Abstract
The objective of the present work was to optimize the operating conditions (P, T cosolvent %) and to study the scale-up and the feasibility of the supercritical fluid extraction (SFE) process for polyphenols from grape pomace, the main solid byproduct of the wine industry. Pilot-scale equipment (1 L extraction vessel) was used to study the scale-up prediction for extraction vessels of 50, 100, 500, and 1000 L capacity. The adopted scale-up criteria consisted of maintaining and keeping constant the solvent mass-to-feed mass ratio and the bed geometry dimension. The results indicated an excellent predictive level obtained by Sovová’s model and success of the adopted scale-up criteria. At industrial scale, yields were close to 2.3 gGAE/100 gDM, a value obtained using the pilot-scale equipment. High concentrations of high-added-value phenols such as cis-resveratrol glucoside, cis-coutaric acid, trans-p-coumaric acid, quercetin, and proanthocyanidins were found in the extract. An economic evaluation of the process indicated the feasibility of an industrial SFE plant with a capacity of 500 L for producing in 60 min an extract with an expected phenolics’ concentration of approximately 133 gGAE/kg extract at an estimated 67€ /kgextract cost of manufacturing. Notably, all values are better than those currently reported in the literature.
1. Introduction
Grape crops are one of the main extended agro-economic activities in the world with more than 40 million tons of wine grape produced every year. In 2021, global wine production reached 260 million hectoliters. Italy (50.2 million hectoliters, 19.3% of all wine produced in the world), France (37.6 million hectoliters, 14.5% of global production), and Spain (35.3 million hectoliters, 13.6% of wine produced in the world) represented 47% of world wine production.1
Grape pomace or marc is the main solid byproduct of the wine industry consisting of skin, seeds, and pulp residues that remain after the crushing, draining, and pressing stages of wine production.1 About 8–9 million tons of grape pomace are produced in the world every year, representing approximately 20% (w/w; fresh weight) of the processed grapes’ weight.2 Approximately 70% of the phenolic content of grapes is preserved in the grape pomace after the winemaking process.2,3 Given the increasing consumer demand for natural compounds, several studies have demonstrated that grape pomace polyphenols could be used whether in food, pharmaceutical, or cosmetic industries.4−9 The recovery of these bioactive compounds from grape pomace enables both the obtainment of high-value-added biomolecules10 and the simultaneous reduction of environmental impact due to high organic matter content and seasonal production.11
However, a crucial challenge in the valorization of grape pomace as an inexpensive source of polyphenols remains the extraction process, which should be efficient, swift, selective, high solute quality (no thermal degradation of labile compounds), and environmentally friendly.
Supercritical fluid extraction (SFE) is a green technology that enables us to overcome the many limitations of conventional extraction methods that are time-, energy-, and solvent-consuming procedures and increase both environmental and health and safety concerns.
The most popular fluid for SFE is CO2 because of its low critical properties, low toxicity, and chemical inertness. Although CO2 is an excellent solvent for nonpolar compounds, a cosolvent at low concentration can be added to CO2 to modify the solvent selectivity toward polar or medium polar molecules.12 The SFE extracts are of superior quality as compared with those obtained by conventional extraction methods.13 SFE process advantages are low temperatures, efficiency in terms of increasing yields and lower extraction time, recyclability, tunable selectivity, reduced energy consumption, prevention of oxidation reactions, and operational flexibility.12,14−17
One of the most serious drawbacks of SFE is represented by the high cost of equipment compared with that required by conventional extraction processes. However, the most common SFE applications performed on a large industrial scale, such as decaffeination of tea and coffee, extraction of hop constituents, and separation of lecithin from oil, demonstrate that the supercritical process can be economically viable.18,19
Among the green or environmental friendly technologies applied for the extraction of bioactive compounds from winery wastes and byproducts,20 several works focused on supercritical fluid extraction of polyphenols from grape pomace.5,21−30 However, to successfully move production from SFE laboratory/pilot scale to SFE industrial scale, each raw material needs the optimization of operational conditions and scale-up study. So far, to the best of our knowledge, there has been no work in the literature regarding SFE scale-up of polyphenols from grape pomace using supercritical carbon dioxide with an ethanol–water mixture as a cosolvent.
Therefore, the aim of the present work is to study SFE of polyphenols from grape pomace, evaluating operational parameters on kinetic and modeling, in order to propose an adequate scale-up for this process and to estimate economic feasibility.
2. Material and Methods
2.1. Grape Pomace Characterization and Preparation
Grape pomace of different white grape varieties (Vitis vinifera L.) were collected after winemaking during September–October, 2020, in the Friuli Venezia-Giulia region (Italy). They were dried in an air circulation oven at 323.15 K for 24 h and stored in dark conditions at 277.15 K until they were used. Prior to supercritical fluid extraction, the grape pomace was milled by a domestic grinder, and the particles were classified according to particle size using a standard sifter with several mesh sizes from 0.5 to 2.0 mm. The mean particle diameter was determined according to Sauter’s equation.31 Moisture content was determined by oven drying to a constant weight at 378.15 K and expressed as a percentage.32 The true density (ρs) of raw material was determined by helium gas pycnometry (Pycnomatic ATC, Thermo Electron Corporation, Milan, Italy). The apparent density (ρa) was calculated by dividing the feed mass by the vessel volume. The porosity of the bed (ε) was calculated as (1 – ρa/ρs).
2.2. Chemicals
Carbon dioxide (mass fraction purity 0.999 in the liquid phase) was supplied by Sapio S.r.l. (Udine, Italy). Sep-Pak Plus tC18 cartridge WAT 036810 and WAT036800 were purchased from Waters (Milan, Italy). The Folin–Ciocalteau reagent, 2,2-difenil-1-picrylhydrazyl, reagents of analytical grade or higher available purity, (−)-epicatechin, (+)-catechin, gallic acid, 3,4-dihydroxybenzoic acid (protocatechuic acid), 4-hydroxy-3,5-dimethoxybenzoic acid (syringic acid), 3,4-dihydroxycinnamic acid (caffeic acid), 4-hydroxy-3-methoxycinnamic acid (ferulic acid), 4-hydroxycinnamicacid (p-coumaric acid), and trans-resveratrol were purchased from Sigma-Aldrich (Milan, Italy).
The other phenolic compounds were quercetin, isoquercitrin (quercetin-3-O-glucoside), kaempferol, kaempferol-3-O-glucoside, rhamnetin, isorhamnetin, isorhamnetin-3-O-glucoside, rutin (quercetin-3-O-rutinoside), and myricetin and were supplied by Extrasynthese (Lyon, France).
2.3. Total Phenolic Content
Purification by C18 cartridge was carried out for the samples to eliminate the interference of sugars, nonvolatile acids, and amino acids in determination of total phenols. The total phenolic content (TPC) values of the grape pomace extracts were measured using the Folin–Ciocalteau reagent, according to Yu et al.33 All analyses were performed in triplicate. Results were expressed as milligrams of equivalent gallic acid per 100 g of dried matter (mgGAE /100 gDM).
2.4. HPLC-DAD-MS Analysis of Polyphenols
The analysis was carried out in a Dionex Ultimate 3000 UPLC system (Thermo Scientific, San Jose, CA, U.S.A.) equipped with a diode array detector (DAD, 280 and 370 nm) coupled to an electrospray ionization mass spectrometer (ESI-MS) detector. The system and analytical procedures were previously described by Lago-Vanzela et al.34 Phenolic compounds were identified based on their chromatographic behavior, UV–vis, and mass spectra by comparing the collected data with standard compounds (when available) and data reported in the literature. A calibration curve based on the UV–vis signal for each available phenolic standard was constructed for quantitative analysis. The results were expressed in μg/100 gDM.
2.5. Fractionation of Proanthocyanidins
Proanthocyanidins present in the extract were fractionated on two C18Sep-Pak cartridges assembled (WAT 36800/WAT36810, top and bottom, respectively) into three fractions by different organic solvents, according to Sun et al.35 Ethyl acetate was used for fraction FI + FII, containing monomeric and oligomeric flavan-3-ols, while methanol was used for fraction FIII, which contains polymeric proanthocyanidins. Results were expressed as mgcatechin/100 gDM
2.6. Antioxidant Activity
The antioxidant activity of proanthocyanidins fractions was evaluated by the total free radical scavenger capacity (RSC) following the methodology describedby Espìn et al.36 using a UV–vis spectrophotometer (Shimadzu UV 1650, Italy). The RSC is the difference of the concentration of DPPH free radical (CDPPH•i) previously dissolved in methanol, after 60 min of reaction with the samples (CDPPH•f). The antioxidant activity of the samples was expressed as mgα-tocopherol/100 gDM. Analyses were performed in triplicate.
2.7. Pilot Plant Supercritical Fluid Extraction
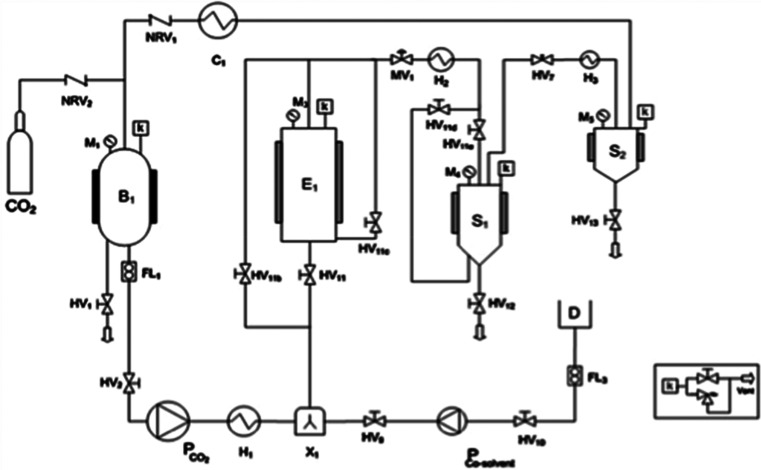
A SFE pilot-plant (SCF100 serie 3 PLC-GR-DLMP, Separeco S.r.l, Pinerolo, Italy) equipped with a 1 L extraction vessel, two 0.3 L separators in series, and a storage tank for CO2 was used (Figure 1).
Figure 1.
SFE pilot plant flow sheet. (B1) Storage tank; (E1) extraction vessel; (S1,S2) separators; (H#) heater exchangers; (C1) condenser; (HV#) Hand valves; (MV1) membrane valve; (NVR#) no return valves; (P) diaphragm pumps; (F1) flowmeter; (M#) manometers; (k) safety devices; (FL1) Coriolis mass flowmeter; (D) cosolvent storage tank; (X#) mixer.
Prior to extracting polyphenols, the removal of nonpolar compounds from grape pomace was carried out by SC-CO2, according to a previous methodology.27,28
The cylindrical extractor basket (H = 0.339 m; D = 0.062 m) was filled with 0.1 kg of ground defatted grape pomace distributed on glass beads (mean diameter of 0.005 m). Supercritical CO2 extractions of grape pomace were carried out at a fixed particle size (0.57 mm), CO2 flow rate (6.0 kg/h), and extraction time (480 min). Different pressures (8, 10, 20 MPa), temperatures (313.15, 323.15, 333.15 K), and percentages (5.0, 7.0, 10.0% w/w) of ethanol–water mixtures at 57% (v/v) were used. Aliquots of grape extract were collected during extractions in a volumetric flask at intervals of 30 min, to assess several data points for the overall extraction curves (OECs). After removal of the cosolvent with a rotary evaporator (Buchi, B465, Switzerland) at 318.15 K, the extracts were weighed and analyzed. All experiments were carried out in triplicate.
2.8. Mathematical Modeling
The broken and intact cells model (BIC), proposed by Sovová et al.37 and Sovová38−40, was used for the SFE process from grape pomace. Sovová’s model divides the solute-extractible content into accessible solutes (from the broken solid particles) and hardly accessible solutes (located inside the unruptured intact solid particle structure). In this model, the overall extraction curve (OEC) is divided into three periods: (1) the constant extraction rate (CER) period, where easily accessible solutes are extracted mainly by convection at a constant rate; (2) the falling extraction rate (FER) period, where the diffusion mechanism starts combined with convection; (3) and the diffusion controlled (DC) period, where the mass transfer occurs mainly by slow diffusion in the bed and inside the solid substrate particles.
The equations of BIC model to calculate the cumulative mass of extract (e) as a function of time (t) in the different periods can be summarized as follow:
| 1 |
| 2 |
| 3 |
where:
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
The adjustable parameters θe, θi, r, ks, and kf can be calculated by minimizing the sum of least-squares between the experimental and calculated values of e.
The application of BIC model needs preliminary determination of several parameters, such as experimental extraction yield (eexp) and the relative amount of passed solvent (q):
| 9 |
| 10 |
where E is the amount of extract (kg), M is the mass of passed solvent (kg).
The mass of insoluble solid, Nm is calculated as
| 11 |
where cu is the solute content in the untreated solid, and N is the solid loaded in the extractor. The value of cu is equal to the asymptotic extraction yield at infinite time. It is calculated by a preliminary fitting of the model equations on experimental data obtained at different pressure and temperature conditions.
The solute weight fraction in the untreated solid (xu) can be calculated as follows:
| 12 |
The bed characteristics, porosity (ε), specific surface area per unit volume of extraction bed (a0), and solvent-to-matrix ratio in the bed (γ) can be calculated with the following equations:
| 13 |
| 14 |
| 15 |
The values of models parameters and graph plots were calculated by Matlab R2016b (MathWorks, Inc., U.S.A.). The agreement between the experimental and model values were assessed by the absolute average relative deviation (AARD), as reported in following equation:
| 16 |
where n is the number of experimental points composing a kinetic curve Xexp,p is the experimental value at point p, and Xcalc,p is the model value at point p.
2.9. Scale-up Method
The scale-up criteria adopted were to keep constant the ratio between solvent flow rate and substrate feed (S/F) and the bed geometry dimension.41−47 The last one was achieved by keeping constant the ratio between the height (H) and diameter (D) of the cylindrical extractor vessel with increasing capacity. Other parameters such as dp, moisture content, true and apparent density of raw material were considered the same of the pilot-scale.
The scale-up prediction was investigated for extraction vessels of 50, 100, 500, and 1000 L capacity. Reynolds number was calculated using eq 17:
| 17 |
where U is the CO2 velocity, dp is the particle diameter, ρf is the CO2 density, and μf is CO2 viscosity.
2.10. Economic Evaluation
Operational data were based on the pilot-scale experiment and the scale-up procedure. The costs of four industrial SFE units as a function of the capacity of extraction vessels (50, 100, 500, and 1000 L) were estimated using eq 18 reported by Rocha-Uribe et al.:48
| 18 |
where C is the cost of the equipment in €, V its capacity in liters, I2019 the cost of the equipment in the year 2019 with a chemical engineering plant cost index (CEPCI) value of 521.9 and I2 refers to the CEPCI for the year 2019 (576.1).
The economic analysis was based on the methodology of Turton et al.49 and Rosa and Meireles.50 It sets the cost of manufacturing (COM) as a function of investment cost (FI) (SFE units), labor cost (COL) related to operators (number and wage) of the extraction unit, utility cost (CUT) which considers the energy used in the solvent cycle for steam generation, refrigeration and electricity requirements, waste treatment cost (CWT), and raw material cost (CRM), using the following relation:
| 19 |
3. Results and Discussion
Physical and chemical characterization of grape pomace are reported in Table 1.
Table 1. Physical and Chemical Characterization of Grape Pomace.
| grape pomace | value |
|---|---|
| moisture (%) | 7.66 ± 0.32 |
| mean particle diameter (dp) (mm) | 0.570 ± 0.008 |
| true density (kg m–3) | 1055 ± 174 |
| apparent density (kg m–3) | 98.42 ± 0.36 |
| porosity (ε) | 0.905 ± 0.015 |
The effects of pressure, temperature, and cosolvent percentage on the extraction yield and total polyphenol contents (TPC) were examined using the SFE pilot scale plant previously described. The performed experiments and the respective operating conditions are summarized in Table 2, where the extraction yields and total polyphenol contents are reported.
Table 2. Operating Conditions, Global Yields, and Total Polyphenol Contents of the Performed Experiments.
| run | pressure | temperature | CO2 flow rate | cosolvent | global yield | total phenols |
|---|---|---|---|---|---|---|
| N° | (MPa) | (K) | (kg/h) | (% w/w) | (% w/w) | (mg GAE/100 g DM) |
| 1 | 8 | 313.15 | 6 | 7.5 | 10.60 ± 0.24 | 1488 ± 3 |
| 2 | 10 | 313.15 | 6 | 7.5 | 4.53 ± 0.12 | 619 ± 5 |
| 3 | 20 | 313.15 | 6 | 7.5 | 2.67 ± 0.05 | 365 ± 4 |
| 4 | 8 | 323.15 | 6 | 7.5 | 8.80 ± 0.18 | 1203 ± 55 |
| 5 | 8 | 333.15 | 6 | 7.5 | 1.60 ± 0.03 | 218 ± 1 |
| 6 | 8 | 313.15 | 6 | 5.0 | 3.32 ± 0.10 | 455 ± 1 |
| 7 | 8 | 313.15 | 6 | 10.0 | 17.72 ± 0.14 | 2245 ± 73 |
3.1. Modeling the OECs
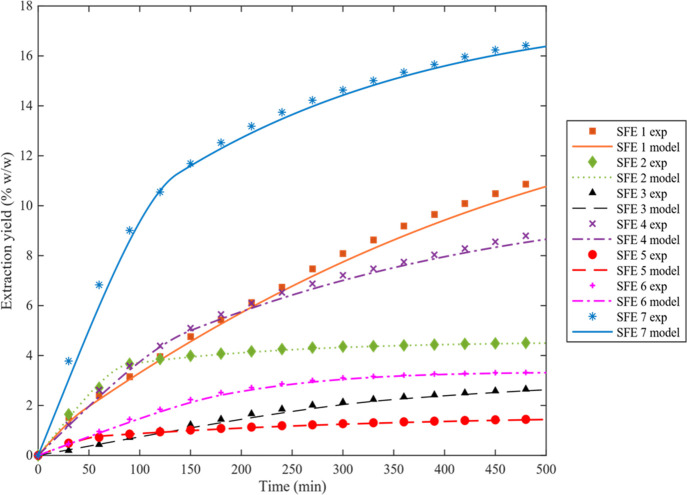
In Figure 2, the experimental extraction curves (OECs) of the seven assays (Table 2) are compared with the predicted profiles from the model. As shown in Figure 2, the OECs present three periods: the first part represents a linear period, characterized by extraction at a constant rate and corresponding to the extracted solute solubility; the second part starts where the extraction rate decreases with time, since diffusion phenomena appear; and the third one is where diffusion is dominant.
Figure 2.
Comparison between experimental and predicted extraction curves (OECs)
The modeling results are reported in Table 3, where it can be noticed that the absolute average relative deviations (AARD) were in the range of 1.01–5.09%. This indicates that the BIC model is suitable for modeling SFE of grape pomace. It was assumed that cosolvent behaves as an incompressible liquid as suggested by Macías-Sánchez et al.51 whereby the density of CO2 plus cosolvent at 5, 7.5 and 10% (w/w) (Table 3) was calculated on the basis of the molar flux composition of each system. The density of ethanol and water at 313.15, 323.15, and 332.15 K for the different pressures was considered the same as that at atmospheric pressure obtained from the literature.52,53
Table 3. Kinetic Parameters of the Sovová40 Model Applied to SFE Experimental Overall Extraction Curves from Grape Pomace.
| run | pressure | temperature | CO2 flow rate | cosolvent | ρf | kfa0 (×10–2) | ksas (×10–5) | ys (×10–4) | θe | θi | tCER | tFER | AARD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N° | (MPa) | (K) | (kg·h–1) | (% w/w) | g mL–1 | (s–1) | (s–1) | (g kgCO2–1) | (−) | (−) | (min) | (min) | (%) |
| 1 | 8 | 313.15 | 6 | 7.5 | 0.356 | 2.474 | 0.408 | 4.699 | 0.2865 | 145.03 | 6.79 | 45.29 | 3.057 |
| 2 | 10 | 313.15 | 6 | 7.5 | 0.664 | 1.043 | 0.843 | 4.108 | 0.2816 | 29.11 | 18.73 | 89.54 | 2.879 |
| 3 | 20 | 313.15 | 6 | 7.5 | 0.842 | 0.794 | 0.602 | 0.758 | 0.2771 | 30.52 | 57.17 | 348.92 | 5.088 |
| 4 | 8 | 323.15 | 6 | 7.5 | 0.304 | 2.807 | 0.487 | 4.058 | 0.3022 | 145.01 | 27.78 | 149.09 | 3.590 |
| 5 | 8 | 333.15 | 6 | 7.5 | 0.279 | 3.528 | 0.528 | 1.674 | 0.2482 | 143.20 | 11.21 | 61.48 | 1.798 |
| 6 | 8 | 313.15 | 6 | 5.0 | 0.331 | 2.792 | 1.488 | 1.534 | 0.2150 | 33.68 | 21.59 | 182.08 | 1.014 |
| 7 | 8 | 313.15 | 6 | 10.0 | 0.381 | 3.499 | 0.929 | 22.013 | 0.2152 | 67.63 | 8.26 | 52.19 | 1.057 |
3.1.1. Effect of Pressure
Increase in pressure from 8 to 20 MPa at 313.15 K and 7.5% (w/w) cosolvent (Run 1, 2 and 3) leads to decrease the extraction yield from 10.60 to 2.67% (w/w) and total polyphenol content from 1488 to 365 mgGAE/100 gDM (Table 2).
As can be observed in Table 3, at higher extraction pressures the volumetric solvent phase mass transfer coefficient kfa0 decreases from 2.474 ·10–2 to 0.794 · 10–2 s–1, and the volumetric solid phase mass transfer coefficient ksas shows a tendency to increase from 0.408 · 10–5 to 0.602· 10–5 s–1. It is interesting to note that kfa0 values are approximately 4 orders of magnitude greater than the ksas ones. This means that convection is more representative than the diffusional mechanism. These results are consistent with those reported for oil extraction of various vegetable matters.31,47,54,55
Although the increase in pressure increases the solvent power through a density increase from 0.336 to 0.842 g mL–1, the solute solubility decreases from 4.699 × 10–4 to 0.758 × 10–4 g kgCO2–1. As suggested by Farias-Campomanes et al.,25 the low mass-transfer observed at the high pressure could be partially due to the low dispersion coefficient of the modified SC-CO2, which accounts for the axial and radial diffusion mechanisms, the characteristics of the raw material which is not homogeneous (skins and seeds), and the high porosity of the extraction bed. The ksas related to the diffusion of the less accessible solute increases due to the damage/destruction of the cell wall under high pressure.56
With regard to the extraction periods, the results in Table 3 show that Sovová model predicted a large decrease of tCER and tFER as pressure decrease. Therefore, the lower the pressure, the shorter the extraction time, for both the compounds present on the surface of the matrix and the less accessible ones. Similar pressure effects were observed by Jia et al.,57 Rezaei et al.,58 Döker et al.,59 Bensebia et al.,60 García-Risco et al.,61 Ciftci et al.,62 and Wagner et al.63
3.1.2. Effect of Temperature
Increase in temperature from 313.15 to 333.15 K at 8 MPa and 7.5% (w/w) cosolvent (Run 1, 4 and 5) decreases the extraction yield from 10.60 to 1.60% w/w and TPC from 1488 to 218 mgGAE/100 gDM (Table 2). Similar results related to extraction temperature effect on phenolic compounds were reported by Zulkafli et al.64 for phenolic compounds extracted from bamboo leaves.
As can be seen in Table 3, kfa0 increases from 2.47 × 10–2 to 3.53 × 10–2 s–1, and ksas increases from 0.408 × 10–5 to 0.508 × 10–5 s–1, whereby both external mass transfer resistance (θe) and internal mass transfer resistance (θi) decrease. In supercritical fluids, a temperature increase can have two opposite effects: it reduces the solvent power through density reduction, and conversely, it enhances solubility by increasing the vapor pressure of the solutes.12 However, at relatively low pressure, a decrease of density and solvent power with increasing temperature prevails, as shown by the decrease of ys values from 4.699 × 10–4 to 1.674 × 10–4 g kgCO2–1 at 313.15 and 333.15 K, respectively. This leads to the observed decrease of the extraction yield due to the decrease in solvent driving force. With respect to extraction periods, the results in Table 3 show that tCER and tFER are extended as temperature increases, and this is due to the slow kinetics.
3.1.3. Effect of Cosolvent
The extraction curves obtained increasing the percentage of cosolvent from 5 to 10% (w/w) (Run 6, 1, and 7) are shown in Figure 2. As can be observed, the cosolvent percentage enhancement leads to an increase in the slope of the linear part of the curve, which means higher solute solubility. The increase of the cosolvent percentage from 5 to 10% (w/w) at 8 MPa and 313.15 K (Run 6, 1 and 7) increased both extraction yield from 3.32 to 17.77% (w/w) and TPC content from 455 to 2245 mgGAE/100 gDM (Table 2). This is not unexpected since as reported by Ting et al.,65,66 Kopcak et al.67 and Zhang et al.,68 the addition of polar cosolvent to supercritical fluids may increase the following: (i) the mixture density leading to the solute solubility enhancement; (ii) the supercritical mixture critical point; (iii) the difference between the local density (around solute molecule) and the bulk density; (iv) the solubility due to specific interactions between the solute and cosolvent molecules.
As can be seen in Table 3, with a cosolvent percentage increase from 5 to 10% (w/w), the kfa0 and ksas values increased due to decrease of mass transfer resistance both in SC-CO2 and grape pomace phases, which determines the increase in driving force and convention. It is worth pointing out the extremely high increase of ys values from 1.334 × 10–4 to 22.013 × 10–4 g kgCO2–1 due to cosolvent increase from 5 to 10% w/w, respectively. Hence, these results clearly indicate that the cosolvent increase considerably promotes the rate of removal of solutes from both the external and the interior of the biomass particles. As far as extraction periods, the Sovová model predicts a large decrease of tCER as the cosolvent percentage increases according to the kfa0 values. Therefore, the higher the cosolvent percentage, the faster the extraction rate. Similar cosolvent effects were observed by Castro-Vargas et al.,69 Akay et al.,70 Andrade et al.,71 and Zulkafli et al.64
Globally, the commented results show that 8 MPa, 313.15 K, and 10% (w/w) cosolvent are the best set experimetal conditions to obtain the highest yield and polyphenol content. It was considered economically advantageous to stop the SFE process at 240 min, which corresponds to 84% of the total extraction yield. Both yield and total phenol content found in this work are higher than those reported in the literature.5,21,23,25,72−75
3.2. Phenolic Composition of the Extract
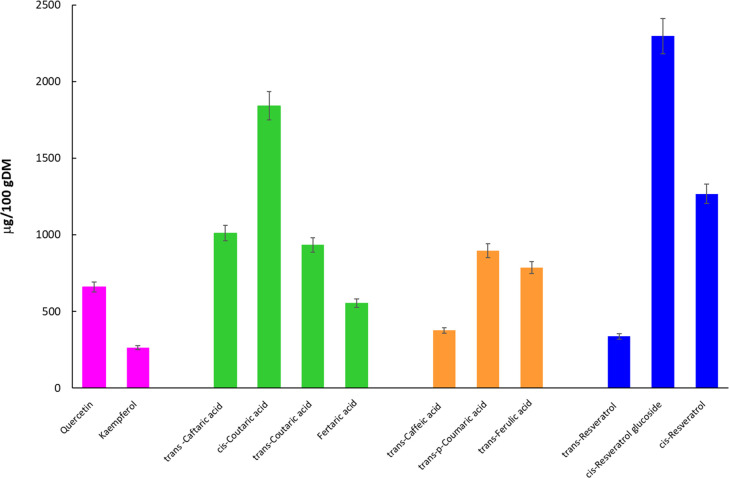
HPLC analysis of the extract obtained at 8 MPa/313.15 K/10% (w/w) cosolvent/240 min extraction time was performed. Flavonols, hydroxycinnamoyl tartaric acids, hydroxycinnamic acids, and stilbenes were identified and quantified as depicted in Figure 3. The major compounds detected were cis-resveratrol glucoside (2296.88 ± 0.02 μg/100 gDM), cis-coutaric acid (1841.16 ± 0.02 μg/100 gDM), trans-p-coumaric acid (896.70 ± 0.05 μg/100gDM), and quercetin (658.69 ± 0.01 μg/100 gDM). Extensive studies have focused on these bioactive compounds due to their health benefits and disease prevention effects.76−78
Figure 3.
Flavonols, hydroxycinnamoyl tartaric acids, hydroxycinnamic acids, and stilbenes identified and quantified in the grape pomace extract obtained at 8 MPa/313.15 K/cosolvent 10% w/w/240 min.
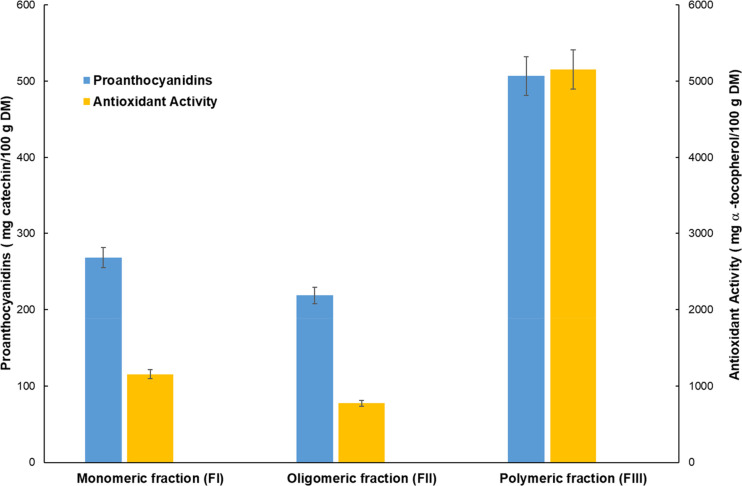
Figure 4 shows the results of proanthocyanidins’ fractionation and antioxidant activity evaluation performed on the grape pomace extract under the operating conditions previously reported. The amount of total proanthocyanidins extracted from grape marc was 0.994 gcatechin /100 gDM, which in comparison with 3.5 g/100 g of grape seed reported by Gu et al.79 can be considered a good recovery taking into account that raw material consists of skin, seeds, and pulp residues. Polymeric proanthocyanidins were the predominant form (506 ± 10.8 mgcatechin/100 gDM) followed by monomeric (268 ± 4.5 mgcatechin/100 gDM) and oligomeric (218 ± 5.2 mgcatechin/100 gDM) ones. The highest antioxidant activity was evaluated for the polymeric fraction (5154 ± 18.5 mgα-tochoferol/100 gDM) due to the high degree of polyphenol polymerization.80
Figure 4.
Proanthocyanidins’ fractions and antioxidant activity of the grape pomace extract obtained at 8 MPa/313.15 K/cosolvent 10% w/w/240 min.
The results show that grape pomace is a potential source for proanthocyanidins. Recently, Unusan81 reported that proanthocyanidins appear to exert pharmacological effects, including antioxidant, antimicrobial, antiobesity, antidiabetic, antineurodegenerative, antiosteoarthritis, anticancer, and cardio- and eye-protective properties. These potential health benefits of proanthocyanidins make them a promising source as nutraceuticals.
3.3. Scale-up Study
As reported in section 2.9, the scale-up criteria adopted were to keep constant both the S/F and the bed geometry dimension. The experimental conditions selected to perform the scale-up were 8 MPa, 313.15 K, 6 kg/h CO2 flow rate, and 10% (w/w) cosolvent. The scale-up prediction was investigated for extraction vessels of 50, 100, 500, and 1000 L capacity,
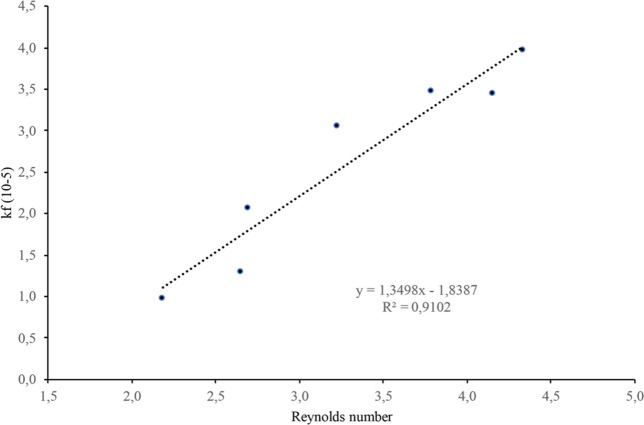
The volumetric mass transfer coefficients (kfa0) determined through modeling of the experimental OACs data were correlated as a function of the dimensionless Reynolds number (Re).46,82,83
Figure 5 shows the good correlation (R2 = 0.9102) obtained. This result allowed to predict the mass transfer coefficients as a function of Re for scaling up.
Figure 5.
Correlation between the volumetric mass transfer coefficients (kfa0) and Reynolds number (Re).
In Table 4 are reported the grape pomace mass (F) to fill the extraction vessels calculated using the apparent density of the raw material determined at the pilot scale, the calculated values of Re and kf for extraction vessel of 50, 100, 500, and 1000 L capacity. The constant ratio of H/D and the values of Re allow us to suppose solvent flow pattern in the extraction vessels as plug flow.
Table 4. Scale-up Predicted Parameters for Extraction Vessel of 50, 100, 500, and 1000 L Capacity.
| extraction vessel | H/D | H | D | S/F | F | S | Re | kf 10 –5 |
|---|---|---|---|---|---|---|---|---|
| (L) | (−) | (m) | (m) | (−) | (kg) | (kg/s) | (−) | (s–1) |
| 50 | 5.47 | 1.249 | 0.228 | 66 | 5 | 0.0917 | 9.941 | 11.580 |
| 100 | 5.47 | 1.573 | 0.288 | 66 | 10 | 0.1833 | 12.525 | 15.068 |
| 500 | 5.47 | 2.691 | 0.492 | 66 | 50 | 0.9167 | 21.418 | 27.071 |
| 1000 | 5.47 | 3.390 | 0.620 | 66 | 100 | 1.8333 | 26.985 | 34.85 |
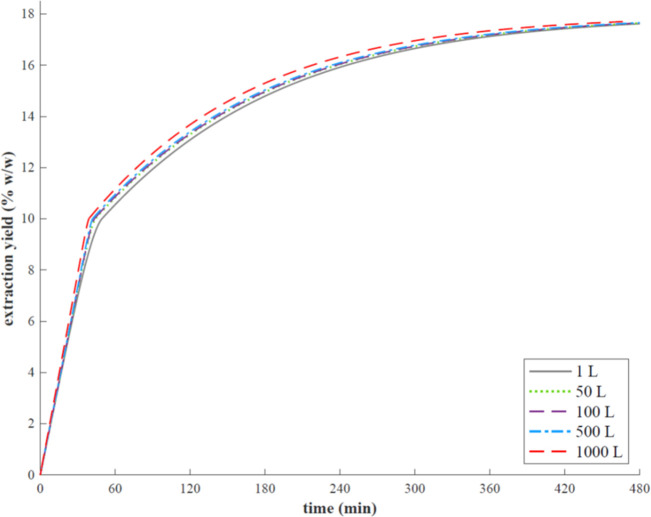
Figure 6 shows the predicted OECs by Sovová model at 50, 100, 500, and 1000 L and the OAC obtained from grape pomace at SFE pilot-scale (1 L). As can be seen, the OECs at industrial scale presented similar shapes, and global yields are close to each other, around 17% (w/w) at 480 min extraction time. These results confirm the excellent predictive level of the BIC model and the success of the scale-up criteria used. Similar findings are in agreement with other scale-up studies conducted by Prado et al.42,43
Figure 6.
Predicted OECs for SFE scale-up from grape pomace.
3.4. Cost Estimation
In order to perform an economic evaluation of the process, four scales of SFE units, all with the same design but with extractor volumes of 50, 100, 500, and 1000 L were evaluated. The SFE extracting conditions were 8 MPa, 313.15 K, S/F ratio of 66, 10% w/w cosolvent, 240 min extraction time. The list of economic parameters used for cost of manufacturing estimation are presented in Table 5.
Table 5. Economic Parameters Needed for COM Estimation.
| FI | 2 extraction vessels of 50 La | 620.000 € |
| 2 extraction vessels of 100 La | 1.000.000 € | |
| 2 extraction vessels of 500 La | 3.000.000 € | |
| 2 extraction vessels of 1000 La | 5.000.000 € | |
| depreciation ratea | 10% year | |
| annual mantenance ratea | 6% year | |
| COL | labor cost | 18 €/h |
| 2 extraction vessels of 50 L | 2 operators | |
| 2 extraction vessels of 100 L | 2 operators | |
| 2 extraction vessels of 500 L | 3 operators | |
| 2 extraction vessels of 1000 L | 3 operators | |
| CRM | grape marc | 0.025 €/kg |
| CWM | cost of waste treatment | 0.015 €/kg |
| CUT | carbon dioxideb | 2.50 €/kg |
| ethanolc | 4.50 €/L | |
| electricityd | 0.15 €/kWh | |
| water | 1.44 €/m3 |
Calculated by the equation proposed by Rocha-Uribe et al;48
Sapio S.r.l..
Sigma-Aldrich.
Sorgenia SPA.
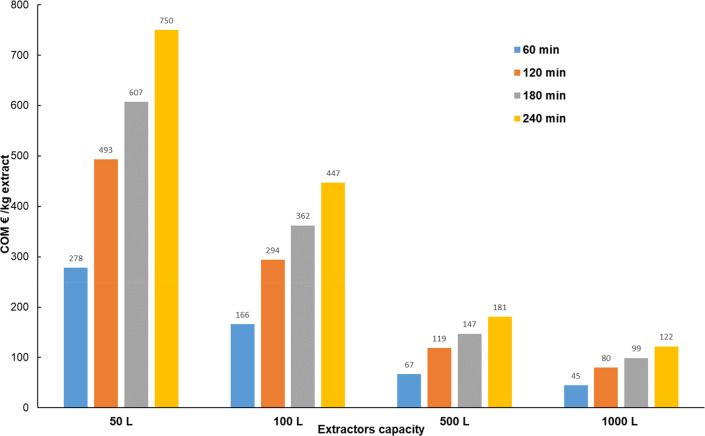
Capital investment or equipment costs (FI) were estimated using eq 18. The SFE unit was considered as working 24 h per day with three daily shifts, for 330 days per year, representing a total of 7920 h per year. The number of operators per shift varies according to the capacity of the plant, at a cost of € 18.00/h. The raw material masses to fill the extraction vessels were calculated from the apparent density of the raw material determined at the pilot scale. The cost of the raw material (CRM) was calculated as the cost of drying and milling, for a total of € 0.025/kg. Also, a loss of 2% of CO2 in the raw material cost according to Leal et al.84 was considered. The cost of the waste treatment (CWT) was considered as € 0.015/kg. In Figure 7, the COM was calculated for SFE units (2 × 50/100/500/1000L) as a function of the extraction time. It can be observed that the COM values exhibited a similar trend in all cases, namely they increased with the extraction time and decreased with plant scale increase. The lowest COMs corresponding to € 67/kg extract and € 45/kg extract were obtained at 60 min for 2 × 500 L unit and 2 × 1000 L unit, respectively.
Figure 7.
COM calculated for SFE units (2 × 50/100/500/1000L) as a function of the extraction time.
Farias-Campomanes et al.25 reported the lowest COM corresponding to US$ 133.16/kgextract for a 2 × 500 L unit, working at 20 MPa, 313.15 K, S (CO2)/F ratio of 47, 10% (w/w) cosolvent (96% ethanol), 180 min extraction time, and achieving 5.5% w/w as grape pomace global yield with an as-expected 23 g/kgextract phenolics’ concentration. Under our experimental conditions, at 60 min extraction time, the global yield was 7.0% w/w and an expected 133 gGAE/kgextract phenolics’ concentration. Therefore, the improvement of the SFE operating conditions led to COM reduction.
The COMs of conventional methods in industrial units for the grape pomace extracts are higher than € 45–67/kgextract25,75
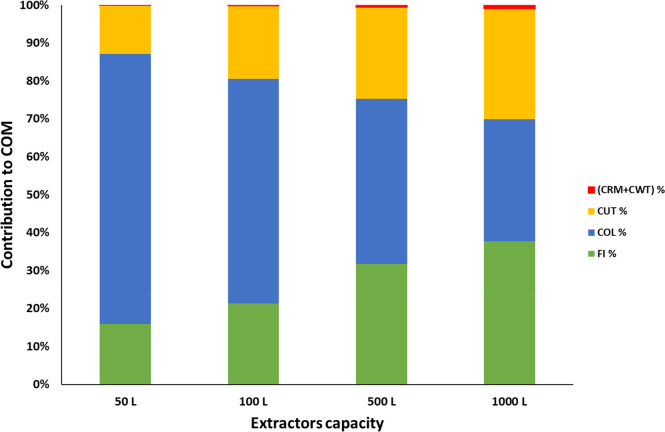
Figure 8 depicts the contribution of each component of COM for grape pomace extracts obtained by SFE units with different extractor capacities. The labor cost (COL) and the fixed cost of investment (FI) comprise the largest portions in the distribution of costs within the COM, followed by the CUT. The labor costs associated with the number of operators required to operate the larger-capacity extractors demonstrate an economy of scale, resulting in a decrease of the influence of the COL (from 71 to 32%) on the COM as the production rate increases. Instead, the FCI increases from 15 to 38% as SFE plant capacity increases, and the CUT increases from 13 to 38%. The other costs, CRM and CWT, had a much smaller influence on the COM, accounting together for less than or equal to 1%. These results are in agreement with the literature.43
Figure 8.
Contribution of each component of COM for grape pomace extracts obtained by SFE units with different extractor capacity.
Conclusions
The results obtained in this study indicate the excellent predictive level obtained by means of Sovová’s model and the successf of the scale-up criteria adopted, proving that industrial-scale SFE could be developed for the separation process of polyphenols from grape pomace at a competitive cost. High quality extracts mantaining their biological activities, suitable to be used by food, cosmetic, and/or pharmaceutical industries, can be produced using the SFE process. SFE at an industrial level could be the technological advancement required to really take off an integrated biorefinery to valorize the winery byproducts so promoting a major sustainability of wine making process.
Glossary
Nomenclature
- CEPCI
chemical engineering plant cost index
- a0
specific surface area per unit volume of extraction bed (m–1)
- as
specific area between the regions of intact and broken cells (m–1)
- cu
(= xu/(1+xu)), solute content in the untreated solid (kg/kg insoluble solid)
- d
extractor diameter (m)
- dp
particle diameter (m)
- e
extraction yield (kg/kg insoluble solid)
- E
extract mass (kg)
- G
initial fraction of extract in open cells
- H
extraction bed length (m)
- D
internal diameter (m)
- kf
fluid-phase mass transfer coefficient (m·s–1)
- ks
solid-phase mass transfer coefficient (m·s –1)
- N
solid charge in the extractor (kg)
- Nm
(= (1–cu)N) charge of insoluble solid (kg)
- q
relative amount of the passed solvent (kgCO2/kginsoluble solid)
- qm
relative amount of passed solvent at the end of CER period (kgCO2/kginsoluble solid)
- qn
relative amount of passed solvent at the end of FER period (kgCO2/kginsoluble solid)
- qs
relative amount of passed solvent at the end of first period when only two periods of extraction are considered (kgCO2/kginsoluble solid)
- Q
solvent flow rate (kg/s)
- r
grinding efficiency (fraction of broken cells)
- t
extraction time (min)
- tCER
(= (qm·Nm)/(Q·60)) extraction time at the end of CER period (min)
- tFER
(= (qn·Nm)/Q·60)) extraction time at the end of FER period (min)
- xu
concentration in the untreated solid (kg/kgCO2)
- y
fluid-phase concentration (kg/kgCO2)
- y0
initial fluid-phase concentration (kg/kgCO2)
- ys
solute solubility (g/kgCO2)
Glossary
Greek letters
- γ
solvent to matrix ratio in the bed (kgCO2/kginsoluble solid)
- ρa
solid apparent density (kg/m3)
- ρf
solvent density (kg/m3)
- ρs
solid real density (kg/m3)
- θe
dimensionless external mass transfer resistance
- θi
dimensionless internal mass transfer resistance
- ε
bed void fraction
The authors declare no competing financial interest.
References
- International Organisation of Vine and Wine (OIV). State of the World Vine and Wine Sector 2021. See the following: https://www.oiv.int.
- Beres C.; Costa G. N. S.; Cabezudo I.; da Silva-James N. K.; Teles A. S. C.; Cruz A. P. G.; Mellinger-Silva C.; Tonon R. V.; Cabral L. M. C.; Freitas S. F. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manage 2017, 68, 581–594. 10.1016/j.wasman.2017.07.017. [DOI] [PubMed] [Google Scholar]
- González-Centeno M. R.; Rosselló C.; Simal S.; Garau M. C.; López F.; Femenia A. Physico-chemical properties of cell wall material obtained from ten grape varieties and their byproducts: grape pomaces and stems. LWT Food Sci. Technol. 2010, 43, 1580–1586. 10.1016/j.lwt.2010.06.024. [DOI] [Google Scholar]
- Kammerer D.; Claus A.; Carle R.; Schieber A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. 10.1021/jf049613b. [DOI] [PubMed] [Google Scholar]
- Oliveira D. A.; Salvador A. A.; Smânia A.; Smânia E. F. A.; Maraschin M.; Ferreira S. R. S. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013, 164, 423–432. 10.1016/j.jbiotec.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Machado N. F. L.; Domínguez-Perles R. Addressing facts and gaps in the phenolics chemistry of winery by-products. Molecules 2017, 22, 286. 10.3390/molecules22020286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira A.; Baenas N.; Dominguez-Perles R.; Barros A.; Rosa E.; Moreno D.; Garcia-Viguera C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. 10.3390/ijms150915638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo P. S.; Massarioli A. P.; Denny C.; dos Santos L. F.; Franchin M.; Pereira G. E.; Vieira T. M. F. d. S.; Rosalen P. L.; Alencar S. M. d. Winery by-products: Extraction optimization. phenolic composition and cytotoxic evaluation to act as a new source of scavenging of reactive oxygen species. Food Chem. 2015, 181, 160–169. 10.1016/j.foodchem.2015.02.087. [DOI] [PubMed] [Google Scholar]
- Monteiro G. C.; Minatel I. O.; Junior A. P.; Gomez-Gomez H. A.; de Camargo J. P. C.; Diamante M. S.; Pereira Basilio L. S.; Tecchio M. A.; Pereira Lima G. P.; et al. Bioactive compounds and antioxidant capacity of grape pomace flours. LWT Food Sci. Technol. 2021, 135, 110053. 10.1016/j.lwt.2020.110053. [DOI] [Google Scholar]
- Ribeiro L. F.; Ribani R. H.; Francisco T. M. G.; Soares A. A.; Pontarolo R.; Haminiuk C. W. I. Profile of bioactive compounds from grape pomace (Vitis vinifera and Vitis labrusca) by spectrophotometric, chromatographic and spectral analyses. J. Chromatography B 2015, 1007, 72–80. 10.1016/j.jchromb.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Chowdhary P.; Gupta A.; Gnansounou E.; Pandey A.; Chaturvedi P. Current trends and possibilities for exploitation of grape pomace as a potential source for value addition. Environ. Pollut. 2021, 278, 116796. 10.1016/j.envpol.2021.116796. [DOI] [PubMed] [Google Scholar]
- Brunner G.Supercritical Gases As Solvents: Phase Equilibria. Gas Extraction: An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes; Steinkopff Heidelberg, 1994; pp 59–146. [Google Scholar]
- Molero Gomez A.; Pereyra Lopez C.; Martinez de la Ossa E. Recovery of grape seed oil by liquid and supercritical carbon dioxide extraction: A comparison with conventional solvent extraction. Chem. Eng. J. and Bioch. Eng. J. 1996, 61, 227–231. 10.1016/0923-0467(95)03040-9. [DOI] [Google Scholar]
- Brunner B. Supercritical fluids: technology and application to food processing. J. Food Eng. 2005, 67, 21–33. 10.1016/j.jfoodeng.2004.05.060. [DOI] [Google Scholar]
- da Silva Porto P.ıc. A. L.; Laranjinha J. A. N.; de Freitas V. A. P. Antioxidant protection of low density lipoprotein by procyanidins: structure/activity relationships. Biochem. Pharmacol. 2003, 66, 947–954. 10.1016/S0006-2952(03)00458-1. [DOI] [PubMed] [Google Scholar]
- Herrero M.; Cifuentes A.; Ibañez E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants. food-by-products, algae and microalgae-A review. Food Chem. 2006, 98, 136–148. 10.1016/j.foodchem.2005.05.058. [DOI] [Google Scholar]
- Reverchon E.; De Marco I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. 10.1016/j.supflu.2006.03.020. [DOI] [Google Scholar]
- Lack E., Seidlitz H.. Commercial scale decaffeination of coffee and tea using supercritical CO2. In Extraction of Natural Products Using Near-Critical Solvents; King M.B., Bott T.R., Eds.; Springer: Amsterdam, 1993; pp 101–139. [Google Scholar]
- Hrncic M. K.; Cor D.; Verboten M. T.; Knez Z. Application of supercritical and subcritical fluids in food processing. Food Qual. Saf. 2018, 2, 59–67. 10.1093/fqsafe/fyy008. [DOI] [Google Scholar]
- Barba F. J.; Zhu Z.; Koubaa M.; Sant'Ana A. S.; Orlien V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. 10.1016/j.tifs.2016.01.006. [DOI] [Google Scholar]
- Pinelo M.; Ruiz-Rodriguez A.; Sineiro J.; Senorans F. J.; Reglero G.; Nunez M. J. Supercritical fluid and solid–liquid extraction of phenolic antioxidants from grape pomace: a comparative study. Eur. Food Res. Technol. 2007, 226, 199–205. 10.1007/s00217-006-0526-3. [DOI] [Google Scholar]
- Passos C. P.; Silva R. M.; Da Silva F. A.; Coimbra M. A.; Silva C. M. Enhancement of the supercritical fluid extraction of grape seed oil by using enzymatically pre-treated seed. J. Supercrit. Fluids 2009, 48, 225–229. 10.1016/j.supflu.2008.11.001. [DOI] [Google Scholar]
- Casas L.; Mantell C.; Rodríguez M.; De La Ossa E. J. M.; Roldán A.; de Ory I.; Caro I.; Blandino A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. 10.1016/j.jfoodeng.2009.08.002. [DOI] [Google Scholar]
- Marqués J. L.; Della Porta G.; Reverchon E.; Renuncio J. A. R.; Mainar A. M. Supercritical antisolvent extraction of antioxidants from grape seeds after vinification. J. Supercrit. Fluids 2013, 82, 238–243. 10.1016/j.supflu.2013.07.005. [DOI] [Google Scholar]
- Farias-Campomanes A. M.; Rostagno M. A.; Meireles A. M. Production of polyphenol extracts from grape bagasse using supercritical fluids: yield, extract composition and economic evaluation. J. Supercrit. Fluids 2013, 77, 70–78. 10.1016/j.supflu.2013.02.006. [DOI] [Google Scholar]
- Murga R.; Ruiz R.; Beltran S.; Cabezas J. L. Extraction of natural complex phenols and tannins from grape seeds by using supercritical mixtures of carbon dioxide and alcohol. J. Agric. Food Chem. 2000, 48, 3408–3412. 10.1021/jf9912506. [DOI] [PubMed] [Google Scholar]
- Da Porto C.; Natolino A.; Decorti D. Extraction of proanthocyanidins from grape marc by supercritical fluid extraction using CO2 as solvent and ethanol – water mixture as cosolvent. J. Supercrit. Fluids 2014, 87, 59–64. 10.1016/j.supflu.2013.12.013. [DOI] [Google Scholar]
- Da Porto C.; Natolino A.; Decorti D. The combined extraction of polyphenols from grape marc: ultrasound assisted extraction followed by supercritical CO2 extraction of ultrasound-raffinate. LWT-Food Sci. Technol. 2015, 61, 98–104. 10.1016/j.lwt.2014.11.027. [DOI] [Google Scholar]
- Martinez G. A.; Rebecchi R.; Decorti D.; Domingos J. M. B.; Natolino A.; Del Rio D.; Bertin L.; Da Porto C.; Fava F. Towards multi-purpose biorefinery platforms for the valorization of agro-industrial wastes: production of polyphenols, volatile fatty acids, polyhydroxyalkanoates and biogas from red grape pomace. Green Chem. 2016, 18, 261–270. 10.1039/C5GC01558H. [DOI] [Google Scholar]
- Da Porto C.; Natolino A. Supercritical fluid extraction of polyphenols from grape seed (Vitis vinifera): study on process variables and kinetics. J. Supercrit. Fluids 2017, 130, 239–245. 10.1016/j.supflu.2017.02.013. [DOI] [Google Scholar]
- Povh N. P.; Marques M. O. M.; Meireles M. A. A. Supercritical CO2 extraction of essential oil and oleoresin from chamomile (Chamomilla recutita [L.] Rauschert). J. Supercrit. Fluids. 2001, 21, 245. 10.1016/S0896-8446(01)00096-1. [DOI] [Google Scholar]
- Association of Official Analytical Chemists (AOAC).Official Methods of Analysis, 17th ed., no 925.40; AOAC, 2000. [Google Scholar]
- Yu L.; Perret J.; Harris M.; Wilson J.; Haley S. Antioxidant properties of bran extracts from “Akron” wheat grown at different locations. J. Agric. Food Chem. 2003, 51, 1566–1570. 10.1021/jf020950z. [DOI] [PubMed] [Google Scholar]
- Lago-Vanzela E. S.; Da-Silva R.; Gomes E.; García-Romero E.; Hermosín-Gutierrez I. Phenolic composition of the brazilian seedless table grape varieties BRS Clara and BRS Morena. J. Agric. Food Chem. 2011, 59, 8314–8323. 10.1021/jf201753k. [DOI] [PubMed] [Google Scholar]
- Sun B.; Leandro C.; Ricardo da Silva J. M.; Spranger I. Separation of grape and wine proanthocyanidins according to their degree of polymerization. J. Agric. Food Chem. 1998, 46, 1390–1396. 10.1021/jf970753d. [DOI] [Google Scholar]
- Espìn J. C.; Soler-Rivas C.; Wichers H. J. Characterization of the total free radical scavenger capacity of vegetable oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. 10.1021/jf9908188. [DOI] [PubMed] [Google Scholar]
- Sovová H.; Kučera J.; Jež J. Rate of the vegetable oil extraction with supercritical CO2-II. Extraction of grape oil. Chem. Eng. Sci. 1994, 49, 415–420. 10.1016/0009-2509(94)87013-6. [DOI] [Google Scholar]
- Sovová H. Rate of the vegetable oil extraction with supercritical CO2-I, Modelling of extraction curves. Chem. Eng. Sci. 1994, 49, 409–414. 10.1016/0009-2509(94)87012-8. [DOI] [Google Scholar]
- Sovová H. Mathematical model for supercritical fluid extraction of natural products and extraction curve evaluation. J. Supercrit. Fluids 2005, 33, 35–52. 10.1016/j.supflu.2004.03.005. [DOI] [Google Scholar]
- Sovová H. Broken-and-intact cell model for supercritical fluid extraction : Its origin and limits. J. Supercrit. Fluids 2017, 129, 3–8. 10.1016/j.supflu.2017.02.014. [DOI] [Google Scholar]
- Clavier J. Y.; Perrut M.. Scale-up issues for supercritical fluid processing in compliance with GMP. In Supercritical Fluid Technology for Drug Product Development; York P., Kompella U. B., Eds.; Marcel Dekker, Inc.: New York, 2004; pp 627–631. [Google Scholar]
- Prado J. M.; Prado G. H. C.; Meireles M. A. A. Scale-up study of supercritical fluid extraction process for clove and sugarcane residue. J. Supercrit. Fluids 2011, 56, 231–237. 10.1016/j.supflu.2010.10.036. [DOI] [Google Scholar]
- Prado J. M.; Dalmolin I.; Carareto N. D. D.; Basso R. C.; Meirelles A. J. A.; Oliveira J. V.; Batista E. A. C.; Meireles M. A. A. Supercritical fluid extraction of grape seed: process scale-up, extract chemical composition and economic evaluation. J. Food Eng. 2012, 109, 249–257. 10.1016/j.jfoodeng.2011.10.007. [DOI] [Google Scholar]
- Zabot G. L.; Moraes M. N.; Meireles M. A. Influence of the bed geometry on the kinetics of rosemary compounds extraction with supercritical CO2. J. Supercrit. Fluids 2014, 94, 234–244. 10.1016/j.supflu.2014.07.020. [DOI] [Google Scholar]
- Zabot G. L.; Moraes M. N.; Petenate A. J.; Meireles A. Influence of the bed geometry on the kinetics of the extraction of clove bud oil with supercritical CO2. J. Supercrit. Fluids 2014, 93, 56–66. 10.1016/j.supflu.2013.10.001. [DOI] [Google Scholar]
- Hassim N.; Markom M.; Rosli M. I.; Harun S. Scale-up criteria and economic analysis for supercritical fluid extraction of Phyllanthus niruri. Chem. Eng. Process.: Process Intensif. 2019, 139, 14–22. 10.1016/j.cep.2019.03.011. [DOI] [Google Scholar]
- López-Padilla A.; Ruiz-Rodriguez A.; Reglero G.; Fornari T. Supercritical carbon dioxide extraction of Calendula officinalis: Kinetic modeling and scaling up study. J. Supercrit. Fluids 2017, 130, 292–300. 10.1016/j.supflu.2017.03.033. [DOI] [Google Scholar]
- Rocha-Uribe J. A.; Novelo-Perez J. I.; Araceli Ruiz-Mercado C. Cost estimation for CO2 supercritical extraction systems and manufacturing cost for habanero chili. J. Supercrit. Fluids 2014, 93, 38–41. 10.1016/j.supflu.2014.03.014. [DOI] [Google Scholar]
- Turton R.; Bailie R. C.; Whiting W. B.; Shaeiwitz J. A.. Analysis. Synthesis and Design of Chemical Processes, 2nd ed.; Prentice Hall: Westford, 2003. [Google Scholar]
- Rosa P. T. V.; Meireles M. A. A. Rapid estimation of the manufacturing cost of extracts obtained by supercritical fluid extraction. J. Food Eng. 2005, 67, 235–240. 10.1016/j.jfoodeng.2004.05.064. [DOI] [Google Scholar]
- Macias-Sanchez M. D.; Serrano C. M.; Rodriguez M. R.; De la Ossa E. M. Kinetics of the supercritical fluid extraction of carotenoids from microalgae with CO2 and ethanol as cosolvent. Chemical Engineering Journal 2009, 150, 104–113. 10.1016/j.cej.2008.12.006. [DOI] [Google Scholar]
- Engineering ToolBox, Ethanol - Density and Specific Weight vs. Temperature and Pressure (2008). https://www.engineeringtoolbox.com/ethanol-ethyl-alcohol-density-specific-weight-temperature-pressure-d_2028.html (accessed Feb. 20, 2022).
- Engineering ToolBox , Water - Density, Specific Weight and Thermal Expansion Coefficients (2003).https://www.engineeringtoolbox.com/water-density-specific-weight-d_595.html (accessed Feb. 20, 2022).
- Huang Z.; Shi X.; Jiang W. Theoretical models for supercritical fluid extraction. J. Chrom. A 2012, 1250, 2–26. 10.1016/j.chroma.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Confortin T. C.; Todero I.; Canabarro N. I.; Luft L.; Ugalde G. A.; Neto J. R. C.; Mazutti M. A.; Zabot G. L.; Tres M. V. Supercritical CO2 extraction of compounds from different aerial parts of Senecio brasiliensis: mathematical modeling and effects of parameters on extract quality. J. Supercrit. Fluids 2019, 153, 104589. 10.1016/j.supflu.2019.104589. [DOI] [Google Scholar]
- Campos L. M. A. S.; Michielin E. M. Z.; Danielski L.; Ferreira S. R. S. Experimental data and modeling the supercritical fluid extraction of marigold (Calendula officinalis) oleoresin. J. Supercrit. Fluids 2005, 34, 163–170. 10.1016/j.supflu.2004.11.010. [DOI] [Google Scholar]
- Jia D. D.; Li S. F.; Xiao L. Supercritical CO2 extraction of Plumula nelumbinis oil: experiments and modeling. J. Supercrit. Fluids 2009, 50, 229–234. 10.1016/j.supflu.2009.06.005. [DOI] [Google Scholar]
- Rezaei K. A.; Temelli F. Using supercritical fluid chromatography to determine diffusion coefficients of lipids in supercritical CO2. J. Supercrit. Fluids 2000, 17, 35–44. 10.1016/S0896-8446(99)00039-X. [DOI] [Google Scholar]
- Doker O.; Salgın U.; Sanal I.; Mehmetoglu U.; Calımlı A. Modeling of extraction of β-carotene from apricot bagasse using supercritical CO2 in packed bed extractor. J. Supercrit. Fluids 2004, 28, 11–19. 10.1016/S0896-8446(03)00006-8. [DOI] [Google Scholar]
- Bensebia O.; Barth D.; Bensebia B.; Dahmani A. Supercritical CO2 extraction of rosemary: effect of extraction parameters and modelling. J. Supercrit. Fluids 2009, 49, 161–166. 10.1016/j.supflu.2009.01.007. [DOI] [Google Scholar]
- García-Risco M. R.; Hernández E. J.; Vicente G.; Fornari T.; Senoráns F. J.; Reglero G. Kinetic study of pilot-scale supercritical CO2 extraction of rosemary (Rosmarinus officinalis) leaves. J. Supercrit. Fluids 2011, 55, 971–976. 10.1016/j.supflu.2010.09.030. [DOI] [Google Scholar]
- Ciftci N.; Calderon J.; Temelli F. Supercritical carbon dioxide extraction of corn distiller’s dried grains with solubles: experiments and mathematical modeling. J. Agric. Food Chem. 2012, 60, 12482–12490. 10.1021/jf302932w. [DOI] [PubMed] [Google Scholar]
- Wagner M. E.; French J.; Rizvi S. S. H. Supercritical fluid extraction of oil from potato chips: two scale comparison and mathematical modeling. J. Food Eng. 2013, 118, 100–107. 10.1016/j.jfoodeng.2013.03.023. [DOI] [Google Scholar]
- Zulkafli Z. D.; Wang H.; Miyashita F.; Utsumi N.; Tamura K. Cosolvent-modified supercritical carbon dioxide extraction of phenolic compounds from bamboo leaves (Sasa palmata). J. Supercrit. Fluids 2014, 94, 123–129. 10.1016/j.supflu.2014.07.008. [DOI] [Google Scholar]
- Ting S. S. T.; Macnaughton S. J.; Tomasko D. L.; Foster N. R. Solubility of naproxen in supercritical carbon dioxide with and without cosolvents. Ind. Eng. Chem. Res. 1993, 32, 1471–1476. 10.1021/ie00019a022. [DOI] [Google Scholar]
- Ting S. S. T.; Tomasko D. L.; Macnaughton S. J.; Foster N. R. Chemical-physical interpretation of cosolvents effects in supercritical fluids. Ind. Eng. Chem. Res. 1993, 32, 1482–1487. 10.1021/ie00019a023. [DOI] [Google Scholar]
- Kopcak U.; Mohamed R. S. Caffeine solubility in supercritical carbon dioxide/cosolvent mixtures. J. Supercrit. Fluids 2005, 34, 209–214. 10.1016/j.supflu.2004.11.016. [DOI] [Google Scholar]
- Zhang M.; Dou M.; Wang M.; Yu Y. Study on the solubility parameter of supercritical carbon dioxide system by molecular dynamics simulation. J. Mol. Liq. 2017, 248, 322–329. 10.1016/j.molliq.2017.10.056. [DOI] [Google Scholar]
- Castro-Vargas H. I.; Rodríguez-Varela L. I.; Ferreira S. R. S.; Parada-Alfonso F. Extraction of phenolic fraction from guava seeds (Psidium guajava L.) using supercritical carbon dioxide and cosolvents. J. Supercrit. Fluids 2010, 51, 319–324. 10.1016/j.supflu.2009.10.012. [DOI] [Google Scholar]
- Akay S.; Alpak I.; Yesil-Celiktas O. Effects of process parameters on supercritical CO2 extraction of total phenols from strawberry (Arbutus unedo L.) fruits: an optimization study. J. Sep. Sci. 2011, 34, 1925–193. 10.1002/jssc.201100361. [DOI] [PubMed] [Google Scholar]
- Andrade K. S.; Gonçalvez R. T.; Maraschin M.; Ribeiro-do-Valle R. M.; Martínez J.; Ferreira S. R. S. Supercritical fluid extraction from spent coffee grounds and coffee husks: antioxidant activity and effect of operational variables on extract composition. Talanta 2012, 88, 544–552. 10.1016/j.talanta.2011.11.031. [DOI] [PubMed] [Google Scholar]
- De Campos L. M.; Leimann F. V.; Pedrosa R. C.; Ferreira S. R. Free radical scavenging of grape pomace extracts from Cabernet sauvingnon (Vitis vinifera). Bioresour. Technol. 2008, 99, 8413–8420. 10.1016/j.biortech.2008.02.058. [DOI] [PubMed] [Google Scholar]
- Vatai T.; Škerget M.; Knez Ž. Extraction of phenolic compounds from elder berry and different grape marc varieties using organic solvents and/or supercritical carbon dioxide. J. Food Eng. 2009, 90, 246–254. 10.1016/j.jfoodeng.2008.06.028. [DOI] [Google Scholar]
- Otero-Pareja M. J.; Casas L.; Fernández-Ponce M. T.; Mantell C.; Ossa E. J. Green extraction of antioxidants from different varieties of red grape pomace. Molecules 2015, 20, 9686–9702. 10.3390/molecules20069686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R.; Baroutian S. A techno-economic comparison of subcritical water. supercritical CO2 and organic solvent extraction of bioactives from grape marc. J. Clean. Prod. 2017, 158, 349–358. 10.1016/j.jclepro.2017.05.043. [DOI] [Google Scholar]
- Barreca D.; Trombetta D.; Smeriglio A.; Mandalari G.; Romeo O.; Felice M. R.; Gattuso G.; Nabavi S. M. Food flavonols: Nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. 10.1016/j.tifs.2021.03.030. [DOI] [Google Scholar]
- Ganguly S.; Arora I.; Tollefsbol T. O. Impact of stilbenes as epigenetic modulators of breast cancer risk and associated biomarkers. Int. J. Mol. Sci. 2021, 22, 10033. 10.3390/ijms221810033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath S. H.; Thimmulappa R. K. Antiviral, immunomodulatory, and anticoagulant effects of quercetin and its derivatives: Potential role in prevention and management of COVID-19. J. Pharm. Anal 2022, 12, 29. 10.1016/j.jpha.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L.; Kelm M. A.; Hammerstone J. F.; Beecher G.; Holden J.; Haytowitz D.; Gebhardt S.; Prior R. L. 2004. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- da Silva R. P.F.F.; Rocha-Santos T. A.P.; Duarte A. C. Supercritical fluid extraction of bioactive compounds. Trends Anal. Chem. 2016, 76, 40–519. 10.1016/j.trac.2015.11.013. [DOI] [Google Scholar]
- Unusan N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. 10.1016/j.jff.2020.103861. [DOI] [Google Scholar]
- Cygnarowicz-Provost M.; O’Brien D. J.; Boswell R. T.; Kurantz M. J. Supercritical-fluid extraction of fungal lipids: Effect of cosolvent on mass-transfer rates and process design and economics. J. Supercrit. Fluids 1995, 8, 51–59. 10.1016/0896-8446(95)90050-0. [DOI] [Google Scholar]
- Hassim N.; Markom M.; Rosli M. I.; Harun S. Scale-up approach for supercritical fluid extraction with ethanol–water modified carbon dioxide on Phyllanthus niruri for safe enriched herbal extracts. Scientific Reports 2021, 11, 15818. 10.1038/s41598-021-95222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal P. F.; Kfouri M. B.; Alexandre F. C.; Fagundes F. H.R.; Prado J. M.; Toyama M. H.; Meireles M. A. A. Brazilian Ginseng extraction via LPSE and SFE: global yields. extraction kinetics. chemical composition and antioxidant activity. J. Supercrit. Fluids 2010, 54, 38–45. 10.1016/j.supflu.2010.03.007. [DOI] [Google Scholar]