Version Changes
Revised. Amendments from Version 2
Version 3 contains an updated version of Figure 3, and a correction to author affiliations.
Abstract
Sexual dimorphism in infectious diseases refers to the different infection susceptibilities and outcomes between males and females, and has been described for many pathogens, including hepatitis B virus (HBV). HBV is a substantial global health problem, with close to 300 million people chronically infected, and accounting for a million deaths each year, with an urgent need for enhanced interventions to support progress towards elimination goals. Sexual dimorphism has a strong influence in HBV infection, with males more likely to be exposed, to develop chronic infection, and to suffer from complications including cirrhosis and hepatocellular carcinoma (HCC) compared to females. Different outcomes are driven by differential immune responses, sexual dimorphism of the liver, and androgen response elements in the HBV genome. The impact of sex may also vary with age, with changes at puberty and influences of menarche, pregnancy and menopause in females. In addition, gender has complex influences on education, beliefs, behaviour and access to / engagement with healthcare services, which may contribute to differences in diagnosis and treatment. Interplay between these complex factors, alongside other attributes of host, virus and the environment, accounts for different outcomes of infection. However, gaps remain in our understanding of sexual dimorphism in HBV, and little effort has previously been made to harness this knowledge for translational gains. In this review, we assimilate human and animal data to consider the mechanism, outcomes and impact of sexual dimorphism, and consider how these insights can be used to inform advances in surveillance, treatment and prevention for HBV infection.
Keywords: hepatitis, HBV, sex, gender, oestrogen, testosterone, androgen, cancer, hepatocellular carcinoma, gender, stigma, epidemiology, outcome, treatment, dimorphism
1. Introduction
Chronic infection with hepatitis B virus (HBV) is estimated to affect 257 million people 1 and accounts for an increasing burden of the 1.34 million yearly deaths due to viral hepatitis 2, 3 . United Nations Sustainable Development Goals (SDGs) underpin ambitious targets for the elimination of hepatitis viruses, with the Global Health Sector Strategy setting out aims to reduce new infections by 90% and mortality by 65% by 2030 4 . Improving our understanding of the natural history of chronic HBV infection (CHB) is central to inform progress through enhanced evidence-based treatment and prevention of liver disease 5 .
Sex, defined as the biological characteristics that differ between males and females 6 , accounts for significant immunological differences leading to disparities in outcomes for a variety of infectious diseases 6, 7 , termed ‘sexual dimorphism’. The effect of host sex on outcomes of infection is complex and multifactorial, influenced by genetics, hormones, and environmental exposures 7 , with trade-offs between protective immune responses (leading to clearance or control of infection) and immunopathology (associated with increased severity and duration of disease). Gender, as a societal and behavioural construct, also plays a role through its influence on perceptions, behaviour, and access to healthcare.
Among chronic viral infections, human immunodeficiency virus (HIV) exemplifies this complex picture, whereby females typically have lower titres of HIV RNA than males 8 , with a 5-fold higher frequency of elite control 9 . However, females also show increased immunopathology, associated with an elevated risk of developing AIDS compared to males with the same HIV RNA levels 6 , and a greater susceptibility to infection, with both a biological and societal basis 10, 11 . UNAIDS data for 10–19 year olds in 2019 reported 33,000 adolescent girls becoming HIV infected compared to 4,200 boys 12 . Hepatitis C virus (HCV) has a higher prevalence in males, with females being more likely to clear the virus and also suffering fewer complications 13, 14 . Sexual dimorphism in HBV has been less rigorously studied, but was first described by Baruch Blumberg in 1972 15 , nine years after his discovery of ‘Australia antigen’ (now termed Hepatitis B surface antigen, HBsAg), underpinning subsequent consistent observations of an increased risk of chronic infection and its complications among males compared to females.
HBV is a partially double-stranded DNA virus which archives itself in the nucleus of hepatocytes as a covalently closed circular (ccc)-DNA ‘mini-chromosome’ accounting for persistent chronic infection, that can lead to inflammatory liver disease, fibrosis, cirrhosis and hepatocellular carcinoma (HCC) 16, 17 . Viral factors, the liver micro-environment, and host attributes all contribute to sexual dimorphism in CHB, as previously reviewed 13, 18, 19 . However, HBV infection has been relatively neglected by research, clinical care, public health interventions, and advocacy 20 , and females are specifically under-represented by translational research 21, 22 .
In this review, we discuss sexual dimorphism in CHB, considering the relevance of sex vs. gender, and the specific influence of menarche, pregnancy and menopause in females. We consider how an improved understanding of differential outcomes between males and females may (i) underpin new insights into the pathophysiology of liver disease, (ii) improve patient stratification for surveillance and treatment, (iii) inform new approaches to personalised therapy, and (iv) optimise public health measures and resource allocation. This would support advances towards elimination goals.
2. Epidemiology of HBV infection according to sex
Blumberg’s 1972 metanalysis of HBV prevalence included studies in 23 disparate populations, including cohorts with leprosy, trisomy 21, and renal dialysis patients. HBsAg carriage was more prevalent in males in 22/23 of the populations studied 15 . The male:female ratio ranged from 3.58 to 0.855 and, interestingly, was found to be greatest in the 0–19 age groups in all but one of the studies where age stratification was possible.
More recently, the male:female sex ratio in CHB has been reported as 1.2 in asymptomatic carriers, increasing to 6.3 in chronic liver disease and 9.8 in HCC 23 , but these estimates vary between settings. Selected studies reporting the prevalence of HBsAg in males and females are presented in Table 1, with odds ratio of infection as high as 1.9 in males (95% CI, 1.2–3.2) 24 .
Table 1. Exemplar studies reporting HBsAg prevalence in males and females.
| Study setting | Number
in study |
Seroprevalence data | Citation |
|---|---|---|---|
| Blood donors, Crete | 65,219 | HBsAg 0.41% in males and 0.28% in females; OR for
males 1.9 (95% CI, 1.2-3.2) |
Koulentaki, M. et al. 24 |
| Migrants and refugees, Southern Italy | 1,212 | HBsAg seroprevalence 9.6% overall. OR for males 1.8
(95% CI, 1.3-2.5) |
Coppola, N. et al. 27 |
| Adults age 35–44, general population,
Taiwan |
45,035 | Seroprevalence 17.8% in males vs. 13.2% in females
(p < 0.001); difference diminished in age >60. HBsAg male to female prevalence ratio 1.49 |
Tsay, P. K. et al. 28 |
| Meta-analysis of 27 studies, China | 5,422,405 | HBsAg prevalence of 5.8% in males (95% CI:5.53–
6.24%) and 5.05% in females (95% CI:4.56–5.88%) |
Wang, H. et al. 29 |
| Meta-analysis of 20 studies in diverse
groups, Pakistan |
81,755 | HBsAg prevalence for general population 2.71%
(95% CI 1.74 to 4.21). Three times more prevalent in males than females (OR not formally presented). |
Khan, N. U. et al. 30 |
| Comparison of pre-vaccine and post-vaccine
studies in Australian Aboriginal and Torres Strait Islander People from 36 studies |
501,622 * | Decrease in the pooled prevalence of HBsAg over
time among women (from 4.2% to 2.2%) and men (from 17.5% to 3.5%). No OR reported. |
Graham, S et al. 31 |
95% CI – 95% confidence interval. HBsAg – Hepatitis B virus surface antigen * Total number tested is not reported in this study, so denominator calculated from study meta-data, but we cannot exclude the possibility of some population groups being represented more than once.
None of the metanalyses we identified ( Table 1) could use data from all of the studies identified due to poor and inconsistent reporting of disaggregated sex data. They also suffered from substantial heterogeneity between studies and potential bias due to inclusion of specific high-risk populations. It is notable that the data included in Table 1 do not include any representation of African populations, as a result of the lack of robust metadata available from the African continent. For example, large scale HBV metanalyses of HBV prevalence from Ethiopia and Burkina Faso were unable to determine a sex ratio 25, 26 . This highlights a consistent difficulty that currently inhibits robust meta-analysis to more accurately delineate sex differences in HBV prevalence.
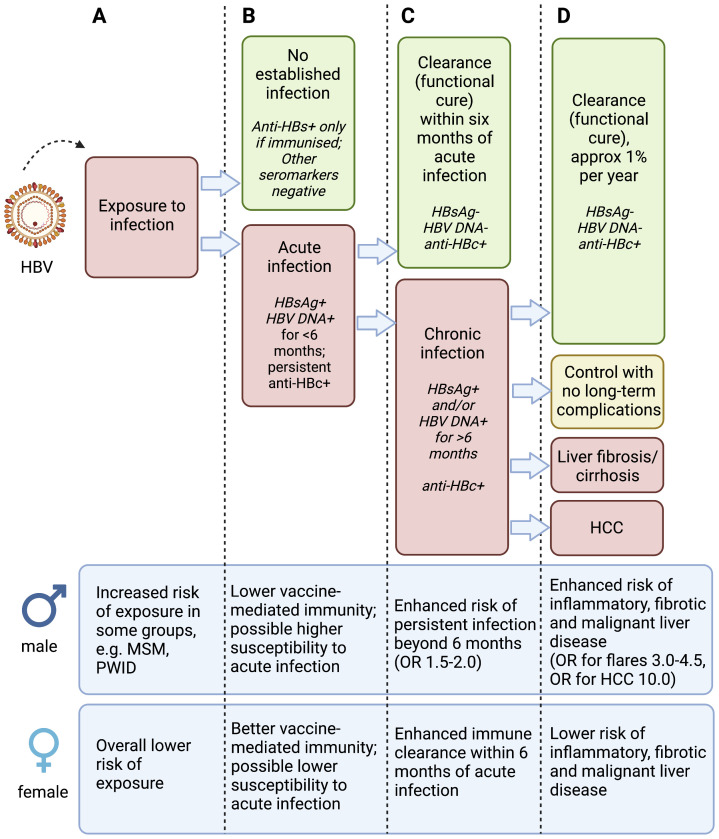
A key question to be addressed is whether CHB prevalence is lower in females due to less exposure events, lower susceptibility to acute infection, or enhanced clearance compared to males, or a combination of all of these ( Figure 1, columns A, B and C respectively). In part, this could be assessed by comparing the prevalence of anti-HBc antibodies (indicating exposure, with or without clearance) and HBsAg (indicating current infection) between males and females. In a cohort of 31,990 blood donors from Crete, HBsAg prevalence was 0.41% in males vs 0.28% in females (OR 1.98, 95% CI 1.2–3.2), and exposure was also higher in males than females, with anti-HBc prevalence of 9.16% vs 5.86% respectively 24 . A large-scale metanalysis in 2019 identified no significant difference in spontaneous HBsAg seroclearance between females and males 32 . However, there was substantial heterogeneity between studies and HBsAg clearance is an uncommon event 33 , so the study may have been underpowered to detect a sex influence.
Figure 1. Schematic to illustrate phases of hepatitis B virus (HBV) infection and relevant sex differences.
Infection is considered according to exposure to the virus (column A), acute infection (column B), chronic infection (column C) and liver disease (column D), with data for males and females presented at the bottom of each column. Anti-HBs – antibody to HBV surface antigen; HBsAg – Hepatitis B virus surface antigen; Anti-HBc – antibody to HBV core protein; HCC – hepatocellular carcinoma; MSM – men who have sex with men; PWID – people who inject drugs. OR (odds ratio) for males is presented based on females as the reference group. There is varied evidence for the specific observations presented in this figure, and the magnitude of increased risk in males varies between populations and settings. Figure created with BioRender.com, with licence to publish.
The epidemiological literature for HBV suffers from over-representation of specific populations that are subject to sex or gender bias. For example, certain defined populations, such as healthcare workers, pregnant women, waste handlers, men who have sex with men (MSM), people who inject drugs, and sex workers are often studied 34, 35 . The increased prevalence of HBsAg in males may reflect a complex combination of exposure risk, response to acute infection, and outcomes in chronic infection. More epidemiological data are needed, disaggregated by age, sex and disease characteristics, in order to determine local requirements for provision of public health and clinical services, and to build a clear picture of the global burden of disease.
3. Relationship between sex and morbidity and mortality in chronic HBV infection
3.1 Sexual dimorphism in liver disease
The liver is a highly sexually differentiated organ, with up to 70% of genes showing differences in expression between male and female mice 19, 36 . In humans, females are relatively protected from chronic liver fibrosis, regardless of aetiology, including lower rates of NAFLD/NASH 37 , a lower rate of cirrhosis and liver transplant 38 and a lower risk of hospitalisation and death from cirrhosis 39 compared to males. Pre-menopausal females with CHB are at a lower risk of chronic liver disease compared to males, as exemplified by a cohort of 672 patients in Switzerland, in which liver-related outcomes were significantly less common in females (OR 0.35, 95% CI 0.20–0.60) 40 . Similarly, in a Canadian study of nearly 6000 patients with HBV infection, male sex was an independent predictor of advanced liver fibrosis 41 . There is increasing recognition that diabetes and metabolic disease are associated with worse outcomes in HBV infection 42 , which may be linked to increased disease in males, as differences in fat biology and metabolism differ by sex 43 . Metabolic-associated fatty liver disease is also significantly more common in males, with a loss of protection in post-menopausal females 44 .
3.2 Hepatocellular carcinoma (HCC)
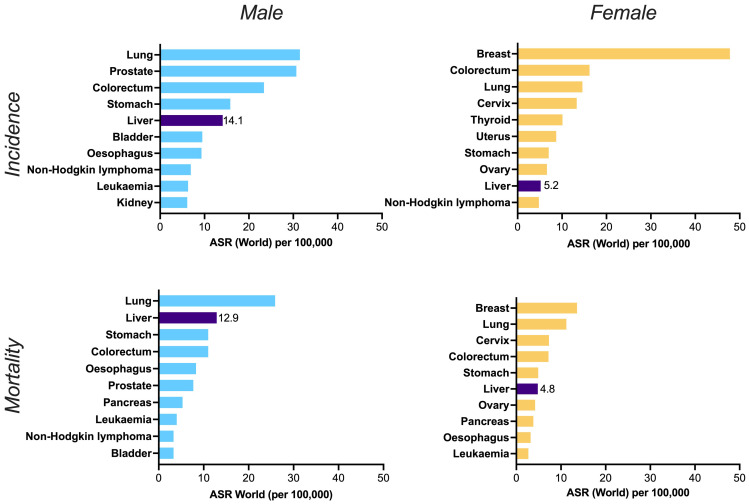
HBV is the single biggest aetiological agent of liver cancer worldwide, causing over half of all cases (point estimate 56%, 95% CI: 52-60), but with substantial regional variation, such that in parts of sub-Saharan Africa and Eastern Asia it is responsible for at least 2/3 of cases 45 , and far more in some settings. Overall, the incidence and mortality of liver cancer is higher in men than in women ( Figure 2). A Global Burden of Disease study estimates annual incidence of primary liver cancer at 591,000 in males vs. 264,000 in females (ratio 2.2); among these, HBV-related HCC was estimated to have caused 203,000 cases in men and 70,000 in women (ratio 2.9) 16 . In specific studies, male:female ratios for HCC are between 2:1 and 9:1. This wide variability is at least partly accounted for by the prevalence of CHB 46 , as in settings in which HBV accounts for a high prevalence of HCC, the male:female ratios are typically high 47 , for example in Senegal, where HBV accounted for almost 70% of HCC cases, the sex ratio was 6.6 48 , and in Vietnam, where >80% of HCC was HBV-associated, the ratio was 8.9:1 49 . HCC outcomes are worse when the aetiology is HBV 50 , and in males compared to females 51 .
Figure 2. Worldwide incidence (top panels) and mortality (bottom panels) of top ten cancers in males (left panels) and females (right panels).
ASR = age standardised rate. Liver cancer is shown in the dark purple bars, alongside the point estimate for ASR in each case, illustrating its place in the top five cancers for incidence and mortality in males, and the top ten for females. Data source: GLOBOCAN 2020 and the Global Cancer Observatory ( http://gco.iarc.fr). Primary liver cancer incidence among men and women is increasing sharply over time, in contrast to the declines for other cancers 53 and over half of this global burden is attributable to HBV infection 45 . Figure created in GraphPad Prism.
These observations in humans are corroborated by evidence from animal models that explore sex-related susceptibility to the development and progression of liver cancer, irrespective of aetiology. For example, following exposure to high dose carcinogens (such as diethylnitrosamine (DEN)), liver inflammation and proliferation are more pronounced in male mice 52 , and males exhibit a higher rate of tumorigenesis 54 , with cancers that progress more rapidly.
3.3 Flares of liver inflammation and acute-on-chronic liver failure
Flares of liver inflammation in HBV infection can be a consequence of the immune response targeting infected hepatocytes (leading to the favourable outcome of HBsAg clearance), or can represent organ damage (with adverse outcomes including liver failure, long-term risk of fibrosis/cirrhosis, and death) 55 . Spontaneous flares of liver inflammation have been reported more commonly in males. For example, in a study of more than 1500 ethnically diverse adults followed over five years by the Hepatitis B Research Network in the US and Canada, flares were significantly commoner in males (OR 3.02; 95% CI: 1.59-5.74) 56 . Smaller CHB cohorts corroborate this finding, with more flares in males in a study of 217 asymptomatic HBeAg-negative patients (OR 4.5; 95% CI: 1.5-20.8) 57 and in 386 patients followed up in China to identify exacerbations 58 . In patients undergoing HBeAg seroconversion, male sex was again a significant risk factor for ALT flares 59 , and likewise in a study exploring flares following treatment withdrawal 60 . A prediction model for mortality in patients with acute on chronic liver failure in the setting of HBV infection includes male sex as a predisposing risk factor, with a view to informing improvements in stratified care 61 .
Based on existing data, it is not possible to determine whether hepatic flares in males are linked to immune control or to progressive organ damage, although given other evidence of enhanced pathology in males it is natural to hypothesise the latter. More data are needed to explore these observations, as liver flares are likely to be under-reported, and are clearly linked to other host and viral factors (HBV treatment, immunosuppression, HBV genotype, age, viral load (VL)) making it complex to disaggregate the overall influence of sex 55, 56 .
4. Mechanistic influence of sex in liver disease associated with HBV infection
4.1 Immunological differences between males and females causing differential outcomes of infection
Females typically have higher magnitude innate, humoral and cytotoxic responses in response to infection, broadly reflecting an immunostimulatory effect of oestrogen 6, 7 , in contrast to the suppressive effect of androgens in males. Across a range of infectious diseases, this potentially explains the increased severity and/or duration of illness in males compared to females 62, 63 , and enhanced vaccine responses in females (discussed in further detail in section 4.2 below). Sex dimorphism may be associated with differential expression of sex hormone receptors by immune cells (lymphocytes, monocytes and dendritic cells) and by hepatocytes. Chemokine and cytokine profiles also differ 64– 66 , with androgens more typically associated with anti-inflammatory cytokines. Sex-specific differences in IL-6 levels have been associated with HCC, with elevated levels in males leading to activation of AR, while oestrogen inhibits IL-6 production in females 67, 68 .
Toll-like receptor 7 (TLR-7) is encoded on the X chromosome, one copy of which is inactivated in females to render TLR-7 expression equivalent to that in males. TLR-7 stimulation activates plasmacytoid dendritic cells, stimulating production of type I interferons and thus promoting T and B cell responses 69 , explaining why TLR-7 expression has been negatively correlated with HBV DNA VL 70 . Escape of the second copy of TLR7 from inactivation can increase TLR-7 expression in females 6, 71 . Furthermore, a SNP in the TLR-7 gene (rs179008) in has been associated with protection from chronic infection in females (but not in males) 72 . Epigenetic modifications, and female X-chromosome mosaicism, may further contribute to this immunological advantage in females.
4.2 Sexual dimorphism in HBV vaccine responses
Males and females respond differently to HBV vaccination, with females mounting higher antibody titres than males 73, 74 , and men more likely to be vaccine ‘non-responders’ (particularly in older age groups). For example, an Italian study reports that girls vaccinated after the age of 1 year mount a 1.2-fold higher median antibody titre than boys 73 . This is in line with trends reported for other childhood vaccines, with females producing increased antibody titres and longer term durability of response 74 . These effects may be due to the enhanced TLR-7 response in females, including a more inflammatory response to vaccine adjuvants. HBV vaccination in mice also demonstrates a higher rate of seroconversion in females, higher titres of anti-HBs antibody, higher magnitude T cell responses, and superior immunological memory 75 . It is uncertain to what extent these differences are significant to the role of the HBV vaccine in supporting elimination efforts, but they illustrate fundamental differences in the quality and quantity of the immune response, and may account for some degree of enhanced male susceptibility, although this is difficult to quantify. Despite the sex differences in vaccine response, vaccination may blunt the overall difference between males and females. Thus, newer studies of younger subjects, in which the prevalence of vaccine-mediated protection is high, may be less powered to detect sex differences compared to pre-vaccine cohorts.
4.3 Interaction between sex hormones and the HBV replication cycle
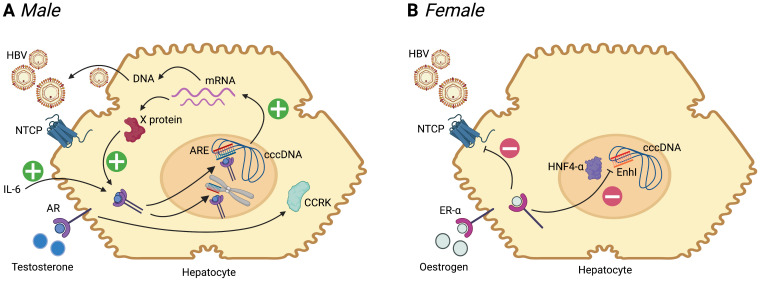
HBV has a complex replication cycle, reviewed elsewhere 76, 77 . The virus has numerous interactions with host cell proteins, and by integration into the host genome can influence fundamental components of the cell cycle. The viral genome also has a direct influence through sex hormone response elements (previously reviewed 18 , and summarised in Figure 3). A pathway to oncogenesis in males occurs through involvement of the androgen receptor (AR) signalling pathway, which is common to cancer evolution caused by diverse viruses including HBV, EBV, HTLV-1, HHV8 and HPV 78 , and is also associated with the evolution of prostate cancer 79 . In HBV infection, AR stimulation is associated with increased expression of all four HBV mRNAs, via two androgen response elements (ARE) in the enhancer 1 (EnhI) region of the HBV genome 80 , leading to an upregulation in production of HBV proteins and DNA. In a mouse liver cell line, HB X protein increases AR activity in a dose-dependent manner 81, 82 . Thus, a positive feedback loop operates between androgen exposure and X protein, which may explain the higher VL in males 18, 83 ( Figure 3). In human CHB infection, higher serum testosterone levels have been correlated with a higher incidence of HCC 84 , consistent with the male/female ratio in HCC. In mouse models, interleukin-6 (IL-6) is elevated in male HCC, leading to activation of signal transduction and activator of transcription 3 (STAT3), which upregulates AR 85 , and IL-6 has been suggested as a biomarker associated with worse outcomes in human HCC 86 .
Figure 3. Summary of the influence of sex hormones on the HBV replication cycle in males and females.
Following entry to the hepatocyte, mediated primarily by interaction with the sodium taurocholate co-transporter polypeptide (NTCP), HBV is trafficked to the nucleus. Host machinery archives the viral genome in the form of a stable covalently closed circular (ccc)-DNA molecule, which is the template for generation of mRNA species. mRNA is translated into new viral proteins (X, surface, capsid and polymerase), and reverse transcribed to DNA. ( A) In males, binding of testosterone to the androgen receptor (AR, a member of the steroid hormone nuclear receptor family) leads to dimerization of the receptor, which then binds to androgen response elements (ARE) in the HBV genome (both in cccDNA and integrated into host DNA). This promotes generation of mRNA species, leading to increased protein production, and pgRNA (pre-genomic RNA) which undergoes reverse transcription to DNA to generate new relaxed-circular DNA genomes. Increase in X protein feeds back on the androgen-AR complex in a positive feedback loop. Androgen-ARE complexes also stimulate cell cycle-related kinases (CCRK) stimulating proliferation. IL-6 is elevated in HCC and leads to activation of AR 18 . ( B) In females, there is evidence that binding of oestrogen to oestrogen receptor alpha (ER-α) leads to down-regulation of the NTCP cell surface protein 19 . Furthermore ER-α inhibits the binding of hepatocyte nuclear factor 4-alpha (HNF4-α) to the Enhancer I (EnhI) region of the HBV genome, reducing mRNA production 87 . In both panels, plus signs indicate stimulation/positive feedback, negative signs indicate blockade. For further details, see reviews 13, 18, 19, 79 . Figure created with BioRender.com, with licence to publish.
In contrast to androgens, oestrogens may actively suppress HBV replication. Activation of the oestrogen receptor alpha (ER-α) can suppress HBV mRNA transcription by reducing expression of the transcription factor hepatocyte nuclear factor 4-alpha (HNF4-α). In a transgenic mouse model, HNF4-α increases HBV transcription by binding the enhancer I (EnhI) region of the HBV genome 87 . Deletion of ER-α in female mice removes the protective influence of oestrogen against hepatocarcinogenesis; relative to wild-type these knock-out animals had a nine-fold increased risk of tumorigenesis on exposure to carcinogens 88 . In cell culture experiments, estradiol reduces expression of the hepatocyte surface protein NTCP (sodium taurocholate co-transporting polypeptide), the main entry receptor for HBV 89, 90 , which is another possible mechanism for protection in females 19 . Whether this mechanism is important in humans remains to be determined, but certain ER-α polymorphisms have also been shown to increase the risk of acute liver failure in patients with HBV 91 .
A longitudinal study of 4155 HBsAg positive individuals aged 30–64 years in the REVEAL-HBV study reported significantly higher HBV DNA VL in males (p<0.001); higher viraemia was associated with a greater risk of both HCC and cirrhosis 83, 92 . In mouse studies, males have higher HBV VL and HBsAg levels 93, 94 compared to females 80, 95– 97 . These effects are more pronounced after puberty, with VL lowered by orchidectomy in male animals and restored by androgen supplementation 80, 93 . Conversely, VL is increased by oophorectomy in females 87 . Both VL and HBsAg are sensitive to androgen receptor mutations, confirming the influence of this pathway in mediating the phenotype 98 .
5. Sex to inform HBV interventions
5.1 Sex to inform stratification for anti-viral therapy
Traditionally, HBV infection has been classified based on serology, VL, and markers of liver damage (such as liver enzyme levels, imaging scores and/or liver biopsy) 99 . Guidelines for nucleoside/nucleotide analogue (NA) therapy, based on these biomarkers together with age and sex, typically consider ~1 in 4 individuals with CHB to be treatment-eligible, although this varies between populations 100 . However, there is increasing recognition that current classification systems over-simplify liver disease by applying a traditional paradigm of linear progression, and that untreated individuals have a risk of progressive liver disease and HCC, and may also be at risk of transmitting infection 100– 103 .
Female sex has been associated with a better response to PEG-IFN-alpha 104 , 105, although this effect is not consistent across all studies 106 . Likewise, a female advantage is observed for nucleos(t)ide analogue treatment: in a study of >2000 HBV patients starting treatment with entecavir, earlier virologic response was observed in treatment-naïve females compared to males 107 . In HIV/HBV coinfected patients starting antiviral therapy, females have a higher rate of functional cure within two years (OR for HBsAg clearance in males 0.54 compared to females as reference group) 108 and male sex is reported in association with failure to suppress HBV viraemia 109 .
An increased understanding of the role of sex as a risk factor for disease could allow refinement of treatment algorithms, for example lowering the threshold for treatment of males and post-menopausal females, such that more individuals are treatment eligible. Treatment with NA agents such as tenofovir (typically prescribed as tenofovir disoproxil fumarate, TDF) is cheap, safe and effective in suppressing viraemia. Expanding CHB treatment is a key intervention to improve progress towards elimination goals 76, 110 by offering therapy to those at greatest risk of long-term disease 111 as well as switching off transmission. Enhanced approaches to risk stratification will also benefit from an improved understanding of the interactions between sex and other attributes of the host (e.g. age, co-morbidity, gravidity, ethnicity) and virus (VL, genotype), which are yet to be fully resolved.
Sex and gender are likely to be significant factors in receipt of treatment. Universal health coverage (UHC) is included in SDG targets, setting a mandate for access to healthcare irrespective of demographic factors including sex, age, and ability to pay. For CHB, sex disaggregated data are currently insufficient to estimate the proportion of the untreated CHB population who are male vs. female 112 . However, treatment coverage may be influenced by biology and natural history of infection, in which higher VL and more advanced disease in males make them more likely to meet eligibility criteria than age-matched females.
Access to screening and engagement with care are also dependent on many other societal and infrastructure considerations, including education, beliefs and behaviour, and the structure of health-care services (discussed further in section 6). This topic has been explored for HIV, in which a higher proportion of women access treatment, have better immunological responses to treatment, and are retained in long-term follow-up 113, 114 , but no such data are available for HBV infection.
5.2 Sex to inform HCC risk assessment and therapy
Risk scores have been proposed to predict HCC risk through measurement of non-invasive parameters such as VL, platelet count, and liver enzyme levels 115 , with risks of HCC increasing with VL >2000 IU/ml 116 . A recent systematic review and metanalysis evaluated the performance of these scores 117 . 12 of the 14 scores used sex as part of the algorithm, but the use of sex as a simple categorical variable may be over simplistic. For example, the REACH-B score assigns a value of 2 to males and 0 to females 118 , without considering enhanced risks in older women. It should also be noted that there was a male predominance in the validation cohorts which may affect the assessment of relative risk between the sexes.
As evidence emerges for the role of sexual dimorphism in metabolic and malignant disease, this may shed light on new therapeutic approaches using hormonal therapy or blockade 119, 120 . Androgen receptor (AR) blockade has thus far not shown therapeutic benefits for HCC 79, 121, 122 , but as we improve understanding of the biological pathways involved in driving cancer, this strategy warrants further exploration.
5.3 Interplay between age and sex
Alongside sex, another factor of considerable importance is age (first noted by Blumberg in his 1972 study, in which sexual dimorphism was most striking in the youngest groups 15 ). Given the strong observed effect of sex, it is to be expected that the natural history of CHB may change throughout life due to changes in the levels of sex steroids. For females, menarche, pregnancy, and menopause are therefore of particular relevance.
Earlier menarche has been correlated with earlier HBeAg seroconversion and a faster rate of HBsAg titre decline 123 . In contrast, early menarche has elsewhere been shown to correlate with an increased risk of HCC in HBsAg carriers 124 . A protective effect of early puberty has also been described in a small cohort of males 125 , perhaps counter-intuitively given that increased testosterone is otherwise identified as a risk factor. This reflects puberty as a period of complex immunological change, and highlights the need for further study. Postmenopausal status has been shown to reduce, or even remove, the protective effect of female sex. A multicentre cross-sectional study in China, involving 17,408 patients with CHB, found that the prevalence of cirrhosis increased at a faster rate after the age of 50 in females than in males 126 , and likewise sex differences diminished among older adults in a large Taiwanese cohort 28 . The risk of liver fibrosis in CHB has similarly been reported as comparable between post-menopausal females and age-matched males 127 . The loss of protection associated with menopause can be mitigated by hormone replacement therapy (HRT), which has a protective effect proportional to the duration of treatment 124 . Similar observations have been made in other chronic infections: in HCV infection, the risk of liver disease is lower in pre-menopausal females and accelerates to match that of males when the protective effect of oestrogen exposure is lost 128 ; in HIV the TLR-7 response and IFN production in women are dampened after the menopause, and restored by oestrogen replacement (reviewed in 129).
Together these data confirm that oestrogen has a protective and dose-dependent effect on the course and characteristics of CHB, which may vary depending on the life history of the individual female. The nuance of hormonal influence in the natural history of CHB in females requires more explicit study, but this is an area in which advanced understanding could impact on simple interventions such as enhanced surveillance, and antiviral and/or HRT prescription for post-menopausal women.
5.4 Maternal and child health
During pregnancy, there is a general tolerization of the immune system, with alterations in the Th1/Th2 ratio (with downregulation of Th1 immunity to avoid rejection of the foetus), such that Th1 cytokines (IFN-gamma and IL-2) are reduced, and there is a relative increase in Th2 cytokines (such as IL-4). These changes may have an impact on HBV infection in the mother 130, 131 : during pregnancy, both increases and decreases in HBV DNA VL have been reported compared to non-pregnant women (reviewed in 132), while ALT flares are well recognised both during pregnancy and post-partum. Post-partum flares are mediated potentially by changes in the immune response (to re-set the non-pregnant Th1/Th2 balance), but can also be related to withdrawal of short-term antiviral therapy instituted to prevent vertical transmission 133– 135 . In the majority of cases, flares are classified as mild (e.g. ALT up to 5 times upper limit of normal, ULN) or moderate (ALT up to 10 times ULN), with no clear detriment to maternal and foetal outcomes 136, 137 , and indeed potentially associated with clearance of HBsAg. Rarely, severe flares (ALT >10 times ULN) arise, which can be associated with fulminant hepatitis 138 . Altered cytokine production and hepatic flares during pregnancy may stimulate clearance of HBeAg, and ultimately also loss of HBsAg (functional cure), although these events are uncommon 131 . In a Turkish cohort, multigravid females had a higher seroprevalence of HBsAg than primigravidae 139 , although this is difficult to interpret, as it may represent differences in exposure rates rather than a biological difference mediated by pregnancy. The risk of HCC in HBsAg positive women has also been inversely correlated with the number of pregnancies and the age of menopause 140 .
HBV infections in children typically occur either at birth (vertical transmission), or in the first few years of life through horizontal transmission from close household contacts 141 . The sex of the child may alter susceptibility, as evidenced by work in the HIV field, in which female fetuses have a 1.5-2-fold increased susceptibility to in utero infection compared to males 10 ; although there are no such data for HBV, this finding is a biological precedent for the impact of sexual dimorphism from the earliest days of life.
HBV vaccination of infants at birth is a simple and effective method of reducing early life infections, which account for most of the long-term burden of CHB. However, the effectiveness of birth vaccination is reduced when mothers have high HBV VL that is not treated during pregnancy 111 . Thus, although females typically have lower VL and a lower risk of long-term liver disease than males, provision of clinical care and interventions for women of child-bearing age and their infants (screening, monitoring, anti-viral therapy, and infant vaccination) are crucial public health interventions warranting sustained investment. The greater risk of persistent viral replication, cirrhosis, and HCC in individuals who have been vertically infected underlines the urgent need for diagnosis and intervention to prevent mother-to-child transmission and other early-life acquisition events 142 . Improved epidemiological data regarding HBV infections occurring in utero, in infancy and during childhood, stratified according to maternal characteristics and the sex of the offspring, could provide important insights into the biology of HBV transmission.
Route of transmission, HBV genotype, host genetics, and environmental factors may all interact with hormonal factors in mediating outcomes. For example, higher rates of vertical transmission in some settings (influenced by genotype) have been suggested to bring the male:female ratio closer to 1:1 in CHB 28 . Regional studies are particularly needed to provide an evidence base for high endemicity populations in the African and Asian subcontinents.
6. The role of gender
The construct of gender - defined as ‘characteristics of women and men that are largely socially created’ 143 - is important, accounting for social and behavioural factors. However, the term gender has often been used interchangeably with sex, and their influence can be difficult to disaggregate. The need to improving reporting of data representing both sex and gender is underpinned by Sex and Gender Equity in Research (‘SAGER’) guidelines, first published in 2016, to promote improved study design, analysis, and interpretation of clinical data 144 . However, progress in this domain has been slow, with little improvement in reporting sex-disaggregated data among organisations reviewed between 2018 and 2021 145 .
Gender roles may influence care-seeking behaviour, particularly through services for women, who typically have more points of contact with health services as a result of child-bearing and childcare, although traditional responsibilities are increasingly changing. Access to healthcare, education and support networks frequently vary by gender, from early in childhood and throughout life. Women are at increased risk of BBV transmission where their access to education is limited, and in the absence of reliable access to sexual and reproductive health services 146 . Access gaps may also be particularly pertinent for adolescent boys and young adult men; based on the HIV literature, these groups are the least likely to seek out or engage with diagnostic and treatment services 147, 148 . For HBV, the long-term risk of complications is highest in these young men due to the combination of pathophysiology of infection in males, combined with the difficulty of providing consistent, accessible and acceptable healthcare. Men are also over-represented among people who inject drugs and the prison population, among whom risks of BBV exposure are higher 149 .
HBV is endemic in some communities of MSM and transgender women (e.g. in Papua New Guinea 150 and Nigeria 151 ), and providing reliable healthcare for these groups is challenging. Access to care for MSM, bisexual and transgender communities may be further complicated in countries where legislation prohibits same-sex relationships. A study in Spain to determine the incidence of acute HBV infection in people living with HIV reported that this was higher in males, particularly in MSM 151 , and HBV infection has also been associated with risky sexual practices and transactional sex 149, 151, 152 . High rates of drug and alcohol misuse increase the risk of exposure to infection with BBV, and accelerate liver disease in those with HBV infection, create barriers to interaction with healthcare services, and are associated with vaccine hesitancy in some populations 153 . Stigma is a barrier to diagnosis and intervention for HBV 154 , with discrimination potentially further enhanced by gender and sexuality. Extreme marginalisation in some of the groups at high risk of HBV infection is a significant barrier to education, diagnosis, treatment and prevention.
7. Conclusion
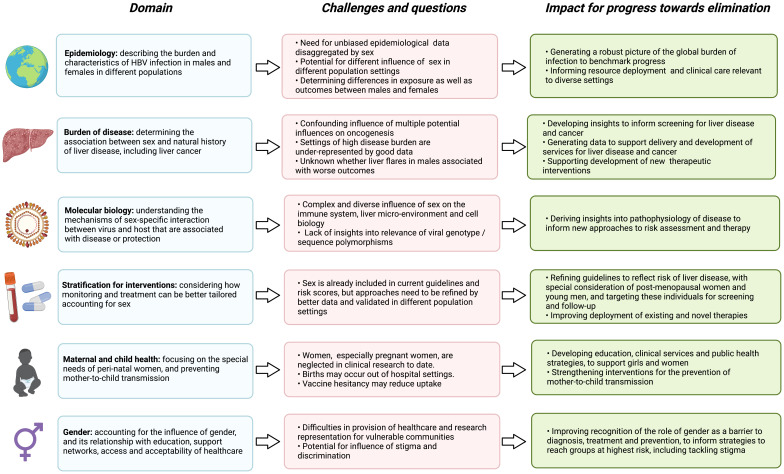
Sex and gender have a fundamental impact on the course and outcomes of HBV infection ( Table 2), and developing insights in this domain has the potential to influence intervention ( Figure 4). The magnitude and nature of the sex effect vary between populations and with age, but - nearly forty years since the phenomenon of sexual dimorphism was first reported - have yet to be robustly quantified on a multiregional scale. Some regions and populations are yet to be represented in epidemiological data; at present there are insufficient data to permit meta-analysis representing the WHO African region. Improving the reporting of sex and gender data is imperative for HBV, as deepening an understanding of the biology and pathophysiology can inform new interventions, while stratification for treatment or surveillance by sex has the potential to improve outcomes for individuals, with associated population benefits in areas of high endemicity.
Figure 4. Summary of the interplay between sex and the outcomes of HBV infection, highlighting gaps and challenges in current understanding, and the impact for advances towards international elimination goals.
Figure created with BioRender.com, with licence to publish.
Table 2. Summary of the influence of sex on HBV infection.
| Attribute of HBV infection | Impact of sex dimorphism |
|---|---|
| Risk of exposure to infection | • Higher in males than females |
| Risk of development of chronic infection | • Higher in males than females |
|
Risk of development of inflammatory/
fibrotic liver disease |
• Higher in males than females
• Female risk may increase post-menopause |
| Development and outcomes of HCC | • Higher incidence of HCC in males
• Worse outcomes on treatment and shorter life expectancy in males |
| Likelihood of receiving treatment | • Higher proportion of males meet treatment eligibility criteria, based on higher VL
and advanced disease • Behaviour and beliefs may prevent males from care seeking (also see gender, below) |
| Representation by existing datasets | • Likely to be better for males than for females, although certain female groups are
better represented (e.g. pregnancy) |
| Access to clinical care | • May be more barriers for younger men
• Women may be able to access screening and interventions through perinatal services • Special focus is required for post-menopausal women • Males are over-represented in certain risk groups for whom there are barriers in access to care (PWID, prison population) |
| Interaction with gender | • Access to diagnosis and care is influenced by gender-specific education, behaviour,
beliefs, role-models and tailoring of health services. • May be particular barriers to care for MSM and trans-gender people • Risk factors overlap with alcohol excess, substance abuse, incarceration, transactional sex |
HCC – hepatocellular carcinoma, MSM – men who have sex with men, PWID – people who inject drugs, VL – viral load
Females with CHB currently suffer from a lack of specific research, that potentially disadvantages HBV elimination efforts as a whole. While women are at lower risk of chronic infection and liver disease, a focus on women’s health is nevertheless a fundamental aspiration for global health interventions for HBV, through reducing mother-to-child transmission, reducing the sex bias in data sets, and focusing attention on post-menopausal women who may be at increased risk of liver disease than younger females.
Enhancing an understanding of the mechanisms through which sex hormones mediate their influence can inform a better understanding of the pathophysiology of liver disease, with potentially important bearing on the use of existing interventions as well as informing the development of new therapies for HBV and the associated complication of HCC. Future research must focus on characterising the influence, impact and mechanisms of sexual dimorphism in HBV.
Data availability
No underlying data are associated with this article.
Acknowledgement
We would like to thank Dr Anna McNaughton for preliminary discussions that informed the basis of this paper.
Funding Statement
This work was supported by Wellcome [110110 to PM] and [104748 to PG], and the National Institutes of Health [RO1-AI133673 to PG]. This work was supported by the Francis Crick Institute which receives its core funding from Cancer Research UK (CC2223), the UK Medical Research Council (CC2223), and Wellcome (CC2223). This research was funded in whole, or in part, by Wellcome [CC2223]. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 3; peer review: 2 approved]
References
- 1. World Health Organization Factsheets: Hepatitis B. (Accessed: 1st June 2021). Reference Source [Google Scholar]
- 2. Global hepatitis report, 2017. (Accessed: 17th June 2021). Reference Source [Google Scholar]
- 3. Stanaway JD, Flaxman AD, Naghavi M, et al. : The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081–1088. 10.1016/S0140-6736(16)30579-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Global Health Sector Strategy on Viral Hepatitis, 2016-2021. 2016; (Accessed: 24th June 2021). Reference Source [Google Scholar]
- 5. Ward JW, Wiktor SZ, Cooke GS: Launch of the Coalition for Global Hepatitis Elimination: a recommendation of the Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2020;5(1):8–10. 10.1016/S2468-1253(19)30319-X [DOI] [PubMed] [Google Scholar]
- 6. vom Steeg LG, Klein SL: SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog. 2016;12(12):e1005374. 10.1371/journal.ppat.1005374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klein SL, Flanagan KL: Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 8. Vieira VA, Zuidewind P, Muenchhoff M, et al. : Strong sex bias in elite control of paediatric HIV infection. AIDS. 2019;33(1):67–75. 10.1097/QAD.0000000000002043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang OO, Cumberland WG, Escobar R, et al. : Demographics and natural history of HIV-1-infected spontaneous controllers of viremia. AIDS. 2017;31(8):1091–1098. 10.1097/QAD.0000000000001443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adland E, Millar J, Bengu N, et al. : Sex-specific innate immune selection of HIV-1 in utero is associated with increased female susceptibility to infection. Nat Commun. 2020;11(1):1767. 10.1038/s41467-020-15632-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Oliveira T, Kharsany ABM, Gräf T, et al. : Transmission networks and risk of HIV infection in KwaZulu-Natal, South Africa: a community-wide phylogenetic study. Lancet HIV. 2017;4(1):e41–e50. 10.1016/S2352-3018(16)30186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Unaids: UNAIDS data.2019. Reference Source [Google Scholar]
- 13. Ruggieri A, Gagliardi MC, Anticoli S: Sex-dependent outcome of hepatitis B and C Viruses infections: Synergy of sex hormones and immune responses? Front Immunol. 2018;9:1–7. 10.3389/fimmu.2018.02302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grattagliano I, Rossi A, Marconi E, et al. : Determinants of HCV-related complications in Italian primary care patients. Liver Int. 2021;41(12):2857–2865. 10.1111/liv.15017 [DOI] [PubMed] [Google Scholar]
- 15. Blumberg BS, Sutnick AI, London WT, et al. : Sex Distribution of Australia Antigen. Arch Intern Med. 1972;130(2):227–231. 10.1001/archinte.1972.03650020057011 [DOI] [PubMed] [Google Scholar]
- 16. Global Burden of Disease Liver Cancer Collaboration, . Akinyemiju T, Abera S, et al. : The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3(12):1683–1691. 10.1001/jamaoncol.2017.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bertuccio P, Turati F, Carioli G, et al. : Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67(2):302–309. 10.1016/j.jhep.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 18. Wang SH, Chen PJ, Yeh SH: Gender disparity in chronic hepatitis B: Mechanisms of sex hormones. J Gastroenterol Hepatol. 2015;30(8):1237–1245. 10.1111/jgh.12934 [DOI] [PubMed] [Google Scholar]
- 19. Buettner N, Thimme R: Sexual dimorphism in hepatitis B and C and hepatocellular carcinoma. Semin Immunopathol. 2019;41(2):203–211. 10.1007/s00281-018-0727-4 [DOI] [PubMed] [Google Scholar]
- 20. O’Hara GA, McNaughton AL, Maponga T, et al. : Hepatitis B virus infection as a neglected tropical disease. PLoS Negl Trop Dis. 2017;11(10):e0005842. 10.1371/journal.pntd.0005842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feldman S, Ammar W, Lo K, et al. : Quantifying Sex Bias in Clinical Studies at Scale With Automated Data Extraction. JAMA Netw Open. 2019;2(7):e196700. 10.1001/jamanetworkopen.2019.6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazure CM, Jones DP: Twenty years and still counting: including women as participants and studying sex and gender in biomedical research. BMC Womens Health. 2015;15:94. 10.1186/s12905-015-0251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chu CM, Liaw YF, Sheen IS, et al. : Sex Difference in Chronic Hepatitis B Virus Infection: An Appraisal Based on the Status of Hepatitis B e Antigen and Antibody. Hepatology. 1983;3(6):947–950. 10.1002/hep.1840030611 [DOI] [PubMed] [Google Scholar]
- 24. Koulentaki M, Spanoudakis S, Kantidaki E, et al. : Prevalence of hepatitis B and C markers in volunteer blood donors in Crete. A 5-year study. J Viral Hepat. 1999;6(3):243–248. 10.1046/j.1365-2893.1999.00155.x [DOI] [PubMed] [Google Scholar]
- 25. Yazie TD, Tebeje MG: An updated systematic review and meta-analysis of the prevalence of hepatitis B virus in Ethiopia. BMC Infect Dis. 2019;19(1):917. 10.1186/s12879-019-4486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lingani M, Akita T, Ouoba S, et al. : High prevalence of hepatitis B infections in Burkina Faso (1996-2017): A systematic review with meta-analysis of epidemiological studies. BMC Public Health. 2018;18(1):551. 10.1186/s12889-018-5432-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coppola N, Alessio L, Gualdieri L, et al. : Hepatitis B virus infection in undocumented immigrants and refugees in Southern Italy: Demographic, virological, and clinical features. Infect Dis Poverty. 2017;6(1):33. 10.1186/s40249-016-0228-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsay PK, Tai DI, Chen YM, et al. : Impact of gender, viral transmission and aging in the prevalence of hepatitis b surface antigen. Chang Gung Med J. 2009;32(2):155–164. [PubMed] [Google Scholar]
- 29. Wang H, Men P, Xiao Y, et al. : Hepatitis B infection in the general population of China: A systematic review and meta-analysis. BMC Infectious Diseases. 2019;19(1):811. 10.1186/s12879-019-4428-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khan NU, Zalan A, Petruzziello A, et al. : Determining the Actual Prevalence of Hepatitis B in Khyber Pakhtunkhwa-Pakistan: A Meta-Analysis. Open Virol J. 2018;12:33–41. 10.2174/1874357901812010033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Graham S, MacLachlan JH, Gunaratnam P, et al. : Chronic hepatitis B prevalence in Australian Aboriginal and Torres Strait Islander people before and after implementing a universal vaccination program: A systematic review and meta-analysis. Sex Health. 2019;16(3):201–211. 10.1071/SH18150 [DOI] [PubMed] [Google Scholar]
- 32. Yeo YH, Ho HJ, Yang HI, et al. : Factors Associated With Rates of HBsAg Seroclearance in Adults With Chronic HBV Infection: A Systematic Review and Meta-analysis. Gastroenterology. 2019;156(3):635–646.e9. 10.1053/j.gastro.2018.10.027 [DOI] [PubMed] [Google Scholar]
- 33. Downs LO, Smith DA, Lumley SF, et al. : Electronic Health Informatics Data To Describe Clearance Dynamics of Hepatitis B Surface Antigen (HBsAg) and e Antigen (HBeAg) in Chronic Hepatitis B Virus Infection. mBio. 2019;10(3):e00699–19. 10.1128/mBio.00699-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Almasi-Hashiani A, Ayubi E, Mansori K, et al. : Prevalence of hepatitis B virus infection among Iranian high risk groups: A systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2018;11(2):91–100. [PMC free article] [PubMed] [Google Scholar]
- 35. Eltom K, Albeely A, El Hussein ARM, et al. : Occult hepatitis B virus infection in Sudan: A systematic review and meta-analysis. JGH Open. 2020;4(5):800–807. 10.1002/jgh3.12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang X, Schadt EE, Wang S, et al. : Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16(8):995–1004. 10.1101/gr.5217506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burra P, Bizzaro D, Gonta A, et al. : Clinical impact of sexual dimorphism in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Liver Int. 2021;41(8):1713–1733. 10.1111/liv.14943 [DOI] [PubMed] [Google Scholar]
- 38. Hagström H, Lindfors A, Holmer M, et al. : Etiologies and outcomes of cirrhosis in a large contemporary cohort. Scand J Gastroenterol. 2021;56(6):727–732. 10.1080/00365521.2021.1912167 [DOI] [PubMed] [Google Scholar]
- 39. Rubin JB, Sundaram V, Lai JC: Gender Differences among Patients Hospitalized with Cirrhosis in the United States. J Clin Gastroenterol. 2020;54(1):83–89. 10.1097/MCG.0000000000001192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vieira Barbosa J, Sahli R, Aubert V, et al. : Demographics and outcomes of hepatitis B and D: A 10-year retrospective analysis in a Swiss tertiary referral center. PLoS One. 2021;16(4):e0250347. 10.1371/journal.pone.0250347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cooper C, Driedger M, Wong D, et al. : Distinct Hepatitis B and HIV co-infected populations in Canada. J Viral Hepat. 2021;28(3):517–527. 10.1111/jvh.13453 [DOI] [PubMed] [Google Scholar]
- 42. Campbell C, Wang T, McNaughton AL, et al. : Risk factors for the development of hepatocellular carcinoma (HCC) in chronic hepatitis B virus (HBV) infection: a systematic review and meta-analysis. J Viral Hepat. 2021;28(3):493–507. 10.1111/jvh.13452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pan R, Chen Y: Fat biology and metabolic balance: On the significance of sex. Mol Cell Endocrinol. 2021;533:111336. 10.1016/j.mce.2021.111336 [DOI] [PubMed] [Google Scholar]
- 44. Chen YL, Li H, Li S, et al. : Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: a cross-sectional comparative study. BMC Gastroenterol. 2021;21(1):212. 10.1186/s12876-021-01782-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maucort-Boulch D, de Martel C, Franceschi S, et al. : Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142(12):2471–2477. 10.1002/ijc.31280 [DOI] [PubMed] [Google Scholar]
- 46. Ruggieri A, Barbati C, Malorni W: Cellular and molecular mechanisms involved in hepatocellular carcinoma gender disparity. Int J Cancer. 2010;127(3):499–504. 10.1002/ijc.25298 [DOI] [PubMed] [Google Scholar]
- 47. Fung J, Cheung KS, Wong DK, et al. : Long-term outcomes and predictive scores for hepatocellular carcinoma and hepatitis B surface antigen seroclearance after hepatitis B e-antigen seroclearance. Hepatology. 2018;68(2):462–472. 10.1002/hep.29874 [DOI] [PubMed] [Google Scholar]
- 48. Diallo I, Ndiaye B, Touré M, et al. : Hepatocellular carcinoma in Senegal: epidemiological, clinical and etiological aspects about 229 cases at Hôpital Principal de Dakar. Pan Afr Med J. 2021;38:99. 10.11604/pamj.2021.38.99.25195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Le VQ, Nguyen VH, Nguyen VH, et al. : Epidemiological Characteristics of Advanced Hepatocellular Carcinoma in the Northern Region of Vietnam. Cancer Control. 2019;26(1): 1073274819862793. 10.1177/1073274819862793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Niño-Ramírez S, Jaramillo-Arroyave D, Ardila O, et al. : Disminuyendo la heterogeneidad en hepatocarcinoma. Análisis de clúster por variables clínicas en pacientes atendidos en una institución de cuarto nivel de complejidad. Rev Gastroenterol México. 2021;86(4):356–362. 10.1016/j.rgmx.2020.07.013 [DOI] [PubMed] [Google Scholar]
- 51. Phipps M, Livanos A, Guo A, et al. : Gender Matters: Characteristics of Hepatocellular Carcinoma in Women From a Large, Multicenter Study in the United States. Am J Gastroenterol. 2020;115(9):1486–1495. 10.14309/ajg.0000000000000643 [DOI] [PubMed] [Google Scholar]
- 52. Hanna D, Sugamori KS, Bott D, et al. : The impact of sex on hepatotoxic, inflammatory and proliferative responses in mouse models of liver carcinogenesis. Toxicology. 2020;442:152546. 10.1016/j.tox.2020.152546 [DOI] [PubMed] [Google Scholar]
- 53. Ryerson AB, Eheman CR, Altekruse SF, et al. : Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–37. 10.1002/cncr.29936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maeda S, Kamata H, Luo JL, et al. : IKKbeta Couples Hepatocyte Death to Cytokine-Driven Compensatory Proliferation that Promotes Chemical Hepatocarcinogenesis. Cell. 2005;121(7):977–990. 10.1016/j.cell.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 55. Ghany MG, Feld JJ, Chang KM, et al. : Serum alanine aminotransferase flares in chronic hepatitis B infection: the good and the bad. Lancet Gastroenterol Hepatol. 2020;5(4):406–417. 10.1016/S2468-1253(19)30344-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brahmania M, Lombardero M, Hansen BE, et al. : Association Between Severe Serum Alanine Aminotransferase Flares and Hepatitis B e Antigen Seroconversion and HBV DNA Decrease in Untreated Patients With Chronic HBV Infection. Clin Gastroenterol Hepatol. 2019;17(12):2541–2551.e2. 10.1016/j.cgh.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kumar M, Chauhan R, Gupta N, et al. : Spontaneous Increases in Alanine Aminotransferase Levels in Asymptomatic Chronic Hepatitis B Virus-Infected Patients. Gastroenterology. 2009;136(4):1272–1280. 10.1053/j.gastro.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 58. Lok AS, Lai CL: Acute exacerbations in Chinese patients with chronic hepatitis B virus (HBV) infection. Incidence, predisposing factors and etiology. J Hepatol. 1990;10(1):29–34. 10.1016/0168-8278(90)90069-4 [DOI] [PubMed] [Google Scholar]
- 59. Chu CM, Liaw YF: Predictive Factors for Reactivation of Hepatitis B Following Hepatitis B e Antigen Seroconversion in Chronic Hepatitis B. Gastroenterology. 2007;133(5):1458–1465. 10.1053/j.gastro.2007.08.039 [DOI] [PubMed] [Google Scholar]
- 60. Liem S, Wong D, Yim C, et al. : FRI-190-Incidence and predictors of flares after discontinuing nucleos (t) ide analogue therapy in HBeAg negative patients with chronic hepatitis B: Results from the randomized controlled STOP study. J Hepatol. 2019;70(1):e474. 10.1016/S0618-8278(19)30935-1 [DOI] [Google Scholar]
- 61. Liu F, Zou Z, Shen L, et al. : A prediction model for outcome in patients with HBV-ACLF based on predisposition, injury, response and organ failure. Sci Rep. 2020;10(1):20176. 10.1038/s41598-020-77235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ben-Batalla I, Vargas-Delgado ME, von Amsberg G, et al. : Influence of Androgens on Immunity to Self and Foreign: Effects on Immunity and Cancer. Front Immunol. 2020;11:1184. 10.3389/fimmu.2020.01184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fish EN: The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737–744. 10.1038/nri2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hewagama A, Patel D, Yarlagadda S, et al. : Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 2009;10(5):509–516. 10.1038/gene.2009.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Furman D, Hejblum BP, Simon N, et al. : Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111(2):869–874. 10.1073/pnas.1321060111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sankaran-Walters S, Macal M, Grishina I, et al. : Sex differences matter in the gut: effect on mucosal immune activation and inflammation. Biol Sex Differ. 2013;4(1):10. 10.1186/2042-6410-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wong VW, Yu J, Cheng AS, et al. : High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124(12):2766–2770. 10.1002/ijc.24281 [DOI] [PubMed] [Google Scholar]
- 68. Naugler WE, Sakurai T, Kim S, et al. : Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–124. 10.1126/science.1140485 [DOI] [PubMed] [Google Scholar]
- 69. Schurz H, Salie M, Tromp G, et al. : The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics. 2019;13(1):2. 10.1186/s40246-018-0185-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xu N, Yao HP, Sun Z, et al. : Toll-like receptor 7 and 9 expression in peripheral blood mononuclear cells from patients with chronic hepatitis B and related hepatocellular carcinoma. Acta Pharmacol Sin. 2008;29(2):239–244. 10.1111/j.1745-7254.2008.00711.x [DOI] [PubMed] [Google Scholar]
- 71. Scotland RS, Stables MJ, Madalli S, et al. : Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118(22):5918–5927. 10.1182/blood-2011-03-340281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Buschow SI, Biesta PJ, Groothuismink ZMA, et al. : TLR7 polymorphism, sex and chronic HBV infection influence plasmacytoid DC maturation by TLR7 ligands. Antiviral Res. 2018;157:27–37. 10.1016/j.antiviral.2018.06.015 [DOI] [PubMed] [Google Scholar]
- 73. Trevisan A, Giuliani A, Scapellato ML, et al. : Sex disparity in response to hepatitis B vaccine related to the age of vaccination. Int J Environ Res Public Health. 2020;17(1):327. 10.3390/ijerph17010327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fischinger S, Boudreau CM, Butler AL, et al. : Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41(2):239–249. 10.1007/s00281-018-0726-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li M, Zhao Y, Chen X, et al. : Contribution of sex‑based immunological differences to the enhanced immune response in female mice following vaccination with hepatitis B vaccine. Mol Med Rep. 2019;20(1):103–110. 10.3892/mmr.2019.10231 [DOI] [PubMed] [Google Scholar]
- 76. McNaughton AL, D'Arienzo V, Ansari MA, et al. : Insights From Deep Sequencing of the HBV Genome-Unique, Tiny, and Misunderstood. Gastroenterology. 2019;156(2):384–399. 10.1053/j.gastro.2018.07.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tsukuda S, Watashi K: Hepatitis B virus biology and life cycle. Antiviral Res. 2020;182:104925. 10.1016/j.antiviral.2020.104925 [DOI] [PubMed] [Google Scholar]
- 78. Kori M, Arga KY: Pathways involved in viral oncogenesis: New perspectives from virus-host protein interactomics. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165885. 10.1016/j.bbadis.2020.165885 [DOI] [PubMed] [Google Scholar]
- 79. Zhang H, Spencer K, Burley SK, et al. : Toward improving androgen receptor-targeted therapies in male-dominant hepatocellular carcinoma. Drug Discov Today. 2021;26(6):1539–1546. 10.1016/j.drudis.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang SH, Yeh SH, Lin WH, et al. : Identification of Androgen Response Elements in the Enhancer I of Hepatitis B Virus: A Mechanism for Sex Disparity in Chronic Hepatitis B. Hepatology. 2009;50(5):1392–402. 10.1002/hep.23163 [DOI] [PubMed] [Google Scholar]
- 81. Chiu CM, Yeh SH, Chen PJ, et al. : Hepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen level. Proc Natl Acad Sci U S A. 2007;104(8):2571–2578. 10.1073/pnas.0609498104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang WJ, Chang CJ, Yeh SH, et al. : Hepatitis B virus X protein enhances the transcriptional activity of the androgen receptor through c-Src and glycogen synthase kinase-3beta kinase pathways. Hepatology. 2009;49(5):1515–24. 10.1002/hep.22833 [DOI] [PubMed] [Google Scholar]
- 83. Chen CJ, Yang HI, Iloeje UH, et al. : Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology. 2009;49(5 Suppl):S72–84. 10.1002/hep.22884 [DOI] [PubMed] [Google Scholar]
- 84. Yip TC, Wong GL, Chan HL, et al. : Elevated testosterone increases risk of hepatocellular carcinoma in men with chronic hepatitis B and diabetes mellitus. J Gastroenterol Hepatol. 2020;35(12):2210–2219. 10.1111/jgh.15079 [DOI] [PubMed] [Google Scholar]
- 85. Sun H, Yang W, Tian Y, et al. : An inflammatory-CCRK circuitry drives mTORC1-dependent metabolic and immunosuppressive reprogramming in obesity-associated hepatocellular carcinoma. Nat Commun. 2018;9(1):5214. 10.1038/s41467-018-07402-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sheng T, Wang B, Wang SY, et al. : The Relationship Between Serum Interleukin-6 and the Recurrence of Hepatitis B Virus Related Hepatocellular Carcinoma after Curative Resection. Medicine (Baltimore). 2015;94(24):e941. 10.1097/MD.0000000000000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang SH, Yeh SH, Lin WH, et al. : Estrogen receptor α represses transcription of HBV genes via interaction with hepatocyte nuclear factor 4α. Gastroenterology. 2012;142(4):989–998.e4. 10.1053/j.gastro.2011.12.045 [DOI] [PubMed] [Google Scholar]
- 88. O’Brien MH, Pitot HC, Chung SH, et al. : Estrogen Receptor-α Suppresses Liver Carcinogenesis and Establishes Sex-Specific Gene Expression. Cancers (Basel). 2021;13(10):2355. 10.3390/cancers13102355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ni Y, Lempp FA, Mehrle S, et al. : Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146(4):1070–83. 10.1053/j.gastro.2013.12.024 [DOI] [PubMed] [Google Scholar]
- 90. Cao J, Wood M, Liu Y, et al. : Estradiol Represses Prolactin-Induced Expression of Na +/Taurocholate Cotransporting Polypeptide in Liver Cells through Estrogen Receptor-alpha and Signal Transducers and Activators of Transcription 5a. Endocrinology. 2004;145(4):1739–49. 10.1210/en.2003-0752 [DOI] [PubMed] [Google Scholar]
- 91. Yan Z, Tan W, Dan Y, et al. : Estrogen receptor alpha gene polymorphisms and risk of HBV-related acute liver failure in the Chinese population. BMC Med Genet. 2012;13:49. 10.1186/1471-2350-13-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen CJ, Iloeje UH, Yang HI: Long-term outcomes in hepatitis B: the REVEAL-HBV study. Clin Liver Dis. 2007;11(4):797–816. 10.1016/j.cld.2007.08.005 [DOI] [PubMed] [Google Scholar]
- 93. Farza H, Salmon AM, Hadchouel M, et al. : Hepatitis B surface antigen gene expression is regulated by sex steroids and glucocorticoids in transgenic mice. Proc Natl Acad Sci U S A. 1987;84(5):1187–1191. 10.1073/pnas.84.5.1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. DeLoia JA, Burk RD, Gearhart JD: Developmental regulation of hepatitis B surface antigen expression in two lines of hepatitis B virus transgenic mice. J Virol. 1989;63(9):4069–4073. 10.1128/JVI.63.9.4069-4073.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Guidotti LG, Matzke B, Schaller H, et al. : High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69(10): 6158–6169. 10.1128/JVI.69.10.6158-6169.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Guidotti LG, Eggers CM, Raney AK, et al. : In vivo regulation of hepatitis B virus replication by peroxisome proliferators. J Virol. 1999;73(12):10377–10386. 10.1128/JVI.73.12.10377-10386.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chen CC, Ko TM, Ma HI, et al. : Long-term inhibition of hepatitis B virus in transgenic mice by double-stranded adeno-associated virus 8-delivered short hairpin RNA. Gene Ther. 2007;14(1):11–19. 10.1038/sj.gt.3302846 [DOI] [PubMed] [Google Scholar]
- 98. Breidbart S, Burk RD, Saenger P: Hormonal Regulation of Hepatitis B Virus Gene Expression: Influence of Androgen Receptor. Pediatr Res. 1993;34(3):300–302. 10.1203/00006450-199309000-00012 [DOI] [PubMed] [Google Scholar]
- 99. Gish RG, Given BD, Lai CL, et al. : Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities. Antiviral Res. 2015;121:47–58. 10.1016/j.antiviral.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 100. McNaughton AL, Lemoine M, van Rensburg C, et al. : Extending treatment eligibility for chronic hepatitis B virus infection. Nat Rev Gastroenterol Hepatol. 2021;18(3):146–147. 10.1038/s41575-020-00398-x [DOI] [PubMed] [Google Scholar]
- 101. Koffas A, Petersen J, Kennedy PT: Reasons to consider early treatment in chronic hepatitis B patients. Antiviral Res. 2020;177:104783. 10.1016/j.antiviral.2020.104783 [DOI] [PubMed] [Google Scholar]
- 102. Koffas A, Kumar M, Gill US, et al. : Chronic hepatitis B: the demise of the ‘inactive carrier’ phase. Hepatol Int. 2021;15(2):290–300. 10.1007/s12072-021-10137-2 [DOI] [PubMed] [Google Scholar]
- 103. Marchio A, Cerapio JP, Ruiz E, et al. : Early-onset liver cancer in South America associates with low hepatitis B virus DNA burden. Sci Rep. 2018;8(1):12031. 10.1038/s41598-018-30229-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yeh ML, Huang JF, Yu ML, et al. : Hepatitis b infection: progress in identifying patients most likely to respond to peginterferon alfa. Expert Rev Gastroenterol Hepatol. 2021;15(4):427–435. 10.1080/17474124.2021.1866985 [DOI] [PubMed] [Google Scholar]
- 105. Chan HLY, Messinger D, Papatheodoridis GV, et al. : A baseline tool for predicting response to peginterferon alfa-2a in HBeAg-positive patients with chronic hepatitis B. Aliment Pharmacol Ther. 2018;48(5):547–555. 10.1111/apt.14862 [DOI] [PubMed] [Google Scholar]
- 106. Charatcharoenwitthaya P, Sukeepaisarnjaroen W, Piratvisuth T, et al. : Treatment outcomes and validation of the stopping rule for response to peginterferon in chronic hepatitis B: A Thai nationwide cohort study. J Gastroenterol Hepatol. 2016;31(11):1874–1881. 10.1111/jgh.13378 [DOI] [PubMed] [Google Scholar]
- 107. Park CH, Kim HY, Lee SW, et al. : On-treatment and off-treatment efficacy of entecavir in a real-life cohort of chronic hepatitis B patients. Eur J Gastroenterol Hepatol. 2016;28(10):1179–1187. 10.1097/MEG.0000000000000691 [DOI] [PubMed] [Google Scholar]
- 108. Chihota BV, Wandeler G, Chilengi R, et al. : High Rates of Hepatitis B Virus (HBV) Functional Cure Among Human Immunodeficiency Virus-HBV Coinfected Patients on Antiretroviral Therapy in Zambia. J Infect Dis. 2020;221(2):218–222. 10.1093/infdis/jiz450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Archampong T, Ojewale O, Bears K, et al. : Brief Report: Relationship Between ABCC4 SNPs and Hepatitis B Virus Suppression During Tenofovir-Containing Antiretroviral Therapy in Patients With HIV/HBV Coinfection. J Acquir Immune Defic Syndr. 2019;82(4):421–425. 10.1097/QAI.0000000000002136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cox AL, El-Sayed MH, Kao JH, et al. : Progress towards elimination goals for viral hepatitis. Nat Rev Gastroenterol Hepatol. 2020;17(9):533–542. 10.1038/s41575-020-0332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Griswold G, Andrieux-Meyer I, Applegate TL, et al. : Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2019;4(2):135–184. 10.1016/S2468-1253(18)30270-X [DOI] [PubMed] [Google Scholar]
- 112. Tan M, Bhadoria AS, Cui F, et al. : Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(2):106–119. 10.1016/S2468-1253(20)30307-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tiendrebeogo T, Messou E, Arikawa S, et al. : Ten-year attrition and antiretroviral therapy response among HIV-positive adults: a sex-based cohort analysis from eight West African countries. J Int AIDS Soc. 2021;24(5):e25723. 10.1002/jia2.25723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Assefa Y, Hill PS, Van Damme W, et al. : Leaving no one behind: lessons from implementation of policies for universal HIV treatment to universal health coverage. Global Health. 2020;16(1):17. 10.1186/s12992-020-00549-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wong VWS, Janssen HLA: Can we use HCC risk scores to individualize surveillance in chronic hepatitis B infection? J Hepatol. 2015;63(3):722–732. 10.1016/j.jhep.2015.05.019 [DOI] [PubMed] [Google Scholar]
- 116. Lin CL, Kao JH: Risk stratification for hepatitis B virus related hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28(1):10–17. 10.1111/jgh.12010 [DOI] [PubMed] [Google Scholar]
- 117. Wu S, Zeng N, Sun F, et al. : Hepatocellular Carcinoma Prediction Models in Chronic Hepatitis B: A Systematic Review of 14 Models and External Validation. Clin Gastroenterol Hepatol. 2021;19(12):2499–2513. 10.1016/j.cgh.2021.02.040 [DOI] [PubMed] [Google Scholar]
- 118. Yang HI, Yuen MF, Chan HLY, et al. : Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12(6):568–74. 10.1016/S1470-2045(11)70077-8 [DOI] [PubMed] [Google Scholar]
- 119. Sanaei M, Kavoosi F, Dehghani F: Comparative Analysis of the Effects of 17-Beta Estradiol on Proliferation, and Apoptosis in Hepatocellular Carcinoma Hep G2 and LCL-PI 11 Cell Lines. Asian Pac J Cancer Prev. 2018;19(9):2637–2641. 10.22034/APJCP.2018.19.9.2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sukocheva OA: Estrogen, estrogen receptors, and hepatocellular carcinoma: Are we there yet? World J Gastroenterol. 2018;24(1):1–4. 10.3748/wjg.v24.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire: Randomized trial of leuprorelin and flutamide in male patients with hepatocellular carcinoma treated with tamoxifen. Hepatology. 2004;40(6):1361–1369. 10.1002/hep.20474 [DOI] [PubMed] [Google Scholar]
- 122. Manesis EK, Giannoulis G, Zoumboulis P, et al. : Treatment of hepatocellular carcinoma with combined suppression and inhibition of sex hormones: A randomized, controlled trial. Hepatology. 1995;21(6):1535–1542. [PubMed] [Google Scholar]
- 123. Wu JF, Tsai WY, Tung YC, et al. : Effect of menarche onset on the clinical course in females with chronic hepatitis B virus infection. J Pediatr. 2014;165(3):534–538. 10.1016/j.jpeds.2014.05.049 [DOI] [PubMed] [Google Scholar]
- 124. Yu MW, Chang HC, Chang SC, et al. : Role of Reproductive Factors in Hepatocellular Carcinoma: Impact on Hepatitis B- and C-Related Risk. Hepatology. 2003;38(6):1393–400. 10.1016/j.hep.2003.09.041 [DOI] [PubMed] [Google Scholar]
- 125. Wu JF, Tsai WY, Hsu HY, et al. : Effect of Puberty Onset on Spontaneous Hepatitis B Virus e Antigen Seroconversion in Men. Gastroenterology. 2010;138(3):942–8.e1. 10.1053/j.gastro.2009.11.051 [DOI] [PubMed] [Google Scholar]
- 126. You H, Kong Y, Hou J, et al. : Female gender lost protective effect against disease progression in elderly patients with chronic hepatitis B. Sci Rep. 2016;6:37498. 10.1038/srep37498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Xiong M, Li J, Yang S, et al. : Influence of Gender and Reproductive Factors on Liver Fibrosis in Patients With Chronic Hepatitis B Infection. Clin Transl Gastroenterol. 2019;10(10):e00085. 10.14309/ctg.0000000000000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Baden R, Rockstroh JK, Buti M: Natural History and Management of Hepatitis C: Does Sex Play a Role? J Infect Dis. 2014;209 Suppl 3:S81–5. 10.1093/infdis/jiu057 [DOI] [PubMed] [Google Scholar]
- 129. Ziegler SM, Altfeld M: Human immunodeficiency virus 1 and type I interferons-where sex makes a difference. Front Immunol. 2017;8:1224. 10.3389/fimmu.2017.01224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Joshi SS, Coffin CS: Hepatitis B and Pregnancy: Virologic and Immunologic Characteristics. Hepatol Commun. 2020;4(2):157–171. 10.1002/hep4.1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Joshi SS, Wong D, Castillo E, et al. : Peripartum cytokine flares in a multiethnic cohort of chronic hepatitis B carriers does not correlate with hepatitis B virus suppression or increased risk of liver disease. Am J Reprod Immunol. 2017;78(4):e12707. 10.1111/aji.12707 [DOI] [PubMed] [Google Scholar]
- 132. Sirilert S, Tongsong T: Hepatitis B Virus Infection in Pregnancy: Immunological Response, Natural Course and Pregnancy Outcomes. J Clin Med. 2021;10(13):2926. 10.3390/jcm10132926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bzowej NH, Tran TT, Li R, et al. : Total Alanine Aminotransferase (ALT) Flares in Pregnant North American Women with Chronic Hepatitis B Infection: Results from a Prospective Observational Study. Am J Gastroenterol. 2019;114(8):1283–1291. 10.14309/ajg.0000000000000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chang CY, Aziz N, Poongkunran M, et al. : Serum Alanine Aminotransferase and Hepatitis B DNA Flares in Pregnant and Postpartum Women with Chronic Hepatitis B. Am J Gastroenterol. 2016;111(10):1410–1415. 10.1038/ajg.2016.296 [DOI] [PubMed] [Google Scholar]
- 135. Chang ML, Liaw YF: Hepatitis B flares in chronic hepatitis B: Pathogenesis, natural course, and management. J Hepatol. 2014;61(6):1407–1417. 10.1016/j.jhep.2014.08.033 [DOI] [PubMed] [Google Scholar]
- 136. Kim HY, Choi JY, Park CH, et al. : Outcome after discontinuing antiviral agents during pregnancy in women infected with hepatitis B virus. J Clin Virol. 2013;56(4):299–305. 10.1016/j.jcv.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 137. Kochaksaraei GS, Castillo E, Osman M, et al. : Clinical course of 161 untreated and tenofovir-treated chronic hepatitis B pregnant patients in a low hepatitis B virus endemic region. J Viral Hepat. 2016;23(1):15–22. 10.1111/jvh.12436 [DOI] [PubMed] [Google Scholar]
- 138. Kochaksaraei GS, Castillo E, Sadler MD, et al. : Real-world clinical and virological outcomes in a retrospective multiethnic cohort study of 341 untreated and tenofovir disoproxil fumarate-treated chronic hepatitis B pregnant patients in North America. Aliment Pharmacol Ther. 2020;52(11–12):1707–1716. [DOI] [PubMed] [Google Scholar]
- 139. Furuncuoglu Y, Bolukbas FF, Bolukbas C, et al. : Changes in the prevalence of HBV infection in pregnant women in Turkey between 1995 and 2015: A 20-year evaluation. Postgrad Med J. 2016;92(1091):510–513. 10.1136/postgradmedj-2015-133876 [DOI] [PubMed] [Google Scholar]
- 140. Yu MW, Chang HC, Chang SC, et al. : Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology. 2003;38(6):1393–1400. 10.1016/j.hep.2003.09.041 [DOI] [PubMed] [Google Scholar]
- 141. Goldstein ST, Zhou F, Hadler SC, et al. : A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34(6):1329–1339. 10.1093/ije/dyi206 [DOI] [PubMed] [Google Scholar]
- 142. Shimakawa Y, Lemoine M, Njai HF, et al. : Natural history of chronic HBV infection in West Africa: a longitudinal population-based study from The Gambia. Gut. 2016;65(12):2007–2016. 10.1136/gutjnl-2015-309892 [DOI] [PubMed] [Google Scholar]
- 143. WHO Gender and Health. Reference Source [Google Scholar]
- 144. Heidari S, Babor TF, Castro PD, et al. : Sex and Gender Equity in Research: rationale for the SAGER guidelines and recommended use. Res Integr peer Rev. 2016;1:2. 10.1186/s41073-016-0007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. 2021 Global Health 50/50 Report - Global Health 50/50.2021; (Accessed: 31st July 2021). Reference Source [Google Scholar]
- 146. Segala FV, Micheli G, Seguiti C, et al. : Prevalence of Sexually Transmitted Infections and Factors Associated with HIV Status Among Vulnerable Women in Northern Uganda: Baseline Results from Pe Atye Kena Cohort Study. Mediterr J Hematol Infect Dis. 2021;13(1):e2021055. 10.4084/MJHID.2021.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Conserve DF, Issango J, Kilale AM, et al. : Developing national strategies for reaching men with HIV testing services in Tanzania: results from the male catch-up plan. BMC Heal Serv Res. 2019;19(1):317. 10.1186/s12913-019-4120-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Unaids: Addressing a blind spot in the response to HIV — Reaching out to men and boys. Reference Source [Google Scholar]
- 149. Kasradze A, Shadaker S, Kuchuloria T, et al. : The burden and epidemiology of hepatitis B and hepatitis D in Georgia: findings from the national seroprevalence survey. Public Health. 2020;185:341– 347. 10.1016/j.puhe.2020.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hakim AJ, Iwamoto C, Badman SG, et al. : High Prevalence of Chlamydia and Gonorrhea and the Need for Sexually Transmitted Infection Testing Among Men Who Have Sex With Men and Transgender Women in Papua New Guinea. Sex Transm Dis. 2021;48(2):109–117. 10.1097/OLQ.0000000000001300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Adeyemi OA, Mitchell A, Shutt A, et al. : Hepatitis B virus infection among men who have sex with men and transgender women living with or at risk for HIV: a cross sectional study in Abuja and Lagos, Nigeria. BMC Infect Dis. 2021;21(1):654. 10.1186/s12879-021-06368-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Messersmith LJ, Adjei R, Beard J, et al. : High levels of used syringe use and unsafe sex among people who inject drugs in Kumasi, Ghana: an urgent call for a comprehensive harm reduction approach. Harm Reduct J. 2021;18(1):62. 10.1186/s12954-021-00510-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Calin R, Massari V, Pialoux G, et al. : Acceptability of on-site rapid HIV/HBV/HCV testing and HBV vaccination among three at-risk populations in distinct community-healthcare outreach centres: the ANRS-SHS 154 CUBE study. BMC Infect Dis. 2020;20(1):851. 10.1186/s12879-020-05601-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Mokaya J, McNaughton AL, Burbridge L, et al. : A blind spot? Confronting the stigma of hepatitis B virus (HBV) infection - A systematic review [version 2; peer review: 2 approved]. Wellcome Open Res. 2018;3:29. 10.12688/wellcomeopenres.14273.2 [DOI] [PMC free article] [PubMed] [Google Scholar]