Abstract
Carbon-based materials are well established as low-cost, easily synthesizable, and low regeneration energy adsorbents against harmful greenhouse gases such as CO2. However, the development of such materials with exceptional CO2 uptake capacity needs well-described research, wherein various factors influencing CO2 adsorption need to be investigated. Therefore, five cost-effective carbon-based materials that have similar textural properties, functional groups, and porous characteristics were selected. Among these materials, biordered ultramicroporous graphitic carbon had shown an excellent CO2 capture capacity of 7.81 mmol/g at 273 K /1 bar with an excellent CO2 vs N2 selectivity of 15 owing to its ultramicroporous nature and unique biordered graphitic morphology. On the other hand, reduced graphene revealed a remarkable CO2 vs N2 selectivity of 57 with a CO2 uptake of 2.36 mmol/g at 273 K/1 bar. In order to understand the high CO2 capture capacity, important properties derived from adsorption/desorption, Raman spectroscopy, and X-ray photoelectron spectroscopy were correlated with CO2 adsorption. This study revealed that an increase in ultramicropore volume and sp2 carbon (graphitic) content of nanomaterials could enhance CO2 capture significantly. FTIR studies revealed the importance of oxygen functionalities in improving CO2 vs N2 selectivity in reduced graphene due to higher quadruple–dipole interactions between CO2 and oxygen functionalization of the material. Apart from high CO2 adsorption capacity, biordered ultramicroporous graphitic carbon also offered low regeneration energy and excellent pressure swing regeneration ability for five consecutive cycles.
Introduction
Environmental deterioration and climate change brought considerable attention from researchers toward the emission of greenhouse gases.1,2 The massive consumption of fossil fuels leading to carbon dioxide (CO2) emission is the main cause of these problems.3 Thus, developing CO2 capture and storage methodologies demands the undivided attention of researchers.4 Currently, the amine-scrubbing methodology is used for industrial CO2 capture.5 However, this approach has several drawbacks, such as high regeneration costs, solvent loss, toxicity, amine degradation, and a tendency to corrode the equipment.6−8 In comparison, solid porous adsorbents are attractive as they offer easy handling, simple processing, and low regeneration costs.9 In this category, porous materials having high surface areas, such as zeolites, metal–organic frameworks (MOFs), silica, nanocarbons, etc., are widely explored as CO2 adsorbents.10 Among these, carbon nanomaterials with narrow pores are of tremendous importance as they offer a high surface area, large pore volume, ease of surface modification, excellent thermal and mechanical stability, and minimal adsorbent regeneration energy.11,12 Moreover, the synthesis methodologies are also low-cost, and hence bulk synthesis of these materials is a viable option.
Activated carbons (ACs) and graphene-based CO2 adsorbents are independently well explored to achieve high CO2 adsorption capacity due to their microporous nature and laminar morphology with functionalized surfaces, respectively.13 Recently, a simple yet innovative approach has emerged wherein graphene-based precursors (GO or rGO) and activated carbon materials are combined to obtain new materials that can enhance the CO2 adsorption capacity. For example, poly(p-phenylenediamine) (PpPDA) as a carbon source and GO as a graphene source were used to prepare an N-doped porous carbon that provided a CO2 uptake of 4.65 mmol/g at 298 K and 5 bar pressure.14 In another study, Yuan and co-workers reported nitrogen-doped porous carbon employing melamine-resorcinol-formaldehyde resin and graphene oxide. This material had shown a CO2 adsorption capacity of 5.21 mmol/g at 298 K at 5 bar pressure.15 In other attempts, graphene-like morphology/graphene-based nanomaterials were also synthesized and explored for CO2 adsorption.16−20 For example, graphene-like multiscale microporous carbon nanosheets were synthesized by Shi et al., which provided a CO2 adsorption capacity of 6.32 mmol/g at 273 K/1 bar.21 A novel three-dimensional B,N co-doped graphene-like carbon nanosheet material was developed, which showed 3.57 mmol/g CO2 adsorption capacity (at 273 K and 1 bar).22 These materials have shown great potential for CO2 adsorption application, which suggests that a detailed study to understand the impact of various structural features of these nanomaterials on CO2 uptake is of great importance. In this context, Merchán and co-workers prepared hybrid hydrogels from graphene oxide and activated carbon fibers and focused on correlating various textural properties with CO2 uptake.23 However, these materials had distinct domains of activated carbon fiber and GO, which resulted in difficulties correlating adsorption characteristics with graphene-like structures. The novel idea of investigating the relation between the properties of materials and CO2 adsorption can help researchers to synthesize new materials that can achieve extraordinary CO2 adsorption capacity and selectivity. Therefore, we opted for low-cost, easily synthesizable carbon materials to establish new insights in correlating the properties with CO2 uptake capacity.
We recently reported a biordered ultramicroporous graphitic carbon (BUGC), which has a high surface area, ultramicropore/micropore volume, and unique graphitization ordering.24 Considering all the above examples, we envisioned that the BUGC’s properties should be beneficial for CO2 uptake and can provide useful insights into the morphological effects on the adsorption properties of carbon nanomaterials. With this objective, we selected five nanomaterials, namely, non-porous carbon (NC), reduced graphene (RG), IMC (immense microporous carbon), BUGC (biordered ultramicroporous graphitic carbon), and MMC (microporous mesoporous carbon).24−26 These materials have distinct porous and functional group characteristics yet similar textures, which allowed us to understand the correlations of their properties with CO2 capture capacity. In the following few sections, we discuss the syntheses, characterizations, and correlation plots for these materials with CO2 uptake.
Experimental Section
Materials and Chemicals
Graphite (150 micron, cat#496588), non-porous carbon (untreated, granular, 4–8 mesh, cat#C2764) (NC), and commercial micro-mesoporous carbon (100 mesh, CMMC) were bought from Sigma-Aldrich. Potassium permanganate (KMnO4), sulfuric acid (H2SO4), formic acid (HCOOH), potassium hydroxide (KOH), acetone, and hydrogen peroxide (H2O2, 50%) were also purchased from Sigma-Aldrich. Acetone and concentrated hydrochloric acid were supplied by Spectrochem and Pallav Chemicals, respectively. Milli-Q water (>18 MΩ) was used for all synthesis and solution preparation.
Synthesis of Reduced Graphene (RG)
RG was synthesized by following a protocol previously reported by our group.25 Briefly, modified Hummer’s method was used wherein 2 g of graphite (150 μm) was dispersed in 46 mL of conc. H2SO4 in a 250 mL round-bottom flask. This dispersion was kept in an ice bath (0 °C), and 6 g of KMnO4 was added carefully. This addition was performed gradually by covering a time span of 20 min in order to keep the temperature of the dispersion below 20 °C. This mixture was stirred for 3 h at 35 °C using a reflux condenser. Thereafter, 92 mL of water was added cautiously over a period of 30 min. Next, in 280 mL of water, all of these contents were poured, and 10 mL of 30% H2O2 was added to destroy excess KMnO4 (the color changed from dark brown to yellow). Finally, this material was filtered and washed with water in a sintered glass filter until the sulfate was completely destroyed (checked with barium chloride addition), and it was named graphite oxide (GO). Then, GO was further washed with 20% formic acid solution to intercalate formic acid into the interlamellar regions of GO. Formic acid-intercalated GO was collected and dried at 60 °C. To obtain RG, 0.5 g of dried GO was exfoliated at 160 °C in a preheated oven.
Synthesis of IMC and BUGC
Both materials were prepared by a chemical activation method using KOH as a porogen, which was reported earlier by our group.24,26 For IMC, an optimized ratio of NC and KOH (1:5) was taken in an alumina boat. This mixture was kept in a temperature-controlled cylindrical tube furnace and ramped to a temperature of 750 °C with a ramp rate of 5 °C/min under a constant flow of N2. The sample was carbonized at 750 °C for 1 h, and then it was allowed to cool down under N2 flow naturally. Thereafter, the obtained sample was washed with 5 M HCl to remove the mineral content. This procedure was followed by washing with distilled water to eliminate chloride ions. The carbon material procured by this procedure was named immense microporous carbon (IMC) since the material is mostly microporous in nature (vide infra).26 For BUGC, a similar procedure was utilized, except that the carbon precursor was NC and RG in a ratio of 8:2, a procedure reported previously by our group.24 The prepared materials were dried at 100 °C for 24 h in a hot air oven.
Synthesis of MMC
MMC was also synthesized by the procedure described above. Herein, instead of NC, CMMC was used as a precursor.26
Characterizations
Scanning electron microscopy (SEM) morphological analysis was performed in a Carl ZEISS (Ultraplus) FE-SEM instrument at 20 kV. For SEM imaging, dried carbon materials were spread on carbon tape and coated with gold for 120 s. Transmission electron microscopy (TEM) experiments were conducted using a TEM FEI TALOS 200S under an accelerating voltage of 200 kV. For TEM sample preparation, sample dispersion was prepared in 2-propanol (0.5 mg in 15 mL), which was drop-casted on a carbon-coated copper grid. Before imaging, these samples were dried overnight at ambient temperature. A Lab RAM HR 800 (Horiba) instrument with an excitation wavelength of 532 nm laser was used to record Raman spectra. Fourier transform infrared (FTIR) spectroscopy experiments were carried out using a Perkin-Elmer Model UATR Spectrum Two Instrument in the range of 500 to 4000 cm–1. Degassed samples were directly used to capture the FTIR spectra. CO2 gas flowed at a rate of 100 mL/min for 12 h to capture the FTIR spectra of CO2-rich samples. During the FTIR characterization, samples were exposed to air for <15 s. X-ray photoelectron spectroscopy (XPS) was performed using a PHI 5000 Versa Prob II, FEI Inc., with a scan time of 1 h per element for a core level scan (energy band of 20 eV, with pass setting of 23.5 eV, 0.025 eV step, 100 ms time per step, 5 cycles). N2 and CO2 adsorption/desorption experiments were carried out using a Quantachrome Autosorb QUA21011 instrument. Prior to the adsorption experiments, samples were degassed under vacuum at 80 °C for 20 h.
Results and Discussion
Morphological Analysis
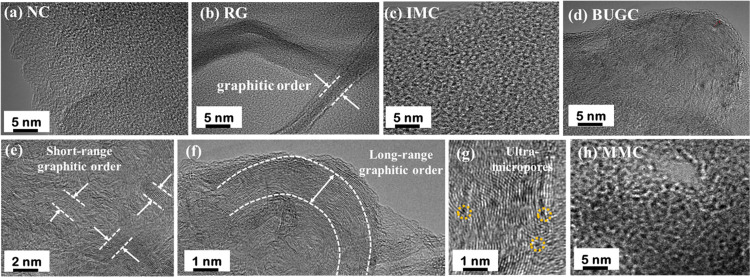
Morphological analyses of all nanomaterials were performed using SEM and TEM. The SEM image of NC showed a nonporous morphology (Figure S1a, SI), whereas RG revealed a layered structure (Figure S1b, SI).25,26 IMC, BUGC, and MMC had shown porous characteristics (Figure S1c–S1e, SI), while BUGC also had layered graphitic regions (Figure S1d, SI).24,26 These observations were further verified by TEM (Figure 1). NC showed non-porous characteristics, while RG showed layered graphitic structures (Figures 1a,b).25,26 IMC, BUGC, and MMC displayed porous characteristics, whereas BUGC had unique layered graphitic morphology (Figures 1c,d,h).24,26 The magnified HRTEM images of BUGC revealed biordered characteristics wherein short-range graphitic orders were visible inside the grain boundary (Figure 1e), and along the grain boundaries, long-range graphitic order was present (Figure 1f).24 HRTEM images further exhibited narrow micropores in BUGC (Figure 1g). An in-depth analysis and discussion about the morphology of BUGC were provided by us previously.24 We proposed that the catalyzation of disordered NC with an ordered RG nanostructure might have occurred for the formation of BUGC, which resulted in the short-range ordered morphological pattern. These short-range ordered graphitic structures reorganize randomly, create a directionally disordered morphology, and generate micropores/ultramicropores. Moreover, the K+ ion from KOH intercalates between graphitic layers and facilitates the exfoliation of multilayered graphitic structure to form a single-layer graphene-like structure under heat treatment.24
Figure 1.
TEM micrographs of (a) NC,26 (b) RG,25 (c) IMC,26 (d) BUGC,24 magnified view of BUGC showing (e) short-range graphitic order,24 (f) long-range graphitic order,24 and (g) ultramicropores,24 and (h) MMC.26
Functional Group Analysis
The FTIR spectra provided in Figure S2 revealed the presence of peaks corresponding to hydroxyl functionalities (C–O) (3300–3700 and 1030 cm–1), alkene/alkane C–H bond (2980–3000 cm–1), ketonic functionalities (C=O) (1695–1720 cm–1), and alkene (C=C) bond (1540–1566 cm–1).24,27 XPS was also performed to determine the surface functional groups of nanomaterials. All materials consisted of carbon and oxygen (Figure S3, SI), and the respective percentages of carbon and oxygen are tabulated in Table S1. The highest percentage of oxygen was present in RG followed by MMC, BUGC, and IMC. Next, the high-resolution C 1s spectra of these materials were deconvoluted to analyze carbon functionalization (Figure S4 and Table S2, SI). Except NC, all other materials had five types of functionalizations situated at 284.6 eV for C=C (sp2 carbon), 285.1 eV for C–C/C–H (sp3 carbon), 286.3 eV for C–O (alcohol functionalities), 287.9 eV for C=O (ketonic functionalities), and 290 eV for O-C=O (carboxylic functionalities) (Figure S4, SI).24,28 Interestingly, a high percentage of sp2 carbon was present in BUGC followed by IMC and MMC, in accordance with the SEM and TEM analysis (vide supra) (Table S2, SI). A relatively low sp2 carbon content in RG does not indicate a low graphitic content; rather, it was due to a higher percentage of oxygen content in comparison to other materials (Table S2, SI). Notably, the alcohol functionality (C–O) content was maximum in BUGC among all materials, whereas the percentage of ketonic functionality (C=O) content was maximum for RG (Table S2, SI).
Raman Analysis
The Raman spectra of all materials were fitted and analyzed (Figure S5, SI). Two major peaks at 1330 and 1590 cm–1 were observed due to defects (D-band) and stretching of sp2 carbons (G-band) in carbon lattice, respectively (Figure S5, SI).27,29 Moreover, D* (∼1110 cm–1), D″ (1500–1540 cm–1), and D′ (1680–1695 cm–1) bands were also present, corresponding to the disordered graphitic lattice due to Csp3 carbon centers, amorphous phases, and defects of the basal plane, respectively (Figure S5, SI).23 The defect ratio (ID/IG) was also determined for all samples and summarized in Table S3, SI. Importantly, the ID/IG ratio was lowest in BUGC, implying that the sp2 carbon character was the highest in BUGC, which validates the results obtained from XPS spectroscopy (vide supra).
Porous Characteristics of Materials
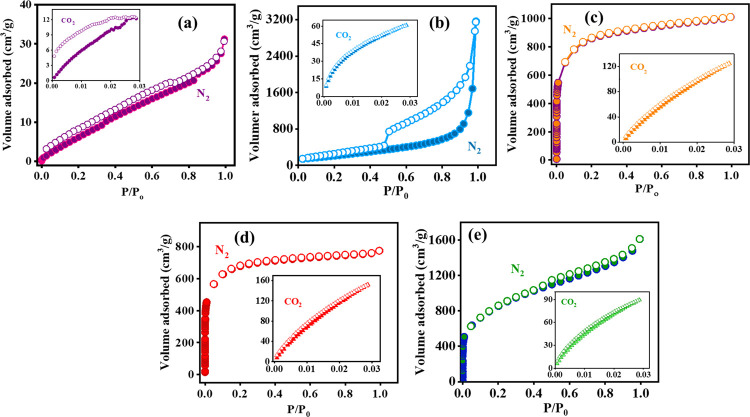
Porous characteristics of all of the materials were analyzed by obtaining N2 adsorption/desorption isotherms at 77 K and CO2 isotherms at 273 K. Figure 2 shows the isotherms for all materials, and the inset provides the CO2 isotherm at 273 K. The isotherm for NC showed a non-porous characteristic (Figure 2a), whereas RG presented type-III and type-IV isotherms according to IUPAC classification, having an H3 type of hysteresis loop, indicating the mesoporous characteristics of the material (Figure 2b).25 IMC and BUGC exhibited a type-I isotherm, highlighting the microporous nature of the nanomaterials (Figures 1c,d).24,26 MMC revealed a mixed type-I and type-IV isotherm, showing the microporous and mesoporous characteristics of the material (Figure 2e).29 BET surface areas (SBET) were analyzed in the range of 0.05–0.2 P/P0 (relative pressure) and are tabulated in Table 1. The surface area of NC was only 37 m2/g, whereas RG exhibited a surface area of 732 m2/g (Table 1). An increasing trend of surface areas in micropore-rich carbons (type-I isotherm) with the highest surface area for MMC (3090 m2/g) followed by IMC (3030 m2/g) and BUGC (2472 m2/g) was obtained (Table 1). The porous characteristics of materials were analyzed using the non-local density functional theory (NLDFT) model. To probe the narrow micropores, CO2 adsorption isotherms were obtained at 273 K and were also analyzed by the NLDFT model (Figure 2 insets). The combined total pore volume and pore size distribution from N2 and CO2 isotherms are provided in Figure S6a–d and Figure S6e–h, respectively. RG was mesoporous in nature, having a sharp pore size distribution peak at 3.9 nm (Figure S6e, SI). On the other hand, IMC and BUGC exhibited completely ultramicroporous/microporous characteristics having pore size distribution in the ranges of 0.33–1.96 and 0.35–1.47 nm, respectively (Figure S6f,g, SI). MMC consisted of bimodal porous characteristics having microporous (0.34–1.32 nm) and mesoporous characteristics (2.56–5.25 nm) (Figure S6h, SI).
Figure 2.
N2 adsorption/desorption isotherm at 77 K for (a) NC, (b) RG,25 (c) IMC,26 (d) BUGC,24 and (e) MMC26 with a CO2 adsorption/desorption isotherm at 273 K as an inset.
Table 1. Porous Characteristics of the Studied Materials Obtained from N2 Adsorption/Desorption Isotherms at 77 K (Superscript a) and CO2 Adsorption/Desorption Isotherms at 273 K (Superscript b).
| CO2 uptake at 273 K (mmol/g) |
|||||||
|---|---|---|---|---|---|---|---|
| material | SBET (m2/g)a | total pore volume (cm3/g)a | ultramicropore volume (cm3/g) (0.3–0.7 nm)b | micropore volume (cm3/g) (0.7–20 nm)a | @1.0 bar | @0.15 bar | CO2 uptake at 298 K (mmol/g) and 1 bar |
| NC | 37 | 0.04 | 0.00 | 0.03 | 0.55 | 0.14 | 0.39 |
| RG | 732 | 3.56 | 0.12 | 0.20 | 2.36 | 1.12 | 1.56 |
| IMC | 3030 | 1.49 | 0.21 | 1.04 | 5.56 | 1.25 | 2.89 |
| BUGC | 2472 | 1.07 | 0.31 | 0.84 | 7.81 | 1.98 | 4.29 |
| MMC | 3090 | 2.30 | 0.20 | 0.77 | 4.00 | 1.06 | 2.82 |
CO2 Capture
CO2 uptake capacity of the materials was performed at two temperatures, i.e., 273 and 298 K (Figure 3). BUGC exhibited an exceptional CO2 adsorption capacity of 7.81 mmol/g at 273 K and relative pressure of 1 bar (Figure 3a and Table 1). Under similar conditions, 5.56, 4.00, 2.63, and 0.55 mmol/g of CO2 were adsorbed by IMC, MMC, RG, and NC, respectively (Figure 3a and Table 1). CO2 adsorption measurements at 298 K and 1 bar relative pressure revealed a decrement in adsorption capacity in all materials (Figure 3b and Table 1). However, the order of uptake was similar, having the highest CO2 uptake of 4.49 mmol/g for BUGC followed by IMC (2.90 mmol/g), MMC (2.81 mmol/g), RG (1.56 mmol/g), and NC (0.39 mmol/g). Uptake capacity at room temperature (298 K) was comparatively lower due to the high kinetic energy of CO2 molecules at 298 K compared to 273 K. At higher temperatures, the adsorption energy decreases, which leads to reduced CO2 adsorption.30 At this point, it is essential to consider the industrial applicability wherein CO2 uptake at a lower pressure (0.15 bar, post-combustion flue gas streams from power plants) needs to be as high as possible (>2 mmol/g at 0.15 bar).8 A high CO2 uptake of 1.98 mmol/g was obtained for BUGC at 273 K, which was close to the industrial requirement (Table 1). Interestingly, although the adsorption capacity of RG was comparatively low at a high pressure (2.56 mmol/g at 1 bar pressure), it still possessed a relatively high adsorption capacity of 1.12 mmol/g at a low pressure of 0.15 bar (Table 1).
Figure 3.
CO2 adsorption isotherms at (a) 273 and (b) 298 K for different materials.
Figure 4 presents the comparison of CO2 and N2 adsorption isotherms for different materials. To understand the adsorption mechanisms, all isotherms were fitted using either Langmuir single-site (eq S1, SI) or Langmuir-dual site adsorption models (eq S2, SI), and fitting parameters are provided in Table S4.31 Except for RG (at 273 K), other isotherms were well fitted with a single-site model describing mostly monolayer adsorption. RG showed the highest accuracy with the Langmuir dual-site model describing two types of favorable sites for adsorption. The mechanism described for RG by the dual-site model presumably reflects the edges/defects as the first adsorption site and pores as second adsorption site.32 The decrement in “k” (Langmuir parameter) value with temperature signifies physical adsorption behavior between CO2 and materials.33 Fitted isotherms were used to calculate the selectivity using ideal adsorbed solution theory (IAST).34 IAST provides ease of determining the selectivity in a gaseous mixture from equilibrium adsorption capacity obtained from the adsorption isotherm of the pure component at a particular pressure and temperature.34 The flue gas consists of more than 70% of N2 in addition to other gases such as water or oxygen gas and only ∼5–15% of CO2. Therefore, the obtained molar loadings from fitted adsorption isotherms (0.15 bar for CO2 and 0.85 bar for N2) of pure component isotherms were used to calculate CO2 vs N2 selectivity. IAST CO2 vs N2 selectivity for BUGC, IMC, and MMC were 15, 11, and 13, respectively (Figure 4d). Fascinatingly, RG displayed the highest selectivity of 57 (Figure 4a,d), which can be attributed to the extent of oxygen functionalities present and a detailed analysis has been provided in next few sections (vide infra).
Figure 4.
CO2 vs N2 adsorption isotherms at 273 K for (a) RG, (b) IMC, (c) BUGC, and (d) MMC. (e) CO2 vs N2 selectivity histogram for all materials.
Scientific Understanding of CO2 Uptake
In order to understand the science behind the high CO2 uptake of BUGC and high selectivity for RG, various materials’ characteristics are analyzed with respect to CO2 uptake (Figure 5). First, the porous characteristics of the materials are compared with uptake capacity in Figure 5a and Figure S8. The ultramicropore (<0.7 nm) volume showed an excellent correlation with CO2 capture having a coefficient of determination (R2) value of 0.99 (Figure 5a). Ultramicropores offer a large adsorption potential due to adjacent pore walls (pore wall–wall interactions). The overlapping of these adsorption potentials generates van der Waals forces to attract CO2 molecules.7,35 Next, the R2 value of 0.73 was obtained upon analyzing the CO2 adsorption capacity with micropore volume (0.7–2.0 nm) (Figure S7a, SI). However, the BET surface area and total pore volume of the materials had very poor correlations with the adsorption capacity and showed R2 values of 0.45 and 0.01, respectively (Figure S7b,c, SI). Hence, these results firmly establish that the narrow micropores/ultramicropores (0.3–0.7 nm) are one of the major driving factors for the high CO2 adsorption capacity of BUGC, which agrees well with the previous reports.20,36,37
Figure 5.
Correlation plots of different materials with CO2 uptake at 273 K and 1 bar with (a) ultramicropore volume, (b) IG/ID ratio from Raman spectra, and (c) % C=C from C 1s XPS spectra.
The high adsorption capacity of BUGC compared to other materials also indicated the importance of unique graphitic regions. Therefore, to correlate sp2 character, Raman spectroscopy inferences were utilized. In this context, the inverse of the defect ratio (IG/ID) calculated from Raman results correlated precisely with the CO2 uptake capacity (R2 = 0.99) (Figure 5b). The IG/ID ratio is directly proportional to the G-band area, which signifies a higher sp2 carbon content for a higher IG/ID ratio. Thus, this analysis suggests that the higher sp2 hybridized carbon content helps to improve CO2 adsorption. To further verify this correlation, the percentage composition of various C 1s species derived from XPS spectra was also evaluated with CO2 uptake at 1 bar (Figure 5c and Figure S8, SI). Fascinatingly, the percentage of sp2 carbon (C=C) correlated perfectly with the CO2 adsorption capacity (R2 = 0.99) (Figure 5c). A glimpse of this correlation was provided by Merchán and co-workers in their recent report, wherein they suggested that this structural property can improve the micropore volume and thus CO2 uptake.23 Carbon in sp2 hybridization states is considered to adsorb CO2 through π–π interactions since sp2 bonds (C=C) are polarizable.38 Therefore, it can be concluded that the BUGC’s short- and long-range graphitic ordering, which is a polarizable π-electron system, has the potential to interact with CO2 molecules effectively through dispersion forces. Additionally, this unique ordering created enormous ultramicropores (vide supra) in BUGC and thus high CO2 uptake capacity was obtained for this material. Furthermore, oxygen-containing carbons such as hydroxyl/ester (C–O) and ketonic (C=O) functional groups had relatively poor correlations with R2 values of 0.81 and 0.84, respectively (Figure S8b,c, SI).
The abovementioned correlations of uptake with respect to the percentage of hydroxyl/ketonic-type functionalities (C–O/C=O) indicate that the presence of oxygen functionalities also affects the adsorption of CO2 molecules. Therefore, in order to confirm the role of these functional groups with CO2 uptake, FTIR experiments were carried out for BUGC (high adsorption capacity) and RG (high selectivity) after purging the materials with CO2. Materials were degassed and purged with CO2 continuously for 12 h, and thereafter, ex situ FTIR spectra were recorded and compared with those before purging (Figure 6 and Figure S9 and Table S5, SI). All the peaks for different functionalities were present after CO2 purging, which indicates the unchanged identity of carbon materials and suggested non-covalent physisorption interactions between CO2 and materials (BUGC and RG) (Figure 6a and Figure S9a, SI).39 However, shifts in peak positions for different functionalities were observed (Figure 6b and Figure S9b, SI). These shifts can be correlated as quadrupole–dipole interactions wherein CO2 acts as a quadrupole and dipoles are the ionic or polar sites of the adsorbent.13 Blue shifts in the wavenumber suggest the electron-donating nature of the functional groups of the materials, whereas red shifts highlight the electron-accepting nature.39 In the case of BUGC, the magnified spectra in Figure 6b suggest a blue shift in the wavenumber related to hydroxyl-type (C–O) (1030 cm–1) and ketone-type (C=O) (1695 cm–1) functionalities, suggesting the electron-donating nature of these oxygen-containing groups in the quadrupole–dipole interactions with CO2 molecules. The peak located at 1564 cm–1 for the graphitic (C=C) bond was also blue-shifted (Figure 6b), which highlights the participation of sp2 hybridized carbon in the CO2 adsorption mechanism in accordance with Raman and XPS correlations (vide supra). A COO– band situated at 2328 cm–1 was red-shifted to 2315 cm–1, indicating the electron-accepting nature of COO– functionalities (Figure 6b). Another shift of this band to a high wavenumber (2342 cm–1, blue shift) was also observed, which corresponds to the electron-donating nature of the functional group (Figure 6b), and this shift symbolizes the interactions with the hydrogen atoms present at the edges of the carbon matrix. Apparently, a red shift in peak corresponding to the C–H bond (2988 cm–1) was observed, indicating the electron-accepting nature of this bond (Figure 6b). Notably, similar shifts were observed in the case of RG, which are displayed in Figure S9a,b. However, the wavenumber shift was higher for RG compared to BUGC (Table S5, SI), which can be directly correlated to the relatively higher oxygen content in RG. Since the oxygen functionalities are electronegative in nature, they act as basic adsorption sites, which results in the attraction of CO2 molecules toward themselves.35,40 Thus, it can be anticipated that the interactions between oxygen functionalities of RG and CO2 molecules could be the major driving force for the high selectivity in RG. BUGC had a lower selectivity compared to RG due to a smaller percentage of oxygen (7.70% in BUGC vs 16.03% in RG, Table S1, SI), which is presumably a requirement for a higher selectivity. At this point, it can be concluded that the high CO2 adsorption for BUGC was due to the ultramicroporous nature, unique sp2 carbon-rich morphology, and presence of oxygen-containing functional groups. In IMC and MMC, a low ultramicroporous volume and undefined morphology lead to comparatively lower CO2 uptakes. Interestingly, in the case of RG, the absence of ultramicropores limits the uptake capacity of the material; nevertheless, a greater amount of oxygen content was responsible for exceptional CO2 vs N2 selectivity.
Figure 6.
FTIR spectral analysis of BUGC before and after purging CO2 for 12 h: (a) full range spectra and (b) magnified regions of spectra. Respective spectral shifts are color matched for easy visibility.
Thermodynamics and Recyclability of CO2 Capture
In order to understand the thermodynamics of the adsorption, isosteric heat of adsorption (QST) was determined using the Clausius–Clapeyron equation at 273 and 298 K (Figure 7a).41QST reflects the interaction of adsorbate molecules with the adsorbent material. For all materials, QST values ranged between 18 and 35 kJ/mol, indicating a predominant physisorption phenomenon (<40 kJ/mol), in accordance with the above fitted “k” (Langmuir parameter) values (vide supra). The smallest QST was in the range of 18–23 kJ/mol for IMC and MMC, which signifies their relatively weaker interactions with CO2 molecules (Figure 7a). RG has shown high values of heat of adsorption, indicating strong interactions with CO2 because of high concentrations of oxygen functional groups, as stated earlier, which validates the high selectivity (Figure 7a). An optimum QST in BUGC (30 kJ/mol) suggests a lower regeneration cost and low-energy desorption (Figure 7a).42 An ease of regeneration in BUGC was further verified by pressure swing regeneration experiments, wherein excellent cyclability after five consecutive cycles of CO2 uptake was observed (Figure 7b). At this juncture, it is important to highlight that the BUGC had shown an excellent performance as a CO2 adsorbent, and the adsorption capacity was significantly higher than that of recently reported carbon-based adsorbents (Table 2).1,30,43−51 Most importantly, the studied correlations of materials’ properties with CO2 capture presented in this work will work as a strong foundation for the scientific community, which will help to further improve CO2 capture by carbon-based materials.
Figure 7.

(a) Heat of adsorption and (b) pressure swing regeneration experiment of BUGC for five cycles of CO2 uptake at 273 K and 1 bar.
Table 2. Literature Comparison of the Latest Carbonaceous Materials Utilized for CO2 Adsorption with BUGC.
| nanomaterial | temperature (K)/pressure (bar) | CO2 uptake (mmol/g) | references |
|---|---|---|---|
| casein-derived porous carbon-2 | 273/1 | 5.30 | (1) |
| chemically activated carbon-S | 273/1 | 5.50 | (34) |
| 298/1 | 4.28 | ||
| oxygen-functionalized mesoporous carbon | 273/1 | 4.16 | (35) |
| 298/1 | 2.32 | ||
| asphaltene-derived activated carbon | 298/4 | 7.56 | (36) |
| amine-functionalized mesoporous silica @ RG | 273/1 | 3.64 | (37) |
| 298/1 | 2.33 | ||
| corn husk oat hull kraft pulp | 298/1 | 0.90 | (38) |
| 298/1 | 1.27 | ||
| 298/1 | 2.11 | ||
| soya-derived doped carbon (900,1000) | 298/1 | 2.7 | (39) |
| 298/1 | 3.2 | ||
| hierarchical porous N-doped carbon | 298/1 | 3.4 | (40) |
| nitrogen-doped activated porous carbon | 273/1 | 5.6 | (41) |
| 298/1 | 4.3 | ||
| N-doped porous CNF (NiO/PCNF) | 273/1 | 2.46 | (42) |
| 298/1 | 1.78 | ||
| N-containing pitch-based activated carbons | 273/1 | 4.93 | (43) |
| 298/1 | 2.57 | ||
| biordered ultramicroporous graphitic carbon (BUGC) | 273/1 | 7.81 | This work |
| 298/1 | 4.46 |
Conclusions
In conclusion, five cost-effective carbon nanomaterials were selected for CO2 capture. Among them, the BUGC nanomaterial prepared from simple KOH activation of two carbon resources (mechanically infused NC and RG) has shown the highest CO2 uptake capacity of 7.81 mmol/g at 273 K and 1 bar pressure. This study revealed that a high ultramicroporous volume helps to improve CO2 capture since the adjacent pore walls of ultramicropores develop a high adsorption potential to interact with CO2 molecules. Next, the higher sp2 carbon content also promotes CO2 adsorption as the polarizable π-electron cloud interacts with CO2 molecules through dispersion forces. Thus, BUGC having high ultramicroporous volume, biordered morphology, and graphitic characteristics had a very high CO2 adsorption capacity. In addition, BUGC revealed a high CO2 vs N2 selectivity of 15, low regeneration energy, and 100% CO2 uptake retention for five consecutive pressure swing cycles. On the other hand, RG has shown a remarkable CO2 vs N2 selectivity of 57 due to the high percentage of different oxygen functionalities present in the nanomaterial, which act as basic adsorption sites due to their electronegative nature and attract CO2 molecules toward themselves, employing a higher quadruple–dipole interaction. Finally, it is important to highlight that the fundamental science developed herein will create a strong foundation for the further development of new materials to improve CO2 uptake in the near future.
Acknowledgments
A.P. acknowledges financial support from DST-SERB New Delhi, (EMR/2016/005999, CRG/2020/002493), infrastructural support from IISER Bhopal, and the FIST supported TEM facility to the Dept. of Chemistry, IISER Bhopal. P.M. acknowledges IISER Bhopal for PhD fellowship. We sincerely thank Dr. Surajit Saha, Department of Physics, IISER Bhopal for Raman spectroscopy experiments and analysis. We thank Dr. Ravi Shankar Singh, Department of Physics, IISER Bhopal for assistance in XPS studies.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c04269.
SEM, TEM, FTIR, XPS, Raman, N2 adsorption, correlation plots, experimental details, and additional results (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Singh G.; Bahadur R.; Ruban A. M.; Davidraj J. M.; Su D.; Vinu A. Synthesis of functionalized nanoporous biocarbons with high surface area for CO2 capture and supercapacitor applications. Green Chem. 2021, 23, 5571–5583. 10.1039/D1GC01376A. [DOI] [Google Scholar]
- Singh G.; Lakhi K. S.; Ramadass K.; Sathish C. I.; Vinu A. High-Performance Biomass-Derived Activated Porous Biocarbons for Combined Pre- and Post-Combustion CO2 Capture. ACS Sustainable Chem. Eng. 2019, 7, 7412–7420. 10.1021/acssuschemeng.9b00921. [DOI] [Google Scholar]
- Singh G.; Lee J.; Karakoti A.; Bahadur R.; Yi J.; Zhao D.; AlBahily K.; Vinu A. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. 10.1039/D0CS00075B. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Wu L.; Bu Z.; Jie S.; Li B.-G. Polyethylenimine-Grafted HKUST-Type MOF/PolyHIPE Porous Composites (PEI@PGD-H) as Highly Efficient CO2 Adsorbents. Ind. Eng. Chem. Res. 2019, 58, 4257–4266. 10.1021/acs.iecr.9b00213. [DOI] [Google Scholar]
- Liang W.; Liu Z.; Peng J.; Zhou X.; Wang X.; Li Z. Enhanced CO2 Adsorption and CO2/N2/CH4 Selectivity of Novel Carbon Composites CPDA@A-Cs. Energy Fuels 2019, 33, 493–502. 10.1021/acs.energyfuels.8b03637. [DOI] [Google Scholar]
- Kupgan G.; Abbott L. J.; Hart K. E.; Colina C. M. Modeling Amorphous Microporous Polymers for CO2 Capture and Separations. Chem. Rev. 2018, 118, 5488–5538. 10.1021/acs.chemrev.7b00691. [DOI] [PubMed] [Google Scholar]
- Oschatz M.; Antonietti M. A search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2018, 11, 57–70. 10.1039/C7EE02110K. [DOI] [Google Scholar]
- Rodríguez-García S.; Santiago R.; López-Díaz D.; Merchán M. D.; Velázquez M. M.; Fierro J. L. G.; Palomar J. Role of the Structure of Graphene Oxide Sheets on the CO2 Adsorption Properties of Nanocomposites Based on Graphene Oxide and Polyaniline or Fe3O4-Nanoparticles. ACS Sustainable Chem. Eng. 2019, 7, 12464–12473. [Google Scholar]
- Zhao Y.; Liu X.; Han Y. Microporous carbonaceous adsorbents for CO2 separation via selective adsorption. RSC Adv. 2015, 5, 30310–30330. 10.1039/C5RA00569H. [DOI] [Google Scholar]
- Choi S. W.; Tang J.; Pol V. G.; Lee K. B. Pollen-derived porous carbon by KOH activation: Effect of physicochemical structure on CO2 adsorption. J. CO2 Util. 2019, 29, 146–155. 10.1016/j.jcou.2018.12.005. [DOI] [Google Scholar]
- Creamer A. E.; Gao B. Carbon-Based Adsorbents for Postcombustion CO2 Capture: A Critical Review. Environ. Sci. Technol. 2016, 50, 7276–7289. 10.1021/acs.est.6b00627. [DOI] [PubMed] [Google Scholar]
- Pang R.; Lu T.; Shao J.; Wang L.; Wu X.; Qian X.; Hu X. Highly Efficient Nitrogen-Doped Porous Carbonaceous CO2 Adsorbents Derived from Biomass. Energy Fuels 2021, 35, 1620–1628. 10.1021/acs.energyfuels.0c03832. [DOI] [Google Scholar]
- Raganati F.; Miccio F.; Ammendola P. Adsorption of Carbon Dioxide for Post-combustion Capture: A Review. Energy Fuels 2021, 35, 12845–12868. 10.1021/acs.energyfuels.1c01618. [DOI] [Google Scholar]
- Wang Y.; Wang H.; Zhang T. C.; Yuan S.; Liang B. N-doped porous carbon derived from rGO-Incorporated polyphenylenediamine composites for CO2 adsorption and supercapacitors. J. Power Sources 2020, 472, 228610. 10.1016/j.jpowsour.2020.228610. [DOI] [Google Scholar]
- Ouyang L.; Xiao J.; Jiang H.; Yuan S. Nitrogen-Doped Porous Carbon Materials Derived from Graphene Oxide/Melamine Resin Composites for CO2 Adsorption. Molecules 2021, 26, 5293. 10.3390/molecules26175293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian R.; Chowdhury S. Recent advances and progress in the development of graphene-based adsorbents for CO2 capture. J. Mater. Chem. A 2015, 3, 21968–21989. 10.1039/C5TA04822B. [DOI] [Google Scholar]
- Shen Z.; Liu C.; Yin C.; Kang S.; Liu Y.; Ge Z.; Xia Q.; Wang Y.; Li X. Facile large-scale synthesis of macroscopic 3D porous graphene-like carbon nanosheets architecture for efficient CO2 adsorption. Carbon 2019, 145, 751–756. 10.1016/j.carbon.2019.01.093. [DOI] [Google Scholar]
- Gong J.; Lin H.; Antonietti M.; Yuan J. Nitrogen-doped porous carbon nanosheets derived from poly(ionic liquid)s: hierarchical pore structures for efficient CO2 capture and dye removal. J. Mater. Chem. A 2016, 4, 7313–7321. 10.1039/C6TA01945E. [DOI] [Google Scholar]
- Hao G.-P.; Jin Z.-Y.; Sun Q.; Zhang X.-Q.; Zhang J.-T.; Lu A.-H. Porous carbon nanosheets with precisely tunable thickness and selective CO2 adsorption properties. Energy Environ. Sci. 2013, 6, 3740–3747. 10.1039/c3ee41906a. [DOI] [Google Scholar]
- Zhang Z.; Cano Z. P.; Luo D.; Dou H.; Yu A.; Chen Z. Rational design of tailored porous carbon-based materials for CO2 capture. J. Mater. Chem. A 2019, 7, 20985–21003. 10.1039/C9TA07297G. [DOI] [Google Scholar]
- Shi W.; Zhang Q.; Liu S.; Su S.; Chang B.; Yang B. Copper ions-assisted inorganic dynamic porogen of graphene-like multiscale microporous carbon nanosheets for effective carbon dioxide capture. J. Colloid Interface Sci. 2021, 600, 670–680. 10.1016/j.jcis.2021.04.146. [DOI] [PubMed] [Google Scholar]
- Shen Z.; Song Y.; Yin C.; Luo X.; Wang Y.; Li X. Construction of hierarchically porous 3D graphene-like carbon material by B, N co-doping for enhanced CO2 capture. Microporous Mesoporous Mater. 2021, 322, 111158. 10.1016/j.micromeso.2021.111158. [DOI] [Google Scholar]
- Ye Y.; Vega Martín L.; Sánchez Montero M. J.; López-Díaz D.; Velázquez M. M.; Merchán M. D. Optimizing the Properties of Hybrids Based on Graphene Oxide for Carbon Dioxide Capture. Ind. Eng. Chem. Res. 2022, 61, 1332–1343. 10.1021/acs.iecr.1c02922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra P.; Singh C.; Cherian I.; Giri A.; Paul A. Deciphering the Incredible Supercapacitor Performance of Conducting Biordered Ultramicroporous Graphitic Carbon. ACS Appl. Energy Mater. 2021, 4, 4416–4427. 10.1021/acsaem.1c00020. [DOI] [Google Scholar]
- Singh C.; Mishra A. K.; Paul A. Highly conducting reduced graphene synthesis via low temperature chemically assisted exfoliation and energy storage application. J. Mater. Chem. A 2015, 3, 18557–18563. 10.1039/C5TA04655F. [DOI] [Google Scholar]
- Singh C.; Paul A. Immense Microporous Carbon@Hydroquinone Metamorphosed from Nonporous Carbon As a Supercapacitor with Remarkable Energy Density and Cyclic Stability. ACS Sustainable Chem. Eng. 2018, 6, 11367–11379. 10.1021/acssuschemeng.8b01239. [DOI] [Google Scholar]
- Mehra P.; Paul A. Covalently Functionalized Hydroxyl-Rich Few-Layer Graphene for Solid-State Proton Conduction and Supercapacitor Applications. J. Phys. Chem. C 2022, 126, 6135–6146. 10.1021/acs.jpcc.1c10829. [DOI] [Google Scholar]
- Mehra P.; Wilson M.; Paul A. Acid-Base Synergism in Nitrogen- and Oxygen-Functionalized Few-Layer Graphene for Low-Activation Barrier Solid-State Proton Conduction. J. Phys. Chem. C 2022, 126, 10534–10545. 10.1021/acs.jpcc.2c02377. [DOI] [Google Scholar]
- Barua A.; Paul A. Synergistic Effect of Oxygen and Nitrogen Co-doping in Metal–Organic Framework-Derived Ultramicroporous Carbon for an Exceptionally Stable Solid-State Supercapacitor via a “Proton Trap” Mechanism. Energy Fuels 2021, 35, 10262–10273. 10.1021/acs.energyfuels.1c00918. [DOI] [Google Scholar]
- Singh G.; Lakhi K. S.; Sathish C. I.; Ramadass K.; Yang J.-H.; Vinu A. Oxygen-Functionalized Mesoporous Activated Carbons Derived from Casein and Their Superior CO2 Adsorption Capacity at Both Low- and High-Pressure Regimes. ACS Appl. Nano Mater. 2019, 2, 1604–1613. 10.1021/acsanm.9b00059. [DOI] [Google Scholar]
- Al-Ghouti M. A.; Da’ana D. A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. 10.1016/j.jhazmat.2020.122383. [DOI] [PubMed] [Google Scholar]
- Nováček M.; Jankovský O.; Luxa J.; Sedmidubský D.; Pumera M.; Fila V.; Lhotka M.; Klímová K.; Matějková S.; Sofer Z. Tuning of graphene oxide composition by multiple oxidations for carbon dioxide storage and capture of toxic metals. J. Mater. Chem. A 2017, 5, 2739–2748. 10.1039/C6TA03631G. [DOI] [Google Scholar]
- Wang Y.; Hu X.; Hao J.; Ma R.; Guo Q.; Gao H.; Bai H. Nitrogen and Oxygen Codoped Porous Carbon with Superior CO2 Adsorption Performance: A Combined Experimental and DFT Calculation Study. Ind. Eng. Chem. Res. 2019, 58, 13390–13400. 10.1021/acs.iecr.9b01454. [DOI] [Google Scholar]
- Myers A. L.; Prausnitz J. M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. 10.1002/aic.690110125. [DOI] [Google Scholar]
- Liu Y.; Wilcox J. Effects of Surface Heterogeneity on the Adsorption of CO2 in Microporous Carbons. Environ. Sci. Technol. 2012, 46, 1940–1947. 10.1021/es204071g. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Zhou J.; Xing W.; Xue Q.; Yan Z.; Zhuo S.; Qiao S. Z. Critical role of small micropores in high CO2 uptake. Phys. Chem. Chem. Phys. 2013, 15, 2523–2529. 10.1039/c2cp44436d. [DOI] [PubMed] [Google Scholar]
- Presser V.; McDonough J.; Yeon S.-H.; Gogotsi Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ. Sci. 2011, 4, 3059–3066. 10.1039/c1ee01176f. [DOI] [Google Scholar]
- Molavi H.; Eskandari A.; Shojaei A.; Mousavi S. A. Enhancing CO2/N2 adsorption selectivity via post-synthetic modification of NH2-UiO-66(Zr). Microporous Mesoporous Mater. 2018, 257, 193–201. 10.1016/j.micromeso.2017.08.043. [DOI] [Google Scholar]
- Lee J. H.; Lee H. J.; Choi J. W. Unveiling anomalous CO2-to-N2 selectivity of graphene oxide. Phys. Chem. Chem. Phys. 2017, 19, 22743–22748. 10.1039/C7CP04318J. [DOI] [PubMed] [Google Scholar]
- Casco M. E.; Morelos-Gómez A.; Vega-Díaz S. M.; Cruz-Silva R.; Tristán-López F.; Muramatsu H.; Hayashi T.; Martínez-Escandell M.; Terrones M.; Endo M.; Rodríguez-Reinoso F.; Silvestre-Albero J. CO2 adsorption on crystalline graphitic nanostructures. J. CO2 Util. 2014, 5, 60–65. 10.1016/j.jcou.2014.01.001. [DOI] [Google Scholar]
- Anas M.; Gönel A. G.; Bozbag S. E.; Erkey C. Thermodynamics of Adsorption of Carbon Dioxide on Various Aerogels. J. CO2 Util. 2017, 21, 82–88. 10.1016/j.jcou.2017.06.008. [DOI] [Google Scholar]
- Chen J.; Yang J.; Hu G.; Hu X.; Li Z.; Shen S.; Radosz M.; Fan M. Enhanced CO2 Capture Capacity of Nitrogen-Doped Biomass-Derived Porous Carbons. ACS Sustainable Chem. Eng. 2016, 4, 1439–1445. 10.1021/acssuschemeng.5b01425. [DOI] [Google Scholar]
- Guo Y.; Tan C.; Sun J.; Li W.; Zhang J.; Zhao C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. 10.1016/j.cej.2019.122736. [DOI] [Google Scholar]
- Kueh B.; Kapsi M.; Veziri C. M.; Athanasekou C.; Pilatos G.; Reddy K. S. K.; Raj A.; Karanikolos G. N. Asphaltene-Derived Activated Carbon and Carbon Nanotube Membranes for CO2 Separation. Energy Fuels 2018, 32, 11718–11730. 10.1021/acs.energyfuels.8b02913. [DOI] [Google Scholar]
- Liu L.; Zou G.; Yang B.; Luo X.; Xu S. Amine-Functionalized Mesoporous Silica @ Reduced Graphene Sandwichlike Structure Composites for CO2 Adsorption. ACS Appl. Nano Mater. 2018, 1, 4695–4702. 10.1021/acsanm.8b00943. [DOI] [Google Scholar]
- Valdebenito F.; García R.; Cruces K.; Ciudad G.; Chinga-Carrasco G.; Habibi Y. CO2 Adsorption of Surface-Modified Cellulose Nanofibril Films Derived from Agricultural Wastes. ACS Sustainable Chem. Eng. 2018, 6, 12603–12612. 10.1021/acssuschemeng.8b00771. [DOI] [Google Scholar]
- Rana M.; Subramani K.; Sathish M.; Gautam U. K. Soya derived heteroatom doped carbon as a promising platform for oxygen reduction, supercapacitor and CO2 capture. Carbon 2017, 114, 679–689. 10.1016/j.carbon.2016.12.059. [DOI] [Google Scholar]
- Li J.; Tian L.; Liang F.; Wang J.; Han L.; Zhang J.; Ge S.; Dong L.; Zhang H.; Zhang S. Molten salt synthesis of hierarchical porous N-doped carbon submicrospheres for multifunctional applications: High performance supercapacitor, dye removal and CO2 capture. Carbon 2019, 141, 739–747. 10.1016/j.carbon.2018.09.061. [DOI] [Google Scholar]
- Wei H.; Chen H.; Fu N.; Chen J.; Lan G.; Qian W.; Liu Y.; Lin H.; Han S. Excellent electrochemical properties and large CO2 capture of nitrogen-doped activated porous carbon synthesised from waste longan shells. Electrochim. Acta 2017, 231, 403–411. 10.1016/j.electacta.2017.01.194. [DOI] [Google Scholar]
- Li Q.; Guo J.; Xu D.; Guo J.; Ou X.; Hu Y.; Qi H.; Yan F. Electrospun N-Doped Porous Carbon Nanofibers Incorporated with NiO Nanoparticles as Free-Standing Film Electrodes for High-Performance Supercapacitors and CO2 Capture. Small 2018, 14, 1704203. 10.1002/smll.201704203. [DOI] [PubMed] [Google Scholar]
- Lee M.-S.; Park M.; Kim H. Y.; Park S.-J. Effects of Microporosity and Surface Chemistry on Separation Performances of N-Containing Pitch-Based Activated Carbons for CO2/N2 Binary Mixture. Sci. Rep. 2016, 6, 23224. 10.1038/srep23224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.