Abstract
Objectives:
Obesity can be an independent predictor of fibrosis in tissues, including the liver, heart, and skin. We evaluated a rural Appalachian cohort of idiopathic pulmonary fibrosis (IPF) for its relation to obesity.
Methods:
Using American Thoracic Society 2018 diagnostic guidelines, an IPF cohort was systematically identified at an Appalachian academic medical center (2015–2019). The cohort was categorized in subgroups of body mass index (BMI) <30 or BMI ≥30 kg/m2. Demographics, clinical variables, and treatment details were collected retrospectively and evaluated for their associations with obesity.
Results:
In our IPF cohort (N = 138), a usual interstitial pneumonia pattern was less prevalent in the obese group (n = 49) relative to the nonobese group (69% vs 85%, respectively). The obese group was younger (mean age 73.27 ± 9.12 vs 77.97 ± 9.59 years) and had a higher prevalence of hypertension (90% vs 72%), hyperlipidemia (83% vs 68%), diabetes mellitus (47% vs 25%), sleep-disordered breathing (47% vs 25%), chronic pain disorders (28% vs 15%), and deep vein thrombosis (19% vs 7%). An increased proportion of obese-IPF patients was seen at a tertiary or an interstitial lung disease center, with more surgical lung biopsies performed and incident diagnosis (ie, within 6 months of presentation) assigned. Only a minority of patients underwent lung transplantation (3.6%), all of them from the obese-IPF subgroup. Approximately 30% of the total IPF cohort died, with a lower mortality observed in the obese group (35% vs 20%, P = 0.017). An increasing BMI predicted a better survival in the total IPF cohort (BMI 25–29.9, 20–24.9, and <20 had mortality rates of 20%, 47%, and 75%, respectively; P < 0.001).
Conclusions:
Our study represents a first known effort to develop an IPF cohort in a rural Appalachian region. Although they shared an increased burden of comorbidities, the obese subgroup showed less advanced fibrosis with a lower mortality rate relative to nonobese subgroup, suggesting a potential “obesity paradox” in IPF. The study findings significantly advance our understanding of challenges posed by IPF in a rural population that also suffers from an alarming rate of obesity. We highlight the need for the multidisciplinary management of these patients and prospective studies to better define this complex relation.
Keywords: body mass index, idiopathic pulmonary fibrosis, mortality, rural Appalachia, usual interstitial pneumonia
Obesity is associated with fibrosis in numerous tissues. It is a risk factor for nonalcoholic fatty liver disease, a spectrum of injuries that includes hepatic steatosis (nonalcoholic fatty liver), nonalcoholic steatohepatitis, fibrosis, and cirrhosis.1 Cardiac (myocardial) fibrosis also is associated with obesity and may contribute to the increased incidence of heart failure, atrial arrhythmias, and sudden cardiac death recognized in this group of patients.2 Finally, scar tissue that grows beyond the confines of the original insult and invades surrounding tissue (ie, keloid formation) demonstrates that fibrosis in the skin similarly can be associated with obesity.3
The most recent American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association (ATS/ERS/JRS/ALAT) guidelines integrate high-resolution computed tomography (HRCT) imaging patterns, surgical lung biopsy patterns, and multidisciplinary discussion to diagnose idiopathic pulmonary fibrosis (IPF), a chronic, progressive lung fibrosis of unknown cause and the most common idiopathic interstitial pneumonia.4 The progression and mortality of IPF depend on a variety of patient characteristics. Single-center reports, clinical trials, and registry data support multiple predictors of IPF, including older age, male sex, usual interstitial pneumonia (UIP) pattern on HRCT, pulmonary function test abnormalities (PFTs), including low or declining forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO), diminished exercise capacity (6-minute walk distance [6MWD]), use of supplemental oxygen, and hospitalization for respiratory etiology.5,6
Fibrotic diseases involving different tissues have distinct etiological and clinical features; however, they can share common pathways to organ injury and failure.7 At the cellular level, different stimuli can affect an injury, followed by inflammatory cell response and fibrogenic-wound healing processes, which involve a complex interplay of multiple cellular, immunological, and molecular mechanisms. With an increased mass of adipose tissue in obesity, hypoxia can be associated with dysregulation of the extracellular matrix.8 Such extracellular matrix dysregulation/remodeling is implicated in metabolic dysfunction with fibrotic injuries in the liver, heart, and kidney.1,2,7,8 An elevated level of leptins, a hormonal product of the adipose cell with a primary function of regulation of fat stores, can correlate with the severity of lung fibrosis and promote collagen production at alveolar epithelial cell levels.9 Obesity also is implicated in endoplasmic reticulum stress, leading to myofibroblast differentiation.10 Obese individuals have higher circulating levels of transforming growth factor-β1 (TGF-β1) expression in the bronchial epithelium, which can participate in the development of lung fibrosis.11 Moreover, obesity and related comorbid conditions of add reflux, pulmonary hypertension, and obstructive sleep apnea can contribute to chronic hypoxemia, leading to the release of proinflammatory cytokines that may propagate clinical deterioration and alter the fibrotic pathway in the lung.12
Despite associations of obesity with fibrosis in various tissues, its role in IPF remains enigmatic. Prior investigation has demonstrated poor survival in malnourished patients with IPF, but evaluation for associations of mortality among IPF patients with an elevated body mass index (BMI) yielded mixed results.13–16Using a systematic approach to identify the cohort of IPF patients at the rural Appalachian academic tertiary center, we characterized relations among HRCT patterns, IPF progression, and mortality with obesity. Considering higher than national average obesity rates in West Virginia,17 we are distinctly challenged by the timely recognition and accurate diagnosis of IPF, which can improve outcomes through the avoidance of potentially harmful therapies and prompt initiation of therapies that are effective even in the early stages of disease.18–20
Methods
A single-center, retrospective, observational cohort study was conducted at the West Virginia University Hospital (WVUH). Before commencement, the study protocol was reviewed and approved by our institutional review board. We identified patients with International Classification of Diseases (ICD) coding for the idiopathic interstitial lung disease (ILD) (ICD 9-CM [Clinical Modification]: 515–516.9; ICD-10-CM J84–84.9) within the electronic medical records (EMRs) of the WVUH system, which included both our tertiary center and peripheral hospitals (2015–2019). Charts were reviewed meticulously to diagnose and categorize patients in accordance with the latest ATS/ERS/JRS/ALAT IPF diagnosis guidelines.4 These diagnostic criteria require the exclusion of other known causes of ILD (domestic and occupational environmental exposures, connective tissue disease, and drug toxicity) and either the presence of the HRCT pattern of UIP or specific combinations of HRCT patterns and histopathology patterns in patients subjected to lung tissue sampling. Multidisciplinary discussion was used to adjudicate cases before inclusion in the study. Patients meeting the diagnosis of IPF were further categorized in subgroups of obese (BMI ≥30 kg/m2 or nonobese (BMI <30 kg/m2, which included underweight BMI<20, normal BMI 20–24.9, and overweight BMI 25–29) (Fig. 1, study approach). For these subgroups, we collected demographic and historical variables of age, sex, comorbid conditions, smoking status, family history, symptom(s) duration prediagnosis and years since diagnosis, and whether seen at the tertiary care or ILD center. In addition, clinical variables of interest, including PFT (maximum of three, which included that at the time of diagnosis, intermediate, and the latest), 6MWD, use of supplemental oxygen, use of antifibrotic agents (pirfenidone and/or nintedanib), and lung transplant status also were recorded. Data were aggregated using REDCap, which is a Health Insurance Portability and Accountability Act–compliant data aggregation tool.21
Fig. 1.
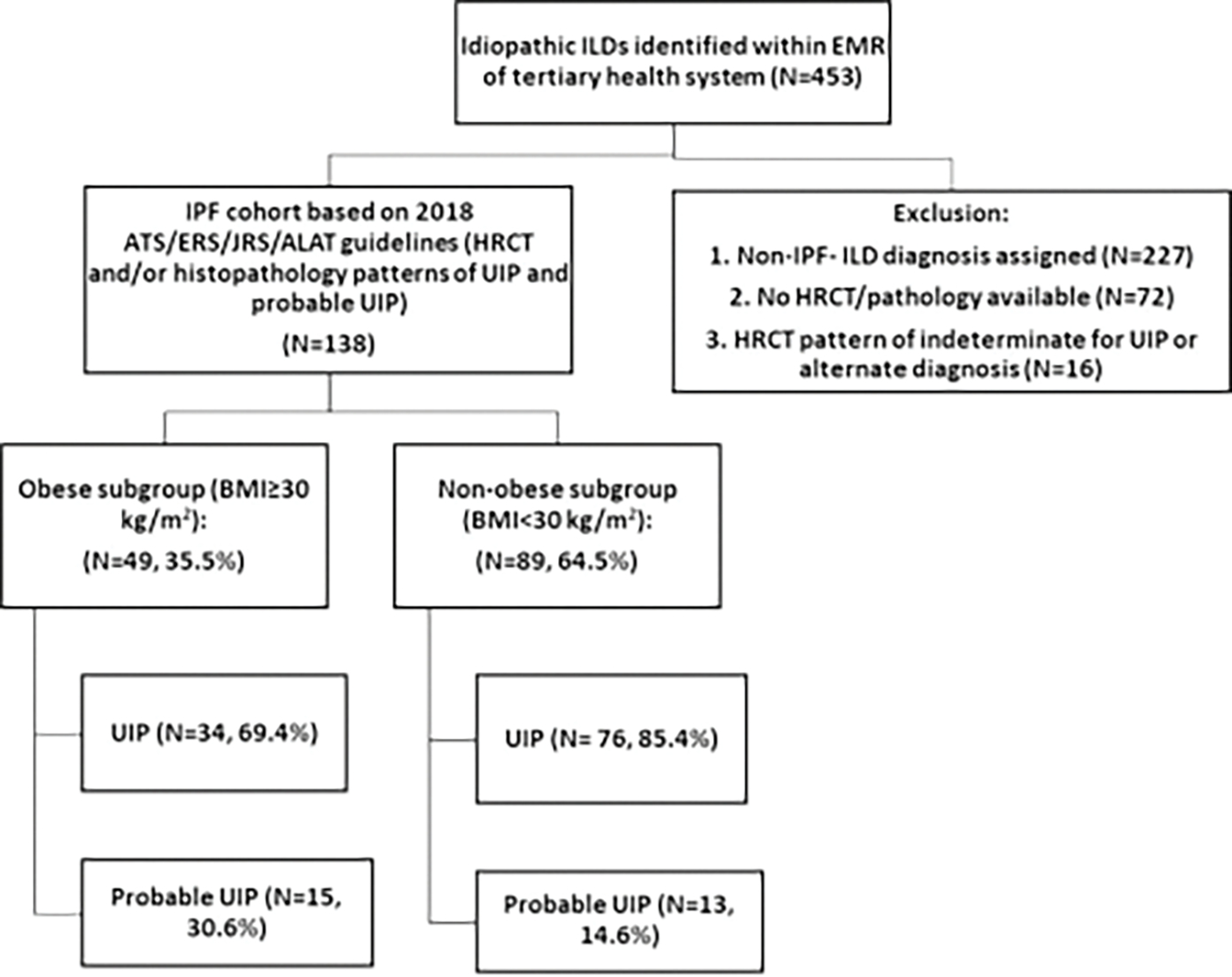
Study approach to create IPF cohort and subgroups. ATS/ERS/JRS/ALAT, American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association; BMI, body mass index; EMR, electronic medical record; HRCT, high-resolution computer tomography; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; UIP, usual interstitial pneumonia.
Statistical Analysis
We generated descriptive statistics, using means, medians, and standard deviations to identify the quantitative variables and frequency distributions to identify the categorical variables. For correlative analysis on outcome variables such as BMI (high/low) and mortality, χ2 tests were used to assess the significance of the association with categorical variables, and independent samples t tests were used for quantitative variables. P values were derived using two-sided tests, and P < 0.05 was considered to be statistically significant. All of the analyses were conducted using statistical software R version 3.6 (R Foundation for Statistical Computing, Vienna, Austria) and SAS version 9.4 (SAS Institute, Cary, NC).
Results
From 2015 to 2019, there was a total of 453 patients who had a diagnostic code of idiopathic ILD within the EMR of the WVUH system (Fig. 1). Applying the most recent ATS/ERS/JRS/ALAT guidelines, 138 of the 453 patients (30.4%) were found to meet the diagnostic criteria for IPF with patterns of UIP and probable UIP (Fig. 1). A total of 227 patients (50%) were determined to have non-IPF ILD diagnosis and 72 patients (15.8%) did not have HRCT in the system to allow an independent review (Fig. 1). UIP was the most common HRCT pattern (n = 110, 79.7%), followed by probable UIP (n = 28, 20.3%). The average duration of symptoms prediagnosis was 20 months; however, it ranged from 1 month to 10 years.
Subgroups were categorized based on obese BMI ≥30 (n = 49, 35.5%) or nonobese BMI <30 (n = 89, 64.5%). A CT chest pattern of UIP was found less frequently in the BMI ≥30 group relative to the BMI <30 group (69.4% vs 85.4%, respectively, P = 0.007). Most of the patients from our cohort had been diagnosed as having IPF for >4 years (4.11 ± 3.03 years in the obese group vs 4.17 ± 3.04 years in the nonobese, P = 0.461). A higher proportion of obese patients underwent evaluation at a tertiary care center and/or specific ILD center (65.3% vs 44.9%, respectively, P = 0.004), had incident (within 6 months of presentation) diagnosis (46.9% vs 38.2%, respectively, P = 0.021), and had surgical lung biopsy (28.6% vs 10.1%, respectively, P = 0.001).
A majority of patients (obese vs nonobese) were oxygen dependent (81.6% vs 77.5% respectively, P = 0.381) at the time of diagnosis. The mean requirement for supplemental oxygen was not different (2.74 ± 1.91 vs. 2.62 ± 1.95 L/min, respectively, P = 0.369). Approximately 20% of patients were offered antifibrotic therapy (either pirfenidone or nintedanib). Only a minority of patients (n = 5, 3.6%) underwent lung transplantation; all were from the BMI ≥30 subgroup. Approximately one-third of patients in the study cohort died and the obese group showed a significantly lower mortality (20.4% vs 34.8%, respectively, P = 0.018). Those with lower BMI in the nonobese group showed the highest mortality (underweight BMI <20 kg/m2—75% vs normal BMI 20–24.9 kg/m2—47% vs overweight BMI 25–29.9 kg/m2—20%, P < 0.001). Table 1 describes the diagnostic, management, and outcome details of the groups.
Table 1.
Diagnostic and management details of the IPF cohort
| Variables N(%) or mean ± SD | Obese, BMI ≥30 kg/m2 (n = 49, 35.5%) | Nonobese, BMI <30 kg/m2 (n = 89, 64.5%) | All IPF cohort (n = 138) | P |
|---|---|---|---|---|
|
| ||||
| Diagnostics | ||||
| 1. HRCT patterns | ||||
| UIP | 34 (69.4) | 76(85.4) | 110(79.7) | 0.007 |
| Probable LIP | 15(30.6) | 13(14.6) | 28 (20.3) | |
| 2. Lung biopsy | 14(28.6) | 9(10.1) | 24(17.4) | 0.001 |
| 3. Seen at tertiary or ILD center | 32 (65.3) | 40 (44.9) | 72 (52.2) | 0.004 |
| 4a. Incident diagnosis (within 6 mo) | 23 (46.9) | 34 (38.2) | 57 (41.3) | 0.021 |
| 4b. Prcsalcnt diagnosis | 27(51) | 54 (60.7) | 81 (58.6) | 0.200 |
| Years since diagnosis | 4.11 ±3.03 | 4.17 ± 3.04 | 4.15 ±3.02 | 0.461 |
| PFT at diagnosis | ||||
| FEV1% predicted | 81.31 ± 14.68 | 78.63 ± 20.01 | 79.74 ± 17.98 | 0.231 |
| FVC% predicted | 75.24 ± 15.60 | 70.47 ± 19.88 | 72.43 ± 18.31 | 0.098 |
| DLCO% predicted | 45.83 ± 17.31 | 45.73 ± 16.88 | 45.77 ± 16.96 | 0.488 |
| 6MWD, m | 253.2 ± 142.57 | 257.64 ± 124.86 | 255.82 ± 13126 | 0.449 |
| Management | ||||
| Supplemental O2 prescribed | 40(81.6) | 69 (77.5) | 109 (79) | 0.381 |
| Supplemental O2 l/min | 2.74 ± 1.91 | 2.62 ± 1.95 | 2.67 ± 1.93 | 0.369 |
| Antifibrotics | ||||
| Nintcdanib | 12(24.5) | 18(20.2) | 30 (21.7) | 0.397 |
| Pirfcnidonc | 12(24.5) | 18(20.2) | 30 (21.7) | 0.397 |
| Lung transplant | 5 00.2) | 0 | 5 (3.6) | |
| Mortality | 10(20.4) | 31 (34.8) | 41 (29.7) | 0.01H |
| Lndcrwcight (BMI <20) 6 (19.4%) | <0.001 | |||
| Normal (BMI 20–24.9) 15 (48.3%) | ||||
| Overweight (BMI 25–29.9) 10 (32.3%) | ||||
Italicized/bold P values refers to significant P values.
6MWD, 6-minute walk distance; BMI, body mass index; DLCO, diffusion capacity for carbon monoxide; FFV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HRCT, high-resolution computer tomography: ILD; interstitial lung disease; IPF, idiopathic pulmonary fibrosis; PFT, pulmonary function test; SD, standard deviation; VIP: usual interstitial pneumonia.
The obese subgroup was younger relative to the nonobese (mean age 73.27 ± 9.12 vs 77.97 ± 9.59, respectively, P = 0.002). Each of the two groups had a higher proportion of males (65% vs 57%, respectively, P = 0.246) and ever-smokers (77.5% vs 68.5%, respectively, P=0.154). Average smoking pack-years were not different between the groups (mean pack-years 33.71 ± 33.49 vs 34.12 ± 24.21, respectively, P = 0.472). The obese group had a higher prevalence of comorbid conditions, including hypertension (89.8% vs 71.9%, respectively, P = 0.001), hyperlipidemia (83.7% vs 68.5%, respectively, P=0.008), sleep-disordered breathing (47% vs 25.8%, respectively, P = 0.002), diabetes mellitus (47% vs 25.8%, respectively, P = 0.002), chronic pain disorders (28.6% vs 15.7%, respectively, P = 0.027), and deep vein thrombosis (20.4% vs 7.9%, respectively, P = 0.014). Interestingly, the nonobese group had a significantly higher proportion of aortic aneurysm (14.6% vs 4%, respectively, P = 0.008). Demographic and historical details can be found in Table 2.
Table 2.
Historical details of IPF cohort
| Variables N(%) or mean ± SD | Obese, BMI ≥30 kg/m2 (n = 49,35.5%) | Nonobese, BMI <30 kg/m2 (n = 89, 64.5%) | Total IPF cohort (n = 138) | P |
|---|---|---|---|---|
|
| ||||
| Age, y | 7327 ±9.12 | 77.97 ± 9.59 | 76.30 ± 9.66 | 0.002 |
| Male sex | 32 (65.3) | 51 (57.3) | 83 (60.1) | 0.246 |
| Family history of IPF | 4(8) | 7 (7.9) | 11(8) | 1.000 |
| Ever-smoking | 38 (77.5) | 61 (68.5) | 99(71.7) | 0.154 |
| Smoking pack-years | 33.71 ±33.49 | 34.12 * 24.21 | 33.96 ±28.12 | 0.472 |
| Comorbidities | ||||
| Hypertension | 44 (89.8) | 64(71.9) | 108(78.3) | 0.001 |
| Hyperlipidemia | 41 (83.7) | 61 (68.5) | 102(73.9) | 0.008 |
| GERD | 35 (71.4) | 62 ((>9.7) | 97 (70.3) | 0.876 |
| CAD | 31 (63.3) | 47 (52.8) | 78 (56.5) | 0.151 |
| COPD | 21 (42.9) | 36 (40.4) | 57(41.3) | 0.667 |
| CHF | 21 (42.9) | 32 (36) | 53 (38.4) | 0.311 |
| Sleep apnea | 23 (47) | 23 (25.8) | 46 (33.3) | 0.002 |
| Diabetes mcllitus | 23 (47) | 23 (25.8) | 46 (33.3) | 0.002 |
| PH | 19(38.8) | 31 (34.8) | 50 (36.2) | 0.462 |
| Atrial arrhythmia | 15(30.6) | 33 (37.1) | 48 (34.7) | 0.294 |
| Anxiety/mood disorders | 15(30.6) | 28(31.5) | 43 (31.2) | 0.879 |
| Chronic pain | 14(28.6) | 14(15.7) | 28 (20.3) | 0.027 |
| Hypothyroidism | 13(26.5) | 32 (36) | 45 (32.6) | 0.126 |
| Anemia | 12(24.5) | 30 (33.7) | 42 (30.4) | 0.119 |
| DVT | 10(20.4) | 7 (7.9) | 17(12.3) | 0.014 |
| CVA | 6(12.2) | 19(21.3) | 25(18.1) | 0.086 |
| PVD | 5(10.2) | 10(11.2) | 15(10.9) | 0.817 |
| Aortic aneurysm | 2(4) | 13(14.6) | 15(10.9) | 0.008 |
| Lung cancer | 2(4) | 5 (5.6) | 7(5.1) | 0.516 |
Italicized/bold P values refers to significant P values.
CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD; chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DVT, deep vein thrombosis; GERD, gastroesophageal reflux disease; IPF, idiopathic pulmonary fibrosis; PH, pulmonary hypertension; PVD, peripheral vascular disease; SD, standani deviation.
Mean forced expiratory volume in 1 second (FEV1) and FVC percentage predicted values at the time of diagnosis were not different between the groups. Mean DLCO percentage predicted and 6MWD values were reduced in both groups; however, these were not statistically different (Table 1). There was a progressive decline in FVC, FEV1, and DLCO values measured sequentially at three distinct time frames of the IPF disease course (at the time of diagnosis, intermediate, and the latest). This was not significantly different between the obese and nonobese groups (at least ≥3 PFT measurements done, n = 21 vs 23, respectively). The distribution of available (sequential) PFTs is shown in Figure 2.
Fig. 2.
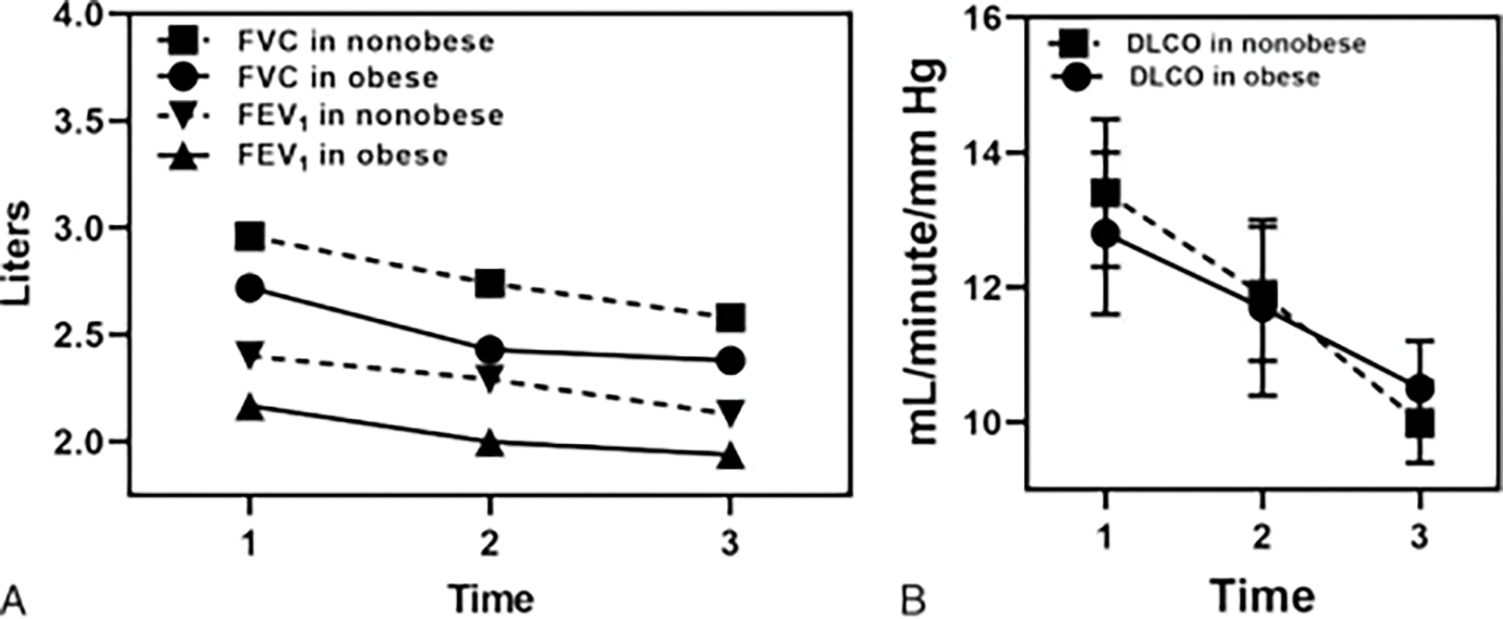
Sequential pulmonary function tests (PFTs) for nonobese (n = 23) and obese IPF (n = 21) groups. (A) Forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) in liters, and (B) diffusion capacity for carbon monoxide (DLCO) in mL/min/mm Hg, respectively, showing progressive decline during the disease course; however, not statistically different between the groups. Time 1,2,3 indicates 3 PFT measurements done at the time of diagnosis, intermediate, and the latest, respectively, within the study duration (2015–2019).
Discussion
Obesity (BMI ≥30 kg/m2) is a global epidemic, with >1 billion overweight adults and at least 300 million obese patients worldwide. In developed countries, more people have obesity than are underweight.22 The effects of obesity on the respiratory system are multifaceted, including the mechanical complication of obstructive sleep apnea and obesity hypoventilation syndrome, airway inflammation and pathogenesis of asthma, implications on chronic obstructive pulmonary disease (COPD) severity, systemic inflammation from adipose tissue endocrine function, and postoperative anesthesia-related complications.23 Despite improved understanding of the effects on the lungs, the association of obesity with fibrotic lung diseases such as IPF remains undefined. The rural Appalachia region continues to face significant health disparities, with challenges of higher than national mortality rates in seven leading causes of death.17 Efforts estimating the burden of chronic lower respiratory diseases in the region have focused largely on COPD and coal worker pneumoconiosis, whereas the chronic progressive ILD (eg, IPF) remains understudied. In such circumstances, developing large patient cohorts can serve as a useful tool to study an infrequent disease such as IPF because it can provide information from a real-world scenario. Using a multidisciplinary approach, we present the first known effort with the latest HRCT diagnostic criteria at a rural academic center.4 With approximately one-third of the WV population being obese, this study afforded us an opportunity to focus on the possible association between obesity and IPF.17
Obesity was prevalent (35.5%) in our IPF cohort in accordance with state statistics.17 The mean BMI (27.92 ± 5.31) was comparable to that previously reported by IPF registries.24 Our subgroup of IPF patients with obesity was younger, had less of a UIP pattern on HRCT, and had better survival as compared with the nonobese patients. An inverse relation between BMI and mortality was evident in our IPF cohort. Differences between the obese and nonobese groups were independent of supplemental oxygen requirement, sequential declining PFT values, reduced 6MWD, and use of antifibrotic agents. Although prior investigation reported poor survival in malnourished IPF patients, this is the first study to demonstrate a significantly greater proportion of nonobese individuals with a pattern and distribution findings of UIP on the HRCT.13,14 In addition to IPF, the UIP pattern on HRCT has been associated with poor survival in connective tissue disease–associated-ILD, interstitial pneumonia with autoimmune features, and idiopathic interstitial pneumonias.25–27
Improvement in diagnostic accuracy has been reported if centers experienced in IPF were involved in the process.28 It has been argued that IPF is overdiagnosed outside specialized centers, with one study observing that community physicians were more likely to assign a final diagnosis of IPF relative to academic physicians.29 Reflecting improved diagnostics in our obese IPF patients, they had more Sequent evaluation at a tertiary and/or specific ILD center, a higher likelihood of incident diagnosis (within 6 months of presentation), and a higher proportion of surgical lung biopsies and lung transplantation. Moreover, this also could have enhanced the therapeutic intervention by avoiding potentially harmful immunosuppressive therapies.19 This is an interesting finding that needs further exploration in prospective studies. Obesity is known to dramatically affect the respiratory mechanics, thus altering lung function, bronchial hyperresponsiveness, and bronchial reversibility.30 Large national databases have suggested that obese individuals are 2.66 times more likely to experience exertional dyspnea.31,32 This increased perception of breathlessness among obese patients may have contributed to an earlier presentation to medical care, resulting in enhanced involvement in diagnostic processes. This would diminish delay in diagnosis, which often challenges IPF care.
Obesity is known to increase the risks of multiple comorbid conditions.33 Obese patients in our cohort had a statistically significant higher prevalence of the commonly associated comorbid conditions of the metabolic syndrome (eg, hypertension, dyslipidemia, diabetes mellitus), sleep-disordered breathing, deep vein thrombosis, and chronic pain disorder. Previous research also has shown that obese patients who receive a bilateral lung transplant for IPF may be at higher risk of 90-day mortality relative to patients of normal weight.34 Obese patients with COPD were observed to have reduced 6MWD, worse dyspnea, a diminished quality of life, and an increased risk for hospitalization for exacerbations.35 Alternatively, nonobese and poor nutrition status is a known predictor of poor outcome in other lung diseases including COPD and cystic fibrosis.36,37A recent meta-analysis suggested a J-shaped association between BMI and mortality after excluding the confounding effects of smoking status and comorbidity.38 In addition, obesity increases the risk factors for a number of cardiovascular diseases, but these patients can demonstrate a better prognosis when overweight or obese, a phenomenon known as the “obesity paradox.”39 A similar phenomenon has been reported in numerous diseases, including hypertension, heart diseases, chronic kidney diseases, chronic liver diseases, cancers, infections, sepsis, acute respiratory distress syndrome, and critical care illness. Among pulmonary diseases, patients diagnosed as having COPD, pneumonia, pulmonary hypertension, and lung cancer have been shown to have the obesity paradox.40–42 Although some investigations suggest methodological concerns as the origin of the obesity paradox, others suggest biochemical and physiological bases. Our demonstration of obese patients having more comorbidities but less fibrosis and mortality also can appear counterintuitive and suggest a potential obesity paradox in IPF. Investigations have suggested the participation of adipose tissue biology in the molecular pathways contributing to cardiovascular diseases 43 Future studies can examine for such relations between adipose tissue biology and IPF.
From the rural health perspective, this IPF cohort demonstrated several interesting observations. The mean age of our cohort was older than previously reported in IPF registries.44 Higher than national smoking rates were observed in our IPF cohort, which predisposed to its development45 Although IPF is defined as an idiopathic disease, cigarette smoking has consistently been shown to be a risk factor.46 A dose-response relation has suggested that the individuals with >20 pack-years of smoking had a notably higher risk for developing IPF.47 Two-thirds of the patients in this cohort were ever-smokers, with the median smoking of 34 pack-years. Although comorbidities are commonly associated with IPF, our cohort demonstrated a significantly greater burden of these diseases relative to previously reported registries.48–50 Comorbidities have been noted to affect a disease course, including survival.51 Given such a higher burden of diseases, this IPF cohort reveals a distinct challenge for rural healthcare providers. Regarding treatment, approximately 80% of patients were hypoxic and the mean requirement of supplemental oxygen was higher than that reported in the IPF-Prospective Outcomes registry; however, the use of antifibrotic agents was low at approximately one-fifth of all patients in the cohort48 A minority of patients underwent lung transplantation likely secondary to the rural community-based hospitals having limited access to a lung transplant center, known socioeconomic limitations, and a higher burden of comorbidities working against transplant eligibility.
There are several limitations to our study. By design, this was a retrospective single-center study in a rural region with some of the highest rates of obesity and smoking in the United States. There are several inherent diagnostic challenges described in developing IPF cohorts and registries, including evolving diagnostic criteria, data collection practices, identifying an accurate time of diagnosis, and effect of missing values.44,46 Certainly, the last was evident in our cohort because 15% of patients had missing data precluding heir inclusion in the study.
Lastly, serial (≥3) PFT measurements in the study required that hey be done at the same laboratory because these were used for the analysis of disease progression. This attempt to control for interlaboratory variability also restricted the number of study subjects.
In conclusion, our experience in developing and characterizing an IPF cohort at a rural academic center provided important insights into an obese IPF phenotype. An advanced fibrotic pattern of UTP was seen less frequently in obese IPF patients with lower mortality rates compared with nonobese IPF patients. Despite having a higher burden of comorbid conditions, we observed improved survival in our obese IPF subgroup, likely signifying a potential obesity paradox in IPF. Further exploration of these findings may provide understanding into the pathogenesis of this phenotype and aid in developing an optimal multidisciplinary management strategy. This study advances our understanding of the respiratory health disparities faced by a rural Appalachian region and furnishes an opportunity to align policies for the management of the chronic progressive lung disease of IPF.
Key Points.
Despite sharing a higher burden of comorbid conditions of the metabolic syndrome, sleep-disordered breathing, chronic pain disorder, and deep vein thrombosis, our obese-idiopathic pulmonary fibrosis (IPF) group (body mass index ≥30 kg/m2) demonstrated less severe fibrosis and had better survival as compared with the nonobese IPF group (body mass index <30 kg/m2).
Obese-IPF patients had a higher proportion of surgical lung biopsies, evaluation at tertiary or specific interstitial lung disease centers, incident diagnosis within 6 months of presentation, and lung transplantation.
Our cohort represents the first effort in developing an IPF cohort in central Appalachia and characterizing a significant effect of obesity, which challenges the residents of the region.
Footnotes
The authors did not report any financial relationships or conflicts of interest
Abstract was presented at American Thoracic Society International Conference, May 2020. Virtual meeting due to COVID-19 pandemic.
References
- 1.Chiang DJ, Pritchard MT, Nagy LE. Obesity, diabetes mellitus, and liver fibrosis. Am J Physiol Gastrointest Liver Physiol 2011;300:G697–G702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavalera M, Wang J, Frangogiannis NG. Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl Res 2014;164:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutherford A, Glass DA 2nd. A case-control study analyzing the association of keloids with hypertension and obesity, Int J Dermatol 2017;56:el87–el89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018;198:e44–e68. [DOI] [PubMed] [Google Scholar]
- 5.Salisbury ML, Tolle LB, Xia M, et al. Possible UIP pattern on high-resolution computed tomography is associated with better survival than definite UIP in IPF patients. Respir Med 2017;131:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder L, Neely ML, Hellkamp AS, et al. Predictors of death or lung transplant after a diagnosis of idiopathic pulmonary fibrosis: insights from the IPF-PRO Registry. Respir Res 2019;20:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 2007;117:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun K, Tordjman J, Clement K, et al. Fibrosis and adipose tissue dysfunction. Cell Metab 2013;18:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gui X, Chen H, Cai H, et al. Leptin promotes pulmonary fibrosis development by inhibiting autophagy via PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun 2018;498:660–666. [DOI] [PubMed] [Google Scholar]
- 10.Hsu HS, Liu CC, Lin JH, et al. Involvement of ER stress, PI3K/AKT activation, and lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Sci Rep 2017;7:14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park YH, Oh EY, Han H, et al. Insulin resistance mediates high-fat diet-induced pulmonary fibrosis and airway hyperresponsiveness through the TGF-beta1 pathway. Exp Mol Med 2019;51:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adegunsoye A, Balachandran J. Inflammatory response mechanisms exacerbating hypoxemia in coexistent pulmonary fibrosis and sleep apnea. Mediators Inflamm 2015;2015:510105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alakhras M, Decker PA, Nadrous HF, et al. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest 2007;131:1448–1453. [DOI] [PubMed] [Google Scholar]
- 14.Jouneau S, Rousseau C, Lederlin M, et al. Low fat-free mass and body mass index are associated with worse survival in patients with idiopathic pulmonary fibrosis (IPF). Eur Resp J 2019;54(suppl 63):PA1337. [Google Scholar]
- 15.Mogulkoc N, Uysaler B, Bishop P. Does body mass index influence survival of patients with idiopathic pulmonary fibrosis? Eur Resp J 2018;52(suppl 62):PA2914. [Google Scholar]
- 16.Patel S, Nolan C, Barker R, et al. Body composition and mortality in idiopathic pulmonary fibrosis (IPF): a prospective cohort study. Eur Resp J 2018;52(suppl 62):PA2056. [Google Scholar]
- 17.Appalachian Research Council. Health disparities in Appalachia, https://www.arc.gov/report/health-disparities-in-appalachia Published August 23, 2017. Accessed August 30, 2020.
- 18.Albera C, Costabel U, Fagan EA, et al. Efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis with more preserved lung function. Eur Respir. J 2016;48:843–851. [DOI] [PubMed] [Google Scholar]
- 19.Idiopathic Pulmonary Fibrosis Clinical Research Network, Raghu G, Anstrom KJ, et al. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 2012;366:1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolb M, Richeldi L, Behr J, et al. Nintedanib in patients with idiopathic pulmonary fibrosis and preserved lung volume. Thorax 2017;72:340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Obesity and overweight, https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Published April 1, 2020. Accessed June 24,2020.
- 23.Zammit C, Liddicoat H, Moonsie I, et al. Obesity and respiratory diseases. Int J Gen Med 2010;3:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jo HE, Glaspole I, Grainge C, et al. Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian Idiopathic Pulmonary Fibrosis Registry. Eur Resp J 2017;49:1601592. [DOI] [PubMed] [Google Scholar]
- 25.Alhamad EH. Clinical characteristics and survival in idiopathic pulmonary fibrosis and connective tissue disease-associated usual interstitial pneumonia. J Thorac Dis 2015;7:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly BT, Moua T. Overlap of interstitial pneumonia with autoimmune features with undifferentiated connective tissue disease and contribution of UIP to mortality. Respirology 2018;23:600–605. [DOI] [PubMed] [Google Scholar]
- 27.Migita K, Arai T, Jiuchi Y, et al. Predictors of mortality in patients with interstitial lung disease treated with corticosteroids: results from a cohort study. Medicine (Baltimore) 2014;93:el75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;164:193–196. [DOI] [PubMed] [Google Scholar]
- 29.Oldham JM, Noth I. Idiopathic pulmonary fibrosis: early detection and referral. Respir Med 2014;108:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med 2018;12:755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernhardt V, Babb TG. Exertional dyspnoea in obesity. Eur Respir Rev 2016;25:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med 2002;162:1477–1481. [DOI] [PubMed] [Google Scholar]
- 33.Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med 2017;5:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gries CJ, Bhadriraju S, Edelman JD, et al. Obese patients with idiopathic pulmonary fibrosis have a higher 90-day mortality risk with bilateral lung transplantation. J Heart Lung Transplant 2015;34:241–246. [DOI] [PubMed] [Google Scholar]
- 35.Lambert AA, Putcha N, Drummond MB, et al. Obesity is associated with increased morbidity in moderate to severe COPD. Chest 2017;151:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005–1012. [DOI] [PubMed] [Google Scholar]
- 37.Courtney JM, Bradley J, McCaughan J, et al. Predictors of mortality in adults with cystic fibrosis. Pediatr Pulmonol 2007;42:525–532. [DOI] [PubMed] [Google Scholar]
- 38.Aune D, Sen A, Prasad M, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016;353:i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elagizi A, Kachur S, Lavie CJ, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis 2018;61:142–150. [DOI] [PubMed] [Google Scholar]
- 40.Spelta F, Fratta Pasini AM, Cazzoletti L, et al. Body weight and mortality in COPD: focus on the obesity paradox. Eat Weight Disord 2018;23:15–22. [DOI] [PubMed] [Google Scholar]
- 41.Papagianni M, Tziomalos K. Effects of obesity on the outcome of pneumonia. Expert Rev Endocrinol Metab 2017;12:315–320. [DOI] [PubMed] [Google Scholar]
- 42.Zafrir B, Adir Y, Shehadeh W, et al. The association between obesity, mortality and filling pressures in pulmonary hypertension patients; the “obesity paradox”. Respir Med 2013;107:139–146. [DOI] [PubMed] [Google Scholar]
- 43.Antonopoulos AS, Tousoulis D. The molecular mechanisms of obesity paradox. Cardiovasc Res 2017;113:1074–1086. [DOI] [PubMed] [Google Scholar]
- 44.Culver DA, Behr J, Belperio JA, et al. Patient registries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2019;200:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. Behavioral risk factor data: tobacco use (2011 to present). https://chronicdata.cdc.gov/Survey-Data/Behavioral-Risk-Factor-Data-Tobacco-Use-201l-to-pr/wsas-xwh5. Accessed May 15, 2020.
- 46.Olson AL, Swigris JJ. Idiopathic pulmonary fibrosis: diagnosis and epidemiology. Clin Chest Med 2012;33:41–50. [DOI] [PubMed] [Google Scholar]
- 47.Baumgartner KB, Samet JM, Stidley CA, et al. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Me 1997;155:242–248. [DOI] [PubMed] [Google Scholar]
- 48.Culver D, Yow E, Neely M, et al. Characteristics of patients with idiopathic pulmonary fibrosis (IPF) in the US: data from the IPF-PRO Registry. Chest 2018;154:397A–398A. [Google Scholar]
- 49.Guenther A, Krauss E, Tello S, et al. The European IPF registry (eurIPFreg): baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir Res 2018;19:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jo HE, Glaspole I, Grainge C, et al. Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian Idiopathic Pulmonary Fibrosis Registry. Eur Respir J 2017;49:1601592. [DOI] [PubMed] [Google Scholar]
- 51.Caminati A, Lonati C, Cassandro R, et al. Comorbidities in idiopathic pulmonary fibrosis: an underestimated issue. Eur Respir Rev 2019;28:190044. [DOI] [PMC free article] [PubMed] [Google Scholar]


