Abstract
Background
Handover of anaesthesia patient care during surgery is common; however, its association with patient outcome is unclear. This systematic review aimed to assess the impact of anaesthesia handover during surgery on patient outcome.
Methods
All prospective and retrospective clinical studies specifically investigating the association of intraoperative transfer of anaesthesia care between anaesthesia providers in the operating room with patient morbidity and mortality were included. Searches were conducted from inception to April 24, 2019 in Medline, Medline in Process, CINAHL, and Embase. Reference lists of included studies were searched. Studies were assessed for eligibility and data were extracted by independent reviewers in duplicate with disagreements resolved by consensus or a third reviewer. Risk of bias was assessed in duplicate using the National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Data were summarised narratively given substantial heterogeneity. An exploratory meta-analysis was conducted using a random-effects model for a subset of comparable studies.
Results
Eight studies met the inclusion criteria. Six studies focused on patients as the unit of analysis (npatients=605 678) and two focused on anaesthesia providers as the unit of analysis (nproviders=307). Seven studies identified a relationship between anaesthesia handovers and adverse patient outcomes, whereas one suggested that handover may be beneficial to error detection or rectification. Included studies were of fair or good quality. Meta-analysis of four studies found a 40% increased risk of patients experiencing an adverse event when an anaesthesia handover occurs during the procedure (pooled risk ratio=1.40; 95% confidence interval, 1.19 to 1.65; P<0.001; I2=98%).
Conclusions
Intraoperative anaesthesia handovers generally increase morbidity and mortality for surgical patients but could have the potential to improve safety in certain contexts. Future research should determine the specific handover characteristics that impact safety.
Keywords: anaesthesia, medical errors, operating rooms, patient care team, patient handoff, patient safety, quality
Editor's key points.
-
•
The authors performed a systematic review and meta-analysis to investigate the effect of intraoperative anaesthesia handover on patient morbidity and mortality.
-
•
Handovers were found largely to increase morbidity and mortality, although one study suggested that handover may be beneficial to error detection or rectification.
-
•
Future research should determine specific handover characteristics that affect patient outcomes.
Communication errors have been identified by the Joint Commission as a leading factor in anaesthesia-related sentinel events.1 These errors may occur during intraoperative anaesthesia handovers, in which the outgoing clinician must transfer all necessary information for continued safe anaesthesia care to the incoming clinician within a complex, dynamic, and distracting environment.2, 3, 4
It is possible that the lack of transfer of relevant and important information about patient care can compromise patient safety.3, 4, 5, 6 At the same time, handover may be an opportunity to correct errors and optimise patient care while mitigating fatigue on healthcare professionals.7,8 Given how common anaesthesia handover is, and the estimated high proportion of suboptimal handover practices,9,10 it is important to precisely quantify its association with patient outcomes. This systematic review aimed to fill this knowledge gap in order to inform future research and practice.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).11 The protocol was published in PROSPERO (CRD42018110120).
Eligibility criteria
All prospective or retrospective quantitative clinical studies specifically investigating the association of intraoperative anaesthesia handovers with patient morbidity or mortality outcomes were eligible for screening. To be included, the handover had to specifically involve transfer of care between anaesthesia providers during a surgical procedure – in other words, providing intraoperative relief or transferring patient care to an incoming anaesthesia provider.10 Anaesthesia provider was defined as any healthcare professional with specific training in anaesthesia, such as physician anaesthetists, postgraduate trainees (residents and fellows), and nurse anaesthetists or anaesthesia assistants. Studies could include patients of any age undergoing any type of surgical procedure requiring anaesthesia. This includes ‘diagnostic or therapeutic treatment of conditions or disease processes by any instruments causing localised alteration or transportation of live human tissue, [such as] lasers, ultrasound, ionising radiation, scalpels, probes, and needles’.12 Date and language restrictions were not applied to the search, although data were extracted only from papers published in English or French. Letters, commentaries, editorials, opinion pieces, and abstracts were excluded.
Search strategy
The search strategy (Supplementary Appendix 1) was designed in collaboration with an information specialist (AD). As per Peer Review of Electronic Search Strategies (PRESS) guidelines, a second trained information scientist subsequently reviewed the strategy.13 Searches were conducted from inception to April 24, 2019 in the electronic databases Medline, Medline in Process (via OVID), CINAHL, and Embase (via OVID). Reference lists of included studies and relevant systematic reviews were also searched.
Study selection
Independent reviewers (SL, FM, IT) screened articles for inclusion in duplicate using a web-based systematic review software, DistillerSR (Evidence Partners, Ottawa, Canada). Consensus or consultation with a third reviewer (HD, NE) was used to resolve disagreements. Titles and abstracts were assessed for eligibility followed by the full texts of those determined to be eligible.
Data extraction
DistillerSR (Evidence Partners, Ottawa, Canada) was used by independent reviewers (SL, FM, IT) to extract information in duplicate, with disagreements resolved via consensus or a third reviewer as needed. Information extracted included: publication details (e.g. author first name, year of study, country of data collection), study design (e.g. observational vs interventional, retrospective vs prospective, method for assessing handovers, sample size), inclusion criteria, exclusion criteria, participant demographic data, patient outcome measures, clinical context (e.g. type of surgery, elective vs crisis situation), intervention details (e.g. handover strategy/tool name and type [e.g. electronic reminder, checklist, protocol], definition of handover, implementation strategy), descriptive characteristics of handovers (e.g. frequency, error rate, associations with other variables).
Risk of bias
Risk of bias was determined using the National Institutes of Health (NIH) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies.14 Reviewers assessed risk of bias independently and in duplicate, using consensus or third reviewer consultation to resolve disagreements (HD, NE).
Data synthesis
We conducted a narrative synthesis of results with specific qualitative and quantitative information from each study summarised in tables. We also conducted a post-hoc exploratory meta-analysis to quantify the effect of handover on patient outcome for a subset of comparable studies. The meta-analysis was conducted using a random-effects model with Review Manager 5.0 (Cochrane Collaboration, London, UK). Effect estimates of dichotomous outcomes were presented as risk ratios (RR) with 95% confidence intervals (CI). Statistical heterogeneity was assessed using the I2 statistic.15 Where data were not available, the original authors of the study were contacted. For studies reporting handovers as continuous, events were summed into a single category of ‘handover’ compared with no (zero) handovers.
Results
Study selection
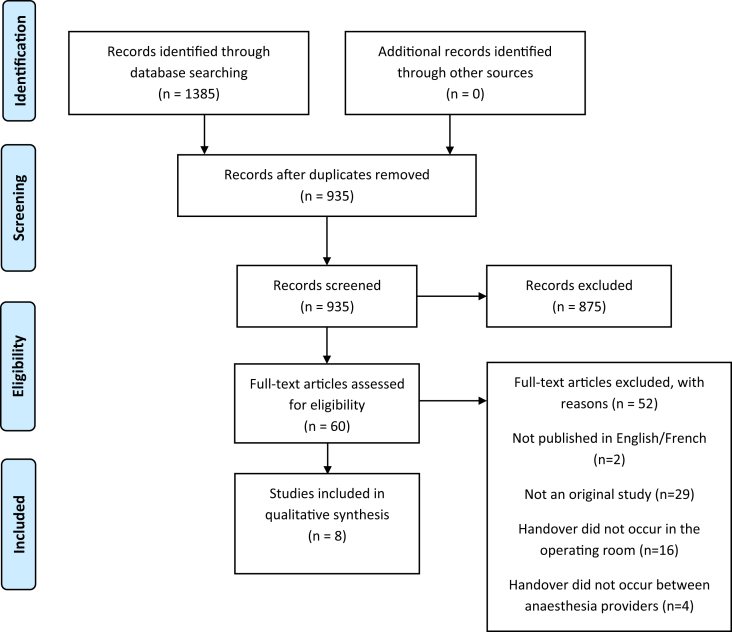
The literature search identified 1385 studies. After removal of duplicates, 935 studies were screened at the title and abstract stage for eligibility, with 875 not meeting the inclusion criteria and subsequently excluded. Of the 60 articles proceeding to full-text screening, 52 were excluded (two were not published in English/French; 29 were not an original study; 16 did not involve an intraoperative handover). Eight studies were ultimately included for data extraction (Fig 1).
Fig. 1.
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Characteristics of included studies
Among the eight studies meeting the inclusion criteria, six studies focused on patients as the unit of analysis (npatients=605 678) and two studies focused on anaesthesia providers as the unit of analysis (nproviders=307). Studies typically defined handover as a transfer of care between anaesthesia providers and collected data on handovers using an electronic documentation system, for example using a billing code.3 Each of the eight studies involved transfer of care between physician anaesthetists. Trainees and nurse anaesthetists were included in four studies (50%). Anaesthesia handovers were assessed across a variety of surgeries, including both cardiac and noncardiac procedures. All but one study16 were conducted between 2014 and 2019. Data collection only occurred in high income countries with the USA being the most frequent country represented (n=5; 63%). Characteristics of included studies are detailed in Table 1.
Table 1.
Study characteristics and results (n=8). ∗Quality was assessed using the NIH Quality Assessment Tool for Observational Cohort and Cross-sectional Studies. NR, not reported; N/A, not applicable; aRD, adjusted risk difference; OR, odds, ratio; CI, confidence interval.
| First author, year, country | Number of patients or providers (type involved in handover) | Study design Type of surgical procedure |
Outcome, timing, primary or secondary | Results | Quality assessment∗ |
|---|---|---|---|---|---|
| Choromanski, 2014, USA | 216 providers (staff, resident and nurse anaesthetists) | Online survey NR |
Situations with insufficient information received during handover, N/A, NR Experience of complications or mismanagement because of poor/incomplete handovers, N/A, NR |
49.1% had no handover protocol at their institution. 88% of respondents who did have institutional handover protocols believed them insufficient for effective patient handover. 84.8% reported situations where there was insufficient information received during a patient handover. 7% reported never experiencing complications or mismanagement because of poor or incomplete handovers. 60% reported rarely having complications, 31% reported sometimes having complications, and 3% reported frequent complications. |
Fair |
| Cooper, 1982, USA | 91 providers (staff, resident and nurse anaesthetists) | Qualitative interviews NR |
1. Error discovered by relief anaesthetist, N/A, NR 2. Cause of error discovered by relief anaesthetist, N/A, NR 3. Negative patient outcomes (e.g. death, cardiac arrest, extended ICU stay), N/A, NR |
1. 96 of 1089 (8.8%) reports of preventable errors and failures associated with anaesthesia management involved a relief anaesthetist. 2. Relief anaesthetist discovered an error or the cause of an error in 28 out of 96 (29.2%). Incidents. 3. No negative patient outcomes caused by a relieving anaesthetist. |
Fair |
| Hudson, 2015, Canada | 14 421 patients (staff anaesthetists) | Single-centre retrospective cohort study Cardiac |
1. Mortality, in-hospital, primary 2. Composite index of postoperative complications, in-hospital, secondary 3. Perioperative MI, in-hospital, secondary 4. CVA, in-hospital, secondary 5. Acute kidney failure requiring haemodialysis, in-hospital, secondary 6. Ventilation postoperatively for longer than 48 hours, in-hospital, secondary |
1. 5.4% in handover group; 4.0% in non-handover group (P=0.04). 2. 8.5% in handover group; 15.6% in non-handover group (P=0.02). 3. 1.9% in handover group; 2.9% in non-handover group (P=0.10). 4. 3.3% in handover group; 1.6% in non-handover group (P=0.0008). 5. 4.3% in handover group; 3.5% in non-handover group (P=0.25). 6. 16.7% in handover group; 13.3% in non-handover group (P=0.007). |
Good |
| Hyder, 2016, USA | 927 patients (staff anaesthetists) | Single-centre retrospective cohort study Colorectal |
Major complication, death, or both, 30 days, primary | Number of attending anaesthetists associated with increased odds of postoperative complication: unadjusted odds ratio (OR) = 1.52, 95% CI 1.18–1.96, P=0.0013; adjusted OR = 1.44, 95% CI 1.09–1.91, P=0.0106. | Fair |
| Jones, 2018, Canada | 313 066 patients (staff anaesthetists) | Population-based retrospective cohort study Multiple (bariatric, cardiac, colorectal, general, neurology, gynaecology, thoracic, urology, vascular) |
1. Composite of all-cause death, hospital readmission and major postoperative complications, 30 days, primary 2. All-cause death, 30 days, primary 3. Major complications, 30 days, primary 4. Readmission to hospital, 30 days, primary 5. Admission to ICU, in-hospital, secondary 6. Length of stay, in-hospital, secondary 7. ED visits, <90 days after index surgery, secondary 8. Any ED visit, <90 days after index surgery, secondary 9. Postoperative ventilation for 48 or more hours 10. Wound dehiscence, NR, secondary 11. Bleeding, NR, secondary 12. Pneumonia, NR, secondary 13. Unplanned return to operating room, NR, secondary 14. New onset atrial fibrillation or flutter, NR, secondary 15. New onset haemodialysis, NR, secondary 16. Myocardial infarction, NR., secondary 17. Acute kidney injury, NR, secondary 18. Cardiac arrest or other life-threatening postoperative incident 19. Shock 20. Stroke 21. Sepsis 22. Pulmonary embolism 23. Deep venous thrombosis 24. Coma |
1. Positive association: aRD=6.8%; 95% CI, 4.5%–9.1%; P<0.001 2. Positive association: aRD=1.2%; 95% CI, 0.5%–2%; P=0.002 3. Positive association: aRD=5.8%; 95% CI 3.6%–7.9%; P<0.001. 4. No association: aRD=1.2%; 95% CI, –0.3%–2.7%; P=0.11 5. Positive association: aRD=3.7%; 95% CI, 1.7%–5.8%; P<0.001 6. Positive association: aRD=1.2%; 95% CI, 0.7%–1.7%; P<0.001 7. No association: aRD=0.03; 95% CI, –0.03%–0.09%; P=0.36 8. No association: aRD=–0.2%; 95% CI, –2.5%–2.1%; P=0.86 9. Positive association: aRD=1.9%; 95% CI, 0.2%–3.6%; P=0.03 10. Positive association: aRD=2.9%; 95% CI, 1.8%–4.1%; P<0.001 11. Positive association: aRD=2.5%; 95% CI, 1.4 to 3.6; P<0.001) 12. Positive association: aRD=0.7%; 95% CI, 0.1%–1.2%; P=0.02 13. Positive association: aRD=1.8%; 95% CI, 0.6%–3.0%; P=0.004 14. No association: aRD=0.3%; 95% CI, –0.2%–0.8%; P=0.29 15. Positive association: aRD=0.8%; 95% CI, 0.3%–1.3%; P=0.002 16. No association: aRD=0.4%; 95% CI, –0.2%–0.9%; P=0.21 17. No association: aRD=0.2%; 95% CI, –0.1%–0.5%; P=0.22 18. No association: aRD=0.04%; 95% CI, –0.2%–0.2%; P=0.66 19. No association: aRD=0.1%; 95% CI, –0.1%–0.4%; P=0.31 20. No association: aRD=0.2%; 95% CI, –0.1%–0.4%; P=0.16 21. No association: aRD=0.08%; 95% CI, –0.1% to –0.2%; P=0.31 22. No association: aRD=0.2%; 95% CI, –0.2%–0.5%, P=0.29 23. No association: aRD=0.04%; 95% CI, -0.1%–0.2%; P=0.56 24. No association: aRD=0.05%; 95% CI, –0.01%–0.1%; P=0.13 |
Good |
| Saager, 2014, USA | 135 810 patients (staff, resident and nurse anaesthetists) | Single-centre retrospective cohort study Noncardiac |
Collapsed composite (any vs none): mortality and major morbidity | Positive association: OR=1.08 (95% CI, 1.05 to 1.10) for an increase of 1 number of handovers, P<0.001 | Fair |
| Liu, 2019, China | 700 patients (staff anaesthetists) | Secondary analysis of database from previous clinical trial Noncardiac |
1. Delirium, 7 days, primary 2. Non-delirium complications, 30 days, primary 3. Length of stay in hospital after surgery, postoperative, primary |
1. Positive association: OR=1.787; 95% CI, 1.012–3.155; P=0.046 2. Positive association: OR; 95% CI, NR; P=0.003 3. Positive association: OR; 95% CI, NR; P=0.001 |
Good |
| Terekhov, 2016, USA | 140 754 patients (staff, resident and nurse anaesthetists) | Single-centre retrospective cohort study NR |
Collapsed composite (any vs none): mortality and six major morbidities considered either life-threatening or potentially resulting in permanent functional disability. Morbidities included serious cardiac, respiratory, gastrointestinal, urinary, bleeding, and infectious complications based on secondary diagnosis codes in addition to the primary diagnosis, in-hospital, primary | The number of anaesthesia handovers was not found to be associated (P=0.19) with increased odds of postoperative mortality and serious complications, as measured by the collapsed composite. | Good |
Association of anaesthesia handovers with patient outcome
Details on the association between anaesthesia handovers and patient outcome are also shown in Table 1. In five of the six (83%) studies collecting data from databases,3, 4, 5, 6,16 anaesthesia handover was associated with increased risk of an adverse patient outcome. Specifically, handover was associated with greater risk of in-hospital mortality,4,5 major morbidity,4,5 postoperative complications,3,6,16 all-cause mortality at 30 days,3 admission to ICU,3 length of stay,3 extended postoperative ventilation,3 wound dehiscence,3 bleeding,3 pneumonia,3 unplanned return to OR,3 new onset haemodialysis,3 and postoperative delirium.17 A list of confounders accounted for by each of these studies is provided in Supplementary Appendix 2.
The remaining two studies involved surveys or interviews with anaesthesia providers. Of the providers surveyed by Choromanski and colleagues,9 93% reported patient complications or mismanagement because of poor or incomplete handovers. Cooper and colleagues16 interviewed 91 providers regarding 1089 reports of preventable errors and failures associated with anaesthesia management. Nearly 10% (n=96) of these reports involved a relief anaesthetist. Of these 96 reports, the relief anaesthetist discovered an error or the cause of an error in approximately 30% of cases (n=28). No negative patient outcomes were reported as caused by a relieving anaesthetist.
Exploratory meta-analysis
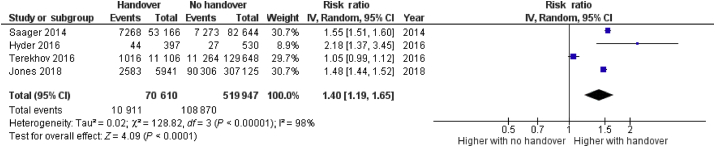
Data from four studies reporting the effect of handover on a composite outcome of mortality and morbidity were evaluated.3,6,18,19 The remaining four studies included in this review did not have a comparable outcome or study design to include in the analysis.
Two of the four studies in the meta-analysis reported the same composite outcome of in-hospital mortality and six major morbidities (cardiac, respiratory, gastrointestinal, urinary, bleeding, and infections complications).18,19 The other two studies reported on death and major complications within 30 days of surgery.3,6 Although all studies used a retrospective cohort design, three were single-centre6,18,19 and one was population-based.3
Results of the meta-analysis are summarised in Fig 2. Overall, there is a 40% increased risk of patients experiencing an adverse event when an anaesthesia handover occurs during the procedure (pooled RR=1.40; 95% CI, 1.19 to 1.65; P<0.001; I2=98%).
Fig. 2.
Descriptive forest plot of handover effect on composite morbidity and mortality outcome. CI, confidence interval.
Risk of bias
The included studies were evaluated using the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies.4, 5, 6,9,15, 16, 17 The overall quality rating was ‘good’ for four of the studies3,5,16,17 and ‘fair’ for the other four studies.4,6,9,15 Ratings are included in Table 1.
Discussion
This systematic review identified eight studies assessing the impact of anaesthesia handovers on patient morbidity and mortality. Seven of the studies identified a negative relationship between anaesthesia handovers and adverse patient outcomes, whereas one study suggested handover may be beneficial for error detection or rectification. Overall, this review suggests that anaesthesia handover is most often associated with patient harm, although it could have the potential to increase safety in some contexts.
Given how common anaesthesia handover is, it may be surprising that so few studies have been conducted to explore its impact on patient outcome. The relative recency of the studies identified by this review suggests there is increasing concern with the implications of intraoperative anaesthesia handovers for surgical patient safety. The limited number of studies may be attributable to the many logistical and methodological challenges associated with obtaining clinical intraoperative data.10 Major adverse events in the OR are rare, requiring a large sample size for sufficient power to detect associations, often making it necessary to rely on retrospective databases. In addition, cases with one or more handovers are typically long and complex, with many potentially confounding factors. It is difficult to capture all of this information with retrospective data or in-person observation. However, with the emergence of new audio–video recording technologies, such as the OR Black Box® (Surgical Safety Technologies, Toronto, Ontario),20 it may be possible for future multicentre studies to prospectively assess intraoperative anaesthesia handovers and investigate their relationship with process of care and near-misses with comprehensive, high-quality data. Similar to black boxes in aviation, the OR Black Box® captures video, audio, environmental, and physiological data. These data are analysed by expert raters and sophisticated artificial intelligence systems to provide a comprehensive surgical timeline, which makes it possible to identify the complete series of events leading to patient harm.21,22
Despite study design limitations (e.g. retrospective accounts, lack of RCTs, self-reported experience), the reported associations between anaesthesia handovers and adverse patient safety are compelling given the large patient sample size (n=605 678) and low risk of bias across studies. In addition, our exploratory meta-analysis revealed an increased risk of morbidity, mortality, or both when handover occurred.
Although most studies included in this review concluded that anaesthesia handover was associated with patient harm, one of the qualitative studies by Cooper and colleagues16 suggests that anaesthesia handovers can actually benefit patients in that it provides an opportunity for the incoming anaesthetist to identify and correct errors. Altogether, this suggests that there may be some specific handover characteristics (e.g. quantity, type of providers involved, quality) that make handover safe or unsafe. It is also important to note that breaks during long shifts may play a role in mitigating the risks of fatigue among anaesthesia providers. This, again, highlights the need to better understand what makes a good quality handover between providers in order to support both provider well-being and patient safety. The studies included in this review primarily indicated only whether a handover took place, rather than a description of specific characteristics of the handover. There remains no clear definition as to how to define an effective or ‘good quality’ handover. Most studies documented whether a handover occurred drawing on billing codes within electronic anaesthesia management systems. Objective tools for assessing handovers quality were not used by any study, suggesting a need to develop an anaesthesia handover assessment tool. This could promote standardised handover measurement across studies to enhance our understanding of current practice and better inform intervention development.23
Strengths and limitations
Results of our study should be interpreted with caution given that these studies are based on observational data and involved diverse types of surgical procedures. Furthermore, there was inconsistent data collection and outcome definitions across studies, and the characteristics of handover in each study were unclear. Substantial heterogeneity was also observed, supporting the need for future standardised measurement and data collection along with more rigorous study designs. There were also a limited number of studies included in the meta-analysis (four).
The strength of this systematic review is that it addresses a very common intraoperative clinical situation with a likely significant impact on patient outcome. Our work shows that anaesthesia handover is associated with patient harm, with most studies finding a negative impact on patient outcome.
Further research is warranted as there is a limited number of studies investigating anaesthesia handovers and variations in design and methodology. It would be helpful to identify specific characteristics of handover that influence patient outcome across different clinical contexts (e.g. surgical specialty, type of anaesthesia, elective vs crisis situation, academic vs community centre). Future studies should also determine under which circumstances handovers benefit or threaten patient safety and whether it is handover quantity, quality, or both that matters most for patient outcomes. Once the key aspects of effective anaesthesia handover are established, specific policies and procedures can then be developed and implemented.
Conclusions
Intraoperative anaesthesia handovers generally increase morbidity and mortality for surgical patients but could have the potential to improve safety in certain contexts. More research is needed to determine the specific handover characteristics that make a difference for patient safety in order to better inform policy and practice.
Authors' contributions
Study design: SB, CE
Analysis: SB, CE
Data interpretation: SB, HD, CE
Coordination of data collection: HD
Data acquisition: SL, IT, FM, CE
Drafting of the article: SB, HD, CE
Revision of the manuscript for intellectual content; SB, HD, SL, IT, HD
All authors approved of the version to be published.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
The Ottawa Hospital Anaesthesia Alternate Funds Association SB. The DistillerSR licenses were funded by the Department of Anaesthesiology and Pain Medicine, The Ottawa Hospital.
Acknowledgements
The authors thank Alexandra Davis, MLIS for her contributions to the literature search and Guillaume Durand for his contribution to the title and abstract screening.
Handling editor: Jonathan Hardman
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.05.062.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.The Joint Commission. Sentinal event data — root causes by event type — 2004 to third quarter 2011. http://www.jointcommission.org/assets/1/18/Root_Causes_Event_Type_2004-3Q2011.pdf. Accessed 11 September 2018.
- 2.Jayaswal S., Berry L., Leopold R., et al. Evaluating safety of handoffs between anesthesia care providers. Ochsner J. 2011;11:99–101. [PMC free article] [PubMed] [Google Scholar]
- 3.Jones P.M., Cherry R.A., Allen B.N., et al. Association between handover of anesthesia care and adverse postoperative outcomes among patients undergoing major surgery. JAMA. 2018;319:143–153. doi: 10.1001/jama.2017.20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saager L., Hesler B.D., You J., et al. Intraoperative transitions of anesthesia care and postoperative adverse outcomes. Anesthesiology. 2014;121:695–706. doi: 10.1097/ALN.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 5.Hudson C.C.C., McDonald B., Hudson J.K.C., Tran D., Boodhwani M. Impact of anesthetic handover on mortality and morbidity in cardiac surgery: a cohort study. J Cardiothorac Vasc Anesth. 2015;29:11–16. doi: 10.1053/j.jvca.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Hyder J.A., Bohman J.K., Kor D.J., et al. Anesthesia care transitions and risk of postoperative complications. Anesth Analg. 2016;122:134–144. doi: 10.1213/ANE.0000000000000692. http://www.ncbi.nlm.nih.gov/pubmed/25794111 [DOI] [PubMed] [Google Scholar]
- 7.Bagian J.P., Paull D.E. Handovers during anesthesia care. JAMA. 2018;319:125. doi: 10.1001/jama.2017.20602. http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2017.20602 [DOI] [PubMed] [Google Scholar]
- 8.Cohen M.D., Hilligoss P.B. 2009. Handoffs in hospitals: a review of the literature on information exchange while transferring patient responsibility or control.https://deepblue.lib.umich.edu/handle/2027.42/61498 [Google Scholar]
- 9.Choromanski D., Frederick J., Michael G., Wang H. Intraoperative patient information handover between anesthesia providers. J Biomed Res. 2014;28:383–387. doi: 10.7555/JBR.28.20140001. http://www.jbr-pub.org/ch/reader/view_abstract.aspx?file_no=JBR140507&flag=1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane-Fall M., Gutsche J.T., Augoustides J.G.T. Are intraoperative anesthesia handovers associated with harm? Getting to the heart of the matter in cardiac surgery: the search for the hat-trick of quality, safety, and continuous improvement. J Cardiothorac Vasc Anesth. 2015;29:8–10. doi: 10.1053/j.jvca.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. http://www.systematicreviewsjournal.com/content/4/1/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American College of Surgeons Definition of surgery legislative toolkit. Am Med Assoc. 2013:1–17. [Google Scholar]
- 13.McGowan J., Sampson M., Salzwedel D., Cogo E., Foerster V., Lefebvre C. 2016. Press – peer review of electronic search Strategies guideline: explanation & elaboration. [DOI] [PubMed] [Google Scholar]
- 14.National Heart, Lung and BI (NHLBI) 2018. Study quality assessment tools.https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools [Google Scholar]
- 15.Moher D., Squires J.E., Kolehmainen N., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264. doi: 10.7326/0003-4819-151-4-200908180-00135. http://annals.org/article.aspx?doi=10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 16.Cooper J.B., Long C.D., Newbower R.S., Philip J.H. Critical incidents associated with intraoperative exchanges of anesthesia personnel. Anesthesiology. 1982;56:456–461. doi: 10.1097/00000542-198206000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Liu G.Y., Su X., Meng Z.T., et al. Handover of anesthesia care is associated with an increased risk of delirium in elderly after major noncardiac surgery: results of a secondary analysis. J Anesth. 2019;33:295–303. doi: 10.1007/s00540-019-02627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurz A., Mascha E.J., You J., et al. Intraoperative transitions of anesthesia care and postoperative adverse outcomes. Anesthesiology. 2014;121:695–706. doi: 10.1097/ALN.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 19.Terekhov M.A., Ehrenfeld J.M., Dutton R.P., Guillamondegui O.D., Martin B.J., Wanderer J.P. Intraoperative care transitions are not associated with postoperative adverse outcomes. Anesthesiology. 2016;125:690–699. doi: 10.1097/ALN.0000000000001246. [DOI] [PubMed] [Google Scholar]
- 20.Goldenberg M.G., Jung J., Grantcharov T.P. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:2017–2018. doi: 10.1001/jamasurg.2017.2888. http://archsurg.jamanetwork.com/article.aspx?doi=10.1001/jamasurg.2017.2888 [DOI] [PubMed] [Google Scholar]
- 21.Jung J.J., Jüni P., Lebovic G., Grantcharov T. First-year analysis of the operating room Black Box study. Ann Surg. 2020;271:122–127. doi: 10.1097/SLA.0000000000002863. [DOI] [PubMed] [Google Scholar]
- 22.Adams-McGavin R.C., Jung J.J., van Dalen A.S.H.M., Grantcharov T.P., Schijven M.P. System factors affecting patient safety in the OR. Ann Surg. 2019 doi: 10.1097/SLA.0000000000003616. [DOI] [PubMed] [Google Scholar]
- 23.Jeffcott S.A., Evans S.M., Cameron P.A., Chin G.S.M., Ibrahim J.E. Improving measurement in clinical handover. Qual Saf Heal Care. 2009;18:272–276. doi: 10.1136/qshc.2007.024570. http://qualitysafety.bmj.com/lookup/doi/10.1136/qshc.2007.024570 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.