should focus on adjunct lifestyle therapies such as exercise, diet and supplements that may provide oncological outcome benefits but also enrich the quality of life for the patient. The role of novel imaging in this field can also be expanded with evidence of disease progression but further randomized control trials are required in this field to validate current findings. Over treatment of low-risk disease may subject patients to treatment-related harms. It is important to remember that AS is a treatment option as well and one that in some situations will cause the least amount of harm to our patients.
Table 2.
Summary of current nonradical treatment trials for localized PCa
| Study | Study type | Men randomized (N) | Cohort pathology | Intervention | Primary outcome | Result for treatment arm |
|---|---|---|---|---|---|---|
| ENACT 2022 | RCT | 227 | Low risk Intermediate risk |
Enzalutamide vs. AS | 1. Time to PCa progression | 1. HR 0.54; 95% CI (0.33-0.89) P = 0.02∗ |
| EPC Programme 2006 | RCT | 8113 | Low risk Intermediate risk |
Bicalutamide + AS vs. Placebo + AS vs. AS alone | 1. Progression free survival 2. Overall survival |
1. HR 1.16; 95% CI (0.99 – 1.37); P = 0.07 2. HR 0.65; 95% CI (0.44-0.95); P = 0.03∗ |
| REDEEM 2010 | RCT | 302 | Low risk | Dutasteride vs. placebo | 1. Time to PCa progression | 1. HR 0.62; 95% CI (0.43 – 0.89); P = 0.009∗ |
| PCM301 2018 | RCT | 413 | Low risk | Focal photodynamic therapy vs. AS | 1. Conversion to radical intervention at 4 years 2. Overall cancer progression |
1. HR 0.31, 95% CI (0.21-0.46); P < 0.05∗ 2. HR 0.42, 95% CI (0.29-0.59); P < 0.05∗ |
| Jarrard et al 2021 | RCT | 30 | Low risk | Pomegranate vs. AS | 1. Altered serum prostate tissue biomarkers 2. Number of positive cores on biopsy |
1. Urothelin A P=<0.01∗ DMEAG P=<0.001∗ 2. Positive core biopsy P = 0.06 |
| PROSTVAC 2018 | RCT | 1297 | Low risk | Immunotherapy vs. AS | 1. Change in CD8+ and CD4+ expression | Results not published |
| ERASE 2019 | RCT | 52 | Low risk | Exercise vs. AS | 1. Cardiorespiratory fitness 2. Immunosurveillance of cancer related biomarkers |
Results to be finalized |
Low risk: cT1c-T2a, PSA <10 ng/mL. Nx/0 M0 and GS 6.
Intermediate risk: cT2B-T2c, PSA <20 ng/mL. N0 M0, and GS 7 (3 + 4 pattern only).
Significant result demonstrating treatment benefit.
Conflict of interest
None to declare.
Acknowledgements
Dr Sachin Perera and Professor Nathan Lawrentschuk are affiliated with the EJ Whitten Foundation Centre for Prostate Cancer Research at Epworth Health Care, Melbourne Australia. No funding to declare.
References
- 1.Willemse P.-P.M., Davis N.F., Grivas N., Zattoni F., Lardas M., Briers E., et al. Systematic Review of active surveillance for clinically localised prostate cancer to develop recommendations regarding inclusion of intermediate-risk disease, biopsy characteristics at inclusion and monitoring, and surveillance repeat biopsy strategy. Eur Urol. 2022;81(4):337–346. doi: 10.1016/j.eururo.2021.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Cowan T., Baker E., McCray G., Reeves F., Houlihan K., Johns-Putra L. Detection of clinically significant cancer in the anterior prostate by transperineal biopsy. BJU Int. 2020;126(S1):33–37. doi: 10.1111/bju.15124. [DOI] [PubMed] [Google Scholar]
- 3.Doan P., Graham P., Lahoud J., Remmers S., Roobol M.J., Kim L., et al. A comparison of prostate cancer prediction models in men undergoing both magnetic resonance imaging and transperineal biopsy: Are the models still relevant? BJU Int. 2021;128(S3):36–44. doi: 10.1111/bju.15554. [DOI] [PubMed] [Google Scholar]
- 4.Neal D.E., Metcalfe C., Donovan J.L., Lane J.A., Davis M., Young G.J., et al. Ten-year mortality, disease progression, and treatment-related side effects in men with localised prostate cancer from the protect randomised controlled trial according to treatment received. Eur Urol. 2020;77(3):320–330. doi: 10.1016/j.eururo.2019.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Wilt T.J., Brawer M.K., Jones K.M., Barry M.J., Aronson W.J., Fox S., et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamdy F.C., Donovan J.L., Lane J.A., Mason M., Metcalfe C., Holding P., et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 7.Ong W.L., Thangasamy I., Murphy D., Pritchard E., Evans S., Millar J., et al. Large variation in conservative management of low-risk prostate cancer in Australia and New Zealand. BJU Int. 2022;130(S1):17–19. doi: 10.1111/bju.15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahal B.A., Butler S., Franco I., Spratt D.E., Rebbeck T.R., D'Amico A.V., et al. Use of active surveillance or watchful waiting for low-risk prostate cancer and management trends across risk groups in the United States, 2010-2015. JAMA. 2019;321(7):704–706. doi: 10.1001/jama.2018.19941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrentschuk N. Sensible government decisions on funding for imaging can change practice and deliver better care for men with prostate cancer. BJU Int. 2022;130(S1):4. doi: 10.1111/bju.15815. [DOI] [PubMed] [Google Scholar]
- 10.Shore N.D., Renzulli J., Fleshner N.E., Hollowell C.M.P., Vourganti S., Silberstein J., et al. Active surveillance plus enzalutamide monotherapy vs active surveillance alone in patients with low-risk or intermediate-risk localized prostate cancer: the ENACT randomized clinical trial. JAMA Oncol. 2022;8(8):1128–1136. doi: 10.1001/jamaoncol.2022.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLeod D.G., Iversen P., See W.A., Morris T., Armstrong J., Wirth M.P. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int. 2006;97(2):247–254. doi: 10.1111/j.1464-410X.2005.06051.x. [DOI] [PubMed] [Google Scholar]
- 12.Fleshner N.E., Lucia M.S., Egerdie B., Aaron L., Eure G., Nandy I., et al. Dutasteride in localised prostate cancer management: the REDEEM randomised, double-blind, placebo-controlled trial. Lancet. 2012;379(9821):1103–1111. doi: 10.1016/S0140-6736(11)61619-X. [DOI] [PubMed] [Google Scholar]
- 13.Andriole G.L., Bostwick D.G., Brawley O.W., Gomella L.G., Marberger M., Montorsi F., et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362(13):1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 14.Gill I.S., Azzouzi A.R., Emberton M., Coleman J.A., Coeytaux E., Scherz A., et al. Randomized trial of partial gland ablation with vascular targeted phototherapy versus active surveillance for low risk prostate cancer: extended followup and analyses of effectiveness. J Urol. 2018;200(4):786–793. doi: 10.1016/j.juro.2018.05.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trottier G., Boström P.J., Lawrentschuk N., Fleshner N.E. Nutraceuticals and prostate cancer prevention: a current review. Nat Rev Urol. 2010;7(1):21–30. doi: 10.1038/nrurol.2009.234. [DOI] [PubMed] [Google Scholar]
- 16.Jarrard D., Filon M., Huang W., Havighurst T., DeShong K., Kim K., et al. A phase II randomized placebo-controlled trial of pomegranate fruit extract in men with localized prostate cancer undergoing active surveillance. Prostate. 2021;81(1):41–49. doi: 10.1002/pros.24076. [DOI] [PubMed] [Google Scholar]
- 17.Shahvazi S., Soltani S., Ahmadi S.M., de Souza R.J., Salehi-Abargouei A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51(1):11–21. doi: 10.1055/a-0774-8809. [DOI] [PubMed] [Google Scholar]
- 18.Parsons J.K., Pinto P.A., Pavlovich C.P., Uchio E., Kim H.L., Nguyen M.N., et al. A randomized, double-blind, phase II trial of PSA-TRICOM (PROSTVAC) in patients with localized prostate cancer: the immunotherapy to prevent progression on active surveillance study. Eur Urol Focus. 2018;4(5):636–638. doi: 10.1016/j.euf.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang D.W., Fairey A.S., Boulé N.G., Field C.J., Courneya K.S. Exercise during active surveillance for prostate cancer-the ERASE trial: a study protocol of a phase II randomised controlled trial. BMJ Open. 2019;9(7) doi: 10.1136/bmjopen-2018-026438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadopoulos E., Gillen J., Moore D., Au D., Kurgan N., Klentrou P., et al. High-intensity interval training or resistance training versus usual care in men with prostate cancer on active surveillance: a 3-arm feasibility randomized controlled trial. Appl Physiol Nutr Metabol. 2021;46(12):1535–1544. doi: 10.1139/apnm-2021-0365. [DOI] [PubMed] [Google Scholar]
- 21.Brassetti A., Ferriero M., Napodano G., Sanseverino R., Badenchini F., Tuderti G., et al. Physical activity decreases the risk of cancer reclassification in patients on active surveillance: a multicenter retrospective study. Prostate Cancer Prostatic Dis. 2021;24(4):1151–1157. doi: 10.1038/s41391-021-00375-8. [DOI] [PubMed] [Google Scholar]