Abstract
Breast cancer remains the most frequently diagnosed cancer in women worldwide. Delayed presentation of the disease, late stage at diagnosis, limited therapeutic options, metastasis, and relapse are the major factors contributing to breast cancer mortality. The development and progression of breast cancer is a complex and multi-step process that incorporates an accumulation of several genetic and epigenetic alterations. External environmental factors and internal cellular microenvironmental cues influence the occurrence of these alterations that drives tumorigenesis. Here, we discuss state-of-the-art information on the epigenetics of breast cancer and how environmental risk factors orchestrate major epigenetic events, emphasizing the necessity for a multidisciplinary approach toward a better understanding of the gene-environment interactions implicated in breast cancer. Since epigenetic modifications are reversible and are susceptible to extrinsic and intrinsic stimuli, they offer potential avenues that can be targeted for designing robust breast cancer therapies.
Keywords: breast cancer, epigenetics, DNA methylation, chromatin modification, metabolism, environment, therapies
Breast cancer overview
Cancers of the breast are the most prevalent malignancy observed in women worldwide. In the year 2022 alone, it is estimated that in the United States, nearly 287,850 new cases of invasive breast cancer and 51,400 new cases of ductal carcinoma in situ (DCIS) would be diagnosed, while 43,250 breast cancer deaths would occur (1). Breast cancers if diagnosed at an early stage, can significantly enhance the effective treatment strategies and improve the survival. The five-year survival rate for early detection is more than 90%, whereas it is reduced to 25% for patients diagnosed at the advanced stages (2).
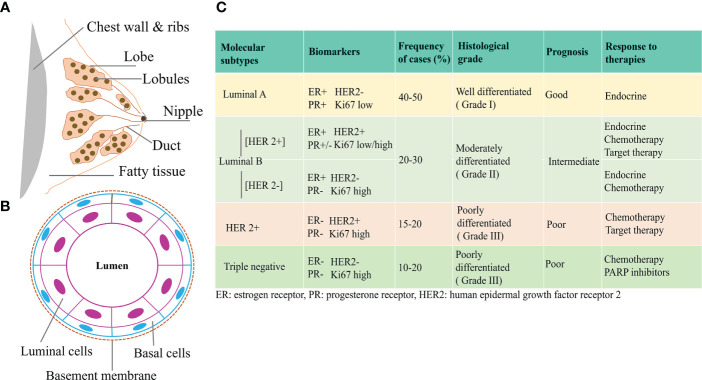
Breast cancer is a highly heterogeneous disease and research is still ongoing to clearly understand its origin and the underlying mechanisms. The breast consists of milk producing glands and the connective tissues comprising the fibrous and fatty tissues. Lobules are the milk producing glands, and ducts carry the milk to the nipples, Figures 1A, B . Most breast cancers begin in the ducts or the lobules and based on the metastatic spread, they can either be benign or invasive. Ductal carcinoma in situ (DCIS) is considered as non-invasive and early-stage breast cancer confined to the milk ducts. If cancer originates in the ducts or lobules and metastasizes, they are considered invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC) respectively. Almost, 80% of breast cancers belong to the IDC category (4, 5).
Figure 1.
Classification of Breast Cancer (A) Breast showing the different tissue types consisting of duct, lobe, lobules, nipples, and fatty tissue. (B) Cross-sectional view of mammary duct, consisting of basal cells and luminal cells. Breast cancer arising from the luminal or basal cells can be further characterized based on the expression of different hormone receptors. (C) Based on the expression of ER, PR, HER2, and proliferation status as assessed by Ki67, different molecular subtypes of breast cancer have been identified that have distinct prognostic features and response to therapies (3).
With the emergence of new high-throughput technologies and gene expression profiling, breast cancer has been molecularly characterized into distinct subtypes based on the expression of hormone receptors and proliferation statuses. Activation of human epidermal growth factor receptor 2 (HER2), estrogen receptor (ER), progesterone receptor (PR), proliferation marker Ki67, and/or mutations in the Breast Cancer (BRCA) gene, has been utilized in the histological and molecular characterization of breast cancer. These molecular subtypes are clinically divided into major forms that include Luminal A, Luminal B, HER2-enriched, and basal/triple negative breast cancer (TNBC). Luminal A cancer can either be ER and/or PR positive (+) or HER2 negative (-). Luminal B tumor can either be ER+ and/or PR+ or PR- and/or HER2+/-. HER2 overexpressed tumors constitute the HER2 enriched group, while TNBC lacks the ER, PR, and HER2 statuses. Luminal A tumors have low Ki 67 levels, are of low grade, and have the best prognosis, compared to Luminal B which have high Ki 67 levels and are usually high grade. Among all, TNBCs, have the worst prognosis and are aggressive due to high metastatic behavior (6–8). Such an existence of multiple subtypes of breast cancer is associated with distinct clinical behaviors/responses and has significant implications in breast cancer therapies (9, 10), Figure 1C .
Genetic predisposition or family history constitutes almost 10% of all breast cancer cases. Mutations in the BRCA gene, BRCA1 and BRCA2 is the most common germline aberrations associated with breast cancer having a collective 70% lifetime risk of developing breast cancer (11, 12). In fact, 15 to 20% of all TNBC cases are linked with the germline mutations in BRCA1 or BRCA2 ( 13) and in US, 12% of breast cancers are contributed by TNBCs with a 5 year survival rate of 8 to 16 percent only (14). Studying a series of early breast cancers revealed that the most frequently amplified genes in the tumors are the p53, Myc, PTEN, PIK3CA, ERBB2, CCND1, GATA 3 and FGFR1 ( 15). The risk of developing breast cancer is high in patients harboring mutations in the BRCA1, BRCA2, TP53, and PTEN genes (16). In addition to the genetic factors, breast cancer microenvironment plays a major role in its development and progression where the immune cell repertoire is cardinal (17).
Heightened or prolonged exposure to estrogen contributes to the major risk factor for breast cancer development. The occurrence of sporadic breast cancers is associated with exposure to estrogen, which is a substantial risk factor for the development of such cancers (18). Other risk factors include old age, obesity, high breast density, alcohol intake, smoking, hormonal therapy, and pregnancy associated factors (19–24). Additionally, early menarche/late menopause, usage of oral contraceptives, hormone replacement therapy, benign lesions, and radiation therapy are some of the known risk factors (25–28). Few of them are modifiable risk factors such as lifestyle and physical activity if adopted successfully, can offer reduction in the disease burden (29).
Epigenetic players in breast cancer
Dynamic and heritable modifications occurring to the genome independently of DNA sequence, is a phenomenon referred to as the “epigenetics”. Interestingly, cancer was the first disease linked to epigenetic changes (30). For the onset of cancer, the activation of oncogenes and/or the suppression of tumor suppressor genes are the key events that are always accompanied with epigenetic changes. These epigenetic changes include DNA methylation, histone posttranslational modifications, expression of micro-RNA, and long non-coding RNA (31, 32).
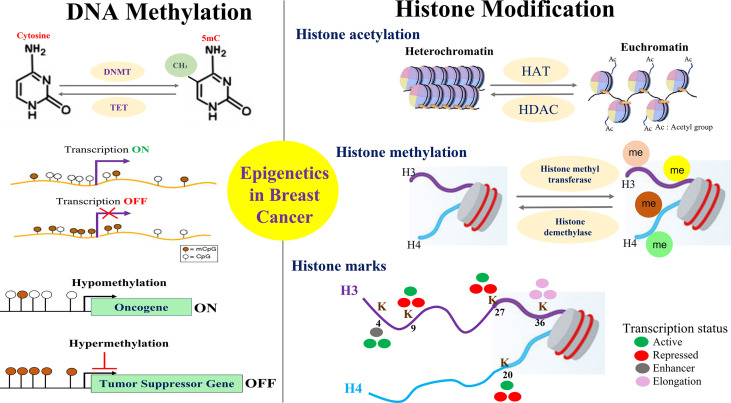
Breast cancer development is a complex and multistep process involving the synergistic crosstalk between genetic and epigenetic alterations which are influenced by a plethora of internal and external factors. Such factors include but not limited to the cell’s intrinsic microenvironment, nutrient supply, cellular stress as well as external environmental exposures to agents that are endocrine disrupters or are of carcinogenic nature. Altogether, critical genes involved in proliferation, apoptosis, cell motility, invasion, etc. are influenced by the epigenetic changes that are implicated in breast cancer development and progression ( Figure 2 ).
Figure 2.
Overview of Key Epigenetic Events in Breast Cancer. Mechanisms for epigenetic alterations in breast cancer are shown focusing on two major players that include the methylation of DNA and the modification of histone proteins. Hypomethylation of oncogenes and hypermethylation of tumor suppressor genes is an important epigenetic phenomenon in breast cancer that affects various cellular processes of proliferation, apoptosis, migration, invasion, drug resistance, etc. Post translation modifications made to histone proteins impact gene expression by altering the chromatin structure towards open or closed conformation. Histone methylation of lysine is implicated in both transcriptional activation and repression depending on the methylation site that constitutes the various histone marks/code.
DNA methylation
One of the most well-known and major epigenetic mechanisms is DNA methylation, which involves the covalent addition of a methyl group (CH3) to the 5′-position of cytosine that resides before the guanine in the DNA sequence. Such methylation within the CpG dinucleotides which are concentrated in large clusters also called the CpG islands, regulates gene expression thereby governing the major biological process implicated in cancer (33, 34). As a result of methylation, a 5-methylcytosine (5mC) structure is formed that can either block the access of transcription factors to the binding sites of the DNA or engage methyl binding domain proteins (MBDs) in conjunction with the modification of histone proteins, so that the expression of methylated genes is prevented. In such a scenario when the promoters of key tumor suppressor genes are densely methylated, leads to their silencing and if oncogenes are less methylated, leads to their aberrant activation (35, 36).
DNA methylation is a reversible process where a specific group of enzymes called the DNA methyltransferase (DNMTs) govern the process. DNMT1, DNMT3a, and DNMT3b are the three active DNA methyltransferases. Demethylation of DNA is catalyzed by an enzyme family belonging to the Ten-eleven translocation methylcytosine dioxygenases also known as ten-eleven translocations (TETs), which can turn 5mC to 5-hydroxymethylcytosine (5-hmC) by the process of hydroxymethylation. TET1, TET2, and TET3 are three such enzymes involved in DNA demethylation thereby recovering the silenced genes that are once affected by the DNMTs. Together, this entire process influences the transcriptional activation of important genes involved in carcinogenesis and genomic stability (37–41). Several other proteins that have DNA demethylase activities and are implicated in breast cancer include the growth arrest and DNA-damage inducible protein (GADD45) and the cytidine deaminases family of proteins, Activation-induced cytidine deaminase (AID) and Apolipoprotein B mRNA editing catalytic polypeptide‐like family (APOBEC). GADD45A has compelling associations between DNA repair and epigenetic gene regulation (42, 43). In breast cancer, the interaction between GADD45 and BRCA1 gene has been suggested to influence the pathogenesis of the disease most likely via triggering the nucleotide excision repair mechanisms (44). Interestingly, GADD45A is abnormally methylated in breast cancer (45). AID proteins have important roles in the active DNA demethylation, where its engagement in the deamination of 5-mC to thymine has been reported (40, 46). Also, AID is known to facilitate DNA demethylation and is essential for the EMT in non-transformed mammary epithelial cells (47). Furthermore, while, APOBEC1 possesses DNA demethylase activity (48–50), APOBEC mutagenesis influencing the tumor evolution in ER+/HER2-breast cancer has been reported (51). Most recently it was shown that the APOBEC mutagenesis prohibited the growth of breast tumors by eliciting immunogenic responses (52).
Several genes in breast cancer exhibit CpG island hypermethylation (53) and in several instances, abnormal activity of DNA methyltransferases led to the hypermethylation and silencing of HOXA5, TMS1, p16, RASSF1A, and BRCA1 genes of tumor suppressor behavior (54–56). Additionally, genes that are silenced due to promoter hypermethylation include E-cadherin, TMS1, GSTP1, and p16 ( 57–59). These genes are involved in major biological processes such as estrogen signaling, pro-apoptosis (HOXA5, TMS1), cell cycle check points (RASSF1A, p16) and DNA repair mechanisms (BRCA1). While one of the best examples of a breast cancer susceptibility gene that is frequently silenced in sporadic breast tumors is the BRCA1 gene, CpG hypermethylation of BRCA1 associated with DNMT 3b overexpression has been reported (60). Early stages of sporadic breast cancer exhibit the loss of cell cycle checkpoint gene p16INK4a via aberrant CpG promoter methylation (61) and nearly 80% of breast tumors also exhibit a decreased expression of another cell cycle inhibitor gene p21/CIP1 /WAF1 via elevated methylation of p21/CIP1 /WAF1 gene (62).
DNA methylation also follows a distinct pattern that is displayed in different subtypes of breast cancer. For example, a high frequency of DNA methylation has been shown in ER+/luminal breast cancer compared to ER−/basal-like tumors (63, 64). Also, well-differentiated tumors have less methylated CpG islands in comparison to poorly differentiated breast tumors which exhibits a greater degree of methylated CpG islands (65). Similarly, increased promoter hypermethylation of the progesterone receptor gene has been observed in the PR negative breast tumors (66). Such a differential methylation pattern in the ER or PR or HER2 gene may affect the expression of these receptors on the breast tumor and hence can significantly impact the responsiveness of such tumors to relevant endocrine/hormonal therapies. In an attempt to study the DNA methylation profiles of the well-known expression subtypes of breast cancer i.e. luminal A, luminal B, and Basal like, 807 cancer associated genes were analyzed and it was revealed that there is variability in the methylation profiles of each of the three breast cancer subtypes and that the profiles are different from each other (64).
DNA methylation alterations in normal breast tissue or normal tissues adjacent to cancer can also give clues towards the likelihood of the occurrence of breast cancer. Interestingly, it is suggested that the detectable methylation variabilities in some of the cancer related genes in normal breast tissues can predate the occurrence of breast cancer (67). Moreover, distinct types of breast cancer can be tracked down back to the specific progenitor population, deploying their unique methylation profiles, thereby addressing the issues owing to their cell of origin or biological heterogeneity as observed in breast cancer (68). More recently, by comparing breast cancer to normal breast, seven breast cancer-specific methylation biomarkers have been identified, while six CpG sites are suggested to predict patient survival (69). Using a genome wide approach to analyze the DNA methylation and expression patterns in breast cancer and normal breast, PRAC2, TDR10, and TMEM132C genes have been identified that can serve as novel DNA methylation-gene markers of diagnostic and prognostic significance in breast cancer (70). Large scale integrative analysis of the DNA methylation profiles across 1538 METABRIC breast tumors with respect to transcriptional, genetic, and clinical aspects, revealed six global trends that affect the DNA methylation profiles of the breast. These trends consist of “contamination of immune and stromal cells”, “replication linked hypomethylation clock”, “X chromosome dosage compensation”, and “epigenetic instability at CpG islands”. Most importantly, this study identified X inactivation as a strong dosage compensation machinery, which can be the causative reason behind the methylation of attained X-associated loci in ER negative tumors (71).
Chromatin modification
DNA is wrapped around histone proteins so that it can fit into the nucleus. Individual histone octamer consists of two copies of H2A/H2B dimer cores and H3/H4 tetramers, that wrap around 146 base pairs of the DNA. Nucleosomes comprise repeating histone units that ultimately make up the chromatin (72, 73). Histone octamer harbors an unstructured N terminal tail of differing lengths that protrudes outward from the nucleosome. This protruding amino terminal tail can be subjected to various kinds of modifications where chemical moieties are added. The addition of various chemical moieties or tags determines whether the DNA wrapped around histones is available for transcription. In case, when the chromatin is tightly folded, the DNA remains inaccessible to the transcription factors and hence the structure is transcriptionally silent, also called heterochromatin. Whereas when the structure is less condensed, more relaxed, and hence more accessible to the transcription factors and thereby remains transcriptionally active, also called euchromatin (74). There are at least four amino acid residues that are subjected to modifications, these include lysine, serine, tyrosine and arginine, and there are more than six kinds of modifications that can occur. These include methylation, acetylation, phosphorylation, ubiquitination, biotinylation, sumoylation, and proline isomerization. The different patterns of histone modifications, also famously referred as the histone code, influences the transition of the chromatin states between the euchromatin and heterochromatin eventually regulating gene expression (75, 76).
Histone acetylation
Post translational modifications made to histone proteins impact gene expression by altering the chromatin structure. Histone acetylation involves the addition of acetyl groups to the lysine residues of histones H3 and H4 by the group of enzymes known as the histone acetyltransferases (HATs) also called as “writers”. As a part of the gene regulatory machinery, such modifications disrupt histone-DNA interactions resulting in the unwinding of the nucleosome. HATs utilize acetyl CoA as a cofactor and catalyze the reaction, and in doing so they neutralize the positive charge on the lysine, thereby weakening the interaction between the histones and the negatively charged phosphate groups of the DNA. As a result, the condensed chromatin is now a more open and relaxed structure that is associated with a higher degree of gene transcription.
Acetylation is a dynamic and reversible process, where the acetyl groups can be removed by the group of enzymes called histone deacetylases (HDACs) also called “erasers”, resulting in the deacetylation of the histone lysine residues thereby making the chromatin more condensed and transcriptionally repressed (74, 76, 77). Acetylation of histone H3 on lysine 9 residue [H3K9], lysine 14 [H3K14], lysine 27 [H3K27], and lysine 122 [H3K122] has been associated with active transcription (78–80). It is interesting to note that DNA methyltransferases can directly interact with the HDACs and the methyl CpG binding domain family of proteins at their promoter regions and ultimately build a complex that is transcriptionally repressive. This repressive complex is critical for the conversion of acetylated histones that is transcriptionally active, to the deacetylated transcriptionally silent form (81).
Enzymes belonging to the category of histone acetylation “writers”, e.g., enzyme harboring the histone acetylation domains P300 is implicated in breast cancer where it is overexpressed and bestow towards an elevated risk of cancer occurrence and lower survival (82). P300/CBP, also modulate several processes associated with proliferation, cell death, epithelial mesenchymal transition (EMT), and metastasis in breast cancer (83–86).
There are important roles exerted by the histone deacetylases “erasers” where they regulate the cell growth, EMT, angiogenesis, and metastasis of breast cancer (87–95). For e.g., Sirtuins, a class III histone deacetylase family regulates the oncogenes and tumor suppressor genes thereby affecting the breast carcinogenesis in a dual fashion. In this context, SIRT1 hindered the TNBC tumorigenesis, whereas fostered the tumorigenesis of luminal subtypes (96, 97). Interestingly, SIRT1 functions downstream of the BRCA1 gene and negatively regulate Survivin, an anti-apoptotic gene. Such transcriptional repression of Survivin is mediated via the deacetylation of histone H3 on lysine 9 on its promoter. Therefore, ablation of BRCA1 via lessened SIRT1 resulted in an upregulation of Survivin that facilitated the growth of breast tumors (98). Other Sirtuin family members are also implicated in breast cancer. For e.g., in TNBC cells, SIRT2 upregulation facilitated the deacetylation of histone H4 at the tumor suppressor gene ARRDC3 and this rendered the aggressiveness of breast cancer (99). Also, SIRT7 is elevated in human breast cancers (100).
Histone methylation
Histone methylation mainly occurs on the side chains of lysine and arginine residues. Unlike acetylation, histone methylation does not alter the charge of the histone protein but involves the addition of the methyl groups. Depending upon the number of methyl moiety added, lysine can be mono, di, or tri methylated whereas arginine can be symmetrically or asymmetrically methylated (101, 102). A special group of enzymes called histone methyltransferases (KMTs) catalyze the transfer of a methyl group from the S-adenosylmethionine (SAM) to a lysine’s ϵ-amino group. Methylation is also a dynamic and reversible process where the removal of the methyl groups is carried out by demethylases (histone demethylases, KDMs). The consequences of histone methylation are more complicated and largely dependent upon the targeted residues. For example, methylation of lysine H3K4, H3K36, and H3K79 at histone H3 contributes to transcriptional activation, while methylation of lysine at H3K9, H3K27 on histone H3 and, H4K20 on histone H4 is associated with transcriptional repression and are considered repressive epigenetic marks (103). Some of the methylated lysine histone marks have a role in DNA repair e.g., H3K36me3 is important for the homologous recombinational repair of the DNA double strand breaks, and H4K20me3 aids the repair via non-homologous end joining process (104). The resulting balance between methyltransferases (also called “writer”) and demethylases (also referred to as “eraser”) determines the methylation status of the cell (105), where DNA methylation and histone acetylation act in coordination to govern the overall gene transcriptional regulation. The balance between the histone acetyltransferases (HATs “writer”) and histone deacetylases (HDACs “eraser”) control the overall chromatin states/structures, hence regulating the gene expression. Histone modifications offer novel targets that can be exploited in breast cancer therapies (106).
In breast cancer, luminal A subtypes are found to exhibit increased global acetylation and methylation of the histone protein in comparison to the basal subtype (107). By measuring the relative levels of seven modified histones proteins including H3K18ac, H3K9ac, H4R3me2, H3K4me2, H4K12ac, H4K16ac, and H4K20me3 in 880 invasive breast cancer patients, it was revealed that the expressions of all seven markers were negatively correlated with tumor grade. While the loss of H4K16ac was suggestive to be an early event in the pathogenesis of invasive breast cancer, reduced levels of H4R3me2, H3K9ac, and H4K16ac were significantly associated with large tumor size. High levels of H4R3me2 and H3K9ac correlated with low lymph node stage (107). Interestingly, the metastatic behavior of breast cancer was correlated to an increased H3K4 histone mark where the dynamics of H3K4 acetylation and methylation exemplify the different breast cancer subtypes. While breast cancer cells representing both early and late cancer cell phenotypes are associated with a genome-wide gain of H3K4ac; late-stage cancer cells exhibited a gain of H3K4me3 (108). PI3K/AKT signaling cascade plays a significant role in breast cancer progression and this signaling was found to regulate the methylation of H3K4 in breast cancer, where an elevated level of H3K4me3 was linked with breast tumors (109). Another histone mark, H3K27ac has an important role in breast cancer progression and is found to regulate the EMT process (110, 111). The loss of a repressive epigenetic mark, the H3K27me3 has been identified as a negative prognostic indicator in breast cancer (112). Strikingly, enrichment of H3K27me3 within the promoter of genes FOXC1, RAD51, CDH1, and RUNX3, resulted in enhanced cell growth and metastasis of breast cancer (113). Loss of Cadherin 1 due to its hypermethylation via DNA methylation and trimethylation of H3K27 has been reported during metastasis (114), where it is important to note that Cadherin 1 is one of the key genes that inhibits metastasis and progression of breast cancer cells. Another mark, H4K20me3 is found to be significantly decreased in breast cancer and, importantly, it was an independent predictor of poor prognosis of the disease. This specific methylation of H4K20 is carried by the KMT5 family of enzymes that ultimately represses the transcription process (115, 116).
Among the enzymes implicated in gene regulation via epigenetic mechanisms, the enhancer of zeste homolog 2 (EZH2) is an important histone methyltransferase that methylates H3K27 leading to the transcriptional silencing of the target genes in breast cancer. Notably, in breast cancer, EZH2 has been found to be upregulated and promoted the EMT process (117, 118). Moreover, the level of EZH2 was gradually increased in breast cancer progression scenarios ranging from normal epithelium to epithelial hyperplasia, DCIS, IDC, and distant metastasis; and the expression of EZH2 was an independent predictor of breast cancer recurrence (119).
Members of the histone methyltransferases family, such as lysine methyltransferase 2 (KMT2) are also involved in the growth and spread of breast cancer cells, where they mediate the active histone methylation of H3K4 at the enhancer and the promoter regions of oncogenes and pro-metastatic genes, thereby facilitating the activation of genes that are estrogen dependent (120–123).
One of the only known histone 3 lysine 79 (H3K79) methyltransferases, is the histone methylase disruptor silencing 1 like (DOT1L) which has critical role in the development of breast cancer and is a potential therapeutic target for invasive breast cancer (124, 125). DOT1L is known to facilitate the aggressiveness of tumors by elevating the metastatic behavior of cancer cells (126) and is implicated in lymph node metastasis of breast cancer (127). In fact, targeting DOT1L by pharmacological interventions inhibited the growth and metastasis of TNBC cancer (128).
Among histone demethylases (erasers) family members are the prominent enzymes that are Fe2+/oxoglutarate-dependent containing a JumonjiC (JmjC) domain (129). Histone demethylase protein LSD1, a non JmjC demethylase has been found to negatively regulate the expression of cell growth and motility genes in breast cancer (130–133). Other JmjC KDMs involved in breast cancer are KDM4A, KDM4B and, KDM4C. Increased levels of KDM4A and KDM4B have been observed in ERα positive breast cancer cells, while TNBC cells showed an increased level of KDM4C (134). KDM4B regulates the cell cycle progression of breast cancer cells and is a direct target of ERα (135). While an increase of KDM3A is concomitant with a reduced H3K9me2/3 during breast tumorigenesis, KDM3A facilitated the activation of genes implicated in breast cancer as MYC, PAX3, Cyclin D1, MMP-9, S100A4, and JUN, thereby enhancing the proliferation and motility of breast cancer cells (136–138). KDM3A also promotes the growth of mammary gland ducts and tumors by positively affecting the proliferation via cyclin D1 (138). KDM4C is also necessary for breast cancer growth and, metastasis, where it serves as a co-activator of HIF-1α, with the underlying epigenetic mechanism of demethylating the H3K9me3 (139). Another histone demethylase PHF8 promoted EMT and breast tumorigenesis (140). PHF20L1, a methyl lysine reader protein containing a TUDOR domain, plays important role in breast cancer metastasis (141). Studies suggested its oncogenic role in response to hypoxic conditions, where it facilitated glycolysis, cell growth and metastasis of breast cancer cells by exerting its direct inhibitory activities on certain genes of tumor suppressive nature like HIC1, KISS1, and BRCA1 (142).
Non-coding RNAs
Functional RNA molecules that cannot be translated into proteins also referred to as non-coding RNA possess important regulatory effects and influence the expression of certain genes implicated in breast cancer. Among these are the long non-coding RNAs (lncRNAs) and micro-RNAs (miR). Micro-RNAs have been widely studied for its epigenetic regulation where they either activate or repress critical biological pathways and mechanisms important for breast tumorigenesis. Interestingly the let-7 family of micro RNAs has a significant role in breast cancer where its silencing has been associated with the development of metastasis and high-grade hormone negative breast tumors (143–145). Other micro-RNAs have important roles too. For. e.g., miR-9-3 activated apoptosis and miR-148a & miR-152 inhibited cell growth and angiogenesis (146, 147). Micro-RNAs involved in invasion and metastasis includes miR-125b, miR-126 and, miR-31 respectively (148–150). Some of the microRNAs whose aberrant hypermethylation has been reported in primary breast tumors include mir-663, mir-148, mir-9-1, mir-152, and mir-124a3 (151). Aberrant hypermethylation of H19, a lncRNA has been observed in invasive breast carcinoma when compared to normal breast tissues, where tumor suppressive functions of H19 have been suggested (152). HOTAIR, is another lncRNA where studies reported the recruitment of several writer proteins such as MLL1, MLL3, and P300/CBP to the HOTAIR’s promoter region thereby resulting in an enrichment of histone acetylation and elevation of H3K4me3, further driving the progression of breast cancer by suppressing the apoptosis (153).
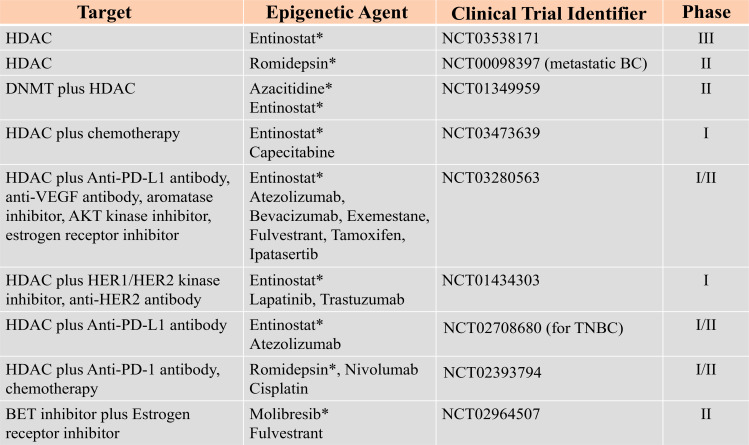
Therefore, epigenetic mechanisms offer many modalities that can be exploited for breast cancer therapies. Considering the fact that epigenetic changes induced by DNMTs and HDACs are transient and reversible, a number of studies are currently ongoing to establish effective, optimal dose and the treatment schedules for several epigenetic agents implicated in breast cancer, Figure 3 . Data adapted from (154).
Figure 3.
Epigenetic Targets and other combined inhibitors for breast cancer therapies under clinical trial. Data adapted from (154). Star (*) represents the specific epigenetic agent.
Environmental triggers of epigenetic aberrations in breast cancer
In addition to family history and genetic predisposition, epidemiological studies unraveled the influence of environmental exposures to hormonal agents and other factors that can increase the risk for breast cancer development. Exposure to endocrine disrupters, indoor and outdoor air pollution, polycyclic aromatic hydrocarbons (PAHs) etc. can induce epigenetic changes in an exposure or disease relation fashion. Xenobiotics such as activators of the aryl hydrocarbon receptor (AHR), dioxin, phthalates, polychlorinated biphenyls (PCB), PAHs, bisphenol A (BPA), arsenic etc. prevalent in the environment, dietary items, soil, water, and other consumable products, are likely to contribute to the epigenetic dysregulation of oncogenes and tumor suppressor genes in breast cancer.
AHR is a well-known sensor and a regulator of toxic and carcinogenic responses to environmental insults (155, 156). In advanced malignant breast carcinomas, AHR is shown to be constitutively active (157) and several studies reveal that targeting AHR can offer a potential treatment option for breast cancer patients (158, 159). Industrial xenobiotics, dietary metabolites etc., serve as agonists of AHR and are ubiquitously present in the environment. AHR-mediated epigenetic repression has been found in the BRCA1 gene which is also a direct target for AHR (160). In fact, CpG hypermethylation, deacetylation of H3K9, upregulation of H3K9me3, DNMT-1, DNMT-3a, DNMT-3b, and methyl-binding protein (MBD)-2 are some of the epigenetic changes linked with AHR mediated repression of BRCA1 gene (161, 162).
BPA is yet another endocrine disrupter and is an epigenetically active xenoestrogen prevalent in plastic and food cans (163, 164) whose exposure has been linked with an increased risk of breast cancer (165). While overexpression of EZH2 is linked to breast cancer, in-utero exposure to BPA is able to alter the EZH2 expression in mammary tissues (166). In fact, exposure of normal breast cells to the environmentally relevant doses of BPA caused the ERα to internalize into the nucleus and also changed the DNA methylation status of a lysosomal associated membrane protein (LAMP3) (167). LAMP3 protein is implicated in metastasis and breast cancer cell motility and is of prognostic significance (168–170).
A very prevalent environmental contaminant of soil, food, and water is arsenic which has been studied widely for its carcinogenic effect. Exposure to arsenic and the risk of developing breast cancer has been reviewed extensively (171). Arsenic is able to transform the normal mammary epithelial cells that were subjected to chronic treatment with low levels. Moreover arsenic facilitated the growth of breast cancer cells that were ERα-positive (172, 173). The involvement of arsenic in the carcinogenesis process comes from the fact that it induces genomic instability mediated by disrupting the Fanconi anemia (FA) and/or breast cancer (BRCA) pathway (174). The epigenetic influence of arsenic has been established in studies reporting that arsenic influences DNA methylation by affecting the pool of available methyl groups. This is because the detoxification of arsenic utilizes methyl group from S-adenosyl-homocysteine (SAM) (175). Therefore, exposure to arsenic and its subsequent metabolism within the cells, impart towards a global hypomethylation owing to the usage of existing methyl stores available from SAM (176). Strikingly, maternal exposure to arsenic not only altered the DNA methylation but also increased the DNA methylation in children (177, 178).
The source of PAHs is myriad, which includes combustion products, automobile exhaust, cigarette smoke, indoor and outdoor air pollution, waste incinerators etc. (179). Tobacco smoking represents one of the important risk factors for breast cancer (180–182). Smoking not only affects the DNA methylation pattern of breast tumors, but it has been a critical factor linking DNA methylation and breast cancer for ER positive cancer subtypes (183, 184). Aberrant methylation alterations have also been observed in breast cancer cells exposed to benzo(a)pyrene, which resulted in the generation of DNA adducts at the CpG dinucleotides, ultimately affecting the epigenetic landscape of the methylation process (185).
External factors are not just limited to toxicants or environmental agents. The cellular microenvironment is sensitive to cues such as nutrient availability, hypoxia and, extracellular pH, and can epigenetically reprogram the metabolic behavior of cancer cells to adapt to the changing environment (186). The fact that metabolic profiles of cancer cells differ from the normal cells, gives us a clear indication of the underlying genetic and epigenetic machinery that are altered in the carcinogenesis process, thereby bestowing growth advantage to cancer cells for their survival. Hence metabolic reprogramming is indispensable for breast cancer and has many therapeutic ramifications (187). Cellular metabolites shuffling from the different cellular compartments such as cytoplasm, mitochondria, nucleus, etc., has the potential to regulate gene expression by altering the availability of enzymatic substrates and co-factors required for the metabolic reactions mediated epigenetic processes, such as DNA and histone modifications. Glucose remains one of the most important metabolites shaping the metabolic profiles of breast cancer by shifting the energy generating mechanisms from glycolysis to oxidative phosphorylation or vice versa. In this context, the availability of glucose affects the estrogen which facilitates glycolysis in a high glucose state but urged oxidative phosphorylation under the conditions of low glucose to meet the energy demands of the breast cancer cells (188). It is noteworthy that in adipose tissues, a major component of the breast, ERα is the vital regulator of a glucose transporter protein expression GLUT4 (189). Glycolysis can also be influenced by ERα, during the conditions of hypoxic stress. Hypoxia inducible factor-1α (HIF-1α) which is an oxygen-dependent transcriptional activator that carries out cellular adaptation to low oxygen and nutrient starved environment, is implicated in the ERα mediated activation of the glycolysis process in breast cancer (190). However, under normoxia and hypoxia conditions, both ERα and HIF-1α regulate histone demethylase JMJD2B and orchestrate breast cancer cell growth by epigenetically regulating the genes implicated in the cell cycle. Moreover, knocking down ERα can compromise the HIF-1α function even under hypoxic circumstances (135). One of the important transcription factors that aid cancer cells in metabolic adaption in a nutrient deprived environment, oxidative or xenobiotic stress is the nuclear factor erythroid 2-related factor 2 (NRF2) (191). Epigenetic modifications including DNA methylation are crucial for the regulation of NRF2 and its adaptor protein KEAP1 (192, 193). In breast cancer patients, elevated NRF2 expression led to decreased overall survival and disease-free survival (194). Elevated NRF2 enhanced the growth and motility of breast cancer cells by upregulating a pivotal enzyme of the pentose phosphate pathway, i.e., the glucose-6-phosphate dehydrogenase (G6PD) (195). In fact, Estradiol (E2) can stimulate NRF2 transcription, leading to an elevation in mitochondrial biogenesis (196).
Mdig, an environment regulated gene in breast cancer
To ascertain the kind of risks and exposures affecting breast carcinogenesis, it is essential to gain an understanding of gene-environment interaction and the genes that are induced and manifested in breast cancer. Since a fraction of breast cancer cases is also sporadic, studying the genetic and epigenetic mechanisms that regulates breast tumor development under environmental and occupational settings, will undoubtedly offer new targets for chemoprevention and therapies.
We have identified one such environmentally induced gene named the Mineral dust-induced gene (mdig), also called MINA53, RIOX2, or NO52. Certain environmental agents such as mineral dust, tobacco smoke, arsenic, silica, etc. induced the expression of mdig (197–200). Mdig has oncogenic and epigenetic roles in a variety of human cancers, where it exhibits elevated expression (201, 202). Mdig promoted cell proliferation, cell cycle transition, and anti-apoptotic behaviors in different cell types, further corroborating its oncogenic role (198, 203). Mdig played key roles in the pathogenesis of arsenic induced lung cancer, where JNK-STAT3 signaling and mi-RNA21 mediate the processes. Further, we found that arsenic exposure induces the phosphorylation of EZH2 at serine 21 via JNK- and STAT3-dependent Akt activation (199, 204). Mdig is also upregulated in smokers in a pack-year dependent fashion, where it predicted poor overall survival in smokers that had lung cancer (205).
More recently, our studies on mdig and environmental factor arsenic revealed crosstalk between mdig and a master regulator of oxidative stress, NRF2, where together they contribute to arsenic induced generation of cancer stem like cells. Normal lung cells treated with arsenic showed an enhancement of HIF1α in the promoter of mdig, which was somehow accredited by activated NRF2 in response to arsenic (206). Since HIF1α is a direct transcriptional target of NRF2 (206) and considering the important role of NRF2 and HIF1 in tumorigenesis, our research further potentiates the importance of mdig on regulating the stress response activities implicated in genomic instability relying on metabolic reprogramming and cancer stem cells (207).
In breast cancer, we have identified that the expression level of mdig predicts the survival outcomes depending upon the different status of lymph node metastasis. A higher level of mdig predicted poor overall survival of patients who had no lymph node metastasis, whereas, in those patients who were positive for lymph node metastasis, high mdig expression predicted better overall survival (208). Dwelling further to assess the role of mdig in breast cancer, our studies revealed a negative correlation of mdig on the migration, invasion, and DNA methylation of breast cancer cells. Mdig not only regulated the chromatin accessibility of the migration/invasion genes but also exhibited a context dependent expression, where its expression was downregulated in invasive and triple negative breast cancer. This supported the notion that mdig is inhibitory for cell motility and spread and that’s why its high expression predicts favorable outcomes in lymph node metastasis positive cases of breast cancer (209). Since mdig is transcriptionally governed by an upstream regulator c-myc (210), which has both tumor accelerator and suppressive roles and can inhibit cancer metastasis (211), our studies are suggestive of the dual roles of mdig in breast cancer, where it is essential for the early stages of cancer development due to its pro-proliferative feature but is inhibitory in the later stages owing to its metastasis inhibitory features.
Mdig protein contains a conserved JmjC domain. Since JmjC domain has been identified as a signature motif of the JmjC family of histone demethylases (129), mdig’s involvement in the epigenetic process of histone modifications is inevitable. Recent studies provide evidence that the oncogenic activity of mdig is presumably achieved via its regulation on the demethylation of histone proteins. Our studies showed a demethylase like activity of mdig towards the repressive histone methylation markers that include H3K9me3, H3K27me3, and H4K20me3. Using the CRISPR-Cas9 gene editing approach coupled with chromatin immunoprecipitation sequencing (ChIP) in human lung epithelial cell line BEAS 2B, lung cancer cell line A549, and breast cancer cell line MDA-MB-231, an antagonistic effect of mdig on repressive histone trimethylation marks were revealed where mdig favored the open conformation of chromatin and permitted active gene transcription. Knocking down mdig resulted in a pronounced enrichment of these repressive trimethylation markers on the genes that are implicated in cell growth, stemness, inflammation, and metastasis (212). With the loss of mdig, there also occurred an increase in the levels of the polycomb repressive complex (PRC2) proteins EZH2 and RBBP4. Strikingly, these proteins are known to catalyze H3K27me3, and our previous studies identified a direct protein-protein interaction between mdig and CBX3, CBX5, RBBP4, and RBBP7 proteins. While RBBP4 and RBBP7 are the regulatory subunits of the PRC2 complex, CBX3 and CBX5 can recognize and bind to H3K9me3 (213).
In breast cancer cells, loss of mdig also enhanced an epigenetic mark of transcription elongation H3K36me3, in addition to H4K20me3 and H3K9me3. In this view, H4K20me3 being a marker for closed chromatin status in the somatic and embryonic stem cells (214), it is suggested that an elevation of H4K20me3 can contribute to growth inhibitory activities in the somatic cells. This notion is further supported by our previous studies where reduced mdig resulted in a decline of the S phase cells (198). It is also indicated that mdig acts as DNA demethylase or indirectly controls DNA methylation via the Tet family of DNA methylases (202). Additionally, a negative correlation was also observed between mdig and H3K9me3 in cellular studies (209, 215, 216). One of the consequences of enriched histone repressive marks H3K9me3 and H3K27me3, is on the transcription of genes implicated in glycan metabolism. Mdig exerted a positive regulatory role on the glycosylation process by inhibiting the repressive histone methylation marks (217).
Altogether, our research on mdig provided a much-needed rationale to explore its activities in several aspects of inflammation, stemness, metabolism, cell growth, metastasis, and epigenetic reprogramming orchestrating the carcinogenesis machinery in breast cancer.
Perspectives
Despite tremendous progress being made in breast cancer research, some challenges still prevail. Metabolic plasticity, epigenetic reprogramming, and altered receptor repertoire lead to the issues of drug resistance and treatment failure. It is yet not fully clear as to what are the remarkable mechanistic programs that are critical for the breast tumor to become metastatic. Although our understanding of the heterogeneity of breast cancers has improved that has led to the generation of novel anti-cancer therapies exploiting the hormone receptor status, epigenetic marks, and other biological machineries, yet, when it comes to the general population there has been very limited success owing to the individual differences among the patients. An efficient personalized therapy would offer rescue to some extent towards combating the setbacks originated due to the heterogeneity and plasticity issues as observed in breast cancer therapies under clinical settings.
Environmental exposure to risk factors for breast cancer require particular attention, where relevant biomarkers related to such exposure need to be identified. Epigenetic mechanisms particularly DNA and histone methylation are involved in the onset of carcinogenesis by modulating the expression of potent oncogenes and tumor suppressors. Thus, dissecting the epigenetic elements would widen our knowledge towards better understanding the causative factors as well as the different routes that cancer cells adopt to attain heterogeneity. Moreover, studying maternal, in utero or pre-conception exposures and unraveling an association between the agents exposed and the different epigenetic repertoires correlating with the disease outcome, will be a promising avenue to explore. Such a strategy would assist in adopting modifiable approaches that can have significant implications in reducing the risk factors as a part of chemoprevention tactics. This demands a multidisciplinary effort that would integrate genomics, proteomics, and metabolomics in examining the different epigenomic profiles and pattern that drive the breast carcinogenesis under the conditions of sporadic and environmental settings. In this context, research on environmentally modulated genes engaged in breast cancer such as mdig, is warranted.
Author contributions
CT and FC conceived the idea and wrote the article. YQ, YF, ZB, WZ, and HJ participated in conducting systemic review of the literature. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Institutes of Health (NIH) grants R01 ES031822, R01 ES028335, R01 ES028263, and Research Start-up fund of the Stony Brook University to FC.
Acknowledgments
We would like to thank all the authors and researchers whose work has been cited here.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
| DCIS | Ductal carcinoma in situ |
| IDC | Invasive ductal carcinoma |
| ILC | Invasive lobular carcinoma |
| HER2 | Human epidermal growth factor receptor 2 |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| BRCA | Breast Cancer gene |
| TNBC | Basal/triple negative breast cancer |
| lncRNAs | Long non-coding RNAs |
| miR | Micro-RNAs |
| MBD | Methyl binding domain |
| 5mC | 5-methylcytosine |
| 5-hmC | 5-hydroxymethylcytosine |
| DNMT | DNA methyltransferase |
| TET | Ten-eleven translocations |
| HAT | Histone acetyltransferases |
| HDAC | Histone deacetylases |
| HKMT | Histone methyltransferases |
| KDM | Histone demethylases |
| SAM | Sadenosylmethionine |
| EMT | Epithelial mesenchymal transition |
| DOT1L | Histone methylase disruptor silencing 1 like |
| JmjC | JumonjiC |
| PAH | Polycyclic aromatic hydrocarbons |
| AHR | Aryl hydrocarbon receptor |
| PCB | Polychlorinated biphenyls |
| BPA | Bisphenol A |
| LAMP3 | Lysosomal associated membrane protein |
| HIF-1α | Hypoxia inducible factor-1α |
| GLUT4 | Glucose transporter protein expressiona |
| G6PD | Glucose-6-phosphate dehydrogenase |
| Mdig | Mineral dust-induced gene |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| ChIP | Chromatin immunoprecipitation sequencing |
| PRC2 | Polycomb repressive complex |
| GADD45 | Growth arrest and DNA-damage inducible protein |
| AID | Activation-induced cytidine deaminase |
| APOBEC | Apolipoprotein B mRNA editing catalytic polypeptide-like family |
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2. Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 4)††These guidelines were developed by the European school of oncology (ESO) and the European society for medical oncology (ESMO). Ann Oncol (2018) 29(8):1634–57. doi: 10.1093/annonc/mdy192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nascimento R, Otoni K. Histological and molecular classification of breast cancer: what do we know? Mastology (2020) 30:1–8. doi: 10.29289/25945394202020200024 [DOI] [Google Scholar]
- 4. Henry NL, Cannon-Albright LA. Breast cancer histologic subtypes show excess familial clustering. Cancer (2019) 125(18):3131–8. doi: 10.1002/cncr.32198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, et al. The 2019 world health organization classification of tumours of the breast. Histopathology (2020) 77(2):181–5. doi: 10.1111/his.14091 [DOI] [PubMed] [Google Scholar]
- 6. Fragomeni SM, Sciallis A, Jeruss JS. Molecular subtypes and local-regional control of breast cancer. Surg Oncol Clinics North America (2018) 27(1):95–120. doi: 10.1016/j.soc.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones RL, Constantinidou A, Reis-Filho JS. Molecular classification of breast cancer. Surg Pathol Clin (2012) 5(3):701–17. doi: 10.1016/j.path.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 8. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res (2007) 13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.CCR-06-3045 [DOI] [PubMed] [Google Scholar]
- 9. Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol (2015) 12(7):381–94. doi: 10.1038/nrclinonc.2015.73 [DOI] [PubMed] [Google Scholar]
- 10. Malhotra GK, Zhao X, Band H, Band V. Histological, molecular and functional subtypes of breast cancers. Cancer Biol Ther (2010) 10(10):955–60. doi: 10.4161/cbt.10.10.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. Jama (2017) 317(23):2402–16. doi: 10.1001/jama.2017.7112 [DOI] [PubMed] [Google Scholar]
- 12. Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol (2007) 25(11):1329–33. doi: 10.1200/JCO.2006.09.1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pohl-Rescigno E, Hauke J, Loibl S, Möbus V, Denkert C, Fasching PA, et al. Association of germline variant status with therapy response in high-risk early-stage breast cancer: A secondary analysis of the GeparOcto randomized clinical trial. JAMA Oncol (2020) 6(5):744–8. doi: 10.1001/jamaoncol.2020.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: A review. Cancer J (2021) 27(1):8–16. doi: 10.1097/PPO.0000000000000500 [DOI] [PubMed] [Google Scholar]
- 15. Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature (2016) 534(7605):47–54. doi: 10.1038/nature17676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walsh T, King MC. Ten genes for inherited breast cancer. Cancer Cell (2007) 11(2):103–5. doi: 10.1016/j.ccr.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 17. Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U.S.A. (2012) 109(8):2796–801. doi: 10.1073/pnas.1104303108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mitrunen K, Hirvonen A. Molecular epidemiology of sporadic breast cancer. the role of polymorphic genes involved in oestrogen biosynthesis and metabolism. Mutat Res (2003) 544(1):9–41. doi: 10.1016/S1383-5742(03)00016-4 [DOI] [PubMed] [Google Scholar]
- 19. Nur U, El Reda D, Hashim D, Weiderpass E. A prospective investigation of oral contraceptive use and breast cancer mortality: findings from the Swedish women’s lifestyle and health cohort. BMC Cancer (2019) 19(1):807. doi: 10.1186/s12885-019-5985-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Del Pup L, Codacci-Pisanelli G, Peccatori F. Breast cancer risk of hormonal contraception: Counselling considering new evidence. Crit Rev Oncol Hematol (2019) 137:123–30. doi: 10.1016/j.critrevonc.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 21. Kehm RD, Genkinger JM, MacInnis RJ, John EM, Phillips KA, Dite GS, et al. Recreational physical activity is associated with reduced breast cancer risk in adult women at high risk for breast cancer: A cohort study of women selected for familial and genetic risk. Cancer Res (2020) 80(1):116–25. doi: 10.1158/0008-5472.CAN-19-1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barrios-Rodríguez R, Toledo E, Martinez-Gonzalez MA, Aguilera-Buenosvinos I, Romanos-Nanclares A, Jiménez-Moleón JJ. Adherence to the 2018 world cancer research Fund/American institute for cancer research recommendations and breast cancer in the SUN project. Nutrients (2020) 12(7):1–12. doi: 10.3390/nu12072076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW Jr., et alet al. Alcohol, tobacco and breast cancer–collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer (2002) 87(11):1234–45.doi: 10.1038/sj.bjc.6600596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seitz HK, Pelucchi C, Bagnardi V, La Vecchia C. Epidemiology and pathophysiology of alcohol and breast cancer: Update 2012. Alcohol Alcohol (2012) 47(3):204–12. doi: 10.1093/alcalc/ags011 [DOI] [PubMed] [Google Scholar]
- 25. Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet (1996) 347(9017):1713–27. doi.org/ 10.1016/s0140-6736(96)90806-5"10.1016/s0140-6736(96)90806-5 [DOI] [PubMed] [Google Scholar]
- 26. Collaborative Group on Hormonal Factors in Breast . C., menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol (2012) 13(11):1141–51. doi.org/ 10.1016/s1470-2045(12)70425-4"10.1016/S1470-2045(12)70425-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldberg M, D’Aloisio AA, O’Brien KM, Zhao S, Sandler DP. Pubertal timing and breast cancer risk in the sister study cohort. Breast Cancer Res (2020) 22(1):112. doi: 10.1186/s13058-020-01326-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doody MM, Freedman DM, Alexander BH, Hauptmann M, Miller JS, Rao RS, et al. Breast cancer incidence in U.S. radiologic technologists. Cancer (2006) 106(12):2707–15. doi: 10.1002/cncr.21876 [DOI] [PubMed] [Google Scholar]
- 29. Chlebowski RT, Luo J, Anderson GL, Barrington W, Reding K, Simon MS, et al. Weight loss and breast cancer incidence in postmenopausal women. Cancer (2019) 125(2):205–12. doi: 10.1002/cncr.31687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature (1983) 301(5895):89–92. doi: 10.1038/301089a0 [DOI] [PubMed] [Google Scholar]
- 31. Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer (2011) 11(10):726–34. doi: 10.1038/nrc3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Esteller M. Epigenetics in cancer. New Engl J Med (2008) 358(11):1148–59. doi: 10.1056/NEJMra072067 [DOI] [PubMed] [Google Scholar]
- 33. Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med (2003) 349(21):2042–54. doi: 10.1056/NEJMra023075 [DOI] [PubMed] [Google Scholar]
- 34. Kulis M, Esteller M. 2 - DNA methylation and cancer. In: Herceg Z, Ushijima T, editors. Advances in genetics. (Barcelona, Catalonia, Spain: Cancer Epigenetics and Biology Program; (2010). 27–56. [DOI] [PubMed] [Google Scholar]
- 35. Jones PA, Baylin SB. The epigenomics of cancer. Cell (2007) 128(4):683–92. doi: 10.1016/j.cell.2007.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature (2007) 447(7143):433–40. doi: 10.1038/nature05919 [DOI] [PubMed] [Google Scholar]
- 37. Kareta MS, Botello ZM, Ennis JJ, Chou C, Chédin F. Reconstitution and mechanism of the stimulation of de novo methylation by human DNMT3L. J Biol Chem (2006) 281(36):25893–902. doi: 10.1074/jbc.M603140200 [DOI] [PubMed] [Google Scholar]
- 38. Okano M, Bell DW, Haber DA, Li E. DNA Methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell (1999) 99(3):247–57. doi: 10.1016/S0092-8674(00)81656-6 [DOI] [PubMed] [Google Scholar]
- 39. Robertson KD. DNA Methylation and human disease. Nat Rev Genet (2005) 6(8):597–610. doi: 10.1038/nrg1655 [DOI] [PubMed] [Google Scholar]
- 40. Scourzic L, Mouly E, Bernard OA. TET proteins and the control of cytosine demethylation in cancer. Genome Med (2015) 7(1):9. doi: 10.1186/s13073-015-0134-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Williams K, Christensen J, Helin K. DNA Methylation: TET proteins-guardians of CpG islands? EMBO Rep (2011) 13(1):28–35. doi: 10.1038/embor.2011.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chandramouly G. Gadd45 in DNA demethylation and DNA repair. In: Zaidi MR, Liebermann DA, editors. Gadd45 stress sensor genes. (Philadelphia, PA, USA: Springer International Publishing: Cham; ) (2022). 55–67. [DOI] [PubMed] [Google Scholar]
- 43. Schäfer A. Gadd45 proteins: key players of repair-mediated DNA demethylation. Adv Exp Med Biol (2013) 793:35–50. doi: 10.1007/978-1-4614-8289-5_3 [DOI] [PubMed] [Google Scholar]
- 44. Pietrasik S, Zajac G, Morawiec J, Soszynski M, Fila M, Blasiak J. Interplay between BRCA1 and GADD45A and its potential for nucleotide excision repair in breast cancer pathogenesis. Int J Mol Sci (2020) 21(3):1–22. doi: 10.3390/ijms21030870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang W, Huper G, Guo Y, Murphy SK, Olson JA Jr., Marks JR, et al. Analysis of methylation-sensitive transcriptome identifies GADD45a as a frequently methylated gene in breast cancer. Oncogene (2005) 24(16):2705–14. doi: 10.1038/sj.onc.1208464 [DOI] [PubMed] [Google Scholar]
- 46. Jeschke J, Collignon E, Fuks F. Portraits of TET-mediated DNA hydroxymethylation in cancer. Curr Opin Genet Dev (2016) 36:16–26. doi: 10.1016/j.gde.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 47. Muñoz DP, Lee EL, Takayama S, Coppé JP, Heo SJ, Boffelli D, et al. Activation-induced cytidine deaminase (AID) is necessary for the epithelial-mesenchymal transition in mammary epithelial cells. Proc Natl Acad Sci U.S.A. (2013) 110(32):E2977–86. doi: 10.1073/pnas.1301021110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morgan HD, Dean W, Coker HA, Reik W, Petersen-Mahrt SK. Activation-induced cytidine deaminase deaminates 5-methylcytosine in DNA and is expressed in pluripotent tissues: IMPLICATIONS FOR EPIGENETIC REPROGRAMMING*. J Biol Chem (2004) 279(50):52353–60. doi: 10.1074/jbc.M407695200 [DOI] [PubMed] [Google Scholar]
- 49. Popp C, Dean W, Feng S, Cokus SJ, Andrews S, Pellegrini M, et al. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature (2010) 463(7284):1101–5. doi: 10.1038/nature08829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell (2011) 145(3):423–34. doi: 10.1016/j.cell.2011.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bos MK, Smid M, Sleijfer S, Martens JWM. Apolipoprotein b mRNA-editing catalytic polypeptide-Like-Induced protein changes in estrogen receptor-positive, human epidermal growth factor receptor 2-negative breast cancer throughout disease progression. JCO Precis Oncol (2022) 6:e2100190. doi: 10.1200/PO.21.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. DiMarco AV, Qin X, McKinney BJ, Garcia NMG, Van Alsten SC, Mendes EA, et al. APOBEC mutagenesis inhibits breast cancer growth through induction of T cell-mediated antitumor immune responses. Cancer Immunol Res (2022) 10(1):70–86. doi: 10.1158/2326-6066.CIR-21-0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, et al. Comprehensive molecular portraits of human breast tumours. Nature (2012) 490(7418):61–70. doi: 10.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Esteller M, Silva JM, Dominguez G, Bonilla F, Matias-Guiu X, Lerma E, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst (2000) 92(7):564–9. doi: 10.1093/jnci/92.7.564 [DOI] [PubMed] [Google Scholar]
- 55. Radpour R, Barekati Z, Kohler C, Schumacher MM, Grussenmeyer T, Jenoe P, et al. Integrated epigenetics of human breast cancer: synoptic investigation of targeted genes, microRNAs and proteins upon demethylation treatment. PloS One (2011) 6(11):e27355. doi: 10.1371/journal.pone.0027355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fujikane T, Nishikawa N, Toyota M, Suzuki H, Nojima M, Maruyama R, et al. Genomic screening for genes upregulated by demethylation revealed novel targets of epigenetic silencing in breast cancer. Breast Cancer Res Treat (2010) 122(3):699–710. doi: 10.1007/s10549-009-0600-1 [DOI] [PubMed] [Google Scholar]
- 57. Shargh SA, Sakizli M, Khalaj V, Movafagh A, Yazdi H, Hagigatjou E, et al. Downregulation of e-cadherin expression in breast cancer by promoter hypermethylation and its relation with progression and prognosis of tumor. Med Oncol (2014) 31(11):1–6. doi: 10.1007/s12032-014-0250-y [DOI] [PubMed] [Google Scholar]
- 58. Mirza S, Sharma G, Prasad CP, Parshad R, Srivastava A, Gupta SD, et al. Promoter hypermethylation of TMS1, BRCA1, ERα and PRB in serum and tumor DNA of invasive ductal breast carcinoma patients. Life Sci (2007) 81(4):280–7. doi: 10.1016/j.lfs.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 59. Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res (2001) 61(8):3225–9. [PubMed] [Google Scholar]
- 60. Butcher DT, Rodenhiser DI. Epigenetic inactivation of BRCA1 is associated with aberrant expression of CTCF and DNA methyltransferase (DNMT3B) in some sporadic breast tumours. Eur J Cancer (2007) 43(1):210–9. doi: 10.1016/j.ejca.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 61. Vallian S, Sedaghat M, Nassiri I, Frazmand A. Methylation status of p16 INK4A tumor suppressor gene in Iranian patients with sporadic breast cancer. J Cancer Res Clin Oncol (2009) 135(8):991–6. doi: 10.1007/s00432-008-0534-8 [DOI] [PubMed] [Google Scholar]
- 62. Askari M, Sobti RC, Nikbakht M, Sharma SC. Aberrant promoter hypermethylation of p21 (WAF1/CIP1) gene and its impact on expression and role of polymorphism in the risk of breast cancer. Mol Cell Biochem (2013) 382(1-2):19–26. doi: 10.1007/s11010-013-1696-5 [DOI] [PubMed] [Google Scholar]
- 63. Győrffy B, Bottai G, Fleischer T, Munkácsy G, Budczies J, Paladini L, et al. Aberrant DNA methylation impacts gene expression and prognosis in breast cancer subtypes. Int J Cancer (2016) 138(1):87–97. doi: 10.1002/ijc.29684 [DOI] [PubMed] [Google Scholar]
- 64. Holm K, Hegardt C, Staaf J, Vallon-Christersson J, Jönsson G, Olsson H, et al. Molecular subtypes of breast cancer are associated with characteristic DNA methylation patterns. Breast Cancer Res (2010) 12(3):R36. doi: 10.1186/bcr2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yan PS, Perry MR, Laux DE, Asare AL, Caldwell CW, Huang TH. CpG island arrays: an application toward deciphering epigenetic signatures of breast cancer. Clin Cancer Res (2000) 6(4):1432–8. [PubMed] [Google Scholar]
- 66. Lapidus RG, Ferguson AT, Ottaviano YL, Parl FF, Smith HS, Weitzman SA, et al. Methylation of estrogen and progesterone receptor gene 5’ CpG islands correlates with lack of estrogen and progesterone receptor gene expression in breast tumors. Clin Cancer Res (1996) 2(5):805–10. [PubMed] [Google Scholar]
- 67. Ennour-Idrissi K, Dragic D, Issa E, Michaud A, Chang S-L, Provencher L, et al. DNA Methylation and breast cancer risk: An epigenome-wide study of normal breast tissue and blood. Cancers (2020) 12(11):3088. doi: 10.3390/cancers12113088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dedeurwaerder S, Desmedt C, Calonne E, Singhal SK, Haibe-Kains B, Defrance M, et al. DNA Methylation profiling reveals a predominant immune component in breast cancers. EMBO Mol Med (2011) 3(12):726–41. doi: 10.1002/emmm.201100801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang M, Wang Y, Wang Y, Jiang L, Li X, Gao H, et al. Integrative analysis of DNA methylation and gene expression to determine specific diagnostic biomarkers and prognostic biomarkers of breast cancer. Front Cell Dev Biol (2020) 8. doi: 10.3389/fcell.2020.529386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. de Almeida BP, Apolónio JD, Binnie A, Castelo-Branco P. Roadmap of DNA methylation in breast cancer identifies novel prognostic biomarkers. BMC Cancer (2019) 19(1):219. doi: 10.1186/s12885-019-5403-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Batra RN, Lifshitz A, Vidakovic AT, Chin S-F, Sati-Batra A, Sammut S-J, et al. DNA Methylation landscapes of 1538 breast cancers reveal a replication-linked clock, epigenomic instability and cis-regulation. Nat Commun (2021) 12(1):5406. doi: 10.1038/s41467-021-25661-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 a resolution. Nature (1997) 389(6648):251–60. doi: 10.1038/38444 [DOI] [PubMed] [Google Scholar]
- 73. Bentley GA, Lewit-Bentley A, Finch JT, Podjarny AD, Roth M. Crystal structure of the nucleosome core particle at 16 a resolution. J Mol Biol (1984) 176(1):55–75. doi: 10.1016/0022-2836(84)90382-6 [DOI] [PubMed] [Google Scholar]
- 74. Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of rna synthesis. Proc Natl Acad Sci U.S.A. (1964) 51(5):786–94. doi: 10.1073/pnas.51.5.786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Audia JE, Campbell RM. Histone Modifications and Cancer Cold Spring Harb Perspect Biol (2016) 8(4):a019521. doi: 10.1101/cshperspect.a019521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res (2011) 21(3):381–95. doi: 10.1038/cr.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ropero S, Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol Oncol (2007) 1(1):19–25. doi: 10.1016/j.molonc.2007.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Koch CM, Andrews RM, Flicek P, Dillon SC, Karaöz U, Clelland GK, et al. The landscape of histone modifications across 1% of the human genome in five human cell lines. Genome Res (2007) 17(6):691–707. doi: 10.1101/gr.5704207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U.S.A. (2010) 107(50):21931–6. doi: 10.1073/pnas.1016071107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pradeepa MM, Grimes GR, Kumar Y, Olley G, Taylor GC, Schneider R, et al. Histone H3 globular domain acetylation identifies a new class of enhancers. Nat Genet (2016) 48(6):681–6. doi: 10.1038/ng.3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lopez-Serra L, Ballestar E, Ropero S, Setien F, Billard LM, Fraga MF, et al. Unmasking of epigenetically silenced candidate tumor suppressor genes by removal of methyl-CpG-binding domain proteins. Oncogene (2008) 27(25):3556–66. doi: 10.1038/sj.onc.1211022 [DOI] [PubMed] [Google Scholar]
- 82. Xiao XS, Cai MY, Chen JW, Guan XY, Kung HF, Zeng YX, et al. High expression of p300 in human breast cancer correlates with tumor recurrence and predicts adverse prognosis. Chin J Cancer Res (2011) 23(3):201–7. doi: 10.1007/s11670-011-0201-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dong H, Wang W, Mo S, Chen R, Zou K, Han J, et al. SP1-induced lncRNA AGAP2-AS1 expression promotes chemoresistance of breast cancer by epigenetic regulation of MyD88. J Exp Clin Cancer Res (2018) 37(1):202. doi: 10.1186/s13046-018-0875-3 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84. Chi Y, Xue J, Huang S, Xiu B, Su Y, Wang W, et al. CapG promotes resistance to paclitaxel in breast cancer through transactivation of PIK3R1/P50. Theranostics (2019) 9(23):6840–55. doi: 10.7150/thno.36338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. He H, Wang D, Yao H, Wei Z, Lai Y, Hu J, et al. Transcriptional factors p300 and MRTF-a synergistically enhance the expression of migration-related genes in MCF-7 breast cancer cells. Biochem Biophys Res Commun (2015) 467(4):813–20. doi: 10.1016/j.bbrc.2015.10.060 [DOI] [PubMed] [Google Scholar]
- 86. Lin C, Liao W, Jian Y, Peng Y, Zhang X, Ye L, et al. CGI-99 promotes breast cancer metastasis via autocrine interleukin-6 signaling. Oncogene (2017) 36(26):3695–705. doi: 10.1038/onc.2016.525 [DOI] [PubMed] [Google Scholar]
- 87. Yu S, Gong X, Ma Z, Zhang M, Huang L, Zhang J, et al. Endocrine resistant breast cancer cells with loss of ERα expression retain proliferative ability by reducing caspase7-mediated HDAC3 cleavage. Cell Oncol (Dordr) (2020) 43(1):65–80. doi: 10.1007/s13402-019-00439-x [DOI] [PubMed] [Google Scholar]
- 88. Hou MF, Luo CW, Chang TM, Hung WC, Chen TY, Tsai YL, et al. The NuRD complex-mediated p21 suppression facilitates chemoresistance in BRCA-proficient breast cancer. Exp Cell Res (2017) 359(2):458–65. doi: 10.1016/j.yexcr.2017.08.029 [DOI] [PubMed] [Google Scholar]
- 89. Liu R, Wang L, Chen G, Katoh H, Chen C, Liu Y, et al. FOXP3 up-regulates p21 expression by site-specific inhibition of histone deacetylase 2/histone deacetylase 4 association to the locus. Cancer Res (2009) 69(6):2252–9. doi: 10.1158/0008-5472.CAN-08-3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tang X, Li G, Su F, Cai Y, Shi L, Meng Y, et al. HDAC8 cooperates with SMAD3/4 complex to suppress SIRT7 and promote cell survival and migration. Nucleic Acids Res (2020) 48(6):2912–23. doi: 10.1093/nar/gkaa039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lu C, Wang X, Zhao X, Xin Y, Liu C. Long non-coding RNA ARAP1-AS1 accelerates cell proliferation and migration in breast cancer through miR-2110/HDAC2/PLIN1 axis. Biosci Rep (2020) 40(4):1–10. doi: 10.1042/BSR20191764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cassandri M, Butera A, Amelio I, Lena AM, Montanaro M, Mauriello A, et al. ZNF750 represses breast cancer invasion via epigenetic control of prometastatic genes. Oncogene (2020) 39(22):4331–43. doi: 10.1038/s41388-020-1277-5 [DOI] [PubMed] [Google Scholar]
- 93. Ray A, Alalem M, Ray BK. Loss of epigenetic kruppel-like factor 4 histone deacetylase (KLF-4-HDAC)-mediated transcriptional suppression is crucial in increasing vascular endothelial growth factor (VEGF) expression in breast cancer. J Biol Chem (2013) 288(38):27232–42. doi: 10.1074/jbc.M113.481184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Roy SS, Gonugunta VK, Bandyopadhyay A, Rao MK, Goodall GJ, Sun LZ, et al. Significance of PELP1/HDAC2/miR-200 regulatory network in EMT and metastasis of breast cancer. Oncogene (2014) 33(28):3707–16. doi: 10.1038/onc.2013.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gong C, Qu S, Lv XB, Liu B, Tan W, Nie Y, et al. BRMS1L suppresses breast cancer metastasis by inducing epigenetic silence of FZD10. Nat Commun (2014) 5:5406. doi: 10.1038/ncomms6406 [DOI] [PubMed] [Google Scholar]
- 96. Rifaï K, Judes G, Idrissou M, Daures M, Bignon YJ, Penault-Llorca F, et al. Dual SIRT1 expression patterns strongly suggests its bivalent role in human breast cancer. Oncotarget (2017) 8(67):110922–30. doi: 10.18632/oncotarget.23006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rifaï K, Judes G, Idrissou M, Daures M, Bignon YJ, Penault-Llorca F, et al. SIRT1-dependent epigenetic regulation of H3 and H4 histone acetylation in human breast cancer. Oncotarget (2018) 9(55):30661–78. doi: 10.18632/oncotarget.25771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang R-H, Zheng Y, Kim H-S, Xu X, Cao L, Lahusen T, et al. Interplay among BRCA1, SIRT1, and survivin during BRCA1-associated tumorigenesis. Mol Cell (2008) 32(1):11–20. doi: 10.1016/j.molcel.2008.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Soung YH, Pruitt K, Chung J. Epigenetic silencing of ARRDC3 expression in basal-like breast cancer cells. Sci Rep (2014) 4:3846. doi: 10.1038/srep03846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ashraf N, Zino S, Macintyre A, Kingsmore D, Payne A, George W, et al. Altered sirtuin expression is associated with node-positive breast cancer. Br J Cancer (2006) 95(8):1056–61. doi: 10.1038/sj.bjc.6603384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-resolution profiling of histone methylations in the human genome. Cell (2007) 129(4):823–37. doi: 10.1016/j.cell.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 102. Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell (2009) 33(1):1–13. doi: 10.1016/j.molcel.2008.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jenuwein T, Allis CD. Translating the histone code. Science (2001) 293(5532):1074–80. doi: 10.1126/science.1063127 [DOI] [PubMed] [Google Scholar]
- 104. Wei S, Li C, Yin Z, Wen J, Meng H, Xue L, et al. Histone methylation in DNA repair and clinical practice: new findings during the past 5-years. J Cancer (2018) 9(12):2072–81. doi: 10.7150/jca.23427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hyun K, Jeon J, Park K, Kim J. Writing, erasing and reading histone lysine methylations. Exp Mol Med (2017) 49(4):e324. doi: 10.1038/emm.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Li W, Wu H, Sui S, Wang Q, Xu S. Targeting histone modifications in breast cancer: A precise weapon on the way. Front Cell Dev Biol (2021) 9. doi: 10.3389/fcell.2021.736935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Elsheikh SE, Green AR, Rakha EA, Powe DG, Ahmed RA, Collins HM, et al. Global histone modifications in breast cancer correlate with tumor phenotypes, prognostic factors, and patient outcome. Cancer Res (2009) 69(9):3802–9. doi: 10.1158/0008-5472.CAN-08-3907 [DOI] [PubMed] [Google Scholar]
- 108. Messier TL, Gordon JA, Boyd JR, Tye CE, Browne G, Stein JL, et al. Histone H3 lysine 4 acetylation and methylation dynamics define breast cancer subtypes. Oncotarget (2016) 7(5):5094–109. doi: 10.18632/oncotarget.6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Spangle JM, Dreijerink KM, Groner AC, Cheng H, Ohlson CE, Reyes J, et al. PI3K/AKT signaling regulates H3K4 methylation in breast cancer. Cell Rep (2016) 15(12):2692–704. doi: 10.1016/j.celrep.2016.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dong H, Hu J, Zou K, Ye M, Chen Y, Wu C, et al. Activation of LncRNA TINCR by H3K27 acetylation promotes trastuzumab resistance and epithelial-mesenchymal transition by targeting MicroRNA-125b in breast cancer. Mol Cancer (2019) 18(1):3. doi: 10.1186/s12943-018-0931-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111. Segelle A, Núñez-Álvarez Y, Oldfield AJ, Webb KM, Voigt P, Luco RF, et al. Histone marks regulate the epithelial-to-mesenchymal transition via alternative splicing. Cell Rep (2022) 38(7):110357. doi: 10.1016/j.celrep.2022.110357 [DOI] [PubMed] [Google Scholar]
- 112. Wei Y, Xia W, Zhang Z, Liu J, Wang H, Adsay NV, et al. Loss of trimethylation at lysine 27 of histone H3 is a predictor of poor outcome in breast, ovarian, and pancreatic cancers. Mol Carcinog (2008) 47(9):701–6. doi: 10.1002/mc.20413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yoo KH, Hennighausen L. EZH2 methyltransferase and H3K27 methylation in breast cancer. Int J Biol Sci (2012) 8(1):59–65. doi: 10.7150/ijbs.8.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chatterjee A, Rodger EJ, Eccles MR. Epigenetic drivers of tumourigenesis and cancer metastasis. Semin Cancer Biol (2018) 51:149–59. doi: 10.1016/j.semcancer.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 115. Jørgensen S, Schotta G, Sørensen CS. Histone H4 lysine 20 methylation: key player in epigenetic regulation of genomic integrity. Nucleic Acids Res (2013) 41(5):2797–806. doi: 10.1093/nar/gkt012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yokoyama Y, Matsumoto A, Hieda M, Shinchi Y, Ogihara E, Hamada M, et al. Loss of histone H4K20 trimethylation predicts poor prognosis in breast cancer and is associated with invasive activity. Breast Cancer Res (2014) 16(3):R66. doi: 10.1186/bcr3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gan L, Yang Y, Li Q, Feng Y, Liu T, Guo W, et al. Epigenetic regulation of cancer progression by EZH2: from biological insights to therapeutic potential. biomark Res (2018) 6(1):10. doi: 10.1186/s40364-018-0122-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Holm K, Grabau D, Lövgren K, Aradottir S, Gruvberger-Saal S, Howlin J, et al. Global H3K27 trimethylation and EZH2 abundance in breast tumor subtypes. Mol Oncol (2012) 6(5):494–506. doi: 10.1016/j.molonc.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ding L, Kleer CG. Enhancer of zeste 2 as a marker of preneoplastic progression in the breast. Cancer Res (2006) 66(19):9352–5. doi: 10.1158/0008-5472.CAN-06-2384 [DOI] [PubMed] [Google Scholar]
- 120. Kim JH, Sharma A, Dhar SS, Lee SH, Gu B, Chan CH, et al. UTX and MLL4 coordinately regulate transcriptional programs for cell proliferation and invasiveness in breast cancer cells. Cancer Res (2014) 74(6):1705–17. doi: 10.1158/0008-5472.CAN-13-1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Su CH, Lin IH, Tzeng TY, Hsieh WT, Hsu MT. Regulation of IL-20 expression by estradiol through KMT2B-mediated epigenetic modification. PloS One (2016) 11(11):e0166090. doi: 10.1371/journal.pone.0166090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Park UH, Kang MR, Kim EJ, Kwon YS, Hur W, Yoon SK, et al. ASXL2 promotes proliferation of breast cancer cells by linking ERα to histone methylation. Oncogene (2016) 35(28):3742–52. doi: 10.1038/onc.2015.443 [DOI] [PubMed] [Google Scholar]
- 123. Jeong KW, Kim K, Situ AJ, Ulmer TS, An W, Stallcup MR. Recognition of enhancer element-specific histone methylation by TIP60 in transcriptional activation. Nat Struct Mol Biol (2011) 18(12):1358–65. doi: 10.1038/nsmb.2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lee JY, Kong G. DOT1L: a new therapeutic target for aggressive breast cancer. Oncotarget (2015) 6(31):30451–2. doi: 10.18632/oncotarget.5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Alexandrova E, Salvati A, Pecoraro G, Lamberti J, Melone V, Sellitto A, et al. Histone methyltransferase DOT1L as a promising epigenetic target for treatment of solid tumors. Front Genet (2022) 13. doi: 10.3389/fgene.2022.864612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cho MH, Park JH, Choi HJ, Park MK, Won HY, Park YJ, et al. DOT1L cooperates with the c-Myc-p300 complex to epigenetically derepress CDH1 transcription factors in breast cancer progression. Nat Commun (2015) 6:7821. doi: 10.1038/ncomms8821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Nassa G, Salvati A, Tarallo R, Gigantino V, Alexandrova E, Memoli D, et al. Inhibition of histone methyltransferase DOT1L silences ERα gene and blocks proliferation of antiestrogen-resistant breast cancer cells. Sci Adv (2019) 5(2):eaav5590. doi: 10.1126/sciadv.aav5590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Byun WS, Kim WK, Han HJ, Chung H-J, Jang K, Kim HS, et al. Targeting histone methyltransferase DOT1L by a novel psammaplin a analog inhibits growth and metastasis of triple-negative breast cancer. Mol Ther - Oncolytics (2019) 15:140–52. doi: 10.1016/j.omto.2019.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature (2006) 439(7078):811–6. doi: 10.1038/nature04433 [DOI] [PubMed] [Google Scholar]
- 130. Zheng Y, Zeng Y, Qiu R, Liu R, Huang W, Hou Y, et al. The homeotic protein SIX3 suppresses carcinogenesis and metastasis through recruiting the LSD1/NuRD(MTA3) complex. Theranostics (2018) 8(4):972–89. doi: 10.7150/thno.22328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Qiu R, Shi H, Wang S, Leng S, Liu R, Zheng Y, et al. BRMS1 coordinates with LSD1 and suppresses breast cancer cell metastasis. Am J Cancer Res (2018) 8(10):2030. [PMC free article] [PubMed] [Google Scholar]
- 132. Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu W, et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell (2009) 138(4):660–72. doi: 10.1016/j.cell.2009.05.050 [DOI] [PubMed] [Google Scholar]
- 133. Yang Y, Huang W, Qiu R, Liu R, Zeng Y, Gao J, et al. LSD1 coordinates with the SIN3A/HDAC complex and maintains sensitivity to chemotherapy in breast cancer. J Mol Cell Biol (2018) 10(4):285–301. doi: 10.1093/jmcb/mjy021 [DOI] [PubMed] [Google Scholar]
- 134. Berry WL, Janknecht R. KDM4/JMJD2 histone demethylases: epigenetic regulators in cancer cells. Cancer Res (2013) 73(10):2936–42. doi: 10.1158/0008-5472.CAN-12-4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Yang J, Jubb AM, Pike L, Buffa FM, Turley H, Baban D, et al. The histone demethylase JMJD2B is regulated by estrogen receptor alpha and hypoxia, and is a key mediator of estrogen induced growth. Cancer Res (2010) 70(16):6456–66. doi: 10.1158/0008-5472.CAN-10-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ramadoss S, Guo G, Wang CY. Lysine demethylase KDM3A regulates breast cancer cell invasion and apoptosis by targeting histone and the non-histone protein p53. Oncogene (2017) 36(1):47–59. doi: 10.1038/onc.2016.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhao QY, Lei PJ, Zhang X, Zheng JY, Wang HY, Zhao J, et al. Global histone modification profiling reveals the epigenomic dynamics during malignant transformation in a four-stage breast cancer model. Clin Epigenet (2016) 8:34. doi: 10.1186/s13148-016-0201-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Qin L, Xu Y, Yu X, Toneff MJ, Li D, Liao Lc, et al. The histone demethylase Kdm3a is required for normal epithelial proliferation, ductal elongation and tumor growth in the mouse mammary gland. Oncotarget (2017) 8(49):84761–75. doi: 10.18632/oncotarget.21380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Luo W, Chang R, Zhong J, Pandey A, Semenza GL, et al. Histone demethylase JMJD2C is a coactivator for hypoxia-inducible factor 1 that is required for breast cancer progression. Proc Natl Acad Sci (2012) 109(49):E3367–76. doi: 10.1073/pnas.1217394109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Shao P, Liu Q, Maina PK, Cui J, Bair TB, Li T, et al. Histone demethylase PHF8 promotes epithelial to mesenchymal transition and breast tumorigenesis. Nucleic Acids Res (2017) 45(4):1687–702. doi: 10.1093/nar/gkw1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sierra-Ramirez JA, Seseña-Mendez E, Godinez-Victoria M, Hernandez-Caballero ME, et al. An insight into the promoter methylation of PHF20L1 and the gene association with metastasis in breast cancer. Adv Clin Exp Med (2021) 30(5):507–15. doi: 10.17219/acem/133426 [DOI] [PubMed] [Google Scholar]
- 142. Hou Y, Liu W, Yi X, Yang Y, Su D, Huang W, et al. PHF20L1 as a H3K27me2 reader coordinates with transcriptional repressors to promote breast tumorigenesis. Sci Adv (2020) 6(16):eaaz0356. doi: 10.1126/sciadv.aaz0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lu L, Katsaros D, Zhu Y, Hoffman A, Luca S, Marion CE, et al. Let-7a regulation of insulin-like growth factors in breast cancer. Breast Cancer Res Treat (2011) 126(3):687–94. doi: 10.1007/s10549-010-1168-5 [DOI] [PubMed] [Google Scholar]
- 144. Yun J, Frankenberger CA, Kuo WL, Boelens MC, Eves EM, Cheng N, et al. Signalling pathway for RKIP and let-7 regulates and predicts metastatic breast cancer. EMBO J (2011) 30(21):4500–14. doi: 10.1038/emboj.2011.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Aure MR, Leivonen SK, Fleischer T, Zhu Q, Overgaard J, Alsner J, et al. Individual and combined effects of DNA methylation and copy number alterations on miRNA expression in breast tumors. Genome Biol (2013) 14(11):R126. doi: 10.1186/gb-2013-14-11-r126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hsu PY, Deatherage DE, Rodriguez BA, Liyanarachchi S, Weng YI, Zuo T, et al. Xenoestrogen-induced epigenetic repression of microRNA-9-3 in breast epithelial cells. Cancer Res (2009) 69(14):5936–45. doi: 10.1158/0008-5472.CAN-08-4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Xu Q, Jiang Y, Yin Y, Li Q, He J, Jing Y, et al. A regulatory circuit of miR-148a/152 and DNMT1 in modulating cell transformation and tumor angiogenesis through IGF-IR and IRS1. J Mol Cell Biol (2013) 5(1):3–13. doi: 10.1093/jmcb/mjs049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhang Y, Yan LX, Wu QN, Du ZM, Chen J, Liao DZ, et al. miR-125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto-oncogene in human invasive breast cancer. Cancer Res (2011) 71(10):3552–62. doi: 10.1158/0008-5472.CAN-10-2435 [DOI] [PubMed] [Google Scholar]
- 149. Augoff K, McCue B, Plow EF, Sossey-Alaoui K. miR-31 and its host gene lncRNA LOC554202 are regulated by promoter hypermethylation in triple-negative breast cancer. Mol Cancer (2012) 11:5. doi: 10.1186/1476-4598-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhang Y, Yang P, Sun T, Li D, Xu X, Rui Y, et al. miR-126 and miR-126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasis. Nat Cell Biol (2013) 15(3):284–94. doi: 10.1038/ncb2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lehmann U, Hasemeier B, Christgen M, Müller M, Römermann D, Länger F, et al. Epigenetic inactivation of microRNA gene hsa-mir-9-1 in human breast cancer. J Pathol (2008) 214(1):17–24. doi: 10.1002/path.2251 [DOI] [PubMed] [Google Scholar]
- 152. Barrow TM, Barault L, Ellsworth RE, Harris HR, Binder AM, Valente AL, et al. Aberrant methylation of imprinted genes is associated with negative hormone receptor status in invasive breast cancer. Int J Cancer (2015) 137(3):537–47. doi: 10.1002/ijc.29419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bhan A, Hussain I, Ansari KI, Kasiri S, Bashyal A, Mandal SS, et al. Antisense transcript long noncoding RNA (lncRNA) HOTAIR is transcriptionally induced by estradiol. J Mol Biol (2013) 425(19):3707–22. doi: 10.1016/j.jmb.2013.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Feng S, De Carvalho DD. Clinical advances in targeting epigenetics for cancer therapy. FEBS J (2022) 289(5):1214–39. doi: 10.1111/febs.15750 [DOI] [PubMed] [Google Scholar]
- 155. Sherr DH. Another important biological function for the aryl hydrocarbon receptor. Arterioscler Thromb Vasc Biol (2011) 31(6):1247–8. doi: 10.1161/ATVBAHA.111.227553 [DOI] [PubMed] [Google Scholar]
- 156. Barouki R, Coumoul X, Fernandez-Salguero PM. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett (2007) 581(19):3608–15. doi: 10.1016/j.febslet.2007.03.046 [DOI] [PubMed] [Google Scholar]
- 157. Eltom SE, Gasmelseed AA, Saoudi-Guentri D. The aryl hydrocarbon receptor is over-expressed and constitutively activated in advanced breast carcinoma. Proceedings of the American Association for Cancer Research, (2006) 47. doi: 10.1002/jcb.21630 [DOI] [Google Scholar]
- 158. Safe S, Cheng Y, Jin U-H. The aryl hydrocarbon receptor (AhR) as a drug target for cancer chemotherapy. Curr Opin Toxicol (2017) 2:24–9. doi: 10.1016/j.cotox.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhang S, Lei P, Liu X, Li X, Walker K, Kotha L, et al. The aryl hydrocarbon receptor as a target for estrogen receptor-negative breast cancer chemotherapy. Endocrine-related Cancer (2009) 16(3):835–44. doi: 10.1677/ERC-09-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hockings JK, Thorne PA, Kemp MQ, Morgan SS, Selmin O, Romagnolo DF. The ligand status of the aromatic hydrocarbon receptor modulates transcriptional activation of BRCA-1 promoter by estrogen. Cancer Res (2006) 66(4):2224–32. doi: 10.1158/0008-5472.CAN-05-1619 [DOI] [PubMed] [Google Scholar]
- 161. Papoutsis AJ, Lamore SD, Wondrak GT, Selmin OI, Romagnolo DF. Resveratrol prevents epigenetic silencing of BRCA-1 by the aromatic hydrocarbon receptor in human breast cancer cells. J Nutr (2010) 140(9):1607–14. doi: 10.3945/jn.110.123422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Papoutsis AJ, et al. BRCA-1 promoter hypermethylation and silencing induced by the aromatic hydrocarbon receptor-ligand TCDD are prevented by resveratrol in MCF-7 cells. J Nutr Biochem (2012) 23(10):1324–32. doi: 10.1016/j.jnutbio.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 163. Jorgensen EM, Alderman MH. Taylor, Preferential epigenetic programming of estrogen response after in utero xenoestrogen (bisphenol-a) exposure. FASEB J (2016) 30(9):3194–201. doi: 10.1096/fj.201500089R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Allard P. Chapter 27 - bisphenol a. In: Gupta RC, editor. Biomarkers in toxicology. Boston: Academic Press; (2014). p. 459–74. [Google Scholar]
- 165. Seachrist DD, Borg JL, Selmin OI, Romagnolo DF. A review of the carcinogenic potential of bisphenol a. Reprod Toxicol (2016) 59:167–82. doi: 10.1016/j.reprotox.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-a (BPA) increases EZH2 expression in the mammary gland: An epigenetic mechanism linking endocrine disruptors to breast cancer. Hormones Cancer (2010) 1(3):146–55. doi: 10.1007/s12672-010-0015-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Weng YI, Hsu PY, Liyanarachchi S, Liu J, Deatherage DE, Huang YW, et al. Epigenetic influences of low-dose bisphenol a in primary human breast epithelial cells. Toxicol Appl Pharmacol (2010) 248(2):111–21. doi: 10.1016/j.taap.2010.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kanao H, Enomoto T, Kimura T, Fujita M, Nakashima R, Ueda Y, et al. Overexpression of LAMP3/TSC403/DC-LAMP promotes metastasis in uterine cervical cancer. Cancer Res (2005) 65(19):8640–5. doi: 10.1158/0008-5472.CAN-04-4112 [DOI] [PubMed] [Google Scholar]
- 169. Nagelkerke A, Bussink J, Mujcic H, Wouters BG, Lehmann S, Sweep FC, et al. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res (2013) 15(1):R2. doi: 10.1186/bcr3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Nagelkerke A, Mujcic H, Bussink J, Wouters BG, van Laarhoven HW, Sweep FC, et al. Hypoxic regulation and prognostic value of LAMP3 expression in breast cancer. Cancer (2011) 117(16):3670–81. doi: 10.1002/cncr.25938 [DOI] [PubMed] [Google Scholar]
- 171. Pullella K, Kotsopoulos J. Arsenic exposure and breast cancer risk: A re-evaluation of the literature. Nutrients (2020) 12(11):3305. doi: 10.3390/nu12113305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Liu Y, Hock JM, Sullivan C, Fang G, Cox AJ, Davis KT, et al. Activation of the p38 MAPK/Akt/ERK1/2 signal pathways is required for the protein stabilization of CDC6 and cyclin D1 in low-dose arsenite-induced cell proliferation. J Cell Biochem (2010) 111(6):1546–55. doi: 10.1002/jcb.22886 [DOI] [PubMed] [Google Scholar]
- 173. Xu Y, Tokar EJ, Waalkes MP. Arsenic-induced cancer cell phenotype in human breast epithelia is estrogen receptor-independent but involves aromatase activation. Arch Toxicol (2014) 88(2):263–74. doi: 10.1007/s00204-013-1131-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Peremartí J, Ramos F, Marcos R, Hernández A. Arsenic exposure disrupts the normal function of the FA/BRCA repair pathway. Toxicol Sci (2014) 142(1):93–104. doi: 10.1093/toxsci/kfu159 [DOI] [PubMed] [Google Scholar]
- 175. Ren X, McHale CM, Skibola CF, Smith AH, Smith MT, Zhang L. An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environ Health Perspect (2011) 119(1):11–9. doi: 10.1289/ehp.1002114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Romagnolo DF, Daniels KD, Grunwald JT, Ramos SA, Propper CR, Selmin OI. Epigenetics of breast cancer: Modifying role of environmental and bioactive food compounds. Mol Nutr Food Res (2016) 60(6):1310–29. doi: 10.1002/mnfr.201501063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Cardenas A, Houseman EA, Baccarelli AA, Quamruzzaman Q, Rahman M, Mostofa G, et al. In utero arsenic exposure and epigenome-wide associations in placenta, umbilical artery, and human umbilical vein endothelial cells. Epigenetics (2015) 10(11):1054–63. doi: 10.1080/15592294.2015.1105424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Chou WC, Chung YT, Chen HY, Wang CJ, Ying TH, Chuang CY, et al. Maternal arsenic exposure and DNA damage biomarkers, and the associations with birth outcomes in a general population from Taiwan. PloS One (2014) 9(2):e86398. doi: 10.1371/journal.pone.0086398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Simon R, Gomez Ruiz JA, von Holst C, Wenzl T, Anklam E. Results of a European inter-laboratory comparison study on the determination of EU priority polycyclic aromatic hydrocarbons (PAHs) in edible vegetable oils. Anal Bioanal Chem (2008) 391(4):1397–408. doi: 10.1007/s00216-007-1771-4 [DOI] [PubMed] [Google Scholar]
- 180. Gaudet MM, Carter BD, Brinton LA, Falk RT, Gram IT, Luo J, et al. Pooled analysis of active cigarette smoking and invasive breast cancer risk in 14 cohort studies. Int J Epidemiol (2017) 46(3):881–93. doi: 10.1002/jcb.21630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Dossus L, Boutron-Ruault MC, Kaaks R, Gram IT, Vilier A, Fervers B, et al. Active and passive cigarette smoking and breast cancer risk: results from the EPIC cohort. Int J Cancer (2014) 134(8):1871–88. doi: 10.1002/ijc.28508 [DOI] [PubMed] [Google Scholar]
- 182. Nicolas M, Grandal B, Dubost E, Kassara A, Guerin J, Toussaint A, et al. Breast cancer (BC) is a window of opportunity for smoking cessation: Results of a retrospective analysis of 1234 BC survivors in follow-up consultation. Cancers (Basel) (2021) 13(10):1–12. doi: 10.3390/cancers13102423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Conway K, Edmiston SN, Parrish E, Bryant C, Tse CK, Swift-Scanlan T, et al. Breast tumor DNA methylation patterns associated with smoking in the Carolina breast cancer study. Breast Cancer Res Treat (2017) 163(2):349–61. doi: 10.1007/s10549-017-4178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Tang H, Yang D, Han C, Mu P. Smoking, DNA methylation, and breast cancer: A mendelian randomization study. Front Oncol (2021) 11. doi: 10.3389/fonc.2021.745918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sadikovic B, Andrews J, Rodenhiser DI. DNA Methylation analysis using CpG microarrays is impaired in benzopyrene exposed cells. Toxicol Appl Pharmacol (2007) 225(3):300–9. doi: 10.1016/j.taap.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 186. Thakur C, Chen F. Connections between metabolism and epigenetics in cancers. Semin Cancer Biol (2019) 57:52–8. doi: 10.1016/j.semcancer.2019.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Gandhi N, Das GM. Metabolic reprogramming in breast cancer and its therapeutic implications. Cells (2019) 8(2):89. doi: 10.3390/cells8020089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. O’Mahony F, Razandi M, Pedram A, Harvey BJ, Levin ER. Estrogen modulates metabolic pathway adaptation to available glucose in breast cancer cells. Mol Endocrinol (2012) 26(12):2058–70. doi: 10.1210/me.2012-1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Barros RP, Gabbi C, Morani A, Warner M, Gustafsson JA. Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab (2009) 297(1):E124–33. doi: 10.1152/ajpendo.00189.2009 [DOI] [PubMed] [Google Scholar]
- 190. Yang J, AlTahan A, Jones DT, Buffa FM, Bridges E, Interiano RB, et al. Estrogen receptor-α directly regulates the hypoxia-inducible factor 1 pathway associated with antiestrogen response in breast cancer. Proc Natl Acad Sci U.S.A. (2015) 112(49):15172–7. doi: 10.1073/pnas.1422015112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Robledinos-Antón N. Activators and inhibitors of NRF2: A review of their potential for clinical development. Oxid Med Cell Longev 2019 (2019) p:9372182. doi: 10.1155/2019/9372182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Guo Y, Yu S, Zhang C, Kong AN. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic Biol Med (2015) 88(Pt B):337–49. doi: 10.1016/j.freeradbiomed.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Cheng D, Wu R, Guo Y, Kong ANc. Regulation of Keap1-Nrf2 signaling: The role of epigenetics. Curr Opin Toxicol (2016) 1:134–8. doi: 10.1016/j.cotox.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Almeida M, Soares M, Ramalhinho AC, Moutinho JF, Breitenfeld L, Pereira L. The prognostic value of NRF2 in breast cancer patients: a systematic review with meta-analysis. Breast Cancer Res Treat (2020) 179(3):523–32. doi: 10.1007/s10549-019-05494-4 [DOI] [PubMed] [Google Scholar]
- 195. Zhang HS, et al. Nrf2 promotes breast cancer cell migration via up-regulation of G6PD/HIF-1α/Notch1 axis. J Cell Mol Med (2019) 23(5):3451–63. doi: 10.1111/jcmm.14241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, Klinge CM. Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol Endocrinol (2008) 22(3):609–22. doi: 10.1210/me.2007-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Wu K, Li L, Thakur C, Lu Y, Zhang X, Yi Z, et al. Proteomic characterization of the world trade center dust-activated mdig and c-myc signaling circuit linked to multiple myeloma. Sci Rep (2016) 6:36305. doi: 10.1038/srep36305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zhang Y, Lu Y, Yuan BZ, Castranova V, Shi X, Stauffer JL, et al. The human mineral dust-induced gene, mdig, is a cell growth regulating gene associated with lung cancer. Oncogene (2005) 24(31):4873–82. doi: 10.1038/sj.onc.1208668 [DOI] [PubMed] [Google Scholar]
- 199. Sun J, Yu M, Lu Y, Thakur C, Chen B, Qiu P, et al. Carcinogenic metalloid arsenic induces expression of mdig oncogene through JNK and STAT3 activation. Cancer Lett (2014) 346(2):257–63. doi: 10.1016/j.canlet.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Thakur C, Wolfarth M, Sun J, Zhang Y, Lu Y, Battelli L, et al. Oncoprotein mdig contributes to silica-induced pulmonary fibrosis by altering balance between Th17 and treg T cells. Oncotarget (2015) 6(6):3722–36. doi: 10.18632/oncotarget.2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Thakur C, Chen F. Current understanding of mdig/MINA in human cancers. Genes Cancer (2015) 6(7-8):288–302. doi: 10.18632/genesandcancer.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Zhang Q, Thakur C, Shi J, Sun J, Fu Y, Stemmer P, et al. New discoveries of mdig in the epigenetic regulation of cancers. Semin Cancer Biol (2019) 57:27–35. doi: 10.1016/j.semcancer.2019.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Yu M, Sun J, Thakur C, Chen B, Lu Y, Zhao H, et al. Paradoxical roles of mineral dust induced gene on cell proliferation and migration/invasion. PloS One (2014) 9(2):e87998. doi: 10.1371/journal.pone.0087998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Chen B, Liu J, Chang Q, Beezhold K, Lu Y, Chen F. JNK and STAT3 signaling pathways converge on akt-mediated phosphorylation of EZH2 in bronchial epithelial cells induced by arsenic. Cell Cycle (Georgetown Tex.) (2013) 12(1):112–21. doi: 10.4161/cc.23030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Shi J, Thakur C, Zhao Y, Li Y, Nie L, Zhang Q, et al. Pathological and prognostic indications of the mdig gene in human lung cancer. Cell Physiol Biochem (2021) 55(S2):13–28. doi: 10.33594/000000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Bi Z, Zhang Q, Fu Y, Seno A, Wadgaonkar P, Qiu Y, et al. Cooperation between NRF2-mediated transcription and MDIG-dependent epigenetic modifications in arsenic-induced carcinogenesis and cancer stem cells. Semin Cancer Biol (2021) 76:310–8. doi: 10.1016/j.semcancer.2021.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Bi Z, Zhang Q, Fu Y, Wadgaonkar P, Zhang W, Almutairy B, et al. Nrf2 and HIF1α converge to arsenic-induced metabolic reprogramming and the formation of the cancer stem-like cells. Theranostics (2020) 10(9):4134–49. doi: 10.7150/thno.42903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Thakur C, Lu Y, Sun J, Yu M, Chen B, Chen F. Increased expression of mdig predicts poorer survival of the breast cancer patients. Gene (2014) 535(2):218–24. doi: 10.1016/j.gene.2013.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Thakur C, Chen B, Li L, Zhang Q, Yang Z-Q, Chen F. Loss of mdig expression enhances DNA and histone methylation and metastasis of aggressive breast cancer. Signal transduction targeted Ther (2018) 3:25–5. doi: 10.1038/s41392-018-0027-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Tsuneoka M, Koda Y, Soejima M, Teye K, Kimura H, et al. A novel myc target gene, mina53, that is involved in cell proliferation. J Biol Chem (2002) 277(38):35450–9. doi: 10.1074/jbc.M204458200 [DOI] [PubMed] [Google Scholar]
- 211. Liu H, Radisky DC, Yang D, Xu R, Radisky ES, Bissell MJA, et al. MYC suppresses cancer metastasis by direct transcriptional silencing of αv and β3 integrin subunits. Nat Cell Biol (2012) 14(6):567–74. doi: 10.1038/ncb2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Zhang Q, Thakur C, Fu Y, Bi Z, Wadgaonkar P, Xu L, et al. Mdig promotes oncogenic gene expression through antagonizing repressive histone methylation markers. Theranostics (2020) 10(2):602–14. doi: 10.7150/thno.36220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Wang W, Lu Y, Stemmer PM, Zhang X, Bi Y, Yi Z, et al. The proteomic investigation reveals interaction of mdig protein with the machinery of DNA double-strand break repair. Oncotarget (2015) 6(29):28269–81. doi: 10.18632/oncotarget.4961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Pannetier M, Julien E, Schotta G, Tardat M, Sardet C, Jenuwein T, et al. PR-SET7 and SUV4-20H regulate H4 lysine-20 methylation at imprinting control regions in the mouse. EMBO Rep (2008) 9(10):998–1005. doi: 10.1038/embor.2008.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Lu Y, Chang Q, Zhang Y, Beezhold K, Rojanasakul Y, Zhao H, et al. Lung cancer-associated JmjC domain protein mdig suppresses formation of tri-methyl lysine 9 of histone H3. Cell Cycle (2009) 8(13):2101–9. doi: 10.4161/cc.8.13.8927 [DOI] [PubMed] [Google Scholar]
- 216. Chen B, Yu M, Chang Q, Lu Y, Thakur C, Ma D, et al. Mdig de-represses H19 large intergenic non-coding RNA (lincRNA) by down-regulating H3K9me3 and heterochromatin. Oncotarget (2013) 4(9):1427–37. doi: 10.18632/oncotarget.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Zhang Q, Wadgaonkar P, Xu L, Thakur C, Fu Y, Bi Z, et al. Environmentally-induced mdig contributes to the severity of COVID-19 through fostering expression of SARS-CoV-2 receptor NRPs and glycan metabolism. Theranostics (2021) 11(16):7970–83. doi: 10.7150/thno.62138 [DOI] [PMC free article] [PubMed] [Google Scholar]