Abstract
OBJECTIVES:
To describe the outcomes of hospitalized patients in a multicenter, international coronavirus disease 2019 registry.
DESIGN:
Cross-sectional observational study including coronavirus disease 2019 patients hospitalized with laboratory-confirmed severe acute respiratory syndrome coronavirus-2 infection between February 15, 2020, and November 30, 2020, according to age and type of organ support therapies.
SETTING:
About 168 hospitals in 16 countries within the Society of Critical Care Medicine’s Discovery Viral Infection and Respiratory Illness University Study coronavirus disease 2019 registry.
PATIENTS:
Adult hospitalized coronavirus disease 2019 patients who did and did not require various types and combinations of organ support (mechanical ventilation, renal replacement therapy, vasopressors, and extracorporeal membrane oxygenation).
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Primary outcome was hospital mortality. Secondary outcomes were discharge home with or without assistance and hospital length of stay. Risk-adjusted variation in hospital mortality for patients receiving invasive mechanical ventilation was assessed by using multilevel models with hospitals as a random effect, adjusted for age, race/ethnicity, sex, and comorbidities. Among 20,608 patients with coronavirus disease 2019, the mean (± sd) age was 60.5 (±17), 11,1887 (54.3%) were men, 8,745 (42.4%) were admitted to the ICU, and 3,906 (19%) died in the hospital. Hospital mortality was 8.2% for patients receiving no organ support (n = 15,001). The most common organ support therapy was invasive mechanical ventilation (n = 5,005; 24.3%), with a hospital mortality of 49.8%. Mortality ranged from 40.8% among patients receiving only invasive mechanical ventilation (n =1,749) to 71.6% for patients receiving invasive mechanical ventilation, vasoactive drugs, and new renal replacement therapy (n = 655). Mortality was 39% for patients receiving extracorporeal membrane oxygenation (n = 389). Rates of discharge home ranged from 73.5% for patients who did not require organ support therapies to 29.8% for patients who only received invasive mechanical ventilation, and 8.8% for invasive mechanical ventilation, vasoactive drugs, and renal replacement; 10.8% of patients older than 74 years who received invasive mechanical ventilation were discharged home. Median hospital length of stay for patients on mechanical ventilation was 17.1 days (9.7–28 d). Adjusted interhospital variation in mortality among patients receiving invasive mechanical ventilation was large (median odds ratio 1.69).
CONCLUSIONS:
Coronavirus disease 2019 prognosis varies by age and level of organ support. Interhospital variation in mortality of mechanically ventilated patients was not explained by patient characteristics and requires further evaluation.
Keywords: big data, coronavirus disease 2019, intensive care unit, organ failure, patients, severe acute respiratory syndrome coronavirus 2, Viral Infection and Respiratory Illness University Study
Coronavirus disease 2019 (COVID-19) was declared a global pandemic on March 11, 2020, and has resulted in almost 2,000,000 deaths worldwide as of January 2021 (1). The most severe forms of COVID-19 often lead to critical illness due to respiratory failure, often requiring invasive mechanical ventilation (IMV) and, potentially, multiorgan system failure (2–6). However, given that most studies of COVID-19 outcomes are single-center case series (2, 5, 7), robust clinically informative prognostic information for hospitalized patients according to types of organ supportive therapies remains unclear. Mortality of mechanically ventilated patients with COVID-19 reported in single- and multicenter studies from Italy, the United States, Germany, and the United Kingdom has ranged from 19% to 75% (8–10). The prognostic information based on the type of organ support required and the likelihood of discharge home in different age groups have not been clear for patients with COVID-19. We sought to describe patient outcomes among hospitalized patients receiving commonly used organ supportive therapies in a multicenter, international COVID-19 registry.
MATERIALS AND METHODS
Study Population, Setting, and Data Collection
In response to the COVID-19 pandemic, Discovery, the Critical Care Research Network formed by the Society of Critical Care Medicine in 2016, provided a centralized platform and resources for clinical investigators to scale up research in critical care. The initiative led to the creation of the Viral Infection and Respiratory Illness Universal Study (VIRUS) (11), which is an HIPAA compliant multinational database developed to capture deidentified clinical information as well as daily physiologic, laboratory, and treatment information collected. Individual study investigators will be able to use the pooled data for ancillary research questions.
We enrolled hospitalized patients 18 years old or older with laboratory-confirmed COVID-19 infection, at 168 (150 from the United States) academic, community, or private hospitals in 16 countries between February 15, 2020, and November 30, 2020 (Supplemental Table 1, http://links.lww.com/CCM/G128). Patients below 18 years old, with no recorded discharge status, and participants that did not have research authorization to access medical records were excluded. Patients were followed until hospital discharge or death whichever came first. A confirmed case of COVID-19 was defined by a positive result on a reverse-transcriptase–polymerase-chain-reaction assay. The registry was granted exempt status for human subject research by the Institutional Review Board at Mayo Clinic (20–002610). The ClinicalTrials.gov number is NCT04323787. Each study site submitted a proposal to their local review boards for approval and signed a data use agreement before being granted permission to extract and enter deidentified data into the registry.
Data Collection
We included data elements common to prior COVID-19 registry work, using the World Health Organization COVID-19 case report forms (CRFs) (12) as a starting template. Data elements for inclusion were selected to capture elements of COVID-19 diagnosis, patient demographics, chronic comorbidities, acute illness characteristics, and details of critical care interventions and outcomes. Electronic CRFs (Supplemental Table 2, http://links.lww.com/CCM/G129) were then constructed using Research Electronic Data Capture (13), a secure web-based software and workflow methodology for electronic collection and management of research data. Study personnel at each site collected data into CRFs by manual review of electronic or paper medical records. All diagnostic and therapeutic management decisions were performed at the discretion of the treating physician. We ensured data prioritization, integrity, and maintained bidirectional open lines of communication between the study sites and central registry organization by having weekly remote training online meetings with clinical research coordinators/data abstractors at participating study sites globally (14). After obtaining patients’ data, we performed quality checks for adherence to the study protocol and accuracy of data collection methods. Then, we reviewed missing data weekly and contacted sites with high rates of missing data points. Finally, we performed multistep data cleaning, looking for field entries out of range or proper data type, with iterations to contact sites for correction of errant data.
Outcome Measures and Exposure of Interest
Primary outcome was hospital mortality. Secondary outcomes were discharge home with or without assistance and total hospital length of stay (LOS). The outcomes are reported based on the types of organ support therapies provided. We focused on the following: no organ support therapies, need for IMV, use of vasopressors and/or inotropes (i.e., vasoactive drugs), new renal replacement therapy (RRT), and extracorporeal membrane oxygenation (ECMO).
Statistical Analysis
Continuous data were summarized as mean (sd) or median (interquartile range [IQR], 25–75%) and categorical variables as n (%).
Due to the strong association between age and prognosis for patients with COVID-19 (15), we stratified analyses of outcomes by age: 18–44 years, 45–59 years, 60–74 years, and 75 years and above. We provided hospital mortality rates by age and different types of organ supportive therapies.
Risk-adjusted variation in hospital mortality (for hospitals entering n > 10 patients into the registry) for patients receiving IMV was assessed by using multilevel models with hospitals as a random effect, adjusted for age, race/ethnicity, sex, and comorbidities (Supplemental Table 3, http://links.lww.com/CCM/G130). The variance from the random effects output was used to calculate the intraclass correlation coefficient —an estimate of the variation explained by the hospital random effect, as well as the median odds ratio (, the median increased odds of death that a patient would have moving from a randomly selected lower to higher risk hospital (16). Variation in hospital mortality was calculated from the random effects output beta estimate for each hospital. In a post hoc exploratory analysis of potential factors associated with hospital variation in mortality among patients requiring mechanical ventilation, we added Sequential Organ Failure Assessment (SOFA) score quartile at ICU admission, hospital case volume of mechanically ventilated patients with COVID-19, and country (United States vs not United States) to the base model adjusted for age, race, sex, and comorbidities. Missing data were coded as missing and included in models as a missing category. Statistical analyses were performed by using SAS (Version 9.1, SAS Institute, Cary, NC). The analyses were conducted by C.R.S. and A.J.W. with input from the VIRUS registry investigators group.
RESULTS
Patient Characteristics at Baseline
Among 49,058 patients enrolled in the VIRUS registry at the time of data extraction, 20,608 met eligibility criteria and were included in the analysis (Fig. 1). Table 1 demonstrates the characteristics of patients included in this study, with mean (± sd) age 60.5 (±17), 54.3% of patients men, and 50.4% White, 25.9% Black, and 5.6% Hispanic; 87% of patients had at least one comorbid condition and 42.4% required ICU admission.
Figure 1.
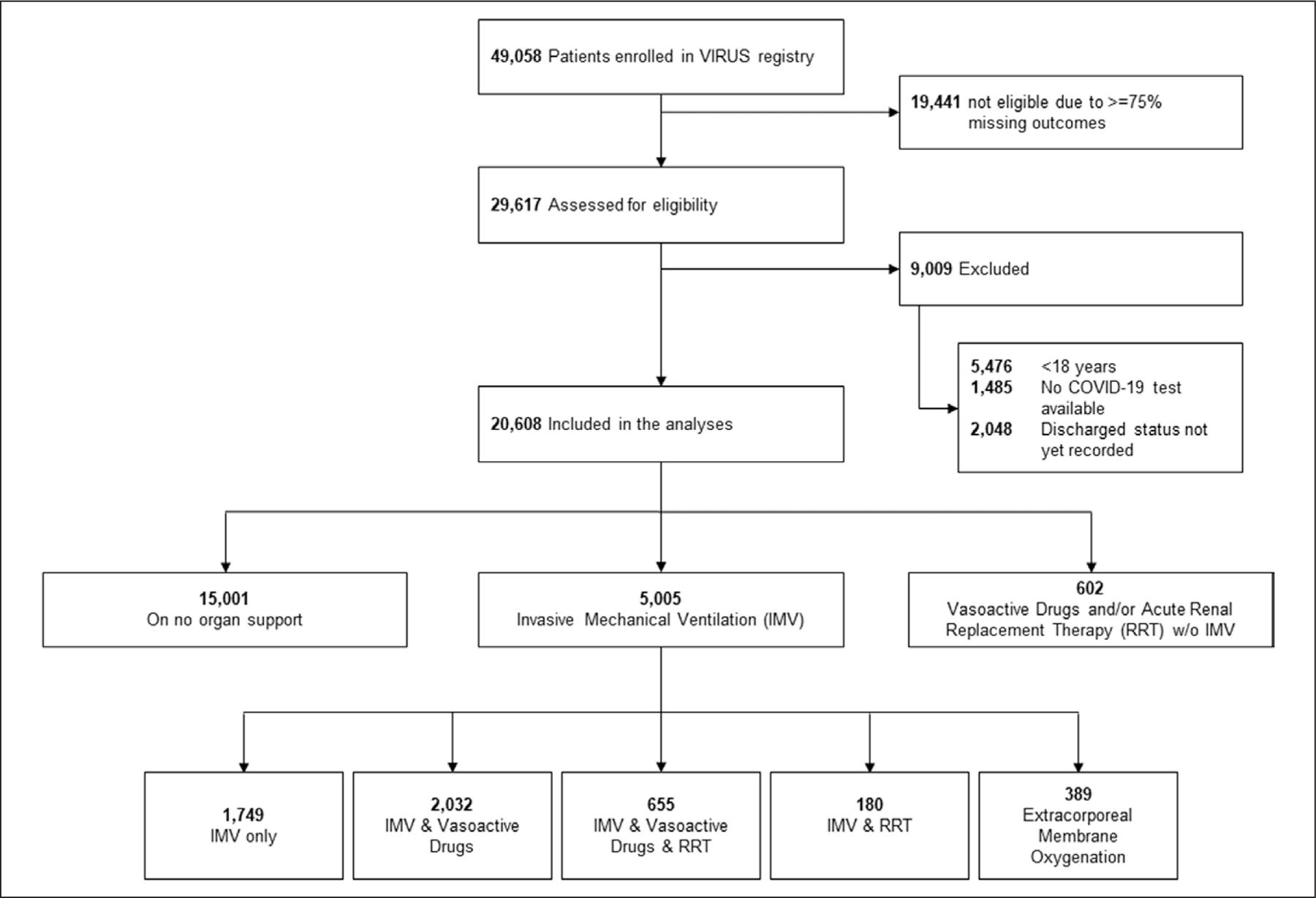
Included patients flowchart. COVID-19 = coronavirus disease 2019, IMV = invasive mechanical ventilation, RRT = renal replacement therapy.
TABLE 1.
Demographic and Clinical Characteristics Among Hospitalized Patients With Coronavirus Disease 2019 Stratified According to Age
| All Adults | 18–44 yr | 45–59 yr | 60–74 y | 75+ yr | |
|---|---|---|---|---|---|
| n | 20,608 | 3,986 | 5,300 | 6,491 | 4,831 |
| Male sex, n (%) | 11,187 (54.3) | 2,016 (50.6) | 3,139 (59.2) | 3,723 (57.4) | 2,309 (47.8) |
| Not reported | 253 (1.2) | 45 (1.1) | 66 (1.3) | 73 (1.1) | 69 (1.4) |
| Race, n (%) | |||||
| White | 10,391 (50.4) | 1,697 (42.6) | 2,392 (45.1) | 3,323 (51.2) | 2,979 (61.7) |
| African American | 5,330 (25.9) | 1,001 (25.1) | 1,423 (26.8) | 1,804 (27.8) | 1,102 (22.8) |
| Native American | 144 (0.7) | 32 (0.8) | 48 (0.9) | 40 (0.6) | 24 (0.5) |
| Asian American | 397 (1.9) | 97 (2.4) | 93 (1.8) | 121 (1.9) | 86 (1.8) |
| East Asian | 247 (1.2) | 33 (0.8) | 60 (1.1) | 68 (1) | 86 (1.8) |
| West Asian | 293 (1.4) | 51 (1.3) | 81 (1.5) | 102 (1.6) | 59 (1.2) |
| South Asian | 911 (4.4) | 330 (8.3) | 296 (5.6) | 238 (3.7) | 47 (1) |
| Southeast Asian | 125 (0.6) | 21 (0.5) | 41 (0.8) | 45 (0.7) | 18 (0.4) |
| Other | 2,641 (12.8) | 698 (17.5) | 830 (15.7) | 711 (11) | 402 (8.3) |
| Not reported | 129 (0.6) | 26 (0.7) | 36 (0.7) | 39 (0.6) | 28 (0.6) |
| Hispanic ethnicity, n (%) | 1,160 (5.6) | 222 (5.6) | 321 (6.1) | 381 (5.9) | 236 (4.9) |
| Not reported | 511 (2.5) | 88 (2.2) | 121 (2.3) | 152 (2.3) | 150 (3.1) |
| Body mass index (median, IQR) | 29 (25.1–34.5) | 30.4 (25.8–37.4) | 30.5 (26.6–36) | 29.1 (25.3–34.4) | 26.4 (22.9–30.7) |
| Not reported | 4,965 (24.1) | 1,045 (26.2) | 1,287 (24.3) | 1,457 (22.5) | 1,176 (24.3) |
| Documented social history, n (%) | |||||
| Current smoker | 677 (3.3) | 171 (4.3) | 214 (4) | 217 (3.3) | 75 (1.6) |
| Former smoker | 2,750 (13.3) | 199 (5) | 499 (9.4) | 1,187 (18.3) | 865 (17.9) |
| Alcohol use disorder | 446 (2.2) | 94 (2.4) | 143 (2.7) | 160 (2.5) | 49 (1) |
| Substance use disorder | 357 (1.7) | 109 (2.7) | 108 (2) | 121 (1.9) | 19 (0.4) |
| Not reported | 8,363 (40.6) | 1,679 (42.1) | 2,079 (39.2) | 2,455 (37.8) | 2,150 (44.5) |
| Comorbid conditions, n (%) | |||||
| Hypertension | 9,893 (48) | 632 (15.9) | 2,231 (42.1) | 3,845 (59.2) | 3,185 (65.9) |
| Diabetes | 6,345 (30.8) | 623 (15.6) | 1,655 (31.2) | 2,515 (38.7) | 1,552 (32.1) |
| Obesity | 3,623 (17.6) | 815 (20.4) | 1,136 (21.4) | 1,207 (18.6) | 465 (9.6) |
| Chronic kidney disease | 2,467 (12) | 144 (3.6) | 397 (7.5) | 942 (14.5) | 984 (20.4) |
| Coronary artery disease | 2,428 (11.8) | 49 (1.2) | 288 (5.4) | 996 (15.3) | 1,095 (22.7) |
| No comorbidities | 2,645 (12.8) | 1,106 (27.7) | 867 (16.4) | 488 (7.5) | 184 (3.8) |
| Not reported | 3,077 (14.9) | 922 (23.1) | 833 (15.7) | 751 (11.6) | 571 (11.8) |
| Sequential Organ Failure Assessment, median (IQR) | 2 (0–5) | 1 (0–3) | 2 (0–4) | 3 (1–6) | 3 (1–6) |
| Not reported, n (%) | 14,038 (68.1) | 2,755 (69.1) | 3,519 (66.4) | 4,314 (66.5) | 3,450 (71.4) |
| Use of steroids, n (%) | 2,818 (13.7) | 521 (13.1) | 790 (14.9) | 995 (15.3) | 512 (10.6) |
| Hospital | |||||
| Length of stay, median (IQR), d | 7 (4–14) | 5 (2.4–9.7) | 7 (3.7–14) | 8.6 (4.7–16.8) | 7.9 (4–14) |
| Not reported, n (%) | 44 (0.2) | 9 (0.2) | 13 (0.3) | 10 (0.2) | 12 (0.3) |
| Discharge disposition, n (%) | |||||
| Homea | 10,264 (49.8) | 2,733 (68.6) | 3,204 (60.5) | 2,973 (45.8) | 1,354 (28) |
| Skilled nursing facility | 835 (4.1) | 67 (1.7) | 145 (2.7) | 336 (5.2) | 287 (5.9) |
| Assisted living | 1,197 (5.8) | 62 (1.6) | 198 (3.7) | 451 (6.9) | 486 (10.1) |
| Other | 1,156 (5.6) | 160 (4) | 287 (5.4) | 368 (5.7) | 341 (7.1) |
| Disposition not reported | 3,250 (15.8) | 781 (19.6) | 884 (16.7) | 880 (13.6) | 705 (14.6) |
| Deceased | 3,906 (19) | 183 (4.6) | 582 (11) | 1,483 (22.8) | 1,658 (34.3) |
| ICU, n (%) | |||||
| Admission | 8,745 (42.4) | 1,349 (33.8) | 2,293 (43.3) | 3,186 (49.1) | 1,917 (39.7) |
| Not reported | 3,567 (17.3) | 698 (17.5) | 847 (16) | 1,015 (15.6) | 1,007 (20.8) |
| Mortality | 3,091 (35.3) | 171 (12.7) | 543 (23.7) | 1,299 (40.8) | 1,078 (56.2) |
| Not reported | 0 (0) | ||||
| IMV, n (%) | 4,810 (55) | 584 (43.3) | 1,279 (55.6) | 1,944 (61) | 1,003 (52) |
| Days on IMV, median (IQR) | 8.8 (3.3–17) | 8 (3–15.6) | 9.4 (4–19) | 9.3 (4–17.9) | 7 (2–13.1) |
IMV = invasive mechanical ventilation, IQR = interquartile range.
Estimates different from Table 2 due to missing data.
Hospital Mortality and Need for Organ Support Therapies
The overall mortality in the hospitalized cohort was 19% (n = 3,906). The most commonly used invasive organ support therapy was IMV (n = 5,005, 24.3%) either alone or in combination with the other organ support therapies; 602 patients (2.9% of the total sample) required vasoactive drugs and/or RRT without IMV. The mortality rate among patients (n = 15,001) who did not receive organ support therapies was 8.2% (n = 1,226). The mortality associated with IMV was 49.8% (n = 2,494) and ranged from 40.8% (n = 714) among patients who only received mechanical ventilation to 71.6% (n = 469) among patients who received IMV, vasoactive drugs, and RRT (Fig. 2; and Supplemental Table 4, http://links.lww.com/CCM/G131). Mortality was 35% (n = 136) among patients who received ECMO. The lowest mortality less than 1% was observed among hospitalized patients younger than 45 years old who did not receive organ supportive therapies, and the highest mortality (78.3%; n = 83) was observed among patients older than 74 years receiving a combination of IMV, vasoactive drugs, and RRT.
Figure 2.
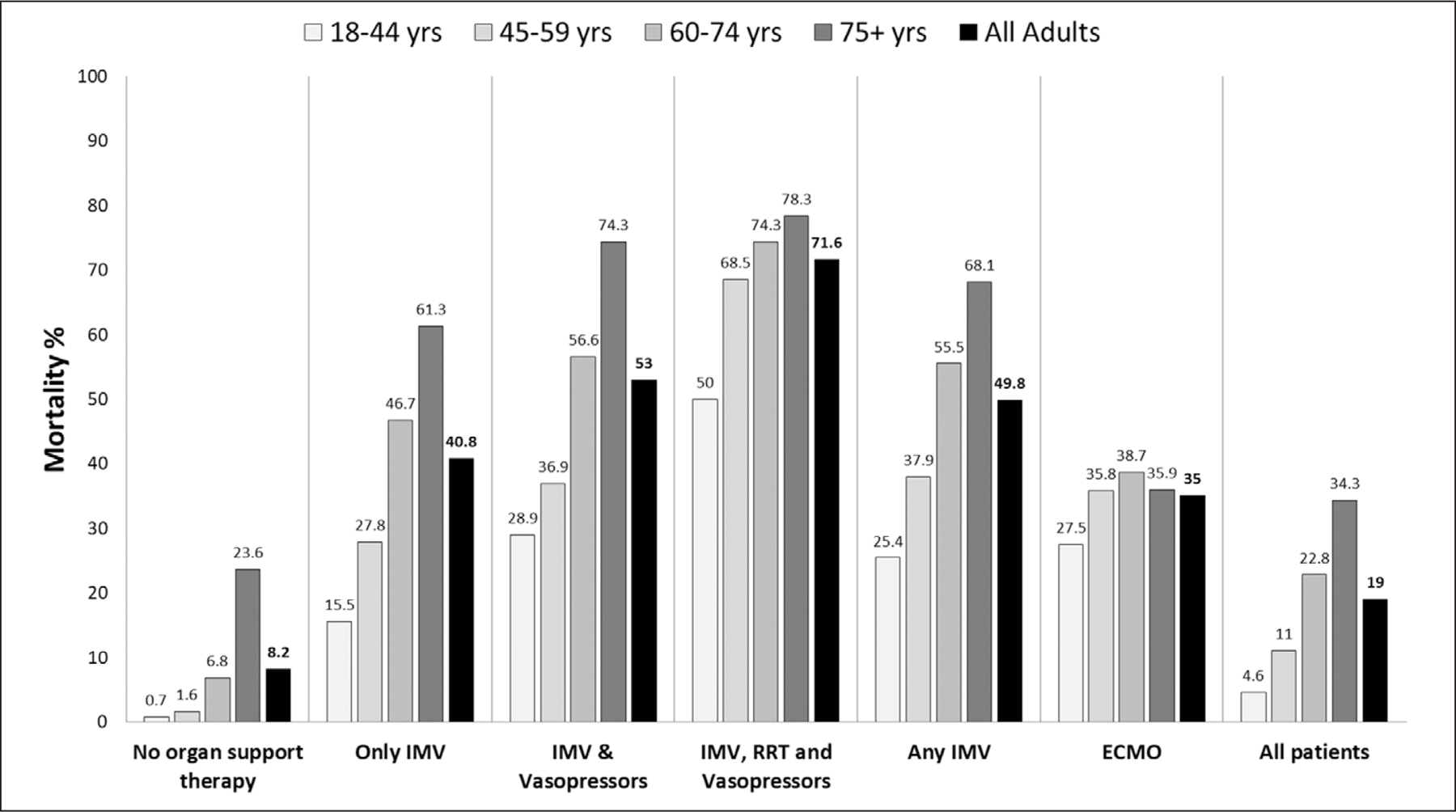
Mortality rate by age and type of organ support therapy. This figure shows the mortality rate by age group from the different invasive organ support therapies received in hospitalized adult patients with coronavirus disease 2019. ECMO = extracorporeal membrane oxygenation, RRT = renal replacement therapy.
Secondary Outcomes
The rate of discharge home (Table 2) from hospitalization with COVID-19 among patients who did not receive organ support therapies was 73.5% (n = 8,900). For patients who received IMV, 24.2% (n = 1,150) were discharged home, with a range of 29.8% (n = 467) for those who only received IMV to 10% (95% CI, 8–14) for those who received the combination of IMV, vasoactive drugs, and RRT, and 41.2% (n = 152) for those who received ECMO. There was a very small number of patients (n = 602) who received vasoactive drugs and/or renal replacement without IMV.
TABLE 2.
Discharged Home Rate by Age Category
| Organ Support Therapy Provideda | All Adults (20,608) | 18–44 yr (3,986) | 45–59 yr (5,300) | 60–74 yr (6,491) | 75+ yr (4,831) |
|---|---|---|---|---|---|
| No organ support required, % | 73.5 (8,900/12,108) | 93.2 (2,413/2,590) | 88.9 (2,728/3,067) | 73.1 (2,544/3,481) | 40.9 (1,215/2,970) |
| IMV only, % | 29.8 (467/1,567) | 60.3 (120/199) | 39.7 (159/401) | 22.3 (139/624) | 14.3 (49/343) |
| IMV and vasopressors (no RRT), % | 22.2 (445/2,007) | 47.5 (103/217) | 34.9 (175/502) | 16.5 (132/799) | 7.2 (35/489) |
| IMV, vasopressors, and RRT, % | 8.8 (57/646) | 27.1 (13/48) | 11.6 (19/164) | 6.4 (21/330) | 3.8 (4/104) |
| Any IMV, % | 24.2 (1,150/4,745) | 50.2 (283/564) | 33.3 (416/1,250) | 17.8 (342/1,925) | 10.8 (109/1,006) |
| Any extracorporeal membrane oxygenation, % | 41.2 (152/369) | 48.1 (37/77) | 36 (54/150) | 40.8 (42/103) | 48.7 (19/39) |
| All patients, % | 59.1 (10,264/17,358) | 85.3 (2,733/3,205) | 72.6 (3,204/4,416) | 53 (2,973/5,611) | 32.8 (1,354/4,126) |
| Disposition not reported | 15.8 (3,250/20,608) | 19.6 (781/3,986) | 16.7 (884/5,300) | 13.6 (880/6,491) | 14.6 (705/4,831) |
IMV = invasive mechanical ventilation, RRT = renal replacement therapy.
Categories are not mutually exclusive.
The median ICU LOS was 7 days (IQR, 4–14 d). The median hospital LOS (Supplemental Table 5, http://links.lww.com/CCM/G132) (IQR) ranged from 6 days (3–10 d) among patients who did not receive organ supportive therapies to 21.4 days (9.6–38 d) among patients receiving ECMO. The median duration of IMV was 8.8 days (IQR,3.3–17 d). The overall hospital LOS for patients on IMV was 17.1 days (IQR, 9.7–28 d).
Mortality Variation of Mechanically Ventilated Patients Across Hospitals
Among the 5,005 patients on IMV (Supplemental Table 6, http://links.lww.com/CCM/G133), we examined the variation in hospital mortality for 4,749 patients receiving IMV across 84 hospitals enrolling more than 10 patients requiring IMV, in 12 countries. Risk-adjusted hospital mortality rates for invasive mechanically ventilated patients ranged from 27.7% to 77.9% (Fig. 3), with an MOR of 1.69 for the adjusted effect of a higher versus lower mortality hospital of admission. An ICC of 8.5% was observed, showing that approximately 10% of the variation in mortality was explained by the hospitals of admission. A post hoc exploratory analysis evaluated effects of severity of acute illness (baseline SOFA Q1 vs Q4: OR, 0.74; 95% CI, 0.54–1.0), and hospital case volume of mechanically ventilated patients with COVID-19 (OR, 0.876; 95% CI, 0.763–1.006) on risk-adjusted hospital mortality among patients requiring mechanical ventilation did not substantively alter results (model ICC, 10%; MOR, 1.69). The effects of country (non United States vs United States; OR, 1.99; 95% CI, 1.26–3.14) was a likely minor contributor to mortality variation, with the United States having lower mortality than non-United States countries, though hospitals remained major contributors to variation in mortality after adjusting for country.
Figure 3.
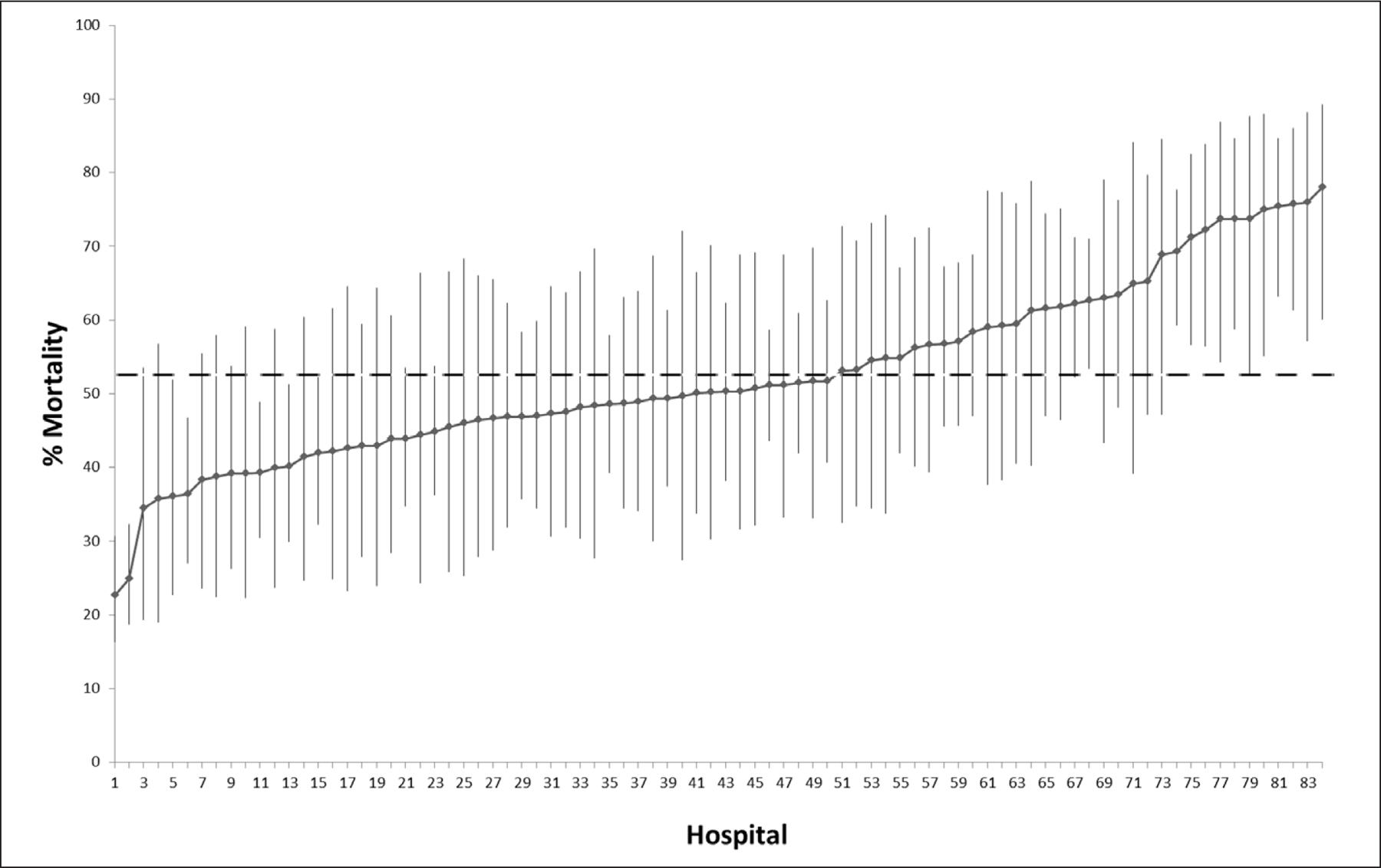
Mortality variation across hospitals adjusted by age, race/ethnicity, sex, and comorbidities. Risk-adjusted hospital mortality rates by comorbidities and demographics for patients on invasive mechanical ventilation from hospitals (n = 84) entering n > 10 patients into the Viral Infection and Respiratory Illness Universal Study (VIRUS) registry. Dotted line indicates the averaged adjusted mortality rate (52.6%) for patients invasive mechanical ventilation from hospitals entering n > 10 patients into the VIRUS registry.
DISCUSSION
We report hospital mortality, discharge home rates, and LOSs for specific organ supportive therapies within a large, multinational registry of patients admitted with laboratory confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Our findings provide novel prognostic estimates for important patient-centered outcomes of survival and probability of discharge home across a wide range of ages and types of organ supportive therapies commonly required for patients with severe COVID-19. Additionally, information presented regarding hospital LOS can assist with patient and hospital planning. Importantly, we provide data that demonstrate wide, unexplained variation in case-mix-adjusted hospital mortality rates of mechanically ventilated patients, presenting an opportunity for future studies to learn from practices at hospitals that achieved low adjusted mortality rates.
Few studies have evaluated COVID-19 outcomes across multiple centers and international hospital settings (17–19). Mortality has varied widely in reports of critically ill patients from 10 European countries (24%) (20), the United States (35.4%) (21), Italy (53.4%) (7), and China (53.8%) (22), but those reports were not stratified by types of organ support. Thus, few prior studies have evaluated outcomes among patients with COVID-19 according to a major prognostic factor: the organ support therapies required by patients (21, 23–25). The mortality rates found in our cohort for patients receiving IMV were similar to that of the control group reported in the meta-analysis of seven randomized trials that evaluated the benefits of corticosteroids on patients with COVID-19 (26); thus, our results also provide information regarding the external validity of clinical trial results to nontrial, routine care settings.
An observational study (27) of hospital claims data of 11,721 hospitalized patients with COVID-19 in the United States with a similar mean age and comorbidities to our cohort reported a hospital mortality rate slightly higher than our overall mortality, but a substantially higher mortality rate for IMV of 70.5%. One potential reason for these differences in outcomes is that our registry enrolled countries other than the United States, some with previously reported lower mortality (28), as well as the possibility that less strained hospitals may have participated in a voluntary registry. The mortality of patients with COVID-19 receiving IMV from our cohort (47%) was similar to the mortality of patients with severe non-COVID-19 Acute Respiratory Distress Syndrome from the Large observational study to understand the Global impact of Severe Acute respiratory Failure (LUNG SAFE) observational study (46%) (29).
We identified a large interhospital variation in mortality for patients receiving IMV, unaccounted for by hospital case-mix, hospital case volume, or patient acute illness severity. Qian et al (30) using data from 5,062 patients with COVID-19 admitted in the ICU—regardless of mechanical ventilation status—found an Acute Physiology and Chronic Health Evaluation II score-adjusted large interhospital variation from hospitals in the England Surveillance System. In the context of a survey (31) of 1,132 ICU specialists showing significant variation in opinions regarding practices for patients with COVID-19, large interhospital variation in outcomes among invasive mechanically ventilated patients provides strong motivation for further studies evaluating links between hospital practice variation—as well as evaluation of ICUs strain—and outcomes.
Strengths of the study include the large, international cohort of hospitalized patients with laboratory confirmed SARS-CoV-2 infection in which complete follow-up of and standardized definitions with data quality monitoring increase data validity and avoid misrepresentation of outcome estimates. Our study includes patients admitted on or before November 30, 2020 with complete discharge status, making our cohort one of the large scale studies with most recent multinational patient data at the time of publication. Another major strength of our cohort is that it captures hospitalized patients on the wards/floors and the ICU, allowing capture of patient outcomes regardless of location within the hospital, especially as hospitals often required expansion of critical care outside of traditional ICUs and ICU admission criteria vary greatly across health systems (32). Our study focused not only on organ support therapies but also on the rates of discharge to home and length of hospital stay, which based on our clinical practice are very important patient centric outcomes for patients and their relatives during their hospitalization; this will help clinicians, patients, and family members to establish data-based expectations and communication strategies during a COVID-19-related hospital admission.
The study’s results should be considered in the context of its limitations. Missing data, local differences in local hospital resources, and capabilities potentially affected the ability to use all registry patients in analyses and may bias analyses if missing data are correlated with outcomes. It is possible that patient factors such as preferences for life-sustaining treatments, and health system factors such staffing, ICU type, and resource constraints related to the surge in pandemics may influence variation in mortality across hospitals; future studies should explore further potential reasons for variation in hospital mortality (33). In the current analyses, there was no accounting for care preferences (comfort measures and do not intubate/do not resuscitate) and will be explored in the subsequent iterations of the registry through ancillary studies. Finally, we could not address specific differences in environment and resources that may have affected mortality estimates.
CONCLUSIONS
We provide novel and clinically applicable patient outcome data based on the type of organ supportive therapies within a large multinational registry. Our findings may be used to assist in prognostication of patients hospitalized with COVID-19; high interhospital mortality variation in patients receiving IMV requires further evaluation that links specific hospital structure and practices to improved outcomes.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ms. Lynn Retford, Ms. Mary Reidy, and Ms. Colleen McNamara for their contributions to the Discovery VIRUS: COVID 19 Registry activities.
Supported, in part, by the Gordon and Betty Moore Foundation, and Janssen Research & Development, LLC.
Drs. Kumar’s, Denson’s, Walkey’s, and Kashyap’s institutions received funding from the Gordon and Betty Moore Foundation. Drs. Kumar’s and Kashyap’s institutions received funding from Janssen Research & Development, LLC. Drs. Kaufman’s and Denson’s institutions received funding from the Society of Critical Care Medicine. Dr. Banner-Goodspeed received partial salary support through her home institution from multiple federal research grants (including from the National Institutes of Health [NIH] and Department of Defense) as key personnel, not the principal investigator. Dr. Anderson III disclosed he is an Advisory Board member for Gift of Life Michigan. Dr. Denson received other support from American Diabetes Association Grant #7-20-COVID-53. Dr. Gajic received support for article research from Gordon and Betty Moore Foundation. Dr. Walkey receives funding from the NIH/National Heart, Lung and Blood Institute grants R01HL151607, R01HL139751, and R01HL136660, Agency of Healthcare Research and Quality, R01HS026485, Boston Biomedical Innovation Center/NIH/NHLBI 5U54HL119145-07, and royalties from UptoDate. They had no influence on acquisition, analysis, interpretation, and reporting of pooled data for this article. Dr. Harhay’s institution receives funding from the NIH/National Heart, Lung and Blood Institute grant R00 HL141678, and he received support for article research from the NIH. They had no influence on acquisition, analysis, interpretation, and reporting of pooled data for this article. Dr. Gajic receives funding from the Agency of Healthcare Research and Quality R18HS 26609-2, NIH/National Heart, Lung and Blood Institute: R01HL 130881 and UG3/UH3HL 141722; Department of Defense W81XWH; American Heart Association Rapid Response Grant—coronavirus disease 2019 (COVID-19); and royalties from Ambient Clinical Analytics. They had no influence on acquisition, analysis, interpretation, and reporting of pooled data for this article. Dr. Kashyap receives funding from the NIH/National Heart, Lung and Blood Institute: R01HL 130881 and UG3/UH3HL 141722; Gordon and Betty Moore Foundation, and Janssen Research & Development, LLC; and royalties from Ambient Clinical Analytics. They had no influence on acquisition, analysis, interpretation, and reporting of pooled data for this article. Dr. St. Hill receives funding from the Minnesota Department of Health. They had no influence on acquisition, analysis, interpretation, and reporting of pooled data for this article. Dr. Denson receives funding for COVID-19 research from the American Diabetes Association grant #7-20-COVID-053, Centers for Disease Control and Prevention BroadAgency Announcement 75D301-20-R-67897, and National Institute of General Medical Sciences/NIH award U54 GM104940, which funds the Louisiana Clinical and Translational Science Center. They had no influence on acquisition, analysis, interpretation, and reporting of pooled data for this article. Dr. Martin receives funding from the Biomedical Advanced Research and Development Authority and NIH through the National Institute of Biomedical Imaging and Bioengineering U54 EB-027690, the National Heart, Lung and Blood Institute: U54 HL-143541-02S2, the National Institute for General Medical Sciences R01 GM-104323, and the Office of the Director OT2 OD-026551; as well as funding from Genentech for clinical trial monitoring. They had no influence on acquisition, analysis, interpretation, and reporting of pooled data for this article. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
For the Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group
Members of the Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study (VIRUS): COVID-19 Registry Investigator Group are listed in the Appendix (http://links.lww.com/CCM/G127).
ClinicalTrials.gov Identifier: NCT04323787.
REFERENCES
- 1.Johns Hopkins University: Coronavirus Resource Center. 2020. Available at: https://coronavirus.jhu.edu/map.html. Accessed January 13, 2020
- 2.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. : Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med 2020; 382:2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gudbjartsson DF, Helgason A, Jonsson H, et al. : Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med 2020; 382:2302–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epidemiology Working Group for NCIP Epidemic Response; Chinese Center for Disease Control Prevention: [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi 2020; 41:145–151 [DOI] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ñamendys-Silva SA, Gutiérrez-Villaseñor A, Romero-González JP: Hospital mortality in mechanically ventilated COVID-19 patients in Mexico. Intensive Care Med 2020; 46: 2086–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G, Greco M, Zanella A, et al. : Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180:1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roedl K, Jarczak D, Thasler L, et al. : Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: A multicentric study in Germany. Aust Crit Care 2020. Oct 27. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auld SC, Caridi-Scheible M, Blum JM, et al. ; Emory COVID-19 Quality and Clinical Research Collaborative: ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med 2020; 48:e799–e804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasselli G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network: Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020; 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walkey AJ, Kumar VK, Harhay MO, et al. : The Viral Infection and Respiratory Illness Universal Study (VIRUS): An International Registry of Coronavirus 2019-related critical illness. Crit Care Explor 2020; 2:e0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization: Global COVID-19 clinical platform: Case report form for suspected cases of multisystem inflammatory syndrome (MIS) in children and adolescents temporally related to COVID-19. 2020. Available at: https://apps.who.int/iris/handle/10665/332121. Accessed June 2, 2020
- 13.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walkey AJ, Sheldrick RC, Kashyap R, et al. : Guiding principles for the conduct of observational critical care research for coronavirus disease 2019 pandemics and beyond: The Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study Registry. Crit Care Med 2020; 48:e1038–e1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallo Marin B, Aghagoli G, Lavine K, et al. : Predictors of COVID-19 severity: A literature review. Rev Med Virol 2020. Sep 9. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merlo J, Chaix B, Ohlsson H, et al. : A brief conceptual tutorial of multilevel analysis in social epidemiology: Using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 2006; 60:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19: Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karagiannidis C, Mostert C, Hentschker C, et al. : Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Respir Med 2020; 8:853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium: Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wendel Garcia PD, Fumeaux T, Guerci P, et al. ; RISC-19-ICU Investigators: Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine 2020; 25:100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S, Hayek SS, Wang W, et al. : Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 2020; 180:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie J, Wu W, Li S, et al. : Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: A retrospective multicenter study. Intensive Care Med 2020; 46:1863–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Contou D, Pajot O, Cally R, et al. : Pulmonary embolism or thrombosis in ARDS COVID-19 patients: A French monocenter retrospective study. PLoS One 2020; 15:e0238413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hua J, Qian C, Luo Z, et al. : Invasive mechanical ventilation in COVID-19 patient management: The experience with 469 patients in Wuhan. Crit Care 2020; 24:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilbers TJ, Koning MV: Renal replacement therapy in critically ill patients with COVID-19: A retrospective study investigating mortality, renal recovery and filter lifetime. J Crit Care 2020; 60:103–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne JAC, Murthy S, Diaz JV, et al. ; WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group: Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA 2020; 324:1330–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fried MW, Crawford JM, Mospan AR, et al. : Patient characteristics and outcomes of 11,721 patients with COVID-19 hospitalized across the United States. Clin Infect Dis 2020. Aug 28. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khafaie MA, Rahim F: Cross-country comparison of case fatality rates of COVID-19/SARS-COV-2. Osong Public Health Res Perspect 2020; 11:74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laffey JG, Bellani G, Pham T, et al. ; LUNG SAFE Investigators and the ESICM Trials Group: Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: The LUNG SAFE study. Intensive Care Med 2016; 42:1865–1876 [DOI] [PubMed] [Google Scholar]
- 30.Qian Z, Alaa AM, van der Schaar M, et al. : Between-centre differences for COVID-19 ICU mortality from early data in England. Intensive Care Med 2020; 46:1779–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azoulay E, de Waele J, Ferrer R, et al. : International variation in the management of severe COVID-19 patients. Crit Care 2020; 24:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seymour CW, Iwashyna TJ, Ehlenbach WJ, et al. : Hospital-level variation in the use of intensive care. Health Serv Res 2012; 47:2060–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabler NB, Ratcliffe SJ, Wagner J, et al. : Mortality among patients admitted to strained intensive care units. Am J Respir Crit Care Med 2013; 188:800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


