Abstract
New 1,4-naphthoquinone derived by triphenylphosphaneylidene (Ph3P) and N-substituted-hydrazine-1-carbothioamides were obtained during a one-pot reaction of 2,3-dichloro-1,4-naphthoquinone with thiosemicarbazides, Ph3P and in the presence of triethyl amine (Et3N) as a catalyst. The structure of the ligands was established by ESI, IR, and NMR spectra, in addition to elemental analyses and X-ray structure analysis. On subjecting the newly prepared ligands with CuCl2 and Ph3P, autoxidation occurs, and (E)-(2-(1,4-dioxo-3-(triphenyl phosphanylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)carbamothioyl)hydrazinyl)-((triphenylphosphanyl)oxy)copper derivatives were formed in very good yields. The structure of the obtained complexes was proved by ESI, IR, NMR, and UV spectra, in addition to elemental analyses and theoretical calculations.
1. Introduction
Natural hydroxy derivatives of 1,4-naphthoquinone lawsone and juglone with hydroxyl groups in the α- and β-positions of the naphthalene core form salts and complexes with cations of various metals and are used as dyes.1 Protein binding, DNA binding/cleavage, and in vitro cytotoxicity studies of 2-((3-(dimethylamino)-propyl)amino)naphthalene-1,4-dione and its four coordinated M(II) complexes [M(II) = Co(II), Cu(II), Ni(II), and Zn(II)] have been investigated. The complexes demonstrated a comparable in vitro cytotoxic activity against two human cancer cell lines (MCF-7 and A-549) with cisplatin. AO/EB and DAPI staining studies suggest an apoptotic mode of cell death in these cancer cells, with the compounds under investigation.2 A series of nine RuII arene complexes bearing tridentate naphthoquinone-based N,O,O-ligands were synthesized and characterized. The cytotoxic profile exhibited much higher cytotoxicity in SW480 colon cancer cells than in the broad chemo- (incl. platinum-) sensitive CH1/PA-1 teratocarcinoma cells. This activity pattern, reduced or slightly enhanced ROS generation, and the lack of DNA interactions indicate a mode of action different from established or previously investigated classes of metallodrugs.3
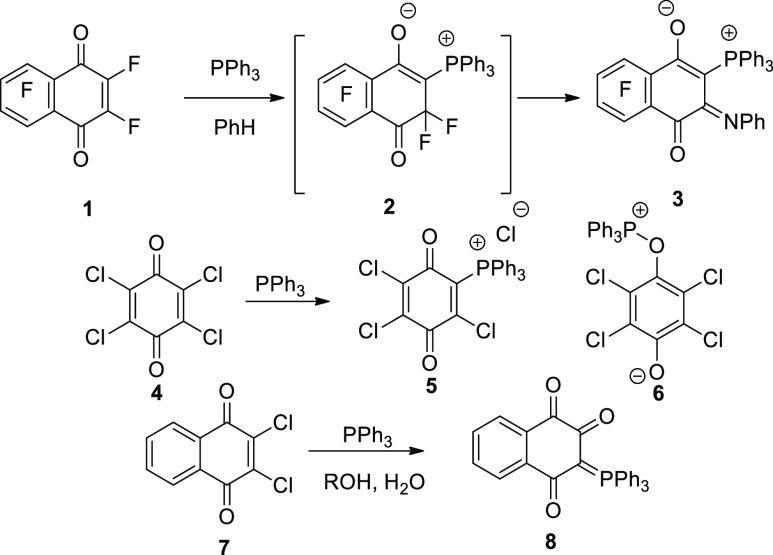
The reactions of 1,4-naphthoquinone with triphenylphosphine (Ph3P) have been previously described. As, for example, in the reaction of fluoronaphthoquinone (1) with triphenylphosphine (Ph3P), derivatives of phosphonium betaines derived from hexafluoro-1,4-naphthoquinone: (triphenyl[5,6,7,8-tetrafluoro-1-oxido-4-oxo-3-(phenylimino)-3,4-dihydro-naphthalen-2-yl]phosphonium) 3 (Scheme 1), were obtained.4 The reaction was explained as due to the formation of intermediate 2 (Scheme 1). Depending upon reaction conditions, it was reported that Ph3P reacted with p-chloranil (4) to produce either Zwitter salts 5 or 6(5) (Scheme 1). However, in aqueous alcoholic medium, 3-(triphenyl-phosphoranylidene)naphthalene-1,2,4(3H)-trione (8)6 was obtained from the reaction of 2,3-dichloro-1,4-naphthoquinone (7) with Ph3P (Scheme 1).
Scheme 1. Effect of Ph3P on Halogenated Naphthoquinones 1, 4, and 7.
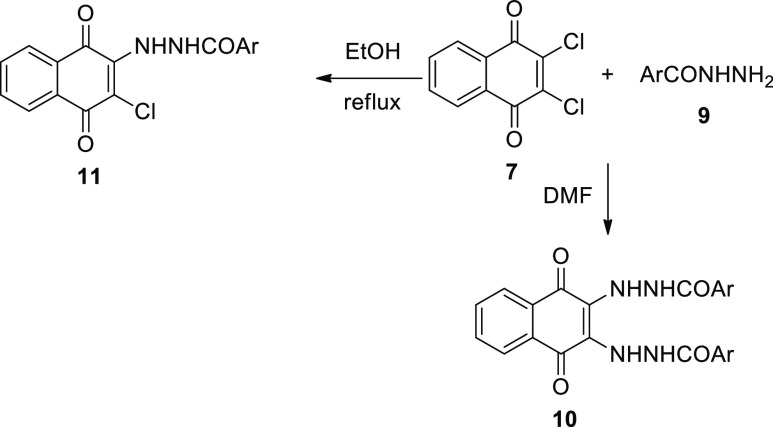
In the same manner, bisphosphonium salt containing a 1,4-dihydroxynaphthyl-substituted moiety was synthesized in high yield by the reaction of 2-methyl-1,4-naphthoquinone with three equivalents of Ph3P and hydrogen bromide.7 Metal-free synthesis of aryltriphenylphosphonium bromides by the reaction of Ph3P with aryl bromides in refluxing phenol was developed.8 With the high reactivity of naphthoquinones, 2,3-dichloro-1,4-naphthoquinone (DCHNQ, 7) reacted with nucleophiles and substituted one or both chlorine atoms.9−13 It was found that the reaction of 7 with p-nitrobenzhydrazide (9) in 1:2 or 2:l ratio in DMF gave only the disubstituted product, bis-(-p-nitrobenzhydrazino)-1,4-naphthoquinone (10). However, when an ethanolic solution of 7 and 9 was refluxed for 21 h, the main product was a bright red precipitate of 11(14) (Scheme 2).
Scheme 2. Nucleophilic Addition of Aroylhydrazide 9 to Compound 7.
On the basis that triphenylphosphine oxide (Ph3P=O) can form facile complexes with some metal salts, Hergueta et al.15 reported the facile removal by complexation with CaBr2, with Ph3P=O in ethereal solvents or toluene. The resulting insoluble precipitated complex was easily eliminated from crude reaction mixtures in high yields by filtration without the need for purification by column chromatography.15
Copper(I) and silver(I) chloride complexes containing PPh3 and 4-phenyl-thiosemicarbazide (4-PTSC) ligands were prepared and structurally analyzed, namely, [CuCl(4-PTSC) (PPh3)2] (12) and [AgCl(4-PTSC) (PPh3)2]CH3CN. Both compounds exhibit a distorted tetrahedral metal coordination environment with two P atoms from two PPh3 ligands, one terminal S atom from the 4-PTSC ligand, and a chloride ion (Figure 1).16
Figure 1.

Copper(I) chloride complex containing PPh3 and 4-phenylthiosemicarbazide (4-PTSC) ligand 12.
The reactions between [CuCl2(PPh3)2] and 3,3-diphenyl-1-(2,4-dichlorobenzoyl)thiourea, 3,3-diisobutyl-1-(2,4-dichlorobenzoyl)thiourea, or 3,3-diethyl-1-(2,4-dichlorobenzoyl)thiourea in benzene gave four-coordinated tetrahedral copper(I) complexes of the type [CuCl(HL) (PPh3)2] [HL = 3,3-dialkyl/aryl-1-(2,4-dichlorobenzoyl)thiourea derivatives].17
Previously, we reacted thiosemicarbazones derived from 2-quinolone with Cu(I), Cu(II), and Ni(II) salts.18 Monodentate Cu(I) quinoloyl-substituted ligands were observed, whereas Ni(II) and Cu(II) gave bidentate-thiosemicarbazone derived by 2-quinolones. Subsequently, molecular docking was used to evaluate each analog’s binding affinity and the inhibition constant (ki) to the RdRp complex of SARS-CoV-2.18 We also synthesized a series of paracyclophane-substituted thiosemicarbazones, thiocarbazones, hydrazones, and thioureas to study their complexation capability toward copper (I) and Cu (II) salts. Tridentate and bidentate of the aforesaid paracyclophane-substituted ligands were observed. Thiosemicarbazonyl, hydrazonyl, and thiourea paracyclophane derivatives formed with Cu(I) and Cu(II) salt tridentate and bidentate structures, whereas no complexes were observed for the prepared thiocarbazone derivatives.19
Copper complexes have numerous properties, including proteasome activity inhibitors,20 DNA intercalation,21 and anticancer chelators. They can also increase the activity of the ligand itself. For example, 4-cyclohexyl-3-(4-nitrophenyl)methyl-1,2,4-triazolin-5-thione has no activity against some selected bacteria, but after obtaining the metal(II) complex, the activity increases to a mild score.22
From this, we here aim to investigate the reaction of 2,3-dichloro-1,4-naphthoquinone (7) together with thiosemicarbazides and Ph3P to achieve the expected bi-nucleophilic substituted naphthoquinone. The newly obtained triphenylphosphine-ylidene-3,4-dihydronaphthalen-2(1H)-ylidene)-N-substituted-hydrazine-1-carbothioamides were then subjected to complexation toward CuCl2 and Ph3P. The stability of these complexes was discussed using a plethora of quantum mechanical calculations and by executing Hirshfeld surface (HS) analysis.
2. Results and Discussion
Thiosemicarbazides 14a-f were prepared by reacting substituted isothiocyanates 13a-f with hydrazine in ethanol as a solvent.23 Upon mixing equimolar amounts of 14a-f with 2,3-dichloro-1,4-naphthoquinone (7), Ph3P, and in the presence of Et3N as a catalyst and acetonitrile as a solvent, triphenylphosphine-ylidene(-3,4-dihydronaphthalen-2(1H)-ylidene)-N-substituted-hydrazine-1-carbothioamides 15a-f were obtained in 72–82% yield (Scheme 3).
Scheme 3. Synthesis of Ligands 15a-f.
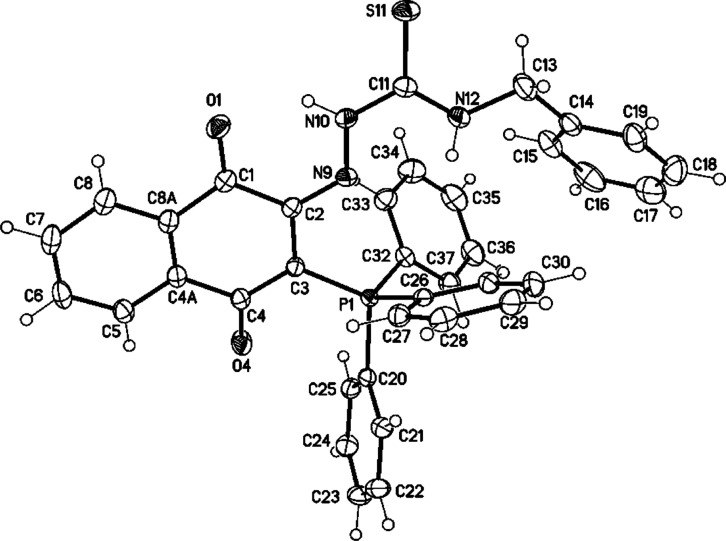
The mass spectrum of compound 15c revealed [M + H]+ at m/z = 598 (75), whereas the molecular ion peak at m/z = 597 (30). HRMS FAB mass confirmed the molecular formula of 15c C36H29N3O2P132S1, [M + H]+) calcd: 598.1718; found: 598.1717. The 1H NMR spectrum displayed the two NH protons at δH = 13.04 (s, 1H, NH1) and at 6.85 (s, 1H, NH2). The benzyl-CH2 resonated as a multiplet, in the 1H NMR spectrum, at δH = 4.38–4.20. The 13C NMR spectrum showed the carbonyl carbon signals at δC = 177.2 and 176.3, whereas the C=S and benzylic-CH2 carbon signals appeared at δC = 162.8 and 46.9 ppm, respectively. IR spectroscopy revealed the NH-stretching, C=P, and C=S groups at ν̃ = 3340–3055, 1153, and 988 cm–1, respectively. X-ray structure analysis confirmed the structure of 15c, as shown in Figure 2.
Figure 2.
Molecular structure of compound 15c (displacement parameters are drawn at 50% probability level). Selected bond distances [Å] and angles [°]: C2–N9 1.3088(16), N9–N10 1.3674(14), N10–C11 1.3546(17), C11–S11 1.6775(13), C11–N12 1.3297(17), N12–C13 1.4557(17); C2–N9 N10 119.45(10), N9–N10–C11 119.65(10), N10–C11–S11 119.43(10), N10–C11–N12116.90(11), C11–N12–C13 122.64(11).
Mass spectroscopy of 15f revealed [M + H]+at m/z = 564 (100), whereas the molecular ion peak appeared at m/z = 563 (30%). HRMS confirmed [M + H]+ of compound 15f as C33H31N3O2P132S1. The 1H NMR spectrum showed the two NH protons at δH = 13.18 and 6.74. In the 1H NMR spectrum, the butyl protons resonated as a double-doublet (J = 7.7, 1.5 Hz, 2H) and three multiplets at δH = 3.58, 1.60–1.49, 1.35–1.26, and 1.02–0.99 ppm, respectively. The 13C NMR spectrum showed the carbonyl carbon signals at δC = 186.1 and 185.9, whereas C=S resonated at 178.4 ppm. The butyl carbon signals resonated in the 13C NMR spectrum at δC = 44.6, 30.4, 20.1, and 14.0 ppm. IR spectroscopy displayed the NH-stretching at ν̃ = 3340–3053 (w, ΝΗ), whereas the two carbonyl groups and C=N appeared at ν̃ = 1586, 1521, and 1434, respectively. Besides, the C=P and the C=S groups appeared as strong bands in the IR spectra at ν̃ = 1170 and 992 cm–1, respectively. The structure of 15f was confirmed by X-ray structural analysis, as shown in Figure 3.
Figure 3.
Molecular structure of one crystallographic independent molecule (with n-butyl substituent) of compound 15f (displacement parameters are drawn at a 30% probability level). Selected bond distances [Å] and angles [°]: C102–N129 1.303(3), N129–N130 1.363(3), N130–C131 1.364(4), C131–S131 1.671(3), C131–N132 1.337(5), N132–C133 1.457(4); C102–N129 N130 120.4(2), N129–N130–C131 119.4(3), N130–C131–S131 119.3(3), N130–C131–N132 115.3(3), C131–N132–C133 126.8(3).
In the crystal structures of 15c and 15f, are the bond distances and angles in the expected range with a slight delocalization of the π-electron in the thiosemicarbazide moiety (see cif-file). The thiosemicarbazide and 1,4-naphthoquinone moieties are coplanar.
The mechanism describes that the formation of 15a-f is based upon the addition of Ph3P to C-1 (or C-2) in 7 to give the Zwitterion 17, which would be in resonance with the intermediate 18 (Scheme 4). The addition of 14a-f in the presence of Et3N would then accompany the elimination of a molecule of HCl and another of Ph3P to give 19a-f (Scheme 4). Subsequently, the extruded Ph3P would again add to halogenated intermediates 19a-f to produce salts 20a-f (Scheme 4). Finally, a molecule of Et3N would facilitate the formation of 20a-fvia the elimination of a molecule of triethylammonium hydrochloride.
Scheme 4. Mechanism Describes the Formation of Ligands 15a-f.
Interestingly, the reaction of equal equivalents of compounds 15a-f with Ph3P and CuCl2·2H2O in ethanol at rt and for 1–3 d produced the Cu-complexes 16a-f (Scheme 5). Based on IR, 1H NMR, and mass spectra, together with elemental analysis, the NH-3 proton of the thiosemicarbazone moiety, in 15a-f, was eliminated, and aerial oxidation occurred, as shown in Scheme 5.
Scheme 5. Mechanism Describes the Formation of Cu-Complexes 16a-f.
Reagents and conditions: 15a-f (1.00 equiv), CuCl2·2H2O (1.00 equiv) and Ph3P (1.00 equiv) in absolute EtOH (40 mL), room temperature (2–4 h). Column chromatography (CC) using EtOAc/hexane (5:1).
2.1. Assignment of the Ligands 15a-f and Their Cu-Complexes 16a-f by Mass Spectroscopy and Elemental Analyses
The molecular formulae of the obtained complexes were proved according to the mass spectroscopic data and the elemental analyses. Physical data from the color, m.p., and yield in g (%) were illustrated, as shown in Table 1.
Table 1. Physical Data and Yield in g (%) of Complexes 16a-f.
| no | complex | color | mp (°C) | yield g (%) |
|---|---|---|---|---|
| 1 | 16a | deep blue | 250–252 (decomp) | 0.718 (82) |
| 2 | 16b | deep blue | 244–246 (decomp) | 0.745 (84) |
| 3 | -16c | deep blue | 262–264 (decomp) | 0.872 (93) |
| 4 | -16d | deep blue | 268–270 (decomp) | 0.712 (80) |
| 5 | -16e | deep blue | 249–251 (decomp) | 0.763 (86) |
| 6 | 16f | deep blue | 257–259 (decomp) | 0.795 (90) |
Elemental analyses and mass spectra indicated that the formed Cu(II)-complexes 16a-f resulted in the sum of the molecular weight of compounds 15a-f with one copper atom together with a Ph3PO molecule. Elemental analyses of compounds 16a-f are shown in Table 2.
Table 2. Stoichiometric Formation and Analytical Data of Cu-Complexes 16a-f.
| ligand | metal salt | complex | stoichiometry | molecular formula | C, H, Cu, N, O, P, S, |
|---|---|---|---|---|---|
| 15a | CuCl2·2H2O | 16a | 1:1 | C49H40CuN3O3P2S | calcd: C, 67.15; H, 4.60; Cu, 7.25; N, 4.79; O, 5.48; P, 7.07; S, 3.66 |
| found: C, 67.17; H, 4.62; Cu, 7.23; N, 4.77; O, 5.50; P, 7.05; S, 3.64 | |||||
| 15b | CuCl2·2H2O | 16b | 1: 1 | C50H40CuN3O3P2S | calcd: C, 67.60; H, 4.54; Cu, 7.15; N, 4.73; O, 5.40; P, 6.97; S, 3.61 |
| found: C, 67.58; H, 4.56; Cu, 7.17; N, 4.71; O, 5.38; P, 6.95; S, 3.63 | |||||
| 15c | CuCl2·2H2O | 16c | 1:1 | C54H42CuN3O3P2S | calcd: C, 69.11; H, 4.51; Cu, 6.77; N, 4.48; O, 5.11; P, 6.60; S, 3.42 |
| found: C, 67.11; H, 4.53; Cu, 6.79; N, 4.50; O, 5.13; P, 6.58; S, 3.44 | |||||
| 15d | CuCl2·2H2O | 16d | 1:1 | C50H42CuN3O3P2S | calcd: C, 67.44; H, 4.75; Cu, 7.14; N, 4.72; O, 5.39; P, 6.96; S, 3.60 |
| found: C, 67.42; H, 4.77; Cu, 7.16; N, 4.70; O, 5.41; P, 6.98; S, 3.62 | |||||
| 15e | CuCl2·2H2O | 16e | 1:1 | C50H40CuN3O3P2S | calcd: C, 67.60; H, 4.54; Cu, 7.15; N, 4.73; O, 5.40; P, 6.97; S, 3.61 |
| found: C, 67.62; H, 4.52; Cu, 7.13; N, 4.75; O, 5.42; P, 6.99; S, 3.59 | |||||
| 15f | CuCl2·2H2O | 16f | 1:1 | C51H44CuN3O3P2S | calcd: C, 67.72; H, 4.90; Cu, 7.03; N, 4.65; O, 5.31; P, 6.85; S, 3.54 |
| found: C, 67.74; H, 4.92; Cu, 7.05; N, 4.63; O, 5.29; P, 6.85; S, 3.52 |
2.2. Assignment by IR Spectra
The significant bands in infrared spectra for both ligands 15a-f and their metal complexes 16a-f are represented in Table 3. The symmetrical stretching of NH bands for compounds 16a-f was absorbed in the region at their expected region of IR spectra. The shift in functional groups from the ligands to the corresponding complexes supported the chelating process. Coordination occurred via N-2 and the oxygen atom of Ph3P. The C=S stretching gave slight shifts in the case of a comparison between 15a-f and 16a-f. Most indicative is the new appearance of P=O, Cu–N, and Cu–O bands during the comparison between 15a-f and 16a-f. For example, IR spectroscopy of 15a showed the following bands at ν = 3345, 3052 for ΝΗ stretching, 1188 (as a strong band) for C=P, and 993 (as a strong band) cm–1 for s, C=S. In the case of 16a, the NH stretching was absorbed as a weak band at ν = 3054, whereas at ν = 1178, as a strong band for the C=P group. New bands were noted at ν = 1019 (m, P=O), 536 (s, Cu–N), and 457 cm–1 (s, Cu–O). Similar values of the previous three groups were previously reported.24,25 The same trend was also observed in all compounds 16b-f (Table 2).
Table 3. IR Absorption Bands (υ, cm–1) of Ligands 15a-f and Their Complexes of Cu(II) 16a-f.
| ligand | absorption of functional groups (ν) in ligands (cm–1) | metal complex | absorption of functional groups (ν) in complexes (cm–1) |
|---|---|---|---|
| 15a | ν = 3345, 3052 (w, ΝΗ), 1587, 1517 (m, C=O), 1435 (s, C=N), 1188 (s, C=P), 993 cm–1 (s, C=S). | 16a | ν = 3054 (w, ΝΗ), 1576, 1505 (m, C=O), 1482 (s, C=N), 1178 (s, C=P), 1019 (m, P=O), 995 (s, C=S), 536 (s, Cu–N), 457 cm–1 (s, Cu–O). |
| 15b | ν = 3347, 3067 (w, ΝΗ), 1585, 1542 (m, C=O), 1431 (s, C=N), 1169 (vs, C=P), 990 cm–1 (vs, C=S). | 16b | ν = 3055 (w, ΝΗ), 1572, 1503 (s, C=O), 1483 (s, C=N), 1187 (s, C=P), 1017 (vs, P=O), 1000 (vs, C=S), 557 (vs, Cu–N), 432 cm–1 (s, Cu–O). |
| 15c | ν = 3340, 3055 (w, ΝΗ), 1582, 1510 (s, C=O), 1433 (s, C=N), 1153 (s, C=P), 988 cm–1 (vs, C=S). | 16c | ν = 3077 (w, ΝΗ), 1577, 1514 (s, C=O), 1480 (s, C=N), 1157 (vs, C=P), 1017 (vs, P=O), 999 (s, C=S), 548 (vs, Cu–N), 453 cm–1 (vs, Cu–O). |
| 15d | ν = 3347, 3052 (w, ΝΗ), 1580, 1507 (w, C=O), 1436 (s, C=N), 1188 (vs, C=P), 996 cm–1 (s, C=S). | 16d | ν = 3051 (w, ΝΗ), 1589, 1513 (m, C=O), 1483 (s, C=N), 1163 (s, C=P), 1026 (w, P=O), 996 (m, C=S), 537 (vs, Cu–N), 449 cm–1 (s, Cu–O). |
| 15e | ν = 3342, 3050 (w, ΝΗ), 1580, 1511(m, C=O), 1432 (s, C=N), 1186 (s, C=P), 996 cm–1 (s, C=S). | 16e | ν = 3056 (w, ΝΗ), 1575, 1496 (m, C=O), 1483 (m, C=N), 1179 (vs, C=P), 1020 (s, P=O), 997 (s, C=S), 540 (vs, Cu–N), 460 cm–1 (s, Cu–O). |
| 15f | ν = 3340, 3053 (w, ΝΗ), 1586, 1521 (m, C=O), 1434 (vs, C=N), 1170 (s, C=P), 992 cm–1 (s, C=S). | 16f | ν = 3063 (w, ΝΗ), 1572, 1494 (m, C=O), 1466 (s, C=N), 1159 (vs, C=P), 1013 (vs, P=O), 1013 (vs, C=S), 547 (vs, Cu–N), 455 cm–1 (s, Cu–O). |
2.3. Assignment of the Complexes 16a-f by NMR Spectra
Together with the elemental analyses, mass, and IR spectra, the chemical shift in NMR spectra, indicating the complexation process of 15a-f with Cu(II) to form 16a-f, as shown in Table 4. As it is known that although most NMR measurements are conducted on diamagnetic compounds, paramagnetic samples are also amenable to analysis and give rise to special effects indicated by a wide chemical shift range and broadened signals.26,27 However, several papers reported the use of the Cu(II) complex with Ph3P of the composition CuCl2(PPh3)2.28−30 The previous studies showed that the oxidation state of II cannot be stabilized for copper in the presence of such a reducing ligand like Ph3P. Therefore, Cu(II) is converted into Cu(I) during complexation with Ph3P and compounds 15a-f. We here note that NMR spectra could be distinguished, and there are remarkable shifts in some values of the chemical shifts (δ) of 1H NMR and 13C NMR spectra of compound 15a, as an example, compared with its complex 16a. In the 1H NMR spectrum, NH-2 resonated at δH = 12.90, whereas NH-1 appeared at δH = 5.77. The ethyl protons appeared as a quartet at δH = 3.19 for CH2ethyl, whereas a triplet at δH = 0.62 ppm (J = 7.1 Hz). In the case of 16a (Table 4), the 1H NMR spectrum of only NH-1 at δH = 5.49, whereas N-2 didn’t reveal any proton. The ethyl protons appeared as a quartet at δH = 4.05 (J = 7.1 Hz) and a triplet at δH = 1.20 ppm (J = 7.1 Hz). The 13C NMR spectrum of 15a showed the carbonyl carbon signals at δC = 183.7 and 183.6, whereas C=S resonated at δC = 176.7 ppm. The ethyl carbons appeared at δC = 37.9 for CH2 and at δC = 13.5 ppm for CH3 (as shown in the Supporting Information). In the case of 16a, in the 13C NMR spectrum, the carbonyl carbon signals resonated at δC = 186.0 and 183.6 ppm. (Cq, CO), whereas the C=S carbon signal appeared at δC = 175.4 ppm. The ethyl carbon signals resonated at δC = 39.0 (CH2ethyl) and 13.6 ppm (CH3ethyl). Significantly, the exocyclic carbon signal (in C=N) appeared in the 13C NMR spectrum of 15a at δC = 142.8, whereas for 16a, it appeared at δC = 143.1.
Table 4. Chemical Shifts (δ), Including 1H And/or 13C NMR Spectroscopic Data for Ligand 15a and Its Complex 16a.
| ligand | 1H and 13C NMR (δ,acetone-d6) | metal complex | 1H and 13C NMR (δ, acetone-d6) |
|---|---|---|---|
| 15a | δH = 12.90 (s, 1H, NH1), 7.93 (dt, J = 44.4, 10.2 Hz, 2H, HAr), 7.84–7.68 (m, 2H, HAr), 7.65–7.31 (m, 15H, HAr), 5.77 (s, 1H, NH2) 3.19–2.56 (m, 2H, CH2ethyl), 0.62 ppm (t, J = 7.1 Hz, 3H, CH3ethyl). δC = 183.7 (Cq, CO), 183.6 (Cq, CO), 176.7 (Cq, CS), 142.8 (Cq, C=N), 137.2 (Cq, CAr), 137.1 (Cq, CAr), 134.5 (Cq, CAr), 134.1 (+, CHAr), 133.7 (+, CHAr), 133.6 (+, CHAr), 133.1 (+, CHAr), 132.8 (+, CHAr), 132.5 (+, CHAr), 132.4 (+, CHAr), 131.8 (+, 2× CHAr), 131.7 (+, 2× CHAr), 130.6 (+, CHAr), 129.1 (+, CHAr), 128.9 (+, CHAr), 128.6 (+, 2× CHAr), 128.5 (+, 2× CHAr), 126.6 (+, CHAr), 125.6 (Cq, CAr), 125.5 (Cq, CAr), 124.5 (Cq, CAr), 37.9 (−, CH2ethyl), 13.5 ppm (+, CH3ethyl). | 16a | δH = 7.73–7.65 (m, 15H, HAr), 7.64–7.58 (m, 6H, HAr), 7.56–7.50 (m, 13H, HAr), 5.49 (s, 1H, NH), 4.05 (q, J = 7.1 Hz, 2H, CH2ethyl), 1.20 ppm (t, J = 7.1 Hz, 3H, CH3ethyl). δC = 170.0 (Cq, CO), 169.6 (Cq, CO), 165.4 (Cq, CS), 143.1 (Cq, C=N), 137.2 (Cq, CAr), 134.1 (+, 2× CHAr), 133.6 (Cq, CAr), 133.5 (Cq, CAr), 133.3 (+, 2× CHAr), 131.9 (+, 6× CHAr), 131.8 (+, 6× CHAr), 131.7 (+, 6× CHAr), 131.6 (+, 6× CHAr), 129.1 (+, 3× CHAr), 129.0 (+, 3× CHAr), 128.6 (Cq, 3× CAr), 128.5 (Cq, 3× CAr), 39.0 (−, CH2ethyl), 13.6 ppm (+, CH3ethyl). |
2.4. UV–Vis Studies
UV–vis absorption spectra of the Cu(II) complexes 16a-f were measured from 200 to 800 nm, using acetonitrile. The blue-colored compounds exhibited bands in UV–vis spectra, ranging from 540 to 548 nm (i.e., n−π*). These bands can be attributed to ligand-to-metal charge transfer transitions from nitrogen to Cu(II). N → Cu ← O bands are common in electronic spectra of metal complexes of thiosemicarbazides. Representative examples are the UV/vis spectra of compounds 16b and 16c (Figure 4). In the field of inorganic chemistry, UV/Vis spectra are usually associated with d–d transitions and colored transition metal complexes.
Figure 4.
UV spectra of 16b and 16c in CH3CN.
2.5. Optimized Geometries
To verify the experimental findings, the complexes under study were optimized and are illustrated in Figure 5. No imaginary frequencies were noticed for the optimized complexes, ensuring that the obtained geometries were true minima. The energy differences (ΔE) between the investigated complex (E) and the most stable one (E1) are also given in Figure 5. The single point energies ensured the further favorability of complex 1 over other studied conformations.
Figure 5.

Optimized geometries of the studied complexes along with the energy difference (ΔE) between the investigated complex (E) and the most stable one (E1).
2.6. HS Analysis
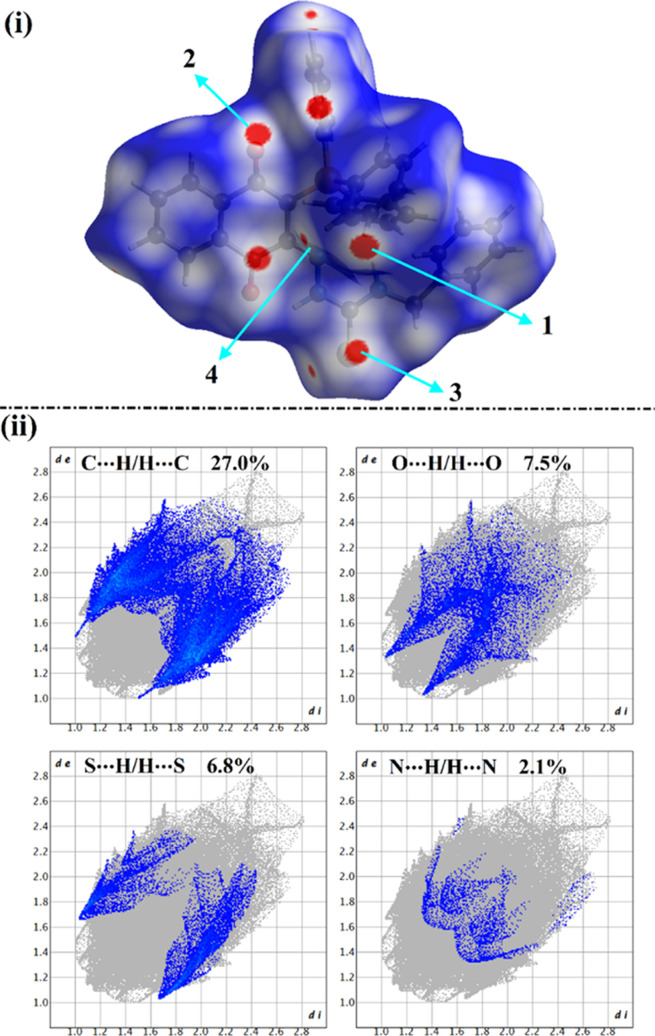
HS analysis was considered a dependable technique to qualitatively elucidate intermolecular interactions within crystal structures and unveil the interactions around the molecules’ surface.31−34 HSs, including the dnorm and its associated 2D fingerprints, shape index, and curvedness, were mapped for the studied complexes. Figure 6 shows the dnorm map and the associated 2D fingerprint plots. The extracted shape index and curvedness maps are illustrated in Figure 7.
Figure 6.
Views of (i) HSs mapped over the dnorm property; the labels 1, 2, 3, and 4 represent C···H/H···C, O···H/H···O, S···H/H···S, and N···H/H···N contacts, respectively; and (ii) 2D fingerprint plots for the interactions above.
Figure 7.

HSs mapped over the shape index and curvedness properties.
As shown in Figure 6(i), the C···H/H···C contacts were noticed with obvious large red regions labeled 1 and exhibited 27.0% of the total HS area. Such contacts were also observed in the 2D fingerprint plots as a pair of symmetrical spikes at (de + di) ∼ 2.6 Å (Figure 6(ii)).
The existence of red regions dubbed 2 in the HSs mapped over the dnorm property could be attributed to the occurrence of the reciprocal O···H/H···O contacts that were found in the 2D fingerprint plots at (de + di) ∼ 2.3 Å. For S···H/H···S contacts (labeled 3), prominent red regions were noticed with 2.1% of the total HS area and characterized by spikes at (de + di) ∼ 2.7 Å. The N···H/H···N contacts were observed with label 4, as white and red regions in the dnorm maps, with a 2.1% contribution.
Conspicuously, the HSs mapped over the shape index and curvedness properties (Figure 7) confirmed the occurrence of the C···H/H···C, O···H/H···O, S···H/H···S, and N···H/H···N interactions by the existence of the complementary pair of red and blue triangles in the shape index and the flat green area in curvedness.
3. Experimental Section
Uncorrected Melting points were taken in a Gallenkamp melting point apparatus (Weiss-Gallenkamp, Loughborough, UK). The infrared spectra were recorded with the Bruker, IFS 88 instrument. Solids were measured by the attenuated total reflection (ATR) method. The positions of the respective transmittance bands are given in wave numbers υ̅ [cm–1] and were measured in the range from 3600 to 500 cm–1. All UV–Vis spectra were recorded on the Specord 50 Plus made by the company Q Analytik Jena (Thuringia, Germany). The NMR spectra of the title compounds described herein were recorded on a Bruker Avance 400 NMR instrument at 400 MHz for 1H NMR and 101 MHz for 13C NMR, and the references used were the 1H and 13C peaks of the solvent, acetone-d6: 2.05 ppm for 1H NMR, and 206.26 ppm for 13C{1H}NMR spectra. For the characterization of centrosymmetric signals, the signal’s median point was chosen; for multiplets, the signal range was given. The following abbreviations were used to describe the proton splitting pattern: d = doublet, t = triplet, m = multiplet, and dd = doublet of a doublet. The following abbreviations were used to distinguish between signals: HAr = aromatic-CH. Signals of the 13C NMR spectra were assigned with the help of DEPT90 and DEPT135 and were specified in the following way: + = primary or tertiary carbon atoms (positive DEPT signal), – = secondary carbon atoms (negative DEPT signal), Cq = quaternary carbon atoms (no DEPT signal). Mass spectra were observed by FAB (Fast atom bombardment) experiments and were recorded using the Finnigan, MAT 90 (70 eV) instrument. Elemental analyses were performed on the Elementar Vario MICRO instrument. TLC silica plates coated with a fluorescence indicator from Merck (silica gel 60 F254, thickness 0.2 mm) were used to purify the crude products, and flash chromatography with Silica gel 60 (0.040 × 0.063 mm, Geduran) (Merck) was used. Solvents, including acetone-d6, were purchased from Merck without further drying.
3.1. General Procedures
Compounds 14a-f were prepared according to the literature.23
3.1.1. General Procedure for the Synthesis of Ligands 15a-f
2,3-Dichloro-1,4-naphthoquinone (7) (250 mg, 1.10 mmol, 1.10 equiv) was added to a stirred solution of substituted hydrazinecarbothioamides (14a-f) (1.00 mmol, 1.00 equiv) in 10 mL of dry CH3CN. The resulting solution was stirred at room temperature for 16 h. After S-alkylation was complete (i.e. the reaction was followed up by TLC), the dried salt was redissolved in dry CH3CN, after which Et3N (1.10 mmol) and Ph3P (1.10 mmol) were added. The resulting mixture was left under reflux for about 13–16 h. The reaction mixture was left to cool at room temperature, distilled H2O (50 mL) was added, and the resulting solution was extracted with CH2Cl2. The organic extracts were dried over anhydrous CaCl2, filtered, and evaporated. The crude material was purified by flash-chromatography using cyclohexane/ethyl acetate (4:1) to give compounds 15a-f.
3.1.1.1. (E)-2-(1,4-Dioxo-3-(triphenyl-λ5-phosphaneylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)-N-ethylhydrazine-1-carbothioamide (15a)
Rf = 0.27 (cyclohexane/ethyl acetate; 4:1). Violet crystals (MeOH), 0.385 g (72%). mp: 210–212 °C. 1H NMR (400 MHz, Acetone-d6): δH = 12.90 (s, 1H, NH1), 7.93 (dt, J = 44.4, 10.2 Hz, 2H, HAr), 7.84–7.68 (m, 2H, HAr), 7.65–7.31 (m, 15H, HAr), 5.77 (s, 1H, NH2) 3.19–2.56 (m, 2H, CH2ethyl), 0.62 ppm (t, J = 7.1 Hz, 3H, CH3ethyl). 13C{1H}NMR (101 MHz, Acetone-d6): δC = 183.7 (Cq, CO), 183.6 (Cq, CO), 176.7 (Cq, CS), 142.8 (Cq, C=N), 137.2 (Cq, CAr), 137.1 (Cq, CAr), 134.5 (Cq, CAr), 134.1 (+, CHAr), 133.7 (+, CHAr), 133.6 (+, CHAr), 133.1 (+, CHAr), 132.8 (+, CHAr), 132.5 (+, CHAr), 132.4 (+, CHAr), 131.8 (+, 2× CHAr), 131.7 (+, 2× CHAr), 130.6 (+, CHAr), 129.1 (+, CHAr), 128.9 (+, CHAr), 128.6 (+, 2× CHAr), 128.5 (+, 2× CHAr), 126.6 (+, CHAr), 125.6 (Cq, CAr), 125.5 (Cq, CAr), 124.5 (Cq, CAr), 37.9 (−, CH2ethyl), 13.5 ppm (+, CH3ethyl). IR (ATR): ν̃ = 3345, 3052 (w, NH), 1587, 1517 (m, C=O), 1435 (s, C=N), 1188 (s, C=P), 993 cm–1 (s, C=S). MS (FAB, 3-NBA): m/z (%) = 536 (100) [M + H]+, 535 (30) [M]+. HRMS (FAB, 3-NBA, C31H27N3O2P132S1, [M + H]+) calcd: 536.1562; found: 536.1563. EA (C31H26N3O2PS) calcd: C, 69.52; H, 4.89; N, 7.85; O, 5.97; P, 5.78; S, 5.99. Found: C, 69.50; H, 4.87; N, 7.87; O, 5.99; P, 5.76; S, 5.97.
3.1.1.2. (E)-N-Allyl-2-(1,4-dioxo-3-(triphenyl-λ5-phosphaneylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)hydrazine-1-carbothioamide (15b)
Rf = 0.29 (cyclohexane/ethyl acetate; 4:1). Violet crystals (MeOH), 0.415 g (76%). – Mp: 190–192 °C. 1H NMR (400 MHz, Acetone-d6): δH = 12.96 (s, 1H, NH1), 8.22–7.97 (m, 2H, HAr), 7.96–7.80 (m, 2H, HAr), 7.80–7.61 (m, 7H, HAr), 7.61–7.29 (m, 8H, HAr), 6.47 (s, 1H, NH2), 5.82–5.71 (m, 1H, CHallyl) 4.95–4.80 (m, 2H, CH2allyl), 4.22–3.61 ppm (m, 2H, CH2allyl). 13C{1H}NMR (101 MHz, acetone-d6): δC = 184.7 (Cq, CO), 184.6 (Cq, CO), 177.9 (Cq, CS), 142.1 (Cq, C=N), 138.1 (Cq, CAr), 135.4 (Cq, CAr), 135.2 (Cq, CAr), 135.0 (+, CHAr), 134.7 (+, CHAr), 134.6 (+, CHAr), 134.5 (+, 2× CHAr), 134.5 (+, CHAr), 133.4 (+, CHAr), 133.3 (+, CHAr), 132.7 (+, CHAr), 132.6 (+, CHAr), 131.5 (+, CHallyl), 130.4 (+, CHAr), 130.0 (+, 2× CHAr), 129.9 (+, CHAr), 129.8 (+, CHAr), 129.6 (+, CHAr), 129.5 (+, CHAr), 129.4 (+, CHAr), 127.5 (+, CHAr), 126.4 (Cq, CAr), 126.2 (Cq, CAr), 125.3 (Cq, CAr), 116.4 (−, CH2allyl), 46.3 ppm (−, CH2allyl).– IR (ATR): ν̃ = 3347, 3067 (w, ΝΗ), 1585, 1542 (m, C=O), 1431 (s, C=N), 1169 (vs, C=P), 990 cm–1 (vs, C=S). MS (FAB, 3-NBA): m/z (%) = 548 (100) [M + H]+, 547 (37) [M]+. HRMS (FAB, 3-NBA, C32H27N3O2P132S1, [M + H]+) calcd: 548.1556; found: 548.1557. EA (C32H26N3O2PS) calcd: C, 70.19; H, 4.79; N, 7.67; O, 5.84; P, 5.66; S, 5.85. Found: C, 70.21; H, 4.77; N, 7.69; O, 5.82; P, 5.68; S, 5.87.
3.1.1.3. (E)-N-Benzyl-2-(1,4-dioxo-3-(triphenyl-λ5-phosphaneylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)hydrazine-1-carbothioamide (15c)
Rf = 0.25 (cyclohexane/ethyl acetate; 4:1). Violet crystals (MeOH), 0.489 g (82%). mp: 220–222 °C. 1H NMR (400 MHz, acetone-d6): δH = 13.04 (s, 1H, NH1), 8.21–7.80 (m, 4H, HAr), 7.80–7.59 (m, 5H, HAr), 7.59–7.33 (m, 10H, HAr), 7.29–7.03 (m, 5H, HAr), 6.85 (s, 1H, NH2), 4.38–4.20 ppm (m, 2H, CH2benzyl). 13C{1H}NMR (101 MHz, acetone-d6): δC = 177.2 (Cq, CO), 176.3 (Cq, CO), 162.8 (Cq, CS), 141.5 (Cq, C=N), 138.0 (Cq, CAr), 137.5 (Cq, CAr), 137.1 (Cq, CAr), 137.0 (Cq, CAr), 134.6 (+, CHAr), 133.7 (+, CHAr), 133.6 (+, 2× CHAr), 133.5 (+, 2× CHAr), 133.4 (+, CHAr), 132.4 (+, CHAr), 132.3 (+, CHAr), 130.6 (+, CHAr), 129.1 (+, 2× CHAr), 128.9 (+, 2× CHAr), 128.7 (+, CHAr), 128.6 (+, CHAr), 128.5 (+, CHAr), 128.3 (+, CHAr), 128.1 (+, CHAr), 127.9 (+, CHAr), 127.7 (+, CHAr), 127.2 (+, CHAr), 126.6 (+, CHAr), 125.5 (+, CHAr), 125.2 (Cq, CAr), 124.5 (Cq, CAr), 124.3 (Cq, CAr), 46.9 ppm (−, CH2benzyl).– IR (ATR): ν̃ = 3340, 3055 (w, ΝΗ), 1582, 1510 (s, C=O), 1433 (s, C=N), 1153 (s, C=P), 988 cm–1 (vs, C=S). MS (FAB, 3-NBA): m/z (%) = 598 (75) [M + H]+, 597 (30) [M]+. HRMS (FAB, 3-NBA, C36H29N3O2P132S1, [M + H]+) calcd: 598.1718; found: 598.1717. EA (C36H28N3O2PS) calcd: C, 72.35; H, 4.72; N, 7.03; O, 5.35; P, 5.18; S, 5.36. Found: C, 72.37; H, 4.74; N, 7.01; O, 5.33; P, 5.16; S, 5.38.
3.1.1.4. (E)-2-(1,4-Dioxo-3-(triphenyl-λ5-phosphaneylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)-N-isopropylhydrazine-1-carbothioamide (15d)
Rf = 0.26 (cyclohexane/ethyl acetate; 4:1). Violet crystals (MeOH), 0.406 g (74%). mp: 200–202 °C. 1H NMR (400 MHz, acetone-d6): δH = 12.82 (s, 1H, NH1), 8.28–7.87 (m, 4H, HAr), 7.87–7.59 (m, 5H, HAr), 7.59–7.16 (m, 10H, HAr), 5.83 (s, 1H, NH2), 4.12–3.75 (m, 1H, CHIsopropyl), 1.50–1.03 ppm (m, 6H, 2× CH3Isopropyl). 13C{1H}NMR (101 MHz, acetone-d6): δC = 184.5 (Cq, CO), 184.4 (Cq, CO), 178.2 (Cq, CS), 147.3 (Cq, C=N), 138.3 (Cq, CAr), 135.1 (Cq, CAr), 134.8 (Cq, CAr), 134.7 (+, CHAr), 134.0 (+, CHAr), 133.5 (+,CHAr), 133.4 (+,CHAr), 132.9 (+, 2× CHAr), 132.8 (+, CHAr), 132.7 (+, CHAr), 132.6 (+, 2× CHAr), 131.5 (+, CHAr), 130.1 (+, CHAr), 129.9 (+, CHAr), 129.5 (+, 2× CHAr), 129.4 (+, 2× CHAr), 127.5 (+, CHAr), 127.3 (+, CHAr), 126.6 (Cq, CAr), 126.1 (Cq, CAr), 125.2 (Cq, CAr), 45.9 (+, CHIsopropyl), 22.2 ppm (+, 2× CH3Isopropyl). IR (ATR): ν̃ = 3347, 3052 (w, ΝΗ), 1580, 1507 (w, C=O), 1436 (s, C=N), 1188 (vs, C=P), 996 cm–1 (s, C=S). MS (FAB, 3-NBA): m/z (%) = 550 (100) [M + H]+, 549 (28) [M]+. HRMS (FAB, 3-NBA C32H29N3O2P132S1, [M + H]+) calcd: 550.1718; found: 550.1719. EA (C32H28N3O2PS) calcd: C, 69.93; H, 5.14; N, 7.65; O, 5.82; P, 5.64; S, 5.83. Found: C, 69.95; H, 5.16; N, 7.63; O, 5.80; P, 5.62; S, 5.81.
3.1.1.5. (E)-N-Cyclopropyl-2-(1,4-dioxo-3-(triphenyl-λ5-phosphaneylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)hydrazine-1-carbothioamide (15e)
Rf = 0.24 (cyclohexane/ethyl acetate; 4:1). Violet crystals (MeOH), 0.426 g (78%). mp: 205–207 °C. 1H NMR (400 MHz, acetone-d6): δH = 13.09 (s, 1H, NH1), 8.41–8.14 (m, 4H, HAr), 8.11–7.80 (m, 3H, HAr), 7.80–7.43 (m, 12H, HAr), 5.99 (s, 1H, NH2), 2.83–2.55 (m, 1H, CHCyclopropyl), 0.71–0.51 ppm (m, 4H, 2× CH2Cyclopropyl). 13C{1H}NMR (101 MHz, acetone-d6): δC = 183.6 (Cq, CO), 183.5 (Cq, CO), 178.2 (Cq, CS), 141.9 (Cq, C=N), 137.2 (Cq, CAr), 137.1 (Cq, CAr), 134.6 (Cq, CAr), 134.3 (+, CHAr), 134.2 (+, CHAr), 133.7 (+, CHAr), 133.6 (+, CHAr), 132.7 (+, CHAr), 132.5 (+, CHAr), 132.0 (+,CHAr), 131.9 (+, 2× CHAr), 131.8 (+, CHAr), 131.7 (+, CHAr), 130.6 (+, CHAr), 129.1 (+, CHAr), 129.0 (+, CHAr), 128.9 (+, CHAr), 128.8 (+, CHAr), 128.6 (+, CHAr), 128.5 (+, 2× CHAr), 126.5 (Cq, CAr), 125.1 (Cq, CAr), 124.2 (Cq, CAr), 25.8 (+, CHCyclopropyl), 6.5 ppm (−, 2× CH2Cyclopropyl). IR (ATR): ν̃ = 3342, 3050 (w, ΝΗ), 1580, 1511 (m, C=O), 1432 (s, C=N), 1186 (s, C=P), 996 cm–1 (s, C=S). MS (FAB, 3-NBA): m/z (%) = 548 (100) [M + H]+, 547 (35) [M]+. HRMS (FAB, 3-NBA, C32H27N3O2P132S1, [M + H]+) calcd: 548.1562; found: 548.1563. EA (C32H26N3O2PS) calcd: C, 70.19; H, 4.79; N, 7.67; O, 5.84; P, 5.66; S, 5.85. Found: C, 70.17; H, 4.81; N, 7.69; O, 5.82; P, 5.64; S, 5.83.
3.1.1.6. (E)-N-Butyl-2-(1,4-dioxo-3-(triphenyl-λ5-phosphaneylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)hydrazine-1-carbothioamide (15f)
Rf = 0.28 (cyclohexane/ethyl acetate; 4:1). Violet crystals (MeOH), 0.450 g (80%). mp: 195–197 °C. 1H NMR (400 MHz, acetone-d6): δH = 13.18 (s, 1H, NH1), 8.10–8.00 (m, 2H, HAr), 7.66–7.55 (m, 8H, HAr), 7.28–7.24 (m, 9H, HAr), 6.74 (s, 1H, NH2), 3.58 (dd, J = 7.7, 1.5 Hz, 2H, CH2butyl) 1.60–1.49 (m, 2H, CH2butyl), 1.35–1.26 (m, 2H, CH2butyl), 1.02–0.99 ppm (m, 3H, CH3butyl). 13C{1H}NMR (101 MHz, acetone-d6): δC = 186.1 (Cq, CO), 185.9 (Cq, CO), 178.4 (Cq, CS), 142.3 (Cq, C=N), 135.1 (Cq, CAr), 134.6 (Cq, CAr), 134.1 (Cq, CAr), 132.7 (+, CHAr), 132.6 (+, CHAr), 132.3 (+, CHAr), 131.7 (+, 3× CHAr), 131.3 (+, CHAr), 131.1 (+, CHAr), 130.7 (+, 3× CHAr), 130.5 (+, CHAr), 130.4 (+, CHAr), 129.9 (+, CHAr), 129.5 (+, CHAr), 129.4 (+, CHAr), 129.3 (+, CHAr), 128.7 (+, 2× CHAr), 128.1 (Cq, CAr), 127.8 (Cq, CAr), 127.7 (Cq, CAr), 44.6 (−, CH2butyl), 30.4 (−, CH2butyl), 20.1 (−, CH2butyl), 14.0 ppm (+, CH3butyl). IR (ATR): ν̃ = 3340, 3053 (w, ΝΗ), 1586, 1521 (m, C=O), 1434 (vs, C=N), 1170 (s, C=P), 992 cm–1 (s, C=S). MS (FAB, 3-NBA): m/z (%) = 564 (100) [M + H]+, 563 (30) [M]+. HRMS (FAB, 3-NBA, C33H31N3O2P132S1, [M + H]+) calcd: 564.1875; found: 564.1873. EA (C33H30N3O2PS) calcd: C, 70.32; H, 5.36; N, 7.46; O, 5.68; P, 5.50; S, 5.69. Found: C, 70.34; H, 5.38; N, 7.44; O, 5.66; P, 5.52; S, 5.67.
3.1.2. General Procedure for the Synthesis of Complexes 16a-f
A mixture of 15a-f (1.00 mmol, 1.00 equiv) with CuCl2·2H2O (0.170 g, 1.00 mmol, 1.00 equiv) and Ph3P (0.262 g, 1.00 mmol, 1.00 equiv) in 40 mL of absolute EtOH was stirred at room temperature for about 2–4 h (the reaction was monitored by thin-layer chromatography). After removal of the solvent under reduced pressure, the crude product was purified by column chromatography using EtOAc/hexane (5:1) to afford compounds 16a–f.
3.1.2.1. (E)-(2-(1,4-Dioxo-3-(triphenyl-λ5-phosphaneylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)-1-(ethylcarbamothioyl)hydrazineyl) ((triphenyl-λ5-phosphaneyl)oxy)copper (16a)
Rf = 0.35 (cyclohexane/ethyl acetate; 4:1). Deep blue crystals (MeOH), 0.718 g (82%). mp: 250–252 °C (decomp). 1H NMR (400 MHz, acetone-d6): δH = 7.73–7.65 (m, 15H, HAr), 7.64–7.58 (m, 6H, HAr), 7.56–7.50 (m, 13H, HAr), 5.49 (s, 1H, NH), 4.05 (q, J = 7.1 Hz, 2H, CH2ethyl), 1.20 ppm (t, J = 7.1 Hz, 3H, CH3ethyl). 13C{1H}NMR (101 MHz, acetone-d6): δC = 170.0 (Cq, CO), 169.6 (Cq, CO), 165.4 (Cq, CS), 143.1 (Cq, C=N), 137.2 (Cq, CAr), 134.1 (+, 2× CHAr), 133.6 (Cq, CAr), 133.5 (Cq, CAr), 133.3 (+, 2× CHAr), 131.9 (+, 6× CHAr), 131.8 (+, 6× CHAr), 131.7 (+, 6× CHAr), 131.6 (+, 6× CHAr), 129.1 (+, 3× CHAr), 129.0 (+, 3× CHAr), 128.6 (Cq, 3× CAr), 128.5 (Cq, 3× CAr), 39.0 (−, CH2ethyl), 13.6 ppm (+, CH3ethyl). IR (ATR): ν̃ = 3054 (w, ΝΗ), 1576, 1505 (m, C=O), 1482 (s, C=N), 1178 (s, C=P), 1019 (m, P=O), 995 (s, C=S), 536 (s, Cu–N), 457 cm–1 (s, Cu–O). MS (FAB, 3-NBA): m/z (%) = 875 (65) [M]+. HRMS (FAB, 3-NBA, C49H40CuN3O3P232S1, [M]+) calcd: 875.1562; found: 875.1563. EA (C49H40CuN3O3P2S) calcd: C, 67.15; H, 4.60; Cu, 7.25; N, 4.79; O, 5.48; P, 7.07; S, 3.66. Found: C, 67.17; H, 4.62; Cu, 7.23; N, 4.77; O, 5.50; P, 7.05; S, 3.64.
3.1.2.2. (E)-(1-(Allylcarbamothioyl)-2-(1,4-dioxo-3-(triphenyl-λ5-phosphaneylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)hydrazineyl) ((triphenyl-λ5-phosphaneyl)oxy)copper (16b)
Rf = 0.33 (cyclohexane/ethyl acetate; 4:1). Deep blue crystals (MeOH), 0.745 g (84%). mp: 244–246 °C (decomp). 1H NMR (400 MHz, acetone-d6): δH = 7.83–7.70 (m, 12H, HAr), 7.67–7.57 (m, 4H, HAr), 7.56–7.49 (m, 12H, HAr), 7.45–7.27 (m, 6H, HAr), 6.02–5.87 (m, 1H, CHallyl), 5.65 (s, 1H, NH), 5.35–5.02 (m, 2H, CH2allyl), 4.42–3.91 ppm (m, 2H, CH2allyl). 13C{1H}NMR (101 MHz, Acetone-d6): δC = 172.7 (Cq, CO), 172.6 (Cq, CO), 167.9 (Cq, CS), 144.4 (Cq, C=N), 138.6 (Cq, CAr), 134.6 (+, 2× CHAr), 134.5 (Cq, CAr), 134.3 (Cq, CAr), 133.8 (+, 2× CHAr), 133.3 (+, 4× CHAr), 132.7 (+, 4× CHAr), 132.4 (+, 4× CHAr), 131.5 (+, 4× CHAr), 130.5 (+, CHallyl), 129.9 (+, 3× CHAr), 129.7 (+, 3× CHAr), 129.5 (+, 2× CHAr), 129.4 (+, 2× CHAr), 128.3 (+, 2× CHAr), 127.4 (+, 2× CHAr), 126.5 (Cq, 3× CAr), 125.2 (Cq, 3× CAr), 116.3 (−, CH2allyl), 46.6 ppm (−, CH2allyl). IR (ATR): ν̃ = 3055 (w, ΝΗ), 1572, 1503 (s, C=O), 1483 (s, C=N), 1187 (s, C=P), 1017 (vs, P=O), 1000 (vs, C=S), 557 (vs, Cu–N), 432 cm–1 (s, Cu–O). MS (FAB, 3-NBA): m/z (%) = 887 (60) [M]+. HRMS (FAB, 3-NBA, C50H40CuN3O3P232S1, [M]+) calcd: 887.1562; found: 887.1563. EA (C50H40CuN3O3P2S) calcd: C, 67.60; H, 4.54; Cu, 7.15; N, 4.73; O, 5.40; P, 6.97; S, 3.61. Found: C, 67.58; H, 4.56; Cu, 7.17; N, 4.71; O, 5.38; P, 6.95; S, 3.63.
3.1.2.3. (E)-(1-(Benzylcarbamothioyl)-2-(1,4-dioxo-3-(triphenyl-λ5-phosphaneylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)hydrazineyl) ((triphenyl-λ5-phosphaneyl)oxy)copper (16c)
Rf = 0.36 (cyclohexane/ethyl acetate; 4:1). Deep blue crystals (MeOH), 0.872 g (93%). mp: 262–264 °C (decomp). 1H NMR (400 MHz, acetone-d6): δH = 8.25–7.75 (m, 4H, HAr), 7.74–7.62 (m, 15H, HAr), 7.61–7.39 (m, 15H, HAr), 7.38–7.07 (m, 5H, HAr), 5.36 (s, 1H, NH), 4.36–4.23 ppm (m, 2H, CH2benzyl). 13C{1H}NMR (101 MHz, acetone-d6): δC = 172.6 (Cq, CO), 172.4 (Cq, CO), 170.9 (Cq, CS), 143.5 (Cq, C=N), 137.1 (Cq, CAr), 134.9 (Cq, CAr), 134.4 (Cq, CAr), 133.9 (Cq, CAr), 133.3 (+, CHAr), 132.6 (+, CHAr), 132.5 (+, CHAr), 132.1 (+, 4× CHAr), 131.9 (+, 4× CHAr), 131.2 (+, CHAr), 130.7 (+, CHAr), 130.4 (+, CHAr), 129.8 (+, 3× CHAr), 129.7 (+, 4× CHAr), 129.4 (+, 4× CHAr), 128.9 (+, CHAr), 128.3 (+, 2× CHAr), 128.2 (+, 2× CHAr), 127.7 (+, 2× CHAr), 127.1 (+, 2× CHAr), 126.8 (+, CHAr), 126.4 (+, CHAr), 126.1 (+, CHAr), 125.9 (+, CHAr), 125.8 (+, CHAr), 125.3 (Cq, 3× CAr), 125.2 (Cq, 3× CAr), 46.3 ppm (−, CH2benzyl). IR (ATR): ν̃ = 3077 (w, ΝΗ), 1577, 1514 (s, C=O), 1480 (s, C=N), 1157 (vs, C=P), 1017 (vs, P=O), 999 (s, C=S), 548 (vs, Cu–N), 453 cm–1 (vs, Cu–O). MS (FAB, 3-NBA): m/z (%) = 937 (75) [M]+. HRMS (FAB, 3-NBA, C54H42CuN3O3P232S1, [M]+) calcd: 937.1718; found: 937.1719. EA (C54H42CuN3O3P2S) calcd: C, 69.11; H, 4.51; Cu, 6.77; N, 4.48; O, 5.11; P, 6.60; S, 3.42. Found: C, 67.11; H, 4.53; Cu, 6.79; N, 4.50; O, 5.13; P, 6.58; S, 3.44.
3.1.2.4. (E)-(2-(1,4-Dioxo-3-(triphenyl-λ5-phosphaneylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)-1-(isopropylcarbamothioyl)hydrazineyl) ((triphenyl-λ5-phosphaneyl)oxy)copper (16d)
Rf = 0.31 (cyclohexane/ethyl acetate; 4:1). Deep blue crystals (MeOH), 0.712 g (80%). mp: 268–270 °C. 1H NMR (400 MHz, acetone-d6): δH = 7.78–7.68 (m, 13H, HAr), 7.63–7.57 (m, 6H, HAr), 7.56–7.47 (m, 15H, HAr), 5.63 (s, 1H, NH), 4.14–3.85 (m, 1H, CHIsopropyl), 1.45–1.23 ppm (m, 6H, 2× CH3Isopropyl). 13C{1H}NMR (101 MHz, acetone-d6): δC = 173.1 (Cq, CO), 172.9 (Cq, CO), 170.1 (Cq, CS), 144.4 (Cq, C=N), 135.2 (Cq, CAr), 134.1 (Cq, CAr), 133.7 (Cq, CAr), 133.4 (+, CHAr), 132.8 (+, 5× CHAr), 132.7 (+, 5× CHAr), 132.6 (+, 5× CHAr), 132.5 (+, 5× CHAr), 132.4 (+, CHAr), 130.4 (+, CHAr), 129.8 (+, CHAr), 129.5 (+, 5× CHAr), 129.3 (+, 5× CHAr), 127.0 (Cq, 3× CAr), 126.7 (Cq, 3× CAr), 46.4 (+, CHIsopropyl), 22.7 ppm (+, 2× CH3Isopropyl). IR (ATR): ν̃ = 3051 (w, ΝΗ), 1589, 1513 (m, C=O), 1483 (s, C=N), 1163 (s, C=P), 1026 (w, P=O), 996 (m, C=S), 537 (vs, Cu–N), 449 cm–1 (s, Cu–O). MS (FAB, 3-NBA): m/z (%) = 889 (55) [M]+. HRMS (FAB, 3-NBA, C54H42CuN3O3P232S1, [M]+) calcd: 889.1713; found: 889.1716. – EA (C50H42CuN3O3P2S) calcd: C, 67.44; H, 4.75; Cu, 7.14; N, 4.72; O, 5.39; P, 6.96; S, 3.60. Found: C, 67.42; H, 4.77; Cu, 7.16; N, 4.70; O, 5.41; P, 6.98; S, 3.62.
3.1.2.5. (E)-(1-(Cyclopropylcarbamothioyl)-2-(1,4-dioxo-3-(triphenyl-λ5-phosphaneylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)hydrazineyl) ((triphenyl-λ5-phosphaneyl)oxy)copper (16e)
Rf = 0.32 (cyclohexane/ethyl acetate; 4:1). Deep blue crystals (MeOH), 0.763 g (86%). mp: 249–251 °C (decomp). 1H NMR (400 MHz, acetone-d6): δH = 8.16–7.84 (m, 13H, HAr), 7.77–7.65 (m, 6H, HAr), 7.63–7.41 (m, 15H, HAr), 5.62 (s, 1H, NH), 1.44–1.17 (m, 1H, CHCyclopropyl), 0.95–0.79 ppm (m, 4H, 2× CH2Cyclopropyl). 13C{1H}NMR (101 MHz, acetone-d6): δC = 176.4 (Cq, CO), 176.1 (Cq, CO), 169.8 (Cq, CS), 144.1 (Cq, C=N), 135.2 (Cq, CAr), 135.1 (Cq, CAr), 135.0 (Cq, CAr), 134.8 (+, CHAr), 134.7 (+, 3× CHAr), 134.6 (+, 3× CHAr), 134.2 (+, 3× CHAr), 134.1 (+, 3× CHAr), 134.0 (+, CHAr), 133.5 (+, CHAr), 133.4 (+, CHAr), 132.9 (+, 4× CHAr), 132.8 (+, 4× CHAr), 132.7 (+, CHAr), 132.6 (+, CHAr), 132.5 (+, CHAr), 130.5 (+, CHAr), 130.3 (+, CHAr), 129.8 (+, CHAr), 129.6 (+, CHAr), 129.5 (+, CHAr), 129.4 (+, CHAr), 127.2 (+, CHAr), 125.5 (Cq, 3× CAr), 124.6 (Cq, 3× CAr), 23.3 (+, CHCyclopropyl), 8.9 ppm (−, 2× CH2Cyclopropyl). IR (ATR): ν̃ = 3056 (w, ΝΗ), 1575, 1496 (m, C=O), 1483 (m, C=N), 1179 (vs, C=P), 1020 (s, P=O), 997 (s, C=S), 540 (vs, Cu–N), 460 cm–1 (s, Cu–O). MS (FAB, 3-NBA): m/z (%) = 887 (70) [M]+. HRMS (FAB, 3-NBA, C50H40CuN3O3P232S1, [M]+) calcd: 887.1562; found: 887.1563. EA (C50H40CuN3O3P2S) calcd: C, 67.60; H, 4.54; Cu, 7.15; N, 4.73; O, 5.40; P, 6.97; S, 3.61. Found: C, 67.62; H, 4.52; Cu, 7.13; N, 4.75; O, 5.42; P, 6.99; S, 3.59.
3.1.2.6. (E)-(1-(Butylcarbamothioyl)-2-(1,4-dioxo-3-(triphenyl-λ5-phosphaneylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)hydrazineyl) ((triphenyl-λ5-phosphaneyl)oxy)copper (16f)
Rf = 0.34 (cyclohexane/ethyl acetate; 4:1). Deep blue crystals (MeOH), 0.795 g (88%). mp: 257–259 °C (decomp). 1H NMR (400 MHz, acetone-d6): δH = δ 8.33–7.99 (m, 13H, HAr), 7.89–7.79 (m, 6H, HAr), 7.75–7.54 (m, 15H, HAr), 5.35 (s, 1H, NH), 3.83–3.74 (m, 2H, CH2butyl) 1.47–1.23 (m, 4H 2× CH2Butyl), 0.93–0.76 ppm (m, 3H, CH3Butyl). 13C{1H}NMR (101 MHz, acetone-d6): δC = 173.72 (Cq, CO), 173.58 (Cq, CO), 171.08 (Cq, CS), 143.61 (Cq, C=N), 136.17 (Cq, CAr), 135.96 (Cq, CAr), 135.71 (Cq, CAr), 135.50 (+, CHAr), 135.36 (+, CHAr), 135.34 (+, 3× CHAr), 135.16 (+, 3× CHAr), 135.12 (+, 4× CHAr), 135.03 (+, CHAr), 134.43 (+, CHAr), 134.01 (+, CHAr), 133.50 (+, 4× CHAr), 133.02 (+, 4× CHAr), 132.64 (+, CHAr), 132.50 (+, CHAr), 132.31 (+, CHAr), 132.12 (+, CHAr), 131.67 (+, CHAr), 131.37 (+, CHAr), 131.16 (+, CHAr), 130.82 (+, CHAr), 130.48 (+, CHAr), 130.27 (+, CHAr), 129.83 (+, CHAr), 129.35 (Cq, 3× CAr), 128.81 (Cq, 3× CAr), 44.9 (−, CH2Butyl), 30.9 (−, CH2Butyl), 20.5 (−, CH2Butyl), 14.4 ppm (+, CH3Butyl). IR (ATR): ν̃ = 3063 (w, ΝΗ), 1572, 1494 (m, C=O), 1466 (s, C=N), 1159 (vs, C=P), 1013 (vs, P=O), 1013 (vs, C=S), 547 (vs, Cu–N), 455 cm–1 (s, Cu–O). MS (FAB, 3-NBA): m/z (%) = 903 (50) [M]+. HRMS (FAB, 3-NBA, C51H44CuN3O3P232S1, [M]+) calcd: 903.1875; found: 903.1876. EA (C51H44CuN3O3P2S) calcd: C, 67.72; H, 4.90; Cu, 7.03; N, 4.65; O, 5.31; P, 6.85; S, 3.54. Found: C, 67.74; H, 4.92; Cu, 7.05; N, 4.63; O, 5.29; P, 6.85; S, 3.52.
3.1.3. Crystal Structure Determinations of 15c and 15f
The single-crystal X-ray diffraction study was carried out on the Bruker D8 Venture diffractometer with a PhotonII detector at 123(2) K or 298(2) K using Cu-Ka radiation (l = 1.54178 Å). Dual space methods (SHELXT)35 were used for the structure solution, and refinement was carried out using the SHELXL-2014 (full-matrix least-squares on F2).36 Hydrogen atoms were refined using a riding model (H(N) for 15c free). Semi-empirical absorption corrections were applied. Compound 15f was refined as a twin with 2 domains; both n-propyl and one n-butyl moiety are disordered (see cif-file for details).
15c: colourless crystals, C36H28N3O2PS, Mr = 597.64, crystal size: 0.18 × 0.06 × 0.03 mm, triclinic, space group P1̅ (no. 2), a = 9.4615(5) Å, b = 12.2923(6) Å, c = 14.1043(7) Å, α = 77.301(2)°, β = 70.645(2)°, γ = 74.755/2)°, V = 1477.26(13) Å3, Z = 2, ρ = 1.344 Mg/m–3, μ(Cu-Kα) = 1.79 mm–1, F(000) = 624, T = 123 K, 2θmax = 144.6°, 28,101 reflections, of which 5812 were independent (Rint = 0.025), 394 parameters, 2 restraints, R1 = 0.031 (for 5558 I > 2σ(I)), wR2 = 0.084 (all data), S = 1.05, largest diff. peak/hole = 0.47/–0.28 e Å–3.
15f: colourless crystals, C32H28N3O2PS·C33H30N3O2PS, Mr = 1113.23, crystal size: 0.18 × 0.12 × 0.03 mm, triclinic, space group P1̅ (no. 2), a = 18.6988(5) Å, b = 18.8744(5) Å, c = 20.8063/6) Å, α = 103.938(2)°, β = 103.303(2)°, γ = 116.775(2)°, V = 5856.8(3) Å3, Z = 4, ρ = 1.262 Mg/m–3, μ(Cu Kα) = 176 mm–1, F(000) = 2336, T = 298 K, 2θmax = 144.6°, 96,411 collected data, merged to 23,046 unique reflections (using a HKLF5 file), 1411 parameters, 2775 restraints (see cif-file for details), R1 = 0.065 (for 18,260 I > 2σ(I)), wR2 = 0.211 (all data), S = 1.03, largest diff. peak/hole = 0.95/–0.47 e Å–3.
CCDC 2182411 (15c) and 2182412 (15f) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
4. Computational Methods
4.1. Geometrical Optimization and Energy Calculations
Various quantum mechanical calculations were carried out to confirm the experimental results. The studied complexes were first optimized at the B3LYP/6-31G* level of theory.37,38 The vibrational frequency and single-point energy calculations were performed upon the optimized geometries. All the adopted quantum mechanical calculations were executed at the B3LYP/6-31G* level of theory with the help of Gaussian 09 software.39
4.2. HS Analysis
In the current study, HS analysis40 was executed to give an in-depth qualitative insight into the role of the main intermolecular interactions. Using HS analysis, the normalized contact distance (dnorm) surface was mapped over a fixed color scale ranging from red (−0.05 au) to blue (+0.75 au). The fingerprint plots were generated using the translated 1.0–2.8 Å range, and reciprocal contacts were considered. Moreover, the shape index and curvedness properties were mapped with the color range of −1.0 au (concave) to 1.0 au (convex) and a range of −4.0 au (flat) to 0.40 au (singular), respectively. The generated HSs and the associated 2D fingerprint plots were extracted using the CrystalExplorer17 software.41
5. Conclusion
New (E)-2-(1,4-dioxo-3-(triphenylphosphaneylidene)-3,4-dihydronaphthalen-ylidene)-N-substituted-hydrazine-1-carbothioamides were obtained during a one-pot reaction of 2,3-dichloro-1,4-naphthoquinone with thiosemicarbazides, triphenylphosphine (Ph3P) in the presence of triethyl amine (Et3N) as a catalyst. The reaction was a type of Eschenmoser nucleophilic addition. Utilizing the newly prepared ligands, their complexation with CuCl2 and Ph3P was investigated. Autoxidation occurs, and (E)-(2-(1,4-dioxo-3-(triphenyl-phosphaneylidene)-3,4-dihydronaphthalen-2(1H)-ylidene)-carbamothioyl)hydrazinyl)-((triphenylphosphanyl)oxy)copper derivatives were formed in very good yields. Quantum mechanical calculations using the DFT method confirmed the stability of the obtained complexes..
Acknowledgments
The authors thank the DFG-funded coordinative research center TR88/3MET, Karlsruhe Institute of Technology, Karlsruhe, Germany, for providing Prof A.A.A. with a two months project, enabling him to carry out analysis and facilities. We also acknowledge support from the KIT-Publication Fund of the Karlsruhe Institute of Technology.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c04113.
NMR (1H NMR and 13C NMR), IR, and mass spectra, in addition to HRMS spectra of compounds 15a-f and 16a-f; figures of UV Spectra of compounds 16a-f; and X-ray figures and structural data and of compounds 15c and 15f (PDF)
Author Contributions
CRediT authorship contribution statement. Mohammed B. Alshammari: editing and revision, Ashraf A. Aly: Conceptualization, writing, and editing, Stefan Bräse: Editing and revision. Martin Nieger: X-ray, Methodology, and editing, Mahmoud A. A. Ibrahim: Software, writing a draft, and editing. Lamiaa E. Abd El-Haleem: Conceptualization, writing, editing, methodology, and writing the draft.
The authors declare no competing financial interest.
Supplementary Material
References
- Aithal K. B.; Kumar M. R. S.; Rao B. N.; Udupa N.; Rao B. S. Juglone, a naphthoquinone from walnut, exerts cytotoxic and genotoxic effects against cultured melanoma tumor cells. Cell Biol. Int. 2009, 33, 1039–1049. 10.1016/j.cellbi.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Kosiha A.; Parthiban C.; Ciattini S.; Chelazzi L.; Elango K. P. Metal complexes of naphthoquinone based ligand: synthesis, characterization, protein binding, DNA binding/cleavage and cytotoxicity studies. J. Biomol. Struct. Dyn. 2018, 36, 4170–4181. 10.1080/07391102.2017.1413423. [DOI] [PubMed] [Google Scholar]
- Geisler H.; Westermayr J.; Cseh K.; Wenisch D.; Fuchs V.; Harringer S.; Plutzar S.; Gajic N.; Hejl M.; Jakupec M. A.; Marquetand P.; Kandioller W. Tridentate 3-Substituted Naphthoquinone Ruthenium Arene Complexes: Synthesis, Characterization, Aqueous Behavior, and Theoretical and Biological Studies. Inorg. Chem. 2021, 60, 9805–9819. 10.1021/acs.inorgchem.1c01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivetyeva S. I.; Zakharova O. D.; Ovchinnikova L. P.; Baev D. S.; Bagryanskaya I. Y.; Shteingarts V. D.; Tolstikova T. G.; Nevinsky G. A.; Tretyakov E. V. Phosphonium betaines derived from hexafluoro-1,4-naphthoquinone: Synthesis and cytotoxic and antioxidant activities. J. Fluorine Chem. 2016, 192, 68–77. 10.1016/j.jfluchem.2016.10.014. [DOI] [Google Scholar]
- Ramirez F.; Dershowitz S. The Structure of Quinone-Donor Adducts. I. The Action of Triphenylphosphine on p-Benzoquinone, 2,5-Dichloro-p-benzoquinone and Chloranil. J. Am. Chem. Soc. 1956, 78, 5614–5622. 10.1021/ja01602a041. [DOI] [Google Scholar]
- Loskustov V. A.; Mamatyuk V. I.; Beregovaya I. V. The structure and properties of 3-(triphenylphosphoranylidene)naphthalene-1,2,4(3H)-trione. Russ. Chem. Bull. 1999, 48, 371–374. 10.1007/bf02494568. [DOI] [Google Scholar]
- Khasiyatullina N. R.; Gubaidullin A. T.; Shinkareva A. M.; Islamov D. R.; Mironov V. F. New bisphosphonium salt containing a 1,4-dihydroxynaphthalene moiety: molecular and supramolecular structure. Russ. Chem. Bull. 2020, 69, 2140–2146. 10.1007/s11172-020-3012-3. [DOI] [Google Scholar]
- Huang W.; Zhong C.-H. Metal-Free Synthesis of Aryltriphenylphosphonium Bromides by the Reaction of Triphenylphosphine with Aryl Bromides in Refluxing Phenol. ACS Omega 2019, 4, 6690–6696. 10.1021/acsomega.9b00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalafy J.; Mohammadlou M.; Mahmoody M.; Salami F.; Poursattar Marjani A. P. Facile synthesis of new 10-substituted-5H-naphtho[1,2-e][1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-5-ones. Tetrahedron Lett. 2015, 56, 1528–1530. 10.1016/j.tetlet.2015.02.002. [DOI] [Google Scholar]
- Al-Alshaikh M. A.; Lahsasni S. A. Synthesis of Novel 2,3-Disubstituted 1,4-Naphthoquinone Derivatives Containing Indole, Quinoline, Thiazole and Imidazole Moieties. Asian J. Chem. 2013, 25, 10199. 10.14233/ajchem.2013.15230. [DOI] [Google Scholar]
- Forezi L. da S. M.; Cardoso M. F. C.; Costa D. C. S.; Silva F. de C. d.; Ferreira V. F. 2,3-Dichloro-1,4-Naphthoquinone in Organic Synthesis: Recent Advances. Mini-Rev. Org. Chem. 2017, 14, 375–390. 10.2174/1570193x14666170518125507. [DOI] [Google Scholar]
- Aly A. A.; Hassan A. A.; AbdEl-latief E.-S. S. M.; El-Sh S. M. An Update of the Use of Thiocarbohydrazides and Thiosemicarbazides in the Preparation of Heterocycles and Their Biological Importance. J. Heterocycl. Chem. 2018, 55, 2196–2223. 10.1002/jhet.3295. [DOI] [Google Scholar]
- Choudhari D.; Lande D. N.; Chakravarty D.; Gejji S. P.; Das P.; Pardesi K. R.; Satpute S.; Salunke-Gawali S. Reactions of 2,3-dichloro-1,4-naphthoquinone with aminophenols: evidence for hydroxy benzophenoxazine intermediate and antibacterial activity. J. Mol. Struct. 2019, 1176, 194–206. 10.1016/j.molstruc.2018.08.066. [DOI] [Google Scholar]
- Carroll F. I.; Miller H. W. The reaction of 2,3-dichloro-1,4-naphthoquinone with p-nitrobenzhydrazide. Org. Prep. Proced. 1970, 2, 259–263. 10.1080/00304947009458625. [DOI] [Google Scholar]
- Hergueta A. R. Easy Removal of Triphenylphosphine Oxide from Reaction Mixtures by Precipitation with CaBr2. Org. Process Res. Dev. 2022, 26, 1845–1853. 10.1021/acs.oprd.2c00104. [DOI] [Google Scholar]
- Wattanakanjana Y.; Janwatthana K.; Romyen T.; Nimthong-Roldán A. Crystal structures of copper(I) and silver(I) chloride complexes containing 4-phenylthiosemicarbazide and triphenylphosphine ligands. ScienceAsia 2021, 47S, 28. 10.2306/scienceasia1513-1874.2021.S001. [DOI] [Google Scholar]
- Gunasekaran N.; Bhuvanesh N. S. P.; Karvembu R. Synthesis, characterization and catalytic oxidation property of copper(I) complexes containing monodentate acylthiourea ligands and triphenylphosphine. Polyhedron 2017, 122, 39–45. 10.1016/j.poly.2016.10.038. [DOI] [Google Scholar]
- Aly A. A.; Abdallah E. M.; Ahmed S. A.; Rabee M. M.; Abdelhafez E. M. N. Metal complexes of thiosemicarbazones derived by 2-quinolones with Cu(I), Cu(II) and Ni(II); Identification by NMR, IR, ESI mass spectra and in silico approach as potential tools against SARS-CoV-2. J. Mol. Struct. 2022, 1265, 133480. 10.1016/j.molstruc.2022.133480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly A. A.; Bräse S.; Weis P. Tridentate and bidentate copper complexes of [2.2]paracyclophanyl-substituted thiosemicarbazones, thiocarbazones, hydrazones and thioureas. J. Mol. Struct. 2019, 1178, 311–326. 10.1016/j.molstruc.2018.10.036. [DOI] [Google Scholar]
- White A. R.; Multhaup G.; Maher F.; Bellingham S.; Camakaris J.; Zheng H.; Bush A. I.; Beyreuther K.; Masters C. L.; Cappai R. The Alzheimer’s Disease Amyloid Precursor Protein Modulates Copper-Induced Toxicity and Oxidative Stress in Primary Neuronal Cultures. J. Neurosci. 1999, 19, 9170–9179. 10.1523/JNEUROSCI.19-21-09170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Zhang X.; Chen J.; Yang Q.; Yang L.; Xu D.; Zhang P.; Wang X.; Liu J. Hinokitiol copper complex inhibits proteasomal deubiquitination and induces paraptosis-like cell death in human cancer cells. Eur. J. Pharmacol. 2017, 815, 147. 10.1016/j.ejphar.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Sangeetha S.; Murali M. Non-covalent DNA binding, protein interaction, DNA cleavage and cytotoxicity of [Cu(quamol)Cl]·H2O. Int. J. Biol. Macromol. 2018, 107, 2501–2511. 10.1016/j.ijbiomac.2017.10.131. [DOI] [PubMed] [Google Scholar]
- Hossain G. M.; Abedin M. d.; Bachar S. C. Synthesis and Characterization of N1-Phenylhydrazine-1,2-bis(carbothioamide) and Its Evaluation for Antimicrobial, Antioxidant, and Brine Shrimp Lethality Bioassay. Org. Chem. Int. 2012, 2012, 278741. 10.1155/2012/278741. [DOI] [Google Scholar]
- Baran E. J. Structural Data and Vibrational Spectra of the Copper(II) Complex of L-Selenomethionine. Z. Naturforsch. 2005, 60, 663–666. 10.1515/znb-2005-0609. [DOI] [Google Scholar]
- Casper J. M.; Remsen E. E. Phosphoryl stretching frequencies of some tris para-substituted phenylphosphine oxides. Spectrochim. Acta, Part A 1978, 34, 1–4. 10.1016/0584-8539(78)80176-7. [DOI] [Google Scholar]
- Köhler F. H.Paramagnetic Complexes in Solution: The NMR Approach. eMagRes; John Wiley & Sons, 2011. [Google Scholar]
- Drago R. S.Physical Methods in Chemistry, 2nd ed; Saunders College Pub: Philadelphia, 1977. [Google Scholar]
- Gnangnon B.; Baran P.. Existence or Nonexistence of Cu(II) Complexes with Triphenylphosphine: Abstracts of Papers; 247th ACS National Meeting & Exposition: Dallas, TX, United States, March 16-20, 2014.
- Bai B.; Wang W.; Wang W.; Sun S.; Chen C. Triphenylphosphine as reducing agent for copper(II)-catalyzed AGET ATRP. Chin. J. Polym. Sci. 2015, 33, 1260–1270. 10.1007/s10118-015-1676-1. [DOI] [Google Scholar]
- Toshiyuki S.; Yoshihiko F.; Koichiro S. Synthesis of Copper (I) triphenyl-phosphine complexes. Chem. Lett. 1972, 1, 163–164. [Google Scholar]
- Anitha K.; Sivakumar S.; Arulraj R.; Rajkumar K.; Kaur M.; Jasinski J. P. Synthesis, crystal structure, DFT calculations and Hirshfeld surface analysis of 3-butyl-2,6-bis(4-fluorophenyl)piperidin-4-one. Acta Crystallogr., Sect. E: Crystallogr. Commun. 2020, 76, 651–655. 10.1107/S2056989020004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arulraj R.; Sivakumar S.; Kaur M.; Thiruvalluvar A.; Jasinski J. P. Crystal structures of three 3-chloro-3-methyl-2,6-diarylpiperidin-4-ones. Acta Crystallogr., Sect. E: Crystallogr. Commun. 2017, 73, 107–111. 10.1107/S2056989016020661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon J. J.; Spackman M. A.; Mitchell A. S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr., Sect. B: Struct. Sci. 2004, 60, 627–668. 10.1107/S0108768104020300. [DOI] [PubMed] [Google Scholar]
- McKinnon J. J.; Jayatilaka D.; Spackman M. A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. 10.1039/b704980c. [DOI] [PubMed] [Google Scholar]
- Sheldrick G. M. SHELXT- Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. 10.1107/s2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. Crystal structure refinement withSHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becke A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B: Condens. Matter Mater. Phys. 1988, 37, 785–789. 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks H. B.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A., Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford CT, 2009.
- Spackman M. A.; Jayatilaka D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. 10.1039/b818330a. [DOI] [Google Scholar]
- Turner M. J.; McKinnon J. J.; Wolff S. K.; Grimwood D. J.; Spackman P. R.; Jayatilaka D.; Spackman M. A.. CrystalExplorer17; University of Western Australia, 2017. http://hirshfeldsurface.net. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.