Abstract
Recent years have witnessed many breakthroughs in research on graphene as well as a significant improvement in the electrochemical synthesis methods of graphene oxide (GO). GO is a derivative of graphene which has attracted the focus of worldwide scientists and researchers because of its hydrophilic and easily functionalized properties. The electrochemical approach is popular because it saves time, creates zero explosion risk, releases no hazardous gases, and avoids environmental pollution. Although recent publications show that the green, rapid, and mass electrochemical synthesis of GO has more advantages as compared with the traditional Hummers method, it is crucial to study the effects of reaction parameters. Herein, we review recent various works regarding the influences of various reaction parameters on the synthesis of GO sheets. The advancement, current challenges, and solutions of electrochemical synthesis methods of GO are also outlined. Through this review, we hope to spark some clear ideas for anyone who wants to scale up the yield of GO.
Introduction
With the presence of hydroxyl, carbonyl, and epoxy groups at the basal plane but carboxyl groups at the edges, graphene oxide (GO) sheets show good dispersibility in numerous solvents.1 In addition, GO can be modified chemically with various functional groups through electrostatic interactions, hydrogen bonds, and van der Waals to fabricate advanced materials such as transparent conductive film, energy storage, sensors, ultralight super elastic aerogels, and others.1−3 Carbon has been used as a main element in electrochemistry4,5 for years, especially graphite and graphene which are popular and successful electrodes due to their excellent electrical properties. Furthermore, graphite intercalation compounds (GICs) have long been synthesized electrochemically with other elements for some applications, and therefore, scientists applied this method to exfoliate graphene from natural graphite.6−11 Brodie, Staudenmaier, and Hummers’ methods have long been used to synthesize graphite oxide before some improvements on oxidation and exfoliation efficiency were made by researchers.12 However, they have long reaction times of hours and even days and release numerous hazardous gases (e.g., NOx and CIO2) in chemical oxidation.1 In addition, the Hummers' method has an explosion risk caused by the presence of highly reactive Mn2O7 intermediates. A huge amount of water is needed to eliminate excessive H2SO4 and KMnO4 after oxidation, which leads to serious environmental pollution. Moreover, the heavy metal ions and Mn2+ can be detected on GO sheets as impurities.1
Besides traditional GO synthesis methods, new synthesis approaches such as electrochemical exfoliation,13,14 dry ball milling,15,16 and ultrasound-assisted synthesis17 have been demonstrated recently. Among them, electrochemical exfoliation has been reported to be as popular as the Hummers' method,18 but the electrochemical method does not utilize strong oxidants and has a long reaction time. By taking advantage of the high electrical conductivity of graphite electrodes, potential current can be connected to graphite to initiate the intercalation of an anion or cation into an oppositely charged electrode, followed by exfoliation. Commercial graphite is used commonly as raw material. Furthermore, coke,19 carbon cloth,20,21 and anthracite coal22 also showed promise as potential candidates for the synthesis of graphene due to the availability of an abundant graphite structure. Therefore, electrochemical exfoliation has many benefits and a huge potential for the mass synthesis of graphene compared to that of conventional chemical methods.23 Besides, the electrochemical approach requires a much lower cost for graphene production as compared with chemical vapor deposition, mechanical exfoliation, reduction of GO, pyrolysis of graphene, and epitaxial growth of graphene.24 Without the use of harsh chemicals, simpler purification steps can be conducted after the electrochemical process. In addition, high quality and functionalized graphene can be synthesized in the electrochemical method which can fulfill the needs of industrial applications in sensor, battery, electronics, composites, and others.25 To enhance the yield of GO synthesis, the electrochemical reaction parameters such as concentration of electrolytes, voltage/current, type of electrode, etc. can be optimized.13,14,26,27 A direction to develop a scalable, green, low-cost, and facile electrochemical exfoliation from graphite is highly desired; thus, a detailed understanding of the parameters to optimize the GO yield is important.
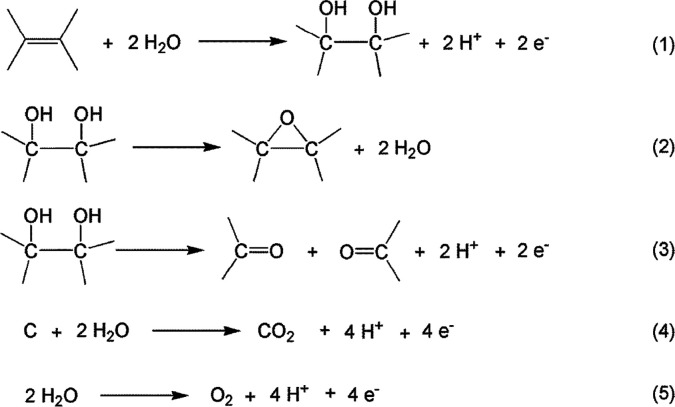
Electrochemical exfoliation involves the intercalation of a cation or anion from the electrolyte under an applied potential. The exfoliation mechanisms depend on the type of potential used: anodic or cathodic. In anodic exfoliation, the accumulation of positive charge at the anode facilitates the intercalation of bulky anions which increase the interlayer spacing between graphite layers. On the other hand, cathodic exfoliation involves the attraction of cations by negative bias at the graphite working electrode. Again, these cations are intercalated and open the graphene layers, causing expansion and exfoliation. As shown in Scheme 1, Müllen and coresearchers28 proposed that when a positive potential is employed to the graphite anode sp2 carbons of the graphite anode were assaulted by nucleophilic OH–, which formed from water molecules. This will create C–OH groups and vicinal OH groups as seen in reaction 1. Next, epoxy rings could be produced (reaction 2), and they can be further oxidized into carbonyl groups (reaction 3). The reaction between C and water induces the formation of CO2 (reaction 4), and self-oxidation of water forms O2 (reaction 5).
Scheme 1. Schematic Representation of the Electrochemical Oxidation Process of Graphite Electrodes.
Reprinted with permission from ref (28). Copyright 2014 American Chemical Society.
This review aims to give an overview of the influences of electrochemical exfoliation parameters on the oxidation degree of exfoliated graphene sheets. This review also intends to inspire all active researchers to improve the current electrochemical methods for the large-scale synthesis of GO flakes to substitute the conventional time-consuming, non-green, and complicated chemical exfoliation and oxidation methods.
Effect of Types of Electrolytes
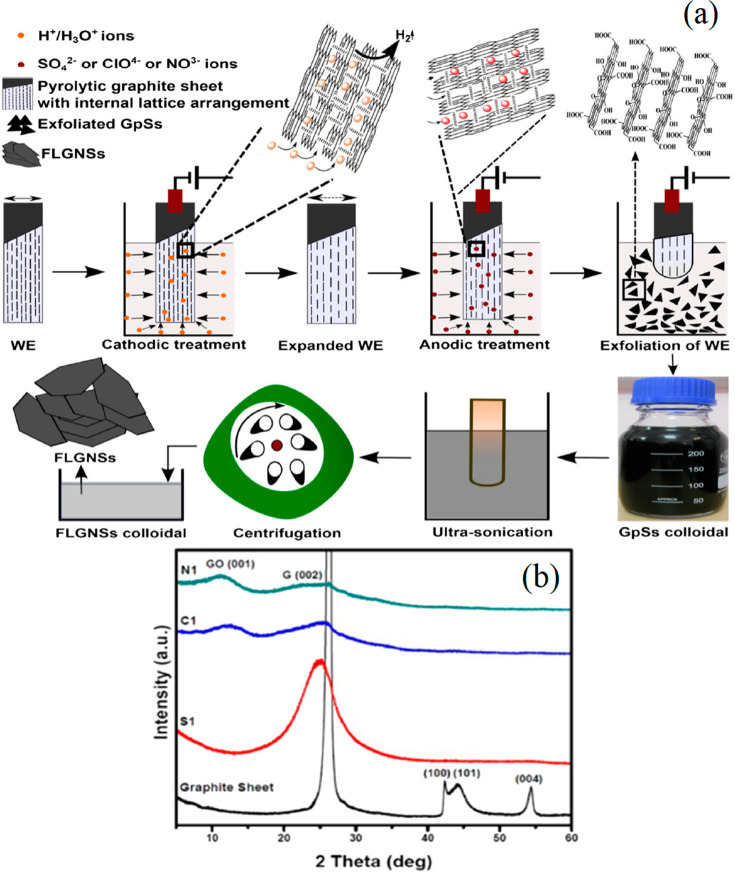
The type of electrolyte is one of the most influential key factors of the yield of GO. Each electrolyte produces a different amount of GO due to its own oxidation capabilities. Molten salts and ionic and inorganic solutions have been reported as popular choices as electrolytes to form GO in the electrochemical process. The composition and structure of GO do not appreciably alter with the addition of an excess oxidizing agent after reaching a specific threshold oxidation degree; sulfuric acid (H2SO4), on the other hand, is a strong and straightforward proton donor (pKa = 10), which displays its advantage as the electrolyte by enhancing the functionality of GO. Therefore, H2SO4 is a highly popular electrolyte27,29 used to exfoliate graphite and to synthesize GO due to the rapid exfoliation rate, the similar size of SO42– with the interlayer distance of graphite, and the formation of SO2 and O2 gases which enhance further the exfoliation rate.30 When a low concentration of H2SO4 was used, Wu et al. synthesized a low oxidation of few-layer graphene due to the fast exfoliation by the strong released gases, which expanded the graphite layer.31 The influence of using 1 M H2SO4, 1 M HClO4, and 1 M HNO3 was reported,32 but GO was formed successfully using the 1 M HClO4 and 1 M HNO3 only (Figure 1a). No GO peak was seen when 1 M H2SO4 was used due to the fast electrochemical exfoliation (Figure 1b). Parvez et al.33 demonstrated the electrochemical exfoliation of graphite into a low oxidation degree of graphene sheets with a high amount (>85%, ≤3 layers) and lateral size up to 44 μm using inorganic salts ((NH4)2SO4, Na2SO4, K2SO4, etc.). However, the acidic electrolytes synthesized a mixture of GO and graphene with moderate quality and bigger lateral size.34 Therefore, the oxidation degree of graphite can be decreased or enhanced by using an inorganic salt or acidic solution as electrolyte, respectively.
Figure 1.
(a) Illustration of electrochemical intercalation and exfoliation by using 1 M H2SO4, 1 M HClO4, and 1 M HNO3. (b) No GO (001) peak was seen in XRD spectrum of 1 M H2SO4 (S1), whereas the GO (001) peak was present in 1 M HClO4 (C1) and 1 M HNO3 (N1). Reprinted with permission from ref (32). Copyright 2016 Springer Nature.
Besides H2SO4, perchloric acid (HClO4) was another electrolyte used to synthesize GO.13 As shown in Figure 2, a T-cell was used where the electrical connectivity between the Pt backing disc and anode graphite was maintained for good batch-to-batch reproducibility. They found that electrochemical duration or the concentration of HClO4 greatly influenced the content of oxygen-containing functional groups, but the carboxyl group was hard to synthesize, although the electrochemical oxidation was extended. In addition, the GO oxidation and structure defect levels were lower than the GO formed using the Hummers' method. Gurzeda’s research group demonstrated the oxidation of a graphite anode through the formation of second-stage HClO4-GIC by using a linear sweep voltammetry method in a 8 M HClO4 solution.35 When 1.4 V was used, the complete oxidation of graphite into GO was achieved as indicated by both XRD and Raman results. Epoxy and alkoxy groups were noticed as the main functional groups which possess many defects on the GO structure. However, the layered GO structure was observed and named as a quasi-graphite structure.
Figure 2.
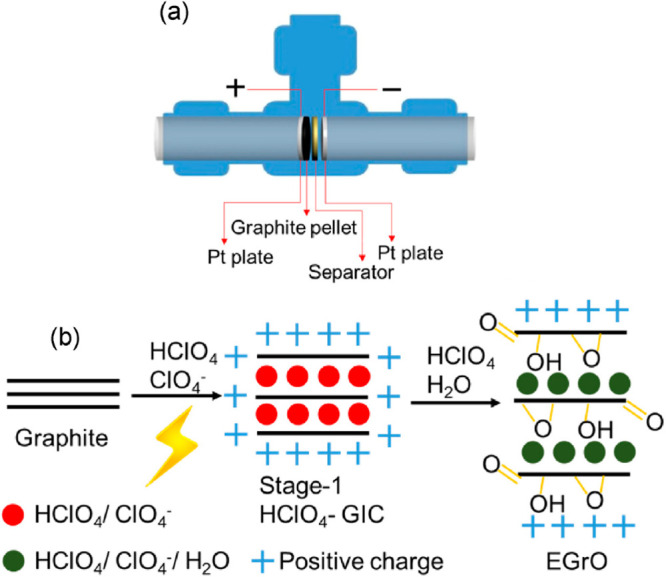
(a) Diagram of electrochemical T-cell setup. (b) In stage 1, graphite was transformed into a graphite intercalation compound (GIC), followed by stage 2 where the GIC was converted into electrochemical graphite oxide. The thick black lines represent graphene layers. Reprinted with permission from ref (13). Copyright 2016 Elsevier Ltd.
Mineral salts such as phosphates and sulfates were also utilized as electrolytes for GO synthesis.36 (NH4)2SO4, Na2SO4, and MgSO4 were able to form GO that dispersed in water easily, but nitrogen and sulfur dopants were attached to the GO synthesized in 1 M (NH4)2SO433 due to unavoidable oxidation and attachment of active species.37 Furthermore, oxone (0.05 M KHSO5·0.5KHSO4·0.5K2SO4) was used as an electrolyte for GO production. The anion SO42– intercalated into the graphite anode, whereas HSO5– and HSO4– created strong oxidization radicals such as SO•– and OH• to form 16.37% oxygen atoms and 7.4% GO with one to three layers (Figure 3).38 GO samples with a C/O atomic ratio of 3.45 were synthesized as well using phosphate buffer solution, which has attracted researchers’ attention along with surfactants, molten salts, and ionic liquids that have been used as electrolytes.36
Figure 3.
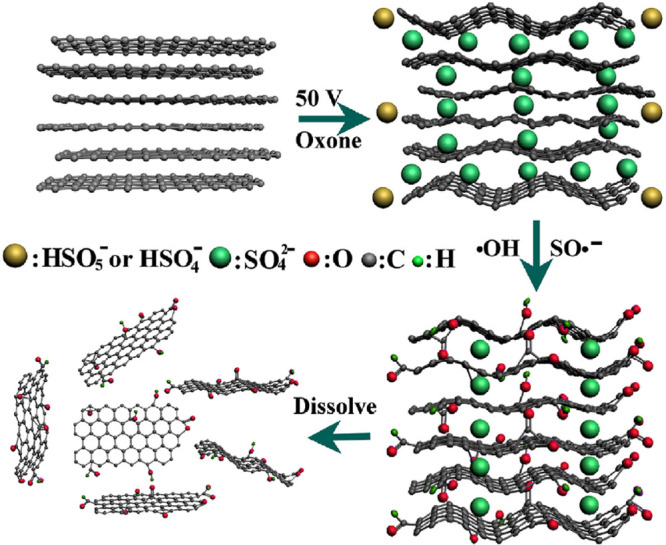
Proposed mechanism of electrochemical exfoliation by choosing ozone as an electrolyte in which exfoliation and oxidation occurred simultaneously. Reprinted with permission from ref (38). Copyright 2017 Elsevier Ltd.
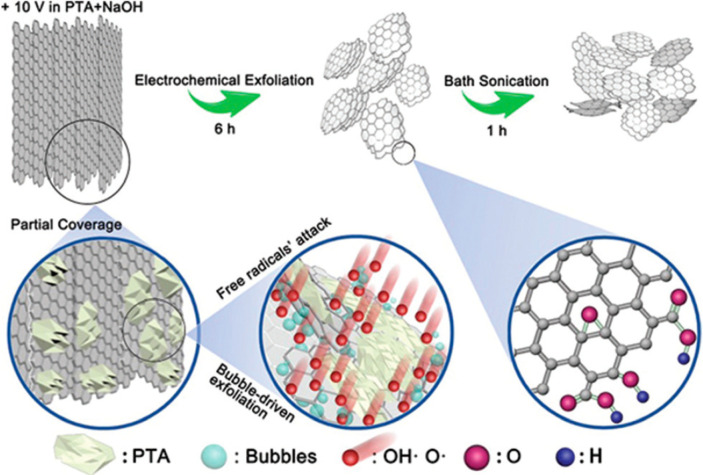
Cetyltrimethylammonium bromide (CTAB) is one of the cationic surfactants used to intercalate into graphite and functionalize GO by grafting alkyl chains onto the edges and yield a stable colloidal suspension.26 In addition, a mixture of urea, acetamide, and ammonium nitrate was utilized to intercalate into graphite due to the high viscosity, high intercalation potential, and low migration speed. The amount of graphene was 76% with one to five layers and showed that a specific surface area (878 m2 g–1) could be archived.39 Besides, the ionic solution is one of the favorable electrolytes to synthesize GO because of two features: superior ionic conductivity and wide-ranging electrochemical potential. As an example, triethyl sulfonium bis(trifluoromethyl sulfonyl) imide was used as an electrolyte, whereas a pencil was used as a graphite anode to synthesize GO and graphene nanosheets. The produced GO was used for the label-free and real-time surface plasmon resonance (SPR) sensing of Salmonella typhi.40 It is important to note that the exfoliation mechanisms of ionic solutions and molten salts are different with aqueous electrolyte due to the absence of water. Furthermore, the oxidation of an anode graphite can be enhanced by adding particular chemicals such as nitric acid as demonstrated by Abdelkader et al.14 A novel method to suppress the fast exfoliation of graphite and to improve the anodic oxidation using p-phthalic acid (PTA) coverage on the anode electrode was reported (Figure 4).41,42 On the other hand, a two-step electrochemical intercalation using concentrated H2SO4 and oxidation using 0.1 M (NH4)2SO4 to synthesize GO on tens of grams scale were demonstrated.43 This method divides the intercalation and oxidation processes into two different steps, which could facilitate the rapid formation of GO in the second step. The GO has an oxygen content of 17.7 at. %, >90% single layer, and high yield (>70 wt %). Table 1 summarizes the characteristics of GO synthesized by using different types of electrolytes.
Figure 4.
Schematic illustration of the exfoliation mechanism where PTA was used to cover the graphite anode surface partially to suppress the exfoliation but enhance the oxidation. The sonication in NaOH helps further multiple exfoliations of big particles to increase the yield of water-dispersible graphene. Reprinted with permission from ref (42). Copyright 2018 The Royal Society of Chemistry.
Table 1. Summary of Types of Electrolytes to Produce GO.
| electrolytes | C/O | ID/IG | GO yield | thickness | lateral sizes | synthesis rate | references |
|---|---|---|---|---|---|---|---|
| H2SO4, H3PO4 | - | 0.71 | - | 3–9 nm | 1–5 μm | - | (29) |
| H2SO4, HCIO4, HNO3 | 1.54–1.76 | 1.005–1.072 | 49–50% | 2.7–5.6 nm | 120–220 nm | - | (32) |
| (NH4)2SO4, H2SO4, Na2SO4, MgSO4 | - | 0.85 | >80% | 1.08 nm | 0.5–3.0 μm | - | (33) |
| HCIO4 | 1.43–11.55 | 0.16–0.75 | 6.63–34.2% | - | - | - | (35) |
| H2SO4 | - | - | ∼5–8 wt % | <3 nm | 1–40 μm | - | (34) |
| HCIO4, H2SO4 | 3.0–5.7 | - | - | 2 nm | 10 μm | - | (13) |
| (NH4)2SO4, NH3·H2O | 7.15 | 1.33 | 34 wt % | 1.9–3.5 nm | - | - | (37) |
| oxone (potassium monopersulfate, KHSO5·0.5KHSO4·0.5K2SO4) | - | ∼1.24 | ∼60.1% | 2.5 nm | 1–5 μm | - | (38) |
| 0.2 M of sodium citrate (pH 3.7), HNO3 | 4 | - | - | 1.1 nm | - | - | (14) |
| NaOH, PTA | 3.15 | - | 99% | 1.8–2.2 nm | 1–50 μm | - | (41) |
| NaOH, PTA | 4.02 | 0.90 | 87.3% | 2.0 nm | 4.2 μm | - | (42) |
| >95% H2SO4, 0.1 M (NH4)2SO4 | 4.6 | 1.48 | 71% | <1.5 nm | - | 10 g, less than 30 min | (43) |
| LiCl, NaCl, KCl, RbCl, CsCl | 57.8 | 0.06–0.12 | - | 5 nm | 1–3 μm | - | (44) |
Effect of Electrolyte Concentration
The electrolyte’s concentration is also one of the key factors for the yield of GO. In other words, the quantity of intercalating ionic species is dependent on the electrolyte’s concentration. When more ionic species are present, a faster rate of intercalation, expansion, exfoliation, and oxidation of the graphene sheets will occur. Furthermore, increasing the content of the electrolyte could result in the improvement of GO dispersion, a coarser surface shape, and a reduction in GO sizes as a reflection of changes in the surface functionality. To enhance the yield of GO, some researchers demonstrated that with an increase of electrolyte concentration the oxidation degree was improved. Nevertheless, a highly concentrated electrolyte contains a small amount of water that is unsuitable for GO synthesis because water is the main source for the formation of oxygen-containing functional groups at the basal and edges of graphene. Thus, the higher the electrolyte concentration, the lower amount of GO will be synthesized. In other words, a high concentration of electrolyte promotes the intercalation rate due to the presence of plentiful anions which act as intercalants, whereas a low concentration facilitates oxidation and exfoliation caused by abundant water molecules.43 Therefore, Dalal et al.44 reported that no GO was synthesized, and exfoliated graphite was mainly collected when the concentrations of H2SO4 were increased. At 2.0 M, the fastest intercalation was observed, followed by a 1.0 M intercalation/exfoliation, which happened at the beginning stage only, and then the process was unsustainable. When the concentration was lower than 0.50 M, light intercalation occurred in a small number of parts, while the rest of the parts continued untouched.44 The tuning of the concentration of H2SO4 in electrolyte, which resulted in the oxidation degree of graphite oxide, was demonstrated where extremely oxidized GO with a C/O ratio of less than 2 was achieved in 40–60 wt % of H2SO4, whereas the top oxidation level with a C/O ratio of 1.5–1.8 was obtained in 50 wt % of H2SO4. Incompletely oxidized samples with a C/O of more than 2 were formed above and below this concentration range.1 However, Lowe et al.45 demonstrated that a high concentration of H2SO4 can facilitate the formation of GO, and the oxidation manner was improved by increasing the concentration from 2 to 16 M. In another article, with an increase of ammonium sulfate concentration, the degree of oxidation of GO was enhanced based on the obtained results, such as an increment of ID/IG ratio, O/C ratio, and C–O content and a decrement of electrical conductivity.46 Furthermore, Sahoo and Mallik also reported the same improvement, where a high concentration of HClO4 formed smaller, better dispersibility, greater oxidation level, and extra defects of GO.47 This is similar to Tian and colleagues’ results too in which oxidation happened when high concentrations of HClO4 (7.0, 9.2, and 11.6 M) were used.13 Nevertheless, mere oxidation was shown by XRD results when a low concentration of HClO4 (4.6 M) was used, and XPS results further proved that electrolyte concentration affected the quantity of oxygen functional groups at the GO significantly. However, the amount of carboxyl and carbonyl was low, from 7.2 to 9.2 M, and the alcohol and epoxy groups became dominant in graphite oxidation at the edge sites before continuing to the basal plane. Table 2 summarizes the influence of the electrolyte’s concentrations on the C/O, ID/IG, yield, thickness, and lateral sizes of GO.
Table 2. Summary of Electrolyte’s Concentrations to Form GO.
| electrolyte’s concentrations | C/O | ID/IG | GO yield | thickness | lateral sizes | synthesis rate | references |
|---|---|---|---|---|---|---|---|
| HCIO4 (0.5, 1.0, 1.5, and 2.0 M) | 1.59–1.67 | 0.96–1.04 | 40–44% | 3–6 layers | 107–140 nm | - | (47) |
| HCIO4 (4.6, 7.0, 9.2, and 11.6 M) | - | - | 19.1–37.3% | - | - | - | (13) |
| H2SO4 (2, 5, 7.1, 10, 11.6, 14, and 16 M) | - | ∼1.2–1.6 | ∼22.5% | 1.7–2 nm | 1–5 μm | - | (45) |
| (NH4)2SO4 (0.5 and 1.0 mol/L) | 3.26–3.31 | 1.66–1.69 | 29.88–32.22% | 10–11 μm | - | - | (46) |
| H2SO4:HNO3 (3:1) (0.5, 1.0, 2.0, and 3.0 M) | - | 1.22–1.24 | 7–9% | 9.16 nm | - | - | (48) |
| H2SO4 (10–100 wt %) | 1.7–5.7 | - | - | 1.0 nm | 1–10 μm | 12 g/h | (1) |
Effect of Anodic Voltages/Currents
Besides the above-mentioned parameters, anodic voltages/currents also act as one of the most influential parameters in oxidation capability. When higher currents are applied, the number of anions attracted to the anode, the intercalation, and the exfoliation and oxidation rates will be increased. A constant voltage or current is widely used in electrochemical exfoliation for certain periods from several minutes to hours. To do the preintercalation of anions into graphite, a minimum voltage (0–2 V) was applied for a short duration of 1–10 min to accumulate charges at the anode and initiate slow intercalation of anions into graphite.29,31,40,49 It is reported that the intercalation of anions requires 1–2 V, where higher voltage leads to the synthesis of graphite intercalation compounds (GICs).13,43 Furthermore, an increase of voltage more than 1.4 V causes the oxidation of GICs and the electrolysis of electrolytes as well.35,43 The production of GO can be accelerated by increasing the voltage or current,48,50 but the graphite electrode can be damaged.48 Two opposite results were reported on the thickness of GO where a H2SO4/HNO3 mixture48 formed GO sheets with the thickness reduced with increasing voltage, whereas a melt composed of acetamide, urea, and ammonium nitrate showed the opposite pattern39 because every electrolyte has unique performance in anion intercalation, exfoliation, and oxidation rates. The effectiveness of intercalation and exfoliation can affect the thickness of graphene sheets with the same electrolyte, but different voltages were used.
Besides, the applied voltages also can influence the oxidation level of GO as reported by Coros et al.48 and Tian et al.,38 which is the reason the presence of reactive radicals accelerated the oxidation process. Nonetheless, an increase of current from 0.5 to 1.0 A resulted in almost the same oxidation amount of GO being reported.33 They explained it was because the oxidation happened mainly at the edges rather than the basal planes of graphene because of the poor intercalation of anions further into the interlayer space of graphite. Therefore, besides applied current/voltage, other factors such as electrolyte, electrode material, and operation temperature can influence the oxidation degree of graphene. The influence of different anodic voltages at 2.5, 3.0, 5.5, and 6.0 V on the morphology of exfoliated graphene sheets prepared in a H2SO4/HNO3 electrolyte was investigated by Coros et al.48 They noticed that multilayered graphene (MLG), GO, and few-layered graphene (FLG) were synthesized in all mentioned voltages. The amount of GO was reduced when applied voltage was decreased, and thus, they concluded that high voltages showed the advantage to form a higher quantity of GO. The same trend also was reported when 14 V was used to form the highest thickness of the GO sample as compared with 3 V due to the piling of the highest number of GO sheets as proven in their FESEM, FTIR, and Raman results.51 Dalal et al. utilized cathodic voltages between −2 and −10 V and noticed that a minimum of −4 V was needed to start the exfoliation; however, −10 V was sufficient to form a significant amount of H2 bubbles to overcome the weak interlayer bonding for highly effective exfoliation. They also reported that a combination of voltage and concentration affected the intercalation and exfoliation significantly. A low concentration of cation failed to initiate intercalation at −10 V, whereas a high cation concentration decreased the exfoliation speed when low voltage was used.44
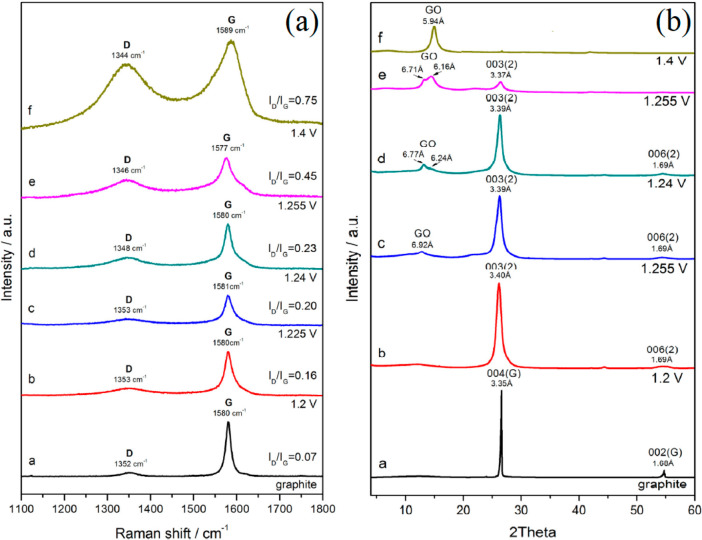
Linear sweep voltammetry (LSV) was used to exfoliate graphite into GO using a potential from rest potential to 1.4 V at a scan rate of 0.01 mV s–1 in 8 M HCIO4 as demonstrated by Gurzęda and coresearchers.35,52 In Figure 5(a), an increase of applied potential leads to the formation of a defect-exfoliated graphite structure as the D band peak intensity (around 1350 cm–1) and ID/IG ratio increased notably. In addition, the XRD results also show that the two-stage HCIO4-GIC was formed at 1.2 V, and when 1.4 V was reached, GIC was fully transformed into GO (Figure 5b). Furthermore, they also used H2SO4 as the electrolyte in LSV to form two-stage H2SO4-GIC at 0.65–1.15 V, and then, the full oxidation was completed at 1.15–1.5 V.53 Therefore, the LSV method has two benefits: (1) controllable oxidation degree of GO by adjusting the end voltage, and (2) highly efficient conversion of GICs into GO was attained. A cyclic voltammetry scan was reported as a rapid electrochemical synthesis of GO (finished about 2–5 min), which was conducted by Zeng and co-workers by using 0–3 V at a scan rate of 50 mV/s in 0.025 M phosphate buffer solution.36
Figure 5.
(a) Intensity of the D band peak (around 1350 cm–1) increased with an increase of applied potential, indicating more disordering of the graphite structure was synthesized. (b) The GO peak intensities increased significantly with increasing applied voltage, as shown in XRD patterns of graphite oxidized in 8 M HCIO4. Reprinted with permission from ref (35). Copyright 2016 Elsevier Ltd.
The switching between two opposite potentials to form GO sheets by exfoliating and oxidizing both graphite electrodes was also reported. Liu and co-workers switched the potentials between +7 and −7 V at each 5–8 min to synthesize GO within a few minutes, and the GO can be reduced effortlessly when negative voltage was applied.29 Similarly, Su and co-workers optimized the exfoliation potential by alternating between +10 and −10 V until a desired quantity of exfoliated samples was obtained. They noticed that exfoliation of the graphite anode and oxidation of graphene sheets happened at +10 V, whereas the GO sheets were reduced when −10 V was utilized.34 The summary of characteristics of GO synthesized by electrochemical exfoliation at different anodic voltages/currents is shown in Table 3.
Table 3. Summary of Anodic Voltages/Currents to Synthesize GO.
| voltages | C/O | ID/IG | GO yield | thickness | lateral sizes | synthesis rate | references |
|---|---|---|---|---|---|---|---|
| between +7 V and –7 V | - | 0.71 | - | 3–9 nm | 1–5 μm | - | (29) |
| 1.2, 1.225, 1.24, 1.255, 1.4 V | 1.43–11.55 | 0.16–0.75 | 6.63–34.2% | - | - | - | (35) |
| 6, 5.5, 5, 4 V | - | 1.09–1.22 | 10–55% | - | - | - | (48) |
| 5, 10, 15, 20, 25 V | - | ∼1.5 | 19% | 3 nm | 3 μm | - | (50) |
| 3, 5, 7, 10, 12, 14 V | - | 0.51–0.99 | - | 1–107 nm | - | - | (51) |
Effect of Volume Fractions of Electrolytes
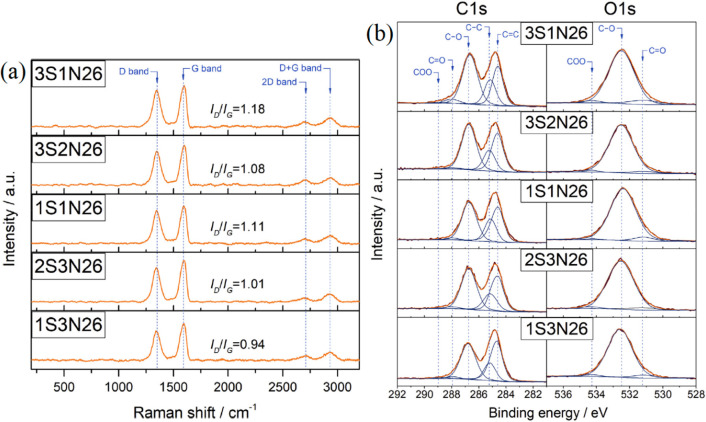
A mixture of two different types of electrolytes in different volume fractions has been shown to improve the oxidation capabilities. However, its influence is less than three key reaction parameters as discussed in a previous section due to much fewer choices of electrolytes that can be mixed. Gurzęda and Krawczyk54 conducted an electrochemical exfoliation of graphite using different volume fractions (1:3, 2:3, 1:1, 3:2, and 3:1 ratio) of 95% H2SO4:65% HNO3 electrolyte. The oxidation degree was affected significantly by the volume ratio of H2SO4/HNO3, where the 3:1 ratio with 26% of water by weight (3S1N26) formed the highest amount of graphite oxide (Figure 6). In addition, the C/O ratio of this sample was 2.09, which is similar to the graphite oxide synthesized by chemical methods. The presence of 26% of water in electrolyte helps the oxidation of a graphite intercalation compound into graphite oxide by reaction between water molecules and the surface of graphene layers. They reported that the alkoxy and/or epoxy groups were formed in the XPS results.
Figure 6.
(a) 3S1N26 had the highest ID/IG ratio (intensity of the D band to intensity of the G band) of 1.18, which implied that the highest oxidation level occurred. (b) The lowest C/O of 2.09 in 3S1N26 indicated the graphite oxide surface contained the richest amount of epoxy and/or alkoxy functional groups. Reprinted with permission from ref (54). Copyright 2019 Elsevier Ltd.
In another project, H2SO4 was mixed with HNO3 in a 3:1 ratio to prepare a 1 M mixture to improve the GO yield to 38.44% (Figure 7a). This number was further enhanced to 50.76% with more C=O and O–C=O groups after the mixture concentration was increased to 3 M48 (Figure 7b). In addition, Gurzeda et al. also showed a similar improvement in GO synthesis when the same ratio between H2SO4 and HNO3 of 3:1 with the addition of 26 wt % water was chosen.54 Aghamohammadi and Eslami-Farsani investigated the influence of different volume ratios of nitric acid/sulfuric acid on the morphology of graphene. They found that GO was formed in the mixture of H2SO4 and HNO3 with a volume ratio of 3:1 and 1:1, whereas no GO was noticed in H2SO4 only and in the mixture of H2SO4 and HNO3 with a volume ratio of 1:3. Furthermore, they highlighted that the highest degree of oxidation of graphene was archived when a volume ratio of 1:1 for H2SO4 and HNO3 was used. However, the degree of exfoliation was reduced by increasing the volume ratio of HNO3 in electrolytes, which indicated that a higher volume fraction of H2SO4 increased the exfoliation efficiency. In addition, the Raman results showed that a volume ratio of 1:1 for H2SO4 and HNO3 synthesized a lower quality of graphene with ID/IG = 0.9 due to the presence of the highest amount of graphene oxide as compared with graphene formed in the mixture of H2SO4 and HNO3 with a volume ratio of 3:1 (ID/IG = 0.77) and 1:3 (ID/IG = 0.63).55 The comparison results are summarized in Table 4 where the detailed volume ratios of electrolytes and the properties of GO are also listed.
Figure 7.
(a) Proportion of 38.44% of oxidized total carbon atoms was achieved when 1 M H2SO4/HNO3 in 3:1 ratio was used. (b) Proportion of 50.76% of oxidized total carbon atoms was achieved when 3 M H2SO4/HNO3 in a 3:1 ratio was utilized. Reprinted with permission from ref (48). Copyright 2016 The Royal Society of Chemistry.
Table 4. Summary of Volume Fractions of Electrolytes to Synthesize GO.
Effect of Graphite Electrode Materials
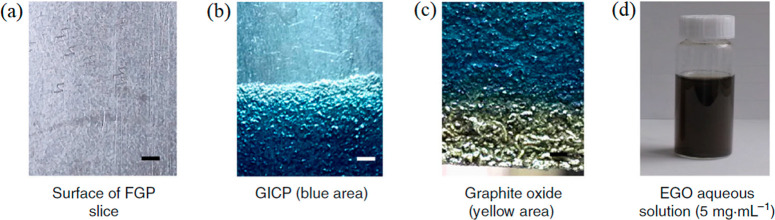
The type of graphite electrode shows the same functionality as the volume fraction of electrolytes in the electrochemical exfoliation and oxidation of graphene sheets because of its few choices of available graphite electrodes for the synthesis of GO. Because carbon is an excellent conductor and has a high melting point, it is a most suitable raw material for the electrochemical exfoliation of graphite. Thus, highly oriented pyrolytic graphite (HOPG), graphites rod, graphite flakes, and graphite foil have been reported as popular electrodes for the electrochemical synthesis of GO. The mechanical strength of graphite electrodes should be well considered for GO synthesis as it can affect the amount of GO, exfoliation rate, and efficiency. Graphite foil and flakes were exfoliated faster than HOPG and graphite rods, and therefore a lower quantity of GO was formed only.33 Moreover, expanded graphite was exfoliated in a higher efficiency, but a lower amount of GO was synthesized as compared with natural graphite.31 In addition, oxygen-containing functional groups were found primarily attached at the edges of GO, whereas the basal planes were almost free from functional groups when high density graphitic electrodes such as graphite rods were used.33 Graphite foil or flexible graphite paper which has high strength, good flexibility, and tolerance to the volume expansion was used for preintercalation in concentrated 98% H2SO4.1 Then, it was followed by oxidation in 50% H2SO4 within a few seconds (Figure 8). The pencil core was also utilized as an electrode in electrochemical exfoliation to form a high purity of GO samples.40
Figure 8.
(a) Flexible graphite paper (FGP). (b) Graphite intercalation compound paper (GICP) (blue area) achieved after preintercalation of FGP at a potential of 1.6 V for 20 min in 98 wt % of H2SO4. (c) Graphite oxide (yellow area) formed after oxidation of GICP in 50 wt % H2SO4 at 5 V for 30 s. (d) Electrochemically synthesized GO (EGO) dispersion attained by sonication of the graphite oxide in water for 5 min. Reprinted with permission from ref (1). Copyright 2018 Springer Nature.
In most articles, a single bulk graphite foil or rod electrode has been used to synthesize graphene sheets, but the oxidation level, amount, and scalable production are limited. The same issues were also highlighted by Chen et al.46 when they noticed that graphite flakes induced the formation of a smaller size and two to three layers of GO as compared with GO exfoliated from graphite foil using the same experimental conditions. These issues can be solved by using graphite flakes with a mechanically assisted electrochemical approach to synthesize GO as reported by Yu and coresearchers.27 The synthesized GO showed a good level of oxidation without much more defect structures than chemically derived GO. The synthesis of GO from graphite rods extracted from unused dry cell batteries has been demonstrated as well, where the degree of oxidation was estimated to be below 24.1% from TGA and SEM-EDS results as compared with ∼43.5% by Hummers’ method.56 It implies that waste graphite rods were suitable as raw materials, which were recycled for the synthesis of GO.57Table 5 summarizes several types of graphite electrode materials used for the synthesis of GO with different characteristics.
Table 5. Summary of Graphite Electrode Materials to Prepare GO.
Effect of Operating Temperature
The operating temperature showed the least effect as compared with other key reaction parameters. The researchers claim that graphene’s negative thermal expansion coefficient causes the high-temperature resonance. Nevertheless, room temperature has been reported as the favorite temperature for the high synthesis of GO, but the temperature can create some side effects in the process. One of the obvious sdie effects is that the interlayer spacing of graphite was increased because more energy was provided by heat to the ions to intercalate into graphite.58 Also, the decrease of the van der Waals interaction after interlayer spacing was increased slightly, and the intercalation of ions was also induced. Another side effect is that the exfoliated synthesis yield clearly increased ∼4.5 times (from ∼17% to ∼77%) as compared with room-temperature synthesis.59 However, the amount of GO can be decreased by the formation of rich bubbles during heating.60 Therefore, further study to reduce heating issues which reduced the synthesis amount of GO is highly needed.61
Advancement in the Synthesis of GO
Electrochemical oxidation shows some advantages as compared with Brodie, Staudenmaier, and Hummers’ method but is still unable to synthesize GO in a large scale at shorter time even though so many efforts to improve the GO yield have been made. In a typical electrochemical exfoliation of graphite, exfoliated graphene acts nearly similar to pristine graphene with very low oxygen-containing functional groups attached at the graphene sheets.43 In view of this problem, some researchers demonstrated the two-step electrochemical exfoliation and oxidation of graphite to encounter the synthesis of a low amount of GO. There are two different concentrations of electrolytes needed in this method. Graphite intercalation compounds (GICs) are formed when electrolyte intercalants are inserted into the tiny space between layers of graphite raw material. In the first stage, concentrated electrolyte is required to form GICs without any oxidation due to a very low amount of water molecules in the electrolyte, whereas a diluted electrolyte with a large quantity of water is needed in the second stage to form graphite oxide because of the presence of oxygen radicals that react with the carbon lattice to build oxygen-containing functional groups. In other words, the GICs are crucial as intermediates to enhance the formation of hydroxyl and epoxide groups, implying less damage to the graphitic structure in the GO.43 Besides, the bubbles oxygen formed from the oxidation of inserted water molecules induce the formation of GO sheets from bulk GIC.62 However, in the traditional electrochemical exfoliation of graphite rods, mild surface oxidation happens at edges in a short time only because at the same time the intercalated water molecules are decomposed into oxygen gas which eases the expansion and exfoliation of graphite by sulfate anions.28 A low oxidation degree has been reported in conventional techniques for two main reasons: (1) GICs are unable to be formed when a high amount of water is used,63 and (2) GICs have an important role as an intermediate in the synthesis of graphite oxide, which was reported in both electrochemical and chemical methods. Therefore, the intercalated water molecules favor a break down into a tremendous quantity of oxygen molecules that expand and exfoliate graphite rapidly, leading to a broken circuit which stops the oxidation immediately.64,16
Pei et al. used concentrated H2SO4 in the first stage, followed by 50 wt % of H2SO4 in the second stage. They observed that a C/O ratio of GO can be changed simply by adjusting the concentration of H2SO4 (Figure 9a), and 50 wt % of H2SO4 formed the highest amount of oxygen-containing functional groups of GO.1 In Figure 9(b), the presence of a 10° peak indicates the success of the two-step method to synthesize GO in 3 min which is over 100 times faster as compared with few days oxidation in Hummers’ method and without any oxidants was used to leave metal ion impurities in the GO samples. Thus, H2SO4 can be fully reused, and the exfoliation showed no explosive risk. In addition, much a simpler cleaning process was conducted because diluted H2SO4 only was used. With these advantages, low-cost and large-scale production for industrial and application demands can be achieved.1
Figure 9.
(a) Changes of exfoliation rate and C/O atomic ratio of GICP with H2SO4 concentrations. (b) XRD patterns after GICP oxidation for different times. Reprinted with permission from ref (1). Copyright 2018 Springer Nature.
Besides, the electrochemical exfoliation process, intercalation, exfoliation, and oxidation are influenced by voltage significantly as demonstrated by Cao et al.43 They noticed that 1.8–2.2 V formed the pure stage 1 GIC, whereas the oxygen-containing functional groups were synthesized in stage 1 GIC by increasing the voltage to 2.4 V as proved by the presence of a wide D band at 1370 cm–1 and a low intensity of the G band at 1608 cm–1. Furthermore, Wu also reported that higher voltage of 5 V can provide a higher oxidation degree than that of 2 V.31 Pet et al.50 demonstrated that the oxygen content increased with increasing voltage and reached 19%, but it still lower as compared with GO synthesized by Hummers’ method (Figure 10).
Figure 10.

Changes of O 1s spectra and oxygen content with applied voltage. Reprinted with permission from ref (50). Copyright 2021 Springer Nature.
The sonication could help to break the bulky size graphite particles into tiny graphene and GO flakes. Kubota et al. reported that after the sonication was conducted the color of the supernatant was changed into brown, indicating the presence of dispersed GO sheets.65 In other words, the two-step electrochemical exfoliation increased the interlayer distances of graphite sheets and thus damaged the interaction between layers. Therefore, with the help of sonication, the GO flakes were separated from exfoliated bulky graphite. It is interesting to know that the combination of electrochemical exfoliation and Hummers’ methods was proved to enhance the amount of GO successfully.66 The graphite anode was exfoliated using a mixture of H2SO4 and H3PO4 in the first step, whereas oxidation was conducted for 1–12 h at ∼60 °C to the exfoliated graphene layers using KMnO4 as an oxidizing solution in the second step. The researchers claimed that 6 h of oxidation synthesized the maximum quantity of GO based on the FTIR, XRD, and Raman results. Sun et al.67 demonstrated the application of tap water as an electrolyte to form GO, therefore preventing the use of strong acids such as sulfuric acid which is not environmentally friendly. The carbon fiber sheets and graphite rod were compared at the same electrical current where carbon fiber sheets showed preferable results such as synthesis of GO in larger amounts, easy control of GO sheets’ sizes, and lesser layers overlapping with each other as compared with graphite rods. However, the number of smaller sizes of GO increased with increasing electrical current, and the size range relies on the types of electrodes.
Conclusion and Perspectives
Over the past many years, the amazing quantity of works dedicated to GO has resulted in a remarkable amount of publications related to the synthesis, modifications, applications, and characterizations of GO. The enduring developments in the formation of GO synthesis have definitely opened up new fascinating chemical modifications of GO for particular applications. With the demand from various applications, the current progress in the synthesis methods of GO is highly needed, and thus, there is a need for a critical review of the effects of each reaction parameter to improve the yield and quality of GO. Regardless of all the promising results achieved so far, whether studies in this field are coming to an end is greatly dependent on the further exploration of GO applications in various field and the ability to keep researchers motivated to synthesize it. The recent and near future market for GO applications is driven by the production strategies for this material. Once the production approach is mature enough, an extensive application of GO can be achieved. The recent trend of GO with less-than-perfect has long been applied in certain applications. In fact, different grades of GO are needed by different applications. Basically, the GO synthesis methods that produce the lowest grade, cheapest production cost, and low quantity of GO will be the first to be sustained for many years, and those methods which form the highest grade and amount of GO may well need decades to develop. The good news is that graphene’s prospects continue to improve after extreme fast developments in the past few years. To find a broader range of applications, the fundamental mechanisms of the electrochemical synthesis improvement of GO must be fully understood. To reach this goal, massive research into the mechanistic details of exfoliation as well as the rapid oxidation is essential. Therefore, we have to study the current challenges and find the solutions to tackle them.
Current challenges and solutions in the electrochemical synthesis of GO:
1. Various electrode materials such as pencil rods, graphite papers, and graphite rods have been used to synthesize GO in a single-step electrochemical process. Sadly, the wanted oxidation degree failed to be completed due to the broken electrical conductivity from the anode electrode when the intercalation of the anion caused the expansion and exfoliation of graphite at an anode rapidly. Thus, the final sample contains a very small quantity of GO which is much less than the quantity of GO prepared using Hummers’ method. If the intercalation and oxidation steps are conducted in two different steps as demonstrated by Pei et al.,1 the yield of GO can be enhanced significantly. Furthermore, it is encouraging that when sonication is conducted in the third step, we get thin GO sheets.
2. It is necessary to point out that the poor mechanical structure of graphite rods is not suitable for the two-step process: intercalation and oxidation due to the graphite rod being easily fragmented into tiny pieces, causing it to not be usable for oxidation in the next step. Furthermore, it also explains the reason that a very low amount of GO is synthesized using graphite rods in a single-step electrochemical exfoliation. Graphite powders or carbon black is also not appropriate because of the tiny particles which needed to be built into a graphite rod before it can be used as an electrode. A study of the synthesis of few-layer graphene-like sheets from powder-based carbon black was conducted, and no GO was formed,68 which indicates graphite powders are not suitable to be used.
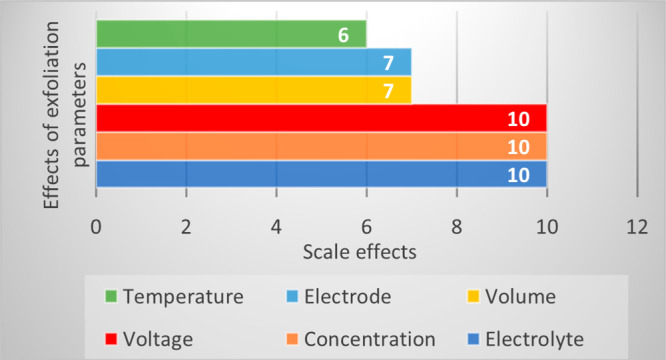
Advancement in research and development opportunities exists for GO in the future. The capability to customize and produce large quantities of GO is necessary, and an understanding and manipulation of the synthesis process will be the main factors to achieve breakthroughs in this frontier research. Therefore, we hope that this review will encourage more exploration and intense efforts from the industry and academia to revolutionize the synthesis methods of GO in a greener way. Lastly, there are six exfoliation parameters that we encounter that can affect the amount of GO: voltage, electrolyte’s concentration, and type of electrolyte represent the most significant effects, whereas the type of electrode and volume fractions have smaller impacts. Thus, by optimizing applied voltage, the electrolyte’s concentration, and type of electrolyte, we believe that the yield of GO can be enhanced easily to fulfill the industries’ demand.
Acknowledgments
The authors would like to acknowledge the support from the Fundamental Research Grant Scheme (FRGS) under two grant numbers, FRGS/1/2015/TK02/UNIMAP/02/5 and FRGS/1/2014/TK06/UNIMAP/02/1, from the Ministry of Higher Education Malaysia for the financial support of this research work. We are grateful to Subash Chandra Bose Gopinath for his advice in helping us to revise the manuscript.
Author Contributions
All authors contributed to manuscript writing and revision. The manuscript was edited by Wei-Wen Liu.
The authors declare no competing financial interest.
References
- Pei S.; Wei Q.; Huang K.; Cheng H. M.; Ren W. Green Synthesis of Graphene Oxide by Seconds Timescale Water Electrolytic Oxidation. Nat. Commun. 2018, 9 (1), 1–9. 10.1038/s41467-017-02479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.; Lin Z.; Sartin M. M.; Huang T. X.; Liu J.; Zhang Q.; Han L.; Li J. F.; Tian Z. Q.; Zhan D. Photosynergetic Electrochemical Synthesis of Graphene Oxide. J. Am. Chem. Soc. 2020, 142 (14), 6516–6520. 10.1021/jacs.0c02158. [DOI] [PubMed] [Google Scholar]
- Chen S.; Qiu L.; Cheng H.-M. Carbon-Based Fibers for Advanced Electrochemical Energy Storage Devices. Chem. Rev. 2020, 120 (5), 2811–2878. 10.1021/acs.chemrev.9b00466. [DOI] [PubMed] [Google Scholar]
- Zhang L. L.; Zhao X. S. Carbon-Based Materials as Supercapacitor Electrodes. Chem. Soc. Rev. 2009, 38 (9), 2520–2531. 10.1039/b813846j. [DOI] [PubMed] [Google Scholar]
- Avouris P.; Chen Z.; Perebeinos V. Carbon-Based Electronics. Nat. Nanotechnol. 2007, 2 (10), 605–615. 10.1038/nnano.2007.300. [DOI] [PubMed] [Google Scholar]
- Ambrosi A.; Chua C. K.; Bonanni A.; Pumera M. Electrochemistry of Graphene and Related Materials. Chem. Rev. 2014, 114 (14), 7150–7188. 10.1021/cr500023c. [DOI] [PubMed] [Google Scholar]
- Chakrabarti M. H.; Low C. T. J.; Brandon N. P.; Yufit V.; Hashim M. A.; Irfan M. F.; Akhtar J.; Ruiz-Trejo E.; Hussain M. A. Progress in the Electrochemical Modification of Graphene-Based Materials and Their Applications. Electrochim. Acta 2013, 107, 425–440. 10.1016/j.electacta.2013.06.030. [DOI] [Google Scholar]
- Li L.; et al. Review-Preparation and Application of Graphene-Based Hybrid Materials through Electrochemical Exfoliation. J. Electrochem. Soc. 2020, 167, 086511. 10.1149/1945-7111/ab933b. [DOI] [Google Scholar]
- Li L.; Zhang D.; Cao M.; Deng J.; Ji X.; Wang Q. Electrochemical Synthesis of 2D Antimony, Bismuth and Their Compounds. J. Mater. Chem. C 2020, 8 (28), 9464–9475. 10.1039/D0TC01960G. [DOI] [Google Scholar]
- Li L.; Zhang D.; Deng J.; Gou Y.; Fang J. Electrochemical Exfoliation of Two-Dimensional Layered Black Phosphorus and Applications. J. Energy Chem. 2020, 49, 365–374. 10.1016/j.jechem.2020.03.010. [DOI] [Google Scholar]
- Li L.; Zhang D.; Gao Y.; Deng J.; Gou Y.; Fang J. Electric Field Driven Exfoliation of MoS2. J. Alloys Compd. 2021, 862, 158551. 10.1016/j.jallcom.2020.158551. [DOI] [Google Scholar]
- Li L.; Zhang D.; Deng J.; Kang Q.; Liu Z.; Fang J.; Gou Y. Review—Progress of Research on the Preparation of Graphene Oxide via Electrochemical Approaches. J. Electrochem. Soc. 2020, 167 (15), 155519. 10.1149/1945-7111/abbbc0. [DOI] [Google Scholar]
- Tian Z.; Yu P.; Lowe S. E.; Pandolfo A. G.; Gengenbach T. R.; Nairn K. M.; Song J.; Wang X.; Zhong Y. L.; Li D. Facile Electrochemical Approach for the Production of Graphite Oxide with Tunable Chemistry. Carbon N. Y. 2017, 112, 185–191. 10.1016/j.carbon.2016.10.098. [DOI] [Google Scholar]
- Abdelkader A. M.; Kinloch I. A.; Dryfe R. A. W. High-Yield Electro-Oxidative Preparation of Graphene Oxide. Chem. Commun. 2014, 50 (61), 8402–8404. 10.1039/C4CC03260H. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. E. D.; Stolle A.; Stelter M. Sustainable Synthesis of High-Surface-Area Graphite Oxide via Dry Ball Milling. ACS Sustain. Chem. Eng. 2018, 6 (5), 6358–6369. 10.1021/acssuschemeng.8b00147. [DOI] [Google Scholar]
- Chen J.; Chen W.; Song D.; Lai B.; Sheng Y.; Yan L. The Solvent-Free Mechanochemical Synthesis of Mildly Oxidized Graphene Oxide and Its Application as a Novel Conductive Surfactant. New J. Chem. 2019, 43 (18), 7057–7064. 10.1039/C9NJ00529C. [DOI] [Google Scholar]
- Muthoosamy K.; Manickam S. State of the Art and Recent Advances in the Ultrasound-Assisted Synthesis, Exfoliation and Functionalization of Graphene Derivatives. Ultrason. Sonochem. 2017, 39, 478–493. 10.1016/j.ultsonch.2017.05.019. [DOI] [PubMed] [Google Scholar]
- Dreyer D. R.; Todd A. D.; Bielawski C. W. Harnessing the Chemistry of Graphene Oxide. Chem. Soc. Rev. 2014, 43 (15), 5288–5301. 10.1039/C4CS00060A. [DOI] [PubMed] [Google Scholar]
- Dou Z.; Qin Z.; Shen Y.; Hu S.; Liu N.; Zhang Y. High–Performance Flexible Supercapacitor Based on Carbon Cloth through in–Situ Electrochemical Exfoliation and Re–Deposition in Neutral Electrolyte. Carbon N. Y. 2019, 153, 617–624. 10.1016/j.carbon.2019.07.073. [DOI] [Google Scholar]
- Zhou Q.; Zhao Z.; Zhang Y.; Meng B.; Zhou A.; Qiu J. Graphene Sheets from Graphitized Anthracite Coal: Preparation, Decoration, and Application. Energy Fuels 2012, 26 (8), 5186–5192. 10.1021/ef300919d. [DOI] [Google Scholar]
- Wang J.; Wang C. Watermelon-like Metallic Co/Graphene-like Nanohybrids from Electrochemical Exfoliation of Anthracite Coal as Superior Oxygen Reduction Reaction Electrocatalyst. ACS Sustain. Chem. Eng. 2019, 7 (14), 12457–12463. 10.1021/acssuschemeng.9b02029. [DOI] [Google Scholar]
- He M.; Guo X.; Huang J.; Shen H.; Zeng Q.; Wang L. Mass Production of Tunable Multicolor Graphene Quantum Dots from an Energy Resource of Coke by a One-Step Electrochemical Exfoliation. Carbon N. Y. 2018, 140, 508–520. 10.1016/j.carbon.2018.08.067. [DOI] [Google Scholar]
- Zhong Y. L.; Tian Z.; Simon G. P.; Li D. Scalable Production of Graphene via Wet Chemistry: Progress and Challenges. Materials Today. 2015, 18, 73. 10.1016/j.mattod.2014.08.019. [DOI] [Google Scholar]
- Novoselov K. S.; Fal’Ko V. I.; Colombo L.; Gellert P. R.; Schwab M. G.; Kim K. A Roadmap for Graphene. Nature 2012, 490 (7419), 192–200. 10.1038/nature11458. [DOI] [PubMed] [Google Scholar]
- Ferrari A. C.; Bonaccorso F.; Fal’ko V.; Novoselov K. S.; Roche S.; Bøggild P.; Borini S.; Koppens F. H. L.; Palermo V.; Pugno N.; Garrido J. A.; Sordan R.; Bianco A.; Ballerini L.; Prato M.; Lidorikis E.; Kivioja J.; Marinelli C.; Ryhänen T.; Morpurgo A.; Coleman J. N.; Nicolosi V.; Colombo L.; Fert A.; Garcia-Hernandez M.; Bachtold A.; Schneider G. F.; Guinea F.; Dekker C.; Barbone M.; Sun Z.; Galiotis C.; Grigorenko A. N.; Konstantatos G.; Kis A.; Katsnelson M.; Vandersypen L.; Loiseau A.; Morandi V.; Neumaier D.; Treossi E.; Pellegrini V.; Polini M.; Tredicucci A.; Williams G. M.; Hee Hong B.; Ahn J. H.; Min Kim J.; Zirath H.; Van Wees B. J.; Van Der Zant H.; Occhipinti L.; Di Matteo A.; Kinloch I. A.; Seyller T.; Quesnel E.; Feng X.; Teo K.; Rupesinghe N.; Hakonen P.; Neil S. R. T.; Tannock Q.; Löfwander T.; Kinaret J. Science and Technology Roadmap for Graphene, Related Two-Dimensional Crystals, and Hybrid Systems. Nanoscale 2015, 7 (11), 4598–4810. 10.1039/C4NR01600A. [DOI] [PubMed] [Google Scholar]
- Kakaei K.; Hasanpour K. Synthesis of Graphene Oxide Nanosheets by Electrochemical Exfoliation of Graphite in Cetyltrimethylammonium Bromide and Its Application for Oxygen Reduction. J. Mater. Chem. A 2014, 2 (37), 15428–15436. 10.1039/C4TA03026E. [DOI] [Google Scholar]
- Yu P.; Tian Z.; Lowe S. E.; Song J.; Ma Z.; Wang X.; Han Z. J.; Bao Q.; Simon G. P.; Li D.; Zhong Y. L. Mechanically-Assisted Electrochemical Production of Graphene Oxide. Chem. Mater. 2016, 28 (22), 8429–8438. 10.1021/acs.chemmater.6b04415. [DOI] [Google Scholar]
- Parvez K.; Wu Z.-S.; Li R.; Liu X.; Graf R.; Feng X.; Müllen K. Exfoliation of Graphite into Graphene in Aqueous Solutions of Inorganic Salts. J. Am. Chem. Soc. 2014, 136 (16), 6083–6091. 10.1021/ja5017156. [DOI] [PubMed] [Google Scholar]
- Liu J.; Yang H.; Zhen S. G.; Poh C. K.; Chaurasia A.; Luo J.; Wu X.; Yeow E. K. L.; Sahoo N. G.; Lin J.; Shen Z. A Green Approach to the Synthesis of High-Quality Graphene Oxide Flakes via Electrochemical Exfoliation of Pencil Core. RSC Adv. 2013, 3 (29), 11745–11750. 10.1039/c3ra41366g. [DOI] [Google Scholar]
- Xia Z. Y.; Pezzini S.; Treossi E.; Giambastiani G.; Corticelli F.; Morandi V.; Zanelli A.; Bellani V.; Palermo V. The Exfoliation of Graphene in Liquids by Electrochemical, Chemical, and Sonication-Assisted Techniques: A Nanoscale Study. Adv. Funct. Mater. 2013, 23 (37), 4684–4693. 10.1002/adfm.201203686. [DOI] [Google Scholar]
- Wu L.; Li W.; Li P.; Liao S.; Qiu S.; Chen M.; Guo Y.; Li Q.; Zhu C.; Liu L. Powder, Paper and Foam of Few-Layer Graphene Prepared in High Yield by Electrochemical Intercalation Exfoliation of Expanded Graphite. Small 2014, 10 (7), 1421–1429. 10.1002/smll.201302730. [DOI] [PubMed] [Google Scholar]
- Sahoo S. K.; Ratha S.; Rout C. S.; Mallik A. Physicochemical Properties and Supercapacitor Behavior of Electrochemically Synthesized Few Layered Graphene Nanosheets. J. Solid State Electrochem. 2016, 20 (12), 3415–3428. 10.1007/s10008-016-3304-6. [DOI] [Google Scholar]
- Parvez K.; Rincón R. A.; Weber N. E.; Cha K. C.; Venkataraman S. S. One-Step Electrochemical Synthesis of Nitrogen and Sulfur Co-Doped, High-Quality Graphene Oxide. Chem. Commun. 2016, 52 (33), 5714–5717. 10.1039/C6CC01250G. [DOI] [PubMed] [Google Scholar]
- Su C. Y.; Lu A. Y.; Xu Y.; Chen F. R.; Khlobystov A. N.; Li L. J. High-Quality Thin Graphene Films from Fast Electrochemical Exfoliation. ACS Nano 2011, 5 (3), 2332–2339. 10.1021/nn200025p. [DOI] [PubMed] [Google Scholar]
- Gurzȩda B.; Florczak P.; Kempiński M.; Peplińska B.; Krawczyk P.; Jurga S. Synthesis of Graphite Oxide by Electrochemical Oxidation in Aqueous Perchloric Acid. Carbon N. Y. 2016, 100, 540–545. 10.1016/j.carbon.2016.01.044. [DOI] [Google Scholar]
- Zeng F.; Sun Z.; Sang X.; Diamond D.; Lau K. T.; Liu X.; Su D. S. In Situ One-Step Electrochemical Preparation of Graphene Oxide Nanosheet-Modified Electrodes for Biosensors. ChemSusChem 2011, 4 (11), 1587–1591. 10.1002/cssc.201100319. [DOI] [PubMed] [Google Scholar]
- Lou F.; Buan M. E. M.; Muthuswamy N.; Walmsley J. C.; Rønning M.; Chen D. One-Step Electrochemical Synthesis of Tunable Nitrogen-Doped Graphene. J. Mater. Chem. A 2016, 4 (4), 1233–1243. 10.1039/C5TA08038J. [DOI] [Google Scholar]
- Tian S.; Yang S.; Huang T.; Sun J.; Wang H.; Pu X.; Tian L.; He P.; Ding G.; Xie X. One-Step Fast Electrochemical Fabrication of Water-Dispersible Graphene. Carbon. 2017, 111, 617. 10.1016/j.carbon.2016.10.044. [DOI] [Google Scholar]
- Zhang Y.; Xu Y.; Zhu J.; Li L.; Du X.; Sun X. Electrochemically Exfoliated High-Yield Graphene in Ambient Temperature Molten Salts and Its Application for Flexible Solid-State Supercapacitors. Carbon N. Y. 2018, 10.1016/j.carbon.2017.11.002. [DOI] [Google Scholar]
- Singh V. V.; Gupta G.; Batra A.; Nigam A. K.; Boopathi M.; Gutch P. K.; Tripathi B. K.; Srivastava A.; Samuel M.; Agarwal G. S.; Singh B.; Vijayaraghavan R. Greener Electrochemical Synthesis of High Quality Graphene Nanosheets Directly from Pencil and Its SPR Sensing Application. Adv. Funct. Mater. 2012, 22 (11), 2352–2362. 10.1002/adfm.201102525. [DOI] [Google Scholar]
- Tang H.; He P.; Huang T.; Cao Z.; Zhang P.; Wang G.; Wang X.; Ding G.; Xie X. Electrochemical Method for Large Size and Few-Layered Water-Dispersible Graphene. Carbon. 2019, 143, 559–563. 10.1016/j.carbon.2018.11.058. [DOI] [Google Scholar]
- Wang H. S.; Tian S. Y.; Yang S. W.; Wang G.; You X. F.; Xu L. X.; Li Q. T.; He P.; Ding G. Q.; Liu Z.; Xie X. M. Anode Coverage for Enhanced Electrochemical Oxidation: A Green and Efficient Strategy towards Water-Dispersible Graphene. Green Chem. 2018, 20 (6), 1306–1315. 10.1039/C7GC03345A. [DOI] [Google Scholar]
- Cao J.; He P.; Mohammed M. A.; Zhao X.; Young R. J.; Derby B.; Kinloch I. A.; Dryfe R. A. W. Two-Step Electrochemical Intercalation and Oxidation of Graphite for the Mass Production of Graphene Oxide. J. Am. Chem. Soc. 2017, 139 (48), 17446–17456. 10.1021/jacs.7b08515. [DOI] [PubMed] [Google Scholar]
- Dalal M. H.; Lee C. Y.; Wallace G. G. Cathodic Exfoliation of Graphite into Graphene Nanoplatelets in Aqueous Solution of Alkali Metal Salts. J. Mater. Sci. 2021, 56 (4), 3612–3622. 10.1007/s10853-020-05468-8. [DOI] [Google Scholar]
- Lowe S. E.; Shi G.; Zhang Y.; Qin J.; Jiang L.; Jiang S.; Al-Mamun M.; Liu P.; Zhong Y. L.; Zhao H. The Role of Electrolyte Acid Concentration in the Electrochemical Exfoliation of Graphite: Mechanism and Synthesis of Electrochemical Graphene Oxide. Nano Mater. Sci. 2019, 1 (3), 215–223. 10.1016/j.nanoms.2019.07.001. [DOI] [Google Scholar]
- Chen J.; Perez-Page M.; Ji Z.; Zhang Z.; Guo Z.; Holmes S. One Step Electrochemical Exfoliation of Natural Graphite Flakes into Graphene Oxide for Polybenzimidazole Composite Membranes Giving Enhanced Performance in High Temperature Fuel Cells. J. Power Sources 2021, 491, 229550. 10.1016/j.jpowsour.2021.229550. [DOI] [Google Scholar]
- Sahoo S. K.; Mallik A. Synthesis and Characterization of Conductive Few Layered Graphene Nanosheets Using an Anionic Electrochemical Intercalation and Exfoliation Technique. J. Mater. Chem. C 2015, 3 (41), 10870–10878. 10.1039/C5TC01893E. [DOI] [Google Scholar]
- Coros M.; Pogacean F.; Rosu M.-C.; Socaci C.; Borodi G.; Magerusan L.; Biris A. R.; Pruneanu S. Simple and Cost-Effective Synthesis of Graphene by Electrochemical Exfoliation of Graphite Rods. RSC Adv. 2016, 6 (4), 2651–2661. 10.1039/C5RA19277C. [DOI] [Google Scholar]
- Ambrosi A.; Pumera M. Electrochemically Exfoliated Graphene and Graphene Oxide for Energy Storage and Electrochemistry Applications. Chem. Eur. J. 2016, 22 (1), 153–159. 10.1002/chem.201503110. [DOI] [PubMed] [Google Scholar]
- Pei J.; Zhang T.; Suo H. Graphene Preparation and Process Parameters by Pre-Intercalation Assisted Electrochemical Exfoliation of Graphite. J. Solid State Electrochem. 2021, 25 (4), 1245–1257. 10.1007/s10008-021-04899-w. [DOI] [Google Scholar]
- Nurhafizah M. D.; Suriani A. B.; Mohamed A.; Soga T. Effect of Voltage Applied for Graphene Oxide/Latex Nanocomposites Produced via Electrochemical Exfoliation and Its Application as Conductive Electrodes. Diam. Relat. Mater. 2020, 101, 107624. 10.1016/j.diamond.2019.107624. [DOI] [Google Scholar]
- Gurzęda B.; Florczak P.; Wiesner M.; Kempiński M.; Jurga S.; Krawczyk P. Graphene Material Prepared by Thermal Reduction of the Electrochemically Synthesized Graphite Oxide. RSC Adv. 2016, 6 (67), 63058–63063. 10.1039/C6RA10903A. [DOI] [Google Scholar]
- Gurzęda B.; Buchwald T.; Nocuń M.; Bakowicz A.; Krawczyk P. Graphene Material Preparation through Thermal Treatment of Graphite Oxide Electrochemically Synthesized in Aqueous Sulfuric Acid. RSC Adv. 2017, 7 (32), 19904–19911. 10.1039/C7RA01678F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurzęda B.; Krawczyk P. Electrochemical Formation of Graphite Oxide from the Mixture Composed of Sulfuric and Nitric Acids. Electrochim. Acta 2019, 310, 96–103. 10.1016/j.electacta.2019.04.088. [DOI] [Google Scholar]
- Aghamohammadi H.; Eslami-Farsani R. An Experimental Investigation on the Sulfur and Nitrogen Co-Doping and Oxidation of Prepared Graphene by Electrochemical Exfoliation of Pencil Graphite Rods. Ceram. Int. 2020, 46 (18), 28860–28869. 10.1016/j.ceramint.2020.08.052. [DOI] [Google Scholar]
- Bandi S.; Ravuri S.; Peshwe D. R.; Srivastav A. K. Graphene from Discharged Dry Cell Battery Electrodes. J. Hazard. Mater. 2019, 366, 358–369. 10.1016/j.jhazmat.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Neog A.; Biswas R.; Bharali N. A Novel Electrochemical Synthesis of Graphene Oxide: Converting Waste Product to Utility. Epl 2022, 137 (1), 16001. 10.1209/0295-5075/ac40ea. [DOI] [Google Scholar]
- Hsieh C.-T.; Hsueh J.-H. Electrochemical Exfoliation of Graphene Sheets from a Natural Graphite Flask in the Presence of Sulfate Ions at Different Temperatures. RSC Adv. 2016, 6 (69), 64826–64831. 10.1039/C6RA15447F. [DOI] [Google Scholar]
- Tripathi P.; Prakash Patel C. R.; Dixit A.; Singh A. P.; Kumar P.; Shaz M. A.; Srivastava R.; Gupta G.; Dhawan S. K.; Gupta B. K.; Srivastava O. N. High Yield Synthesis of Electrolyte Heating Assisted Electrochemically Exfoliated Graphene for Electromagnetic Interference Shielding Applications. RSC Adv. 2015, 5 (25), 19074–19081. 10.1039/C4RA17230B. [DOI] [Google Scholar]
- Hossain S. T.; Wang R. Electrochemical Exfoliation of Graphite: Effect of Temperature and Hydrogen Peroxide Addition. Electrochim. Acta 2016, 216, 253–260. 10.1016/j.electacta.2016.09.022. [DOI] [Google Scholar]
- Fang S.; Lin Y.; Hu Y. H. Recent Advances in Green, Safe, and Fast Production of Graphene Oxide via Electrochemical Approaches. ACS Sustain. Chem. Eng. 2019, 7 (15), 12671–12681. 10.1021/acssuschemeng.9b02794. [DOI] [Google Scholar]
- Dimiev A. M.; Tour J. M. Mechanism of Graphene Oxide Formation. ACS Nano 2014, 8 (3), 3060–3068. 10.1021/nn500606a. [DOI] [PubMed] [Google Scholar]
- Beck F.; Jiang J.; Krohn H. Potential Oscillations during Galvanostatic Overoxidation of Graphite in Aqueous Sulphuric Acids. J. Electroanal. Chem. 1995, 389 (1), 161–165. 10.1016/0022-0728(95)03870-M. [DOI] [Google Scholar]
- Yang S.; Brüller S.; Wu Z.-S.; Liu Z.; Parvez K.; Dong R.; Richard F.; Samorì P.; Feng X.; Müllen K. Organic Radical-Assisted Electrochemical Exfoliation for the Scalable Production of High-Quality Graphene. J. Am. Chem. Soc. 2015, 137 (43), 13927–13932. 10.1021/jacs.5b09000. [DOI] [PubMed] [Google Scholar]
- Kubota W.; Utsunomiya T.; Ichii T.; Sugimura H. Local Current Mapping of Electrochemically-Exfoliated Graphene Oxide by Conductive AFM. Jpn. J. Appl. Phys. 2020, 59, SN1001. 10.35848/1347-4065/ab80df. [DOI] [Google Scholar]
- Kumar N.; Srivastava V. C. Simple Synthesis of Large Graphene Oxide Sheets via Electrochemical Method Coupled with Oxidation Process. ACS Omega 2018, 3 (8), 10233–10242. 10.1021/acsomega.8b01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.; Xu G.; Lian W.; Kastiukas G.; Zhang J.; Zhang X.; Liu W.; Xing F.; Ren J. Electrochemical Synthesis and Property Characterisation of Graphene Oxide Using Water as Electrolyte. Chem. Phys. Lett. 2022, 786 (3688), 139206. 10.1016/j.cplett.2021.139206. [DOI] [Google Scholar]
- Sharief S. A.; Susantyoko R. A.; Alhashem M.; Almheiri S. Synthesis of Few-Layer Graphene-like Sheets from Carbon-Based Powders via Electrochemical Exfoliation, Using Carbon Black as an Example. J. Mater. Sci. 2017, 52 (18), 11004–11013. 10.1007/s10853-017-1275-3. [DOI] [Google Scholar]